Summary
Mastication as we all know has always been related to its primary function of digestion, but little do we know that it produces an enhancing effect on general health, especially the cognitive performance related aspects of memory. Recent studies have shown its association with activation of various brain regions, however little is known about its effects on neuronal activity in these specified regions. According to the enormous evidences collected so far, mastication has proved to be effective in conducting huge amount of sensory information to the brain, and maintaining learning and memory functions of hippocampus. Therefore it is essential that we maintain normal occlusion and preserve the masticatory function as long as possible to prevent the attenuation of hippocampus, caused by occlusal disharmony and reduced mastication. We provide an overview on how mastication activates various cortical areas of the brain and how an increase in the cerebral blood oxygen level of hippocampus and prefrontal cortex (PFC) accentuates the learning and memory process. We also justify why maintaining and establishing a normal occlusion is important from neurological point of view.
Keywords: Mastication, Brain functions, Stress, Occlusion, Occlusal disharmony
1. Introduction
The process which involves crushing and grinding of food by teeth in order to increase the surface area for effective breakdown of food by enzymes is termed as mastication. Though mastication is a semi autonomic activity, the motor program governing it is a central nervous system (CNS) function which involves neural networks in brainstem and several regions of the brain that controls and creates the complex masticatory patterns. After the confirmation of a positive association between mastication and cognitive functions through many animal and human experimental studies, oral health disorders have attracted further attention on its association with cognitive defects [1], [2].
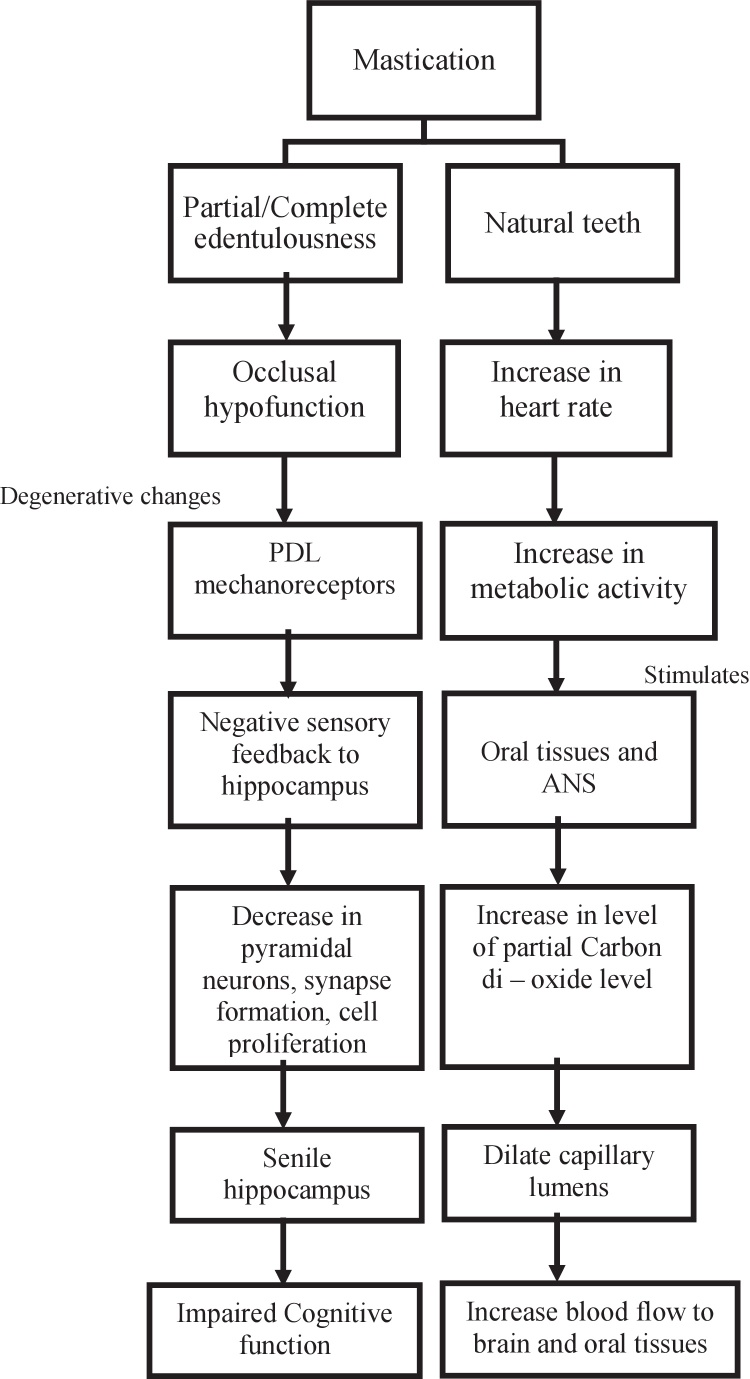
Hollingworth [3] in 1939 demonstrated the relationship between mastication and cognitive functions through electroencephalography and stated that there is an increase in performance of cognitive function during mastication. In 2002, Wilkinson et al. [4] carried out an experimental study between chewing and non-chewing groups, and found an increase in episodic, spatial and numeric working memory in chewing group when compared with that of the non-chewing control group. Numerous such studies have been carried out which associate the reduced number of residual teeth and decreased use of dentures to cognitive deficits [5], [6]. This review provides a critical analysis and a comprehensive evaluation on how mastication induces neuronal activities in various regions of the brain [7], [8] thereby increasing the cerebral blood oxygen level of hippocampus and prefrontal cortex (PFC) which accentuates the learning and memory process, it also provides an insight into masticatory disharmony and its association with cognitive deficits (Fig. 1).
Figure 1.
Relation between mastication and cognitive functions; PDL — periodontal ligament, ANS— Autonomic Nervous System.
2. Search methodology and evaluative criteria
Scopus, PubMed, ScienceDirect, Clinical Key and Google Scholar were used as search engines. The search term “Mastication” was used along with “stress”, “cognition”, “working memory”, and “occlusal disharmony” separately. Articles published from the year 1939 to 2015 were reviewed and cross references were checked for further useful research. In addition to these, only standardized results of cognitive valuation were considered. Self-evaluation measures or any other form of questionnaires were excluded from this review.
3. Pathways that convey masticatory information to hippocampus
Hippocampus is a central nervous system region which is vital for learning, maintaining spatial memory, formation and retrieval of episodic memories in human [9]. Literature has proved that there are multiple neural circuits which connect the masticatory organ to the hippocampus and these neural circuits help in maintaining the cognitive functions in hippocampus during mastication, but precise interaction amongst these areas have not yet been exhibited. On the other hand, it is postulated that there are two likely indirect pathways: neuronal and humoral pathways.
3.1. Neuronal pathway
The nociceptive and proprioceptive sensations that are transmitted through primary sensory somata, which carry the sensory input from the oral cavity to the CNS, are located in the mesencephalic trigeminal nucleus and trigeminal ganglion. The central axons of mesencephalic trigeminal nucleus that are responsible for voluntary mastication, end at trigeminal motor nuclei and also at supra and infra trigeminal regions while those of trigeminal ganglion terminate at spinal and principal sensory nuclei of trigeminal nerve [10] and the trigeminal sensory nuclei. The mesencephalic primary sensory neurons also project their afferent fibers to the brainstem reticular formation [11], [12], which regulates sensory input as RAS (reticular activating system) to the higher brain centers. These reticular formation and RAS together are necessary for stimulation of the brain for conscious learning, perception and attention. Thus the sense of perception of oral cavity may influence memory and learning ability.
3.2. Humoral pathway
Various growth factors like epidermal and nerve growth factor (NGF) which are produced in salivary glands [13], [14], [15] are increased during mastication [16], and occlusal disharmony may alter the levels. Interestingly, the presence of NGF in HPA axis at the level of median eminence has been reported [17] and any alteration in the level of NGF due to malocclusion might alter the negative feedback system of HPA axis in hippocampal region leading to increase in secretion of corticosterone, which will suppress the excitability of neurons and also decrease its number in hippocampus ultimately leading to death of neurons [18].
Momose et al. [19] carried out an experiment to study the effects of mastication on regional cerebral blood flow by positron emission tomography (PET) and discovered that there is an increase in metabolic activity which stimulates autonomic nervous system and the oral tissues. This increase in metabolic activity in turn causes an increase in the partial pressure of carbon dioxide in the cortical sensorimotor neurons which dilate the capillary lumens and results in an upsurge of blood flow to the brain and oral tissues.
After the documented reports of neuroimaging studies [7], [8] carried out using functional magnetic resonance imaging (fMRI) and in humans, which showed multiple regions of the brain becoming activated during mastication, including the primary motor cortex, primary somatosensory cortex, premotor area, supplementary motor area, insula, prefrontal cortex, thalamus, posterior parietal cortex, cerebellum and striatum, it can be said that mastication influences hippocampal functions through various neural pathways.
Thus it can be said that effects of mastication on CNS may be attributed to multiple pathways.
4. Effects of mastication on cognitive functions and human working memory
Dementia is defined as an impairment in memory along with a decreased cognitive functioning which results in impairment of daily activities. Dementia substantially decreases the quality of life of an individual and also has a considerable influence on his mortality and disability.
Luo carried out a study on 3063 Chinese adult population of age more than 60 years to analyze the relationship between missing teeth and cognitive functions. He found out that individuals who had an average of 18.7 teeth missing were diagnosed with dementia, those with a loss of 10.2 teeth on average had mild cognitive impairment and those with an average of 9.2 were cognitive normal [20].
Wilkinson et al. [21] carried out an experiment to study the effects of mastication on the cognitive functions and memory. They formed two groups which consisted of chewers and non-chewers (control group) and presented both the groups to a series of words, which they had to recall immediately and after 24 h. The results showed that there was a substantial improvement in both delayed and immediate recall of formerly learned words in the chewers compared to those of the control group. There was an increase in learning performance of a minimum of 30% that was claimed after mastication
On the contrary, a similar study was carried out by Houcan and Li [22] on 4th and 5th grade elementary school students to study the positive effects of chewing (students were provided with chewing gum) during a comprehension test. The students were subjected to short stories and were asked to learn as much as possible. After 5 min and 24 h they were asked to recall and write down as much they could remember. Data analysis revealed that there was no influence of gum chewing on their performance after 24 h delay trial, but after 5 min delay trial, students subjected to gum chewing could recall and write better than those students without gum chewing.
Folstein et al. [23] carried out an assessment of cognitive functioning using Mini-Mental State Examination. The highest score was set to be 30, and individuals with a score of 25 or higher along with normal cognitive status were included in the study. He categorized these individuals into two groups, complete denture wearers and another group consisted of full complement of natural teeth. The mean Mini-Mental State Examination score for older adults with natural teeth were remarkably higher than that of the complete denture wearers.
Watanabe et al. [24] performed a study on 195 healthy adult individuals who were in age related cognitive decline group to investigate the relationship between number of remaining teeth with that of the gray matter volume in brain. Individuals with reduced number of teeth had decreased volume of gray matter around hippocampus, also the frontal lobe volumes which are associated with thought and violation were reduced.
Several experimental studies performed on rats showed that mastication increased the survival rate of newly formed cells in dentate gyrus of hippocampus. Aoki et al. [25] performed an experimental study on male Wistar rats and found that there was a decrease in the number of dentate gyrus cells of hippocampus in those rats which were subjected to soft diet feeding as compared with that of hard diet feeding rats.
Kato et al. [26] concluded through his experimental study that when all the molar teeth were removed in a group of mice, their ability to learn decreased. He also discovered that Fos protein which is an indicator of neural plasticity was also reduced, in the hippocampal region, in these mice as compared with that of the control group.
Bordji et al. along with Gladding and Raymond [27] elucidated through their animal study that, when multiple teeth are lost, the consequent rewiring, remapping and rebuilding of the motor and sensory information’s to their original and precise neuromuscular pathways becomes difficult. Additionally, constant rebuilding and rewiring of neural pathways, may result in over-detoured or non-ideal connections which can accumulate abnormal levels of Beta-Amyloid, thus causing loss of extrasynaptic and synaptic function. Considerable data to demonstrate the pathway in humans is lacking.
5. Effects of mastication on stress
Stress is a psychological and physiological response to noxious stimuli and environmental changes that activates the autonomic and neuroendocrine system via hypothalamic–pituitary–adrenal axis (HPA) axis, thereby releasing hormones and corticosteroids. Mastication alters the activity of the HPA axis and autonomic nervous system. It decreases the catecholamine and plasma corticosterone level that increase in stressful conditions and also attenuates nitric oxide and neurotrophic factors, which are examples of stress linked substances.
Therefore, mastication can be considered as a stress coping behavior that can reduce stress induced diseases.
6. Effects of occlusal disharmony
Occlusion and brain must coordinate to efficaciously carry out the important functions of mastication and stress management. There have been reports of suppressed memory ability and learning caused by abnormal sensory input in case of reduced mastication due to occlusal disharmony.
In an experiment carried out by Iinuma et al. a significant reduction in c-Fos expression associated with learning was noticed due to occlusal disharmony [28]. This occlusal disharmony represented chronic stress, and the longer it continued, the worse became their ability to learn. Experiments carried out on monkeys and rodents by placing occlusal splints on maxillary arch, fabricating acrylic caps on incisors or by applying adhesives to molar teeth increased cortisol level in urine and plasma corticosterone level as a response to acute stress caused by occlusal disharmony.
Further Budtz-Jorgensen [29] examined that when occlusal disharmony was corrected, the raised cortisol and corticosterone levels returned to basal values, which suggested that occlusal disturbances activated the HPA axis that increased the corticosterone level thereby suppressing the c-Fos expression associated with learning ability in hippocampal region.
Multiple experiments were carried out on mice using Morris Water Maze test to study the effects of occlusal disharmony on brain function. These studies reported a radical decrease in the density of dendritic spines and pyramidal neurons along with an increase in astrocytes and hypertrophy of hippocampal CA1 field in mice. Further, these alterations in the morphology of cells, were correlated with spatial memory impairment.
Sakatani et al. [30] used a splint to displace the mandible and studied the effects of artificial occlusal disharmony (AOD) on PFC activity and working memory function on elderly population. They used the modified Sternberg test to evaluate the working memory function and NIRS (near infrared spectroscopy) to measure the bilateral PFC activity during AOD. There was a steady increase in oxyhemoglobin (oxy-Hb) level in bilateral PFC also the increase in oxy-Hb after AOD was smaller which validate the decrease in working memory function and PFC activity in elderly population due to AOD.
Thus to quantify, mastication preserves the cognitive functions of memory and learning when there is normal occlusion, but with occlusal disharmony it may adversely impair those functions.
7. Conclusion
In conclusion, mastication maintains the peripheral sensory input along with general health and increases blood supply in different brain regions rendering physiological benefits to CNS cognitive areas. To additionally elucidate the neural involvement of other brain regions with masticatory function, further studies correlating masticatory functions and hippocampal involvement need to be done. Considering the ameliorative effect of mastication on cognitive functions, it may be negligible in the young population who have a favorable functioning hippocampus, but it becomes apparent in the hippocampus whose performance is decreased due to age or stress. Therefore it is essential that we maintain normal occlusion and preserve the masticatory function as long as possible to prevent the attenuation of hippocampus caused by occlusal disharmony and reduced mastication.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
The authors declare that they have contributed significantly to preparation of the manuscript and that all authors are in agreement with the content of the manuscript.
Role of funding source
Nil.
Footnotes
Scientific field of dental science: Prosthodontics and Occlusion.
References
- 1.Reyes-Ortiz C.A., Luque J.S., Eriksson C.K., Soto L. Self-reported tooth loss and cognitive function: data from the Hispanic Established Populations for Epidemiologic Studies of the Elderly (Hispanic EPESE) Colomb Medica. 2013;44(3):139–145. [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart R., Hirani V. Dental health and cognitive impairment in an English national survey population. J Am Geriatr Soc. 2007;55(9):1410–1414. doi: 10.1111/j.1532-5415.2007.01298.x. [DOI] [PubMed] [Google Scholar]
- 3.Hollingworth H.L. Chewing as a technique of relaxation. Science. 1939;90:385–387. doi: 10.1126/science.90.2339.385. [DOI] [PubMed] [Google Scholar]
- 4.Wilkinson L., Scholey A., Wesnes K. Chewing gum selectively improves aspects of memory in healthy volunteers. Appetite. 2002;38(3):235–236. doi: 10.1006/appe.2002.0473. [DOI] [PubMed] [Google Scholar]
- 5.Nakata M. Masticatory function and its effects on general health. Int Dent J. 1998;48:540–548. doi: 10.1111/j.1875-595x.1998.tb00489.x. [DOI] [PubMed] [Google Scholar]
- 6.Stein P.S., Desrosiers M., Donegan S.J., Yepes J.F., Kryscio R.J. Tooth loss, dementia and neuropathology in the Nun study. J Am Dent Assoc. 2007;138:1314–1322. doi: 10.14219/jada.archive.2007.0046. [DOI] [PubMed] [Google Scholar]
- 7.Onozuka M., Fujita M., Watanabe K., Hirano Y., Niwa M., Nishiyama K. Mapping brain region activity during chewing: a functional magnetic resonance imaging study. J Dent Res. 2002;81:743–746. doi: 10.1177/0810743. [DOI] [PubMed] [Google Scholar]
- 8.Onozuka M., Fujita M., Watanabe K., Hirano Y., Niwa M., Nishiyama K. Age-related changes in brain regional activity during chewing: a functional magnetic resonance imaging study. J Dent Res. 2003;82:657–660. doi: 10.1177/154405910308200817. [DOI] [PubMed] [Google Scholar]
- 9.Henke K. A model for memory systems based on processing modes rather than consciousness. Nat Rev Neurosci. 2010;11:523–532. doi: 10.1038/nrn2850. [DOI] [PubMed] [Google Scholar]
- 10.Takemura M., Sugiyo S., Moritani M., Kobayashi M., Yonehara N. Mechanisms of orofacial pain control in the central nervous system. Arch Histol Cytol. 2006;69:79–100. doi: 10.1679/aohc.69.79. [DOI] [PubMed] [Google Scholar]
- 11.Lazarov N.E. Comparative analysis of the chemical neuroanatomy of the mammalian trigeminal ganglion and mesencephalic trigeminal nucleus. Prog Neurobiol. 2002;66:19–59. doi: 10.1016/s0301-0082(01)00021-1. [DOI] [PubMed] [Google Scholar]
- 12.Capra N.F., Dessem D. Central connections of trigeminal primary afferent neurons: topographical and functional considerations. Crit Rev Oral Biol Med. 1992;4:1–52. doi: 10.1177/10454411920040010101. [DOI] [PubMed] [Google Scholar]
- 13.Gresik E., Barka T. Immunocytochemical localization of epidermal growth factor in mouse submandibular gland. J Histochem Cytochem. 1997;25:1027–1035. doi: 10.1177/25.9.333017. [DOI] [PubMed] [Google Scholar]
- 14.Bothwell M.A., Wilson W.H., Shooter E.M. The relationship between glandular kallikrein and growth factor-processing proteases of mouse submaxilliary gland. J Biol Chem. 1979;254:7287–7294. [PubMed] [Google Scholar]
- 15.Frederickson C.J., Perez-Clausell J., Danscher G. Zinc-containing 7s-NGF complex, evidence from zinc histochemistry for localization in salivary secretory granules. J Histochem Cytochem. 1987;35:579–583. doi: 10.1177/35.5.2435783. [DOI] [PubMed] [Google Scholar]
- 16.Bardow A., Pedersen A.M.L., Miles T.S., Svensson P., Nautofte B. Clinical oral physiology. Copenhagen Quintessence; 2004. Saliva; pp. 17–51. [Google Scholar]
- 17.Yan Q., Johnson E.M., Jr. Immuno histochemical localization and biochemical characterization of nerve growth factor receptor in adult rat brain. J Comp Neurol. 1989;290:585–598. doi: 10.1002/cne.902900411. [DOI] [PubMed] [Google Scholar]
- 18.Meaney M.J., Lupian S. Hippocampus, overview. In: Fink G., editor. Encyclopedia of stress. Academic Press; California: 2000. pp. 379–386. [Google Scholar]
- 19.Momose T., Nishikawa J., Watanabe T., Sasaki Y., Senda M., Kubota K. Effect of mastication on regional cerebral blood flow in humans examined by positron-emission tomography with 15O-labelled water and magnetic resonance imaging. Arch Oral Biol. 1997;42:57–61. doi: 10.1016/s0003-9969(96)00081-7. [DOI] [PubMed] [Google Scholar]
- 20.Luo J., Wu B., Zhao Q., Guo Q., Meng H., Yu L. Association between tooth loss and cognitive function among 3063 Chinese older adults: a community-based study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkinson L., Scholey A., Wesnes K. Chewing gum selectively improves aspects of memory in healthy volunteers. Appetite. 2002;38(3):235–236. doi: 10.1006/appe.2002.0473. [DOI] [PubMed] [Google Scholar]
- 22.Houcan Z., Li W. Effects of chewing gum on learning and memory. China J Health Psychol. 2007;15:518–520. [Google Scholar]
- 23.Folstein M.F., Folstein S.E., Mchugh P.R. Mini-mental state—practical method for grading cognitive state of patients for clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe M., Tsuboi A., Ohi T. Dental treatment and oral care for patients with dementia. Dement Jpn. 2008;22:269–278. [Google Scholar]
- 25.Aoki H., Kimoto K., Hori N., Toyoda M. Cell proliferation in the dentate gyrus of rat hippocampus is inhibited by soft diet feeding. Gerontology. 2005;51:369–374. doi: 10.1159/000088700. [DOI] [PubMed] [Google Scholar]
- 26.Kato T., Usami T., Noda Y., Hasegawa M., Ueda M., Nabeshima T. The effect of the loss of molar teeth on spatial memory and acetylcholine release from the parietal cortex in aged rats. Behav Brain Res. 1997;83:239–242. doi: 10.1016/s0166-4328(97)86078-0. [DOI] [PubMed] [Google Scholar]
- 27.Gladding C.M., Raymond L.A. Mechanisms underlying NMDA receptor synaptic/extrasynaptic distribution and function. Mol Cell Neurosci. 2011;48:308–320. doi: 10.1016/j.mcn.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Iinuma M., Ichihashi Y., Hioki Y., Kurata C., Tamura Y., Kubo K.Y. Malocclusion induces chronic stress. Okajimas Folia Anat Jpn. 2008;85:35–42. doi: 10.2535/ofaj.85.35. [DOI] [PubMed] [Google Scholar]
- 29.Budtz-Jørgensen E. Occlusal dysfunction and stress an experimental study in macaque monkeys. J Oral Rehabil. 1981;8:1–9. doi: 10.1111/j.1365-2842.1981.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 30.Sakatani K., Tsujii T., Hirayama T., Katayama Y., Takeda T., Amemiya A. Effects of occlusal disharmony on working memory performance and prefrontal cortex activity induced by working memory tasks measured by NIRS. Adv Exp Med Biol. 2013;765:239–244. doi: 10.1007/978-1-4614-4989-8_33. [DOI] [PubMed] [Google Scholar]