Summary
In recent decades, the eating habits of children and adolescents have undergone many changes due to the diversification of lifestyles worldwide. Reduced masticatory function in growing animals results in changes in the mandible, including a decrease in bone mass. However, the influence of different eating behaviors on jaw bone metabolism (e.g., the palatal palate) during the growth period is not fully understood. In addition, recent clinical studies reported that masticatory performance is positively related to tongue pressure in adults, but no consensus has been reached regarding whether tongue pressure is related to masticatory performance in children. This review summarizes current findings related to these issues, focusing on the influence of different feeding behaviors on jaw bone metabolism, including the development of tongue pressure. Consumption of a soft diet had a negative impact on jaw bone metabolism in the maxilla and mandible of rats; however, mastication of a hard diet recovered the collapsed equilibrium of bone turnover caused by a soft diet during growth. Tongue pressure is closely associated with an increase in masticatory performance in children. Peak maximum tongue pressure is reached earlier in women than in men. Before reaching adulthood, women require intervention to increase their peak tongue pressure.
Keywords: Soft diet, Masticatory performance, Jaw bone, Mid palatal suture, Tongue pressure
1. Introduction
Masticatory performance increases during childhood and adolescence, peaks in young adulthood, plateaus, and finally declines. Therefore, to inhibit any decrease in masticatory performance, it is important to attain as high a level of masticatory performance as possible during the growth period. However, in recent decades, the eating habits of children and adolescents have undergone many changes due to the diversification of lifestyles worldwide [1], [2], [3], [4]. A previous study reported that masticatory frequencies and eating times have decreased due to the appearance of soft modern foods, including processed foods, which can be swallowed and digested quickly [2]. Clinicians are concerned that the decrease in masticatory force due to the increased consumption of soft (primarily processed) foods could affect jaw bone growth, resulting in malocclusion [5], [6]. Reduced masticatory performance results in smaller mandibles, lower bone mass, and thinner condylar cartilage in growing animals [7], [8], [9], [10]. However, the influence of a soft diet on jaw bone metabolism, including the palatal palate, during growth is not fully understood.
Body size [11], [12], tooth number [13], [14], [15], and tongue movement [16] are positively related to masticatory performance. There is a relationship between masticatory performance and tongue pressure in adults, and the tongue plays an important role in mastication [17], [18]. However, no consensus has been reached regarding whether the development of tongue pressure is related to an increase in masticatory performance in children.
In this review, we summarize the influence of different feeding behaviors on jaw bone metabolism and the relationship between masticatory performance and the development of tongue pressure based on human and animal studies.
2. Impact of eating habits on masticatory performance
Food choice is generally influenced not only by the preferences of individuals and the characteristics and availability of food but also by social factors, including financial status, and oral characteristics such as tooth loss or pain [19]. In Japan, the National Health and Nutrition Survey reported that 80% of men and women in their twenties regard ‘preference’ as an important factor when selecting a food [20]. In modern life, eating while watching television is common among children. There is reportedly a relationship between television viewing and weight gain [21], [22], and this may increase the quantity of food, and thus the number of calories, consumed [19]. Moreover, television viewing is associated with increased fat and sugar consumption [23]. Also, insufficient chewing and television viewing for more than 2 h per day were strongly correlated with the incidence of underweight or obesity in children [24].
In clinical studies, poorer masticatory performance was associated with a higher body mass index (BMI) in 3–5-year-old children [25], [26], and poor masticatory performance was associated with a greater frequency of daily ingestion of liquid foods among children with a high BMI [26].
Ichikawa et al. [27] developed a self-administered questionnaire related to the preference and hardness of 25 foodstuffs with different viscosity and brittleness values to measure the subjective masticatory ability (SMA) of 6–12-year-old children. The examiner described the foods, and the subjects were asked to assign each food item to one of five categories (i.e., dislike or have never eaten, hard, slightly hard, slightly soft, and soft). To calculate the SMA score, mastication ability was characterized using a 4-point Likert scale as follows: soft (4 points), slightly soft (3 points), slightly hard (2 points), hard (1 points), and dislike or have never eaten (0 points) [28]. The SMA score was significantly correlated with objective measurements of mastication of jelly-based chewable materials (Kamuzokun®, Mamarisshimo Ltd, Tokyo, Japan). These chewable samples had dimensions of 15 mm × 15 mm × 15 mm, and consisted of maltitol, gelatin, powdery wafer, sweetener (xylitol) and thickener (Arabian gum). The ability of individuals to chew hard foodstuffs, and the frequency with which they chewed such foodstuffs, in their daily life directly affected masticatory performance. These findings suggest that inappropriate eating habits affect body composition and masticatory performance in children.
3. Impact of different feeding behaviors on jaw bone growth
A soft diet has been used to induce growth retardation of the mandible in animal studies [7], [8], [10], [29], [30], [31]. A 14-week soft diet affected the height of the mandibular ramus by suppressing condylar cartilage growth in 3-week-old rats [7]. Additionally, a 6-week powdered diet and kneaded diet significantly suppressed the vertical growth of the mandibular ramus [29]; also, a 6-week powdered diet significantly suppressed the vertical growth of the coronoid process in 3-week-old rats. Furthermore, a 4-week powdered diet suppressed growth in the posterior direction in the corpus, growth in the superior direction in the ramus, growth in the buccal direction in the gonial angle, and growth in the buccal direction in the zygomatic arch (insertion points of the masseter muscles) in 3-week-old rats [30]. Generally, jaw muscle activity is required during chewing to induce movement of the lower jaw and exert the forces required to cut or grind food [32]. Kiliaridis et al. suggested that vertical jaw relationships are affected by muscular activity during development [33]. Whether masticatory effort influences the size of the dental arches and the amount of space for the teeth is unclear. However, in our study, an 8-week powdered diet significantly suppressed growth in the buccal direction in the gonial angle and in the buccal direction in alveolar bone in 3-week-old rats [31]. These data suggest that an 8-week powdered diet extended the range of inhibition of bone growth from the insertions of the masseter muscles to the dental arch in the mandible of growing rats. However, the growth retardation in the superior direction in the ramus, in the buccal direction in the gonial angle, and in the buccal direction in the zygomatic improved when rats were switched from a 4-week soft diet to a 4-week hard diet [31].
In humans, Proffit et al. [34] suggested that dietary consistency affects dental arch dimensions, and they questioned whether a preadolescent child’s masticatory effort plays a major role in determining dental arch dimensions. The precise relationship, however, remains unknown.
4. Impact of differences in feeding behavior on jaw bone metabolism from animal studies
Current knowledge supports the idea that a decrease in muscular activity caused by a soft diet leads to decreased mandibular bone mass in growing animals [7], [9], [10], [29], [30], [31], [35]. Additionally, a significant decrease in bone mineral density in the mandibular condyle was shown in growing rats fed a soft diet for 20 days compared with rats fed a hard diet for 20 days [36]. However, another study reported that purpose-made soft pellets had little effect on the degree of cortical bone mineralization in the mandibles of rabbits when compared with standard pellets [37]. Also, a 6-week powdered diet resulted in significantly reduced bone mineral contents in the coronoid process and angle of the mandible in 3-week-old rats, but not in rats fed a kneaded diet, compared with rats fed a hard diet [29]. These data suggest that differences in feeding behavior (e.g., chewing or sucking), rather than differences in diet hardness, are related to changes in mandibular bone mass.
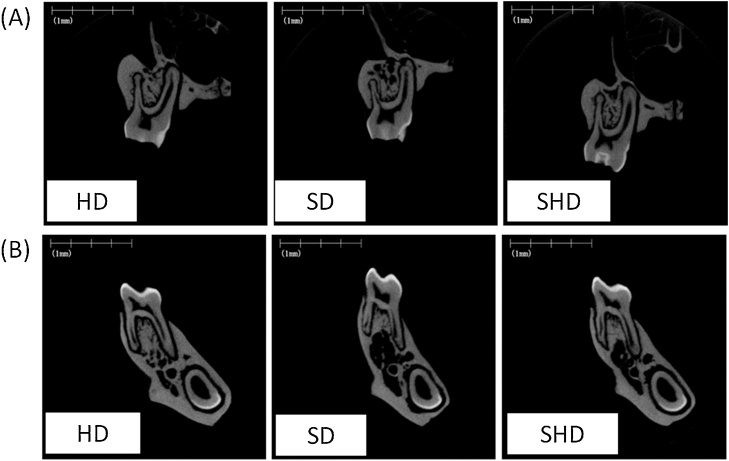
Few studies have evaluated the effects of a soft diet on the maxilla. Recently, an animal study using micro-computed tomography (micro-CT) reported that a 4-week powdered diet significantly affected the average cortical thickness (Ct.Th) and cortical bone area fraction (Ct.Ar/Tt.Ar), as well as the trabecular bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular separation (Tb.Sp), in the maxilla of 3-week-old rats [30]. In addition, reductions in Ct.Ar/Tt.Ar and Ct.Th and a deterioration in Tb.Th in the maxilla and mandible were observed in rats fed a powdered diet for 8 weeks compared with rats fed a hard diet for 8 weeks (Table 1, Table 2; Fig. 1A and B). Bone histomorphometric analyses showed that the 8-week powdered diet accelerated the formation of osteoid surface/bone surface (OS/BS) on the periosteal surface of the maxilla and mandible of 3-week-old rats, suggesting that the soft diet delayed bone formation by inhibiting cortical bone mineralization (Table 3, Table 4). However, 4 weeks after a return to the hard diet from the 4-week powdered diet, the reductions in Ct.Ar/Tt.Ar in the maxilla recovered, as did the increases in the OS/BS on the periosteal surface and bone resorption on the endosteal surface (Table 1, Table 3; Fig. 1A). In contrast to the maxilla, 4 weeks after the return to a hard diet from the 4-week powdered diet, the reductions in Ct.Th and Ct.Ar/Tt.Ar in the mandible had not recovered (Table 2; Fig. 1B), suggesting that mastication of the hard diet had a greater effect on the cortical bone in the maxilla than in the mandible. In the trabecular bone, an increase in bone resorption due to an increased number of osteoclasts/bone surface (N.Oc/BS) in the maxilla and mandible was found in rats fed a powdered diet for 8 weeks compared with rats fed a hard diet for 8 weeks (Table 3, Table 4). However, the deterioration in trabecular bone structure in the maxilla and mandible recovered 4 weeks after a return to the hard diet from the 4-week powdered diet; the increase in bone resorption also improved (Table 1, Table 2; Fig. 1A and B). Moreover, 4 weeks after the return to a hard diet from the 4-week powdered diet, we observed a significant increase in mineralizing surface/bone surface (MS/BS) in the maxilla and a tendency toward an increase in MS/BS in the mandible, to above-normal levels (Table 3, Table 4) [31]. We believe that these processes contributed to the rapid compensation for the loss of bone mass caused by the soft diet. Thus, these data suggest that mastication of the hard diet recovered the collapsed equilibrium of jaw bone turnover caused by a soft diet during growth.
Table 1.
micro-CT analysis of cortical and trabecular bones in the maxilla.
| Cortical bone |
Trabecular bone |
|||
|---|---|---|---|---|
| Ct.Th (μm) | Ct.Ar/Tt.Ar (%) | BV/TV (%) | Tb.Th (μm) | |
| Groups | ||||
| HD | 626.49 ± 11.94 | 45.39 ± 3.22 | 22.15 ± 4.47 | 177.46 ± 8.88 |
| SD | 414.99 ± 20.26* | 38.81 ± 2.83* | 23.48 ± 3.35 | 141.25 ± 24.14* |
| SHD | 531.68 ± 39.98*, † | 44.04 ± 2.28† | 23.31 ± 1.75 | 167.31 ± 35.22 |
HD, hard diet. SD, powdered (soft) diet. SHD, switch from soft to hard diets. Ct.Th, average cortical thickness. Ct.Ar/Tt.Ar, cortical bone area fraction. BV/TV, trabecular bone volume fraction. Tb.Th, trabecular thickness.
P < 0.05 vs HD group (ANOVA and Tukey test).
P < 0.05 vs SD group (ANOVA and Tukey test).
Table 2.
Micro-CT analysis of cortical and trabecular bones in the mandible.
| Cortical bone |
Trabecular bone |
|||
|---|---|---|---|---|
| Ct.Th (μm) | Ct.Ar/Tt.Ar (%) | BV/TV (%) | Tb.Th (μm) | |
| Groups | ||||
| HD | 437.26 ± 21.08 | 36.36 ± 1.11 | 27.19 ± 3.99 | 151.00 ± 15.98 |
| SD | 318.77 ± 17.55* | 29.93 ± 0.97* | 21.24 ± 2.68* | 128.59 ± 8.71* |
| SHD | 363.81 ± 19.85*, † | 32.77 ± 1.75*, † | 24.02 ± 4.01 | 141.20 ± 19.67 |
Data are mean ± standard deviations. HD, hard diet. SD, powdered (soft) diet. SHD, switch from soft to hard diets. Ct.Th, average cortical thickness. Ct.Ar/Tt.Ar, cortical bone area fraction. BV/TV, trabecular bone volume fraction. Tb.Th, trabecular thickness.
P < 0.05 vs HD group (ANOVA and Tukey test).
P < 0.05 vs SD group (ANOVA and Tukey test).
Figure 1.
Representative micro-CT images of frontal cross-section of the first molar region in the maxilla (A) and mandible (B).
HD, hard diet. SD, powdered (soft) diet. SHD, switch from soft to hard diets.
Table 3.
Histomorphometric analysis of cortical and trabecular bones in maxilla.
| Cortical bone |
Trabecular bone |
|||
|---|---|---|---|---|
| OS/BS (%) | MS/BS (%) | MS/BS (%) | N.Oc/BS (N/100 mm) |
|
| Groups | ||||
| HD | 24.57 ± 6.06 | 41.16 ± 4.90 | 9.78 ± 3.37 | 0.00 ± 0.00 |
| SD | 67.47 ± 14.61* | 40.86 ± 5.68 | 11.29 ± 3.72 | 44.02 ± 12.76 |
| SHD | 35.68 ± 9.99 | 39.88 ± 11.86 | 13.79 ± 0.83* | 0.00 ± 0.00 |
Data are mean ± standard deviations. HD, hard diet. SD, powdered (soft) diet. SHD, switch from soft to hard diets. BS, bone surface. OS, osteoid surface. MS, mineralizing surface. N.Oc, number of osteoclasts.
P < 0.05 vs HD group (ANOVA and Tukey test).
Table 4.
Histomorphometric analysis of cortical and trabecular bones in mandible.
| Cortical bone |
Trabecular bone |
|||
|---|---|---|---|---|
| OS/BS (%) | MS/BS (%) | MS/BS (%) | N.Oc/BS (N/100 mm) |
|
| Groups | ||||
| HD | 27.39 ± 8.15 | 46.65 ± 5.20 | 10.91 ± 4.10 | 27.36 ± 10.93 |
| SD | 55.20 ± 10.89* | 48.39 ± 8.49 | 12.42 ± 2.81 | 55.85 ± 34.95 |
| SHD | 43.29 ± 5.69* | 51.87 ± 1.49 | 13.07 ± 6.72 | 46.39 ± 11.14 |
Data are mean ± standard deviations. HD, hard diet. SD, powdered (soft) diet. SHD, switch from soft to hard diets. BS, bone surface. OS, osteoid surface. MS, mineralizing surface. N.Oc, number of osteoclasts.
P < 0.05 vs HD group (ANOVA and Tukey test).
A 4-week powdered diet exerted a greater effect on the trabecular bone architecture in the mandible than in the maxilla in 3-week-old rats [30], suggesting that alveolar osteopenia was more extensive in the mandible than in the maxilla in growing rats that experienced low masticatory loading [38]. These findings were likely caused by the trabeculae in areas distant from the periodontal ligament being more abundant in the mandible than in the maxilla. Generally, masticatory forces exerted from the teeth are transmitted through the periodontal ligament to the alveolar bone, and trabeculae are arranged in response to stress lines on the bone surface [34]. Another study suggested that the alveolar trabecular bone loss induced by occlusal hypofunction is correlated with a decrease in the number of osteoclasts, and this bone resorption causes a reduction in bone volume to the minimum necessary in the absence of forces stimulating the alveolar bone, such as occlusal force, in growing rats [39]. Also, a soft diet affected the turnover of periodontal ligament collagen and altered the bone dynamics of the tooth furcation area in young rats [40]. In a histological study, a 4-week powdered diet resulted in trabecular bone loss and enlargement of the bone marrow cavity; however, trabeculae attached to the periodontal ligament remained in the maxilla and mandible of the rats [30]. Therefore, bone resorption spreads mainly along the trabeculae after the cessation of mechanical stimulation.
5. Impact of differences in feeding behavior on midpalatal suture development in animal studies
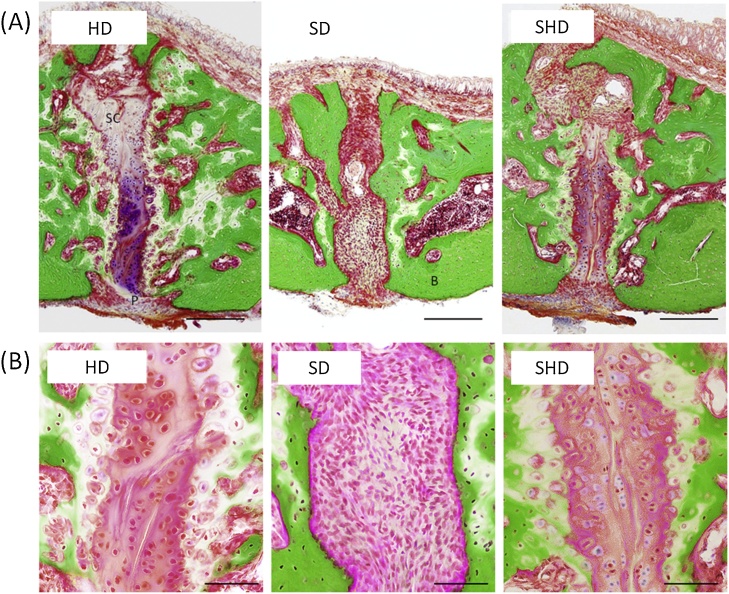
Little stress is transferred to the upper and middle portions of the facial bones during mastication, except for the mandible, in macaques and baboons [41], [42]. A recent study showed that in the midpalatal suture of growing rats fed a hard diet for 4 weeks, typical cartilaginous ossification, such as a growth plate structure, showing cell multiplication, hypertrophy, and matrix calcification were observed. However, a 4-week powdered diet resulted in narrower cartilage cell layers and disappearance of the cartilaginous areas [30]. Moreover, the suture region in 3-week-old rats was filled with fibrous tissue after consuming a powdered diet for 8 weeks (Fig. 2A and B) [31]. These findings are consistent with those of studies in growing rats [43] and growing pigs [44]. We suggest that a soft diet inhibits the differentiation of mesenchymal cells into chondrocytes, as well as chondrocyte development and maturity in the midpalatal suture region. Similarly, a rapid expansion of orthodontic force increased the migration of cellular fibrous tissue, including periosteal cells and blood vessels, to the central portion of the midpalatal suture from the oral and nasal cavity sides in small animals [45], [46]. However, the expansion forces resulted in rapid replacement of cartilage tissues with newly formed bone tissues at the oral and nasal cavity sides, which differs from the effects of the soft diet. These findings suggest that physiological forces are effective in terms of inducing gradual endochondral ossification in the midpalatal suture. In fact, 4 weeks after returning to a hard diet from a 4-week powdered diet, chondrocyte development and maturity were reactivated in the midpalatal suture region, and membranous ossification of the deteriorated bone structure was promoted in the palatal bone of rats (Fig. 2A and B) [31]. Thus, we suggest that mechanical stimulation, such as tongue pressure on the palate, with a solid diet, and the transmission of force from the periodontal support through the hard palate during food processing, are closely related to chondrocyte development and maturity in the midpalatal suture region.
Figure 2.
Representative histological images of the hard plate by Villanueva Goldner staining.
HD, hard diet. SD, powdered (soft) diet. SHD, switch from soft to hard diets. Frontal cross-section of the area of midpalatal suture region (A). P, periosteum within the oral region of the midpalatal suture, SC, suture cartilages, B, bone. Scale bar = 200 μm. Higher magnifications of the midpalatal suture (B). Scale bar = 50 μm.
6. Relationship between masticatory performance and tongue pressure during growth
Movement of the tongue contributes to food reduction by exerting a shearing force on the food between the tongue and hard palate during processing, and the duration of this force with hard food (peanut) was significantly longer than that with soft food (banana) [47]. A clinical study reported that crushing ability is significantly correlated with maximum tongue pressure in dentate adults [17]. During mastication and squeezing, the work performed by the tongue increases with increasing initial consistency of the test food (jelly), by modulating both the magnitude and duration of tongue pressure mainly at the posterior part of the hard palate [16]. Generally, the tongue is located in the forward suckling position for nursing during the neonatal period, and the swallowing pattern is infantile. Proprioception causes postural and functional changes in the tongue over 12–18 months, which is followed by a transitional period. Between 2 and 4 years, functionally balanced mature swallowing prevails; however, the tongue thrust, which is part of the infantile swallowing pattern, may be found in children older than 4 years, and even sometimes in adolescents and adults [48]. In several studies, maximum tongue pressure was directly associated with masticatory performance among children aged 6–12 years [27], [49]. In addition, tongue pressure and masticatory performance were also positively correlated in young adults [18], [49]. These data suggest that the development of masticatory performance is closely associated with an increase in tongue pressure.
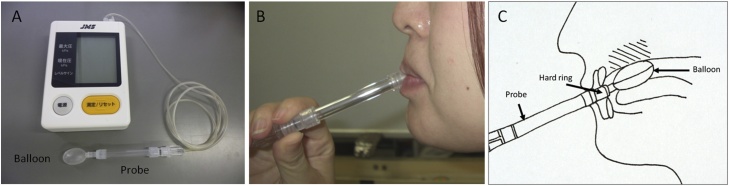
The JMS tongue pressure manometer (JMS Co. Ltd., Hiroshima, Japan), which is a simple chair-side tongue pressure measurement device, is useful for children and adults (Fig. 3A). Subjects were asked to place a balloon on the anterior part of their palate, and to close their lips, biting a hard ring with the upper and lower incisors (Fig. 3B). Then, the subjects were asked to raise their tongues and compress the balloon onto the palate with maximal voluntary muscular effort for approximately 7 s (Fig. 3C). The pressure was measured (in kilopascals) using a digital voltmeter attached to the tongue pressure manometer [18], [27], [49], [50], [51]. A recent study reported that the maximum tongue pressure increased with age (although there were some vertical variations) in 6–12-year-old children [27]. The mean maximum tongue pressure of 12-year-olds was less than that for adults aged 20–30 years [18], [27], [50]. Several studies showed that maximum tongue pressure remained constant from twenties to fifties, and started to decrease from the sixties in men, however, maximum tongue pressure started to decrease from the seventies in women [52], [53]. In addition, the mean maximum tongue pressure of children aged 6 years was similar to that of adults aged >70 years [27], [50], [51]. These data suggest that tongue pressure increases during the growth period, peaks during early adulthood, and decreases in old age. However, the maximum tongue pressure in healthy adult women (mean age 24 ± 9 years) was approximately 35 kPa, compared to approximately 50 kPa in men [18]. Our recent study reported that the mean maximum tongue pressure in girls (mean age 10.0 years) was 36.9 kPa, although that in adult women (mean age 25.9 ± 2.7 years) was 30.5 kPa. In men, the mean maximum tongue pressure increased with dental developmental stage [49]. These data suggest that peak maximum tongue pressure is reached earlier in women than in men. Therefore, before reaching adulthood, women require intervention to increase their peak tongue pressure.
Figure 3.
The maximum tongue pressure test.
An image of the measurement device (A). An image of the patient during measurement of the maximum tongue pressure (B). Intra-oral positioning of the balloon (C).
Oral chondrocyte therapy has for several decades been used to treat and prevent poor oral habits [54]. Various exercises are used to encourage mature swallowing in children, including pushing the tip of the tongue against the hard palate [55], [56]. Another researchers advocated tongue-strengthening exercises to improve tongue functions for speech and swallowing in dysarthric and dysphagic patients [57]. In addition, a tongue rotation exercise significantly increased the maximum tongue pressure in accordance with the elongated period in adults [58]. However, these studies involved young people with normal occlusion and no dysfunction of the stomatognathic system. A recent study reported that orofacial myofunctional therapy positively influenced tongue behavior in children aged 7.1–10.6 years with an anterior open bite and visceral swallowing pattern, and influenced the tongue elevation strength, tongue posture at rest, and tongue position during swallowing of solid food after therapy for 6 months [56], suggesting that orofacial myofunctional therapy can improve tongue habits, including tongue thrust, in children. However, whether tongue thrust is improved by orofacial myofunctional therapy after the critical period is unclear.
7. Conclusions
A soft diet has a negative impact on metabolism in the maxilla and mandible of growing rats. A soft diet delayed bone formation by inhibiting mineralization of the cortical bone periosteal surface in the maxilla and mandible. Additionally, a soft diet increased trabecular bone resorption by increasing the number of osteoclasts in the maxilla and mandible. However, mastication of a hard diet recovered the collapsed equilibrium of jaw bone turnover caused by a soft diet.
Furthermore, tongue pressure is closely associated with an increase in masticatory performance in children and may be related to chondrocyte development and maturity in the midpalatal suture. It is important that girls receive training in tongue function before reaching adulthood. Furthermore, children with tongue habits should exert effort to improve their masticatory performance.
Funding
This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan (25713064 and 17K11967).
Conflict of interest
None.
References
- 1.Bauer K.W., Larson N.I., Nelson M.C., Story M., Neumark-Sztainer D. Fast food intake among adolescents: secular and longitudinal trends from 1999 to 2004. Prev Med. 2009;48:284–287. doi: 10.1016/j.ypmed.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 2.Yanagisawa Y., Tamura A., Akasaka M., Teramoto Y. Physical properties of food and eating functions. 1: An objective method for the measurement of the physical properties of foods, and classification of foods. Shoni Shikagaku Zasshi. 1985;23:962–983. [in Japanese] [PubMed] [Google Scholar]
- 3.Kotecha P.V., Patel S.V., Baxi R.K., Mazumdar V.S., Shobha M., Mehta K.G. Dietary pattern of schoolgoing adolescents in urban Baroda, India. J Health Popul Nutr. 2013;31:490–496. doi: 10.3329/jhpn.v31i4.20047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poti J.M., Duffey K.J., Popkin B.M. The association of fast food consumption with poor dietary outcomes and obesity among children: is it the fast food or the remainder of the diet? Am J Clin Nutr. 2014;99:162–171. doi: 10.3945/ajcn.113.071928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.English J.D., Buschang P.H., Throckmorton G.S. Does malocclusion affect masticatory performance? Angle Orthod. 2002;72:21–27. doi: 10.1043/0003-3219(2002)072<0021:DMAMP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Henrikson T., Ekberg E.C., Nilner M. Masticatory efficiency and ability in relation to occlusion and mandibular dysfunction in girls. Int J Prosthodont. 1998;11:125–132. [PubMed] [Google Scholar]
- 7.Hichijo N., Kawai N., Mori H., Sano R., Ohnuki Y., Okumura S. Effects of the masticatory demand on the rat mandibular development. J Oral Rehabil. 2014;41:581–587. doi: 10.1111/joor.12171. [DOI] [PubMed] [Google Scholar]
- 8.Kiliaridis S., Engstrom C., Thilander B. The relationship between masticatory function and craniofacial morphology. I. A cephalometric longitudinal analysis in the growing rat fed a soft diet. Eur J Orthod. 1985;7:273–283. doi: 10.1093/ejo/7.4.273. [DOI] [PubMed] [Google Scholar]
- 9.Mavropoulos A., Kiliaridis S., Bresin A., Ammann P. Effect of different masticatory functional and mechanical demands on the structural adaptation of the mandibular alveolar bone in young growing rats. Bone. 2004;35:191–197. doi: 10.1016/j.bone.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Enomoto A., Watahiki J., Yamaguchi T., Irie T., Tachikawa T., Maki K. Effects of mastication on mandibular growth evaluated by microcomputed tomography. Eur J Orthod. 2010;32:66–70. doi: 10.1093/ejo/cjp060. [DOI] [PubMed] [Google Scholar]
- 11.Julien K.C., Buschang P.H., Throckmorton G.S., Dechow P.C. Normal masticatory performance in young adults and children. Arch Oral Biol. 1996;41:69–75. doi: 10.1016/0003-9969(95)00098-4. [DOI] [PubMed] [Google Scholar]
- 12.Toro A., Buschang P.H., Throckmorton G., Roldan S. Masticatory performance in children and adolescents with class I and II malocclusions. Eur J Orthod. 2006;28:112–119. doi: 10.1093/ejo/cji080. [DOI] [PubMed] [Google Scholar]
- 13.Ikebe K., Matsuda K., Kagawa R., Enoki K., Yoshida M., Maeda Y. Association of masticatory performance with age, gender, number of teeth, occlusal force and salivary flow in Japanese older adults: is ageing a risk factor for masticatory dysfunction? Arch Oral Biol. 2011;56:991–996. doi: 10.1016/j.archoralbio.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Barbosa Tde S., Tureli M.C., Nobre-dos-Santos M., Puppin-Rontani R.M., Gaviao M.B. The relationship between oral conditions, masticatory performance and oral health-related quality of life in children. Arch Oral Biol. 2013;58:1070–1077. doi: 10.1016/j.archoralbio.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 15.de Onis M., Onyango A.W., Borghi E., Siyam A., Nishida C., Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoyama S., Hori K., Tamine K., Fujiwara S., Inoue M., Maeda Y. Tongue pressure modulation for initial gel consistency in a different oral strategy. PLoS One. 2014;9:e91920. doi: 10.1371/journal.pone.0091920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada A., Kanazawa M., Komagamine Y., Minakuchi S. Association between tongue and lip functions and masticatory performance in young dentate adults. J Oral Rehabil. 2015;42:833–839. doi: 10.1111/joor.12319. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi M., Koide K., Arakawa I., Mizuhashi F. Association between perioral muscle pressure and masticatory performance. J Oral Rehabil. 2013;40:909–915. doi: 10.1111/joor.12105. [DOI] [PubMed] [Google Scholar]
- 19.Pereira L.J., van der Bilt A. The influence of oral processing, food perception and social aspects on food consumption: a review. J Oral Rehabil. 2016;43:630–648. doi: 10.1111/joor.12395. [DOI] [PubMed] [Google Scholar]
- 20.The National Health and Nutrition Survey in Japan. Ministry of Health, Labour and Welfare; 2014 [in Japanese]. http://www.mhlw.go.jp/bunya/kenkou/eiyou/dl/h26-houkoku.pdf.
- 21.Blass E.M., Anderson D.R., Kirkorian H.L., Pempek T.A.P.I., Koleini M.F. On the road to obesity: television viewing increases intake of high-density foods. Physiol Behav. 2006;88:597–604. doi: 10.1016/j.physbeh.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 22.Liang T., Kuhle S., Veugelers P.J. Nutrition and body weights of Canadian children watching television and eating while watching television. Public Health Nutr. 2009;12:2457–2463. doi: 10.1017/S1368980009005564. [DOI] [PubMed] [Google Scholar]
- 23.Ramos E., Costa A., Araujo J., Severo M., Lopes C. Effect of television viewing on food and nutrient intake among adolescents. Nutrition. 2013;29:1362–1367. doi: 10.1016/j.nut.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Ohsuka K. The association between lifestyle and body proportion in primary school children. Nihon Koshu Eisei Zasshi. 2013;60:128–137. [in Japanese with English abstract] [PubMed] [Google Scholar]
- 25.Soares M.E., Ramos-Jorge M.L., de Alencar B.M., Oliveira S.G., Pereira L.J., Ramos-Jorge J. Influence of masticatory function, dental caries and socioeconomic status on the body mass index of preschool children. Arch Oral Biol. 2017;81:69–73. doi: 10.1016/j.archoralbio.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 26.Consolacao Soares M.E., Ramos-Jorge M.L., de Alencar B.M., Marques L.S., Pereira L.J., Ramos-Jorge J. Factors associated with masticatory performance among preschool children. Clin Oral Investig. 2017;21:159–166. doi: 10.1007/s00784-016-1768-5. [DOI] [PubMed] [Google Scholar]
- 27.Ichikawa M., Fujita Y., Hamaguchi A., Chaweewannakorn W., Maki K. Association of tongue pressure with masticatory performance and dental conditions in Japanese children. Ped Dent J. 2016;26:51–59. [Google Scholar]
- 28.Likert R. A technique for the measurement of attitudes. Arch Phychol. 1932;140:5–55. [Google Scholar]
- 29.Maki K., Nishioka T., Shioiri E., Takahashi T., Kimura M. Effects of dietary consistency on the mandible of rats at the growth stage: computed X-ray densitometric and cephalometric analysis. Angle Orthod. 2002;72:468–475. doi: 10.1043/0003-3219(2002)072<0468:EODCOT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Goto S., Fujita Y., Hotta M., Sugiyama A., Maki K. Influence of differences in the hardness and calcium content of diets on the growth of craniofacial bone in rats. Angle Orthod. 2015;85:969–979. doi: 10.2319/102214-765.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujita Y., Goto S., Ichikawa M., Hamaguchi A., Maki K. Effect of dietary calcium deficiency and altered diet hardness on the jawbone growth: a micro-CT and bone histomorphometric study in rats. Arch Oral Biol. 2016;72:200–210. doi: 10.1016/j.archoralbio.2016.08.036. [DOI] [PubMed] [Google Scholar]
- 32.Agrawal K.R., Lucas P.W., Bruce I.C., Prinz J.F. Food properties that influence neuromuscular activity during human mastication. J Dent Res. 1998;77:1931–1938. doi: 10.1177/00220345980770111101. [DOI] [PubMed] [Google Scholar]
- 33.Kiliaridis S. Masticatory muscle influence on craniofacial growth. Acta Odontol Scand. 1995;53:196–202. doi: 10.3109/00016359509005972. [DOI] [PubMed] [Google Scholar]
- 34.Proffit W.R., Fields H.W., Sarver D.M. The etiology of orthodontic problems. In: Proffit W.R., Fields H.W., Sarver D.M., editors. Contemporary orthodontics. 5th ed. Elsevier Inc; St. Louis: 2013. pp. 114–146. [Google Scholar]
- 35.Mavropoulos A., Ammann P., Bresin A., Kiliaridis S. Masticatory demands induce region-specific changes in mandibular bone density in growing rats. Angle Orthod. 2005;75:625–630. doi: 10.1043/0003-3219(2005)75[625:MDIRCI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 36.Kufley S., Scott J.E., Ramirez-Yanez G. The effect of the physical consistency of the diet on the bone quality of the mandibular condyle in rats. Arch Oral Biol. 2017;77:23–26. doi: 10.1016/j.archoralbio.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 37.Grunheid T., Langenbach G.E., Brugman P., Everts V., Zentner A. The masticatory system under varying functional load. Part 2: effect of reduced masticatory load on the degree and distribution of mineralization in the rabbit mandible. Eur J Orthod. 2011;33:365–371. doi: 10.1093/ejo/cjq084. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu Y., Ishida T., Hosomichi J., Kaneko S., Hatano K., Ono T. Soft diet causes greater alveolar osteopenia in the mandible than in the maxilla. Arch Oral Biol. 2013;58:907–911. doi: 10.1016/j.archoralbio.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu Y., Hosomichi J., Kaneko S., Shibutani N., Ono T. Effect of sympathetic nervous activity on alveolar bone loss induced by occlusal hypofunction in rats. Arch Oral Biol. 2011;56:1404–1411. doi: 10.1016/j.archoralbio.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Jang A.T., Merkle A.P., Fahey K.P., Gansky S.A., Ho S.P. Multiscale biomechanical responses of adapted bone-periodontal ligament-tooth fibrous joints. Bone. 2015;81:196–207. doi: 10.1016/j.bone.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouvier M., Hylander W.L. Effect of bonestrain on cortical bone structure in macaques (Macacamulatta) J Morphol. 1981;167:1–12. doi: 10.1002/jmor.1051670102. [DOI] [PubMed] [Google Scholar]
- 42.Hylander W.L., Picq P.G., Johnson K.R. Masticatory-stress hypotheses and the supraorbital region of primates. Am J Phys Anthropol. 1991;86:1–36. doi: 10.1002/ajpa.1330860102. [DOI] [PubMed] [Google Scholar]
- 43.Katsaros C., Kiliaridis S., Berg R. Functional influence on sutural growth: a morphometric study in the anterior facial skeleton of the growing rat. Eur J Orthod. 1994;16:353–360. doi: 10.1093/ejo/16.5.353. [DOI] [PubMed] [Google Scholar]
- 44.Burn A.K., Herring S.W., Hubbard R., Zink K., Rafferty K., Lieberman D.E. Dietary consistency and the midline sutures in growing pigs. Orthod Craniofac Res. 2010;13:106–113. doi: 10.1111/j.1601-6343.2010.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hou B., Fukai N., Olsen B.R. Mechanical force-induced midpalatal suture remodeling in mice. Bone. 2007;40:1483–1493. doi: 10.1016/j.bone.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma J., Wu Y., Zhang W., Smales R.J., Huang Y., Pan Y. Up-regulation of multiple proteins and biological processes during maxillary expansion in rats. BMC Musculoskelet Disord. 2008;9:37. doi: 10.1186/1471-2474-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hiiemae K. Mechanisms of food reduction, transport and deglutition: how the texture of food affects feeding behavior. J Texture Stud. 2004;35:171–200. [Google Scholar]
- 48.Peng C.L., Jost-Brinkmann P.G., Yoshida N., Chou H.H., Lin C.T. Comparison of tongue functions between mature and tongue-thrust swallowing—an ultrasound investigation. Am J Orthod Dentofacial Orthop. 2004;125:562–570. doi: 10.1016/j.ajodo.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 49.Fujita Y., Ichikawa M., Hamaguchi A., Maki K. Comparison of masticatory performance and tongue pressure between children and young adults. Clinical and Experimental Dental Research. 2018;4:52–58. doi: 10.1002/cre2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hiramatsu T., Kataoka H., Osaki M., Hagino H. Effect of aging on oral and swallowing function after meal consumption. Clin Interv Aging. 2015;10:229–235. doi: 10.2147/CIA.S75211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsuga K., Yoshikawa M., Oue H., Okazaki Y., Tsuchioka H., Maruyama M. Maximal voluntary tongue pressure is decreased in Japanese frail elderly persons. Gerodontology. 2012;29:e1078–e1085. doi: 10.1111/j.1741-2358.2011.00615.x. [DOI] [PubMed] [Google Scholar]
- 52.Vanderwegen J., Guns C., Van Nuffelen G., Elen R., De Bodt M. The influence of age, sex, bulb position, visual feedback, and the order of testing on maximum anterior and posterior tongue strength and endurance in healthy belgian adults. Dysphagia. 2013;28:159–166. doi: 10.1007/s00455-012-9425-x. [DOI] [PubMed] [Google Scholar]
- 53.Utanohara Y., Hayashi R., Yoshikawa M., Yoshida M., Tsuga K., Akagawa Y. Standard values of maximum tongue pressure taken using newly developed disposable tongue pressure measurement device. Dysphagia. 2008;23:286–290. doi: 10.1007/s00455-007-9142-z. [DOI] [PubMed] [Google Scholar]
- 54.Moeller J.L. Orofacial myofunctional therapy: why now? Cranio. 2012;30:235–236. doi: 10.1179/crn.2012.035. [DOI] [PubMed] [Google Scholar]
- 55.Saccomanno S., Antonini G., D'Alatri L., D'Angelantonio M., Fiorita A., Deli R. Patients treated with orthodontic-myofunctional therapeutic protocol. Eur J Paediatr Dent. 2012;13:241–243. [PubMed] [Google Scholar]
- 56.Van Dyck C., Dekeyser A., Vantricht E., Manders E., Goeleven A., Fieuws S. The effect of orofacial myofunctional treatment in children with anterior open bite and tongue dysfunction: a pilot study. Eur J Orthod. 2016;38:227–234. doi: 10.1093/ejo/cjv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lazarus C., Logemann J.A., Huang C.F., Rademaker A.W. Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatr Logop. 2003;55:199–205. doi: 10.1159/000071019. [DOI] [PubMed] [Google Scholar]
- 58.Arakawa I., Koide K., Takahashi M., Mizuhashi F. Effect of the tongue rotation exercise training on the oral functions in normal adults – Part 1 investigation of tongue pressure and labial closure strength. J Oral Rehabil. 2015;42:407–413. doi: 10.1111/joor.12271. [DOI] [PubMed] [Google Scholar]