Abstract
The Fas receptor/ligand system plays a prominent role in hepatic apoptosis and hepatocyte death. Although hepatitis B virus (HBV) surface Ag (HBsAg) is the most abundant HBV protein in the liver and peripheral blood of patients with chronic HBV infection, its role in Fas-mediated hepatocyte apoptosis has not been disclosed. In this study, we report that HBsAg sensitizes HepG2 cells to agonistic anti-Fas Ab CH11-induced apoptosis through increasing the formation of SDS-stable Fas aggregation and procaspase-8 cleavage but decreasing both the expression of cellular FLIPL/S and the recruitment of FLIPL/S at the death-inducing signaling complex (DISC). Notably, HBsAg increased endoplasmic reticulum stress and consequently reduced AKT phosphorylation by deactivation of phosphoinositide-dependent kinase-1 (PDPK1) and mechanistic target of rapamycin complex 2 (mTORC2), leading to enhancement of Fas-mediated apoptosis. In a mouse model, expression of HBsAg in mice injected with recombinant adenovirus-associated virus 8 aggravated Jo2-induced acute liver failure, which could be effectively attenuated by the AKT activator SC79. Based on these results, it is concluded that HBsAg predisposes hepatocytes to Fas-mediated apoptosis and mice to acute liver failure via suppression of AKT prosurviving activity, suggesting that interventions directed at enhancing the activation or functional activity of AKT may be of therapeutic value in Fas-mediated progressive liver cell injury and liver diseases.
Introduction
Hepatitis B virus (HBV) infection remains a major health problem worldwide as ∼350 million people are chronically infected with HBV who are at a high risk of developing hepatitis, cirrhosis, and hepatocellular carcinoma (HCC). However, the molecular mechanisms underlying chronic HBV infection and its pathological consequences have not been fully understood. Hepatocytic apoptosis is one of the factors prolonging inflammation in chronic hepatitis B (CHB). It appears to be mediated by Fas, a 45-kDa cell surface glycoprotein, which is expressed in the liver and transduces apoptotic signals to the liver cells when agonistic anti-Fas Ab or Fas ligand (FasL) binds with it (1). Fas-mediated apoptosis has been shown to be a major effector of the cytotoxic immune response (2) and should be an important pathogenic mechanism during CHB infection. Indeed, Fas expression in liver tissues of patients with CHB infection was closely correlated with the activity of viral hepatitis (3). Moreover, the serum concentration of the soluble form of Fas (sFas) in patients chronically infected by HBV was significantly higher in comparison with healthy HBV surface Ag (HBsAg) carriers and healthy persons (4). Interestingly, an in situ investigation of Fas/FasL expression in CHB infection and related liver diseases revealed that the Fas/FasL expression level was closely correlated with the inflammatory activity, which may initiate disease and promote its progression as a result of apoptosis following Fas–FasL interaction (5).
AKT, a serine/threonine protein kinase with antiapoptotic activity, is one of the major downstream targets of the PI3K signaling pathway. AKT is a crucial mediator of cell survival, and its deactivation is implicated in various types of stress-induced pathological cell death, including hepatocyte injury (6). Activation of AKT was reported to block Fas aggregation and procaspase-8 cleavage at the death-inducing signaling complex (DISC), and inhibition of AKT phosphorylation promotes Fas DISC assembly (7).
HBsAg is the most abundant viral envelope protein produced during HBV replication (8). Although excess HBsAg subviral particles have been suggested to sequester the neutralizing Ab against HBV and contribute to a state of immune tolerance, thereby enabling the survival of infectious virions and leading to persistent infections (9), the biological and pathological significance of HBsAg remains elusive. The aim of this study was to determine whether HBsAg is involved in modulating the Fas/FasL apoptotic pathway. We found that HBsAg exaggerated Fas/FasL-mediated apoptosis of hepatocytes and shortened survival of mice specifically by inhibition of AKT phosphorylation.
Materials and Methods
Ethics statement
Cryopreserved primary human hepatocytes (PHH) were purchased from BioreclamationIVT (Brussels, Belgium), who obtains and distributes consented human material from a network of Institutional Review Board–approved collection sites under adherence to effective ethical and regulatory guidelines.
Plasmid construction
pHBsAg was constructed by inserting a PCR-generated HBsAg gene fused with FLAG tag sequences (10) into the HindIII and NotI sites (New England BioLabs, Beverly, MA) of the plasmid pcDNA3.1/Hygro(+) (Invitrogen, Carlsbad, CA). HBV DNA used as a template was described previously (11), and the primers were as follows: forward, 5′-CCCAAGCTTGCCACCATGGAGAACATCGCATCAGGACTCCTA-3′, reverse, 5′-ATAAGAATGCGGCCGCTTACTTGTCGTCATCGTCTTTGTAGTCAATGTATACCCAAAGACA-3′. A total of 14 HBsAg mutants with amino acid substitutions at position Q30K, N40S, T45K, T45N, T45S, L49I, L49P, L49T, M133I, G145R, S204R, L205V, or M213I were constructed by PCR-based mutagenesis using pHBsAg as a template.
pcDNA3.1-AKT was constructed by inserting a PCR-generated AKT gene from HepG2 cDNA into the KpnI and XhoI sites (New England BioLabs) of the plasmid pcDNA3.1/Hygro(+). The primers were as follows: forward, 5′-CGGGGTACCGCCACCATGAGCGACGTGGCTAT TGTGAAGGA-3′, reverse, 5′-CCGCTCGAGCTAGGCCGTGCCGCTGGCCGAGTAGGAGAA CTGG-3′.
pRep-HBV harboring 1.2-U lengths of the HBV genome and control empty plasmid pRep-Sal1 were described previously (12). The pRep-HBV-HBc and HBx(−) plasmid contains a stop codon at HBx codon 8 (CAA to UAA) accompanied with HBc initiation codon ATG to ACG. The pRep-HBV-HBsAg(−) mutation plasmid contains ACG instead of ATG. These two mutants were generated by site-directed mutagenesis as described previously (13).
Cell lines and cell culture
HepG2 cells were cultured in DMEM containing 10% FBS (Invitrogen). Cryopreserved PHH were purchased from BioreclamationIVT, who obtains and distributes consented human material from a network of Institutional Review Board–approved collection sites under adherence to effective ethical and regulatory guidelines. PHH were thawed according to the manufacturer’s instructions and cultured in William’s medium E (Sigma-Aldrich, St. Louis, MO) supplemented with 10% FBS, 2 mM l-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin.
Generation of stable HBsAg-expressing cell lines
HepG2 cells were transfected with HBsAg-expressing or empty plasmid and selected in the presence of 400 μg/ml hygromycin for 4 wk. The hygromycin-resistant clones were expanded together into cell lines and screened for expression of HBsAg by Western blot analysis.
Production of HBsAg-expressing recombinant adenoviruses
The detailed procedure of construction and generation of the recombinant adenoviruses were described previously (14).
Adenovirus-associated virus injection
The adenovirus-associated virus 8 (AAV8) expression system was used to achieve overexpression of wild-type and mutant HBsAg in liver. High-titer AAV8 particles were produced and supplied by Obio Technology (Shanghai, China). For these particles, 2 × 1011 viral genomes of AAV8 in 100 μl of PBS was i.v. injected into age-matched (6–8 wk old) C57BL/6 mice weighing 16–18 g. Mice were subsequently treated for 3 wk.
HBsAg quantitative analysis
Levels of cell medium and serum HBsAg were tested quantitatively by an Architect assay (Abbott Laboratories, Chicago, IL).
Treatment of cells with chemicals and Abs
In HepG2 or PHH, Fas receptor stimulation was performed with recombinant human FasL (no. 5452; Cell Signaling Technology, Danvers, MA) or the agonistic human mAb anti-Fas CH11 (SY-001; MBL, Nagoya, Japan) at 1 μg/ml plus 0.5 μg/ml actinomycin D (ActD; A1410; Sigma-Aldrich). For inhibition of anti-Fas Ab-induced apoptosis, cells were pretreated with Fas receptor antagonistic mAb anti-Fas ZB4 (MD-11-3; MBL) at 1 and 4 μg/ml AKT activator SC79 (no. 123871; Calbiochem, La Jolla, CA) or 5 mM 4-phenylbutyric acid (4-PBA; P21005; Sigma-Aldrich). All of these pretreated cells were then treated with anti-Fas CH11 at 1 μg/ml for an additional 8 h. IgM (M079-3; MBL) was used as a control for anti-Fas CH11, and IgG (M075-3; MBL) was used for anti-Fas ZB4.
Animal experiments
All work performed with animals was in accordance with and approved by the Institutional Animal Care and Use Committee at Fujian Medical University. Male age-matched (6–8 wk old) C57BL/6 mice (Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China) weighing 16–18 g were maintained in ventilated cages under a 12-h light/dark cycle. Mice at 3 wk after injection with HBsAg-expressing or empty control AAV8 were pretreated i.p. with 10 μg/g SC79 or DMSO at 0.5 h before i.p. administration of an agonistic anti-Fas Jo2 Ab (no. 554255; BD Pharmingen) diluted in 100 μl of PBS at 0.5 μg/g, a dose that could kill fully 100% of mice within 12 h. After this lethal challenge, mice were monitored for mortality for 14 d. Forty SC79-pretreated or untreated control mice with 10 mice per group were analyzed with mortality as the endpoint. Liver and blood samples were taken from SC79-treated or untreated groups at 3, 6, and 12 h after injection of the Ab. Serum activities of alanine transaminase (ALT) and aspartate transaminase (AST) were determined using a standard clinical automatic analyzer (Hitachi 7020; Hitachi, Kyoto, Japan). Immediately after taking the blood samples retroorbitally, mice were sacrificed by cervical dislocation. The liver was excised, sectioned, and fixed overnight at 4°C in 10% formalin solution. The sections were then dehydrated, paraffin embedded, and cut at 3-mm thickness and stained with H&E for histological examination.
Western blot analysis
Western blotting was performed as described previously (10, 15). The subcellular fractions were prepared with a mitochondria/cytosol fractionation kit (BioVision, Milpitas, CA) according to the manufacturer’s instructions. The specific Abs used in this study included anti–Fas-associated death domain (FADD; ab24533, 1:1000 dilution; Abcam); anti-HBc (sc-23947), anti-HBsAg (sc-23944, recognizing the epitopes of N-terminal 77–82 aa), and anti-HBx (sc-57760) from Santa Cruz Biotechnology (Santa Cruz, CA); and anti-Flag (no. 14793), anti-Fas (no. 4233), anti-AKT (no. 4691), anti-pAKT (Thr308) (no. 13038), anti-pAKT (Ser473) (no. 4060), anti-Fyn (no. 4023), anti-Bad (no. 9292), anti-pBad (Ser136) (no. 4366), anti-Bax (no. 5032), anti-FLIPL (no. 8510), anti-FLIPS (no. 56343), anti–procaspase-8 (no. 9746, recognizing full-length procaspase-8 and the cleaved intermediate p43/p41), anti-cleaved caspase-8 (no. 9496, recognizing the active p18 fragment), anti–β-tubulin (no. 2128), anti–pPDPK1; Ser241) (no. 3438), anti-phosphoinositide-dependent kinase-1 (PDPK1; no. 3062), anti-pmTOR (Ser2481) (no. 2974), anti-mTOR (no. 2972), anti-PTEN (no. 9188), anti-Rictor (no. 2114), anti-active caspase-3 (no. 9664), anti-Bid/tBid (no. 2002), and anti–cytochrome c (no. 11940) from Cell Signaling Technology. Secreted HBsAg was concentrated from culture supernatant or blood serum by ultracentrifugation at 40,000 × g for 16 h. The pellet was diluted 1:10 and resuspended in protein loading buffer, followed by Western blot analysis.
Coimmunoprecipitation assay
Lysates from HepG2 cells were immunoprecipitated with anti-FLAG M2 agarose (Sigma-Aldrich) or AKT, PDPK1, mTOR, and PTEN Abs, and after incubation with protein A/G agarose (Santa Cruz Biotechnology) at 4°C overnight, the immune complexes were eluted and subjected to Western blot analysis with the respective Abs.
DISC analysis
HepG2 cells were incubated with anti-Fas CH11 for 4 h at 37°C, then lysed in immunoprecipitation (IP) lysis buffer (150 nM NaCl, 50 mM Tris [pH 7.5], 1 mM EDTA, 1 mM DTT, 1% Nonidet P-40, 10 mM β-phosphoglycerate, 0.1 mM NaF, 1 mM NaVO4, and small peptide inhibitors). Lysates were precleared by incubation with 30 μl of protein G–agarose beads (50% slurry in IP lysis buffer). The DISC was immunoprecipitated for 12 h at 4°C using anti-Fas Ab (no. 8023; Cell Signaling Technology) and protein A/G–agarose (Santa Cruz Biotechnology). After IP, the beads were washed four times with IP lysis buffer and the immunoprecipitates were eluted from beads with 2× Laemmli sample buffer (2.1% SDS, 26.3% glycerol, 10% 2-ME, 0.01% bromphenol blue, 65.8 mM Tris HCl [pH 6.8]) at 95°C for 5 min and subjected to SDS-PAGE, followed by immunoblotting.
Fas palmitoylation detection by the acyl-biotinyl exchange technique
Protein palmitoylation was detected by a previously described method adapted from the acyl-biotinyl exchange protocol of Hueber and colleagues (16).
CCK-8 assay
Cell proliferation and viability was measured using a CCK-8 assay as previously described (10).
TUNEL assay
Liver tissues were dissected and fixed in 4% paraformaldehyde overnight, dehydrated, embedded in paraffin, and then subjected to a TUNEL assay as previously reported (10).
Annexin V binding analysis
Cells were treated with proapoptotic chemicals and/or Abs. Apoptosis was detected using an FITC annexin V apoptosis detection kit (BD Pharmingen) according to the manufacturer’s instructions, and analyzed by flow cytometry (FACSVerse; BD Biosciences) using FACSuite software (BD Biosciences).
Quantitative real-time PCR analysis of Fas, FasL, and p53
Quantitative real-time PCR was performed with the same pairs of primers as previously reported (10).
Semiquantitative RT-PCR analysis of mFas, sFas, and XBP1
Semiquantitative RT-PCR analyses of mFas and sFas were performed as we previously reported (10). For XBP1 amplification, the pair of forward (5′-CTGGAAAGCAAGTGGTAGA-3′) and reverse (5′-CTGGGTCCTT CTGGGTAGAC-3′) primers were used. The amplified products of the spliced (XBP1S; 398 bp) and unspliced (XBP1U; 424 bp) XBP1 were obtained.
Fas splicing assay
Procedures for the Fas splicing assay were followed exactly as we previously described (10).
ELISA for sFas
sFas in cell culture supernatants was detected using a human sFas ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. The absorbance of each well was measured at 450 nm using a microplate reader (Bio-Rad Laboratories). The concentration of sFas was calibrated from a dose response curve based on reference standards.
Caspase enzymatic activities assay
Activities of caspase-3/7, -8, and -9 were measured using the Apo-ONE Homogeneous Caspase-Glo 3/7, 8, and 9 assay kits, respectively (G8091, G8201, G8211; Promega, Madison, WI), according to the manufacturer’s instructions.
Statistical analysis
ANOVA was adopted to analyze differences between groups in experiments in which more than two groups were compared at a single time. Two-tailed Student t tests were used for analyzing all other experiments. Statistical software, such as GraphPad Prism and SPSS (IBM), was used for all analysis. A p value ≤0.05 was considered significant for all statistical analyses.
Results
HBsAg sensitizes hepatoma cells and primary hepatocytes to Fas-mediated apoptosis
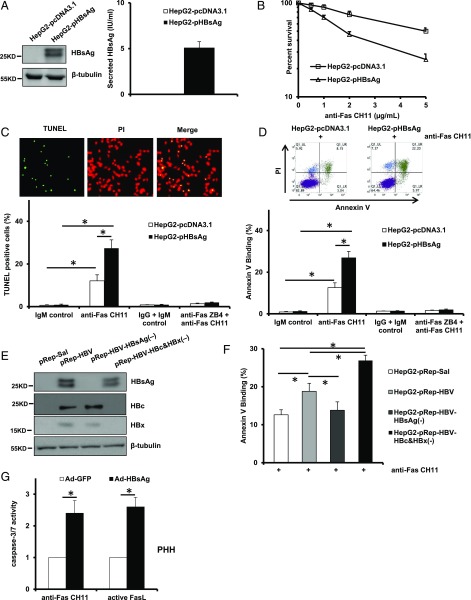
To investigate the role of HBsAg as a regulator of apoptosis, we generated HepG2 sublines stably transfected with empty vector pcDNA3.1/Hygro(+) or HBsAg-expressing pcDNA3.1-HBsAg and tested their sensitivity to agonistic anti-Fas Ab CH11, known to kill cells by the process of apoptosis. The successful expression of intracellular and secreted HBsAg was confirmed by Western blot analysis and an Architect assay, respectively (Fig. 1A). The CH11 concentration–survival curves show that HBsAg-expressing cells (HepG2-pHBsAg) were more sensitive to the cytotoxic effect of CH11 as compared with the empty vector–transfected cells (HepG2- pcDNA3.1) (Fig. 1B). To determine whether the increased sensitivity was due to more induction of apoptosis, we assessed the apoptotic rate of cells by a TUNEL assay. As shown in Fig. 1C, a significant increase in the number of apoptotic cells after CH11 treatment was observed in an HepG2-pHBsAg cell population compared with the control HepG2-pcDNA3.1 cells. Notably, pretreatment with antagonistic anti-Fas Ab ZB4 completely abolished the proapoptotic effects of CH11, and there was no significant difference in the apoptotic rate between HepG2-pHBsAg and HepG2-pcDNA3.1 cells. Furthermore, the increased apoptosis of HepG2 cells augmented by HBsAg was also verified by annexin V binding (Fig. 1D). A similar result was obtained with HepG2 cells transfected with 1.2-U lengths of the HBV genome (pRep-HBV), showing the enhanced sensitivity to Fas-induced apoptosis (Fig. 1E, 1F). Interestingly, transfection of the HBsAg mutant pRep-HBV-HBsAg(−) into HepG2 reduced Fas-induced apoptosis compared with cells transfected with pRep-HBV (Fig. 1E, 1F). We and others have reported that both HBc and HBx inhibit Fas-mediated apoptosis (10, 17). As expected, transfection of HBc and HBx double-mutant pRep-HBV-HBc&HBx(−) into HepG2 did greatly increase Fas-induced apoptosis compared with cells transfected with pRep-HBV and pRep-Sal1 (Fig. 1E, 1F). Because hepatoma cells may have abnormal death regulatory pathways, we employed PHH to test whether HBsAg could also potentiate Fas-mediated apoptosis triggered by either CH11 or FasL. As shown in Fig. 1G, infection of PHH with an adenovirus expressing HBsAg significantly augmented CH11- or FasL-induced caspase-3/7 activity as compared with the control virus-infected cells. These results clearly indicate that HBsAg is able to render hepatocytes hypersensitive to Fas/FasL-mediated apoptotic cell death.
FIGURE 1.
Expression of HBsAg renders hepatoma cells and PHH hypersensitive to Fas-mediated apoptosis. (A) Forced expression of HBsAg in HepG2 cells was confirmed by Western blot analysis and Architect assay. (B) In vitro cytotoxicity of anti-Fas CH11 to HepG2-pcDNA and HepG2-pHBsAg cells. The cells were treated with increasing concentrations of anti-Fas CH11 in the presence of 0.5 μg/ml ActD for 24 h, and a CCK-8 assay was used to quantify cell viability. Values are mean ± SD; n = 5. (C) Quantification of apoptotic cell proportions by TUNEL staining. Cells were treated with 1 μg/ml anti-Fas CH11 in the presence of ActD for 4 h with or without pretreatment with antagonistic anti-Fas ZB4. Data are expressed as percentage of the number of TUNEL-positive cells to the total number of HepG2-pcDNA3.1 or HepG2-pHBsAg cells counted. Original magnification ×200. Values are mean ± SD; n = 5. *p < 0.05. (D) Quantification of apoptotic cell fractions by annexin V/propidium iodide (PI) staining. Cells received the same treatments as indicated in (C) and were subjected to flow cytometric analysis. Values are mean ± SD; n = 3. *p < 0.05. (E) Expression of HBsAg, HBc, and HBx in HepG2-pRep HBV, HepG2-pRep HBV HBsAg(−), and HepG2-pRep HBV HBc and HBx(−) cells was assessed by Western blot analysis. (F) Flow cytometric analysis of the fraction of apoptotic cells 4 h after the indicated cells were treated with 1 μg/ml CH11 in the presence of ActD. Values are mean ± SD; n = 3. *p < 0.05. (G) Measurements of caspase-3/7 enzymatic activity of Ad-GFP– or Ad-HBsAg–infected PHH after CH11 and FasL stimulation. Values are mean ± SD; n = 3. *p < 0.05.
Fas/FasL apoptosis-inducing function is regulated by a number of mechanisms, including the levels of the ligand and receptor, efficiency of submembrane localization for receptor signaling complex assembly and activation, as well as p53 in some circumstances. Therefore, we measured the relative expression levels of p53, mFas, sFas, and FasL as well as palmitoylation status of Fas between HepG2-pcDNA3.1 and HepG2-pHBsAg cells. To our surprise, HBsAg does not affect the expression levels of p53, mFas, sFas, and FasL as well as Fas palmitoylation (Supplemental Fig. 1).
HBsAg facilitates procaspase-8 cleavage by enhancing the formation of SDS-stable Fas aggregation and reducing the recruitment of FLIPL/S at the DISC
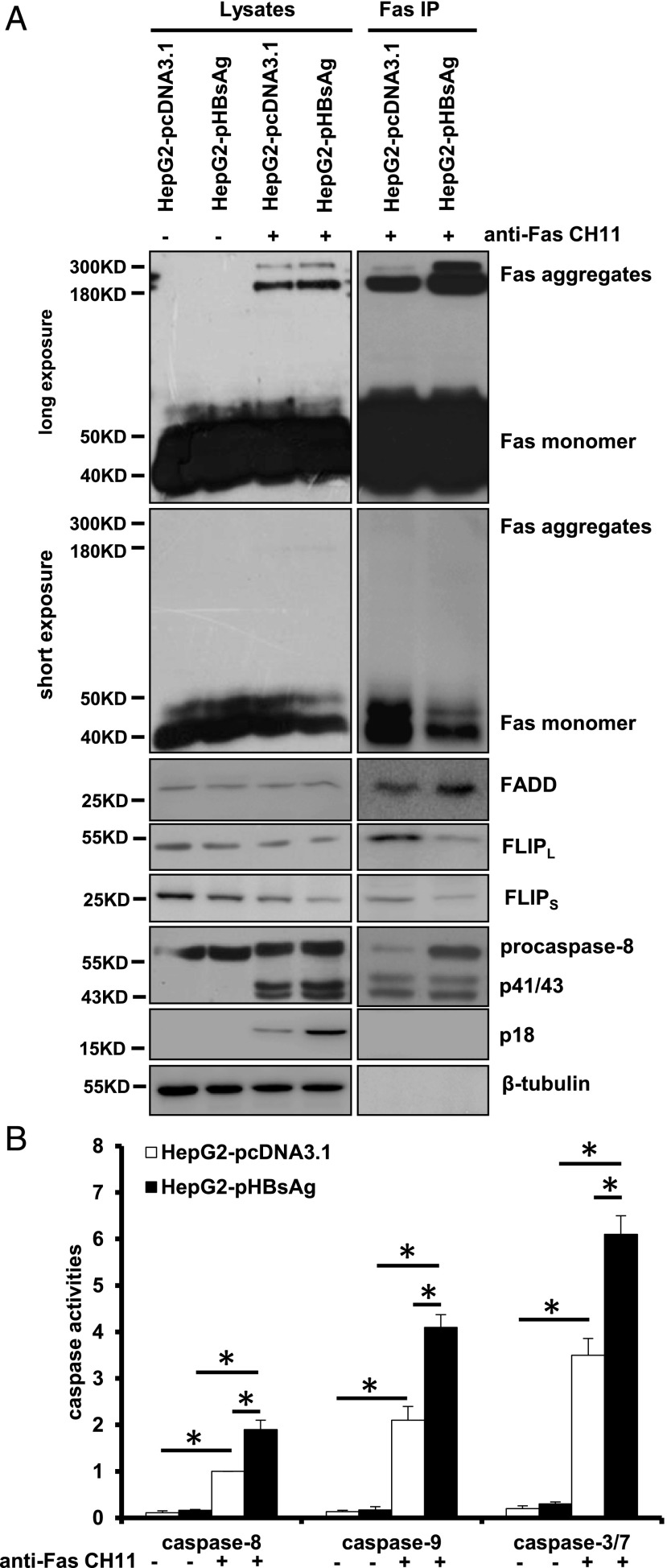
Binding of FasL to Fas transmits cell death signals via proapoptotic adapter protein FADD that mediates the activation of procaspase-8, also called FLICE, to form a DISC and subsequent activation of downstream executioner caspase-3 and caspase-7 contributing to hepatocyte apoptosis (18). To examine whether HBsAg is involved in modulating the formation of DISC, the DISC components were determined by IP and Western blot analysis in HepG2 cells (Fig. 2A). Fas preexists on the cell surface as noncovalently associated homotrimers of the 45-kDa Fas protein. Upon stimulation by agonistic anti-Fas Ab, Fas receptors appear to oligomerize rapidly to form high molecular masses that migrate at ∼200–250 kDa in SDS-PAGE (19–23). As anticipated, SDS-stable tetrameric Fas aggregates with a high molecular mass of ∼200–250 kDa were present in the cells treated with CH11 (Fig. 2A, row 1). An increase of Fas aggregates was accompanied by a decrease of Fas monomers in HepG2-HBsAg cells, opposite to what occurred in HepG2-pcDNA3.1 cells (Fig. 2A, rows 1 and 2, lanes 3 and 4). FADD was not changed between these two cell lines (Fig. 2A, row 3). FLIP is an antiapoptotic cytoplasmic protein with sequence homology to caspase-8, functioning as a dominant-negative inhibitor of caspase-8 to prevent Fas-induced apoptosis (24). We found that FLIPL/S protein levels were decreased in HepG2-pHBsAg cells compared with HepG2-pcDNA3.1 cells (Fig. 2A, rows 4 and 5, lanes 1 and 2), and treatment of CH11 enlarged the magnitude of this effect (Fig. 2A, rows 4 and 5, lanes 3 and 4). Furthermore, whereas CH11 treatment efficiently cleaved procaspase-8, leading to generation of the p41/43 fragment and active p18 prodomain in both cells, HepG2-pHBsAg cells produced a more active p18 subunit (Fig. 2A, rows 6 and 7, lanes 3 and 4). Analysis of DISC components revealed that CH11-treated HepG2-pHBsAg cells recruited more Fas aggregates and FADD but less FLIPL/S, allowing recruitment of sufficient procaspase-8 to be cleaved as compared with HepG2-pcDNA3.1 cells (Fig. 2A, lanes 5 and 6). Consistently, the cascade activation of sequential caspase-8, -9, and -3/7 after CH11 treatment was greater in HepG2-pHBsAg cells than in HepG2-pcDNA3.1 cells (Fig. 2B). Notably, ActD is often combined with Fas agonist to increase the sensitivity of tumor cells to the Fas apoptotic signal (25), as we did in this study. We found that ActD alone did not affect the levels of those DISC-associated proteins such as FADD, FLIP, and procaspase-8 (Supplemental Fig. 2). Taken together, these results indicate that HBsAg promotes procaspase-8 cleavage by enhancing the formation of SDS-stable Fas aggregation and reducing the recruitment of FLIPL/S at the DISC.
FIGURE 2.
HBsAg facilitates procaspase-8 cleavage via enhancing the formation of SDS-stable Fas aggregation and reducing the recruitment of FLIPL/S at the DISC. (A) Analysis of the DISC components from the immunoprecipitated complexes with anti-Fas Ab. (B) Measurements of caspase-3/7, -8, and -9 enzymatic activity expressed as the fold change relative to that in the HepG2-pcDNA3.1 control cells. Values are mean ± SD; n = 3. *p < 0.05.
HBsAg restores the reduction of Fas-mediated apoptosis caused by AKT activation
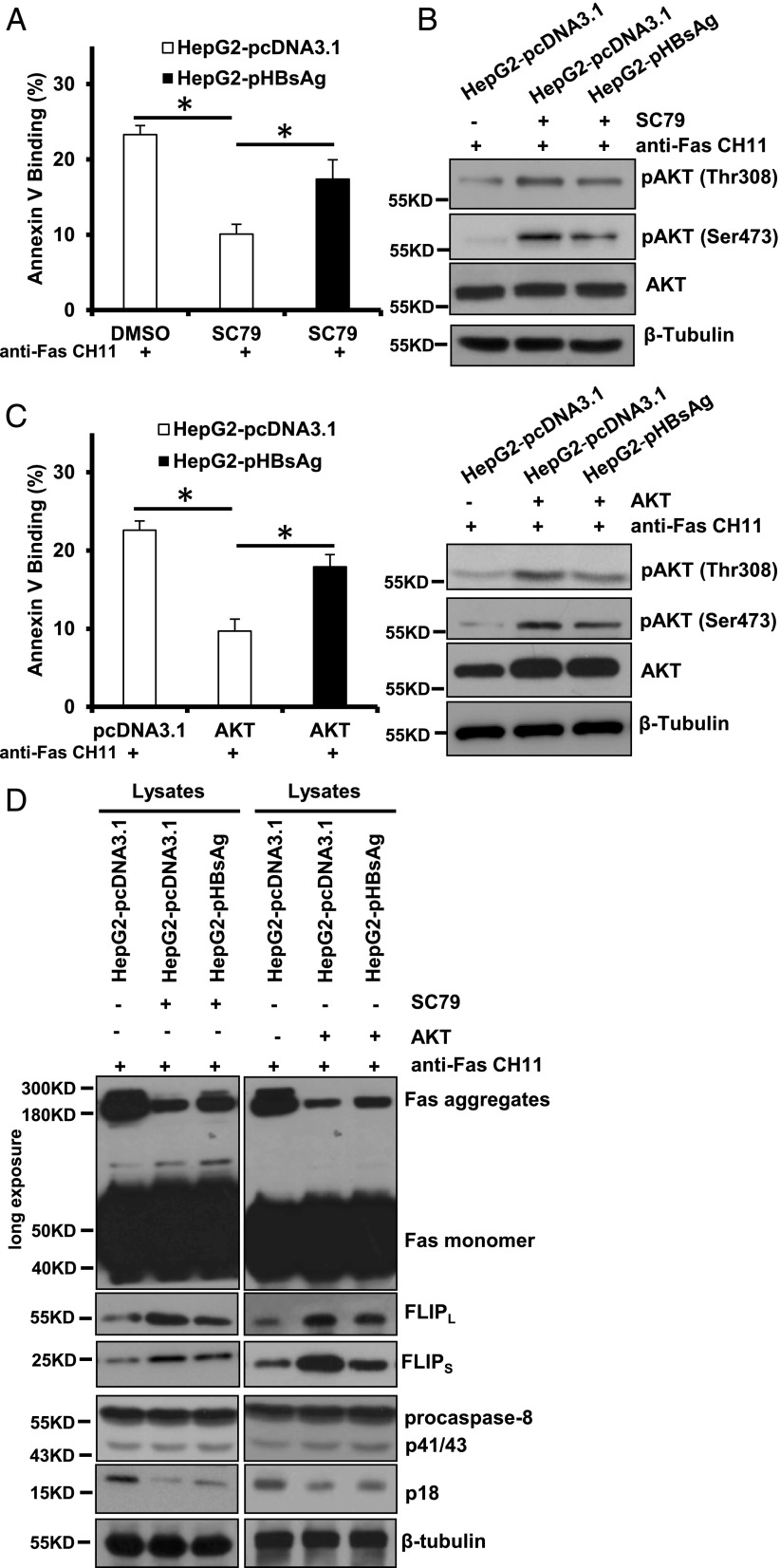
AKT is a crucial prosurviving factor of the cell, and its deactivation has been implicated in various kinds of stress-induced pathological cell death, including pathogenesis of hepatocytes injury (6, 26–29). More specifically, activation of AKT has been reported to abolish Fas aggregation and procaspase-8 cleavage at the DISC, whereas inhibition of AKT phosphorylation promotes Fas DISC assembly (7). Our recent work has also documented that activation of AKT by a novel AKT-specific activator, SC79, prevented hepatocytes from Fas-induced apoptosis (30). Based on these observations we reasoned that the effect of HBsAg on modulating the formation of DISC may be AKT-dependent, and therefore SC79 was introduced as an antiapoptotic tool to explore the probable cause of the observed enhancement of Fas-triggered apoptosis by HBsAg. We found that SC79 did protect HepG2-pcDNA3.1 cells from CH11-induced apoptotic cell death; however, such a protective effect was significantly diminished in HBsAg-overexpressed HepG2-pHBsAg cells (Fig. 3A). Consistently, there was less AKT activation in HepG2-pHBsAg cells as compared with HepG2-pcDNA3.1 cells when both cells were treated with SC79 (Fig. 3B). Similar results were obtained when AKT was overexpressed (Fig. 3C). Then, we further determined whether the reversal of HBsAg on SC79 effects was primarily due to alterations in Fas aggregation and DISC formation. As shown in Fig. 3D (lanes 1–3), SC79 substantially reduced Fas aggregation, and this reduction was partially restored when HBsAg was expressed in the HepG2-pHBsAg cells. Upregulation of FLIPL/S expression by AKT activation has been reported to inhibit receptor-induced apoptosis (31), and decreased FLIPL/S expression sensitized hepatocytes to Fas-mediated apoptosis (32). As expected, FLIPL/S expression was upregulated by SC79 treatment, and upregulation of FLIPL/S expression by SC79 was attenuated in the presence of HBsAg expression. Furthermore, CH11 treatment efficiently cleaved procaspase-8 to generate the active p18 prodomain. In contrast, SC79 pretreatment before CH11 stimulation only processed procaspase-8 to the p41/43 fragment and produced a less active p18 subunit. Again, expression of HBsAg reversed this effect and caused an increase of caspase-8 activation. Similar results were obtained when AKT was overexpressed in HepG2 cells (Fig. 3D, lanes 4–6). Collectively, these results indicate that HBsAg is able to restore the reduction of Fas-mediated apoptosis caused by AKT activation.
FIGURE 3.
HBsAg restored Fas-mediated apoptosis initially attenuated by enhanced AKT phosphorylation. (A) Quantification of apoptotic cell fractions by annexin V/propidium iodide staining. DMSO- or SC79-pretreated HepG2-pcDNA3.1 and HepG2-pHBsAg cells were exposed to 1 μg/ml agonistic Fas CH11 for 6 h and subjected to flow cytometric analysis. Values are mean ± SD; n = 3. *p < 0.05. (B) HBsAg abrogated SC79-induced Akt activation in HepG2 cells. (C) Quantification of apoptotic cell fractions by annexin V/PI staining in the AKT-overexpressed cells 6 h after treatment with 1 μg/ml agonistic Fas CH11. Values are mean ± SD; n = 3. *p < 0.05. Western blot analysis shows that HBsAg abrogated AKT activation by ectopic expression of AKT in HepG2 cells. (D) Analysis of the DISC components from the lysates stimulated with anti-Fas CH11.
HBsAg downregulates AKT phosphorylation by endoplasmic reticulum stress–induced deactivation of PDPK1 and mechanistic target of rapamycin complex 2
Given the observation that HBsAg enhanced CH11-induced and Fas-mediated apoptosis, which was dependent on AKT deactivation, the phosphorylation status of AKT at Thr308 and Ser473 sites was examined. As shown in Fig. 4A, under CH11 treatment, phosphorylation of both AKT and its upstream signaling molecules PDPK1 and mTOR was significantly reduced in HepG2-HBsAg cells as compared with HepG2-pcDNA3.1 cells. HBsAg could trigger endoplasmic reticulum (ER) stress (33), and ER stress negatively regulates AKT activation through several mechanisms (34, 35). To determine whether ER stress is involved in HBsAg modulating AKT phosphorylation and activation, we evaluated the effects of ER stress inhibitor 4-PBA on AKT phosphorylation attenuated by HBsAg. As shown in Fig. 4B, exposure of HepG2-pHBsAg cells to 4-PBA increased AKT phosphorylation at the sites of Thr308 and Ser473, resulting from increased phosphorylation of PDPK1 (Ser241) and mTOR (Ser2481). To further ascertain an important role of 4-PBA in HBsAg-regulated and Fas-mediated apoptosis, HepG2-pHBsAg cells were treated with 4-PBA followed by exposure to CH11 and then assayed for annexin V binding. As expected, pretreatment with 4-PBA partially abrogated CH11-induced apoptotic cell death in the presence of HBsAg (Fig. 4C). To assess whether HBsAg could physically interact with endogenous AKT, PDPK1, mTOR, or the PI3K negative regulator PTEN, we performed a coimmunoprecipitation study with HepG2-pHBsAg cells. The results showed that FLAG-tagged HBsAg was not immunoprecipitated with AKT, PDPK1, mTOR, or PTEN, and no interactions between them were further confirmed by reverse IP using AKT, PDPK1, mTOR, or PTEN Abs (Fig. 4D, 4E). Mechanistic target of rapamycin complex 2 (mTORC2) consists of phosphorylated mTOR and the Rictor subunit, which binds and activates AKT through S473 phosphorylation (36). When mTOR was immunoprecipitated, we observed a significant decrease in the amount of Rictor and AKT in HepG2-pHBsAg cells as compared with HepG2-pcDNA3.1 cells. However, 4-PBA partially enhanced the interaction in HepG2-pHBsAg cells (Fig. 4F). These data suggest that HBsAg could attenuate AKT phosphorylation, likely through deactivation of PDPK1 and mTORC2 by inducing ER stress.
FIGURE 4.
HBsAg downregulates AKT phosphorylation via deactivation of PDPK1 and mTORC2. (A) Representative Western blot showing the effect of HBsAg on expression of total and phosphorylated forms of key molecules in the AKT signaling pathway in the HepG2-pcDNA3.1 and HepG2-pHBsAg cells. (B) Representative Western blot showing the effect of 4-PBA on expression of total and phosphorylated forms of key molecules in the AKT signaling pathway in the HepG2-pHBsAg cells. (C) Quantification of apoptotic cell fractions by annexin V/propidium iodide staining in the 4-PBA–pretreated HepG2-pcDNA3.1 and HepG2-pHBsAg cells 6 h after treatment with 1 μg/ml agonistic Fas CH11. Values are mean ± SD; n = 3. *p < 0.05. (D and E) Interaction between HBsAg and AKT, PDPK1, mTOR, and PTEN as determined by coimmunoprecipitation assay. FLAG-tagged HBsAg was immunoprecipitated with anti-FLAG Ab, and AKT, PDPK1, mTOR, or PTEN was detected by Western blotting with specific Abs. Reciprocal coimmunoprecipitation was carried out using AKT, PDPK1, mTOR, and PTEN Abs, and HBsAg was detected by Western blotting with anti-FLAG Ab. Normal IgG served as a control. (F) The effect of 4-PBA on interaction between mTOR and pmTOR (Ser2481), Rictor, AKT, and pAKT (Ser473) in HepG2-pcDNA3.1 and HepG2-pHBsAg cells as determined by coimmunoprecipitation assay. mTOR was immunoprecipitated with the specific Ab, and pmTOR (Ser2481), Rictor, AKT, or pAKT (Ser473) was detected by Western blotting with specific Abs.
Secretion-deficient HBsAg variants enhance their proapoptotic abilities via induction of ER stress
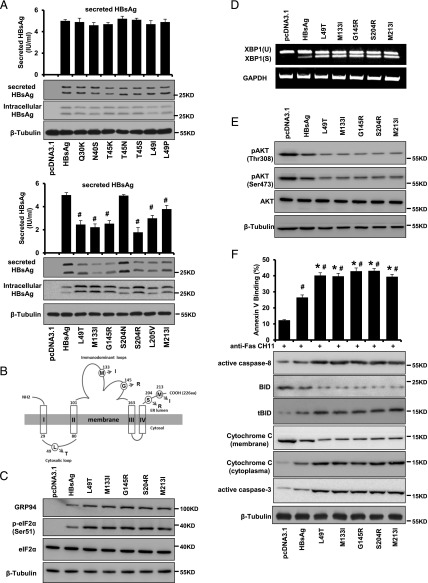
Given the observation that HBsAg could enhance Fas-mediated hepatocyte apoptosis through increasing ER stress, one might speculate that certain types of S gene mutations could further aggravate the ER retention, consequently leading to more severe hepatocytic injury. In fact, clusters of S gene mutations have been found in patients with HBV infection at various clinical stages (37). In this regard, we generated 14 commonly occurring HBsAg mutants, including Q30K, N40S, T45K, T45N, T45S, L49I, L49P, L49T, M133I, G145R, S204N, S204R, L205V, and M213I (38), and assessed their biological relevance in the context of ER stress and Fas-mediated apoptosis in hepatocytes. We found that there was a distinct intracellular retention in the cells expressing L49T, M133I, G145R, S204R, and M213I HBsAg mutants, and the extracellular level of HBsAg was simultaneously reduced (Fig. 5A). Additionally, ER stress marker GRP94, p-eIF2α, and XBP1 were also examined by Western blot analysis and semiquantitative RT-PCR. The results showed that these five HBsAg mutants (the respective mutation sites are illustrated in Fig. 5B) markedly induced the expression of GRP94 and the phosphorylation of eIF2α as compared with wild-type HBsAg (Fig. 5C). Additionally, the spliced form of XBP1 was also higher in the five HBsAg mutant-expressing cells as compared with wild-type HBsAg-transfected cells (Fig. 5D). Moreover, pAKT was all decreased with a relatively lower level seen in the mutated HBsAg-transfected cells (Fig. 5E). The frequency of apoptosis was substantially increased, with the effect being greater in the five HBsAg mutant-expressing HepG2 cells compared with the wild-type HBsAg-transfected cells (Fig. 5F). The mutated HBsAg-transfected HepG2 cells also had a higher cytoplasmic level of active caspase-8, tBID, and cytochrome c than did wild-type HBsAg-transfected cells (Fig. 5F). These data suggest that HBsAg intracellular retention promotes apoptosis by induction of ER stress.
FIGURE 5.
HBsAg intracellular retention as a result of secretion deficiency enhances its proapoptotic activity by induction of ER stress. (A) Expression of secreted HBsAg from HepG2 cells stably transfected with wild-type and various mutated HBsAg was assessed by Western blot analysis and Architect assay. (B) Schematic illustration of HBsAg mutations sites responsible for intracellular retention of HBsAg and its proapoptotic activity. (C) Representative Western blot showing the expression of total and phosphorylated forms of key molecules involved in ER stress in HepG2 cells stably transfected with wild-type and mutated forms of HBsAg. (D) Semiquantitative RT-PCR showing the expression of XBP1(U) and XBP1(S) in HepG2 cells stably transfected with wild-type and mutated forms of HBsAg. (E) Representative Western blot showing the expression of total and phosphorylated forms of AKT in HepG2 cells stably transfected with wild-type and mutated forms of HBsAg. (F) Quantification of apoptotic cell fractions by annexin V/propidium iodide staining (upper panel) and Western blot analysis of expression of apoptosis-related mediators in HepG2 cells stably transfected with wild-type and mutated forms of HBsAg. Values are mean ± SD. n = 3. #p < 0.05, *p < 0.05.
HBsAg predisposes mice to acute liver failure, which could be effectively attenuated by SC79
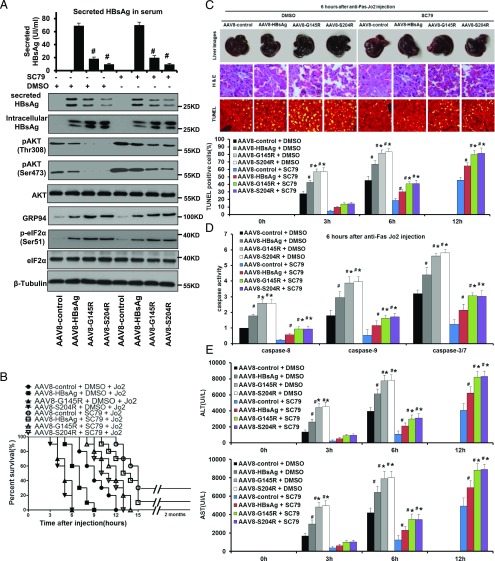
To determine whether the effects of HBsAg on Fas-mediated apoptosis in vitro could translate into responsiveness in vivo, anti-Fas Jo2 was injected i.p. into mice to establish an acute liver failure (ALF) model. We expressed HBsAg and G145R/S204R mutants, which demonstrated the highest ability of causing ER retention in vitro, in the livers of mice using a liver-targeted AAV8. As shown in Fig. 6A, G145R and S204R mutations significantly reduced the extracellular level of HBsAg, and a distinct retention of HBsAg was observed in mice. Mice successfully expressing HBsAg had an increased ER stress but a decreased AKT activation, to a greater extent in G145R and S204R groups as compared with AAV8-HBsAg and empty control groups. No significant morphological changes and histological lesions were observed between HBsAg, G145R, S204R, and the AAV8-control group, indicating that HBsAg alone did not impair liver histology (Supplemental Fig. 3). When Jo2 was injected i.p. into DMSO-pretreated groups, early acute hepatic failure leading to 100% mortality rate occurred in all DMSO-pretreated littermates within 12 h. In spectacular contrast, SC79 pretreatment significantly extended the survival time (Fig. 6B). Livers of Ab-treated mice were taken for histological examination at 3, 6, and 12 h after i.p. injection. Severe histological lesions of the liver were observed in DMSO-pretreated control mice, including morphological changes characteristic of apoptosis. As shown in Fig. 6C, in the control mice, early onset of massive apoptosis was observed 3 h after the injection of Jo2. At 6 h, the AAV8-HBsAg group had a significant increase of hepatic apoptosis, whereas the effect was greatest for the AAV8-G145R and S204R mutant groups with the apoptosis rate >80%. In contrast, histological analysis of livers from SC79-pretreated mice revealed delayed and far less severe apoptosis with a faster recovery for the AAV8-HBsAg group compared with the AAV8-G145R and S204R groups. Consistent with histological data, a remarkable elevation of caspases-8, -9, and -3/7 activities was detected in the liver extracts of DMSO-treated mice at 6 h after Jo2 treatment, with a greater effect for the AAV8-G145R and S204R groups relative to the AAV8-control group, whereas all caspase activities were significantly reduced in SC79-pretreated mice (Fig. 6D).
FIGURE 6.
Expression of HBsAg in mice renders them susceptible to ALF, and this effect is attenuated by administration of SC79. (A) SC79 treatment led to AKT hyperactivation in the livers of mice expressing wild-type and variant HBsAg by the AAV8 system. Protein extracts collected from the liver of untreated and SC79-treated mice were resolved on SDS-PAGE and immunoblotted with indicated Abs. SC79 was applied via i.p. injection at a concentration of 10 mg/kg body weight. (B) The protective effects of SC79 on an acute and lethal apoptotic hepatic injury induced by an anti-Fas agonist, Jo2 Ab. SC79-pretreated mice markedly extended the survival time from 6 to 13 h for AAV8-S204R, 6 to 14 h for AAV8-G145R, 9 to 15 h for AAV8-HBsAg, and 12 to 15 h for AAV8-control (all p < 0.01, n = 10 for all groups). (C) Macroscopic appearance of representative liver samples with H&E staining, and TUNEL staining 6 h after Jo2 treatment of the different groups as indicated. The histogram summarizes the average percentage of apoptotic cells in the livers of the indicated groups at various time points after Jo2 treatment. Original magnification ×200. (D) Caspase-8, -9, and -3/7 activity assay and (E) serum ALT and AST analyses, showing that significantly less severity with regard to apoptosis, caspase activities, and serum aminotransferase levels was observed in SC79-pretreated mice. Values are mean ± SD. n = 3. #p < 0.05, *p < 0.05.
Serum levels of ALT and AST were also measured and the results showed that ALT and AST were dramatically increased within 3 h after Jo2 injection in AAV8-HBsAg mice and at a higher level in the AAV8-G145R and S204R groups as compared with the AAV8-control group (Fig. 6E). In contrast, delayed and significantly lower levels of ALT and AST were recorded for SC79 pretreatment mice at 3 and 6 h after injection of anti-Fas Jo2. ALT and AST were dramatically increased at 12 h after Jo2 injection in AAV8-HBsAg mice and at a higher level in the AAV8-G145R and S204R groups after SC79 pretreatment. Taken together, these results suggest that HBsAg is capable of predisposing mice to ALF, and the specific HBsAg mutations could further augment this effect via increasing their ER retention. Additionally, SC79 can protect the mice from HBsAg-induced ALF.
Discussion
Hepatocyte apoptosis, mainly induced by death receptor ligands such as TNF-α and FasL, is implicated in several experimental and human liver diseases, including viral hepatitis and ALF. Therefore, identification of pro- and antiapoptotic pathways related to death receptor–mediated hepatocyte apoptosis would contribute importantly to understanding the pathophysiological role of apoptosis in major liver diseases. In this study, we demonstrated for the first time, to our knowledge, that HBsAg is a strong death-inducing factor capable of rendering hepatocytes susceptible to Fas-induced apoptosis through the AKT-dependent Fas/FasL signaling pathway both in vitro and in vivo.
It is now generally accepted that HBV infection could interfere with the apoptosis signaling for viral proliferation, and alterations in the control of apoptosis mediated through the Fas system contribute significantly to the pathogenesis of severe liver diseases such as liver cirrhosis and HCC (39). In CHB patients, upregulation of Fas expression was found to correlate with the degree of liver inflammation (3, 5). Fas expression was highly upregulated in hepatocytes of patients with HBV-related cirrhosis, the most serious consequence of chronic liver injury (40). In contrast, Fas expression in HBV-associated HCC specimens was found to be less frequent and weaker in HCC tissues than in the corresponding noncancerous tissues (41). Acquired resistance to Fas-mediated apoptosis is common in HBV-infected cells, which may offer them critical survival advantages, ultimately resulting in virus chronicity and malignancy (42). Additionally, such selection for resistance to the apoptosis-inducing signal may eventually shield the virus-infected hepatocytes from elimination by the host immune surveillance (43). Thus, disorder in Fas expression and its mediated hepatocyte apoptosis is now considered to be an important pathway for major HBV-related liver diseases.
Fas signaling originates from Fas aggregation and DISC formation (18). The DISC consists of oligomerized receptors, FADD, procaspase-8, procaspase-10, and FLIPL/S, and activation of procaspase-8 at the DISC leads to the induction of Fas-mediated apoptosis (44) whereas the FLIPL/S functions to block procaspase-8 activation (24). Our observation that HBsAg not only enhances the formation of SDS-stable Fas aggregation but also diminishes the recruitment of FLIPL/S at the DISC may explain, at least in part, why HBsAg augments Fas-mediated apoptosis.
We found that reduction of the formation of SDS-stable Fas aggregation and procaspase-8 cleavage by enhanced AKT phosphorylation is reversible by expression of HBsAg. Therefore, Fas signaling enhanced by HBsAg is AKT-dependent. Fas has been reported to either reside in or to be recruited into membrane rafts (45) where caspase-8 is activated (46). The role of lipid rafts and receptor internalization regulated by AKT in Fas signaling is also linked to DISC assembly and apoptosis (47), although the detailed mechanism of how HBsAg regulates AKT-related redistribution of Fas and DISC into lipid rafts needs to be further elucidated.
The possibility that HBsAg may function as a signal transduction pathway regulatory protein arises from its ER stress–inducing properties (33) that include the process of being matured in ER and Golgi apparatus and accumulating in large quantities in the persistence of the virus infection (48). Accumulation of misfolded proteins in the ER may negatively regulate the AKT signaling pathway, producing toxic effects (49) and ultimately leading to cell death (35). Important roles for ER-initiated cell death pathways have been recognized in several liver diseases (50). In the present study, we demonstrated that HBsAg expression reversed the effect of enhanced AKT phosphorylation on reduction of Fas-mediated apoptosis. Furthermore, HBsAg downregulated AKT phosphorylation by ER stress–induced deactivation of PDPK1 and mTORC2. ER stress has been implied in intrinsic or mitochondrial apoptosis (51), and this study points toward the concept that the extrinsic or death receptor pathway of apoptosis was also linked to ER stress.
Note that a negative regulation of AKT activity by HBsAg can be further proved from its regulation on PI3K/AKT downstream targets BAD and BAX, the two major proapoptotic members of the Bcl-2 family. BAD promotes cell death, whereas phosphorylation of BAD at Ser136 by growth factor–activated AKT suppresses apoptosis and promotes cell survival (52, 53). BAX localizes largely in the cytoplasm but redistributes to mitochondria upon a wide variety of apoptotic stimuli such as Fas, where it induces cytochrome c release for apoptosis (3). PI3K/AKT activity is known to play a critical role in inhibiting Bax translocation to mitochondria, thus promoting survival and preventing cell death (3). As shown in Supplemental Fig. 4, expression of HBsAg not only abolished BAD phosphorylation at Ser136, which was initially enhanced by the growth factor IGF-1, but it also facilitated the translocation of BAX from cytoplasm to mitochondria in response to CH11 stimulation. These observations might further support the notion that HBsAg potentiates Fas-mediated hepatocyte apoptosis likely through negatively regulating PI3K/AKT signaling.
HBV mutants with substitutions within the coding region for HBsAg have been found naturally in chronic carriers. It has been estimated that 75.9% of HBV reactivated patients were carriers of more than one HBsAg mutation (54). We found that the L49T, M133I, G145R, S204R, and M213I mutations significantly reduced the extracellular level of HBsAg but increased a distinct retention within the cell, thus enhancing its proapoptotic activity by strong induction of ER stress. Intriguingly, not all HBsAg mutants could result in the enhancement of ER stress and apoptosis. The details of how specific mutations in HBsAg caused the retention within the ER of the hepatocytes remain to be determined. To gain further insight into the contribution of HBsAg to ALF, a mouse model was established by expressing the sequence encoding wild-type and HBsAg mutants in the livers of mice. We demonstrated that in vivo expression of HBsAg caused a significant increase of ER stress, severe apoptosis, and liver injury, with the effect being greater for the HBsAg mutants (G145R and S204R). Note that whereas the ER stress reliever 4-PBA could also partially attenuate HBsAg-induced dephosphorylation of AKT, it had little effect on HBsAg-related and Fas-induced ALF (data not shown).
A large number of healthy HBsAg carriers or chronic HBV-infected patients of high HBsAg tiers may present with no signs of spontaneous liver disease. The reason why HBsAg is unable to trigger a Fas-mediated hepatocyte apoptosis and possible liver damage under such circumstances is open to debate. A study showed that the serum concentration of sFas among healthy HBsAg carriers was low and comparable to healthy persons (4). Another possible explanation of this fact is that HBsAg may induce immune tolerance during chronic HBV infection. Presentation of HBsAg by hepatocytes in the absence of proinflammatory response may induce a defective T cell response that could be further depleted due to prolonged exposure to large quantities of such Ags (55). Furthermore, persistently high HBsAg Ag load has been shown to allow cross-presentation by liver professional APCs (Kupffer cells, liver endothelial cells) that could induce tolerance in chronic HBV patients (56). Such direct presentation of foreign Ag by hepatocytes preferentially induces CD8+ T cell tolerance, as evidenced by a reduction of CD8+ T cell clonal expansion and an increase of T cell apoptosis (56).
In summary, to the best of our knowledge, this report is the first demonstration to date that HBsAg sensitizes hepatocytes to Fas-mediated apoptosis and increases susceptibility to ALF in mice via suppression of AKT phosphorylation. Our results also suggest that SC79 may prevent hepatocytes from apoptosis induced by HBsAg-related enhancement of Fas/FasL activity, thus increasing the survival of mice with ALF.
Supplementary Material
This work was supported by National Natural Science Foundation of China Grant 81601776, State Key Project Specialized for Infectious Diseases Grant 2017ZX10202203-005-002, and Joint Funds for the Innovation of Science and Technology, Fujian Province Grant 2016Y91030022.
The online version of this article contains supplemental material.
- AAV8
- adenovirus-associated virus 8
- ActD
- actinomycin D
- ALF
- acute liver failure
- ALT
- alanine transaminase
- AST
- aspartate transaminase
- CHB
- chronic hepatitis B
- DISC
- death-inducing signaling complex
- ER
- endoplasmic reticulum
- FADD
- Fas-associated death domain
- FasL
- Fas ligand
- HBsAg
- HBV surface Ag
- HBV
- hepatitis B virus
- HCC
- hepatocellular carcinoma
- IP
- immunoprecipitation
- mTORC2
- mechanistic target of rapamycin complex 2
- 4-PBA
- 4-phenylbutyric acid
- PDPK1
- phosphoinositide-dependent kinase-1
- PHH
- primary human hepatocyte
- sFas
- soluble form of Fas.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Nagata S. 1997. Apoptosis by death factor. Cell 88: 355–365. [DOI] [PubMed] [Google Scholar]
- 2.Kondo T., Suda T., Fukuyama H., Adachi M., Nagata S. 1997. Essential roles of the Fas ligand in the development of hepatitis. Nat. Med. 3: 409–413. [DOI] [PubMed] [Google Scholar]
- 3.Mochizuki K., Hayashi N., Hiramatsu N., Katayama K., Kawanishi Y., Kasahara A., Fusamoto H., Kamada T. 1996. Fas antigen expression in liver tissues of patients with chronic hepatitis B. J. Hepatol. 24: 1–7. [DOI] [PubMed] [Google Scholar]
- 4.Lapinski T. W., Kowalczuk O., Prokopowicz D., Chyczewski L. 2004. Serum concentration of sFas and sFasL in healthy HBsAg carriers, chronic viral hepatitis B and C patients. World J. Gastroenterol. 10: 3650–3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo K. X., Zhu Y. F., Zhang L. X., He H. T., Wang X. S., Zhang L. 1997. In situ investigation of Fas/FasL expression in chronic hepatitis B infection and related liver diseases. J. Viral Hepat. 4: 303–307. [DOI] [PubMed] [Google Scholar]
- 6.Schulze-Bergkamen H., Brenner D., Krueger A., Suess D., Fas S. C., Frey C. R., Dax A., Zink D., Büchler P., Müller M., Krammer P. H. 2004. Hepatocyte growth factor induces Mcl-1 in primary human hepatocytes and inhibits CD95-mediated apoptosis via Akt. Hepatology 39: 645–654. [DOI] [PubMed] [Google Scholar]
- 7.Pizon M., Rampanarivo H., Tauzin S., Chaigne-Delalande B., Daburon S., Castroviejo M., Moreau P., Moreau J. F., Legembre P. 2011. Actin-independent exclusion of CD95 by PI3K/AKT signalling: implications for apoptosis. Eur. J. Immunol. 41: 2368–2378. [DOI] [PubMed] [Google Scholar]
- 8.Lok A. S., McMahon B. J. 2009. Chronic hepatitis B: update 2009. Hepatology 50: 661–662. [DOI] [PubMed] [Google Scholar]
- 9.Chai N., Chang H. E., Nicolas E., Han Z., Jarnik M., Taylor J. 2008. Properties of subviral particles of hepatitis B virus. J. Virol. 82: 7812–7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu W., Lin Y. T., Yan X. L., Ding Y. L., Wu Y. L., Chen W. N., Lin X. 2015. Hepatitis B virus core protein inhibits Fas-mediated apoptosis of hepatoma cells via regulation of mFas/FasL and sFas expression. FASEB J. 29: 1113–1123. [DOI] [PubMed] [Google Scholar]
- 11.Lin X., Yuan Z. H., Wu L., Ding J. P., Wen Y. M. 2001. A single amino acid in the reverse transcriptase domain of hepatitis B virus affects virus replication efficiency. J. Virol. 75: 11827–11833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y. L., Peng X. E., Zhu Y. B., Yan X. L., Chen W. N., Lin X. 2015. Hepatitis B virus X protein induces hepatic steatosis by enhancing the expression of liver fatty acid binding protein. J. Virol. 90: 1729–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Y. J., Huang L. R., Yang H. C., Tzeng H. T., Hsu P. N., Wu H. L., Chen P. J., Chen D. S. 2010. Hepatitis B virus core antigen determines viral persistence in a C57BL/6 mouse model. Proc. Natl. Acad. Sci. USA 107: 9340–9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y. L., Wang D., Peng X. E., Chen Y. L., Zheng D. L., Chen W. N., Lin X. 2013. Epigenetic silencing of NAD(P)H:quinone oxidoreductase 1 by hepatitis B virus X protein increases mitochondrial injury and cellular susceptibility to oxidative stress in hepatoma cells. Free Radic. Biol. Med. 65: 632–644. [DOI] [PubMed] [Google Scholar]
- 15.Liu W., Guo T. F., Jing Z. T., Yang Z., Liu L., Yang Y. P., Lin X., Tong Q. Y. 2018. Hepatitis B virus core protein promotes hepatocarcinogenesis by enhancing Src expression and activating the Src/PI3K/Akt pathway. FASEB J. 32: 3033–3046. [DOI] [PubMed] [Google Scholar]
- 16.Rossin A., Durivault J., Chakhtoura-Feghali T., Lounnas N., Gagnoux-Palacios L., Hueber A. O. 2015. Fas palmitoylation by the palmitoyl acyltransferase DHHC7 regulates Fas stability. Cell Death Differ. 22: 643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diao J., Khine A. A., Sarangi F., Hsu E., Iorio C., Tibbles L. A., Woodgett J. R., Penninger J., Richardson C. D. 2001. X protein of hepatitis B virus inhibits Fas-mediated apoptosis and is associated with up-regulation of the SAPK/JNK pathway. J. Biol. Chem. 276: 8328–8340. [DOI] [PubMed] [Google Scholar]
- 18.Algeciras-Schimnich A., Shen L., Barnhart B. C., Murmann A. E., Burkhardt J. K., Peter M. E. 2002. Molecular ordering of the initial signaling events of CD95. Mol. Cell. Biol. 22: 207–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirschnek S., Paris F., Weller M., Grassme H., Ferlinz K., Riehle A., Fuks Z., Kolesnick R., Gulbins E. 2000. CD95-mediated apoptosis in vivo involves acid sphingomyelinase. J. Biol. Chem. 275: 27316–27323. [DOI] [PubMed] [Google Scholar]
- 20.Kamitani T., Nguyen H. P., Yeh E. T. 1997. Activation-induced aggregation and processing of the human Fas antigen. Detection with cytoplasmic domain-specific antibodies. J. Biol. Chem. 272: 22307–22314. [DOI] [PubMed] [Google Scholar]
- 21.Papoff G., Hausler P., Eramo A., Pagano M. G., Di Leve G., Signore A., Ruberti G. 1999. Identification and characterization of a ligand-independent oligomerization domain in the extracellular region of the CD95 death receptor. J. Biol. Chem. 274: 38241–38250. [DOI] [PubMed] [Google Scholar]
- 22.Legembre P., Beneteau M., Daburon S., Moreau J. F., Taupin J. L. 2003. Cutting edge: SDS-stable Fas microaggregates: an early event of Fas activation occurring with agonistic anti-Fas antibody but not with Fas ligand. J. Immunol. 171: 5659–5662. [DOI] [PubMed] [Google Scholar]
- 23.Feig C., Tchikov V., Schütze S., Peter M. E. 2007. Palmitoylation of CD95 facilitates formation of SDS-stable receptor aggregates that initiate apoptosis signaling. EMBO J. 26: 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scaffidi C., Schmitz I., Krammer P. H., Peter M. E. 1999. The role of c-FLIP in modulation of CD95-induced apoptosis. J. Biol. Chem. 274: 1541–1548. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki A., Tsutomi Y., Akahane K., Araki T., Miura M. 1998. Resistance to Fas-mediated apoptosis: activation of caspase 3 is regulated by cell cycle regulator p21WAF1 and IAP gene family ILP. Oncogene 17: 931–939. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki A., Hayashida M., Kawano H., Sugimoto K., Nakano T., Shiraki K. 2000. Hepatocyte growth factor promotes cell survival from fas-mediated cell death in hepatocellular carcinoma cells via Akt activation and Fas-death-inducing signaling complex suppression. Hepatology 32: 796–802. [DOI] [PubMed] [Google Scholar]
- 27.Moumen A., Ieraci A., Patané S., Solé C., Comella J. X., Dono R., Maina F. 2007. Met signals hepatocyte survival by preventing Fas-triggered FLIP degradation in a PI3k-Akt-dependent manner. Hepatology 45: 1210–1217. [DOI] [PubMed] [Google Scholar]
- 28.Xiao G. H., Jeffers M., Bellacosa A., Mitsuuchi Y., Vande Woude G. F., Testa J. R. 2001. Anti-apoptotic signaling by hepatocyte growth factor/Met via the phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways. Proc. Natl. Acad. Sci. USA 98: 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osawa Y., Banno Y., Nagaki M., Brenner D. A., Naiki T., Nozawa Y., Nakashima S., Moriwaki H. 2001. TNF-alpha-induced sphingosine 1-phosphate inhibits apoptosis through a phosphatidylinositol 3-kinase/Akt pathway in human hepatocytes. J. Immunol. 167: 173–180. [DOI] [PubMed] [Google Scholar]
- 30.Liu W., Jing Z. T., Wu S. X., He Y., Lin Y. T., Chen W. N., Lin X. J., Lin X. 2018. A novel AKT activator, SC79, prevents acute hepatic failure induced by Fas-mediated apoptosis of hepatocytes. Am. J. Pathol. 188: 1171–1182. [DOI] [PubMed] [Google Scholar]
- 31.Uriarte S. M., Joshi-Barve S., Song Z., Sahoo R., Gobejishvili L., Jala V. R., Haribabu B., McClain C., Barve S. 2005. Akt inhibition upregulates FasL, downregulates c-FLIPs and induces caspase-8-dependent cell death in Jurkat T lymphocytes. Cell Death Differ. 12: 233–242. [DOI] [PubMed] [Google Scholar]
- 32.Okano H., Shiraki K., Inoue H., Kawakita T., Yamanaka T., Deguchi M., Sugimoto K., Sakai T., Ohmori S., Fujikawa K., et al. 2003. Cellular FLICE/caspase-8-inhibitory protein as a principal regulator of cell death and survival in human hepatocellular carcinoma. Lab. Invest. 83: 1033–1043. [DOI] [PubMed] [Google Scholar]
- 33.Li J., Liu Y., Wang Z., Liu K., Wang Y., Liu J., Ding H., Yuan Z. 2011. Subversion of cellular autophagy machinery by hepatitis B virus for viral envelopment. J. Virol. 85: 6319–6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin L., Wang Z., Tao L., Wang Y. 2010. ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy 6: 239–247. [DOI] [PubMed] [Google Scholar]
- 35.Chen C. H., Shaikenov T., Peterson T. R., Aimbetov R., Bissenbaev A. K., Lee S. W., Wu J., Lin H. K., Sarbassov D. D. 2011. ER stress inhibits mTORC2 and Akt signaling through GSK-3β-mediated phosphorylation of rictor. Sci. Signal. 4: ra10. [DOI] [PubMed] [Google Scholar]
- 36.Copp J., Manning G., Hunter T. 2009. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res. 69: 1821–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mina T., Amini Bavil Olyaee S., Tacke F., Maes P., Van Ranst M., Pourkarim M. R. 2015. Genomic diversity of hepatitis B virus infection associated with fulminant hepatitis B development. Hepat. Mon. 15: e29477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrosillo N., Ippolito G., Solforosi L., Varaldo P. E., Clementi M., Manzin A. 2000. Molecular epidemiology of an outbreak of fulminant hepatitis B. J. Clin. Microbiol. 38: 2975–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammam O., Mahmoud O., Zahran M., Aly S., Hosny K., Helmy A., Anas A. 2012. The role of Fas/Fas ligand system in the pathogenesis of liver cirrhosis and hepatocellular carcinoma. Hepat. Mon. 12: e6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galle P. R., Hofmann W. J., Walczak H., Schaller H., Otto G., Stremmel W., Krammer P. H., Runkel L. 1995. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J. Exp. Med. 182: 1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higaki K., Yano H., Kojiro M. 1996. Fas antigen expression and its relationship with apoptosis in human hepatocellular carcinoma and noncancerous tissues. Am. J. Pathol. 149: 429–437. [PMC free article] [PubMed] [Google Scholar]
- 42.Barreiros A. P., Sprinzl M., Rosset S., Höhler T., Otto G., Theobald M., Galle P. R., Strand D., Strand S. 2009. EGF and HGF levels are increased during active HBV infection and enhance survival signaling through extracellular matrix interactions in primary human hepatocytes. Int. J. Cancer 124: 120–129. [DOI] [PubMed] [Google Scholar]
- 43.Hayashi N., Mita E. 1999. Involvement of Fas system-mediated apoptosis in pathogenesis of viral hepatitis. J. Viral Hepat. 6: 357–365. [DOI] [PubMed] [Google Scholar]
- 44.Medema J. P., Scaffidi C., Kischkel F. C., Shevchenko A., Mann M., Krammer P. H., Peter M. E. 1997. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J. 16: 2794–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gajate C., Mollinedo F. 2015. Lipid rafts and raft-mediated supramolecular entities in the regulation of CD95 death receptor apoptotic signaling. Apoptosis 20: 584–606. [DOI] [PubMed] [Google Scholar]
- 46.Muppidi J. R., Siegel R. M. 2004. Ligand-independent redistribution of Fas (CD95) into lipid rafts mediates clonotypic T cell death. Nat. Immunol. 5: 182–189. [DOI] [PubMed] [Google Scholar]
- 47.Bénéteau M., Pizon M., Chaigne-Delalande B., Daburon S., Moreau P., De Giorgi F., Ichas F., Rebillard A., Dimanche-Boitrel M. T., Taupin J. L., et al. 2008. Localization of Fas/CD95 into the lipid rafts on down-modulation of the phosphatidylinositol 3-kinase signaling pathway. Mol. Cancer Res. 6: 604–613. [DOI] [PubMed] [Google Scholar]
- 48.Huovila A. P., Eder A. M., Fuller S. D. 1992. Hepatitis B surface antigen assembles in a post-ER, pre-Golgi compartment. J. Cell Biol. 118: 1305–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu C., Bailly-Maitre B., Reed J. C. 2005. Endoplasmic reticulum stress: cell life and death decisions. J. Clin. Invest. 115: 2656–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malhi H., Kaufman R. J. 2011. Endoplasmic reticulum stress in liver disease. J. Hepatol. 54: 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tabas I., Ron D. 2011. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 13: 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zha J., Harada H., Yang E., Jockel J., Korsmeyer S. J. 1996. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-XL. Cell 87: 619–628. [DOI] [PubMed] [Google Scholar]
- 53.Datta S. R., Dudek H., Tao X., Masters S., Fu H., Gotoh Y., Greenberg M. E. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91: 231–241. [DOI] [PubMed] [Google Scholar]
- 54.Salpini R., Colagrossi L., Bellocchi M. C., Surdo M., Becker C., Alteri C., Aragri M., Ricciardi A., Armenia D., Pollicita M., et al. 2015. Hepatitis B surface antigen genetic elements critical for immune escape correlate with hepatitis B virus reactivation upon immunosuppression. Hepatology 61: 823–833. [DOI] [PubMed] [Google Scholar]
- 55.Bertoletti A., Ferrari C. 2012. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Gut 61: 1754–1764. [DOI] [PubMed] [Google Scholar]
- 56.Bertoletti A., Tan A. T., Gehring A. J. 2009. HBV-specific adaptive immunity. Viruses 1: 91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.