Summary
In our aging society, the number of patients with dysphagia, which is associated with disease and aging, is rapidly increasing. The swallowing reflex is a complex process that involves coordinated contractions of swallowing muscles. Many researchers have reported that age-related changes, such as frailty and sarcopenia, affect swallowing muscles and contribute to the decline in the swallowing function. Thus, simple, non-invasive evaluation methods and exercises for swallowing muscles in elderly patients with dysphagia are important.
Anterior–superior hyolaryngeal elevation during swallowing results from contractions of the suprahyoid muscle, which plays a primary role in opening the upper esophageal sphincter, along with relaxation of the cricopharyngeal muscle and laryngeal closure. Thus, many researchers have studied methods for evaluating and augmenting suprahyoid muscles. On the other hand, some researchers have reported on dysphagia rehabilitation focused on jaw-opening actions, because hyolaryngeal elevation muscles correspond with jaw-opening muscles. In this study, we describe a new dysphagia evaluation method and an exercise that focuses on suprahyoid muscles with application of jaw-opening actions.
Abbreviations: SH, suprahyoid; sEMG, surface electromyography; 320-ADCT, 320-row area detector computed tomography; JOF, jaw-opening force; JOE, jaw-opening exercise; JOFT, jaw-opening force test; JOR, jaw-opening against resistance
Keywords: Dysphagia, Suprahyoid muscle, Swallowing, Hyoid, Jaw-opening, Aging
1. Introduction
Dysphagia or swallowing difficulty is a common problem in elderly. In Japan, the prevalence of dysphagia has been reported at 13.8% in community-dwelling elderly individuals [1], and 63.8% in nursing home residents [2]. Symptoms of dysphagia occur not only due to cerebrovascular and neuromuscular disease, but also due to frailty [3], [4] and sarcopenia [5], [6]. Frailty has been defined by Fried et al. [7] as “a clinical state of increased vulnerability and decreased ability to maintain homeostasis that is age-related and centrally characterized by declines in functional reserves across multiple physiologic systems.” Sarcopenia is a geriatric syndrome characterized by progressive and generalized loss of skeletal mass, strength, and function [8]. The swallowing reflex is a complex process that involves coordinated contraction of swallowing muscles. The swallowing muscles, including the tongue [9], [10], suprahyoid (SH) [11], [12], and pharyngeal muscles [13], are affected by frailty and sarcopenia, which contribute to the decline of the swallowing function. The relationships between dysphagia and frailty or sarcopenia are interdependent. The presence of dysphagia, including malnutrition and aspiration pneumonia, contributes to the development of frailty and sarcopenia, while frailty and sarcopenia contribute to dysphagia. Thus, dysphagia rehabilitation should include a swallowing evaluation, and exercises should be recommended to patients with age-related dysphagia to prevent aspiration pneumonia and improve swallowing function.
Jaw-movement, a common phrase in gerodontology, is comprised of closing movement, lateral movement, and opening movement. Jaw-opening is achieved by contraction of the SH muscles and the lateral pterygoid. Some SH muscles are involved not only in hyoid elevation but also in jaw opening, by virtue of pulling the lower jaw down via contraction of the muscles. These muscles include the mylohyoid muscle, the anterior belly of the digastric muscles, and the geniohyoid muscle. SH muscles also play a primary role in elevating hyolaryngeal structures during swallowing. In other words, the jaw-opening muscles correspond with certain hyoid elevation muscles. In this review article, we describe a newly-developed dysphagia evaluation method and an exercise that focuses on SH muscles with application of a jaw-opening actions.
2. Suprahyoid muscle contraction during swallowing: significance and traditional evaluation
Bolus transport from oral cavity into pharynx is archived by dynamical lingual deformation and movement. As the bolus is propelled into the upper esophagus, the pharynx is typically completely obliterated by the tongue pushing against the contracting posterior pharyngeal wall [14]. In addition, the upper esophageal sphincter (UES) is opened via anterior–superior traction of the hyoid and larynx, due to SH muscle contraction on the relaxed cricopharyngeal muscle [15], [16], [17]. Decreased elevation of the hyoid and larynx causes insufficient opening of the UES, resulting in an increased amount of pharyngeal residue and risk of aspiration [18], [19]. Superior hyolaryngeal excursion during swallowing is thought to contribute to airway protection prevented aspiration. Anterior hyolaryngeal excursion is thought to be more related to upper esophageal sphincter opening [16], [17], [19], [20]. The mylohyoid and geniohyoid muscles attach to the body of the hyoid [21]. The digastric and stylohyoid muscles, which have no direct attachments to the hyoid bone, are connected with SH muscles and tendinous or fibrous connective tissue [21]. SH muscles play a primary role in controlling hyoid bone movement during swallowing due to their attachment to the hyoid bone [22], [23]. Various techniques have been used to study the physiological and biomechanical aspects of SH muscles. A well-known videofluorographic study can evaluate SH muscle strength indirectly by verifying and quantifying the upward and subsequent forward movement of the hyoid bone during swallowing [24], [25]. Logemann reported that older men exhibited significantly reduced maximal superior and anterior hyoid movement, as compared to younger men. These data support the hypothesis of reduced muscular reserve [26]. Electromyography (EMG) recordings have been used to assess SH muscle activity patterns [27], [28], [29]. Submental surface electromyography (sEMG) recordings are commonly used in the investigation of swallowing disorders and are recorded simultaneously from the submental muscles which consist of mylohyoid, anterior belly of the digastric, geniohyoid, genioglossus, and platysma [30]. However, the primary contributions to submental surface recordings were made by the mylohyoid, anterior belly of the digastric, and geniohyoid muscles [30]. Contributions from the genioglossus and platysma muscles were minimal [30]. Electromyography studies have been used to analyze the temporal characteristics and amplitude aspects of SH muscle contraction, as well as activity patterns of the SH muscle. The duration of submental sEMG activities are affected by sensory inputs such as volume and viscosity of the bolus swallowed [27], [28], [29]. Furthermore, Pearson et al. reported that, based on physiological cross-sectional areas of muscles taken from cadavers, the geniohyoid has the most potential to move the hyoid anteriorly, and the mylohyoid has the most potential to move the hyoid superiorly [32]. The use of 320-row area detector computed tomography (320-ADCT) facilitates quantitative kinematic analysis of dynamic changes in SH muscle contraction [33]. Okada’s 320-ADCT study proposed that the stylohyoid, posterior digastric, and mylohyoid muscles were related to upward movement of the hyoid bone [33]. The geniohyoid plays a primary role in the forward movement of the hyoid bone. In addition, other various methods, such as muscle functional magnetic resonance imaging [34] and ultrasonography [35], have been used to study SH muscles during swallowing.
3. Dysphagia evaluation focused on suprahyoid muscle strength
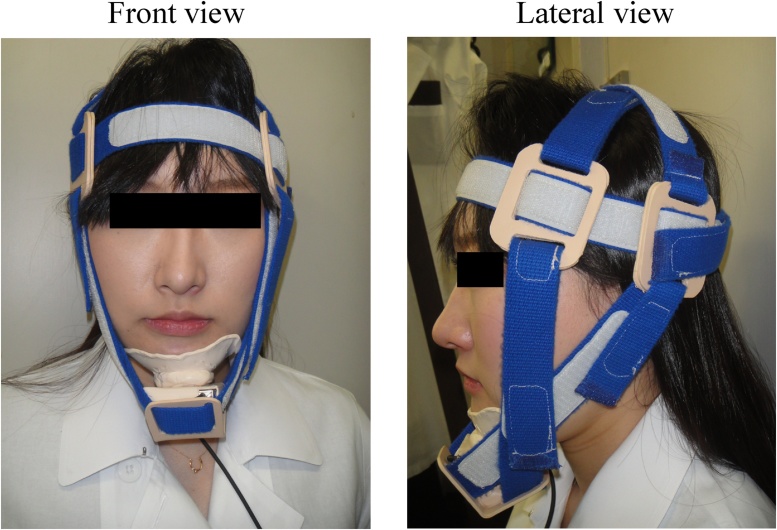
Detailed evaluations of eating disorders and dysphagia involve videofluoroscopic swallowing studies and fiberoptic endoscopic evaluation of swallowing. However, these modalities require specialized knowledge, equipment, and technical expertise. Many simplified dysphagia screening tests have been developed [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49] to predict aspiration while swallowing liquids [37], [38], [39], [40], [41], [42], [45] or semisolids [39], [46], [47] and silent aspiration via cough reflexes after inhalation of an irritant [48], [49]. Although these screening methods look for abnormal symptoms, such as loss of the swallowing reflex, presence or absence of the cough reflex, and presence or absence of wet voice, after consumption of test food or inhalation of an irritant, they do not focus on the strength of swallowing muscles to evaluate swallowing function. Many studies have also investigated the magnitude of swallowing muscle strength, including tongue pressure and pharyngeal pressure, which can be used as indicators of dysphagia. To date, no reports have focused on SH muscle strength as it relates to swallowing function. Tohara et al. [50] developed the jaw-opening sthenometer to measure the strength of SH muscles, because the jaw-opening muscles correspond to certain SH muscles. The jaw-opening sthenometer consists of fabric belts, hook and loop fasteners, thermoplastic splint material (LMB Blend: a), and a mini-isometric dynamometer (μTas F1: b) (Fig. 1). The device includes one head-encircling belt (see Fig. 1, Circle 1), two belts to secure the top of the head to the head-encircling belt (see Fig. 1, Circle 2), and two belts to secure the mandible to the head-encircling belt (see Fig. 1, Circle 3). Thermoplastic splint material is used to secure the belts (see Fig. 1, Circle 4) and chin cap (see Fig. 1, Circle 5). To assess jaw-opening strength, a dynamometer (see Fig. 1, Circle 6) is placed directly beneath the chin cap and attached to a monitor (see Fig. 1, Circle 7). The chin cap and dynamometer are secured to the head-encircling belt via the mandible belt. Fig. 2 depicts an individual wearing the jaw-opening sthenometer. Because the distance between the head and mandible and head circumference differ between subjects, the belts are adjustable with a hook and loop fastener. The belts are secured to ensure that they remain tight and that the jaw remains closed during measurements. Belts at the top of the head are placed such that they cross over each other (see Fig. 2, Circle 1). Chin cap belts are positioned to sandwich the ears, secure the head-encircling belt, and cross over each other just above the chin cap (see Fig. 2, Circle 2). The belts are fixed to clips on each side.
Figure 1.
Jaw-opening sthenometer. The jaw-opening sthenometer consists of fabric belts, hook and loop fasteners, thermoplastic splint material (LMB Blend), and a mini-isometric dynamometer (μTas F1). (1) One head-encircling belt. (2) Two belts to secure the top of the head to the head-encircling belt. (3) Two belts to secure the mandible to the head-encircling belt. (4) Thermoplastic splint material to secure the belts, (5) Chin cap. (6) Dynamometer. (7) Monitor.
Figure 2.
Front and lateral views of the jaw-opening sthenometer. (1) Belts at the top of the head are placed such that they cross over each other. (2) Chin cap belts are positioned to sandwich the ears, secure the head-encircling belt, and cross over each other just above the chin cap.
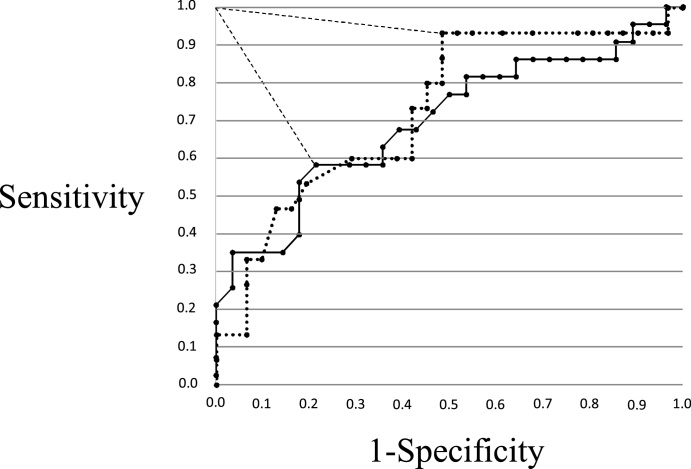
Measurements using the jaw-opening sthenometer had high intra- (interclass correlation coefficient: ICC = 0.96) and inter-subject (ICC = 0.94) reliability in average data when measured twice [51]. In healthy adult subjects (mean age, 44.7 ± 12.6 years), the mean jaw-opening force (JOF) was approximately 8 kg (mean male JOF, approximately 10 kg; mean female JOF, approximately 6 kg) [50]. Furthermore, JOF was significantly correlated with handgrip strength (r = 0.69, p < 0.01), but not with age (r = 0.16, p < 0.21) [50]. Iida et al. [12] reported on the relationship between JOF and aging. In 150 healthy volunteers, JOF was measured and compared between an adult group (mean age, 48.8 ± 13.8 years; range, 23–69) and an elderly group (mean age, 78.1 ± 4.8 years; range, 70–92). The mean JOF of the adult group was approximately 10 kg in men and approximately 6 kg in women, which was significantly greater than the mean JOF of the elderly group (approximately 7 kg in men and approximately 4 kg in women) [12] (Table 1). Feng et al. [11] reported that, based on CT images of geniohyoid muscles, fatty infiltration increases and muscle fibers decrease with aging, leading to decreased muscle mass. Consistent with this report, we considered all SH muscles, including the geniohyoid muscle, as contributing to decreases in JOF. There were not, however, dysphagia screening tests that focused on SH muscle strength and predicted pharyngeal residue, which can lead to aspiration after swallowing [52]. Hara et al. [53] studied patients complaining of dysphagia with chronic underlying causes, and assessed JOF in an attempt to predict dysphagia (jaw-opening force test: JOFT) (Table 2). Forces of 3.2 kg for men and 4.0 kg for women were used as cutoff values for predicting aspiration, with a sensitivity and specificity of 0.57 and 0.79 for men, and 0.93 and 0.52 for women, respectively (Fig. 3). For prediction of pharyngeal residue, forces of 5.3 kg in men and 3.9 kg in women were used as cutoff values, with a sensitivity and specificity of 0.80 and 0.88 for men, and 0.83 and 0.81 for women, respectively (Fig. 4). This study suggested that the JOFT could predict pharyngeal residue. Pharyngeal residue is a risk factor of aspiration after swallowing and influenced by various parameters, including movement at the base of the tongue [54], pharyngeal constriction [55], hyoid laryngeal elevation [18], [19], and pharyngeal shortening [56], [57].
Table 1.
Jaw-opening force in healthy and dysphagic subjects.
| Men |
Women |
|||
|---|---|---|---|---|
| Jaw-opening force (kg) | Mean age (y) | Jaw opening force (kg) | Mean age(y) | |
| Healthy adults | 9.7 ± 2.8 | 48.5 ± 13.4 (N = 38) | 5.9 ± 1.6 | 49.2 ± 14.4 (N = 38) |
| Healthy elderly | 7.0 ± 2.4 | 78.1 ± 5.2 (N = 37) | 4.4 ± 1.1 | 78.1 ± 4.5 (N = 37) |
| Disphagic patients | 5.0 ± 2.9 | 75.4 ± 9.7 (N = 49) | 4.1 ± 1.8 | 79.3 ± 9.6 (N = 46) |
Figure 3.
The solid line represents men, and the dotted line represents women. The optimal cutoff point is defined as the closest point to the upper left-hand corner of the graph, and the closest distance from the upper left-hand corner is shown as a large dotted line (0.48 for men, 0.49 for women). The data points correspond to jaw-opening strengths of 3.2 kg for men and 4.0 kg for women.
Figure 4.
The solid line represents men, and the dotted line represents women. The optimal cutoff point is defined as the closest point to the upper left-hand corner of the graph, and the closest distance from the upper left-hand corner is shown as a large dotted line (0.23 for men, 0.25 for women). The data points correspond to jaw-opening strengths of 5.3 kg for men and 3.9 kg for women.
Hyolaryngeal elevation and pharyngeal shortening are related to SH muscle function. A pharyngeal pressure study using a pharyngeal manometry catheter indicated that the amplitude of pharyngeal contraction did not differ significantly in groups with and without pharyngeal residue [54]. The strength of SH muscles, therefore, is related more to pharyngeal residue than other muscles.
JOF can be used as an indicator of dysphagia, as can tongue pressure and pharyngeal pressure. However, measurement of JOF has some limitations. First, despite the high reliability of JOF measurements, the validity of these measurements, including the relationship between JOF and SH muscle strength, has not yet been studied. In future studies, JOF should be measured simultaneously with hyoid bone movement, as observed through VF or SH muscle activities recorded by sEMG. Second, JOF is controlled by three SH muscles (geniohyoid, anterior belly of the digastric, and mylohyoid), as well as the lateral pterygoid (non-SH) muscle. Conversely, the posterior belly of the digastric muscle and stylohyoid muscle are involved with SH muscles, but do not affect jaw opening. The JOF is therefore affected by the lateral pterygoid, which is not involved in swallowing, but is not affected by the posterior belly of the digastric muscle and stylohyoid. We consider it unlikely that these muscles would greatly influence JOF measurement because of their size [32]. Third, JOF is a maximal force, created by the lateral pterygoid and three SH muscles. Swallowing does not require maximal SH muscle strength in hyoid elevation. Regarding tongue pressure, maximal tongue strength decreases gradually with aging [9], [10], whereas swallowing tongue strength does not. Therefore, healthy elderly individuals can maintain swallowing tongue strength, even if maximal tongue strength is decreased [55]. It is noteworthy that JOF differs with the strength of the three involved SH muscles during swallowing. A detailed study on the relationship between JOF and SH muscle strength will be required to address these issues.
4. Dysphagia rehabilitation to augment suprahyoid muscles
Decreased hyolaryngeal elevation contributes to insufficient UES opening, which results in aspiration and pharyngeal residue [19]. To improve hyolaryngeal elevation, many researchers have reported on various types of exercise and maneuvers that augment SH muscles. The Mendelsohn maneuver and effortful swallow are specific swallowing maneuvers designed to enhance SH muscle activation and improve hyolaryngeal elevation [14], [56]. Surface electrical stimulation of SH muscles has been gaining attention for its muscle-strengthening effect by motor stimulation and facilitation of swallowing reflex by sensory stimulation [57], [58]. Expiratory muscle strength training increases motor unit recruitment of the SH muscle complex [59]. On the other hand, the Shaker Exercise [60] may be the most well-known training technique for dysphagia patients with abnormal UES opening to improve hyolaryngeal elevation. The Shaker Exercise needs head-lift in the supine position and that consists of isometric and isokinetic exercise a day for six weeks. Patients raise their head as they observe their toes with contacting their shoulders on the ground (Fig. 5). The isometric exercise component involves three consecutive sustained head raisings for 1-min each with a 1-min rest period between each head raising. The isokinetic component involves 30 consecutive head raising motions performed in the same supine position. This head-lifting exercise strengthens not only the SH muscles, but also the thyrohyoid muscle [61]. Application of this exercise in normal elderly [60] and tube feeding patients with severe dysphagia [62] resulted in improved hyolaryngeal elevation and wider UES opening. Although substantial evidence demonstrates the efficiency of the Shaker Exercise, we consider some problems regarding protocol and compliance. Some researchers believe that the rather strenuous protocol decreases compliance due to sternocleidomastoid muscle discomfort, especially in elderly, frail patients [63], [64]. Easterling et al. [65] concluded that a structured and gradually progressive program is necessary for the elderly to follow in order to achieve Shaker Exercise goals. In their study of 26 elderly adults (aged 66–93 years) without swallowing problems, only 50% of participants completed the prescribed isometric goals and only 70% completed the prescribed isokinetic goals in an exercise regimen that spanned six weeks. Regarding the times per day the Shaker Exercise was performed, similar effects have been observed in those who performed the Shaker Exercise once a day and those who performed it three times a day [66]. Patients who cannot lift their heads and flex their necks, such as those with cervical spondylosis, cannot perform this exercise. Therefore, application of the Shaker Exercise should be reconsidered.
Figure 5.
Shaker Exercise.
Patients raise their head able to observe their toes with contacting their shoulders on the ground.
A few years later, Wada et al. [67] reported the jaw-opening exercise (JOE) for improving hyolaryngeal elevation and UES opening. The JOE is an isometric exercise performed in the seated position twice per day for four weeks. The protocol is as follows: first, subjects open their jaws to the maximum extent and maintain this position for 10 s (Fig. 6). During the exercise, each patient is made aware that the SH muscles are strongly contracted. This open-and-hold exercise is performed five times with 10 s of rest, which is counted as one set. Application of this exercise in mild and moderate dysphagia patients resulted in improved upward hyoid elevation, width of UES, and pharyngeal passage time [67]. There are also reports on jaw-opening for dysphagia rehabilitation. Watts [68] compared SH muscle activity in JOE against resistance and a head-lift exercise for a duration of 10 s. In their study, isometric JOE against resistance resulted in greater activation of the SH muscles than an isometric head-lift exercise. Furthermore, Kraaijenga et al. [69] developed a jaw-opening against resistance (JOR) exercise using a Swallowing Exercise Aid, in which a device loads on mandibular against jaw-opening to strengthen SH muscles. Application of JOR in healthy elderly individuals improved jaw-opening strength and SH muscle volume. Moreover, jaw movement actions, including non-isometric jaw-opening actions, improved trismus-related symptoms effectively after surgery for head and neck cancer [70]. These reports demonstrate the efficacy of jaw-opening exercises for dysphagia rehabilitation, because jaw-opening in the seated position allows more simple movement than head-lifting in the supine position, where jaw-opening actions can directly overload SH muscles. However, there have not yet been enough reports or evidence to support dysphagia rehabilitation focused on jaw-opening actions. JOE was unable to improve anterior hyoid movement despite improved upward hyoid movement and UES width [67]. Upper esophageal sphincter opening is correlated with anterior hyoid movement, rather than upward moment. Patients with a history of mandibular arthritis should avoid dysphagia rehabilitation using jaw-opening due to excessive overloading of the mandibular joint. However, dysphagia rehabilitation using jaw-opening can be applied to patients who cannot lift their heads and flex their necks to perform the Shaker Exercise. Further studies of dysphagia rehabilitation, focusing on jaw-opening movements, may contribute to advancements in dysphagia rehabilitation.
Figure 6.
Jaw-opening exercise.
Patients open their jaws to the maximum extent and maintain this position for 10 s.
Suppliers
a. Hogy Medical Co., Ltd., 2-7-7 Akasaka, Minato-ku, Tokyo, Japan.
b. Anima Inc., 3-65-1 Shimoishihara, Chofu-shi, Tokyo, Japan.
5. Conclusion
We described dysphagia rehabilitation techniques, focusing on SH muscles and jaw-opening actions. The jaw-opening sthenometer can be used to evaluate SH muscle strength quickly and noninvasively, and JOF can be used as an indicator of dysphagia. Jaw-opening exercises can be used to directly strengthen SH muscles and improve swallowing function for abnormal UES opening. Further study on SH muscles, focusing on jaw-opening actions, may contribute to advances not only in dysphagia rehabilitation, but also in gerodontology.
Footnotes
Scientific field of dental science: Gerodontology.
References
- 1.Kawashima K., Motohashi Y., Fujishima I. Prevalence of dysphagia among community-dwelling elderly individuals as estimated using a questionnaire for dysphagia screening. Dysphagia. 2004;19:266–271. doi: 10.1007/s00455-004-0013-6. [DOI] [PubMed] [Google Scholar]
- 2.Sugiyama M., Takada K., Shinde M., Matsumoto N., Tanaka K., Kuzuya M. National survey of the prevalence of swallowing difficulty and tube feeding use as well as implementation of swallowing evaluation in long-term care settings in Japan. Geriatr Gerontol Int. 2014;14:577–581. doi: 10.1111/ggi.12137. [DOI] [PubMed] [Google Scholar]
- 3.Wakabayashi H., Sashika H., Matsushima M. Head lifting strength is associated with dysphagia and malnutrition in frail older adults. Geriatr Gerontol Int. 2015;15:410–416. doi: 10.1111/ggi.12283. [DOI] [PubMed] [Google Scholar]
- 4.Hathaway B., Vaezi A., Egloff A.M., Smith L., Wasserman-Wincko T., Johnson J.T. Frailty measurements and dysphagia in the outpatient setting. Ann Otol Rhinol Laryngol. 2014;123:629–635. doi: 10.1177/0003489414528669. [DOI] [PubMed] [Google Scholar]
- 5.Maeda K., Akagi J. Sarcopenia is an independent risk factor of dysphagia in hospitalized older people. Geriatr Gerontol Int. 2015 doi: 10.1111/ggi.12486. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Maeda K., Akagi J. Decreased tongue pressure is associated with sarcopenia and sarcopenic dysphagia in the elderly. Dysphagia. 2015;30:80–87. doi: 10.1007/s00455-014-9577-y. [DOI] [PubMed] [Google Scholar]
- 7.Fried L.P., Walston J.D., Ferrucci Frailty L. In: Hazzard’s geriatric medicine and gerontology. 6th ed. Halter J.B., Ouslander J.G., Tinetti M.E., Studenski S., High K.P., Asthana S., editors. McGraw-Hill; New York, NY: 2009. [Google Scholar]
- 8.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Utanohara Y., Hayashi R., Yoshikawa M., Yoshida M., Tsuga K., Akagawa Y. Standard values of maximum tongue pressure taken using newly developed disposable tongue pressure measurement device. Dysphagia. 2008;23:286–290. doi: 10.1007/s00455-007-9142-z. [DOI] [PubMed] [Google Scholar]
- 10.Youmans S.R., Stierwalt Julie A.G. Measures of tongue function related to normal swallowing. Dysphagia. 2006;21:102–111. doi: 10.1007/s00455-006-9013-z. [DOI] [PubMed] [Google Scholar]
- 11.Feng X., Todd T., Lintzenich C.R., Ding J., Carr J.J., Ge Y. Aging-related geniohyoid muscle atrophy is related to aspiration status in healthy older adults. J Gerontol A Biol Sci Med Sci. 2013;68:853–860. doi: 10.1093/gerona/gls225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iida T., Tohara H., Wada S., Nakane A., Sanpei R., Ueda K. Aging decreases the strength of suprahyoid muscles involved in swallowing movements. Tohoku J Exp Med. 2013;231:223–228. doi: 10.1620/tjem.231.223. [DOI] [PubMed] [Google Scholar]
- 13.Herwaarden M.A., Katz P.O., Gideon M., Barrett J., Castell J.A., Achem S. Are manometric parameters of the upper esophageal sphincter and pharynx affected by age and gender? Dysphagia. 2003;18:211–217. doi: 10.1007/s00455-002-0099-7. [DOI] [PubMed] [Google Scholar]
- 14.Logemann J.A. 2nd ed. Pro-Ed; Austin: 1993. Manual for the videofluorographic study of swallowing. [Google Scholar]
- 15.Sivarao D.V., Goyal R.K. Functional anatomy and physiology of the upper esophageal sphincter. Am J Med. 2000;108(Suppl. 4a):27S–37S. doi: 10.1016/s0002-9343(99)00337-x. [DOI] [PubMed] [Google Scholar]
- 16.Jacob P., Kahrilas P.J., Logemann J.A., Shah V., Ha T. Upper esophageal sphincter opening and modulation during swallowing. Gastroenterology. 1989;97:1469–1478. doi: 10.1016/0016-5085(89)90391-0. [DOI] [PubMed] [Google Scholar]
- 17.Cook I.J., Dodds W.J., Dantas R.O. Opening mechanism of the human upper esophageal sphincter. Am J Physiol. 1989;257:G748–G759. doi: 10.1152/ajpgi.1989.257.5.G748. [DOI] [PubMed] [Google Scholar]
- 18.Murray J. Singular Publication Group; San Diego: 1999. Manual of dysphagia assessment in adults. [Google Scholar]
- 19.Steele C.M., Bailey G.L., Chau T., Molfenter S.M., Oshalla M., Waito A.A. The relationship between hyoid and laryngeal displacement and swallowing impairment. Clin Otolaryngol. 2011;36:30–36. doi: 10.1111/j.1749-4486.2010.02219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim Y., McCullough G.H. Maximum hyoid displacement in normal swallowing. Dysphagia. 2008;23:274–279. doi: 10.1007/s00455-007-9135-y. [DOI] [PubMed] [Google Scholar]
- 21.Sonoda N., Tamatsu Y. Observation on the attachment of muscles onto the hyoid bone in human adults. Okajimas Folia Anat Jpn. 2008;85:79–90. doi: 10.2535/ofaj.85.79. [DOI] [PubMed] [Google Scholar]
- 22.Ludlow C.L., Humbert I., Saxon K., Poletto C., Sonies B., Crujido L. Effects of surface electrical stimulation both at rest and during swallowing in chronic pharyngeal dysphagia. Dysphagia. 2007;22:1–10. doi: 10.1007/s00455-006-9029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuo K., Palmer J.B. Anatomy and physiology of feeding and swallowing: normal and abnormal. Phys Med Rehabil Clin N Am. 2008;19:691–707. doi: 10.1016/j.pmr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dodds W.J., Stewart E.T., Logemann J.A. Physiology and radiology of the normal oral and pharyngeal phases of swallowing. AJR Am J Roentgenol. 1990;154:953–963. doi: 10.2214/ajr.154.5.2108569. [DOI] [PubMed] [Google Scholar]
- 25.Perlman A.L., VanDaele D.J., Otterbacher M.S. Quantitative assessment of hyoid bone displacement from video images during swallowing. J Speech Hear Res. 1995;38:579–585. doi: 10.1044/jshr.3803.579. [DOI] [PubMed] [Google Scholar]
- 26.Logemann J.A., Pauloski B.R., Rademaker A.W., Colangelo L.A., Kahrilas P.J., Smith C.H. Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. J Speech Lang Hear Res. 2000;43:1264–1274. doi: 10.1044/jslhr.4305.1264. [DOI] [PubMed] [Google Scholar]
- 27.Miyaoka Y., Ashida I., Kawakami S., Tamaki Y., Miyaoka S. Activity patterns of the suprahyoid muscles during swallowing of different fluid volumes. J Oral Rehabil. 2010;37:575–582. doi: 10.1111/j.1365-2842.2010.02081.x. [DOI] [PubMed] [Google Scholar]
- 28.Ertekin C., Aydoğdu I., Yüceyar N., Pehlivan M., Ertaş M., Celebi G. Effects of bolus volume on oropharyngeal swallowing: an electrophysiologic study in man. Am J Gastroenterol. 1997;92:2049–2053. [PubMed] [Google Scholar]
- 29.Ashida I., Iwamori H., Kawakami S.Y., Miyaoka Y., Murayama A. Analysis of the pattern of suprahyoid muscle activity during pharyngeal swallowing of foods by healthy young subjects. J Med Eng Technol. 2010;34:268–273. doi: 10.3109/03091901003646096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer P.M., Luschei E.S., Jaffe D., McCulloch T.M. Contributions of individual muscles to the submental surface electromyogram during swallowing. J Speech Lang Hear Res. 1999;42:1378–1391. doi: 10.1044/jslhr.4206.1378. [DOI] [PubMed] [Google Scholar]
- 32.Pearson W.G., Langmore S.E., Zumwalt A.C. Evaluating the structural properties of suprahyoid muscles and their potential for moving the hyoid. Dysphagia. 2011;26:345–351. doi: 10.1007/s00455-010-9315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okada T., Aoyagi Y., Inamoto Y., Saitoh E., Kagaya H., Ueda K. Dynamic change in hyoid muscle length associated with trajectory of hyoid bone during swallowing: analysis using 320-row area detector computed tomography. J Appl Physiol. 2013;115:1138–1145. doi: 10.1152/japplphysiol.00467.2013. [DOI] [PubMed] [Google Scholar]
- 34.Pearson W.G., Jr., Hindson D.F., Langmore S.E., Zumwalt A.C. Evaluating swallowing muscles essential for hyolaryngeal elevation by using muscle functional magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2013;85:735–740. doi: 10.1016/j.ijrobp.2012.07.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chi-Fishman G., Sonies B.C. Effects of systematic bolus viscosity and volume changes on hyoid movement kinematics. Dysphagia. 2002;17:278–287. doi: 10.1007/s00455-002-0070-7. [DOI] [PubMed] [Google Scholar]
- 36.Nishiwaki K., Tsuji T., Liu M., Hase N., Fujiwara T. Identification of a simple screening tool for dysphagia in patients with stroke using factor analysis of multiple dysphagia variable. J Rehabil Med. 2005;37:247–251. doi: 10.1080/16501970510026999. [DOI] [PubMed] [Google Scholar]
- 37.DePippo K.L., Holas M.A., Reding M.J. Validation of the 3-oz water swallow test for aspiration following stroke. Arch Neurol. 1992;49:1259–1261. doi: 10.1001/archneur.1992.00530360057018. [DOI] [PubMed] [Google Scholar]
- 38.Wu M.C., Chang Y.C., Wang T.G., Lin L.C. Evaluating swallowing dysfunction using a 100-ml water swallowing test. Dysphagia. 2004;19:43–47. doi: 10.1007/s00455-003-0030-x. [DOI] [PubMed] [Google Scholar]
- 39.Tohara H., Saitoh E., Mays K.A., Kuhlemeier K., Palmer J.B. Three tests for predicting aspiration without videofluorography. Dysphagia. 2003;18:126–134. doi: 10.1007/s00455-002-0095-y. [DOI] [PubMed] [Google Scholar]
- 40.Teramoto S., Fukuchi Y. Detection of aspiration and swallowing disorder in older stroke patients: simple swallowing provocation test versus water swallowing test. Arch Phys Med Rehabil. 2000;81:1517–1519. doi: 10.1053/apmr.2000.9171. [DOI] [PubMed] [Google Scholar]
- 41.Osawa A., Maeshima S., Tanahashi N. Water-swallowing test: screening for aspiration in stroke patients. Cerebrovasc Dis. 2013;35:276–281. doi: 10.1159/000348683. [DOI] [PubMed] [Google Scholar]
- 42.Patterson J.M., Hildreth A., McColl E., Carding P.N., Hamilton D., Wilson J.A. The clinical application of the 100mL water swallow test in head and neck cancer. Oral Oncol. 2011;47:180–184. doi: 10.1016/j.oraloncology.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 43.Martino R., Silver F., Teasell R., Bayley M., Nicholson G., Streiner D.L. The Toronto Bedside Swallowing Screening Test (TOR-BSST): development and validation of a dysphagia screening tool for patients with stroke. Stroke. 2009;40:555–561. doi: 10.1161/STROKEAHA.107.510370. [DOI] [PubMed] [Google Scholar]
- 44.DePippo K.L., Holas M.A., Reding M.J. The Burke dysphagia screening test: validation of its use in patients with stroke. Arch Phy Med Rehabil. 1994;75:1284–1286. [PubMed] [Google Scholar]
- 45.Mari F., Matei M., Ceravolo M.G., Pisani A., Montesi A., Provinciali L. Predictive value of clinical indices in detecting aspiration in patients with neurological disorders. J Neurol Neurosurg Psychiatry. 1997;63:456–460. doi: 10.1136/jnnp.63.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chong M.S., Lieu P.K., Sitoh Y.Y., Meng Y.Y., Leow L.P. Bedside clinical methods useful as screening test for aspiration in elderly patients with recent and previous stroke. Ann Acad Med Singapore. 2003;32:790–794. [PubMed] [Google Scholar]
- 47.Lim S.H., Lieu P.K., Phua S.Y., Seshadri R., Venketasubramanian N., Lee S.H. Accuracy of bedside clinical methods compared with fiberoptic endoscopic examination of swallowing (FEES) in determining the risk of aspiration in acute stroke patients. Dysphagia. 2001;16:1–6. doi: 10.1007/s004550000038. [DOI] [PubMed] [Google Scholar]
- 48.Wakasugi Y., Tohara H., Hattori F., Motohashi Y., Nakane A., Goto S. Screening test for silent aspiration at the bedside. Dysphagia. 2008;23:364–370. doi: 10.1007/s00455-008-9150-7. [DOI] [PubMed] [Google Scholar]
- 49.Sato M., Tohara H., Iida T., Wada S., Inoue M., Ueda K. A simplified cough test for screening silent aspiration. Arch Phy Med Rehabil. 2012;93:1982–1986. doi: 10.1016/j.apmr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 50.Tohara H., Wada S., Sanpei R., Inoue M., Sato M., Ueda K. Development of a jaw-opening sthenometer to assess swallowing functions first report: jaw opening muscle strength of healthy volunteers. Jpn J Gerodontol. 2011;26:78–84. [in Japanese] [Google Scholar]
- 51.Hara K., Tohara H., Wada S., Nakane A., Minakuchi S., Ansai T. Development of a jaw-opening sthenometer to assess swallowing functions third report: the reliability of a jaw opening sthenometer for jaw opening strength. Jpn J Gerodontol. 2013;28:361–365. [in Japanese] [Google Scholar]
- 52.Eisenhuber E., Schima W., Schober E., Pokieser P., Stadler A., Scharitzer M. Videofluoroscopic assessment of patient with dysphagia: pharyngeal retention is a predictive factor for aspiration. Am J Roentgenol. 2002;78:393–398. doi: 10.2214/ajr.178.2.1780393. [DOI] [PubMed] [Google Scholar]
- 53.Hara K., Tohara H., Wada S., Iida T., Ueda K., Ansai T. Jaw-opening force test to screen for Dysphagia: preliminary results. Arch Phys Med Rehabil. 2014;95:867–874. doi: 10.1016/j.apmr.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Dejaeger E., Pelemans W., Ponette E., Joosten E. Mechanism involved in postdeglutition in the elderly. Dysphagia. 1997;12:63–67. doi: 10.1007/PL00009520. [DOI] [PubMed] [Google Scholar]
- 55.Ekberg O., Nylander G. Pharyngeal constrictor paresis in patients with dysphagia: a cineradiographic study. Clin Radiol. 1982;33:253–258. doi: 10.1016/s0009-9260(82)80253-5. [DOI] [PubMed] [Google Scholar]
- 56.Olsson R., Castell J., Johnston B., Ekberg O., Castell D.O. Combined videomanometric identification of abnormalities related to pharyngeal retention. Acad Radiol. 1997;4:349–354. doi: 10.1016/s1076-6332(97)80116-x. [DOI] [PubMed] [Google Scholar]
- 57.Kahrilas P.J., Lin S., Logemann J.A., Ergun G.A., Facchini F. Deglutitive tongue action: volume accommodation and bolus propulsion. Gastroenterology. 1993;104:152–162. doi: 10.1016/0016-5085(93)90847-6. [DOI] [PubMed] [Google Scholar]
- 58.Ludlow C.L., Humbert I., Saxon K., Poletto C., Sonies B., Crujido L. Effects of surface electrical stimulation both at rest and during swallowing in chronic pharyngeal dysphagia. Dysphagia. 2007;22:1–10. doi: 10.1007/s00455-006-9029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wheeler K.M., Chiara T., Sapienza C.M. Surface electromyographic activity of the submental muscles during swallow and expiratory pressure threshold training tasks. Dysphagia. 2007;22:108–116. doi: 10.1007/s00455-006-9061-4. [DOI] [PubMed] [Google Scholar]
- 60.Shaker R., Kern M., Bardan E., Taylor A., Stewart E.T., Hoffmann R.G. Augmentation of deglutitive upper esophageal sphincter opening in the elderly by exercise. Am J Physiol. 1997;272:G1518–G1522. doi: 10.1152/ajpgi.1997.272.6.G1518. [DOI] [PubMed] [Google Scholar]
- 61.Mepani R., Antonik S., Massey B., Kern M., Logemann J., Shaker R. Augmentation of deglutitive thyrohyoid muscle shortening by the Shaker Exercise. Dysphagia. 2009;24:26–31. doi: 10.1007/s00455-008-9167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shaker R., Easterling C., Kern M., Nitschke T., Massey B., Daniels S. Rehabilitation of swallowing by exercise in tube-fed patients with pharyngeal dysphagia secondary to abnormal UES opening. Gastroenterology. 2002;122:1314–1321. doi: 10.1053/gast.2002.32999. [DOI] [PubMed] [Google Scholar]
- 63.Easterling C., Grande B., Kern M., Sears K., Shaker R. Attaining and maintaining isometric and isokinetic goals of the Shaker exercise. Dysphagia. 2005;20:133–138. doi: 10.1007/s00455-005-0004-2. [DOI] [PubMed] [Google Scholar]
- 64.Yoon W.L., Khoo J.K., Rickard Liow S.J. Chin tuck against resistance (CTAR): new method for enhancing suprahyoid muscle activity using a Shaker-type exercise. Dysphagia. 2014;29:243–248. doi: 10.1007/s00455-013-9502-9. [DOI] [PubMed] [Google Scholar]
- 65.Easterling C., Grande B., Kern M., Sears K., Shaker R. Attaining and maintaining isometric and isokinetic goals of the Shaker exercise. Dysphagia. 2005;20:133–138. doi: 10.1007/s00455-005-0004-2. [DOI] [PubMed] [Google Scholar]
- 66.Woo H.S., Won S.Y., Chang K.Y. Comparison of muscle activity between two adult groups according to the number of Shaker exercise. J Oral Rehabil. 2014;41:409–415. doi: 10.1111/joor.12165. [DOI] [PubMed] [Google Scholar]
- 67.Wada S., Tohara H., Iida T., Inoue M., Sato M., Ueda K. Jaw opening exercise for insufficient opening of upper esophageal sphincter. Arch Phys Med Rehabil. 2012;93:1995–1999. doi: 10.1016/j.apmr.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 68.Watts C.R. Measurement of hyolaryngeal muscle activation using surface electromyography for comparison of two rehabilitative dysphagia exercises. Arch Phys Med Rehabil. 2013;94:2542–2548. doi: 10.1016/j.apmr.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 69.Kraaijenga S.A., van der Molen L., Stuiver M.M., Teertstra H.J., Hilgers F.J., van den Brekel M.W. Effects of strengthening exercises on swallowing musculature and function in senior healthy subjects: a prospective effectiveness and feasibility study. Dysphagia. 2015;30:392–403. doi: 10.1007/s00455-015-9611-8. [DOI] [PubMed] [Google Scholar]
- 70.Pauli N., Andréll P., Johansson M., Fagerberg-Mohlin B., Finizia C. Treating trismus: a prospective study on effect and compliance to jaw exercise therapy in head and neck cancer. Head Neck. 2014 doi: 10.1002/hed.23818. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]