Abstract
Enhancing the antitumor effect of radiation, while reducing damage to organs, is a significant challenge in radiation therapy for head and neck malignancies. One promising radiosensitizer is gold. The present study aimed to determine whether gold nanoparticles (AuNPs) have the potential to enhance the effects of X-ray irradiation on head and neck cancer cells. The human head and neck carcinoma cell line HSC-3 was used. Total cell number and the levels of cell proliferation and apoptosis were compared between control cells and cells treated with 5-nm AuNPs alone at four concentrations (0.1, 0.4, 1.0 and 10.0 nM), X-ray irradiation alone at three doses (2, 4 and 8 Gy), or a combination of 4 Gy X-ray irradiation and 1.0 nM AuNPs. Analysis of variance and Tukey-Kramer testing were performed to compare the different groups. The total number of cells significantly decreased following 4 and 8 Gy X-ray irradiation, compared with in the control group (control vs. 4Gy, P=2.19×10−4; control vs. 8Gy, P=1.28×10−6). The combination of 4 Gy X-ray irradiation and 1.0 nM AuNPs significantly reduced the total number of cells compared with 4 Gy X-ray irradiation alone (P=2.95×10−4). Cell proliferation was not affected by AuNP treatment alone, 4 Gy X-ray irradiation alone or the combination of X-ray irradiation and AuNPs. The combination of 4 Gy irradiation and 1.0 nM AuNPs significantly increased the number of apoptotic cells compared with 4 Gy irradiation alone (P=0.0261). In conclusion, AuNPs combined with X-ray irradiation enhanced the cytotoxic effect on human head and neck cancer cells in vitro, through the induction of apoptosis, but not inhibition of cell proliferation.
Keywords: gold nanoparticle, X-ray irradiation, head and neck squamous cell carcinoma, apoptosis, cell proliferation
Introduction
Head and neck cancer is a major global health issue, with >500,000 new cases diagnosed worldwide each year (1). The most common site for head and neck cancer is the oral cavity, and the most common pathological type is squamous cell carcinoma (2). Radiation therapy has a vital role in current treatment strategies for head and neck squamous cell carcinoma.
The most challenging aspects of radiation therapy are enhancing antitumor effect and reducing damage to surrounding healthy tissue. There are a number of approaches aimed at preferentially sensitizing tumors to radiation whilst minimizing the effects in healthy tissue, including the use gold, which is considered a promising radiosensitizer (3). The biological application of nanoparticles (NPs; particles with dimensions in the range 1–100 nm), has increased, and has potential in both the diagnosis and treatment of human cancers (4–6). The main mechanisms in the biological response of cells to gold nanoparticle (AuNP) radiosensitization reportedly include the production of reactive oxygen species (ROS) and oxidative stress, DNA damage induction, cell cycle effects and potential interference with bystander effects (3). The surface of gold is electronically active, which is the basis for its catalysis of chemical reactions and promotion of ROS production (3). The interaction of the surfaces of NPs with O2 is a reason for the cytotoxicity of NPs (3). Donor electrons are transferred from the NP surfaces to oxygen (O2) molecules generating superoxide (O2−) that leads to ROS production through dismutation reactions (3). Radiosensitization using AuNPs can occur due to the high absorbance property of gold, which results in the deposition of photoelectron and auger electron energy in surrounding tissue (7). When dosed alone, AuNPs may inhibit the proliferation of cancer cells and reverse epithelial-mesenchymal transition by upregulating E-cadherin (as a key molecule required for mechanical adhesion in epithelial cells) and downregulating Snail (a key transcriptional factor required for epithelial-mesenchymal transition) (8).
Although there have been numerous studies on radiosensitization using AuNPs in various cell lines (3), few studies have used human head and neck cancer cells. However, one study did indicate localization-dependent (extracellular, cytoplasmic and nuclear localization of AuNPs) cytotoxicity in human oral squamous cell carcinoma cells (9). Another study reported that AuNPs enhanced chemosensitization in human oral squamous cell carcinoma cells via cell cycle regulation (10). However, to the best of our knowledge, no study has evaluated AuNP-induced radiosensitization of human head and neck squamous cell carcinoma cells. The present study aimed to determine whether AuNPs have the potential to enhance the effects of X-ray irradiation against human head and neck cancer cells in vitro. The underlying mechanisms for the cytotoxicity of X-ray irradiation combined with AuNPs were also investigated.
Materials and methods
Cell culture and reagents
The human head and neck carcinoma cell line HSC-3 (tongue carcinoma), provided by the Japanese Collection of Research Bioresources Cell Bank (Tokyo, Japan) was used. HSC-3 cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Wako Pure Chemical Industries, Ltd., Osaka, Japan) supplemented with 10% (v/v) fetal bovine serum (Biowest, Nuaillé, France) and 1% penicillin/streptomycin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C in a humidified atmosphere of 5% (v/v) CO2 in air. Cells were incubated in DMEM for 48 h prior to treatment. Early passages of cells (between 2 and 10 passages from the stage of primary culture) were used. AuNPs were purchased from Cytodiagnostics, Inc. (Burlington, ON, Canada). Standard Gold Nanoparticles (5 nm) were used (lot number: 2456206_5).
Gold nanoparticle and X-ray irradiation exposure
Cells were grown in 2 ml DMEM on micro cover glass slips in 35×10 mm polystyrene tissue culture dishes at a density of 1×105 cells/ml. After 24 h of culture at 37°C, AuNP was diluted with phosphate-buffered saline (PBS) at different concentrations [0 nM, 0.1 nM (3.9×10−5 µg), 0.4 nM (1.6×10−4 µg), 1.0 nM (3.9×10−4 µg) and 10.0 nM (3.9×10−3 µg)] were added to each dish. Assays to assess cell growth and apoptosis were performed after cells and AuNPs had incubated for 48 h (37°C).
In untreated cells, X-ray irradiation was performed using an MBR-1505R2 X-ray generator (Hitachi, Tokyo, Japan) under the following conditions: 150 kV, 4 mA, 1 mm aluminum filter, at room temperature. Cells were exposed to a fixed X-ray dose (2, 4 or 8 Gy). Experiments were performed 48 h after X-ray irradiation with incubation at 37°C.
To further evaluate the effect of AuNPs, DMEM and AuNP concentrations of 1.0 nM were added to cells and the cells were immediately exposed to X-ray irradiation. The cells were incubated at 37°C for 48 h, after which experiments were conducted.
Cell counting
At 48 h post-treatment (AuNPs addition or X-ray irradiation), cells were washed with PBS and fixed with 1% formaldehyde in PBS for 10 min at room temperature. Fixed cells were permeabilized with 0.2% Triton X-100 in PBS for 5 min at room temperature, and washed with PBS. Cells were blocked with 1% bovine serum albumin (Sigma-Aldrich; Merck KGaA) in PBS for 30 min at room temperature, and then stained with 4′,6-diamidino-2-phenylindole (DAPI; Thermo Fisher Scientific, Inc., Waltham, MA, USA). After rinsing with PBS, specimens were embedded in FluorSave™ (Calbiochem; EMD Millipore, Billerica, MA, USA). Acquisition of images was performed using a BZ-X 700 fluorescence microscope (Keyence Corporation, Osaka, Japan). The total cell number was calculated by counting the number of DAPI-positive nuclei in five randomly selected independent microscopy images (magnification, ×40). Results were representative of three independent experiments.
Determination of cell survival
Growth inhibitory effects of X-ray irradiation and AuNPs were evaluated. The number of viable cells was determined by measuring cell metabolic activity using a Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan) according to the manufacturer's instructions. A total of 5×103 cells in complete medium were suspended in a 96-well plate (100 µl/well) at 37°C. After 24 h of cell growth, nanoparticle solutions with the stated concentrations were added to each well. After 48 h incubation, cells were exposed to X-rays. CCK-8 solution (10 µl) was added to cells 48 h after X-ray irradiation. Plates were incubated for 1–4 h at room temperature, after which absorbance was measured at 450 nm using a microplate reader.
Measurement of apoptosis
To detect apoptotic cells, a terminal deoxynucleotidyl transferase (TdT)-mediated nick-end labeling (TUNEL) assay using an In Situ Apoptosis Detection kit (Takara Bio, Inc., Otsu, Japan) was performed, labeling 3′-OH DNA ends generated by DNA fragmentation (11). Cells were fixed with 4% paraformaldehyde/PBS solution (pH 7.4) at room temperature for 30 min, and washed with PBS. Endogenous peroxidase was inactivated using methanol containing 0.3% hydrogen peroxide at room temperature for 30 min. A total of 100 µl permeabilization buffer was added to the cells on ice for 5 min to promote permeation of the labeling reaction mixture, followed by washing with PBS. Then, 50 µl labeling reaction mixture [consisting of TdT enzyme (5 µl) and labeling safe buffer (45 µl), prepared and cooled on ice prior to use] were added to the cells on a slide, which were then incubated at room temperature for 1.5 h, and washed with PBS. Specimens were embedded in FluorSave™ and observed using a fluorescence microscope. The number of apoptotic cells was divided by the total number of cells and expressed as a percentage. The method used to count TUNEL-positive apoptotic cells and total cell number was performed as described in the cell counting section above.
Statistical analysis
Data were presented as means ± standard deviation. Statistical analyses were performed using Ekuseru-Toukei 2012 version 1.11 (Social Survey Research Information Co., Ltd. Tokyo, Japan). One-way analysis of variance and Tukey-Kramer multiple comparisons tests were used. P<0.05 was considered to indicate statistical significance.
Results
Cell counting assay
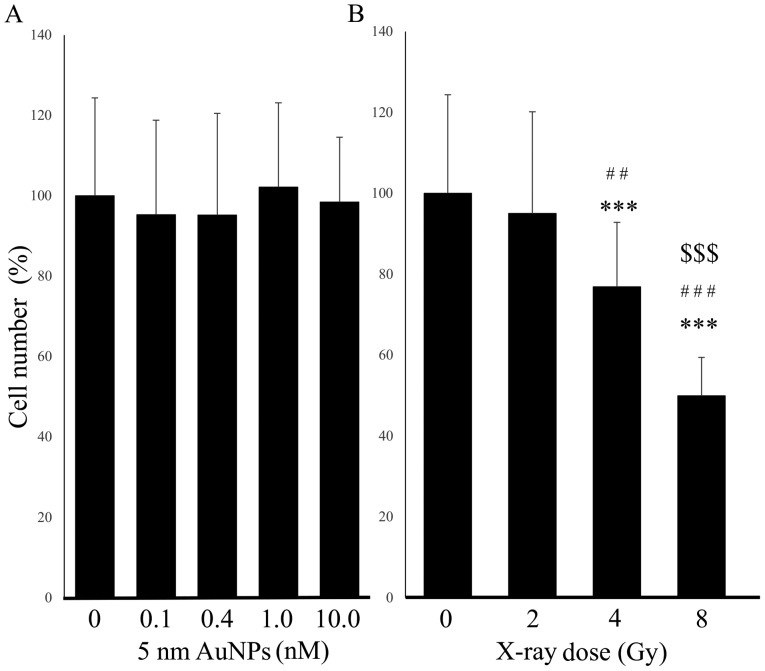
First the effect of four concentrations of 5-nm AuNPs without X-ray treatment was assessed in HSC-3 cells. No significant difference was identified between the total cell number of the control cells and the cells treated with 0.1, 0.4, 1.0 or 10.0 nM AuNPs (Fig. 1A). By contrast, X-ray irradiation alone reduced the total cell number, in an apparent dose-dependent manner, relative to the control cells (2 Gy, P=0.869; 4 Gy, P=2.19×10−4; 8 Gy, P=1.28×10−6; Fig. 1B). Dose-dependence was indicated by significant differences between the dosed groups (2 vs. 4 Gy, P=0.00342; 2 vs. 8 Gy, P=1.28×10−6; 4 vs. 8 Gy, P=5.60×10−6). This result indicated that the lowest radiation dose to have cytotoxicity was 4 Gy. Furthermore, previous studies analyzing whether molecular blockade could enhance radiosensitivity applied 4 Gy (12,13). Therefore, the radiation dose of 4 Gy was selected for subsequent experiments.
Figure 1.
Effect of single treatment with AuNPs or X-ray irradiation on HSC-3 cell number. (A) The total number of HSC-3 cells was not affected by addition of 5-nm AuNPs at any concentration; (B) but was significantly decreased following 4 and 8 Gy X-ray irradiation, relative to control cells. The cell number was counted in five randomly selected independent microscopy images. The data are representative of three independent experiments and presented as the means ± standard deviation. Y-axis values indicate percentage with the control as 100%. ***P<0.001 vs. Control; ##P<0.01 and ###P<0.001 vs. 2 Gy; $$$P<0.001 vs. 4 Gy. AuNPs, gold nanoparticles.
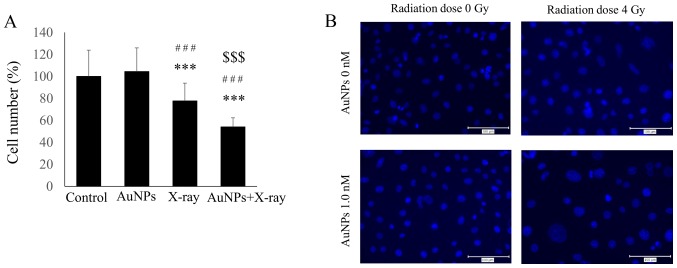
The combination of 1.0 nM AuNPs and 4 Gy irradiation significantly reduced the total cell number compared with 4 Gy irradiation alone (P=2.95×10−4; Fig. 2A). The results of immunofluorescent staining with DAPI are presented in Fig. 2B. There was no obvious difference between control treatment and administration of AuNPs, but the number of cell nuclei appeared to decrease with irradiation.
Figure 2.
(A) Combined treatment with 4 Gy X-ray irradiation and 1.0 nM AuNPs significantly reduced the total number of cells compared with in the control and 4 Gy X-ray irradiation alone groups. Y-axis values indicate percentage with the control as 100%. (B) DAPI staining of HSC-3 cells treated as controls (top left); with 4 Gy X-ray irradiation (top right); with 1.0 nM AuNPs alone (bottom left); and with 4 Gy irradiation plus 1.0 nM AuNPs (bottom right). Magnification, ×40. All data are representative of three independent experiments and presented as means ± standard deviation. ***P<0.001 vs. Control; ###P<0.001 vs. AuNPs; $$$P<0.001 vs. X-ray. AuNPs, gold nanoparticles.
Proliferation assay
To establish the underlying cause for the significant reduction in total cell number with the combination of X-ray irradiation and AuNPs, proliferation and apoptosis assays were performed. Cell proliferation was not affected by treatment with 1.0 nM AuNPs alone (Fig. 3). Furthermore, 4 Gy irradiation alone and the combination of X-ray irradiation and AuNPs also had no effect on cell proliferation in HSC-3 cells (Fig. 3).
Figure 3.
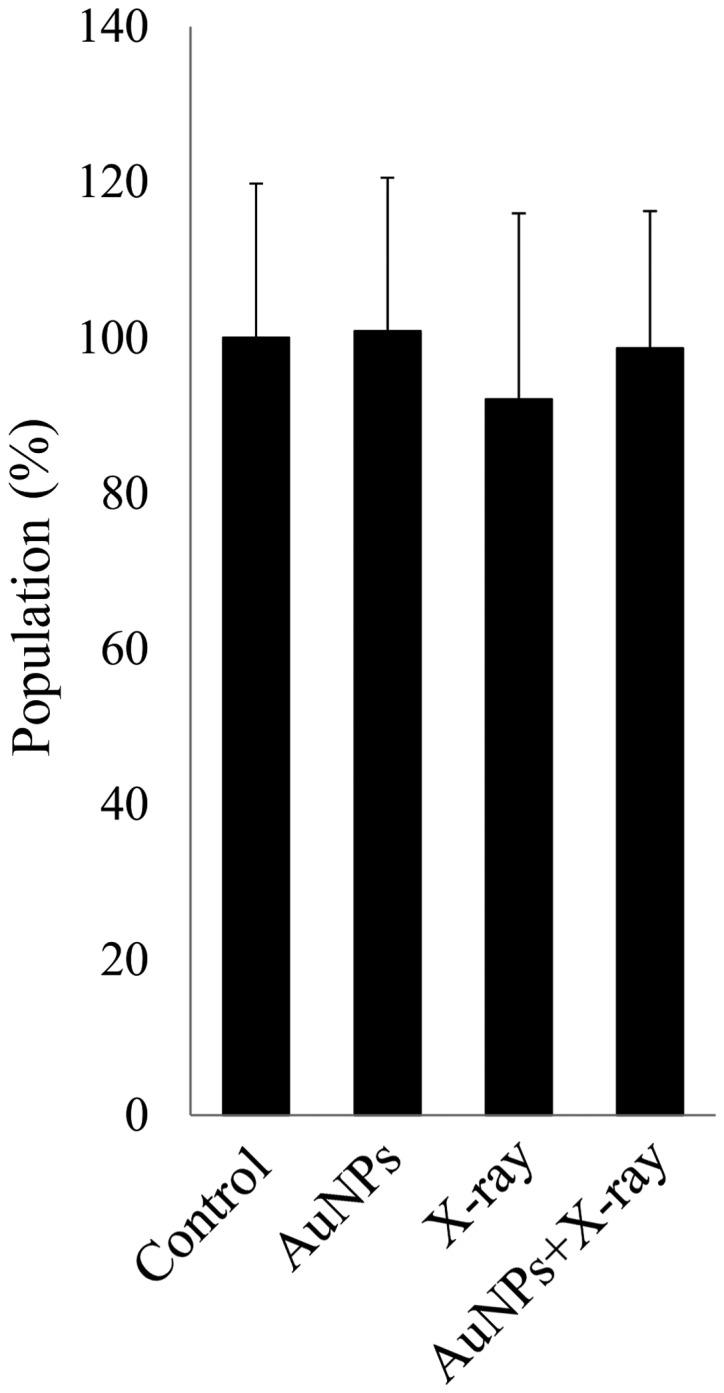
Addition of AuNPs did not inhibit cell proliferation. Cell Counting Kit-8 assays demonstrated that 1.0 AuNP treatment alone, 4 Gy X-ray irradiation alone and the combination of 4 Gy irradiation and 1.0 nM AuNPs did not affect cell proliferation. Y-axis values indicate percentage with the control as 100%. The data are representative of three independent experiments and presented as means ± standard deviation. AuNPs, gold nanoparticles.
TUNEL assay
By contrast, the combination of 1.0 nM AuNPs and 4 Gy X-ray irradiation induced apoptosis, as demonstrated by the significantly increased number of TUNEL-positive apoptotic cells, compared with 4 Gy irradiation alone (P=0.0261; Fig. 4).
Figure 4.
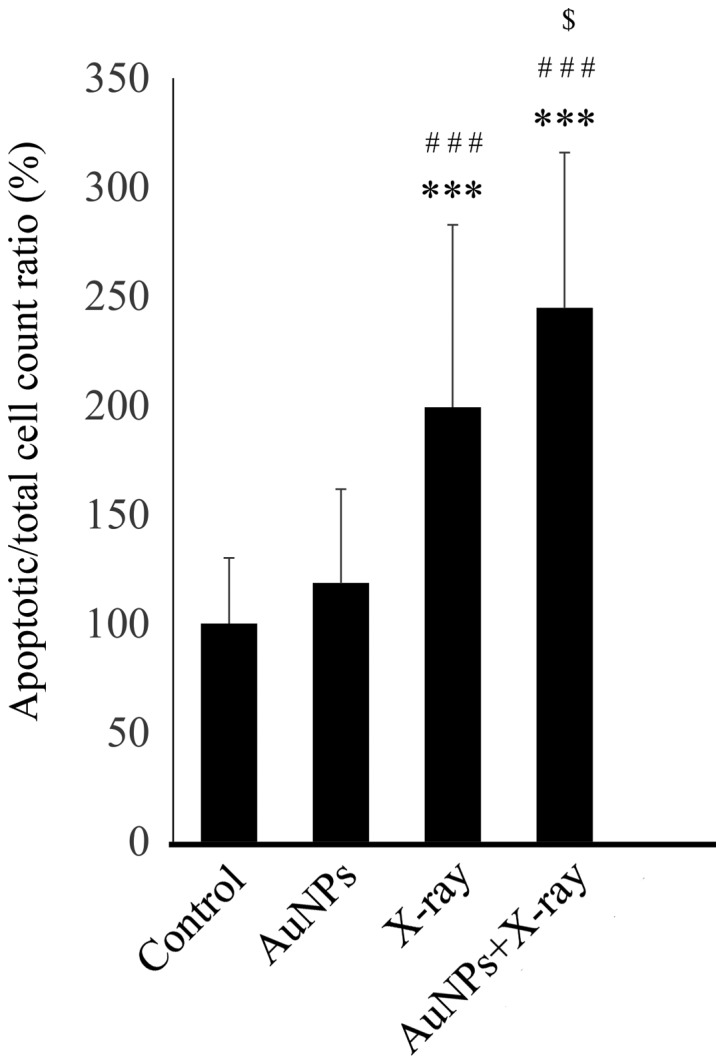
Addition of AuNPs significantly enhanced the apoptotic effect of X-ray irradiation. The combination of 4 Gy irradiation and 1.0 nM AuNPs significantly increased the percentage of TUNEL-positive apoptotic cells compared with 4 Gy X-ray irradiation alone. Y-axis values are expressed as percentage with the control as 100%. The data are representative of three independent experiments and presented as the means ± standard deviation. ***P<0.001 vs. control; ###P<0.001 vs. AuNPs; $P<0.05 vs. X-ray. AuNPs, gold nanoparticles; TUNEL, terminal deoxynucleotidyl transferase-mediated nick-end labeling.
Discussion
The current study indicated that X-ray irradiation was able to significantly reduce total cell number in cultures of HSC-3 cells, and that the addition of AuNPs enhanced this suppressive effect on total cell number caused by X-ray irradiation. The reduction of total cell number by X-ray irradiation alone and when combined with AuNPs was apparently due to the induction of apoptosis, but not inhibition of cell proliferation. To the best of our knowledge, although there have been reports on localization-dependent cytotoxicity of AuNPs alone (9) and the chemosensitization effect of AuNPs (specifically, nuclear-targeted 30-nm AuNPs enhanced 5-fluorouracil drug efficacy in HSC-3 cells via regulation of the cell cycle) (10), this is the first report indicating a radiosensitization effect of AuNPs against human head and neck squamous cell carcinoma cells in vitro.
Radiation therapy has a vital role in current therapeutic strategies for cancer. Since 2000, human papillomavirus (HPV)-positive head and neck squamous cell carcinoma has increased in incidence worldwide (1). Notably, the prevalence of HPV-positive head and neck cancer in recent reports exceeded 60%, while the incidence in the early 1980s was approximately 16% (1). Radiation therapy is most commonly used in patients with early-stage oropharyngeal carcinoma (14). HPV-positive oropharyngeal cancer is considered to be more radiosensitive than HPV-negative tumors (15). Recent advances in radiotherapy techniques have enabled selective sparing of structures critical for oral and oropharyngeal functions, such as including saliva production and swallowing, leading to improvements in xerostomia, dysphagia and overall quality of life following treatment (16). However, further emphasis on organ sparing techniques is required, since long-term survivors of oropharyngeal cancer generally experience a deterioration in quality of life (16,17). One promising solution to minimize effects in healthy surrounding tissues is to use a radiosensitizer (18). Head and neck carcinoma is considered to be one of the diseases that would significantly benefit from the development of such radiosensitizers (17).
AuNPs dosed alone have been reported to affect cell properties (8). If AuNPs themselves are cytotoxic, they pose concerns when considering potential clinical applications. There have therefore been studies focusing on the cytotoxic potential of NPs themselves. These papers indicate that AuNPs have different toxicities in cells depending on concentration and shape, and spherical AuNPs at low concentrations have no or weak cytotoxicity against endothelial cells (19–22). Indeed, cytotoxicity has been identified to be influenced by NP properties including size and surface chemistry (19,20). Although smaller sized particles are often considered to be more toxic, the effect of size may not be so direct, as NP toxicity has also been associated with cellular uptake, which is influenced by particle size. Optimal uptake has been reported for particles with a diameter of ~50 nm (20). In a previous study, AuNPs of four different sizes (~5, 20, 50 or 100 nm) were used to determine the relationship between NP size and biological response. After 72 h incubation, the 20-nm AuNPs exhibited the highest efficacy for inhibiting proliferation of ovarian cancer cell lines (A2780 and SKOV3-ip); while 5-nm AuNPs exhibited only a modest inhibition, and 50 and 100-nm AuNPs had no inhibitory effect (8). Based on these findings the current study selected 5 nm AuNPs, as it was postulated that these particles may also have minimal cytotoxic effects in HSC-3 cells. The effect of 5-nm AuNPs at four different concentrations in HSC-3 cells was first determined. The 48-h incubation of 5-nm AuNPs with cells did not affect the total cell number, indicating that 48-h treatment with 5 nm AuNPs at all tested concentrations had minimal cytotoxicity in HSC-3 cells.
To determine whether AuNPs have potential as a radiosensitizer, the effect of X-ray irradiation alone was evaluated. Samples irradiated with 4 and 8 Gy of X-rays exhibited significantly reduced total cell number in a dose-dependent manner; however the samples that received 2 Gy did not. The combination of 1.0 nM AuNPs and 4 Gy X-ray irradiation significantly reduced the total cell number compared with 4 Gy X-ray irradiation alone. The reduction in the total cell number may be attributed principally to the induction of apoptosis, and not a decrease in cell viability. This is based on the observation that 4 Gy X-ray irradiation alone and the combination of 4 Gy X-ray irradiation and AuNPs increased the percentage of apoptotic HSC-3 cells without affecting the proliferation rate. A previous study using HSC-3 cells reported that cell growth analyzed using a CCK-8 assay 48 h after 4 Gy irradiation alone, did not significantly decrease in comparison with control cells (13). By contrast, another study using human breast cancer cells observed that 8 Gy X-ray irradiation alone could inhibit cell proliferation compared with controls (23). X-ray irradiation alone at 8 Gy may also induce apoptosis through increasing caspase-3 expression in human breast cancer cells (23). Another in vivo study reported that the number of apoptotic cells in tumors excised from mice was significantly higher in a group subjected to combined treatment with 200 nM of 13-nm AuNPs and 25 Gy radiation, than that in a group dosed with radiation alone (24). The current results indicated that the combination of 4 Gy X-ray irradiation and 1.0 nM AuNPs enhanced the effect in inducing apoptosis, compared with 4 Gy X-ray irradiation alone, indicating that the addition of 1.0 nM AuNPs may be sufficient to enhance apoptosis. Radiation-induced apoptosis is considered to be one of the main cell death mechanisms following exposure to radiation (25). However, the underlying mechanisms of tissue-specific responses to irradiation, cellular factors determining the regulation of apoptosis activated by irradiation, and irradiation-induced cell deaths under specific circumstances, have not been established. Nonetheless, it is established that radiation-induced cell death is associated with DNA reparation, cell cycle arrest and senescence, as well as apoptosis (25).
A limitation of the current study is that no other factors than cell viability and apoptosis, such as the production of ROS and oxidative stress, DNA damage induction, cell cycle effects and potential interference with bystander effects, were not evaluated. Therefore, further investigations into DNA reparation, cell-cycle arrest and senescence with X-ray irradiation and AuNP treatment are required. Although the current study identified that the addition of AuNPs enhanced the cytotoxicity of AuNPs, a comparison with the combination of X-ray irradiation and chemotherapy is required. Additionally, attempts were made to confirm the results of the TUNEL assay via western blotting with caspase 3, but results were ambiguous (data not shown).
In conclusion, AuNPs had the potential to enhance the cytotoxic effects of X-ray irradiation against human head and neck cancer cells in vitro. The underlying cause of this cytotoxicity was observed to involve the induction of apoptosis, and not inhibition of cell proliferation.
Acknowledgements
Not applicable.
Funding
The current work was supported by the Japan Society for the Promotion of Science, KAKENHI, Grant-in-Aid for Young Scientists (B) (grant no. 16K20574).
Availability of data and materials
All data generated and/or analysed in the current article are available upon request from the corresponding author.
Authors' contributions
ST, YK, MA and RS contributed to conception and design of the study, and acquisition, analysis and interpretation of the data. ST and MA drafted the manuscript and aided in revision of the manuscript. YK, EI and TH contributed to acquisition of the data and to revision of the manuscript. DM, RS and TK contributed to analysis and interpretation of the data as well as to revision of the manuscript. All authors read and approved the final version of the manuscript to be published.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wang MB, Liu IY, Gornbein JA, Nguyen CT. HPV-Positive Oropharyngeal Carcinoma: A Systematic Review of Treatment and Prognosis. Otolaryngol Head Neck Surg. 2015;153:758–769. doi: 10.1177/0194599815592157. [DOI] [PubMed] [Google Scholar]
- 2.Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26:123–141. doi: 10.1016/j.coms.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosa S, Connolly C, Schettino G, Butterworth KT, Prise KM. Biological mechanisms of gold nanoparticle radiosensitization. Cancer Nanotechnol. 2017;8:2. doi: 10.1186/s12645-017-0026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim BY, Rutka JT, Chan WC. Nanomedicine. N Engl J Med. 2010;363:2434–2443. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 5.Service RF. Materials and biology. Nanotechnology takes aim at cancer. Science. 2005;310:1132–1134. doi: 10.1126/science.310.5751.1132. [DOI] [PubMed] [Google Scholar]
- 6.Yezhelyev MV, Gao X, Xing Y, Al-Hajj A, Nie S, O'Regan RM. Emerging use of nanoparticles in diagnosis and treatment of breast cancer. Lancet Oncol. 2006;7:657–667. doi: 10.1016/S1470-2045(06)70793-8. [DOI] [PubMed] [Google Scholar]
- 7.Nakayama M, Sasaki R, Ogino C, Tanaka T, Morita K, Umetsu M, Ohara S, Tan Z, Nishimura Y, Akasaka H, et al. Titanium peroxide nanoparticles enhanced cytotoxic effects of X-ray irradiation against pancreatic cancer model through reactive oxygen species generation in vitro and in vivo. Radiat Oncol. 2016;11:91. doi: 10.1186/s13014-016-0666-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arvizo RR, Saha S, Wang E, Robertson JD, Bhattacharya R, Mukherjee P. Inhibition of tumor growth and metastasis by a self-therapeutic nanoparticle; Proc Natl Acad Sci USA; 2013; pp. 6700–6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austin LA, Ahmad S, Kang B, Rommel KR, Mahmoud M, Peek ME, El-Sayed MA. Cytotoxic effects of cytoplasmic-targeted and nuclear-targeted gold and silver nanoparticles in HSC-3 cells-a mechanistic study. Toxicol In Vitro. 2015;29:694–705. doi: 10.1016/j.tiv.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Mackey MA, El-Sayed MA. Chemosensitization of cancer cells via gold nanoparticle-induced cell cycle regulation. Photochem Photobiol. 2014;90:306–312. doi: 10.1111/php.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Portera-Cailliau C, Sung CH, Nathans J, Adler R. Apoptotic photoreceptor cell death in mouse models of retinitis pigmentosa; Proc Natl Acad Sci USA; 1994; pp. 974–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shintani S, Li C, Mihara M, Terakado N, Yano J, Nakashiro K, Hamakawa H. Enhancement of tumor radioresponse by combined treatment with gefitinib (Iressa, ZD1839), an epidermal growth factor receptor tyrosine kinase inhibitor, is accompanied by inhibition of DNA damage repair and cell growth in oral cancer. Int J Cancer. 2003;107:1030–1037. doi: 10.1002/ijc.11437. [DOI] [PubMed] [Google Scholar]
- 13.Hoshikawa H, Indo K, Mori T, Mori N. Enhancement of the radiation effects by D-allose in head and neck cancer cells. Cancer Lett. 2011;306:60–66. doi: 10.1016/j.canlet.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 14.Urban D, Corry J, Rischin D. What is the best treatment for patients with human papillomavirus-positive and -negative oropharyngeal cancer? Cancer. 2014;120:1462–1470. doi: 10.1002/cncr.28595. [DOI] [PubMed] [Google Scholar]
- 15.Chen AM, Li J, Beckett LA, Zhara T, Farwell G, Lau DH, Gandour-Edwards R, Vaughan AT, Purdy JA. Differential response rates to irradiation among patients with human papillomavirus positive and negative oropharyngeal cancer. Laryngoscope. 2013;123:152–157. doi: 10.1002/lary.23570. [DOI] [PubMed] [Google Scholar]
- 16.Vainshtein JM, Moon DH, Feng FY, Chepeha DB, Eisbruch A, Stenmark MH. Long-term quality of life after swallowing and salivary-sparing chemo-intensity modulated radiation therapy in survivors of human papillomavirus-related oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2015;91:925–933. doi: 10.1016/j.ijrobp.2014.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Høxbroe Michaelsen S, Grønhøj C, Høxbroe Michaelsen J, Friborg J, von Buchwald C. Quality of life in survivors of oropharyngeal cancer: A systematic review and meta-analysis of 1366 patients. Eur J Cancer. 2017;78:91–102. doi: 10.1016/j.ejca.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Retif P, Pinel S, Toussaint M, Frochot C, Chouikrat R, Bastogne T, Barberi-Heyob M. Nanoparticles for Radiation Therapy Enhancement: The Key Parameters. Theranostics. 2015;5:1030–1044. doi: 10.7150/thno.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sultana S, Djaker N, Boca-Farcau S, Salerno M, Charnaux N, Astilean S, Hlawaty H, de la Chapelle ML. Comparative toxicity evaluation of flower-shaped and spherical gold nanoparticles on human endothelial cells. Nanotechnology. 2015;26:055101. doi: 10.1088/0957-4484/26/5/055101. [DOI] [PubMed] [Google Scholar]
- 20.Soenen SJ, Manshian B, Montenegro JM, Amin F, Meermann B, Thiron T, Cornelissen M, Vanhaecke F, Doak S, Parak WJ, et al. Cytotoxic effects of gold nanoparticles: A multiparametric study. ACS Nano. 2012;6:5767–5783. doi: 10.1021/nn301714n. [DOI] [PubMed] [Google Scholar]
- 21.Tarantola M, Pietuch A, Schneider D, Rother J, Sunnick E, Rosman C, Pierrat S, Sönnichsen C, Wegener J, Janshoff A. Toxicity of gold-nanoparticles: Synergistic effects of shape and surface functionalization on micromotility of epithelial cells. Nanotoxicology. 2011;5:254–268. doi: 10.3109/17435390.2010.528847. [DOI] [PubMed] [Google Scholar]
- 22.Zhang XD, Wu HY, Wu D, Wang YY, Chang JH, Zhai ZB, Meng AM, Liu PX, Zhang LA, Fan FY. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int J Nanomedicine. 2010;5:771–781. doi: 10.2147/IJN.S8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li DL, Wei L, Wen XM, Song H, Li Q, Lv JW, Kuang CC, Wei ZZ, Zhang JW. The effects of X-ray irradiation on the proliferation and apoptosis of MCF-7 breast cancer cells. Ultrastruct Pathol. 2014;38:211–216. doi: 10.3109/01913123.2013.861569. [DOI] [PubMed] [Google Scholar]
- 24.Chang MY, Shiau AL, Chen YH, Chang CJ, Chen HH, Wu CL. Increased apoptotic potential and dose-enhancing effect of gold nanoparticles in combination with single-dose clinical electron beams on tumor-bearing mice. Cancer Sci. 2008;99:1479–1484. doi: 10.1111/j.1349-7006.2008.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eriksson D, Stigbrand T. Radiation-induced cell death mechanisms. Tumour Biol. 2010;31:363–372. doi: 10.1007/s13277-010-0042-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and/or analysed in the current article are available upon request from the corresponding author.