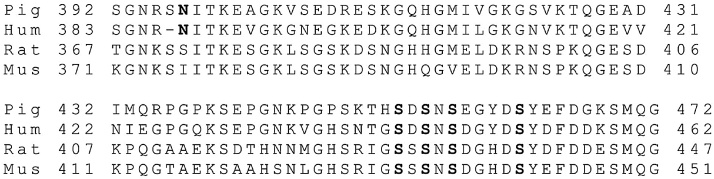Summary
Dentin sialophosphoprotein (DSPP) plays an important role in the formation of dentin. Understanding its structure and function would provide important insights into the regulation of dentin mineralization. For the past 15 years, we have been studying DSPP-derived proteins isolated from pig dentin. Porcine DSPP is synthesized and secreted by odontoblasts and processed into three proteins, i.e., dentin sialoprotein (DSP), dentin glycoprotein (DGP), and dentin phosphoprotein (DPP), by bone morphogenetic protein 1 and matrix metalloproteinase-20 and -2. DSP is a proteoglycan that forms covalent dimers, DGP is a phosphorylated glycoprotein, and DPP is a highly phosphorylated intrinsically disordered protein with genetic polymorphisms. Furthermore, DPP is not detected in dental pulp. This is possibly due to the existence of two mRNA variants of the DSPP gene: one that encodes the DSP region alone and another that encodes full-length DSPP. The mRNA variant encoding DSP alone is expressed in dental pulp and odontoblasts, but the variant encoding full-length DSPP is predominantly expressed in odontoblasts and barely in dental pulp.
Keywords: Dentin, Dentin sialophosphoprotein, Protease, Gene, Proteoglycan, Tooth
1. Introduction
Dentin is the hard tissue that constitutes the body of a tooth and is involved in the protection of the dental pulp within and the support of the overlying enamel and cementum. On a weight basis, dentin consists of approximately 70% inorganic substances, 20% organic substances, and 10% water. Of the organic substances, approximately 90% is type I collagen [1] and the remaining 10% comprises non-collagenous proteins. The largest component of the non-collagenous proteins is the protease-processed products of dentin sialophosphoprotein (DSPP) [2]. Porcine DSPP comprises three proteins, namely, dentin sialoprotein (DSP), dentin glycoprotein (DGP), and dentin phosphoprotein (DPP) [3], [4], [5], [6]. Genetic studies have shown that type I collagen and DSPP are critical for the formation of human dentin. Presently, hereditary dentin defects are classified as dentin dysplasia (types I and II) and dentinogenesis imperfecta (types I–III) [7]. Mutation of the DSPP gene (4q21) is especially known to cause dentin dysplasia type II and dentinogenesis imperfecta types II and III [8], [9], [10], [11], [12], [13], [14], [15], making the pursuit of studies on DSPP at both the genetic and protein levels very meaningful. However, most protein-level studies of DSPP have been performed with rodent teeth thus far, leaving many issues unresolved, such as (1) the expression levels of DSP and DPP in dentin are different (DSP: 5–8%, DPP: 60%), even though they are both synthesized from the same DSPP gene; (2) DGP has not been identified in rodent teeth, as opposed to DPP; (3) polymorphisms of DPP have not been characterized; (4) the processing mechanism of DSPP has not been elucidated; (5) the function of DSP has not been explained; and (6) no information is available on the linker regions. Therefore, we decided to perform protein-level studies of DSPP using second molars from approximately 5-month-old pigs as an experimental model (Figure 1), as they: (1) yield larger quantities of each DSPP-derived protein than rodents; (2) display high homology to the human DSPP gene; and (3) can be obtained cheaply. In this review, our observations based on our results so far on the structures and processing mechanisms of DSPP-derived proteins and their gene expression will be presented, as well as their possible functions and usefulness in future studies.
Figure 1.
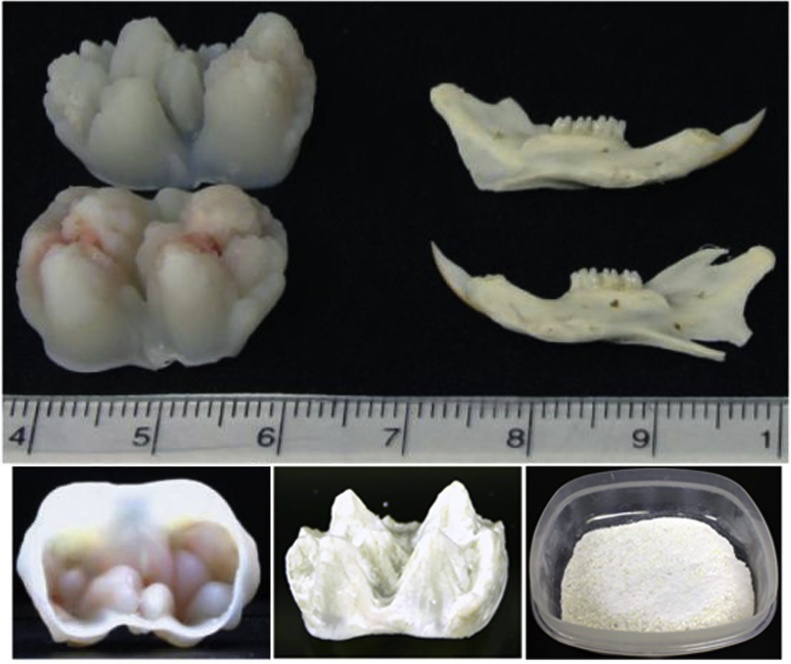
Tooth size and dentin powder sample. The developing molars used in our studies are just finishing crown formation, but have not yet started root development. They are about 2 cm in mesialdistal dimension, compared with an adult rat jaw, which is about 3 cm long. On the lower left is the view of the porcine 2nd molar from a 5- to 6-month-old pig. Note the open pulp chamber allows easy removal of the pulp at the time of extraction, minimizing contamination by blood components. Following the removal of enamel (lower center), the molar is pulverized by Jaw crusher to prepare the dentin powder (lower right).
2. DSPP-derived proteins
2.1. Dentin sialoprotein (DSP)
DSP was discovered approximately 35 years ago as the most prominent electrophoretic band among the rat dentin non-collagenous proteins [16]. Its molecular weight was approximately 95 kDa on electrophoresis, but was measured as 52.57 kDa with a sedimentation equilibrium method, containing approximately 29.6% carbohydrates [17]. On the basis of the N-terminal amino acid sequence of rat DSP (IPVPQLV), cDNA cloning of DSP and DPP was performed. It was then reported that the translation of DSP terminated at the 366th amino acid [18], and the DSP and DPP domains were proposed to be of a so-called “bicistronic nature,” where one gene encodes two different proteins [19], [20], [21]. However, it was later proven by cloning mouse DSPP cDNA that DSP and DPP are in fact expressed from a single open reading frame, revealing DSP and DPP to be chimeric proteins whose genes form a single entity and are transcribed and expressed together as a single protein [2]. Studies on rat DSP protein were advanced further after this discovery, revealing DSP and DPP to be DSPP-derived proteins that are synthesized from the DSPP gene expressed in odontoblasts, secreted, and then subjected to protease processing [22]. However, many issues remained unresolved, as mentioned in the Introduction. My research group has launched our own DSPP protein investigations using pigs as our experimental model. As there was no information available on porcine DSPP genomic and cDNA sequences when we started, we constructed a unidirectional cDNA library derived from the pulp organ of developing pig teeth and screened for the DSPP gene. As a result, we succeeded in isolating a cDNA clone that only encodes DSP [23]. Using a glutathione-S-transferase (GST) fusion protein expression vector in Escherichia coli, we produced a recombinant DSP protein (GST-DSP) based on the isolated cDNA sequence, followed by the generation of an anti- DSP polyclonal antibody. In order to detect DSP in western blots with this antibody, we prepared pulverized dentin samples (40 g) from second molars of 5-month-old pigs. Proteins were extracted from these samples by sequential extraction with a tris-guanidine buffer (G1), an acetic acid solution (A), and an acetic acid-sodium chloride solution (AN). Each protein fraction was subjected to western blotting using our anti-DSP antibody. DSP was detected as smeared bands in the molecular weight range of 120–280 kDa in fraction AN and 20–35 kDa in fraction A (Figure 2C) [24]. Three DSP isoforms were obtained from the purification of DSP from fraction AN (Figure 3), and the following were revealed from structural analysis of the most abundant isoform (DSP-2) (Figure 4) [24], [25], [26]: (1) Porcine DSP forms dimers via a disulfide bridge at Cys205, as the addition of β-mercaptoethanol during electrophoresis dissipates the band at approximately 280 kDa and unifies it with the band at approximately 120 kDa. (2) The estimated molecular weight of recombinant DSP from E. coli was 39 kDa, while the DSP that was actually detected was far larger, indicating the possibility that porcine DSP is glycosylated. Glycopeptidase and O-glycosidase treatment revealed the presence of N-glycosylation and O-glycosylation, respectively, as these enzymes reduced the molecular weight of the DSP band on an electrophoretic gel. (3) Chondroitinase ABC treatment dissipated the DSP smear band and a positive band was found on western blot analysis using an anti-chondroitin 6-sulfate antibody. We thus revealed porcine DSP to be a proteoglycan, making our group world-famous. Chondroitin 4-sulfate attachment was also detected in later studies, revealing that chondroitin 4- and 6-sulfates are variably linked to Ser253 and Ser265. It also became clear that of the three DSP isoforms, DSP-1 does not have the N-terminal region but rather only possesses the chondroitin sulfate attachment domain. (4) Amino acid sequence analysis was performed on DSP fragment peptides prepared with various endopeptidases. As a result, Arg391 was identified as the C-terminal amino acid. Thus, it became clear that the main porcine DSP isoform (DSP-2) comprises 376 amino acids from Ile16 to Arg391 (the numbering in this review takes into account the 15 signal peptides Met1–Ala15).
Figure 2.
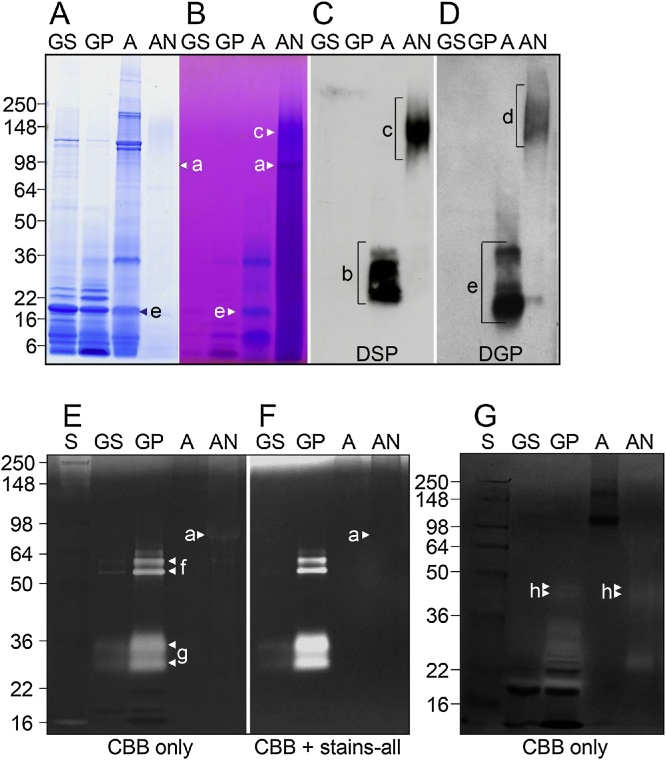
Primary dentin extracts. The four primary dentin extracts are GS, GP, A, and AN extract. (A and B) Porcine dentin extracts analyzed by SDS-PADE stained with Coomassie Brilliant Blue (CBB) (A) and Stains-all (B). DPP is the CBB-negative, Stains-all-positive band migrating at 98 kDa in the AN extract (a, arrowheads). (C and D) Western blots of SDS-PAGE using the DSP, and DGP antibodies. Lower molecular weight (LMW) DSP cleavage products from 22 to 38 kDa are observed in the A extract (b, bracket). Higher molecular weight (HMW) DSP-positive (c, bracket) and DGP-positive (d, bracket) proteins are observed in the AN extract. Most of lower molecular weight DGP-positive bands in the A extract are also DSP-positive except for the only DGP-positive proteins from 16 to 21 kDa (e, bracket). (E) Gelatin zymogram of dentin extracts. Strong gelatinase-positive activities are evident at 60–65 kDa (MMP-2; f, arrowheads) and 30–36 kDa (KLK4; g, arrowheads) in the GP extract. A relatively weak band at 98 kDa was observed in the AN extract (a, arrowhead). (F) Gelatin zymogram of dentin extracts stained with Stains-all. Stains-all staining eliminated the weak, 98-kDa band (a, arrowhead), suggesting that this gelatinase-positive band was an artifact caused by the DPP band at 98 kDa, which does not stain with CBB. (G) Casein zymogram of dentin extracts, which shows a weak doublet at 41 and 44 kDa (MMP-20; h, arrowheads). GS: guanidine–water soluble fraction, GP: guanidine–water insoluble fraction, A: acetic acid soluble fraction, AN: acetic acid–NaCl soluble fraction.
Figure 3.
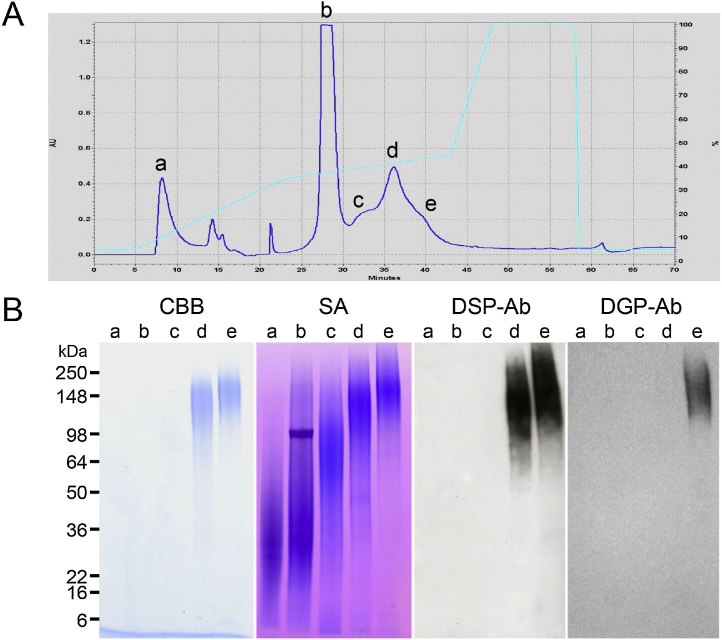
Isolation of DSP, DSP–DGP, and DPP. (A) Chromatogram of the AN extract being separated into five parts (peak a through peak e) by RP-HPLC. (B) SDS-PAGE stained with Coomassie Brilliant Blue (CBB) and Stains-all (SA) and Western blots immunostained with the DSP (DSP-Ab) and DGP (DGP-Ab) antibodies. Peaks a: DPP degradation products, b: DPP, c: DSP-core (DSP-1), d: DSP (DSP-2), e: DSP–DGP complex (DSP-3).
Figure 4.
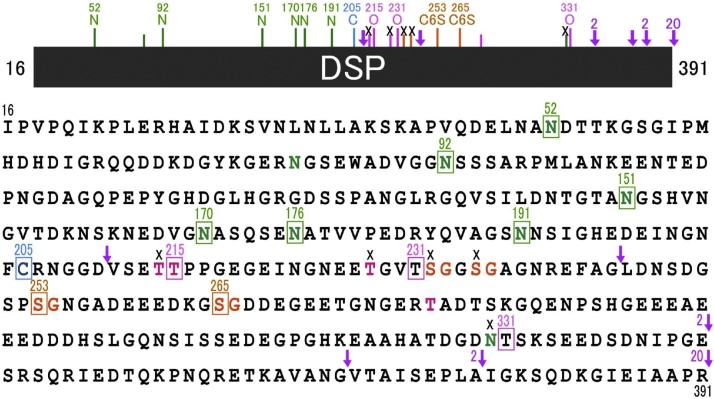
Amino acid sequence and posttranslational modification sites in porcine DSP. Potential and known carbohydrate attachment and cleavage sites by MMP-2 and MMP-20 in porcine DSP. In the primary amino acid sequence of porcine DSP there are eight predicted N-linked glycosylation (N at positions 52, 82, 92, 151, 170, 176, 191 and 330) (green), four potential O-linked glycosylations (T at positions 214, 215, 228, and 279) (pink), and four potential glycan attachment sites (S at positions 232, 235, 253, and 265) (orange). We have now demonstrated at the protein level that six of the potential N-linked sites (N52, N92, N151, N170, N176 and N191) (green squares) are glycosylated, but one (N330) is not (black cross). Two of the four predicted O-linked glycosylation sites (T214 and T228) are not glycosylated (black crosses), but one predicted site (T215) and two unpredicted sites (T231 and T331) are O-glycosylated (pink squares); and two of the four potential glycan attachment sites (S253 and S265) (orange squares) are used, but other two (S232 and S235) are not. The arrows indicate the locations of cleavage sites by proteinases. We have demonstrated that three sites are cleaved by MMP-2 (2) and MMP-20 (20). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.2. Dentin glycoprotein (DGP)
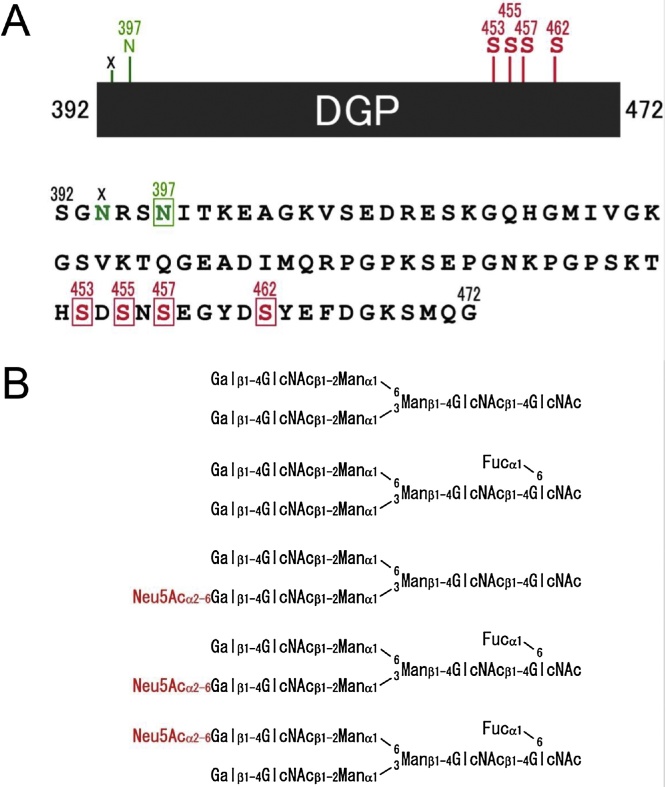
After the determination of the C-terminus of porcine DSP, the presence of an 81-amino acid polypeptide (Ser392–Gly472) was revealed before the N-terminus (Asp473) of DPP. This region between DSP and DPP was defined as a linker in rodent DSPP studies, but its structure had not been elucidated. Thus, we synthesized a 16-amino acid peptide deduced from the cDNA of the linker found in pig, produced an antibody against it, and tested it on western blots of dentin protein fractions. This revealed positive bands of approximately 19, 25, and 38 kDa in fraction A, and bands of 130–250 kDa in fraction AN (Figure 2D). Moreover, of the three isoforms of DSP, DSP-3 was also detected using this antibody, indicating that this isoform is a complex containing DSP and the linker region (Figure 3). After purification, the primary structure and posttranslational modifications of the 19 kDa linker protein in fraction A that reacted most strongly to our linker antibody were analyzed. We showed that this linker protein is a phosphorylated glycoprotein, and designated it as DGP [27] (Figure 5). Of the two predicted N-glycosylation sites of DGP (Asn394 and Asn397), Asn397 was variably bound to bi- and triantennary glycan. Furthermore, all of the four C-terminal phosphorylation sites (Ser453, Ser455, Ser457, and Ser462) were phosphorylated.
Figure 5.
Amino acid sequence and structures of oligosaccharides of porcine DGP. (A) DGP is consisted of 81 amino acids. We have demonstrated that one potential N-linked oligosaccharide binding site (N397) is glycosylated (green square) and four serines (S453, S455, S457 and S462) are phosphorylated (red squares). (B) we also have identified biantennary glycan structures by analyses of oligosaccharides released from DGP and fluorescent labeled. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
DGP was also identified recently in human dentin [28], but not yet in rodents. This is because the cleavage sites for matrix metalloprotease-20 (MMP20) processing that gives rise to DGP from DSP-3 (DSP–DGP complex) differ between rodents and other mammals (Figure 6), as will be explained later. DGP is generally thought to be a protein of little functional importance that is merely cleaved from DSP. However, its strong affinity to hydroxyapatite indicates a possible function that is different from that of DSP, which will hopefully be revealed with further studies.
Figure 6.
Comparison of amino acid sequences of non-rodent and rodent DGP. The four known mammalian DGP sequences are aligned from pig (Pig) (NP_998942.1), human (Hum) (AAF42472.1), rat (Rat) (AAL79813.1), and mouse (Mus) (AAC12787.1). The number of identities with pig DGP 81 amino acids for human is 58 (81%), for rat is 40 (49%), and for mouse is 38 (47%). Note that only one dash is needed to maintain the alignment. Amino acids modified in pig DGP and conserved in other species are shown in bold.
2.3. Dentin phosphoprotein (DPP)
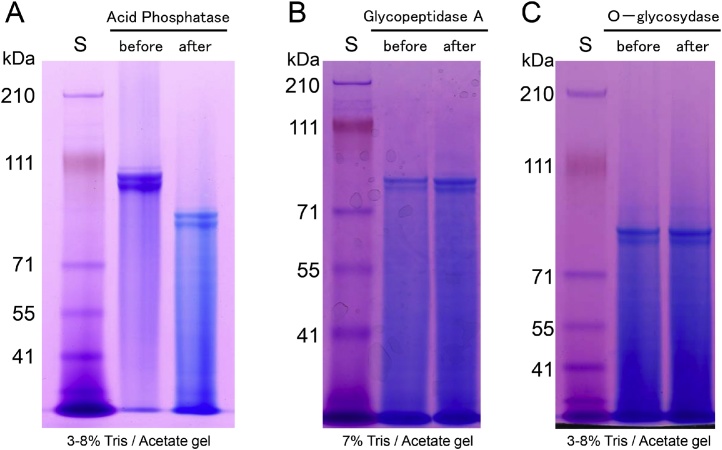
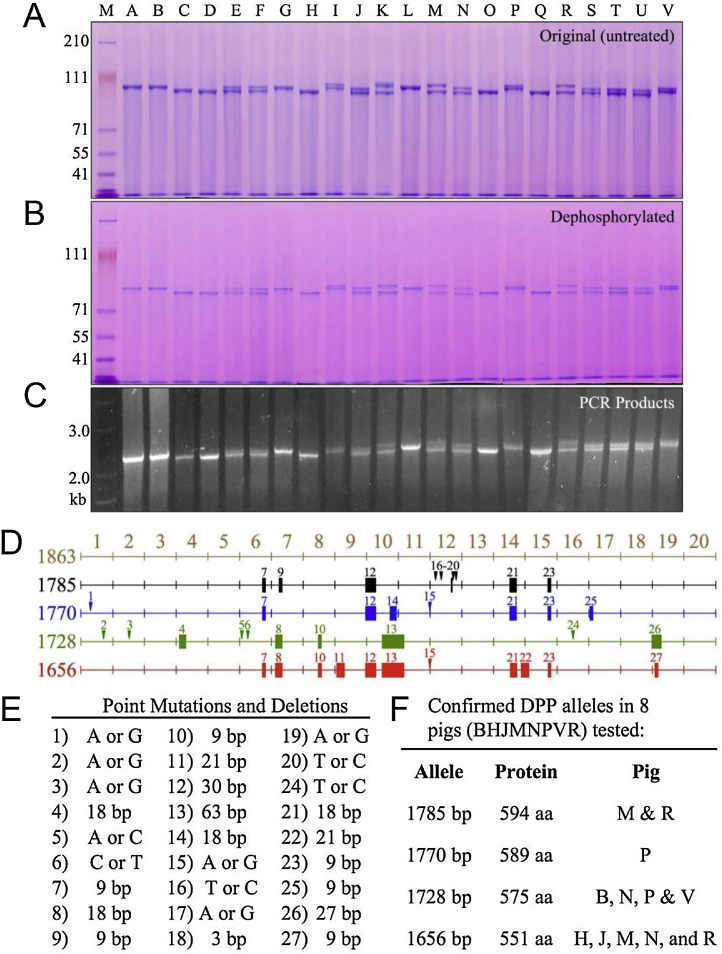
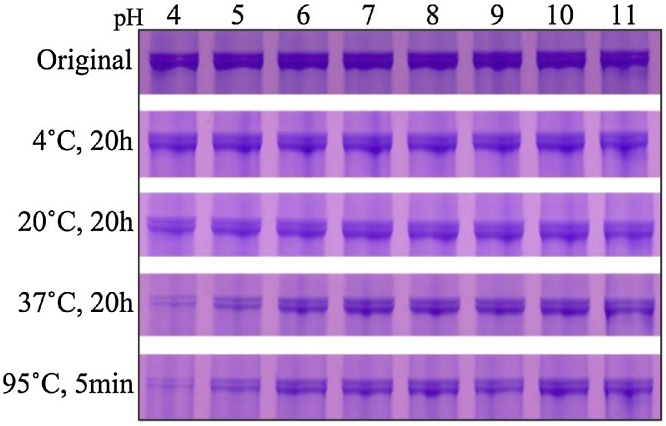
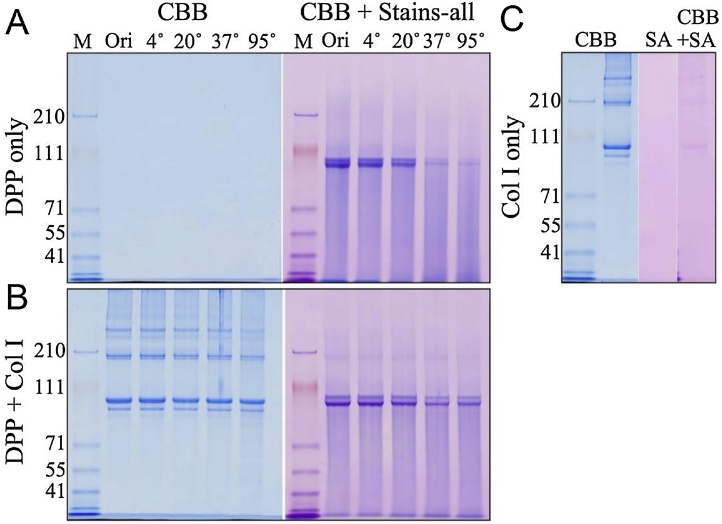
DPP, which is also known as phosphophoryn, is a dentin-specific non-collagenous protein that has been studied for approximately 50 years. DPP has a characteristic amino acid composition consisting of 70–80% aspartic acid and serine residues. Moreover, DPP is a strongly acidic protein with an isoelectric point close to 1.1, as approximately 80% of its serine residues are phosphorylated (porcine DPP contains an average of 155 phosphate residues per molecule [29]). This makes DPP unstainable with Coomassie Brilliant Blue, which is commonly used in electrophoresis; rather, it is detected using Stains-all. Due to its particular amino acid composition, the structure of DPP displays many aspartic acid-serine repeats (SDD, DSS, DDS, etc.). We have observed the following from our structural analyses of porcine DPP [29]: (1) DPP is detected mainly as two Stains-all-positive bands in the molecular weight range of 95–100 kDa. (2) It was proposed that it appears as a double band due to different degrees of phosphorylation [30]; however, dephosphorylation by acidic phosphatase shifted the molecular weights of both bands down without affecting their general pattern (Figure 7A). We then investigated their potential differences in glycosylation, which also did not alter the band pattern (Figure 7B and C). We then isolated DPP individually from 22 pigs and performed electrophoresis and were able to observe variations in the size of DPP. Subsequent PCR of the coding region of DPP revealed that the length variation of the protein was closely correlated with genetic-level variation (Figure 8A–C). Further studies of the DPP coding region revealed four allelic variations; 27 sequence variations caused by 16 polymorphisms occurring in the range of 3 and 63 bp have been confirmed among these alleles (Figure 8D and E). We found that DPP molecules consisting of 551, 575, 589, or 594 amino acids are translated from the four alleles, of which the 551 and 575 amino acid variants are the predominant types in pigs. (3) As mentioned above, DPP contains aspartic acid-serine repeats in its structure. This makes it a so-called “intrinsically disordered protein,” which is unstable on its own under physiological conditions and prone to morphological changes such as aggregation and interactions [31]. Indeed, porcine DPP disintegrates easily upon changes in pH and temperature (Figure 9). However, we have observed that DPP is stabilized in the presence of collagen (Figure 10) [29].
Figure 7.
Multiple DPP bands are not due to variable levels of phosphorylation or glycosylation. (A) DPP in large scale preparations (from 8 pigs) typically shows 2 prominent bands and 1 or 2 additional faint bands (lane 1). Dephosphorylation with acidphosphatase shifts the multiple bands down the gel, but the pattern of multiple bands is preserved. (B) N-Deglycosylation of the dephosphorylated DPP with glycopeptidase A (lane 2) does not significantly alter the mobility of DPP or its pattern of multiple bands (lane 1). (C) O-Deglycosylation of dephosphorylated and N-deglycosylated DPP (lane 2) does not significantly alter the mobility of DPP or its pattern of multiple bands (lane 1). The 3 DPP bands from lane 1 in panel A were excised from the gel and characterized by N-terminal sequencing. All gave the same result: DDPNS, which is the conserved DPP N-terminal sequence.
Figure 8.
Length polymorphisms of DPP protein and coding region, and DPP allelic variations. (A) SDS-PAGE showing variations in the mobility of DPP protein isolated from 22 individual pigs. Nine of the 22 pigs showed only a single band of DPP (A, B, C, D, G, H, L, O, Q). Thirteen pigs showed two DPP bands (E, F, I, J, K, M, N, P, R, S, T, U, V). Although no individual pig showed more than 2 DPP bands, six bands of non-identical size could be discerned among the 22 pigs. (B) Dephosphorylation of DPP from the 22 pigs shifted the bands lower on the gel but did not alter the pattern of bands. (C) DPP PCR products using genomic DNA as template generated an identical pattern of 1 or 2 bands. (D) Mapping of sequence variations in the porcine DPP coding region. Point mutations are indicated by numbered arrowheads; deletions are indicated by solid rectangles. On the left is the number of basepairs in each DPP allele. The first map (1863 bp, brown) is not an allele, but a merged reference sequence that contains all of the DPP code found on the four DPP alleles characterized. The four DPP alleles had 1785 (black), 1770 (blue), 1728 (green) and 1656 (red) basepairs (bp). (E) Key to the 27 sequence variations observed in the 4 DPP alleles characterized. (F) Listing of the pigs analyzed for sequence variations, the number of coding bp in each allele, the number of amino acids (aa) in the DPP protein, and the pigs shown to host each allele. The predominant alleles in the 22 pigs were 1656 and 1728 bp. Based upon the mobility of the proteins (A), and the cloning results, these alleles were likely present in 15 or 14 of the 22 pigs, respectively (1656: C2, D2, E, F, H2, J, K, M, N, O2, Q2, R, S, T, U; 1728: A2, B2, E, F, G2, I, J, L2, N, P, S, T, U, V). Only 6 of the 22 pigs hosted other alleles (I, K, M, P, R, V). The 1770 allele in pig P also appeared to be present in pigs I and V. The 1785 allele in pigs M and R also appeared to be in pig K. The frequency of the four characterized alleles in the 22 pigs (44 alleles) were 1656: 20 alleles; 1728: 18 alleles; 1770: 3 alleles; 1785: 3 alleles. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Figure 9.
Instability of DPP at low pH and high temperature. SDS-PAGE of DPP bands stained with Stains-all that have been incubated at the pH shown at the top and the temperature and length of time shown on the left. DPP is particularly unstable if heated at low pH.
Figure 10.
Stability of DPP in the solution at pH 7 under the presence or absence of collagen. (A) SDS-PAGE stained with CBB or CBB plus Stains-all. DPP was not stained with CBB but can be visualized with Stains-all. DPP is not stable when incubated at higher temperature. (B) SDS-PAGE of Col I and DPP after being mixed together and incubated at 4, 20, 37, or 95 °C. The presence of collagen reduces the instability of DPP at 37 and 95 °C. (C) SDS-PAGE showing type I collagen (Col I). Col I stains with CBB but does not stain with Stains-all (SA).
3. Processing mechanism of DSPP
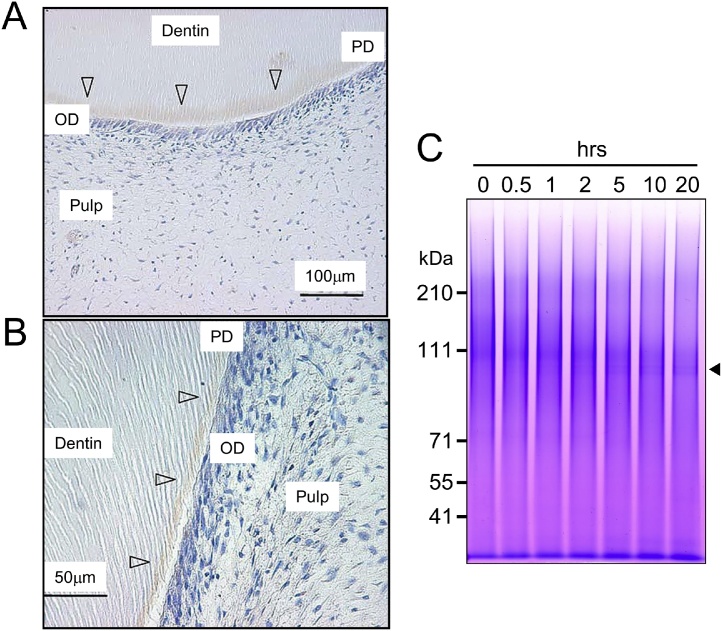
Full-length DSPP has never been isolated from rodents or other mammals. Indeed, we were able to isolate DSP–DGP complex (Ile16–Gly472) and DPP (Asp473–C-terminus) from our 5-month-old pig second molar dentin samples, but have been unable to identify full-length DSPP. It was thus hypothesized that the processing of DSPP into DSP–DGP complex and DPP occurs either immediately after DSPP secretion from odontoblasts or intracellularly before secretion. However, the amino acid consensus sequence of bone morphogenetic protein 1 (BMP1) target sites was published by Hopkins et al. in 2007 [32], which we found to be a match to the boundary between DSP–DGP complex and DPP. Immunohistochemistry with an anti-BMP1 antibody revealed that BMP1 is distributed evenly in predentin [33] (Figure 11A and B). Thus, alongside our effort to isolate full-length DSPP, we created a fluorescent resonance energy transfer peptide containing the boundary between DSP–DGP complex and DPP and tested various proteases thought to be present in dentin. As a result, only BMP1 worked on the appropriate site, releasing DPP and revealing that BMP1 is the initial DSPP processing protease [33].
Figure 11.
Localization of BMP-1 in odontoblasts and predentin by immunohistochemistry, and digestion of porcine DSPP with BMP-1. (A, B) Decalcified sections of developing third molars from a 6-month-old pig showing positive immunostaining (arrowheads) of odontoblasts and in predentin using a commercial antibody raised against recombinant human (rh) BMP-1. OD: odontoblasts; PD: predentin. (C) SDS-PAGE (3%–8% tris/acetate gel) showing the digestions of the porcine DSPP proteogly can by rhBMP-1 after 0, 0.5, 1, 2, 5, 10, and 20 h. DPP bands generated by rhBMP-1 during the digestion (arrowhead).
In our pursuit to isolate full-length DSPP, we changed the 0.5 M acetic acid that we had been using for demineralization to 0.17 N HCl/0.98% formic acid, and reduced the demineralization step from 3 days to 1 day. As a result, we succeeded in isolating full-length DSPP. Treating the purified protein with BMP1 released DPP, confirming the role of BMP1 in the initial step of extracellular DSPP processing [33] (Figure 11C). To elucidate further the processing of DSP–DGP complex, which is produced during the initial step of DSPP processing, we analyzed the proteases contained in each dentin protein fraction with gelatin and casein zymography. This revealed the presence of MMP20 and MMP2 as the predominant matrix metalloproteases in dentin (Figure 2E–G); thus, we used these in our in vitro cleavage experiment on DSP–DGP complex (DSP-3). As a result, we found that the action of MMP20 releases DGP, and MMP2 produces the low molecular weight DSP of 20–35 kDa observed in fraction A [25].
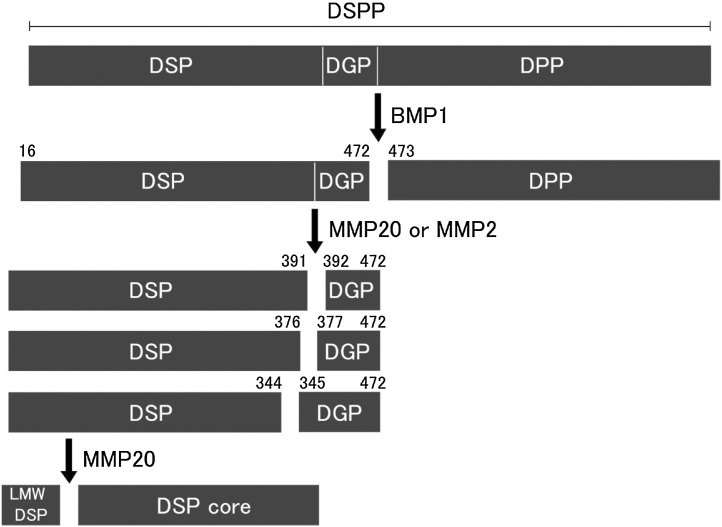
To summarize the above-mentioned DSPP processing mechanism (Figure 12), full-length DSPP synthesized and secreted by odontoblasts is cleaved into DSP–DGP complex and DPP by BMP1 at an early stage. Subsequent processing forms the DSPP-derived proteins, each with their own functions; DGP is formed by the action of MMP20 and is further processed by MMP2 into extended DGP by the addition of a few of the C-terminal amino acids of DSP to the N-terminus of DGP. Furthermore, the N-terminal region of DSP is processed by MMP20.
Figure 12.
Proteolysis of porcine DSPP. Numbers indicate cleavage sites discovered by characterizing DSPP-derived cleavage products. DSPP is first processed by BMP-1 to generate DSP–DGP complex and DPP. Then, DSP–DGP is further cleaved in the dentin extracellular matrix by MMP-2 and MMP-20, generating DSPs and DGPs. MMP-20 performs the cleavage that separates DSP and DGP (at Ser392) and generates N-terminal DSP cleavage products. On the other hand, MMP-2 cleaves DSP–DGP primarily within the C-terminal region of the DSP (at Ser345 and Ile377), releasing DGP-containing cleavage products. The mechanism of DPP degradation in vivo is unknown.
4. DSPP gene splice variants
Recently, we performed electrophoresis with proteins extracted from dental pulp tissue and discovered that DPP is absent from this tissue (Figure 13) [34]. This reminded us of our previous discovery of a cDNA encoding DSP alone in porcine dental pulp tissue. Thus, we prepared total RNA from incisor pulp and odontoblasts of 5-month-old pigs [35] and performed real-time PCR using a forward primer based on the C-terminal region of DSP and reverse primers based on (1) the C-terminal region of DSP (DSPP-1A), (2) N-terminal region of DPP (DSPP-1B), and (3) DSP-only region (DSPP-2), in an attempt to analyze at least two of the mRNA variants (full-length DSPP [DSP, DGP, and DPP] and DSP-only) from the DSPP gene (Figure 14A and B) [34].
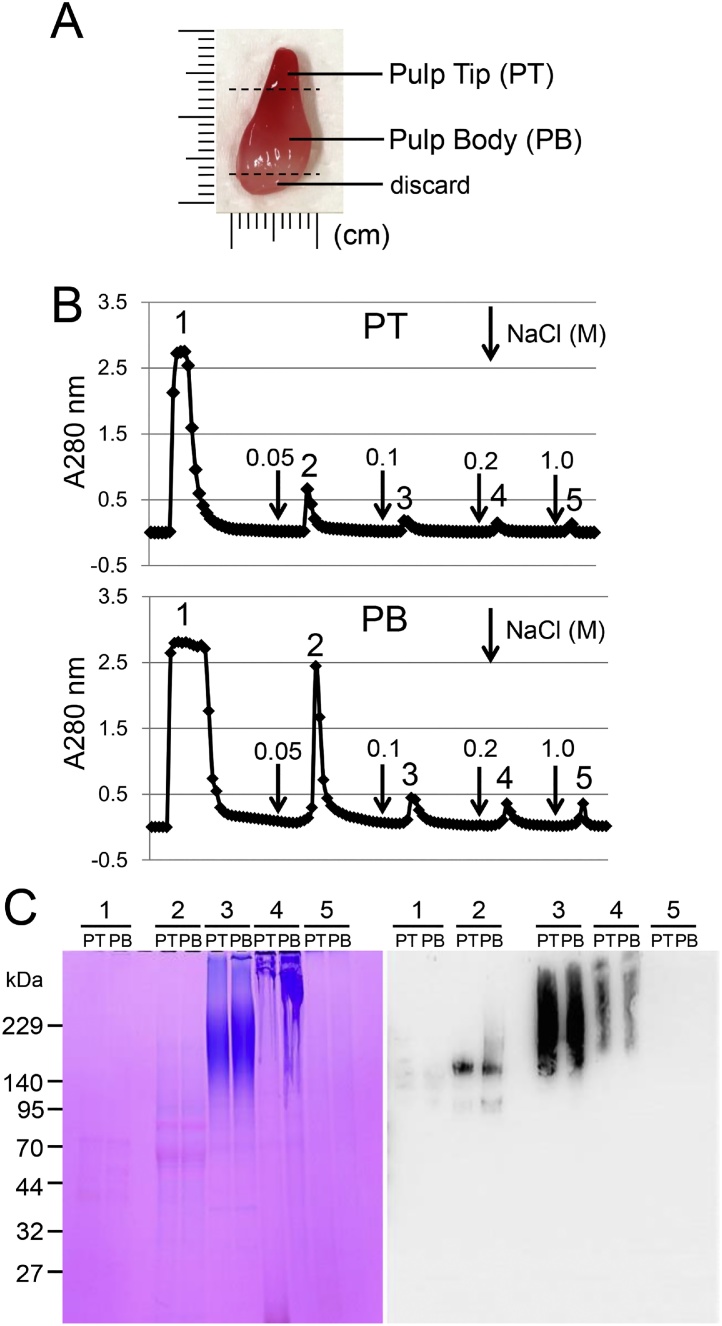
Figure 13.
Isolation of DSPP–derived proteins in porcine incisor pulp and dentin. (A) Permanent incisor pulp from 5-month-old pig. The pulp tip (PT), pulp body (PB), and bottom part were excised off a razor blade. (B) Heparin chromatograms showing absorbance at 280 nm for extracts from pulp tip (PT; top) and pulp body (PB; bottom). Downward-pointing arrows are the starting point of the step gradient with 0.05, 0.1, 0.2, and 1 M NaCl. (C, left) SDS-PAGE (5% to 20% gradient gel) stained with Stains-all showing each fraction on a Heparin chromatogram. (C, right) Western blots showing each fraction on a Heparin chromatogram with specific antibodies against N-terminal dentin sialoprotein.
Figure 14.
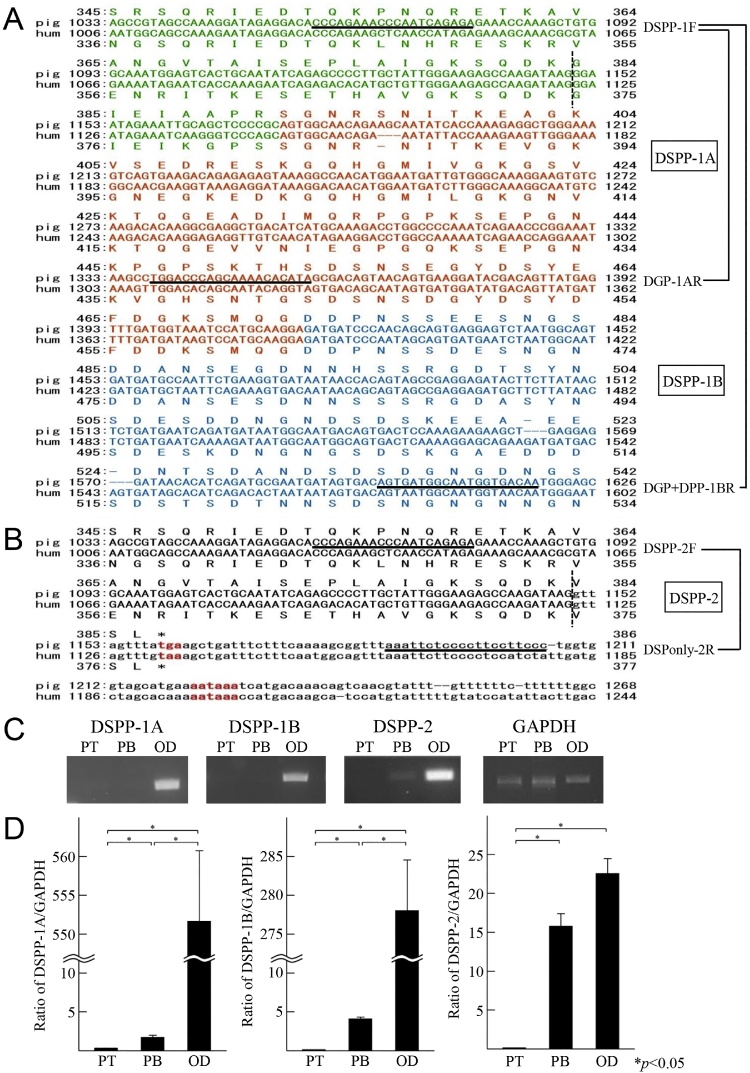
Expression of porcine DSPP splicing variants in pulp and odontoblasts. (A) Alignment of the 3′ end of the porcine DSP cDNA (green), the whole DGP cDNA (orange), and the 5′ beginning of the DPP cDNA (light blue), as well as (B) the 3′ end of the porcine DSP-only cDNA. The porcine full-length and DSP-only cDNA sequences have GenBank accession numbers NM_213777.1 and AF332578.1, respectively. The number of the last nucleotide or amino acid in each row is provided on the right. The 3′ ends of the exon 4 segments are indicated by a vertical dotted line. The primer annealing sites (A) for DSPP variant 1 (DSPP-1F, in exon 4; DSPP-1AR and DSPP-1BR, in exon 5) and (B) for DSPP variant 2 (DSPP-2F, in exon 4; DSPP-2R, in intron 4) are underlined. (B) The uppercase character of nucleotide indicates the 3′ end of the porcine DSP cDNA (exon 4), while the lowercase character of nucleotide indicates the beginning of intron 4. In-frame translation stop codons are indicated as red color, as are the polyadenylation signals. (C) The mRNA expression by RT-PCR analysis of DSPP variants 1A (DSPP-1A), 1B (DSPP-1B), and 2 (DSPP-2) in pulp tip (PT), pulp body (PB), and odontoblast (OD). Each PCR product was based on an equal amount of GAPDH and separated by electrophoresis on 2% agarose gel. Their expected sizes were 300 base pairs (bp) for DSPP-1A, 562 bp for DSPP-1B, 149 bp for DSPP-2, and 346 bp for GAPDH. (D) The mRNA expression by quantitative real-time PCR analysis of DSPP-1A (left), DSPP–1B (middle), and DSPP-2 (right) in PT, PB, and OD. Each ratio was normalized per the relative quantification data of DSPP-1A, DSPP-1B, and DSPP-2 in pulp and odontoblast in comparison to a reference gene (GAPDH), which was generated on the basis of a mathematical model for relative quantification in quantitative real-time PCR system. All values were represented as means ± standard error. Statistical significance (*) was determined with an unpaired Student’s t test. In all cases, P < 0.05 was regarded as statistically significant. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The DSPP gene comprises 5 exons. The DSP-only transcript is produced using a polyadenylation signal in intron 4 located between exons 4 and 5; thus, exon 5, which encodes DGP and DPP, is not included. As a translation termination signal appears as the fourth codon after the end of exon 4, translated DSP has three extra amino acids at its C-terminus (Figure 14B) [23].
Real-time PCR showed the prominent expression of full-length DSPP mRNA (DSPP-1A and DSPP-1B) in odontoblasts rather than in dental pulp, while the expression level of DSP-only mRNA (DSPP-2) was similar in both. However, a comparison between full-length DSPP and DSP-only mRNAs in odontoblasts revealed the higher expression of full-length DSPP compared to DSP-only (Figure 14C and D) [34].
5. Future prospects
DSP is thought to be involved in the early mineralization of dentin and DPP during the maturation of dentin [36]. Considering that DPP reportedly localizes in the so-called “mineralization front” between dentin and predentin, it is plausible that DPP is involved in intertubular dentin formation as a strongly acidic phosphoprotein. Immunohistochemistry using an anti-DSP antibody has shown that DSP localizes in peritubular dentin, suggesting the involvement of DSP in its formation as a proteoglycan; further studies are required to confirm this hypothesis.
DSPP is produced by odontoblasts. The differentiation of dental papilla to an odontoblast is caused by the expression of various signaling molecules, transcription factors, and growth factors in the inner enamel epithelium [37]. In mouse studies, the C-terminal region of DSP is reportedly involved in the differentiation and mineralization of human periodontal membrane stem cells and mouse dental papilla mesenchymal cells by controlling the expression of teeth and bone markers, growth factors, and transcription factors [38], [39]. Moreover, the DSPP gene is used widely as a marker of the differentiation of dental pulp-derived stem cells into odontoblasts [40], [41], [42]. These studies may progress even further by considering the DSPP splice variants found in our studies.
Ethical approval
All animal experiments were approved by the Institutional Animal Care Committee and the Recombination DNA Experiment and Biosafety Committee of the Tsurumi University School of Dental Medicine and the Institutional Animal Care and Use Program at the University of Michigan.
Conflicts of interest
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Acknowledgements
We thank Drs. James P. Simmer and Jan C-C. Hu from the Department of Biologic Materials Sciences at the University of Michigan School of Dentistry, who served as scientific advisors. We also thank Drs. Takanori Iwata from the Institute of Advanced Biomedical Engineering and Science at Tokyo Women’s Medical University, Kazuyuki Kobayashi from the Department of Dental Hygiene at Tsurumi Junior College, Takatoshi Nagano from the Department of Periodontology at Tsurumi University School of Dental Medicine, Shuhei Tsuchiya from Division of Oral and Maxillofacial Surgery at Nagoya University, Ryuji Yamamoto from the Department of Biochemistry and Molecular Biology at Tsurumi University School of Dental Medicine, and Ichiro Saito from the Department of Pathology at Tsurumi University School of Dental Medicine for their helpful support. This study was supported by National Institute of Dental and Craniofacial Research (NIDCR), of the U.S. National Institute of Health, grant DE018020, JSPS KAKENHI Grant-in-Aid for Scientific Research (C26462982), Grant-in-Aid for Young Scientists (B25861901) and the MEXT-Supported Program for the Strategic Research Foundation at Private Universities (S1511018).
Contributor Information
Yasuo Yamakoshi, Email: yamakoshi-y@tsurumi-u.ac.jp.
James P. Simmer, Email: jsimmer@umich.edu.
References
- 1.Linde A., Lussi A., Crenshaw M.A. Mineral induction by immobilized polyanionic proteins. Calcif Tissue Int. 1989;44:286–295. doi: 10.1007/BF02553763. [DOI] [PubMed] [Google Scholar]
- 2.MacDougall M., Simmons D., Luan X., Nydegger J., Feng J., Gu T.T. Dentin phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4. Dentin phosphoprotein DNA sequence determination. J Biol Chem. 1997;272:835–842. doi: 10.1074/jbc.272.2.835. [DOI] [PubMed] [Google Scholar]
- 3.Veis A., Perry A. The phosphoprotein of the dentin matrix. Biochemistry. 1967;6:2409–2416. doi: 10.1021/bi00860a017. [DOI] [PubMed] [Google Scholar]
- 4.Leaver A.G., Triffitt J.T., Holbrook I.B. Newer knowledge of non-collagenous protein in dentin and cortical bone matrix. Clin Orthop Rel Res. 1975;110:269–292. doi: 10.1097/00003086-197507000-00039. [DOI] [PubMed] [Google Scholar]
- 5.Dimuzio M.T., Veis A. Phosphophoryns-major noncollagenous proteins of rat incisor dentin. Calcif Tissue Res. 1978;25:169–178. doi: 10.1007/BF02010765. [DOI] [PubMed] [Google Scholar]
- 6.Linde A., Bhown M., Butler W.T. Noncollagenous proteins of dentin: a re-examination of proteins from rat incisor dentin utilizing techniques to avoid artifacts. J Biol Chem. 1980;255:5931–5942. [PubMed] [Google Scholar]
- 7.Shields E.D., Bixler D., El-Kafrawy A.M. A proposed classification for heritable human dentine defects with a description of a new entity. Arch Oral Biol. 1973;18:543–553. doi: 10.1016/0003-9969(73)90075-7. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X., Zhao J., Li C., Gao S., Qiu C., Liu P. DSPP mutation in dentinogenesis imperfecta Shields type II. Nat Genet. 2001;27:151–152. doi: 10.1038/84765. [DOI] [PubMed] [Google Scholar]
- 9.Xiao S., Yu C., Chou X., Yuan W., Wang Y., Bu L. Dentinogenesis imperfecta 1 with or without progressive hearing loss is associated with distinct mutations in DSPP. Nat Genet. 2001;27:201–204. doi: 10.1038/84848. [DOI] [PubMed] [Google Scholar]
- 10.Rajpar M.H., Koch M.J., Davies R.M., Mellody K.T., Kielty C.M., Dixon M.J. Mutation of the signal peptide region of the bicistronic gene DSPP affects translocation to the endoplasmic reticulum and results in defective dentine biomineralization. Hum Mol Genet. 2002;11:2559–2565. doi: 10.1093/hmg/11.21.2559. [DOI] [PubMed] [Google Scholar]
- 11.Malmgren B., Lindskog S., Elgadi A., Norgren S. Clinical, histopathologic, and genetic investigation in two large families with dentinogenesis imperfecta type II. Hum Genet. 2004;114:491–498. doi: 10.1007/s00439-004-1084-z. [DOI] [PubMed] [Google Scholar]
- 12.Kim J.W., Nam S.H., Jang K.T., Lee S.H., Kim C.C., Hahn S.H. A novel splice acceptor mutation in the DSPP gene causing dentinogenesis imperfecta type II. Hum Genet. 2004;115:248–254. doi: 10.1007/s00439-004-1143-5. [DOI] [PubMed] [Google Scholar]
- 13.Kim J.W., Hu J.C., Lee J.I., Moon S.K., Kim Y.J., Jang K.T. Mutational hot spot in the DSPP gene causing dentinogenesis imperfecta type II. Hum Genet. 2005;116:186–191. doi: 10.1007/s00439-004-1223-6. [DOI] [PubMed] [Google Scholar]
- 14.McKnight D.A., Suzanne Hart P., Hart T.C., Hartsfield J.K., Wilson A., Wright J.T. A comprehensive analysis of normal variation and disease-causing mutations in the human DSPP gene. Hum Mutat. 2008;29:1392–1404. doi: 10.1002/humu.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song Y.L., Wang C.N., Fan M.W., Su B., Bian Z. Dentin phosphoprotein frameshift mutations in hereditary dentin disorders and their variation patterns in normal human population. J Med Genet. 2008;45:457–464. doi: 10.1136/jmg.2007.056911. [DOI] [PubMed] [Google Scholar]
- 16.Butler W.T., Bhown M., Dimuzio M.T., Linde A. Nonocollagenous proteins of dentin. Isolation and partial characterization of rat dentin proteins and proteoglycans using a three-step preparative method. Coll Relat Res. 1981;1:187–199. doi: 10.1016/s0174-173x(81)80019-2. [DOI] [PubMed] [Google Scholar]
- 17.Butler W.T. Dentin extracellular matrix and dentinogenesis. Oper Dent. 1992;5(Suppl):18–23. [PubMed] [Google Scholar]
- 18.Ritchie H.H., Hou H., Veis A., Butler W.T. Cloning and sequence determination of rat dentin sialoprotein, a novel dentin protein. J Biol Chem. 1994;269:3698–3702. [PubMed] [Google Scholar]
- 19.Ritchie H.H., Wang L.H. Sequence determination of an extremely acidic rat dentin phosphoprotein. J Biol Chem. 1996;271:21695–21698. doi: 10.1074/jbc.271.36.21695. [DOI] [PubMed] [Google Scholar]
- 20.Ritchie H., Wang L.H. A mammalian bicistronic transcript encoding two dentin-specific proteins. Biochem Biophys Res Commun. 1997;231:425–428. doi: 10.1006/bbrc.1997.6126. [DOI] [PubMed] [Google Scholar]
- 21.Ritchie H.H., Ritchie D.G., Wang L.H. Six decades of dentinogenesis research. Historical and prospective views on phosphophoryn and dentin sialoprotein. Eur J Oral Sci. 1998;106(Suppl. 1):211–220. doi: 10.1111/j.1600-0722.1998.tb02178.x. [DOI] [PubMed] [Google Scholar]
- 22.Qin C., Cook R.G., Orkiszewski R.S., Butler W.T. Identification and characterization of the carboxyl-terminal region of rat dentin sialoprotein. J Biol Chem. 2001;276:904–909. doi: 10.1074/jbc.M006271200. [DOI] [PubMed] [Google Scholar]
- 23.Yamakoshi Y., Hu J.C., Liu S., Zhang C., Oida S., Fukae M. Characterization of porcine dentin sialoprotein (DSP) and dentin sialophosphoprotein (DSPP) cDNA clones. Eur J Oral Sci. 2003;111:60–67. doi: 10.1034/j.1600-0722.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- 24.Yamakoshi Y., Hu J.C., Fukae M., Iwata T., Kim J.W., Zhang H. Porcine dentin sialoprotein is a proteoglycan with glycosaminoglycan chains containing chondroitin 6-sulfate. J Biol Chem. 2005;280:1552–1560. doi: 10.1074/jbc.M409606200. [DOI] [PubMed] [Google Scholar]
- 25.Yamakoshi Y., Hu J.C., Iwata T., Kobayashi K., Fukae M., Simmer J.P. Dentin sialophosphoprotein is processed by MMP-2 and MMP-20 in vitro and in vivo. J Biol Chem. 2006;281:38235–38243. doi: 10.1074/jbc.M607767200. [DOI] [PubMed] [Google Scholar]
- 26.Yamakoshi Y., Nagano T., Hu J.C., Yamakoshi F., Simmer J.P. Porcine dentin sialoprotein glycosylation and glycosaminoglycan attachments. BMC Biochem. 2011;12:6. doi: 10.1186/1471-2091-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamakoshi Y., Hu J.C., Fukae M., Zhang H., Simmer J.P. Dentin glycoprotein: the protein in the middle of the dentin sialophosphoprotein chimera. J Biol Chem. 2005;280:17472–17479. doi: 10.1074/jbc.M413220200. [DOI] [PubMed] [Google Scholar]
- 28.Yamakoshi Y. Dentin sialophosphoprotein (DSPP): protein structure & post-translational modifications. In: Goldberg M., editor. Phosphorylated extracellular matrix proteins of bone and dentin. Bentham Sciences Publisher; 2012. pp. 203–220. [Google Scholar]
- 29.Yamakoshi Y., Lu Y., Hu J.C., Kim J.W., Iwata T., Kobayashi K. Porcine dentin sialophosphoprotein: length polymorphisms, glycosylation, phosphorylation, and stability. J Biol Chem. 2008;283:14835–14844. doi: 10.1074/jbc.M800633200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butler W.T., Bhown M., DiMuzio M.T., Cothran W.C., Linde A. Multiple forms of rat dentin phosphoproteins. Arch Biochem Biophys. 1983;225:178–186. doi: 10.1016/0003-9861(83)90021-8. [DOI] [PubMed] [Google Scholar]
- 31.Yamakoshi Y. Dentin sialophosphoprotein (DSPP) and gentin. J Biosci. 2008;50:33–44. [Google Scholar]
- 32.Hopkins D.R., Keles S., Greenspan D.S. The bone morphogenetic protein 1/tolloid-like metalloproteinases. Matrix Biol. 2007;26:508–523. doi: 10.1016/j.matbio.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuchiya S., Simmer J.P., Hu J.C., Richardson A.S., Yamakoshi F., Yamakoshi Y. Astacin proteases cleave dentin sialophosphoprotein (Dspp) to generate dentin phosphoprotein (Dpp) J Bone Miner Res. 2011;26:220–228. doi: 10.1002/jbmr.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto R., Oida S., Yamakoshi Y. Dentin sialophosphoprotein-derived proteins in the dental pulp. J Dent Res. 2015;94:1120–1127. doi: 10.1177/0022034515585715. [DOI] [PubMed] [Google Scholar]
- 35.Oida S., Nagano T., Yamakoshi Y., Ando H., Yamada M., Fukae M. Amelogenin gene expression in porcine odontoblasts. J Dent Res. 2002;81:103–108. [PubMed] [Google Scholar]
- 36.Suzuki S., Sreenath T., Haruyama N., Honeycutt C., Terse A., Cho A. Dentin sialoprotein and dentin phosphoprotein have distinct roles in dentin mineralization. Matrix Biol. 2009;28:221–229. doi: 10.1016/j.matbio.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamashiro T., Zheng L., Shitaku Y., Saito M., Tsubakimoto T., Takada K. Wnt10a regulates dentin sialophosphoprotein mRNA expression and possibly links odontoblast differentiation and tooth morphogenesis. Differentiation. 2007;75:452–462. doi: 10.1111/j.1432-0436.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- 38.Yuan G.H., Yang G.B., Wu L.A., Chen Z., Chen S. Potential role of dentin sialoprotein by inducing dental pulp mesenchymal stem cell differentiation and mineralization for dental tissue repair. Dental Hypotheses. 2010;1:69–75. doi: 10.5436/j.dehy.2010.1.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozer A., Yuan G., Yang G., Wang F., Li W., Yang Y. Domain of dentine sialoprotein mediates proliferation and differentiation of human periodontal ligament stem cells. PLoS One. 2013;8:e81655. doi: 10.1371/journal.pone.0081655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tziafas D., Kodonas K. Differentiation potential of dental papilla, dental pulp, and apical papilla progenitor cells. J Endod. 2010;36:781–789. doi: 10.1016/j.joen.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Bakopoulou A., Leyhausen G., Volk J., Tsiftsoglou A., Garefis P., Koidis P. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP) Arch Oral Biol. 2011;56:709–721. doi: 10.1016/j.archoralbio.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 42.Liu M., Sun Y., Liu Y., Yuan M., Zhang Z., Hu W. Modulation of the differentiation of dental pulp stem cells by different concentrations of β-glycerophosphate. Molecules. 2012;17:1219–1232. doi: 10.3390/molecules17021219. [DOI] [PMC free article] [PubMed] [Google Scholar]