Abstract
The sonic hedgehog (Shh) signaling pathway has been reported to protect cells against hypoxia/reoxygenation (H/R) injury; however, the role of Shh and relevant molecular mechanisms remain unclear. In the present study, the rat cardiomyoblast cell line H9C2 was subjected to hypoxia and serum-starvation for 4 h. Cells were subsequently reoxygenated using 95% O2 and 5% CO2. Reverse transcription-quantitative polymerase chain reaction was performed to quantify the expression of Shh mRNA, while cell apoptosis was assessed using flow cytometry. Caspase-3 activity and p53 expression were measured by western blotting and an MTT assay was subsequently used to assess cell viability. In addition, reactive oxygen species levels were measured using dichlorofluorescein and H/R-induced changes in the activation of superoxide dismutase, catalase, phosphorylated-endothelial nitric oxide synthase, phosphorylated-protein kinase B (Akt) and mammalian target of rapamycin activation were assessed using western blotting. H/R treatment decreased the cell viability of H9C2 cells, but activated endogenous Shh signaling. The activation of Shh signaling protected H9C2 myocardial cells from H/R-induced apoptosis and restored cell viability. In the present study, Shh signaling was demonstrated to serve a protective role against H/R by activating the phosphoinositol 3-kinase (PI3K)/Akt pathway and promoting the expression of anti-oxidant enzymes to ameliorate oxidative stress. In summary, Shh signaling attenuated H/R-induced apoptosis through via the PI3K/Akt pathway.
Keywords: sonic hedgehog signaling pathway, hypoxia/reoxygenation, H9C2 myocardial cells, apoptosis
Introduction
Cardiovascular disease associated with obesity, including coronary heart disease, is caused by dysfunctions of the heart and blood vessels (1,2). Dysregulated myocardial structures and functions are associated with myocardial hypertrophy and cardiovascular disease (3,4). Hypoxia and reoxygenation-induced injuries, such as coronary syndrome, are commonly observed in the clinic (5). Early studies have demonstrated the effects of hypoxia and reoxygenation induced oxidative stress and tissue damage in heart disease (5,6). Additionally, cardiac reoxygenation injury promotes apoptosis, alters enzyme activity, induces mitochondrial dysfunction and is often accompanied by myocardial injury (1,7). Cardiomyocyte apoptosis is associated with caspase-3 and −9 activity (8) and is accompanied by the release of mitochondrial cytochrome c (Cyto c) (9,10). The activity of a number of mitochondrial enzymes, including cytochrome oxidase, catalase and manganese superoxide dismutase (SOD2), is decreased under hypoxic conditions (11). Impaired cytochrome oxidase and anti-oxidant enzyme activity results in the overproduction of reactive oxygen species (ROS) (12). At present, oxidative stress is considered to be a key intermediary step of the hypoxia/reoxygenation (H/R)-induced apoptosis process.
H/R-induced vascular remodeling involves a number of signaling pathways, including the phosphoinositol 3-kinase (PI3K)/protein kinase B (Akt) and mitogen-activated protein kinase (MAPK) pathways (13–16). MAPK pathways are activated by oxidative stress and have a number of important downstream molecules, including extracellular signal-regulated kinase (ERK) and PI3K (17,18). The PI3K/Akt pathway regulates multiple biological processes and mediates apoptosis to regulate cellular metabolism and cell growth (19,20). Furthermore, Akt and its downstream effector mammalian target of rapamycin (mTOR) have been reported to enhance cardiac protection under oxidative stress (21).
Signaling factors in the Hedgehog family include sonic hedgehog (Shh), Indian hedgehog, desert hedgehog and three glioma-associated (GLI) proteins; GLI1, GLI2 and GLI3 (22,23). The Shh and PI3K/Akt pathways have been reported to regulate cell migration, proliferation and apoptosis in a number of cell lines (24). It has previously been reported that the Shh signaling pathway is activated in ischemia to regulate a number of biological processes, exerting anti-apoptosis and anti-oxidative stress effects (25) while also promoting the proliferation of neural progenitors and muscle regeneration under hypoxic conditions (26,27). However, the precise molecular mechanism of the Shh signaling pathway in H/R-induced apoptosis remains unclear. The aim of the present study was to clarify the effects of Shh signaling on H/R-induced apoptosis and investigate the potential downstream targets of Shh. An H9C2 myocardial cell model was used for in vitro investigation.
Materials and methods
Cell culture
The rat cardiomyoblast H9C2 cell line was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) and maintained in DMEM (Sigma-Aldrich; Merck KGaA) supplemented with 10% fetal bovine serum (FBS; Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C in a humidified atmosphere containing 5% CO2. Cells were grown to 80–90% confluence and then exposed to hypoxic conditions as follows: Incubation in an atmosphere containing 0.1% O2 and 5% CO2 in 1% FBS serum-starvation medium for 4 h. After hypoxia, the cells were reoxygenated in an atmosphere containing 95% O2 and 5% CO2. In the present study, cells were treated with H/R only (group I), H/R + 20 mM purmorphamine (group II; Sigma-Aldrich; Merck KGaA), H/R + 10 µΜ cyclopamine (group III; Sigma-Aldrich; Merck KGaA), or H/R + 20 mM purmorphamine + 5 µM AKT inhibitor AZD 5363 (group IV; Cayman Chemical Company, Ann Arbor, MI, USA). H9C2 cells were pre-treated with 20 mM purmorphamine or 10 µΜ cyclopamine or 20 mM purmorphamine + 5 µM AKT inhibitor AZD 536 and then exposed to H/R for 4 h at 37°C.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's protocol. Single-strand cDNA was synthesized from total RNAs using the Reverse Transcription System (Promega Corp., Madison, WI, USA) at 70°C for 10 min. cDNA was amplified by qPCR with SYBR Green using a StepOne Plus real-time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). Thermocycling conditions were as follows: 10 min polymerase activation at 94°C followed by 40 cycles at 95°C for 15 sec and 60°C for 60 sec. The following primers were used: Shh, forward 5′-TTCTGTGAAAGCAGAGAACTCC-3′ and reverse 5′-GGGACGTAAGTCCTTCACCA-3′; Shh, forward 5′-AGTGGACATCACCACGTCTG-3′ and reverse 5′-CACCGAGTTCTCTGCTTTCA-3′; GAPDH: Forward 5′-TGTCCGTCGTGGATCTGAC-3′ and reverse 5′-CCTGCTTCACCACCTTCTTG-3′. Products were separated on 2% agarose gels and results were normalized against GAPDH and quantified using SynGene software (1.6.1; Syngene Europe, Cambridge, UK) using the 2−ΔΔCq method. Experiments were performed in triplicate.
Western blotting
H9C2 myocardial cells were lysed in lysis buffer (50 mM Tris-base, 0.5 M NaCl, 1 mM EDTA, 1% NP40, 1% Glycerol, 1 mM β-mercaptoethanol, proteinase k inhibitor) from each experiment (30 mg per lane) and separated by 10% SDS-PAGE. Proteins were then transferred to a nitrocellulose membrane. Membranes were then incubated for 1 h at room temperature with 3% non-fat dried milk in PBS followed by incubation with the following primary antibodies: Anti-Shh (sc-373779), anti-p-mTOR (sc-101738), anti-SOD (sc-17767), anti-catalase (sc-50508; all 1:5,000; all Santa Cruz Biotechnology, Inc., Dallas, TX, USA), anti-p53 (ab17990), anti-eNOS (ab5589), anti-Gli-1 (ab49314; all 1:5,000; all Abcam, Cambridge, UK), anti-total Akt (9272S; 1:5,000; Cell Signaling Technology, Inc., Danvers, MA, USA), and anti-GAPDH (ab37168; 1:10,000; Abcam) at 4°C overnight. Membranes were washed and subsequently incubated with the horseradish peroxidase-conjugated secondary antibodies [goat anti-rabbit IgG (sc-2004), 1:5,000; goat anti-mouse IgG (sc-2005), 1:5,000; Santa Cruz Biotechnology Inc.] for 1 h at room temperature. The membranes were developed using an enhanced chemiluminescence system (Thermo Fisher Scientific, Inc.). The band intensities were normalized to GAPDH and quantified using SynGene software (1.6.1; Syngene Europe). Experiments were performed in triplicate.
Cell viability assay
In the present study, H9C2 myocardial cells that had not been exposed to hypoxia served as the negative control group (group 0). Hypoxia-treated cells were pre-incubated in an atmosphere containing 5% CO2 in 1% FBS serum-starvation medium for 12 h at 37°C. Following treatment, H9C2 myocardial cells were washed with PBS and incubated in fresh DMEM medium containing 1 g/l MTT (BioVision, Inc., Milpitas, CA, USA) for 4 h at 37°C and MTT crystals were dissolved in dimethyl sulfoxide. MTT was removed and the absorption was measured at 490 nm using an ELISA reader.
Apoptosis assay
Cells were harvested and centrifuged at 5,000 × g for 5 min at 4°C, following which the supernatant was aspirated. Normal or apoptotic cells were distinguished using the staining buffer (Sigma-Aldrich; Merck KGaA), which was then mixed with 2 µl Annexin-V and PI (Alexa Fluor® 488 Annexin V/Dead Cell Apoptosis kit; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Cells were analyzed using a flow cytometer and ~20,000 counts were acquired from each sample (BD FACSuite™ software; version, 1.0; Becton-Dickinson; BD Biosciences, Franklin Lakes, NJ, USA).
Caspase-3 activity assay
Caspase-3 activity was analyzed using a caspase-3 assay kit (Caspase 3 Assay kit; Sigma-Aldrich; Merck KGaA). H9C2 myocardial cells were seeded in 96-well plates at a density of 5×104 cells/well. Cells were trypsinized, washed with PBS and centrifuged (1,000 × g; 5 min; 4°C). The caspase-3 assay buffer was added, cells were centrifuged (1,000 × g; 5 min; 4°C) and the supernatant was transferred to another tube. The cell lysates were mixed with the caspase-3 assay buffer in each well and then incubated for 30 min in the dark at 37°C. The relative fluorescence of each well was detected using a fluorescence plate reader at 450 nm (A450) within 30 min.
Monitoring ROS generation
Dichlorofluorescein dye (non-fluorescent CM-H2DCFDA) is able to diffuse through the cell membrane and the fluorescence intensity is indicative of intracellular ROS contents. ROS levels in H9C2 myocardial cells were measured using flow cytometry following incubation under H/R conditions, with or without Shh activator, Shh inhibitor or Akt inhibitor. Cells were trypsinized, washed and re-suspended in Hanks' Balanced Salt Solution for 48 h. Cells were subsequently incubated with 5 µM CM-H2DCFDA for 30 min at 37°C in a humidified atmosphere containing 5% CO2. DCFDA florescence was measured using a BD FACSCanto Flow cytometer at 520 nm. At least 10,000 events were acquired.
Statistical analysis
Data are presented as the mean ± standard deviation. Statistical significance was assessed using unpaired two-tailed Student's t-test for comparisons between two groups or using one-way analysis of variance followed by Dunnett's multiple comparison for more than three groups using. Analyses were performed using SPSS 19.0 statistical software (IBM Corp., Armonk, NY, USA) and P<0.05 was considered to indicate a statistically significant difference.
Results
H/R induced Shh expression in the H9C2 myocardial cell model
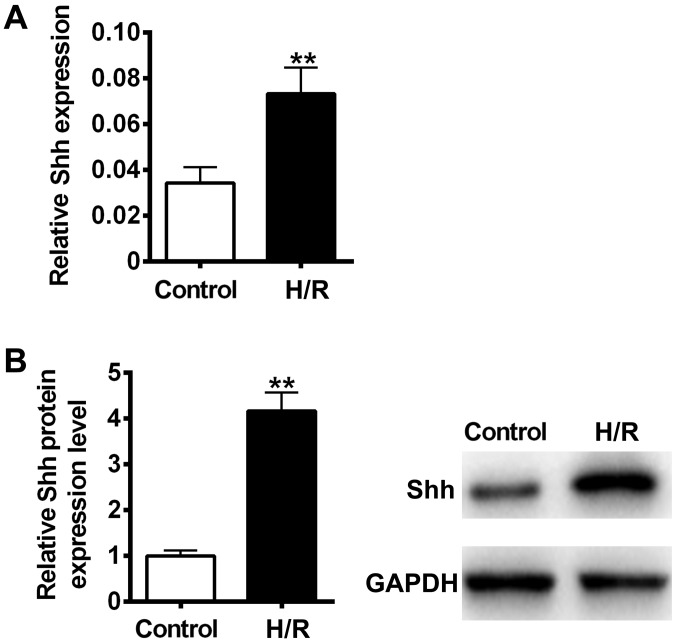
It has previously been reported that cellular hypoxia and reoxygenation are significant elements of ischemia-reperfusion injury (28). To determine whether H/R could activate the Shh signaling pathway in H9C2 myocardial cells, Shh mRNA and protein expression was measured using RT-qPCR and immunoblotting. The results revealed that Shh mRNA and protein levels were significantly increased following H/R treatment compared with the control (P<0.01; Fig. 1). These results suggest that the Shh signaling pathway may participate in H/R-induced cellular injury in H9C2 myocardial cells.
Figure 1.
Effects of H/R on Shh signaling. H9C2 myocardial cells were exposed to hypoxic conditions (atmosphere containing 0.1% O2 and 5% CO2) in 1% fetal bovine serum serum-starvation medium for 4 h. Cells were reoxygenated in an atmosphere containing 95% O2 and 5% CO2. Shh (A) mRNA and (B) protein expression was measured using reverse transcription-quantitative polymerase chain reaction and western blotting, respectively. Data are presented as the mean ± standard deviation. **P<0.01 vs. control. Shh, sonic hedgehog; H/R, hypoxia reoxygenation.
Shh signaling is activated to protect against H/R-induced apoptosis
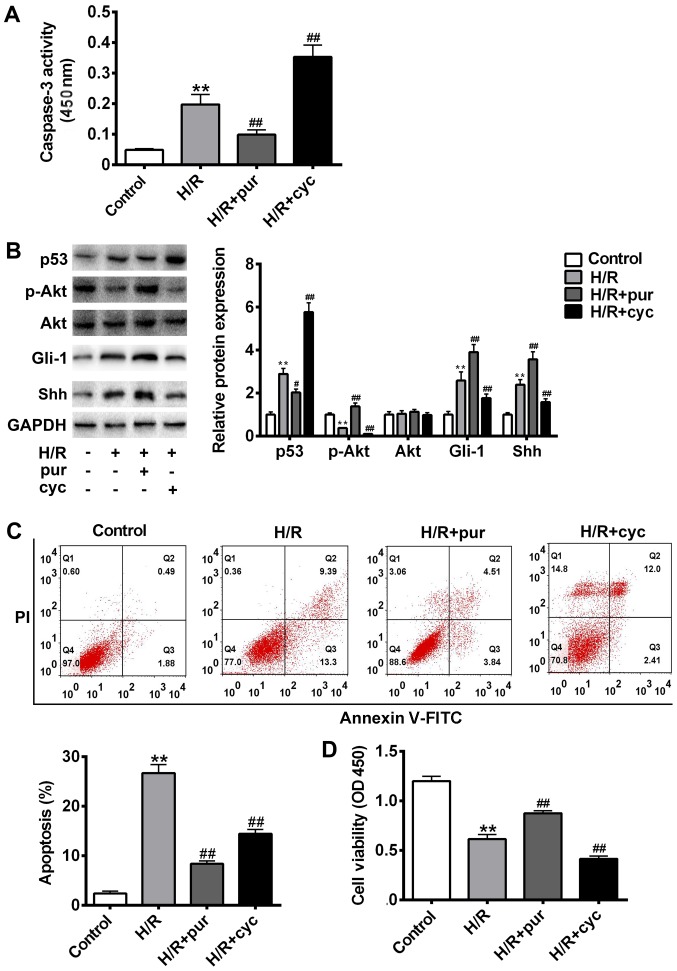
It has previously been reported that the morphological nuclear changes associated with apoptosis are triggered by the activation of caspase proteins (29). In the caspase family, caspase-3 serves as the executor of apoptosis (30). To determine whether the Shh signaling pathway serves a role in H/R-induced caspase-3 cleavage, H9C2 myocardial cells were exposed to H/R conditions and treated with the Shh activator purmorphamine or Shh inhibitor cyclopamine. The results revealed that combined treatment with H/R and purmorphamine significantly ameliorated H/R-induced caspase-3 cleavage compared with the control (P<0.01; Fig. 2A). However, caspase-3 cleavage significantly increased following treatment with H/R and cyclopamine compared with the H/R group (P<0.01; Fig. 2A).
Figure 2.
Effects of the Shh signaling pathway on H/R-induced apoptosis. Cells were treated with H/R only, H/R + 20 mM pur or HR + 10 µΜ cyc. (A) Cleaved caspase-3 expression was assessed using a kit and the absorbance was measured at 450 nm. (B) p53, Gli-1, Akt, p-Akt, Shh and GAPDH protein expression was measured using western blotting. (C) Apoptosis was assessed using Annexin V-FITC and PI staining with a flow cytometer. (D) Cell viability was assessed using an MTT assay and measured at 450 nm. Data are presented as the mean ± standard deviation. **P<0.01 vs. control group; #P<0.05 and ##P<0.01 vs. H/R group. Shh, sonic hedgehog; H/R, hypoxia reoxygenation; pur, purmorphamine; cyc, cyclopamine; Gli-1, glioma-associated oncogene 1; Akt, protein kinase B; p, phosphorylated; FITC, fluorescein isothiocyanate; PI, propidium iodide; OD, optical density.
To assess the role of Shh signaling in H/R-induced apoptosis, cell apoptosis was measured using flow cytometry with Annexin V-FITC and PI staining (Fig. 2B), while the expression of p53, Gli-1 and p-Akt was measured using western blotting (Fig. 2C). Compared with the untreated control group, cell apoptosis was significantly increased following H/R treatment (P<0.01). The expression of p-Akt was significantly decreased following H/R treatment compared with the control (P<0.01; Fig. 2C); however co-treatment with purmorphamine reversed this effect (P<0.01 Fig. 2C). Immunohistochemistry revealed that the expression of Gli1 was upregulated following combined treatment with H/R and purmorphamine compared with the control (Fig. 2), suggesting that purmorphamine inhibits H/R-induced apoptosis and activates the Akt pathway. However, Gli1 upregulation could be blocked by cyclopamine. Compared with the H/R alone group, cell viability was significantly increased following treatment with purmorphamine (P<0.01; Fig. 2D). Collectively, these results suggest that the Shh activator purmorphamine decreases H/R-induced apoptosis.
Shh signaling is critical for H/R-induced apoptosis via the PI3K/AKT/mTOR pathway
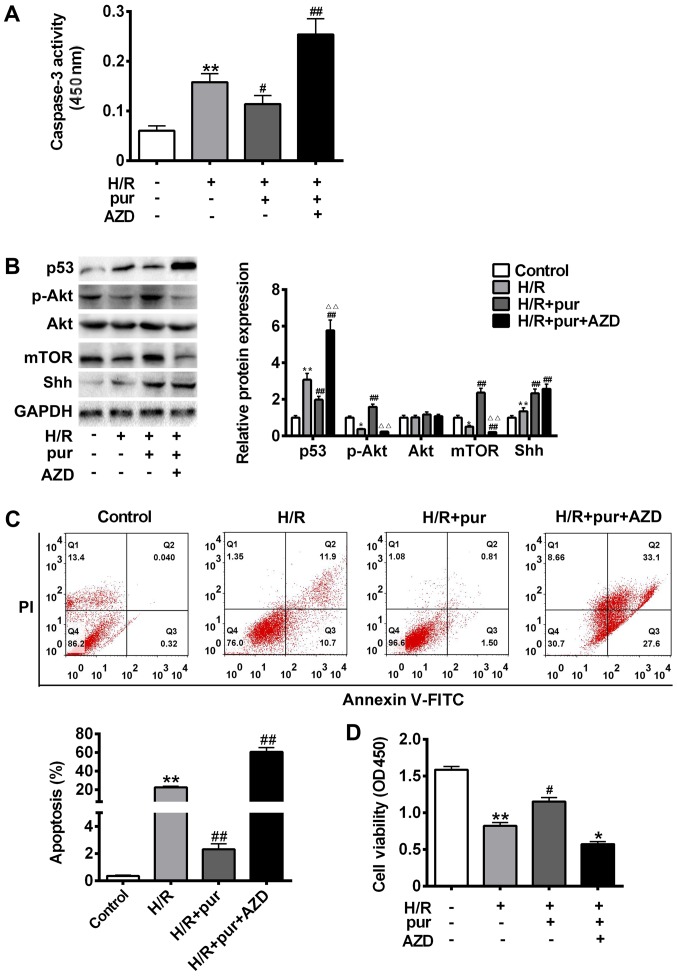
The Akt/mTOR pathway serves a critical protective role against apoptosis, and so it was investigated whether the mechanism of purmorphamine involves this pathway. It H9C2 myocardial cells were pre-treated with H/R and then treated with purmorphamine alone or purmorphamine and Akt inhibitor AZD 5363 (Fig. 3).
Figure 3.
Effects of Shh and Akt signaling on H/R-induced apoptosis. Cells were treated with H/R only, H/R + 20 mM pur for 24 h or H/R + 5 µΜ Akt inhibitor AZD 5363 for 24 h. (A) Cleaved caspase-3 expression was assessed using a kit and the absorbance was measured at 450 nm. (B) p53, mTOR, Akt, p-Akt, Shh and GAPDH protein expression was measured using western blotting. (C) Apoptosis was assessed using Annexin V-FITC and PI staining with a flow cytometer. (D) Cell viability was assessed using an MTT assay and measured at 450 nm. Data are presented as the mean ± standard deviation. **P<0.01 vs. control group; #P<0.05 and ##P<0.01 vs. H/R group. Shh, sonic hedgehog; H/R, hypoxia reoxygenation; pur, purmorphamine; Akt, protein kinase B; AZD, AZD 5363; Gli-1, glioma-associated oncogene 1; p, phosphorylated; FITC, fluorescein isothiocyanate; PI, propidium iodide; OD, optical density.
Treatment with purmorphamine treatment alone significantly protected H9C2 myocardial cells from H/R-induced apoptosis (P<0.05; Fig. 3C). However, AZD 5363 inhibited the expression of p-Akt and mTOR, leading to a significant increase in caspase-3 cleavage (P<0.05; Fig. 3A) compared with the purmorphamine alone group (Fig. 3B-C). p53 protein expression was also significantly upregulated in H9C2 myocardial cells treated with purmorphamine and AZD 5363 compared with the H/R group (P<0.01; Fig. 3B). Cell viability was significantly decreased compared with the H/R group following co-treatment with purmorphamine and AZD 5363 (P<0.05; Fig. 3D). These results suggest that Shh may serve a protective role against H/R injury by activating the PI3K/Akt pathway.
Shh activation restored oxidative damage induced by H/R
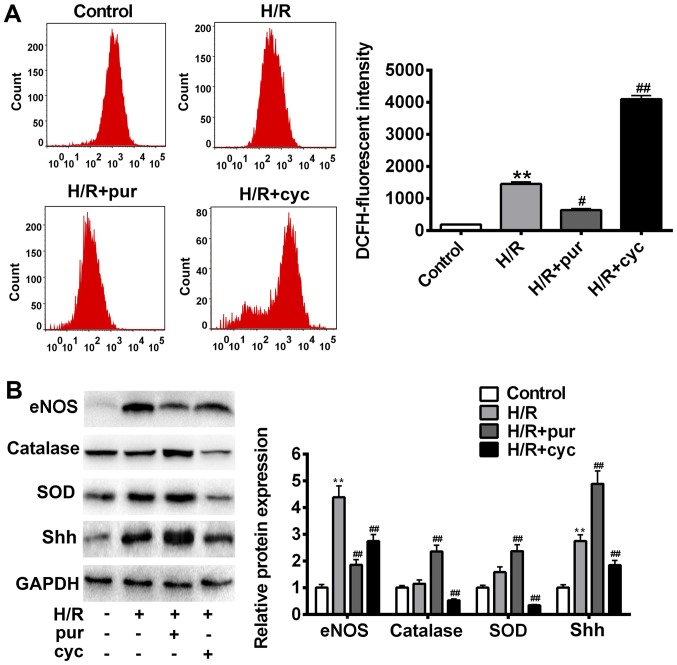
The effects of Shh signaling activation in H/R-induced oxidative stress were investigated. Intracellular ROS production was assessed after H/R treatment using flow cytometry (Fig. 4A). H9C2 myocardial cells co-treated with H/R and purmorphamine showed a significant decrease in fluorescence intensity compared with control cells treated with H/R alone (P<0.05). In addition, a significant overall increase in ROS production was observed in the cyclopamine group (P<0.01).
Figure 4.
Effects of Shh activation on ROS production following H/R treatment. (A) Intracellular ROS accumulation was determined by measuring the DCF-derived fluorescence following incubation with DCFH-D. (B) eNOS, catalase, SOD, Shh and GAPDH expression was assessed using western blotting. Data are presented as the mean ± standard deviation. *P<0.05 and **P<0.01 vs. control group; #P<0.05 and ##P<0.01 vs. H/R group. Shh, sonic hedgehog; ROS, reactive oxygen species; H/R, hypoxia reoxygenation; p, phosphorylated; eNOS, endothelial nitric oxide synthase; SOD, superoxide dismutase; NO, nitric oxide.
In order to identify candidate antioxidant enzymes for H/R treatment, intracellular SOD, catalase and eNOS levels were measured using western blotting (Fig. 4B). It was demonstrated that H/R caused cellular oxidative stress and increased eNOS expression (Fig. 4B). Purmorphamine significantly reversed H/R-induced cell damage and inhibited eNOS expression (P<0.05; Fig. 4B). Similarly, co-treatment with purmorphamine resulted in a significant increase in intracellular SOD and catalase compared with the H/R group (P<0.01; Fig. 4B). In comparison with the H/R group, eNOS expression significantly increased following cyclopamine treatment (P<0.05; Fig. 4B). These data suggest that the activation of Shh signaling results in ROS scavenging, thereby protecting cells from H/R-induced oxidative stress.
Discussion
It has previously been reported that the Shh signaling pathway acts as a key mediator of cardioprotection in cardiomyocytes (31,32). Hypoxic injury after reoxygenation is a significant cause of cellular injury in the myocardial tissue (33,34). In the present study, it was first determined whether the Shh signaling pathway could be activated by H/R. The results demonstrated that the expression of Shh and downstream factors was increased following H/R treatment, which is consistent with a previous study in which activation of the Shh signaling pathway has been reported to be associated with hypoxic conditions (35). Shh activators and inhibitors were used to enhance or suppressed the expression of Shh. The results indicated that combined treatment with H/R and Shh activator reversed H/R-induced apoptosis, while the opposite was observed with the Shh inhibitor. These results suggest that the Shh signaling pathway may serve an important role in a H9C2 myocardial cell model of H/R-induced cellular injury.
Cellular H/R generally typically results in cell death due to necrosis or apoptosis (36). Shh signaling contributes to cell survival and is able to partially ameliorate stress-induced apoptosis in cells (37). It has been reported that the activation of Shh signaling promotes coronary neovascularization and protects myocardial tissues from ischemia (38,39). However, the role of Shh signaling in H/R induced cellular injury remains unclear.
In the present study, the effects of SHH signaling activation on H/R-induced cell apoptosis were assessed. Apoptosis was measured using a number of assays, including annexin V-binding, caspase-3 activity and p53 expression. Subsequently, the effects of Shh signaling activation via the PI3K/Akt pathway were also investigated. In the present study, the PI3K/Akt pathway was revealed to contribute to cell viability and inhibit cell apoptosis. However, treatment with the Akt inhibitor disrupted the protective effect of Shh signaling in H/R-induced cell injury. In the present study, it was speculated that the PI3K/Akt pathway may be a downstream target of the Shh pathway. Given that Shh signaling and the PI3K/Akt pathway are associated with cell survival, it was postulated that stimulating PI3K/Akt with insulin-like growth factor-I potentiated Gli might be essential for Shh signaling (40,41). PI3K/Akt activation allowed cells to combat oxidative stress, while specific inhibitors of the PI3K/Akt pathway blocked the Shh-mediated protective effects in H/R conditions.
Oxidative stress is a major cause of cellular injury and has been reported in many diseases, including cancer, neurodegeneration and cardiovascular and cerebrovascular diseases (42,43). Apoptosis may be activated by increased intracellular ROS production (44,45), which typically occurs after H/R injury. Oxidative stress contributes to mitochondrial permeability and release of Cyto c (46), while reoxygenation-induced cardiomyocyte apoptosis is associated with the activation of caspases-3 and Cyto c (28,47). Inhibiting ROS production in H/R injury requires the protection of various reperfused tissues using anti-oxidant enzymes, including SOD (48,49). Anti-oxidant systems function as ROS scavengers that limit the damage caused by reoxygenation-induced cellular injury (28,50). Cellular ROS are produced via mitochondrial electron transport complexes under hypoxia pre-exposure conditions (51). Interestingly, the number of mitochondrial enzymes was decreased following H/R injury, indicating the downregulation of anti-oxidant defenses in hypoxia, which in turn may result in increased ROS production by reoxygenated mitochondria. In the present study, it was revealed that the Shh activator could significantly ameliorate H/R-induced cell damage and inhibit the expression of eNOS. These data suggest that the activation of Shh signaling protects cardiomyocytes from oxidative stress. Previous studies have demonstrated that extracellular SOD and catalase are able to completely prevent reoxygenation injury (52). In the present study, SOD and catalase were upregulated in response to pre-treatment with the Shh activator. Furthermore, it has been reported that Shh expression stimulates cellular SOD and catalase expression, resulting in cardiac protection against oxidative stress (53,54).
In summary, activation of the Shh signaling pathway significantly increases the expression of cellular anti-oxidant factors and protects H9C2 myocardial cells against H/R-induced oxidative stress. The Shh signaling pathway regulates the PI3K/Akt pathway to attenuate H/R-induced apoptosis and enhance the activity of cellular antioxidant enzymes to combat oxidative stress. The results of the present study provide a novel insight into the protective effects of the Shh signaling pathway and may serve as a basis for the development of effective treatments for cardiovascular disease.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
RZ designed the study. JM collected the data. HL and ZQ analyzed the data and ZQ drafted the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, Yuan J, Moskowitz MA. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosc. 1998;18:3659–3668. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 3.Van Gaal LF, Mertens IL, Christophe E. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 4.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88:389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reiter RJ, Tan DX. Melatonin: A novel protective agent against oxidative injury of the ischemic/reperfused heart. Cardiovasc Res. 2003;58:10–19. doi: 10.1016/S0008-6363(02)00827-1. [DOI] [PubMed] [Google Scholar]
- 6.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 7.Braunwald E, Kloner RA. Myocardial reperfusion: A double-edged sword? J Clin Invest. 1985;76:1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu W, Lee WL, Wu YY, Chen D, Liu TJ, Jang A, Sharma PM, Wang PH. Expression of constitutively active phosphatidylinositol 3-kinase inhibits activation of caspase 3 and apoptosis of cardiac muscle cells. J Biol Chem. 2000;275:40113–40119. doi: 10.1074/jbc.M004108200. [DOI] [PubMed] [Google Scholar]
- 9.Chang J, Xie M, Shah VR, Schneider MD, Entman ML, Wei L, Schwartz RJ. Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc Natl Acad Sci USA. 2006;103:14495–14500. doi: 10.1073/pnas.0601911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narula J, Pandey P, Arbustini E, Haider N, Narula N, Kolodgie FD, Dal Bello B, Semigran MJ, Bielsa-Masdeu A, Dec GW, et al. Apoptosis in heart failure: Release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc Natl Acad Sci USA. 1999;96:8144–8149. doi: 10.1073/pnas.96.14.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci. 2000;25:502–508. doi: 10.1016/S0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- 13.Radhakrishnan Y, Maile LA, Ling Y, Graves LM, Clemmons DR. Insulin-like growth factor-I stimulates Shc-dependent phosphatidylinositol 3-kinase activation via Grb2-associated p85 in vascular smooth muscle cells. J Biol Chem. 2008;283:16320–16331. doi: 10.1074/jbc.M801687200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosner D, Stoneman V, Littlewood T, McCarthy N, Figg N, Wang Y, Tellides G, Bennett M. Interferon-gamma induces Fas trafficking and sensitization to apoptosis in vascular smooth muscle cells via a PI3K- and Akt-dependent mechanism. Am J Pathol. 2006;168:2054–2063. doi: 10.2353/ajpath.2006.050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen KH, Guo X, Ma D, Guo Y, Li Q, Yang D, Li P, Qiu X, Wen S, Xiao RP, Tang J. Dysregulation of HSG triggers vascular proliferative disorders. Nat Cell Biol. 2004;6:872–883. doi: 10.1038/ncb1161. [DOI] [PubMed] [Google Scholar]
- 16.Campbell M, Trimble ER. Modification of PI3K- and MAPK-dependent chemotaxis in aortic vascular smooth muscle cells by protein kinase CbetaII. Circ Res. 2005;96:197–206. doi: 10.1161/01.RES.0000152966.88353.9d. [DOI] [PubMed] [Google Scholar]
- 17.Ueda S, Masutani H, Nakamura H, Tanaka T, Ueno M, Yodoi J. Redox control of cell death. Antioxid Redox Signal. 2002;4:405–414. doi: 10.1089/15230860260196209. [DOI] [PubMed] [Google Scholar]
- 18.Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. Biofactors. 2003;17:287–296. doi: 10.1002/biof.5520170128. [DOI] [PubMed] [Google Scholar]
- 19.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 21.Maiese K, Chong ZZ, Hou J, Shang YC. Oxidative stress: Biomarkers and novel therapeutic pathways. Exp Gerontol. 2010;45:217–234. doi: 10.1016/j.exger.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hooper JE, Scott MP. Communicating with hedgehogs. Nat Rev Mol Cell Bio. 2005;6:306–317. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- 23.Riobo NA, Manning DR. Pathways of signal transduction employed by vertebrate Hedgehogs. Biochem J. 2007;403:369–379. doi: 10.1042/BJ20061723. [DOI] [PubMed] [Google Scholar]
- 24.Sharma N, Nanta R, Sharma J, Gunewardena S, Singh KP, Shankar S, Srivastava RK. PI3K/AKT/mTOR and sonic hedgehog pathways cooperate together to inhibit human pancreatic cancer stem cell characteristics and tumor growth. Oncotarget. 2015;6:32039–32069. doi: 10.18632/oncotarget.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghanizadeh A. Malondialdehyde, Bcl-2, superoxide dismutase and glutathione peroxidase may mediate the association of sonic hedgehog protein and oxidative stress in autism. Neurochem Res. 2012;37:899–901. doi: 10.1007/s11064-011-0667-z. [DOI] [PubMed] [Google Scholar]
- 26.Sims JR, Lee SW, Topalkara K, Qiu J, Xu J, Zhou Z, Moskowitz MA. Sonic hedgehog regulates ischemia/hypoxia-induced neural progenitor proliferation. Stroke. 2009;40:3618–3626. doi: 10.1161/STROKEAHA.109.561951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Surace EM, Balaggan KS, Tessitore A, Mussolino C, Cotugno G, Bonetti C, Vitale A, Ali RR, Auricchio A. Inhibition of ocular neovascularization by hedgehog blockade. Mol Ther. 2006;13:573–579. doi: 10.1016/j.ymthe.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002;282:C227–C241. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- 29.Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi T, Masumoto J, Tada T, Nomiyama T, Hongo K, Nakayama J. Prognostic significance of the immunohistochemical staining of cleaved caspase-3, an activated form of caspase-3, in gliomas. Clin Cancer Res. 2007;13:3868–3874. doi: 10.1158/1078-0432.CCR-06-2730. [DOI] [PubMed] [Google Scholar]
- 31.Ueda K, Takano H, Niitsuma Y, Hasegawa H, Uchiyama R, Oka T, Miyazaki M, Nakaya H, Komuro I. Sonic hedgehog is a critical mediator of erythropoietin-induced cardiac protection in mice. J Clin Invest. 2010;120:2016–2029. doi: 10.1172/JCI39896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulis L, Fauconnier J, Cazorla O, Thireau J, Soleti R, Vidal B, Ouillé A, Bartholome M, Bideaux P, Roubille F, et al. Activation of Sonic hedgehog signaling in ventricular cardiomyocytes exerts cardioprotection against ischemia reperfusion injuries. Sci Rep. 2015;5:7983. doi: 10.1038/srep07983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schäfer C, Ladilov Y, Inserte J, Schäfer M, Haffner S, Garcia-Dorado D, Piper HM. Role of the reverse mode of the Na+/Ca2+ exchanger in reoxygenation-induced cardiomyocyte injury. Cardiovascu Res. 2001;51:241–250. doi: 10.1016/S0008-6363(01)00282-6. [DOI] [PubMed] [Google Scholar]
- 34.Buja LM. Myocardial ischemia and reperfusion injury. Cardiovascu Pathol. 2005;14:170–175. doi: 10.1016/j.carpath.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saikumar P, Dong Z, Weinberg JM, Venkatachalam M. Mechanisms of cell death in hypoxia/reoxygenation injury. Oncogene. 1998;17:3341–3349. doi: 10.1038/sj.onc.1202579. [DOI] [PubMed] [Google Scholar]
- 37.Mazumdar T, DeVecchio J, Shi T, Jones J, Agyeman A, Houghton JA. Hedgehog signaling drives cellular survival in human colon carcinoma cells. Cancer Res. 2011;71:1092–1102. doi: 10.1158/0008-5472.CAN-10-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kusano KF, Pola R, Murayama T, Curry C, Kawamoto A, Iwakura A, Shintani S, Ii M, Asai J, Tkebuchava T, et al. Sonic hedgehog myocardial gene therapy: Tissue repair through transient reconstitution of embryonic signaling. Nat Med. 2005;11:1197–1204. doi: 10.1038/nm1313. [DOI] [PubMed] [Google Scholar]
- 39.Lavine KJ, White AC, Park C, Smith CS, Choi K, Long F, Hui CC, Ornitz DM. Fibroblast growth factor signals regulate a wave of Hedgehog activation that is essential for coronary vascular development. Genes Dev. 2006;20:1651–1666. doi: 10.1101/gad.1411406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riobó NA, Lu K, Ai X, Haines GM, Emerson CP., Jr Phosphoinositide 3-kinase and Akt are essential for Sonic Hedgehog signaling. Proc Natl Acad Sci USA. 2006;103:4505–4510. doi: 10.1073/pnas.0504337103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koh SH, Kim SH, Kwon H, Park Y, Kim KS, Song CW, Kim J, Kim MH, Yu HJ, Henkel JS, Jung HK. Epigallocatechin gallate protects nerve growth factor differentiated PC12 cells from oxidative-radical-stress-induced apoptosis through its effect on phosphoinositide 3-kinase/Akt and glycogen synthase kinase-3. Brain Res Mol Brain Res. 2003;118:72–81. doi: 10.1016/j.molbrainres.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Mariani E, Polidori M, Cherubini A, Mecocci P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: An overview. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827:65–75. doi: 10.1016/j.jchromb.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 43.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matés JM, Sánchez-Jiménez FM. Role of reactive oxygen species in apoptosis: Implications for cancer therapy. Int J Biochem Cell Biol. 2000;32:157–170. doi: 10.1016/S1357-2725(99)00088-6. [DOI] [PubMed] [Google Scholar]
- 45.Giorgio M, Trinei M, Migliaccio E, Pelicci PG. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol. 2007;8:722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- 46.Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci USA. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar D, Jugdutt B. Apoptosis and oxidants in the heart. J Lab Clin Med. 2003;142:288–297. doi: 10.1016/S0022-2143(03)00148-3. [DOI] [PubMed] [Google Scholar]
- 48.Zhang W, Wang M, Xie HY, Zhou L, Mseng XQ, Shi J, Zheng S. Role of reactive oxygen species in mediating hepatic ischemia-reperfusion injury and its therapeutic applications in liver transplantation. Transplant Proc. 2007;39:1332–1337. doi: 10.1016/j.transproceed.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 49.Dhalla NS, Elmoselhi AB, Hata T, Makino N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovasc Res. 2000;47:446–456. doi: 10.1016/S0008-6363(00)00078-X. [DOI] [PubMed] [Google Scholar]
- 50.Yamada J, Yoshimura S, Yamakawa H, Sawada M, Nakagawa M, Hara S, Kaku Y, Iwama T, Naganawa T, Banno Y, et al. Cell permeable ROS scavengers, Tiron and Tempol, rescue PC12 cell death caused by pyrogallol or hypoxia/reoxygenation. Neurosci Res. 2003;45:1–8. doi: 10.1016/S0168-0102(02)00196-7. [DOI] [PubMed] [Google Scholar]
- 51.Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem. 1998;273:18092–18098. doi: 10.1074/jbc.273.29.18092. [DOI] [PubMed] [Google Scholar]
- 52.Sorescu D, Griendling KK. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail. 2002;8:132–140. doi: 10.1111/j.1527-5299.2002.00717.x. [DOI] [PubMed] [Google Scholar]
- 53.Al-Ayadhi LY. Relationship between Sonic hedgehog protein, brain-derived neurotrophic factor and oxidative stress in autism spectrum disorders. Neurochem Res. 2012;37:394–400. doi: 10.1007/s11064-011-0624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghanizadeh A, Akhondzadeh S, Hormozi M, Makarem A, Abotorabi-Zarchi M, Firoozabadi A. Glutathione-related factors and oxidative stress in autism, a review. Curr Med Chem. 2012;19:4000–4005. doi: 10.2174/092986712802002572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.