Abstract
Plant secondary compounds (PSCs), also called secondary metabolites, have high chemical and structural diversity and appear as non-volatile or volatile compounds. These compounds may have evolved to have specific physiological and ecological functions in the adaptation of plants to their growth environment. PSCs are produced by several metabolic pathways and many PSCs are specific for a few plant genera or families. In forest ecosystems, full-grown trees constitute the majority of plant biomass and are thus capable of producing significant amounts of PSCs. We summarize older literature and review recent progress in understanding the effects of abiotic and biotic factors on PSC production of forest trees and PSC behavior in forest ecosystems. The roles of different PSCs under stress and their important role in protecting plants against abiotic and biotic factors are also discussed. There was strong evidence that major climate change factors, CO2 and warming, have contradictory effects on the main PSC groups. CO2 increases phenolic compounds in foliage, but limits terpenoids in foliage and emissions. Warming decreases phenolic compounds in foliage but increases terpenoids in foliage and emissions. Other abiotic stresses have more variable effects. PSCs may help trees to adapt to a changing climate and to pressure from current and invasive pests and pathogens. Indirect adaptation comes via the effects of PSCs on soil chemistry and nutrient cycling, the formation of cloud condensation nuclei from tree volatiles and by CO2 sequestration into PSCs in the wood of living and dead forest trees.
Keywords: CO2, drought, ozone, phenolics, temperature, terpenes, UV-B, VOCs
Introduction
Plant secondary compounds (PSCs) have high chemical diversity with an estimated 200,000 compounds (Dixon and Strack, 2003). In higher plants, terpenoids (approximately 30,000 known compounds, Lämke and Unsicker, 2018), alkaloids (21,000) (Wink, 2010), and phenolic compounds (8,000) (Munné-Bosch, 2012) are the most diverse PSC groups. The majority of PSCs are related to plant chemical defense and herbivore pressure, which are considered the main drivers of PSC diversification (Lämke and Unsicker, 2018). Terpenoids originate directly from glycolysis of glucose via the mevalonate pathway or the methylerythritol phosphate (MEP) pathway (Bartram et al., 2006). Phenolic compounds originate from the shikimic acid pathway, which is related to the metabolism of carbohydrates and aromatic amino acids (Seigler, 1998; Lindroth, 2012). Most of the alkaloids are derived from amino acid precursors (Wink, 2010), but coniferous alkaloids are synthesized via the polyketide pathway (Seigler, 1998). Terpenes and phenolics are the most extensively studied PSCs in forest trees (Lindroth, 2012; Lämke and Unsicker, 2018), while alkaloids have been considered in relatively few studies (Virjamo et al., 2014). Alkaloids and phenolics may compete more with protein synthesis than terpenoids (Koricheva, 2002). Up to 10% of recently fixed carbon can be allocated to volatile organic compounds (VOCs) in stressed plants (Peñuelas and Staudt, 2010), while stored terpenoids typically require secretory structures such as resin canals and glandular trichomes to avoid autotoxicity (Goodger et al., 2013).
At the global level, climate change (CC) is strongly linked to increasing CO2 concentration in the atmosphere, which affects temperature globally (IPCC, 2014). Depletion of the stratospheric ozone (O3) layer is partly affected by elevated CO2 and leads to increased UV-B radiation (IPCC, 2014). In the lower atmosphere (troposphere) O3 acts as a greenhouse gas and is phytotoxic to plants (Vapaavuori et al., 2009; Lindroth, 2010). At the regional scale, global warming affects arctic and boreal areas most strongly and will include drastic changes, e.g., in precipitation, drought, and cloudiness (shading), affecting temperature-dependent ecosystem processes in forests such as nutrient availability (IPCC, 2014).
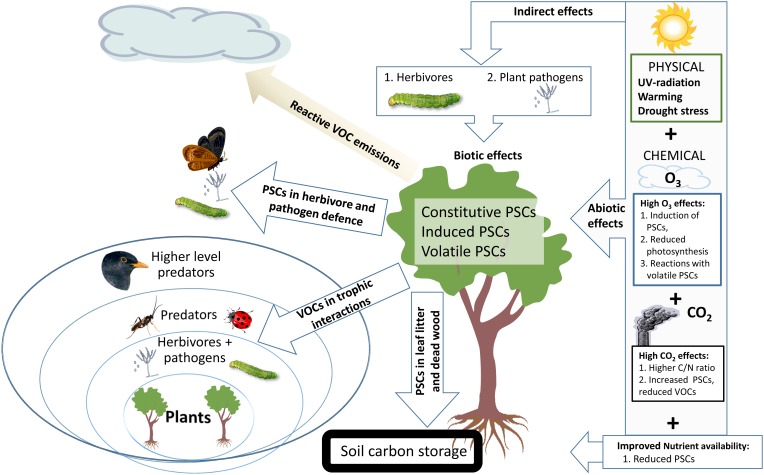
Climate change directly affects the abiotic conditions of forest trees and their growth, physiology and defense including induced PSC production (Metlen et al., 2009; Figure 1). The responses of other organisms, such as microbial plant pathogens, herbivorous insects and arrival of exotic invasive herbivores and pathogens will alter the biotic stress burden of trees (Donnelly et al., 2012). The richness of chemical compounds originating from PSCs in forest ecosystems is affected by PSC reactivity (Kundu et al., 2012). Many of the volatile reaction products form secondary organic aerosols (SOA), which may affect cloud formation (Zhao et al., 2017; Rose et al., 2018; Scott et al., 2018) and provide important ecosystem – atmosphere feedback, thus helping vegetation to adapt to CC (e.g., Niinemets, 2018). SOA particles may become wet or dry deposited on forest vegetation and accumulate in soil (Holopainen et al., 2017) together with litter PSCs (Smolander et al., 2012).
FIGURE 1.
Global climate change – related abiotic and biotic stresses and their influence on types of plant secondary compounds (PSCs) in forest trees. Ecosystem level feedbacks transmitted by leaf PSCs are indicated with arrows on the left from the target tree. PSCs in foliage provide chemical defenses against herbivores and pathogens (Lämke and Unsicker, 2018). PSCs of leaf and needle litter affect mostly on tree nutrient uptake (Smolander et al., 2012) and rhizosphere organisms while PSCs in deadwood are part of important carbon storage (Pan et al., 2011) and stored PSCs could mitigate wood decay by decomposer organisms (Nerg et al., 2004; Karppanen et al., 2007) and release of CO2 to the atmosphere. Volatile PSCs (VOCs) affect the trophic interactions in the forest ecosystem where the tree is growing (Blande et al., 2014), while reactive VOCs affect the atmosphere and may have atmosphere-biosphere level feedbacks in the surrounding ecosystems (Joutsensaari et al., 2015).
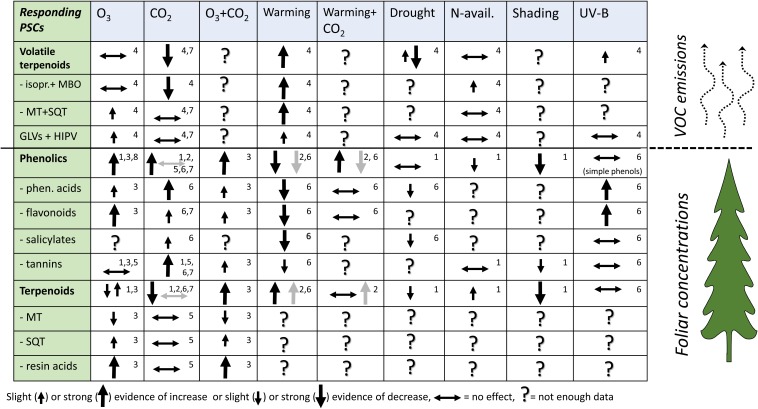
We summarize the major results of literature reviews and meta-analyses on the quantitative responses of tree PSCs to specific CC-related abiotic factors, with restriction to literature up to 2015 in Figure 2. Recent results of PSC (after 2015) and plant VOC (after 2012) research are then reviewed. We then discuss the roles of multiple CC-related stresses on PSCs of forest trees and the potential of non-volatile and volatile PSCs to modulate plant adaptation to CC and their involvement in climate feedback.
FIGURE 2.
Summary of the results in comprehensive reviews and meta-analyses of plant PSCs responses in their concentration or emissions in forest trees under climate change related stresses. This tabulation covers nearly 400 original scientific articles. Type of stresses: O3 = elevated ozone, CO2 = elevated carbon dioxide, UV-B = elevated UV-B radiation. The tabulation gives the direction of PSC responses to single environmental factors, except for the combined O3+CO2 and CO2+warming effects. Arrow direction (increase or decrease) shows the effect size (significant or highly significant effect in meta-analysis) or the amount of evidence in reviews. Horizontal bidirectional arrow indicates the studies with non-significant results and up and down arrows in the same cell indicate significant results in both directions dominate. Question mark indicates that the reviews and meta-analyses did not provide enough data. Gray arrows in CO2, warming and CO2+warming columns indicate woody tissue responses as specified in reference 2. Small numbers in the upper-right corner of reach cell indicate the source reference: 1 = Koricheva et al., 1998; 2 = Zvereva and Kozlov, 2006; 3 = Valkama et al., 2007; 4 = Peñuelas and Staudt, 2010; 5 = Lindroth, 2012; 6 = Julkunen-Tiitto et al., 2015; 7 = Robinson et al., 2012; 8 = Li et al., 2017. References 1–3 and 7–8 are meta-analyses and 4–6 literature reviews. Meta-analyses give the size of the effect, and the reviews give the frequency of observations in each response categories (–, 0, +).
Summary of Older Reviews
Terpenoids and phenolic compounds in leaves and terpenoids in emissions are the most frequently studied PSCs of forest trees in response to CC-related factors (Figure 2). Major CC factors such as elevated CO2 and warming have the most distinctive, and often contrasting effects on PSCs. Greater availability of CO2 increases phenolics in foliage and reduces terpenoids both in foliage and in emissions, while warming reduces phenolics in foliage and increases terpenoids in foliage and emissions (Zvereva and Kozlov, 2006; Peñuelas and Staudt, 2010). CO2+warming increased phenolics in foliage, but reduced phenolics in woody tissues of woody plants (Zvereva and Kozlov, 2006). O3 effects on foliar concentrations of terpenes and volatile emissions have been variable, but increased phenolic compounds have been found. One reason for variable responses of terpenes could be within-species variation in O3 sensitivity (Valkama et al., 2007) and variable O3 reactivity of terpenes (Blande et al., 2014). Major terpenoid groups such as monoterpenes (MT) and sesquiterpenes (SQT) in foliage have been less studied separately under most of the other CC related stresses.
Responses to Abiotic Factors – PSC Concentrations in Trees
Certain plant PSC groups such as flavonoids (Lavola et al., 2013) and MTs (Semiz et al., 2007) are under strong genotypic control. This may increase the potential for forest tree chemical defenses to evolutionarily adapt to environmental changes (Lavola et al., 2013). However, high within-species variation in PSCs is problematic for short-term experimental set-ups and may mask the effects of environmental factors on PSC concentrations and emissions.
Elevated CO2 and Warming
Total leaf phenolics, particularly condensed tannins (Julkunen-Tiitto et al., 2015), follow the traditional carbon-nutrient balance hypothesis (Bryant et al., 1983), which predicts that photosynthesised carbon will be diverted to carbon-rich PSCs, if photosynthesis or CO2 availability are at a high level and nutrient availability limits carbon allocation to plant growth (Koricheva et al., 1998). However, many flavonoids and other phenolics, and particularly terpenoids, do not support that hypothesis (Lindroth, 2012). Recent studies have confirmed a trend with foliar phenolics; doubled ambient CO2 increased salicylates and phenolic acids (Sobuj et al., 2018), anthocyanins and flavonoids (Vanzo et al., 2015; Nissinen et al., 2016), while less than doubled CO2 did not affect phenolics (McKiernan et al., 2012; Randriamanana et al., 2018). Furthermore, some phenolic groups, such as phenolic glycosides, are not as responsive (Jamieson et al., 2017). In Eucalyptus spp., there was no observed effect of elevated CO2 (McKiernan et al., 2012) or elevated CO2 decreased (Bustos-Segura et al., 2017) stored foliar terpenes.
Reduction in foliar phenolics at elevated temperatures (Julkunen-Tiitto et al., 2015) has been explained by a dilution effect whereby more carbon was allocated to growth supporting structures with turnover of phenolics slow or even absent (Kosonen et al., 2012). Recently, it has been reported that warming reduces flavonoids (Nissinen et al., 2016; Zhang et al., 2018), salicylates and phenolic acids (Sivadasan et al., 2018) in deciduous trees and total phenolics in a conifer (Zhang et al., 2018). Flavonoids, alkaloids, and saponins (triterpenes) were increased in Robinia pseudoacacia grown in warmer open-top chambers compared to seedlings grown in an open field (Zhao et al., 2016). However, enclosure may significantly affect foliar PSCs (Peltonen et al., 2005).
Warming induced increase in foliar terpenes (Valkama et al., 2007; Julkunen-Tiitto et al., 2015) is possibly explained by improved plant growth and larger storage space for stored terpenes such as MTs and resin acids (Sallas et al., 2003). Recent results showing higher MT concentrations in xylem resin (Ferrenberg et al., 2017) and increased number of needle resin canals (Kivimäenpää et al., 2016) in Pinus spp. in warmer environments supports this view. Warming (+2°C) increased concentrations of Picea abies needle alkaloids (Virjamo et al., 2014) and parental temperature range correlated with conifer alkaloid concentrations (Gerson et al., 2009; Virjamo and Julkunen-Tiitto, 2016).
O3
O3 is phytotoxic and reduces plant photosynthesis and growth (Bueker et al., 2015; Li et al., 2017) and induces substantial changes in protein activity, gene expression, signaling pathways, and metabolism even before any tissue damage can be detected (Bueker et al., 2015; Vainonen and Kangasjärvi, 2015). Activated PSC metabolism (increased biosynthesis and reduced turnover) explains the increased concentrations of phenolic and terpenoid PSCs in tree foliage found in a meta-analysis (Valkama et al., 2007). In response to O3 treatment, elevated levels of total phenolics (Gao et al., 2016), condensed tannins (Couture et al., 2017) and flavonoids (Cotrozzi et al., 2018) were found in foliage of deciduous trees, but this was not the case in needles of coniferous trees (Riikonen et al., 2012).
Drought
Drought in warm periods is expected to become the most frequent widespread climatic extreme that negatively affects terrestrial ecosystems (Schwalm et al., 2017). Forests in southern Europe will face stronger summer drought than those in the north (Ruosteenoja et al., 2018). The observed trends in PSCs are not strong, although reduction of some terpenes and phenolics have been reported (Koricheva et al., 1998; Julkunen-Tiitto et al., 2015). In Quercus ilex leaves, moderate drought periods resulted in higher concentrations of total polyphenolics (Nogues et al., 2014; Rivas-Ubach et al., 2014). Drought stress did not affect alkaloid concentrations in conifers (Gerson and Kelsey, 2004). Increasing severity of drought increased resin canal density and resin acid content in the stem wood of Pinus sylvestris (Heijari et al., 2010). Contents of most MTs and sesquiterpenoids in needles increased with increasing severity of water deficit, but resin acids showed the opposite trend and polyphenols did not respond (Sancho-Knapik et al., 2017). Drought slightly increased the needle MT contents of the coastal, but not of the interior Pseudotsuga menziesii provenance (Kleiber et al., 2017).
Shading
Shading limits photosynthesis and it is predicted that less carbon is allocated to PSCs in shaded conditions (Koricheva et al., 1998). Giertych et al. (2015) tested the hypothesis with light-demanding and shade-tolerant deciduous tree species. All species had higher total phenolic content of leaves in sunny conditions, but shade tolerant species invested more carbon into growth when more light was available.
UV-B
Short-wave (280–315 nm) UV-B radiation induces tree foliage to produce more phenolic acids and flavonoids as protective pigments (Julkunen-Tiitto et al., 2015; Kaling et al., 2015). In Populus tremula seedlings, two flavonoid compounds increased under enhanced UV-B radiation (Nissinen et al., 2017). Filtering out solar UV-B radiation from reaching P. tremula in mountains lead to lower flavonoid concentration, but increased salicin in leaves, and salicylates and total phenolics in stem (Stromme et al., 2018). However, a three-year exposure of Salix myrsinifolia to 32% elevated UV-B in a field site did not result in significant changes in flavonoid concentrations, and in male trees total salicylates were even reduced (Ruuhola et al., 2018). Enhanced levels of UV-B radiation did not affect alkaloid concentrations in conifers (Virjamo et al., 2014).
Responses to Abiotic Factors – Emission of Volatile PSCs
Warming
Warming is one of the most intensively studied factors in volatile PSC studies (Peñuelas and Staudt, 2010), due to VOC emissions being strongly light and temperature dependent and some, e.g., isoprene not being stored in leaves (Sharkey and Yeh, 2001). At the global scale, isoprene represents nearly 50% of the estimated biogenic VOC emission, and emissions of MTs and SQTs together comprise about 15 and 3%, respectively (Guenther et al., 2012). Warming increased emissions of volatile terpenoids most consistently (Peñuelas and Staudt, 2010) by stimulating biosynthesis (Loreto and Schnitzler, 2010; Fini et al., 2017) and temperature-dependent release from leaf storage structures (Grote and Niinemets, 2008; Peñuelas and Staudt, 2010). VOC emissions from storage structures are high in conifers, e.g., over 40% in Pinus, and very low in deciduous trees (Ghirardo et al., 2010). Differences in emission responses of MTs and SQTs to temperature might be caused by temporal storage of less volatile SQTs in leaf surface waxes (Joensuu et al., 2016), and their temperature-dependent adherence and release (Himanen et al., 2015).
Warming of +3°C increased (∼70%) emission rates of isoprene-emitting and MT-emitting Quercus spp. (Staudt et al., 2017). Isoprene-emitting Populus spp. have an emission maximum at +45°C even if grown in elevated CO2 (Potosnak et al., 2014; Niinemets and Sun, 2015). Long-term warming of 1°C+ambient increased MT (fivefold), SQT (fourfold) and green leaf volatile (40%) emissions of Betula pendula (Hartikainen et al., 2012). Ontogeny of needle development in terpene-storing conifers affects variability in MT emission rate responses to warming (Esposito et al., 2016; Kivimäenpää et al., 2016; Ghimire et al., 2017; Tiiva et al., 2018). Some specific MTs of forest trees, particularly β-ocimene are known to be heat-stress indicators (Jardine et al., 2017).
CO2
There is clear evidence that exposure to elevated CO2 reduces terpenoid-based plant VOC emissions (Peñuelas and Staudt, 2010; Potosnak et al., 2014; Niinemets and Sun, 2015; Mochizuki et al., 2017) and uncouples isoprene emissions from photosynthesis. Inhibition of isoprene synthesis becomes stronger with increasing CO2 concentration (Wilkinson et al., 2009; Rasulov et al., 2018) and isoprene is more responsive than MTs (Peñuelas and Staudt, 2010). There is still no consensus of the mechanisms that lead to the contrasting responses of photosynthesis and isoprene biosynthesis (Fini et al., 2017) and emission at elevated CO2 (Rasulov et al., 2018). In MT-storing gymnosperms the reduced MT needle concentration at elevated CO2 (Sallas et al., 2003) may partly explain reduced MT emissions.
O3
Plant VOCs easily react with O3 and samples may be destroyed if ozone scrubbers are not used in sampling (Blande et al., 2014). O3 exposure experiments have shown that plant VOC responses are variable and depend on the O3 concentration (Peñuelas and Staudt, 2010). Acute ozone exposure can induce similar VOCs to herbivore damage (Blande et al., 2014). Moderately elevated O3 concentrations increase emissions of MTs in coniferous (Kivimäenpää et al., 2013, 2016; Ghimire et al., 2017) and deciduous trees (Carriero et al., 2016), but decrease isoprene emission in poplar (Yuan et al., 2016) and MT emissions in Larix were not affected (Mochizuki et al., 2017).
Drought
Warm and dry periods lead to drought stress; mild drought may increase VOC emissions (Peñuelas and Staudt, 2010; Bourtsoukidis et al., 2014; Yuan et al., 2016), not affect (Nogues et al., 2014) or have increasing-decreasing trend (Simpraga et al., 2011), while severe drought results in a reduction of emissions (Peñuelas and Staudt, 2010; Rodriguez-Calcerrada et al., 2013; Luepke et al., 2016; Marino et al., 2017; Saunier et al., 2017; Staudt et al., 2017). Drought stimulated herbivore-induced GLV and MT emissions in Alnus glutinosa (Copolovici et al., 2014).
Shading
Shading by branch position can reduce isoprene and MT emissions from forest trees (Esposito et al., 2016; Juran et al., 2017; van Meeningen et al., 2017). This suggests that cloudiness that reduces penetration of solar radiation to the canopy (Niinemets, 2018) may reduce carbon allocation to volatile PSCs. However, in the canopy of Fagus sylvatica, MT emissions were highest in the semi-shaded leaves (Simpraga et al., 2013).
Combined Abiotic Factors Versus Non-Volatile and Volatile PSCs
The effects of eutrophication [nitrogen (N) deposition and forest fertilization] on tree VOCs are poorly known, although it appears to slightly increase isoprene emissions (Figure 2; Peñuelas and Staudt, 2010). Generally, exposure to additional abiotic factors may mitigate, intensify or not affect foliar chemical defense (Sallas et al., 2001; Zvereva and Kozlov, 2006; Vapaavuori et al., 2009) or VOC (Kivimäenpää et al., 2016; Ghimire et al., 2017; Tiiva et al., 2017) responses to the first factor. For example elevated O3 and N availability had additive effects on terpenoid emissions from a deciduous tree (Carriero et al., 2016) and conifer (Kivimäenpää et al., 2016), whereas N in addition to warming suppressed the emissions of non-oxygenated MTs from a conifer (Ghimire et al., 2017). In Populus sp. N addition (Yuan et al., 2017) or drought (Yuan et al., 2016) did not mitigate the negative effects of O3 on isoprene emission.
An elevated CO2-related decrease of terpenoids in conifer needles was mitigated by elevated temperature and CO2-induced increase in phenolics was intensified by elevated temperature (Zvereva and Kozlov, 2006). Furthermore, elevated CO2 may mitigate a typical O3 response such as ozone-induced increase of some phenolics in B. pendula (Vapaavuori et al., 2009), but in Larix CO2 and O3 did not have interactive effects on volatiles (Mochizuki, et al., 2017). Along altitudinal gradients, the total phenolics in Quercus robur foliage were found to decrease at lower elevations where temperature was warmer (Abdala-Roberts et al., 2016). Our analysis shows that in addition to a warming-induced reduction in total phenolics, elevated UV-B radiation may increase flavonoids and phenolic acids in foliage (Julkunen-Tiitto et al., 2015). However, recent combined warming and UV-B exposure outdoors has confirmed that warming has a stronger increasing effect on leaf phenolics (Nissinen et al., 2017) and terpenoid emissions (Maja et al., 2016) than elevated UV-B radiation.
Plant PSCs and Biotic Stress
Abiotic stresses affect growth, reproduction and behavior of herbivorous insects and severity of microbial plant pathogens and their biotic stress on trees (Figure 1). Furthermore, arrival of exotic invasive herbivores and pathogens with warming related to CC may add another threat, because local tree species have not adapted to use PSCs to defend against these invasive species (Donnelly et al., 2012). Increased accumulation of PSCs in plant tissues under elevated atmospheric CO2 resulted in compensation feeding and more severe defoliation (Docherty et al., 1996). Warming may reduce constitutive PSCs in foliage (Stark et al., 2015) and improve (Grainger and Gilbert, 2017) or reduce (Ghimire et al., 2017) insect performance and lead to induced production of non-volatile (Rubert-Nason et al., 2015) or volatile PSCs (Blande et al., 2010; Holopainen and Gershenzon, 2010).
PSC Transmitted Ecosystem Feedbacks to Climate Change
Forests are a globally important sink of atmospheric CO2 (Pan et al., 2011; Pukkala, 2018). Carbon is sequestered in soil carbon (44%), living biomass (42%), deadwood (8%), and litter (5%) (Pan et al., 2011). Reducing forest harvesting and adding dead wood as large trees is the fastest way to increase CO2 sequestration in boreal forests (Pukkala, 2018). Reduced decay rate of dead wood decreases release of CO2 back to the atmosphere. Concentrations of both resin acids (Karppanen et al., 2007) and stilbenes (Ioannidis et al., 2017) in heartwood of Pinus sp. can exceed 10% of dry weight and significantly reduce decay rate of dead wood by fungal rot (Karppanen et al., 2007) and by woodborers (Nerg et al., 2004).
The PSCs are released into the soil (Kainulainen et al., 2003) and atmosphere (Aaltonen et al., 2011) faster from the needle litter than dead wood. In conifer forests, MTs contribute over 90% of litter emissions peaking in early summer and during the needle fall in autumn (Aaltonen et al., 2011). After 19-months of decomposition, pine needles have lost 96, 79, and 86 percent of initial concentrations of MTs, resin acids and total phenolics, respectively (Kainulainen et al., 2003). Warm periods in the boreal conifer forests increase reactive MT emissions to the forest atmosphere and stimulate formation of SOA, particularly in evenings (Rose et al., 2018) and potentially stimulate cloudiness (Joutsensaari et al., 2015; Zhao et al., 2017), thereby reducing penetration of solar radiation to vegetation and mitigating warming and drought stress (Niinemets, 2018).
Volatile PSCs have several functions in the species interactions affecting herbivores and their natural enemies and activating defenses in healthy plants (Blande et al., 2014). CC factors also affect herbivore-induced volatiles, but studies on forest trees and woody plants remain limited (Holopainen and Gershenzon, 2010). Non-volatile PSCs released from plant leaf and needle litter accumulate in soil and affect C or net N mineralization in forest soils (e.g., Smolander et al., 2012). Growing three seasons at elevated CO2 and O3 did not affect concentrations of total phenolics and MTs in P. sylvestris needles, but at elevated O3 resin acids were increased. However, during decomposition this difference in needle litter was lost (Kainulainen et al., 2003). In leaf litter of B. pendula growing at elevated CO2 and O3 concentrations, several phenolic compounds were elevated, and the growth of juvenile earthworms was reduced (Kasurinen et al., 2007) suggesting that CC reduced quality of food for soil animals. In Alnus incana litter, UV-A and UV-B exclusion affected concentrations of phenolic groups variably, whereas in birch litter there were no significant differences in phenolic compounds (Kotilainen et al., 2009).
Concluding Remarks
There is strong evidence that CO2 increases phenolic compounds in foliage, but limits terpenoids in foliage and emissions, while warming decreases phenolic compounds in foliage, but increases terpenoids in foliage and emissions. CO2 in combination with other abiotic stresses has more variable effects. As current PSC information is mostly from tree seedlings, long-term experiments covering the whole ontogeny of trees in forest ecosystems are needed to better understand CC effects on PSCs in trees. Assessment of the role of volatile PSCs in sun-screening and non-volatile PSCs in control of decay rate of carbon storage in dead wood will be necessary to improve our knowledge of the capacity of PSCs to mitigate CC. Transgenic trees with silenced or over-expressed genes for key volatile PSCs (Kaling et al., 2015; Vanzo et al., 2015) will be important tools in this task.
Author Contributions
JH drafted the manuscript. All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was funded by University of Eastern Finland BORFOR Top Research Area and Academy of Finland, projects nos. 278424, 267360, 305343, and 309425.
References
- Aaltonen H., Pumpanen J., Pihlatie M., Hakola H., Hellen H., Kulmala L., et al. (2011). Boreal pine forest floor biogenic volatile organic compound emissions peak in early summer and autumn. Agric. For. Meteorol. 151 682–691. 10.1016/j.agrformet.2010.12.010 [DOI] [Google Scholar]
- Abdala-Roberts L., Rasmann S., Berny-Mier Y., Teran J. C., Covelo F., Glauser G., et al. (2016). Biotic and abiotic factors associated with altitudinal variation in plant traits and herbivory in a dominant oak species. Am. J. Bot. 103 2070–2078. 10.3732/ajb.1600310 [DOI] [PubMed] [Google Scholar]
- Bartram S., Jux A., Gleixner G., Boland W. (2006). Dynamic pathway allocation in early terpenoid biosynthesis of stress-induced lima bean leaves. Phytochemistry 67 1661–1672. 10.1016/j.phytochem.2006.02.004 [DOI] [PubMed] [Google Scholar]
- Blande J. D., Holopainen J. K., Niinemets Ü. (2014). Plant volatiles in polluted atmospheres: stress responses and signal degradation. Plant Cell Environ. 37 1892–1904. 10.1111/pce.12352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blande J. D., Korjus M., Holopainen J. K. (2010). Foliar methyl salicylate emissions indicate prolonged aphid infestation on silver birch and black alder. Tree Physiol. 30 404–416. 10.1093/treephys/tpp124 [DOI] [PubMed] [Google Scholar]
- Bourtsoukidis E., Kawaletz H., Radacki D., Schuetz S., Hakola H., Hellen H., et al. (2014). Impact of flooding and drought conditions on the emission of volatile organic compounds of Quercus robur and Prunus serotina. Trees Struct. Funct. 28 193–204. 10.1007/s00468-013-0942-5 [DOI] [Google Scholar]
- Bryant J., Chapin F., Klein D. (1983). Carbon nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40 357–368. 10.2307/3544308 [DOI] [Google Scholar]
- Bueker P., Feng Z., Uddling J., Briolat A., Alonso R., Braun S., et al. (2015). New flux based dose-response relationships for ozone for European forest tree species. Environ. Pollut. 206 163–174. 10.1016/j.envpol.2015.06.033 [DOI] [PubMed] [Google Scholar]
- Bustos-Segura C., Dillon S., Keszei A., Foley W. J., Kulheim C. (2017). Intraspecific diversity of terpenes of Eucalyptus camaldulensis (Myrtaceae) at a continental scale. Aust. J. Bot. 65 257–269. 10.1071/BT16183 [DOI] [Google Scholar]
- Carriero G., Brunetti C., Fares S., Hayes F., Hoshika Y., Mills G., et al. (2016). BVOC responses to realistic nitrogen fertilization and ozone exposure in silver birch. Environ. Pollut. 213 988–995. 10.1016/j.envpol.2015.12.047 [DOI] [PubMed] [Google Scholar]
- Copolovici L., Kännaste A., Remmel T., Niinemets Ü. (2014). Volatile organic compound emissions from Alnus glutinosa under interacting drought and herbivory stresses. Environ. Exp. Bot. 100 55–63. 10.1016/j.envexpbot.2013.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrozzi L., Campanella A., Pellegrini E., Lorenzini G., Nali C., Paoletti E. (2018). Phenylpropanoids are key players in the antioxidant defense to ozone of European ash, Fraxinus excelsior. Environ. Sci. Pollut. Res. 25 8137–8147. 10.1007/s11356-016-8194-8 [DOI] [PubMed] [Google Scholar]
- Couture J. J., Meehan T. D., Rubert-Nason K. F., Lindroth R. L. (2017). Effects of elevated atmospheric carbon dioxide and tropospheric ozone on phytochemical composition of trembling aspen (Populus tremuloides) and paper birch (Betula papyrifera). J. Chem. Ecol. 43 26–38. 10.1007/s10886-016-0798-4 [DOI] [PubMed] [Google Scholar]
- Dixon R., Strack D. (2003). Phytochemistry meets genome analysis, and beyond. Phytochemistry 62 815–816. 10.1016/S0031-9422(02)00712-4 [DOI] [PubMed] [Google Scholar]
- Docherty M., Hurst D., Holopainen J., Whittaker J., Lea P., Watt A. (1996). Carbon dioxide-induced changes in beech foliage cause female beech weevil larvae to feed in a compensatory manner. Glob. Change Biol. 2 335–341. 10.1111/j.1365-2486.1996.tb00085.x [DOI] [Google Scholar]
- Donnelly A., Caffarra A., Kelleher C. T., O’Neill B. F., Diskin E., Pletsers A., et al. (2012). Surviving in a warmer world: environmental and genetic responses. Clim. Res. 53 245–262. 10.3354/cr01102 [DOI] [Google Scholar]
- Esposito R., Lusini I., Vecerova K., Holisova P., Pallozzi E., Guidolotti G., et al. (2016). Shoot-level terpenoids emission in Norway spruce (Picea abies) under natural field and manipulated laboratory conditions. Plant Physiol. Biochem. 108 530–538. 10.1016/j.plaphy.2016.08.019 [DOI] [PubMed] [Google Scholar]
- Ferrenberg S., Langenhan J. M., Loskot S. A., Rozal L. M., Mitton J. B. (2017). Resin monoterpene defenses decline within three widespread species of pine (Pinus) along a 1530-m elevational gradient. Ecosphere 8:e01975 10.1002/ecs2.1975 [DOI] [Google Scholar]
- Fini A., Brunetti C., Loreto F., Centritto M., Ferrini F., Tattini M. (2017). Isoprene responses and functions in plants challenged by environmental pressures associated to climate change. Front. Plant Sci. 8:1281. 10.3389/fpls.2017.01281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F., Calatayud V., Garcia-Breijo F., Reig-Arminana J., Feng Z. (2016). Effects of elevated ozone on physiological, anatomical and ultrastructural characteristics of four common urban tree species in China. Ecol. Ind. 67 367–379. 10.1016/j.ecolind.2016.03.012 [DOI] [Google Scholar]
- Gerson E., Kelsey R. (2004). Piperidine alkaloids in North American Pinus taxa: implications for chemosystematics. Biochem. Syst. Ecol. 32 63–74. 10.1016/S0305-1978(03)00174-1 [DOI] [Google Scholar]
- Gerson E. A., Kelsey R. G., St Clair J. B. (2009). Genetic variation of piperidine alkaloids in Pinus ponderosa: a common garden study. Ann. Bot. 103 447–457. 10.1093/aob/mcn228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghimire R. P., Kivimäenpää M., Kasurinen A., Häikiö E., Holopainen T., Holopainen J. K. (2017). Herbivore-induced BVOC emissions of Scots pine under warming, elevated ozone and increased nitrogen availability in an open-field exposure. Agric. For. Meteorol. 242 21–32. 10.1016/j.agrformet.2017.04.008 [DOI] [Google Scholar]
- Ghirardo A., Koch K., Taipale R., Zimmer I., Schnitzler J., Rinne J. (2010). Determination of de novo and pool emissions of terpenes from four common boreal/alpine trees by 13CO2 labelling and PTR-MS analysis. Plant Cell Environ. 33 781–792. 10.1111/j.1365-3040.2009.02104.x [DOI] [PubMed] [Google Scholar]
- Giertych M. J., Karolewski P., Oleksyn J. (2015). Carbon allocation in seedlings of deciduous tree species depends on their shade tolerance. Acta Physiol. Plant. 37:216 10.1007/s11738-015-1965-x [DOI] [Google Scholar]
- Goodger J. Q. D., Heskes A. M., Woodrow I. E. (2013). Contrasting ontogenetic trajectories for phenolic and terpenoid defences in Eucalyptus froggattii. Ann. Bot. 112 651–659. 10.1093/aob/mct010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger T. N., Gilbert B. (2017). Multi-scale responses to warming in an experimental insect metacommunity. Glob. Change Biol. 23 5151–5163. 10.1111/gcb.13777 [DOI] [PubMed] [Google Scholar]
- Grote R., Niinemets Ü. (2008). Modeling volatile isoprenoid emissions - a story with split ends. Plant Biol. 10 8–28. 10.1055/s-2007-964975 [DOI] [PubMed] [Google Scholar]
- Guenther A. B., Jiang X., Heald C. L., Sakulyanontvittaya T., Duhl T., Emmons L. K., et al. (2012). The model of emissions of gases and aerosols from nature version 2.1 (MEGAN2.1): an extended and updated framework for modeling biogenic emissions. Geosci. Model Dev. 5 1471–1492. 10.5194/gmd-5-1471-2012 [DOI] [Google Scholar]
- Hartikainen K., Riikonen J., Nerg A., Kivimäenpää M., Ahonen V., Tervahauta A., et al. (2012). Impact of elevated temperature and ozone on the emission of volatile organic compounds and gas exchange of silver birch (Betula pendula Roth). Environ. Exp. Bot. 84 33–43. 10.1016/j.envexpbot.2012.04.014 [DOI] [Google Scholar]
- Heijari J., Nerg A., Holopainen J. K., Kainulainen P. (2010). Wood borer performance and wood characteristics of drought-stressed Scots pine seedlings. Entomol. Exp. Appl. 137 105–110. 10.1111/j.1570-7458.2010.01046.x [DOI] [Google Scholar]
- Himanen S. J., Bui T. N. T., Maja M. M., Holopainen J. K. (2015). Utilizing associational resistance for biocontrol: impacted by temperature, supported by indirect defence. BMC Ecol. 15:16. 10.1186/s12898-015-0048-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holopainen J. K., Gershenzon J. (2010). Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 15 176–184. 10.1016/j.tplants.2010.01.006 [DOI] [PubMed] [Google Scholar]
- Holopainen J. K., Kivimäenpää M., Nizkorodov S. A. (2017). Plant-derived secondary organic material in the air and ecosystems. Trends Plant Sci. 22 744–753. 10.1016/j.tplants.2017.07.004 [DOI] [PubMed] [Google Scholar]
- Ioannidis K., Melliou E., Alizoti P., Magiatis P. (2017). Identification of black pine (Pinus nigra Arn.) heartwood as a rich source of bioactive stilbenes by qNMR. J. Sci. Food Agric. 97 1708–1716. 10.1002/jsfa.8090 [DOI] [PubMed] [Google Scholar]
- IPCC (2014). “Climate change. synthesis report,” in Proceedings of the Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds Core Writing Team R. K., Pachauri, Meyer L. A. (Geneva: IPCC; ), 151. [Google Scholar]
- Jamieson M. A., Burkle L. A., Manson J. S., Runyon J. B., Trowbridge A. M., Zientek J. (2017). Global change effects on plant-insect interactions: the role of phytochemistry. Curr. Opin. Insect Sci. 23 70–80. 10.1016/j.cois.2017.07.009 [DOI] [PubMed] [Google Scholar]
- Jardine K. J., Jardine A. B., Holm J. A., Lombardozzi D. L., Negron-Juarez R. I., Martin S. T., et al. (2017). Monoterpene “thermometer’ of tropical forest-atmosphere response to climate warming. Plant Cell Environ. 40 441–452. 10.1111/pce.12879 [DOI] [PubMed] [Google Scholar]
- Joensuu J., Altimir N., Hakola H., Rostas M., Raivonen M., Vestenius M., et al. (2016). Role of needle surface waxes in dynamic exchange of mono- and sesquiterpenes. Atmos. Chem. Phys. 16 7813–7823. 10.5194/acp-16-7813-2016 [DOI] [Google Scholar]
- Joutsensaari J., Yli-Pirilä P., Korhonen H., Arola A., Blande J. D., Heijari J., et al. (2015). Biotic stress accelerates formation of climate-relevant aerosols in boreal forests. Atmos. Chem. Phys. 15 12139–12157. 10.5194/acp-15-12139-2015 [DOI] [Google Scholar]
- Julkunen-Tiitto R., Nybakken L., Randriamanana T., Virjamo V. (2015). “Boreal woody species resistance affected by climate change,” in Climate Change and Insect Pests, eds Björkman C., Niemelä P. (Wallingford: CAB International: ), 54–73. 10.1079/9781780643786.0054 [DOI] [Google Scholar]
- Juran S., Pallozzi E., Guidolotti G., Fares S., Sigut L., Calfapietra C., et al. (2017). Fluxes of biogenic volatile organic compounds above temperate Norway spruce forest of the Czech Republic. Agric. For. Meteorol. 232 500–513. 10.1016/j.agrformet.2016.10.005 [DOI] [Google Scholar]
- Kainulainen P., Holopainen T., Holopainen J. (2003). Decomposition of secondary compounds from needle litter of Scots pine grown under elevated CO2 and O3. Glob. Change Biol. 9 295–304. 10.1046/j.1365-2486.2003.00555.x [DOI] [Google Scholar]
- Kaling M., Kanawati B., Ghirardo A., Albert A., Winkler J. B., Heller W., et al. (2015). UV-B mediated metabolic rearrangements in poplar revealed by non-targeted metabolomics. Plant Cell Environ. 38 892–904. 10.1111/pce.12348 [DOI] [PubMed] [Google Scholar]
- Karppanen O., Venalainen M., Harju A. M., Willfor S., Pietarinen S., Laakso T., et al. (2007). Knotwood as a window to the indirect measurement of the decay resistance of Scots pine heartwood. Holzforschung 61 600–604. 10.1515/HF.2007.091 [DOI] [Google Scholar]
- Kasurinen A., Peltonen P. A., Julkunen-Tiitto R., Vapaavuori E., Nuutinen V., Holopainen T., et al. (2007). Effects of elevated CO2 and O3 on leaf litter phenolics and subsequent performance of litter-feeding soil macrofauna. Plant Soil 292 25–43. 10.1007/s10886-009-9731-4 [DOI] [PubMed] [Google Scholar]
- Kivimäenpää M., Ghimire R. P., Sutinen S., Haikio E., Kasurinen A., Holopainen T., et al. (2016). Increases in volatile organic compound emissions of Scots pine in response to elevated ozone and warming are modified by herbivory and soil nitrogen availability. Eur. J. For. Res. 135 343–360. 10.1007/s10342-016-0939-x [DOI] [Google Scholar]
- Kivimäenpää M., Riikonen J., Ahonen V., Tervahauta A., Holopainen T. (2013). Sensitivity of Norway spruce physiology and terpenoid emission dynamics to elevated ozone and elevated temperature under open-field exposure. Environ. Exp. Bot. 90 32–42. 10.1016/j.envexpbot.2012.11.004 [DOI] [Google Scholar]
- Kleiber A., Duan Q., Jansen K., Junker L. V., Kammerer B., Rennenberg H., et al. (2017). Drought effects on root and needle terpenoid content of a coastal and an interior Douglas fir provenance. Tree Physiol. 37 1648–1658. 10.1093/treephys/tpx113 [DOI] [PubMed] [Google Scholar]
- Koricheva J. (2002). Meta-analysis of sources of variation in fitness costs of plant antiherbivore defenses. Ecology 83 176–190. 10.1890/0012-9658(2002)083[0176:MAOSOV]2.0.CO;2 [DOI] [Google Scholar]
- Koricheva J., Larsson S., Haukioja E., Keinanen M. (1998). Regulation of woody plant secondary metabolism by resource availability: hypothesis testing by means of meta-analysis. Oikos 83 212–226. 10.2307/3546833 [DOI] [Google Scholar]
- Kosonen M., Keski-Saari S., Ruuhola T., Constabel C. P., Julkunen-Tiitto R. (2012). Effects of overproduction of condensed tannins and elevated temperature on chemical and ecological traits of genetically modified hybrid aspens (Populus tremula x P. tremuloides). J. Chem. Ecol. 38 1235–1246. 10.1007/s10886-012-0193-8 [DOI] [PubMed] [Google Scholar]
- Kotilainen T., Haimi J., Tegelberg R., Julkunen-Tiitto R., Vapaavuori E., Aphalo P. J. (2009). Solar ultraviolet radiation alters alder and birch litter chemistry that in turn affects decomposers and soil respiration. Oecologia 161 719–728. 10.1007/s00442-009-1413-y [DOI] [PubMed] [Google Scholar]
- Kundu S., Fisseha R., Putman A. L., Rahn T. A., Mazzoleni L. R. (2012). High molecular weight SOA formation during limonene ozonolysis: insights from ultrahigh-resolution FT-ICR mass spectrometry characterization. Atmos. Chem. Phys. 12 5523–5536. 10.5194/acp-12-5523-2012 [DOI] [Google Scholar]
- Lämke J. S., Unsicker S. B. (2018). Phytochemical variation in treetops: causes and consequences for tree-insect herbivore interactions. Oecologia 187 377–388. 10.1007/s00442-018-4087-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavola A., Nybakken L., Rousi M., Pusenius J., Petrelius M., Kellomaki S., et al. (2013). Combination treatment of elevated UVB radiation, CO2 and temperature has little effect on silver birch (Betula pendula) growth and phytochemistry. Physiol. Plant. 149 499–514. 10.1111/ppl.12051 [DOI] [PubMed] [Google Scholar]
- Li P., Feng Z., Catalayud V., Yuan X., Xu Y., Paoletti E. (2017). A meta-analysis on growth, physiological, and biochemical responses of woody species to ground-level ozone highlights the role of plant functional types. Plant Cell Environ. 40 2369–2380. 10.1111/pce.13043 [DOI] [PubMed] [Google Scholar]
- Lindroth R. L. (2010). Impacts of elevated atmospheric CO2 and O3 on forests: phytochemistry, trophic interactions, and ecosystem dynamics. J. Chem. Ecol. 36 2–21. 10.1007/s10886-009-9731-4 [DOI] [PubMed] [Google Scholar]
- Lindroth R. L. (2012). “Atmospheric change, plant secondary metabolites, and ecological interactions,” in The Ecology of Plant Secondary Metabolites: From Genes to Global Processes, eds Iason G. R., Dicke M., Hartley S. E. (Cambridge: Cambridge University Press; ), 120–153. 10.1017/CBO9780511675751.008 [DOI] [Google Scholar]
- Loreto F., Schnitzler J. P. (2010). Abiotic stresses and induced BVOCs. Trends Plant Sci. 15 154–166. 10.1016/j.tplants.2009.12.006 [DOI] [PubMed] [Google Scholar]
- Luepke M., Leuchner M., Steinbrecher R., Menzel A. (2016). Impact of summer drought on isoprenoid emissions and carbon sink of three Scots pine provenances. Tree Physiol. 36 1382–1399. 10.1093/treephys/tpw066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maja M. M., Kasurinen A., Holopainen T., Julkunen-Tiitto R., Holopainen J. K. (2016). The effect of warming and enhanced ultraviolet radiation on gender-specific emissions of volatile organic compounds from European aspen. Sci. Total Environ. 547 39–47. 10.1016/j.scitotenv.2015.12.114 [DOI] [PubMed] [Google Scholar]
- Marino G., Brunetti C., Tattini M., Romano A., Biasioli F., Tognetti R., et al. (2017). Dissecting the role of isoprene and stress-related hormones (ABA and ethylene) in Populus nigra exposed to unequal root zone water stress. Tree Physiol. 37 1637–1647. 10.1093/treephys/tpx083 [DOI] [PubMed] [Google Scholar]
- McKiernan A. B., O’Reilly-Wapstra J. M., Price C., Davies N. W., Potts B. M., Hovenden M. J. (2012). Stability of plant defensive traits among populations in two eucalyptus species under elevated carbon dioxide. J. Chem. Ecol. 38 204–212. 10.1007/s10886-012-0071-4 [DOI] [PubMed] [Google Scholar]
- Metlen K. L., Aschehoug E. T., Callaway R. M. (2009). Plant behavioural ecology: dynamic plasticity in secondary metabolites. Plant Cell Environ. 32 641–653. 10.1111/j.1365-3040.2008.01910.x [DOI] [PubMed] [Google Scholar]
- Mochizuki T., Watanabe M., Koike T., Tani A. (2017). Monoterpene emissions from needles of hybrid larch F-1 (Larix gmelinii var. japonica x Larix kaempferi) grown under elevated carbon dioxide and ozone. Atmos. Environ. 148 197–202. 10.1016/j.atmosenv.2016.10.041 [DOI] [Google Scholar]
- Munné-Bosch S. (2012). Phenolic Acids: Composition, Applications and Health Benefits. New York, NY: Nova Science Publishers, Inc. [Google Scholar]
- Nerg A., Heijari J., Noldt U., Viitanen H., Vuorinen M., Kainulainen P., et al. (2004). Significance of wood terpenoids in the resistance of Scots pine provenances against the old house borer, Hylotrupes bajulus, and brown-rot fungus, Coniophora puteana. J. Chem. Ecol. 30 125–141. 10.1023/B:JOEC.0000013186.75496.68 [DOI] [PubMed] [Google Scholar]
- Niinemets Ü. (2018). “What are plant-released biogenic volatiles and how they participate in landscape- to global-level processes?,” in Ecosystem Services from Forest Landscapes, eds Perera A. H., Peterson U., Martínez Pastur G., Iverson L. R. (Cham: Springer; ), 29–56. [Google Scholar]
- Niinemets Ü., Sun Z. (2015). How light, temperature, and measurement and growth [CO2] interactively control isoprene emission in hybrid aspen. J. Exp. Bot. 66 841–851. 10.1093/jxb/eru443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissinen K., Nybakken L., Virjamo V., Julkunen-Tiitto R. (2016). Slow-growing Salix repens (Salicaceae) benefits from changing climate. Environ. Exp. Bot. 128 59–68. 10.1016/j.envexpbot.2016.04.006 [DOI] [Google Scholar]
- Nissinen K., Virjamo V., Randriamanana T., Sobuj N., Sivadasan U., Mehtatalo L., et al. (2017). Responses of growth and leaf phenolics in European aspen (Populus tremula) to climate change during juvenile phase change. Can. J. For. Res. 47 1350–1363. 10.1139/cjfr-2017-0188 [DOI] [Google Scholar]
- Nogues I., Llusia J., Ogaya R., Munne-Bosch S., Sardans J., Peñuelas J., et al. (2014). Physiological and antioxidant responses of Quercus ilex to drought in two different seasons. Plant Biosyst. 148 268–278. 10.1080/11263504.2013.768557 [DOI] [Google Scholar]
- Pan Y., Birdsey R. A., Fang J., Houghton R., Kauppi P. E., Kurz W. A., et al. (2011). A large and persistent carbon sink in the world’s forests. Science 333 988–993. 10.1126/science.1201609 [DOI] [PubMed] [Google Scholar]
- Peltonen P., Vapaavuori E., Julkunen-tiitto R. (2005). Accumulation of phenolic compounds in birch leaves is changed by elevated carbon dioxide and ozone. Glob. Change Biol. 11 1305–1324. 10.1111/j.1365-2486.2005.00979.x [DOI] [Google Scholar]
- Peñuelas J., Staudt M. (2010). BVOCs and global change. Trends Plant Sci. 15 133–144. 10.1016/j.tplants.2009.12.005 [DOI] [PubMed] [Google Scholar]
- Potosnak M. J., LeStourgeon L., Nunez O. (2014). Increasing leaf temperature reduces the suppression of isoprene emission by elevated CO2 concentration. Sci. Total Environ. 481 352–359. 10.1016/j.scitotenv.2014.02.065 [DOI] [PubMed] [Google Scholar]
- Pukkala T. (2018). Carbon forestry is surprising. For. Ecosyst. 5:11 10.1186/s40663-018-0131-5 [DOI] [Google Scholar]
- Randriamanana T. R., Nissinen K., Ovaskainen A., Lavola A., Peltola H., Albrectsen B., et al. (2018). Does fungal endophyte inoculation affect the responses of aspen seedlings to carbon dioxide enrichment? Fungal Ecol. 33 24–31. 10.1016/j.funeco.2017.12.002 [DOI] [Google Scholar]
- Rasulov B., Talts E., Bichele I., Niinemets Ü. (2018). Evidence that isoprene emission is not limited by cytosolic metabolites. exogenous malate does not invert the reverse sensitivity of isoprene emission to high [CO2]. Plant Physiol. 176 1573–1586. 10.1104/pp.17.01463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riikonen J., Kontunen-Soppela S., Ossipov V., Tervahauta A., Tuomainen M., Oksanen E., et al. (2012). Needle metabolome, freezing tolerance and gas exchange in Norway spruce seedlings exposed to elevated temperature and ozone concentration. Tree Physiol. 32 1102–1112. 10.1093/treephys/tps072 [DOI] [PubMed] [Google Scholar]
- Rivas-Ubach A., Gargallo-Garriga A., Sardans J., Oravec M., Mateu-Castell L., Perez-Trujillo M., et al. (2014). Drought enhances folivory by shifting foliar metabolomes in Quercus ilex trees. New Phytol. 202 874–885. 10.1111/nph.12687 [DOI] [PubMed] [Google Scholar]
- Robinson E. A., Ryan G. D., Newman J. A. (2012). A meta-analytical review of the effects of elevated CO2 on plant-arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytol. 194 321–336. 10.1111/j.1469-8137.2012.04074.x [DOI] [PubMed] [Google Scholar]
- Rodriguez-Calcerrada J., Buatois B., Chiche E., Shahin O., Staudt M. (2013). Leaf isoprene emission declines in Quercus pubescens seedlings experiencing drought - Any implication of soluble sugars and mitochondrial respiration? Environ. Exp. Bot. 85 36–42. 10.1016/j.envexpbot.2012.08.001 [DOI] [Google Scholar]
- Rose C., Zha Q., Dada L., Yan C., Lehtipalo K., Junninen H., et al. (2018). Observations of biogenic ion-induced cluster formation in the atmosphere. Sci. Adv. 4:eaar5218. 10.1126/sciadv.aar5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubert-Nason K. F., Couture J. J., Major I. T., Constabel C. P., Lindroth R. L. (2015). Influence of genotype, environment, and gypsy moth herbivory on local and systemic chemical defenses in trembling aspen (Populus tremuloides). J. Chem. Ecol. 41 651–661. 10.1007/s10886-015-0600-z [DOI] [PubMed] [Google Scholar]
- Ruosteenoja K., Markkanen T., Venalainen A., Raisanen P., Peltola H. (2018). Seasonal soil moisture and drought occurrence in Europe in CMIP5 projections for the 21st century. Clim. Dyn. 50 1177–1192. 10.1007/s00382-017-3671-4 [DOI] [Google Scholar]
- Ruuhola T., Nybakken L., Randriamanana T., Lavola A., Julkunen-Tiitto R. (2018). Effects of long-term UV-exposure and plant sex on the leaf phenoloxidase activities and phenolic concentrations of Salix myrsinifolia (Salisb.). Plant Physiol. Biochem. 126 55–62. 10.1016/j.plaphy.2018.02.025 [DOI] [PubMed] [Google Scholar]
- Sallas L., Kainulainen P., Utriainen J., Holopainen T., Holopainen J. K. (2001). The influence of elevated O3 and CO2 concentrations on secondary metabolites of Scots pine (Pinus sylvestris L.) seedlings. Glob. Change Biol. 7 303–311. 10.1046/j.1365-2486.2001.00408.x [DOI] [Google Scholar]
- Sallas L., Luomala E. M., Utriainen J., Kainulainen P., Holopainen J. K. (2003). Contrasting effects of elevated carbon dioxide concentration and temperature on Rubisco activity, chlorophyll fluorescence, needle ultrastructure and secondary metabolites in conifer seedlings. Tree Physiol. 23 97–108. 10.1093/treephys/23.2.97 [DOI] [PubMed] [Google Scholar]
- Sancho-Knapik D., Angeles Sanz M., Peguero-Pina J. J., Niinemets Ü., Gil-Pelegrin E. (2017). Changes of secondary metabolites in Pinus sylvestris L. needles under increasing soil water deficit. Ann. For. Sci. 74:24 10.1007/s13595-017-0620-7 [DOI] [Google Scholar]
- Saunier A., Ormeno E., Boissard C., Wortham H., Temime-Roussel B., Lecareux C., et al. (2017). Effect of mid-term drought on Quercus pubescens BVOCs’ emission seasonality and their dependency on light and/or temperature. Atmos. Chem. Phys. 17 7555–7566. 10.5194/acp-17-7555-2017 [DOI] [Google Scholar]
- Schwalm C. R., Anderegg W. R. L., Michalak A. M., Fisher J. B., Biondi F., Koch G., et al. (2017). Global patterns of drought recovery. Nature 548 202–205. 10.1038/nature23021 [DOI] [PubMed] [Google Scholar]
- Scott C. E., Monks S. A., Spracklen D. V., Arnold S. R., Forster P. M., Rap A., et al. (2018). Impact on short-lived climate forcers increases projected warming due to deforestation. Nat. Commun. 9:157. 10.1038/s41467-017-02412-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigler D. S. (1998). Plant Secondary Metabolism. Dordrecht: Kluwer Academic Publishers: 10.1007/978-1-4615-4913-0 [DOI] [Google Scholar]
- Semiz G., Heijari J., Isik K., Holopainen J. K. (2007). Variation in needle terpenoids among Pinus sylvestris L. (Pinaceae) provenances from Turkey. Biochem. Syst. Ecol. 35 652–661. 10.1016/j.bse.2007.05.013 [DOI] [Google Scholar]
- Sharkey T., Yeh S. (2001). Isoprene emission from plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52 407–436. 10.1146/annurev.arplant.52.1.407 [DOI] [PubMed] [Google Scholar]
- Simpraga M., Verbeeck H., Bloemen J., Vanhaecke L., Demarcke M., Joo E., et al. (2013). Vertical canopy gradient in photosynthesis and monoterpenoid emissions: an insight into the chemistry and physiology behind. Atmos. Environ. 80 85–95. 10.1016/j.atmosenv.2013.07.047 [DOI] [Google Scholar]
- Simpraga M., Verbeeck H., Demarcke M., Joo E., Pokorska O., Amelynck C., et al. (2011). Clear link between drought stress, photosynthesis and biogenic volatile organic compounds in Fagus sylvatica L. Atmos. Environ. 45 5254–5259. 10.1016/j.atmosenv.2011.06.075 [DOI] [Google Scholar]
- Sivadasan U., Chenhao C., Nissinen K., Randriamanana T., Nybakken L., Julkunen-Tiitto R. (2018). Growth and defence of aspen (Populus tremula) after three seasons under elevated temperature and ultraviolet-B radiation. Can. J. For. Res. 48 629–641. 10.1139/cjfr-2017-0296 [DOI] [Google Scholar]
- Smolander A., Kanerva S., Adamczyk B., Kitunen V. (2012). Nitrogen transformations in boreal forest soils-does composition of plant secondary compounds give any explanations? Plant Soil 350 1–26. 10.1007/s11104-011-0895-7 [DOI] [Google Scholar]
- Sobuj N., Virjamo V., Zhang Y., Nybakken L., Julkunen-Tiitto R. (2018). Impacts of elevated temperature and CO2 concentration on growth and phenolics in the sexually dimorphic Populus tremula (L.). Environ. Exp. Bot. 146 34–44. 10.1016/j.envexpbot.2017.08.003 [DOI] [Google Scholar]
- Stark S., Vaisanen M., Ylanne H., Julkunen-Tiitto R., Martz F. (2015). Decreased phenolic defence in dwarf birch (Betula nana) after warming in subarctic tundra. Polar Biol. 38 1993–2005. 10.1007/s00300-015-1758-0 [DOI] [Google Scholar]
- Staudt M., Morin X., Chuine I. (2017). Contrasting direct and indirect effects of warming and drought on isoprenoid emissions from Mediterranean oaks. Reg. Environ. Chang. 17 2121–2133. 10.1007/s10113-016-1056-6 [DOI] [Google Scholar]
- Stromme C. B., Julkunen-Tiitto R., Olsen J. E., Nybakken L. (2018). The dioecious Populus tremula displays interactive effects of temperature and ultraviolet-B along a natural gradient. Environ. Exp. Bot. 146 13–26. 10.1016/j.envexpbot.2017.09.013 [DOI] [Google Scholar]
- Tiiva P., Häikiö E., Kasurinen A. (2018). Impact of warming, moderate nitrogen addition and bark herbivory on BVOC emissions and growth of Scots pine (Pinus sylvestris L.) seedlings. Tree Physiol. 10.1093/treephys/tpy029 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Tiiva P., Tang J., Michelsen A., Rinnan R. (2017). Monoterpene emissions in response to long-term night-time warming, elevated CO2 and extended summer drought in a temperate heath ecosystem. Sci. Total Environ. 580 1056–1067. 10.1016/j.scitotenv.2016.12.060 [DOI] [PubMed] [Google Scholar]
- Vainonen J. P., Kangasjärvi J. (2015). Plant signalling in acute ozone exposure. Plant Cell Environ. 38 240–252. 10.1111/pce.12273 [DOI] [PubMed] [Google Scholar]
- Valkama E., Koricheva J., Oksanen E. (2007). Effects of elevated O3, alone and in combination with elevated CO2, on tree leaf chemistry and insect herbivore performance: a meta-analysis. Glob. Change Biol. 13 184–201. 10.1111/j.1365-2486.2006.01284.x [DOI] [Google Scholar]
- van Meeningen Y., Schurgers G., Rinnan R., Holst T. (2017). Isoprenoid emission response to changing light conditions of English oak, European beech and Norway spruce. Biogeosciences 14 4045–4060. 10.1111/gcb.12980 [DOI] [PubMed] [Google Scholar]
- Vanzo E., Jud W., Li Z., Albert A., Domagalska M. A., Ghirardo A., et al. (2015). Facing the future: effects of short-term climate extremes on isoprene-emitting and nonemitting poplar. Plant Physiol. 169 560–575. 10.1104/pp.15.00871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vapaavuori E., Holopainen J. K., Holopainen T., Julkunen-Tiitto R., Kaakinen S., Kasurinen A., et al. (2009). Rising atmospheric CO2 concentration partially masks the negative effects of elevated O3 in silver birch (Betula pendula Roth). Ambio 38 418–424. 10.1579/0044-7447-38.8.418 [DOI] [PubMed] [Google Scholar]
- Virjamo V., Julkunen-Tiitto R. (2016). Variation in piperidine alkaloid chemistry of Norway spruce (Picea abies) foliage in diverse geographic origins grown in the same area. Can. J. For. Res. 46 456–460. 10.1139/cjfr-2015-0388 [DOI] [Google Scholar]
- Virjamo V., Sutinen S., Julkunen-Tiitto R. (2014). Combined effect of elevated UVB, elevated temperature and fertilization on growth, needle structure and phytochemistry of young Norway spruce (Picea abies) seedlings. Glob. Change Biol. 20 2252–2260. 10.1111/gcb.12464 [DOI] [PubMed] [Google Scholar]
- Wilkinson M. J., Monson R. K., Trahan N., Lee S., Brown E., Jackson R. B., et al. (2009). Leaf isoprene emission rate as a function of atmospheric CO2 concentration. Glob. Change Biol. 15 1189–1200. 10.1111/pce.12787 [DOI] [PubMed] [Google Scholar]
- Wink M. (2010). Introduction: biochemistry, physiology and ecological functions of secondary metabolites. Annu. Plant Rev. 40 1–19. [Google Scholar]
- Yuan X., Calatayud V., Gao F., Fares S., Paoletti E., Tian Y., et al. (2016). Interaction of drought and ozone exposure on isoprene emission from extensively cultivated poplar. Plant Cell Environ. 39 2276–2287. 10.1111/pce.12798 [DOI] [PubMed] [Google Scholar]
- Yuan X., Shang B., Xu Y., Xin Y., Tian Y., Feng Z., et al. (2017). No significant interactions between nitrogen stimulation and ozone inhibition of isoprene emission in Cathay poplar. Sci. Total Environ. 601 222–229. 10.1016/j.scitotenv.2017.05.138 [DOI] [PubMed] [Google Scholar]
- Zhao D. F., Buchholz A., Tillmann R., Kleist E., Wu C., Rubach F., et al. (2017). Environmental conditions regulate the impact of plants on cloud formation. Nat. Commun. 8:14067. 10.1038/ncomms14067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. H., Jia X., Wang W. K., Liu T., Huang S. P., Yang M. Y. (2016). Growth under elevated air temperature alters secondary metabolites in Robinia pseudoacacia L. seedlings in Cd- and Pb-contaminated soils. Sci. Total Environ. 565 586–594. 10.1016/j.scitotenv.2016.05.058 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Virjamo V., Du W., Yin Y., Nissinen K., Nybakken L., Guo H., Julkunen-Tiitto R. (2018). Effects of soil pyrene contamination on growth and phenolics in Norway spruce (Picea abies) are modified by elevated temperature and CO2. Environ. Sci. Pollut. Res. 25 12788–12799. 10.1007/s11356-018-1564-7 [DOI] [PubMed] [Google Scholar]
- Zvereva E., Kozlov M. (2006). Consequences of simultaneous elevation of carbon dioxide and temperature for plant-herbivore interactions: a metaanalysis. Glob. Change Biol. 12 27–41. 10.1111/j.1365-2486.2005.01086.x [DOI] [Google Scholar]