Abstract
The p53 tumor suppressor plays a major role in controlling the initiation and development of cancer by regulating cell cycle arrest, apoptosis, senescence, and DNA repair. The MDM2 oncogene is a major negative regulator of p53 that inhibits the activity of p53 and reduces its protein stability. MDM2, p53, and the p53-MDM2 pathway represent well-documented targets for preventing and/or treating cancer. Natural products, especially those from medicinal and food plants, are a rich source for the discovery and development of novel therapeutic and preventive agents against human cancers. Many natural product-derived MDM2 inhibitors have shown potent efficacy against various human cancers. In contrast to synthetic small-molecule MDM2 inhibitors, the majority of which have been designed to inhibit MDM2-p53 binding and activate p53, many natural product inhibitors directly decrease MDM2 expression and/or MDM2 stability, exerting their anticancer activity in both p53-dependent and p53-independent manners. More recently, several natural products have been reported to target mutant p53 in cancer. Therefore, identification of natural products targeting MDM2, mutant p53, and the p53-MDM2 pathway can provide a promising strategy for the development of novel cancer chemopreventive and chemotherapeutic agents. In this review, we focus our discussion on the recent advances in the discovery and development of anticancer natural products that target the p53-MDM2 pathway, emphasizing several emerging issues, such as the efficacy, mechanism of action, and specificity of these natural products.
Keywords: MDM2, Natural products, Oncogene, p53, Tumor suppressor
Introduction
Human cancer is associated with alterations in a number of oncogenes and tumor suppressor genes that occur at various stages, from carcinogenesis to tumor growth, progression and metastasis.1, 2 Proto-oncogenes normally control cell division and growth; molecular alterations, including gain-of-function mutations, amplification, and overexpression, can trigger the activation of oncogenes, leading to uncontrolled cell division, finally causing cancer.3, 4 In contrast, tumor suppressor genes are “good” genes that normally slow cell division and growth. When loss-of-function mutations and the deletion of tumor suppressor genes occur, tumor suppressors are inactivated. Consequently, cell division and growth become out of control, resulting in cancer.5, 6 Therefore, the balance between the oncogenes and tumor suppressor genes critically controls the initiation and progression of cancer. Inhibiting oncogenes and activating tumor suppressor genes are promising approaches for preventing or treating malignancies.
Natural products from edible and medicinal plants have shown potent cancer chemopreventive and chemotherapeutic activity in both preclinical and clinical studies.7, 8 Many natural products mediate their anticancer activities by targeting oncogenes and/or tumor suppressors (Fig. 1). In comparison to the synthetic anticancer compounds designed for a single molecular target, most anticancer natural products have a broader range of targets, including the MDM2 oncogene and p53 tumor suppressor gene.7 The p53 tumor suppressor has a vital role in regulating cancer cell death, cell cycle arrest, apoptosis, senescence, and DNA repair.9 p53 forms a negative feedback loop with MDM2, which directly interacts with p53 and inhibits its function and expression.10 Many natural products have been reported to target the p53-MDM2 pathway, including chalcones, genistein, curcumin, sesquiterpenoids, ginsenosides, etc.; some of these have been summarized in a 2012 review paper published by our group.11 In the present review, we focus on the newly-reported natural products that target the p53-MDM2 pathway, as well as the in vitro and in vivo activity and mechanisms of action of natural product-derived p53/MDM2 inhibitors. We also discuss the emerging issues, especially those related to the efficacy, bioavailability, and toxicity of these natural product inhibitors.
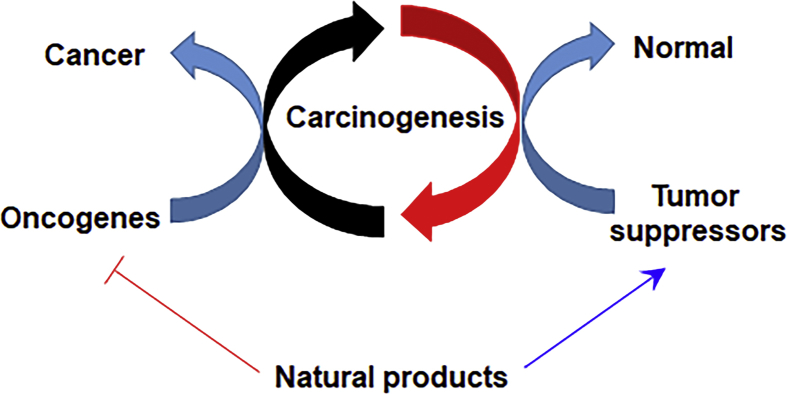
Figure 1.
Natural products that target tumor suppressors and oncogenes in malignant cells. The tumor suppressor genes and oncogenes play critical roles in various stages of cancer development, from carcinogenesis to progression and metastasis. A number of natural products target tumor suppressor genes and oncogenes and have shown potential for cancer prevention and therapy.
The interplay between p53 and MDM2
The dysregulation of the p53-MDM2 pathway, including p53 mutations and deletions and/or MDM2 amplification and overexpression, is the most frequently observed molecular alteration in various human cancers.12, 13, 14, 15 Several recent reviews comprehensively discuss the roles of the p53-MDM2 pathway in the initiation, progression, and metastasis of human cancer.10, 16, 17 In this section, we briefly introduce the p53-MDM2 interaction and the p53-independent functions of MDM2 in human cancer.
The p53 tumor suppressor and MDM2 interact with each other and participate in an autoregulatory feedback loop, which is critical for controlling their expression levels in both normal and cancer cells (Fig. 2). First, p53 binds to the MDM2 P2 promoter and activates MDM2 expression at the transcriptional level.18 Second, MDM2 binds to the transactivation domain of p53 and inhibits p53-mediated transcription of the downstream target genes, including MDM2 itself.19 Third, MDM2 functions as an E3 ubiquitin ligase and promotes p53 ubiquitination.20, 21 The MDM2-mediated p53 ubiquitination facilitates the recognition and interaction of p53 by the proteasome, resulting in enhanced p53 degradation.22 MDM2 is also responsible for p53 sumoylation and nuclear export through its interaction with a SUMO E3 ligase, PIASy.23, 24 In addition, many p53-MDM2 binding modulators are involved in regulating the functions, expression, and protein stability of MDM2 and p53, and have been comprehensively discussed in recent reviews.10, 11, 25, 26
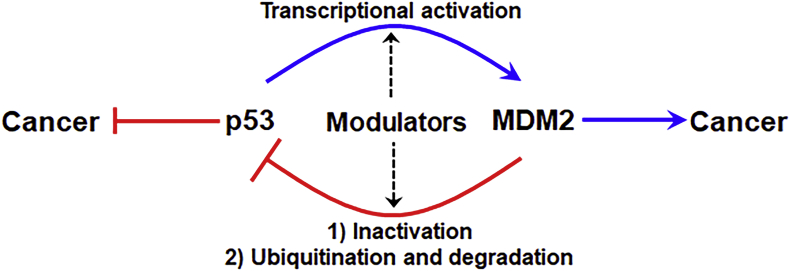
Figure 2.
The p53-MDM2 pathway and natural products inhibitors. The p53 tumor suppressor and MDM2 oncogene form an autoregulatory feedback loop. The p53 protein binds to the MDM2 P2 promoter and increases MDM2 expression. MDM2, in turn, binds to the transactivation domain of p53 and inhibits its ability to activate the transcription of its target genes. MDM2 also acts as an E3 ligase and promotes p53 ubiquitination and degradation. A number of natural products have been reported to exert their anticancer activity by inhibiting MDM2 and/or activating p53.
MDM2 also has various p53-independent functions, which have been discussed in several reviews.27, 28, 29 Much attention has been paid to the p53-independent effects of MDM2 due to the frequent observations of MDM2 amplification and overexpression in human cancers harboring mutant p53.26, 30, 31, 32 Our lab have discovered that MDM2 plays an important role in regulating the expression of p21, Bax, pRb, ppRb, and E2F1 in p53 null PC3 cells.33 Further studies have indicated that MDM2 binds to p21 and induces its degradation by causing a conformation change.34, 35, 36 MDM2 inhibits the ubiquitination of E2F1 and enhances its protein stability and activity.37, 38 MDM2 also destabilizes the Rb, FOXO3a, FOXO4, and E-cadherin proteins, which are important for the p53-independent activity of MDM2 in regulating cancer development and metastasis.39, 40, 41, 42, 43 Therefore, direct inhibition of MDM2 may have preventive and therapeutic potential because it can inhibit both the p53-dependent and p53-independent functions of MDM2.
Natural products targeting the p53-MDM2 pathway
Natural products that target the p53-MDM2 pathway typically fall into three categories: 1) natural products that directly inhibit MDM2 expression and/or protein stability, 2) natural products that inhibit p53-MDM2 binding and activate wild-type p53, and 3) natural products that inhibit MDM2's E3 ligase activity and stabilize p53. Numerous natural product MDM2 inhibitors (Table 1) have previously been discussed in our 2012 review paper.11 There are many newly-discovered natural product MDM2 inhibitors (Fig. 3), which mainly fall into categories 1 and 2, that have shown potent cancer preventive and therapeutic activities in vitro and in vivo. In this section, we focus our discussion on these new natural product MDM2 inhibitors, as well as their efficacy and mechanisms of action (Table 1).
Table 1.
Natural products as preventive and therapeutic agents that target the p53-MDM2 pathway.
| Natural product | Cancer type | In vitro activity | In vivo efficacy | Mechanism(s) of action | Reference(s) |
|---|---|---|---|---|---|
| 1. Natural products that inhibit MDM2 expression and/or decrease its protein stability | |||||
| Flavonoids and isoflavonoids | |||||
| Genistein | Prostate, colon, and breast cancer | Inhibits cell proliferation, arrests cells at G2/M phase, and induces cell apoptosis, regardless of p53 status | Inhibits tumor growth in PC3 xenograft model and sensitizes tumors to gemcitabine | Inhibits NFAT1-mediated MDM2 transcription and promotes MDM2 autoubiquitination and degradation | 44 |
| Apigenin | Ovarian cancer | Inhibits tube formation | Not reported | Inhibits MDM2 phosphorylation and decreases MDM2 protein level | 45 |
| Oroxylin A | Liver, cervical, breast, ovarian, and colon cancer, leukemia | Inhibits the growth of cancer cells (at 10–200 μM) and induces cell apoptosis | Not reported | Decreases MDM2 protein expression level | 46 |
| Flavopiridol (1) | Glioma | Inhibits the growth of glioma cells (200–500 nM), arrests cells at G2/M phase, and induces cell apoptosis | Not reported | Inhibits MDM2 expression at mRNA level | 47 |
| Lung, prostate, and colon cancer, leukemia | Inhibits the growth of glioma cells (at 50–500 nM), arrests cells at G2/M phase, and induces cell apoptosis | Not reported | Decreases MDM2 protein expression level | 48 | |
| Ginsenosides and saponins | |||||
| 25-OCH3-PPD | Prostate, pancreatic and lung cancer | Inhibits cell growth (IC50 = 4.9–19.1 μM) and proliferation, induces cell cycle arrest at G1 phase and apoptosis, regardless of p53 | Inhibits tumor growth in PC3, Panc-1 and A549 xenograft models and enhances the antitumor effects of taxotere, gemcitabine and radiation. | Decreases MDM2 protein level | 49, 50, 51 |
| Breast cancer | Inhibits cell migration | Inhibits tumor growth in MCF7 and MDA-MB-469 xenograft models and inhibits lung metastasis in MDA-MB-231 metastatic model | Inhibits MDM2 transcription and promotes MDM2 ubiquitination and degradation | 53 | |
| 25-OH-PPD | Prostate and pancreatic cancer | Inhibits cell growth (IC50 = 21–60 μM) and proliferation, induces cell cycle arrest at G1 phase and apoptosis, regardless of p53 | Inhibits tumor growth in PC3 and Panc-1 xenograft models and enhances the antitumor effects of taxotere, gemcitabine and radiation. | Decreases MDM2 protein level | 50, 52 |
| 20(S)-Ginsenoside Rg3 (2) | Gallbladder cancer | Inhibits cell growth (IC50 = ∼100 μM) and colony formation, and induces cell cycle arrest at G1 phase, apoptosis, and senescence | Not reported | Decreases MDM2 protein level | 54 |
| Nandrolone (3) | Not reported | Not reported | Not reported | Decreases MDM2 protein level | 55 |
| Platycodin D (4) | Breast cancer | Inhibits MDA-MB-231 cell growth (IC50 = 7.8 μM) and proliferation and induces cell cycle arrest at G0/G1 phase | Inhibits tumor growth in MDA-MB-231 xenograft model | Decreases the protein levels of MDM2, MDMX, and mutant p53 | 56 |
| Alkaloids | |||||
| Berberine | Acute lymphoblastic leukemia | Induces cell death and apoptosis | Not reported | Increases MDM2 self-ubiquitination by disrupting MDM2–DAXX–HAUSP interactions | 57 |
| Leukemia | Induces cell death and apoptosis | Not reported | Decreases MDM2 protein level | 58 | |
| FBA-TPQ | Breast, prostate, ovarian, and pancreatic cancer | Inhibits cell growth (IC50 = 0.1–1.8 μM) and proliferation, induces cell cycle arrest and apoptosis, regardless of p53 | Inhibits tumor growth in MCF7, OVCAR-3, and Panc-1 xenograft models. | Decreases MDM2 protein level | 88, 89, 90, 91 |
| PEA-TPQ | Breast cancer | Inhibits cell growth (IC50 = 0.1–2.5 μM) and proliferation, induces cell cycle arrest and apoptosis, regardless of p53 | Not reported | Decreases MDM2 protein level | 88 |
| MPA-TPQ | Breast cancer | Inhibits cell growth (IC50 = 0.6–4.9 μM) and proliferation, induces cell cycle arrest and apoptosis, regardless of p53 | Not reported | Decreases MDM2 protein level | 88 |
| DPA-TPQ | Breast cancer | Inhibits cell growth (IC50 = 0.3–24.4 μM) and proliferation, induces cell cycle arrest and apoptosis, regardless of p53 | Not reported | Decreases MDM2 protein level | 88 |
| BA-TPQ | Breast cancer | Inhibits cell growth (IC50 = 0.1–0.4 μM) and induces cell cycle arrest and apoptosis, regardless of p53 | Inhibits tumor growth in MCF7 and MDA-MB-468 xenograft models | Decreases MDM2 protein level | 92 |
| TCBA-TPQ | Lung cancer | Inhibits cell growth (IC50 = 0.39–1.41 μM) and induces cell cycle arrest and apoptosis, regardless of p53 | Not reported | Decreases MDM2 protein level | 93 |
| Matrine (5) | Liver cancer | Induces cell apoptosis, independent of p53 | Not reported | Decreases MDM2 mRNA synthesis | 60 |
| Melatonin (6) | Breast cancer | Not reported | Not reported | Inhibits MDM2 transcription, decreases MDM2p (Ser166) level, and enhances p53 acetylation | 61 |
| Xanthones, naphthoquinones, and polyphenols | |||||
| Gambogic acid | Breast and non-small cell lung cancer | Inhibits the growth of MCF7 (IC50 = 3.5 μM) and H1299 (IC50 = 3.5 μM) cells, arrests cells at G2/M phase, and induces cell apoptosis, regardless of p53 status | Inhibits tumor growth in H1299 xenograft model | Inhibits MDM2 transcription and promotes MDM2 ubiquitination and degradation | 62 |
| Plumbagin (7) | Osteosarcoma | Inhibits the growth of U2OS (IC50 = 2.5 μM) cells, arrests cells at S phase, and induces cell apoptosis | Not reported | Decreases MDM2 protein expression level | 63 |
| Gossypol (8) | Breast cancer | Induces cell death and apoptosis in MCF7 and MDA-MB-468 cells | Suppresses the tumor growth in MCF7 and MDA-MB-468 xenograft models | Inhibits the binding of MDM2 to VEGF mRNA and induces MDM2 self-ubiquitination and protein degradation | 64 |
| Terpenoids | |||||
| Triptolide | Acute lymphoblastic leukemia | Inhibits cell growth (IC50 = 47–73 nM) and induces cell apoptosis | Not reported | Inhibits MDM2 at the transcriptional level by suppressing its mRNA synthesis | 65 |
| Gastric cancer | Induces cell apoptosis | Not reported | Decreases MDM2 protein level | 66 | |
| Parthenolide | Colon cancer | Inhibits the growth of HCT116 (IC50 = 6 μM) and HCT116 p53−/− (IC50 = 10 μM) cells and induces cell apoptosis | Not reported | Promotes MDM2 ubiquitination and degradation | 67 |
| Japonicone A (9) | Breast cancer | Inhibits cell growth (IC50 = 0.5–2 μM), proliferation, and colony formation and induces cell cycle arrest at G2/M phase and apoptosis, regardless of p53 | Inhibits tumor growth in MCF7 and MDA-MB-231 xenograft models | Inhibits NFAT1-mediated MDM2 transcription and promotes MDM2 ubiquitination and degradation | 68, 69 |
| Inulanolide A (10) | Breast cancer | Inhibits cell growth (IC50 = 0.9–4.1 μM), proliferation, and colony formation, induces cell cycle arrest at G2/M phase and apoptosis, and prevents cell migration and invasion, regardless of p53 | Inhibits tumor growth in MDA-MB-231 orthotopic model | Inhibits NFAT1-mediated MDM2 transcription and promotes MDM2 ubiquitination and degradation | 70 |
| Prostate cancer | Inhibits cell growth (IC50 = 1.3–4 μM), proliferation, and colony formation and prevents cell migration and invasion, regardless of p53 | Not reported | Inhibits NFAT1-mediated MDM2 transcription, disrupts MDM2-MDMX binding, and promotes ubiquitination and degradation of both MDM2 and MDMX | 71 | |
| Lineariifolianoid A (11) | Breast cancer | Inhibits cell growth (IC50 = 4.4–9.1 μM), proliferation, and colony formation, induces cell cycle arrest at G2/M phase and apoptosis, and prevents cell migration and invasion, regardless of p53 | Not reported | Inhibits NFAT1-mediated MDM2 transcription and promotes MDM2 ubiquitination and degradation | 72 |
| Curcumin and derivatives | |||||
| Curcumin | Prostate, lung, and breast cancer | Inhibits cell proliferation and colony formation and induces cell apoptosis, regardless of p53 status | Inhibits tumor growth in PC3 xenograft model and enhances the antitumor effects of gemcitabine and irradiation. | Inhibits MDM2 transcription through the PI3K/mTOR/ETS2 pathway | 73 |
| Curcumin derivative 1 | Neuroblastoma | Inhibits SH-SY5Y cell growth (IC50 = 8 μM), arrests cells at S phase, and induces cell apoptosis | Not reported | Decreases the MDM2 protein level | 74 |
| Hispolon (12) | Liver and breast cancer | Induces autophagy | Not reported | Enhances the binding of MDM2 with HSP90, HSP70, HSC70, and LAMP2A and decreases the MDM2 protein level | 75 |
| Chalcone and derivatives | |||||
| Chalcone N9 (13) | Glioma | Inhibits U87-MG cell growth (IC50 = 0.72 μg/mL) and colony formation, arrests cells at G1 phase, and induces cell apoptosis | Inhibits tumor growth in U87-MG xenograft model. | Decreases MDM2 protein level | 76 |
| 2. Natural products that inhibit MDM2-p53 binding | |||||
| Chalcone and derivatives | |||||
| Chalcone derivative A | Not reported | Not reported | Not reported | Inhibits MDM2-p53 binding (Ki = 206 μM) | 77 |
| Chalcone derivative B | Not reported | Not reported | Not reported | Inhibits MDM2-p53 binding (Ki = 49 μM) | 77 |
| Chalcone derivative B-1 | Not reported | Not reported | Not reported | Inhibits MDM2-p53 binding (Ki = 117 μM) | 77 |
| Chalcone derivative C | Not reported | Not reported | Not reported | Inhibits MDM2-p53 binding (Ki = 250 μM) | 77 |
| Hexylitaconic acid | |||||
| Hexylitaconic acid | Not reported | Not reported | Not reported | Inhibits MDM2-p53 binding (Ki = 50 μg/mL) | 78 |
| Natural peptide chlorofusin and derivatives | |||||
| Chlorofusin | Liver cancer | No cytotoxicity against HepG2 cells at 4 μM | Not reported | Inhibits MDM2-p53 binding (Ki = 4.6 μM) | 79 |
| Chlorofusin derivative 17 (14) | Osteosarcoma | Inhibits SJSA-1 cell growth (IC50 = 33.1 μM) | Not reported | Inhibits MDM2-p53 binding (Ki = 3.1 μM) | 81 |
| Chlorofusin derivative 18 (15) | Osteosarcoma | Inhibits the growth of SJSA-1 (IC50 = 31.2 μM) and A375 (IC50 = 49.3 μM) cells | Not reported | Inhibits MDM2-p53 binding (Ki = 7.0 μM) | 81 |
| Hoiamide D | Non-small cell lung cancer | Inhibits H460 cell growth (IC50 = 40 μM) | Not reported | Inhibits MDM2-p53 binding (Ki = 4.5 μM) | 80 |
| Flavonoids | |||||
| Tricetin (16) | Breast cancer | Inhibits MCF7 cell growth (IC50 = 32.2 μM) and colony formation, and induces cell cycle arrest at G2/M phase and apoptosis. | Not reported | Inhibits MDM2-p53 binding and induces p53 phosphorylation at Ser15 and Ser392 | 82 |
| Alkaloids | |||||
| Indole-3-carbinol (17) | Breast | Induces MCF10A cell cycle arrest at G1 phase | Not reported | Inhibits MDM2-p53 binding and induces p53 phosphorylation at Ser15 | 83 |
| Fluspirilene (18) | Colon cancer | Inhibits HCT116 cell growth at 10 μM | Not reported | Inhibits MDM2-p53 binding | 84 |
| Lithocholic acid, Isokotomolide A, and Leptomycin B | |||||
| Lithocholic acid (19) | Colon cancer | Induces HCT116 cell apoptosis at 300 μM | Not reported | Dually inhibits MDM2-p53 (Ki = 66.0 μM) and MDMX-p53 (Ki = 15.4 μM) binding | 85 |
| Isokotomolide A (20) | Lung cancer | Inhibits A549 cell growth (IC50 = 4.4 μM) and colony formation, and induces cell cycle arrest at G0/G1 phase and apoptosis | Not reported | Inhibits MDM2-p53 binding | 86 |
| Leptomycin B (21) | Osteosarcoma and lung cancer | Not reported | Not reported | Protects p53 from MDM2-mediated degradation | 87 |
| Marine compounds | |||||
| Sempervirine | Osteosarcoma | Induces U2OS cell apoptosis | Not reported | Inhibits MDM2 E3 ligase activity (IC50 = 8 μg/mL) | 94 |
| Isolissoclinotoxin B | Not reported | Not reported | Not reported | Inhibits MDM2 E3 ligase activity (IC50 = 58.6 μM) | 95 |
| Varacin | Not reported | Not reported | Not reported | Inhibits MDM2 E3 ligase activity (IC50 > 295 μM) | 95 |
| N,N-dimethyl-5-methylvaracin | Not reported | Not reported | Not reported | Inhibits MDM2 E3 ligase activity (IC50 = 120.8 μM) | 95 |
| Diplamine B | Not reported | Not reported | Not reported | Inhibits MDM2 E3 ligase activity (IC50 = 101.3 μM) | 95 |
| Lissoclinidine B | Osteosarcoma | Induces U2OS cell death | Not reported | Inhibits MDM2 E3 ligase activity (IC50 = 98.1 μM) | 95 |
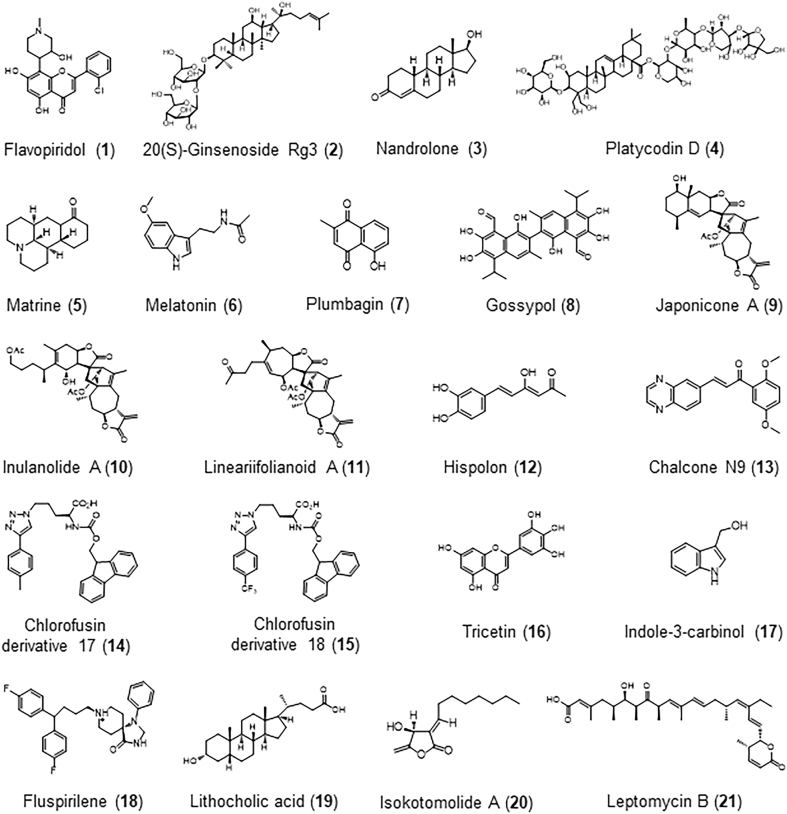
Figure 3.
The structures of newly-discovered natural product MDM2 inhibitors.
Natural products that inhibit MDM2 expression and/or decrease its protein stability
Nature-derived flavonoids and isoflavonoids, including genistein,44 apigenin,45 and oroxylin A,46 have shown excellent anticancer activity in vitro and in vivo, and these effects are at least partly mediated by inhibiting MDM2 expression, as discussed previously.11 Recent studies have discovered a new flavonoid MDM2 inhibitor, flavopiridol (1) (Fig. 3 and Table 1), which has displayed broad-spectrum cytotoxicity against glioma, leukemia, lung, prostate, and colon cancer cell lines in vitro (IC50 values in the range of 50–500 nM).47, 48 Flavopiridol arrests cells at the G2/M phase and induces cell apoptosis, independent of p53. Mechanistically, flavopiridol decreases the MDM2 mRNA level without affecting the half-life of the MDM2 protein, regardless of the p53 status of the cells.44 However, the anticancer activity of flavopiridol and its inhibitory effects on MDM2 have not been examined in any in vivo models yet, which is critical for the further development of this natural product.
We have identified a novel class of ginsenosides, including 25-OCH3-PPD and 25-OH-PPD, which inhibit cancer cell growth in vitro and in vivo by decreasing the MDM2 protein levels.49, 50, 51, 52 Our recent studies have further demonstrated that 25-OCH3-PPD prevents breast cancer cell migration in vitro and inhibits tumor metastasis in vivo by inhibiting MDM2 transcription and promoting MDM2 ubiquitination and degradation.53 Another ginsenoside MDM2 inhibitor, 20(S)-Ginsenoside Rg3 (2) (Fig. 3 and Table 1), has been shown to inhibit gallbladder cancer cell growth and colony formation, arrest the cell cycle at the G1 phase, and promote senescence and apoptosis by decreasing the MDM2 protein levels.54 An anabolic steroid, nandrolone (3) (Fig. 3 and Table 1) has also been found to reduce MDM2 expression.55 However, all of these studies were performed in in vitro cell models, and further in vivo evaluation is needed for both 20(S)-Ginsenoside Rg3 and nandrolone. Studies of the molecular mechanisms underlying the inhibitory effects of these agents on MDM2 are still required.
More recently, platycodin D (4) (Fig. 3 and Table 1), a triterpenoid saponin, has been shown to inhibit triple negative breast cancer (MDA-MB-231) cell growth in vitro and xenograft tumor growth in vivo, which are attributed to platycodin D's inhibitory effects on MDM2 and MDMX.56 MDMX is also an important regulator of p53 and has been demonstrated as a potential drug target for treating and preventing human cancer.17 Interestingly, platycodin D also decreases the expression level of mutant p53 in MDA-MB-231 cells, although the molecular mechanism(s) is unclear.
Natural alkaloids represent an important class of natural products that target the p53-MDM2 pathway in human cancer. It has been known that the alkaloid berberine induces MDM2 self-ubiquitination and degradation by inhibiting MDM2-DAXX-HAUSP interactions.57 A recent study has further indicated that berberine down-regulates MDM2 expression, resulting in a reduction in XIAP expression and leukemia cell apoptosis, independent of p53.58 Our lab has identified a class of tricyclic pyrroloquinone alkaloid analogs as novel MDM2 inhibitors, including FBA-TPQ, PEA-TPQ, MPA-TPQ, DPA-TPQ, BA-TPQ, and TCBA-TPQ (Table 1). All of these compounds have shown excellent in vitro and in vivo activities in various cancer models with different p53 status (wild-type, mutant, and null), as were discussed in a recent review.59 Matrine (5), an alkaloid derived from the traditional Chinese medical herb Sophora flavescens Ait, has been shown to inhibit MDM2 expression by decreasing MDM2 mRNA synthesis in liver cancer cells (Fig. 3 and Table 1).60 Matrine also sensitizes MDM2-overexpressing liver cancers to etoposide-induced apoptosis, independent of p53. Melatonin (6), a monoamine alkaloid, inhibits MDM2 at the transcriptional level in MCF7 breast cancer cells (Fig. 3 and Table 1). Melatonin also inhibits MDM2 phosphorylation and enhances p53 acetylation, resulting in the disruption of p53-MDM2 binding and p53 stabilization.61 The anticancer activity of matrine and melatonin, and their inhibitory effects on MDM2, need to be further investigated in in vivo cancer models.
Gambogic acid, a natural xanthone, has been reported to inhibit MDM2 at both the transcriptional and post-translational levels and exerts anticancer activity in vitro and in vivo, regardless of the p53 status of the cells/tumors.62 Several natural products with similar structural features have been identified to inhibit MDM2 and exert cancer preventive and therapeutic effects. For example, plumbagin (7), a natural naphthoquinone derivative, has been identified as a new MDM2 inhibitor that decreases MDM2 protein expression levels, independent of p53 (Fig. 3 and Table 1).63 Plumbagin also inhibits the growth of osteosarcoma U2OS cells (IC50 = 2.5 μM), arrests cells at the S phase, and induces cell apoptosis in vitro, regardless of the p53 status of the cells. The in vivo efficacy and safety profiles of plumbagin have not yet been reported. Gossypol (8) is a natural polyphenol that has been identified as an inhibitor of MDM2-VEGF mRNA binding via a high-throughput screening assay (Fig. 3 and Table 1).64 Gossypol induces MDM2 self-ubiquitination and protein degradation and inhibits VEGF translation in breast cancer cell lines harboring wild-type p53 and mutant p53. Consequently, gossypol treatment causes cancer cell death and apoptosis in vitro and suppresses the xenograft tumor growth in vivo in a p53-independent manner.
Natural terpenoids, especially diterpenoids and sesquiterpenoids, represent a promising source of cancer preventive and therapeutic agents; some of which have entered clinical trials.8 Triptolide, a diterpene triepoxide, has been found to inhibit MDM2 mRNA synthesis in leukemia and gastric cancer cells.65, 66 Triptolide inhibits cancer cell growth and induces apoptosis at nanomolar concentrations in vitro, and these effects are dependent on its inhibiting MDM2. Parthenolide, a sesquiterpene lactone, has been demonstrated to induce MDM2 ubiquitination and protein degradation and to inhibit cell growth and induce apoptosis in colon cancer cells in a p53-independent manner.67 However, the inhibitory effects of triptolide and parthenolide on MDM2 have not been reported in any in vivo tumor models yet.
We have recently discovered a novel class of dimeric sesquiterpene lactones, including Japonicone A (JapA, 9),68, 69 Inulanolide A (InuA, 10),70, 71 and Lineariifolianoid A (LinA, 11)72 as MDM2 inhibitors (Fig. 3 and Table 1), which have been reviewed in recent papers.8, 59 JapA, InuA, and LinA have been demonstrated to directly bind to the MDM2 protein and promote MDM2 ubiquitination and degradation. These natural products also bind to the transcription factor NFAT1 and inhibit NFAT1-mediated MDM2 transcription. More recently, we have discovered that InuA binds to the RING domain of MDMX and induces MDMX ubiquitination and degradation, which is important for InuA-induced MDM2 degradation.71
Curcumin, a dietary polyphenol, has shown promising cancer chemopreventive and therapeutic efficacy in preclinical and clinical studies.73 We have demonstrated that curcumin inhibits MDM2 transcription through the PI3K/mTOR/ETS2 pathway, which is important for its anti-prostate cancer activity in vitro and in vivo.73 Many curcumin derivatives have been developed and shown inhibitory effects on MDM2 in various cancer models. A curcumin derivative has been reported to induce cell cycle arrest at the S phase and apoptosis in neuroblastoma cells by decreasing MDM2 protein expression and increasing p53 expression.74 Hispolon (12), a natural phenol derivative, induces autophagy in liver and breast cancer cells by decreasing the MDM2 protein levels (Fig. 3 and Table 1).75 Further studies have indicated that hispolon enhances the binding of MDM2 with HSP90, HSP70, HSC70, and LAMP2A. The mechanisms underlying the binding to these various targets and the in vivo efficacy of hispolon have not yet been determined. A chalcone derivative, N9 (13), has been reported to inhibit glioma cell growth and colony formation, arrest cells at the G1 phase, and induce cell apoptosis in vitro, and also inhibits xenograft tumor growth in vivo by decreasing the MDM2 expression levels (Fig. 3 and Table 1).76 Further studies are needed to determine the mechanism(s) underlying N9's inhibitory effects on MDM2.
Natural products that inhibit MDM2-p53 binding
The major focus of the discovery and development of synthetic small molecule MDM2 inhibitors is still inhibiting MDM2-p53 binding to activate p53 and protect p53 from MDM2-mediated p53 ubiquitination and degradation. There are a number of natural products that have shown significant inhibitory effects on p53-MDM2 binding, including chalcone derivatives,77 hexylitaconic acid and its derivatives,78 natural peptide chlorofusin,79 and hoiamide D (Table 1).80 Recently, two new chlorofusin derivatives (14, 15) have been reported to inhibit osteosarcoma SJSA-1 cell growth by inhibiting p53-MDM2 binding (Fig. 3 and Table 1), although the in vivo efficacy is still being investigated.81
Tricetin (16), a dietary flavonoid, has recently been reported to inhibit cell growth and colony formation, induce cell cycle arrest at the G2/M phase, and lead to apoptosis in breast cancer MCF7 cells (p53 wild-type) in vitro.82 Tricetin directly inhibits the p53-MDM2 interaction and stabilizes p53, which is critical for the anticancer activity of this natural product (Fig. 3 and Table 1). The dietary phytochemical, indole-3-carbinol (17), has been considered a promising cancer preventive natural product. A recent study demonstrated that indole-3-carbinol induces p53 phosphorylation at Ser15, resulting in the inhibition of p53-MDM2 binding and p53 stabilization and cell cycle arrest at the G1 phase.83 A virtual screening of p53-MDM2 binding inhibitors has recently been performed, and fluspirilene (18) was identified as a new natural product MDM2 inhibitor.84 Similar to other inhibitors that inhibit MDM2-p53 binding, fluspirilene significantly inhibited the growth of HCT116 p53+/+ colon cancer cells, but not HCT116 p53−/− cells in vitro.84 In another in silico screening for dual inhibitors of MDM2-p53 and MDMX-p53 binding, lithocholic acid (19) was identified and showed inhibitory effects at micromolar concentrations.85 Lithocholic acid has also been shown to induce apoptosis in p53 wild-type HCT116 cells in vitro.
Isokotomolide A (20) (Fig. 3 and Table 1), a natural butanolide, has been reported to inhibit cancer cell growth and colony formation and induce cell cycle arrest at the G0/G1 phase and apoptosis, in lung cancer A549 (p53 wild-type) cells by activating p53 and p21.86 Further studies have indicated that isokotomolide A directly inhibits the binding of MDM2 to p53 and prohibits MDM2-mediated p53 degradation. Leptomycin B (21), a natural product that inhibits nuclear export, protects p53 from MDM2-mediated degradation by inducing a modification at the amino-terminal half of the full-length MDM2 protein.87 Interestingly, this MDM2 modification also protects the amino 32 kDa fragment of MDM2 from complete degradation. However, all of these studies have only been done in in vitro cancer models, and the further in vivo evaluation of these natural p53-MDM2 binding inhibitors is needed to confirm these effects.
Natural products targeting mutant p53
In addition to targeting the p53-MDM2 pathway, targeting mutant p53 with natural products represents another promising approach to treating human cancers. Mutations of p53 are common in human cancers, although the mutation rate varies widely among different types of cancers.96 These mutations often result in the loss of p53 function, i.e., the inability of the protein to bind to p53-binding sites on DNA and to exert its functions in signaling checkpoint arrest and apoptosis. Moreover, mutant p53 (e.g., p53R273H) may exert oncogenic functions through transdominant repression of wild-type p53 activities.97 Mutant p53 proteins have been reported to regulate cancer cell survival, proliferation, migration, invasion, chemo-resistance, inflammation, etc., which have been discussed in several reviews.98, 99 Therefore, mutant p53 has been proposed as a preventive and therapeutic target in cancer. Many molecules have been developed to reactivate or reinstate the wild-type functions of mutant p53 or inhibit its oncogenic functions.9 Considerable effort has also been expended to identify natural products that target mutant p53 in cancer. In this section, we focus our discussion on the natural products targeting mutant p53 and their anticancer activities and molecular mechanisms (Fig. 4 and Table 2).
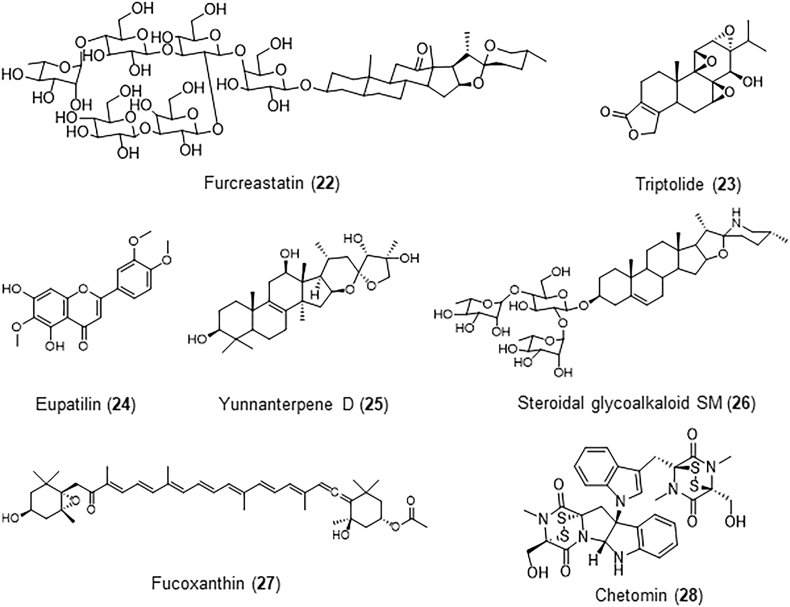
Figure 4.
The structures of natural products targeting mutant p53 in cancer.
Table 2.
Natural products as preventive and therapeutic agents that target mutant p53.
| Natural product | Cancer type | In vitro activity | In vivo efficacy | Mechanisms of action | References |
|---|---|---|---|---|---|
| Furcreastatin (22) | Oral squamous cell carcinoma and breast cancer | Selectively inhibits the growth of p53-mutant cancer cells (IC50 = 2.6–6.3 μg/mL) | Not reported | Not reported | 100 |
| Green tea or caffeine | Skin cancer | Not reported | Prevents UVB irradiation-induced tumor formation in female SKH-1 hairless mice | Changes the mutation profile of p53 in early mutant p53- positive epidermal patches | 101, 102 |
| Triptolide (23) | Breast cancer | Inhibits cell growth and induces S phase cell cycle arrest | Not reported | Decreases the protein expression level of mutant p53 | 103 |
| N37063 | Osteosarcoma, lung and colon cancer | Inhibits cancer cell growth and induces apoptosis in a mutant p53-dependent manner | Not reported | Restores wild-type p53 function to His175 and His273 mutant p53 proteins and induces the expression of p53 target genes | 104 |
| Eupatilin (24) | Endometrial cancer | Inhibits cell growth and induces cell cycle arrest at the G2/M phase | Not reported | Decreases the protein expression level of mutant p53 | 105 |
| Yunnanterpene D (25) | Various cancer types | Selectively inhibits the growth of cancer cells (IC50 = 5.5 μM) | Not reported | Decreases the protein expression level of mutant p53 | 106 |
| Origanum majorana extract | Breast cancer | Inhibits cell growth and colony formation in MDA-MB-231 cells and induces cell cycle arrest at the G2/M phase and apoptosis | Not reported | Decreases the protein expression level of mutant p53 | 107 |
| Steroidal glycoalkaloid SM (26) | Gastric cancer | Inhibits the growth of MGC-803 cells and induces cell cycle arrest at the S phase and apoptosis | Not reported | Decreases the protein expression level of mutant p53 | 108 |
| Fucoxanthin (27) | Bladder cancer | Inhibits T24 cell growth and colony formation and induces cell cycle arrest at the G0/G1 phase and apoptosis | Not reported | Inhibits the mortalin-p53 complex and reactivates mutant p53 | 109 |
| Turmeric and curcumin | Epidermoid cancer | Induces apoptosis and autophagy in A431 cells | Not reported | Induces the degradation of mutant p53 | 110 |
| Chetomin (28) | Pancreatic, colon, ovarian, lung, prostate, breast, epidermoid, bile duct and tongue cancer, renal cell carcinoma | Selectively inhibits the growth of cancer cells with p53 R175H | Specifically inhibits tumor growth in TOV-112D (p53 R175H) and CAL-33 (p53 R175H) xenograft models without significant effects on A431 (R273H) and H1299 (p53 null) xenograft tumor growth | Reactivates mutant p53 R175H by increasing the binding capacity of Hsp40 to mutant p53 R175H and causing a potential conformational change to a wild-type-like p53 | 111 |
In a screen to identify compounds that selectively inhibit the growth of mutant p53-expressing cells, furcreastatin (22), a steroidal saponin, has been identified and shown selective cytotoxicity against oral squamous cell carcinoma and breast cancer cell lines with mutant p53.100 However, the molecular mechanisms underlying furcreastatin's selective cytotoxicity against mutant-p53 cancer cells and its in vivo efficacy are still unclear. Green tea and caffeine have been reported to prevent UVB irradiation-induced tumor formation in female SKH-1 hairless mice by inhibiting the formation of mutant p53-positive cellular patches.101, 102 Mechanistic studies have indicated that oral exposure to green tea and caffeine changes the mutation profile of p53 in early mutant p53-positive epidermal patches in vivo.101, 102 The direct effects of green tea and caffeine on mutant p53 need to be further investigated.
Triptolide (23), a previously-reported MDM2 inhibitor,65, 66 has recently been reported to decrease the protein expression level of mutant p53 in MDA-MB-231 breast cancer cells. The decrease in mutant p53 was partially responsible for triptolide's inhibitory effects on cell growth and cell cycle progression.103 An in silico screen of the NCI natural product database has been performed to identify compounds and plant extracts that inhibit the growth of mutant p53-expressing cancer cells. An extract from the terrestrial plant Brachylaena ramiflora, named N37063, has been found to inhibit cell growth and induce apoptosis in osteosarcoma, lung, and colon cancer cells in a mutant p53-dependent manner.104 Further studies have shown that N37063 restores wild-type p53 function to His175 and His273 mutant p53 proteins and induces the expression of p53 target genes. Eupatilin (24), a dietary flavonoid, has been found to induce G2/M cell cycle arrest in Hec1A (mutant p53) endometrial cancer cells by activating p21.105 Further studies have shown that eupatilin decreases the expression level of mutant p53, resulting in the upregulation of p21. However, neither N37063 nor eupatilin has been examined in any animal models containing specific mutant p53.
A class of new triterpene derivatives has recently been identified to target mutant p53 N236S, and yunnanterpene D (25) has shown great selective cytotoxicity against p53 N236S-expressing cells.106 However, the specific effects of yunnanterpene D on the mutant p53 N236S protein have not been examined yet. Origanum majorana ethanolic extract (OME) has been shown significant effects against MDA-MB-231 breast cancer cells by inhibiting cell growth and colony formation and inducing G2/M cell cycle phase arrest and apoptosis.107 In studies of its mechanism of action, OME has been found to inhibit the expression of mutant p53, resulting in the activation of p21, which is mainly responsible for the OME-induced G2/M phase arrest. Six steroidal glycoalkaloids, including SM (26), have been shown to inhibit the growth of MGC-803 gastric cancer cells and induce S phase arrest and apoptosis.108 SM has been also found to decrease the expression level of mutant p53, which is important for its anticancer activity.108
Fucoxanthin (27), a natural carotenoid, inhibits cell growth and colony formation, induces cell cycle arrest at the G0/G1 phase, and leads to apoptosis in T24 bladder cancer cells.109 Fucoxanthin inhibits the mortalin-p53 complex and reactivates mutant p53 in these cells, resulting in the upregulation of p21. The crude extract of turmeric (Curcuma longa) and its bioactive component, curcumin, have been found to induce apoptosis and autophagy in A431 epidermoid cancer cells, which express mutant p53 R273H.110 Both turmeric and curcumin induce macroautophagy, resulting in the degradation of mutant p53. In a cell-based, high-throughput small-molecule screening study, chetomin (28) has been identified as a mutant p53 R175H reactivator.111 Chetomin has been further tested in several human cancer cell models with different p53 status, and the compound only selectively inhibited the growth of cancer cells with p53 R175H. Chetomin has also been shown to specifically inhibit the tumor growth in TOV-112D (p53 R175H) and CAL-33 (p53 R175H) xenograft models without affecting the growth of A431 (R273H) and H1299 (p53 null) xenograft tumors. Further mechanistic studies have shown that chetomin increases the binding capacity of Hsp40 to mutant p53 R175H and causes a potential conformational change to a wild-type-like p53, resulting in reactivation of the mutant p53 R175H.
Future research directions
The p53-MDM2 pathway is commonly dysregulated and involved in cancer initiation, progression, and metastasis.10, 17 The tumor suppressor role of p53 and the oncogenic functions of MDM2 and mutant p53 are well characterized in various cancers. Considerable efforts have been made to discover and develop inhibitors of the p53-MDM2 pathway for cancer prevention and treatment.11, 112, 113 Several synthetic small molecules that specifically inhibit p53-MDM2 binding or MDM2 E3 ligase activity have entered clinical trials as cancer chemotherapeutic drugs.114 However, all of these inhibitors exert their anticancer activity in a p53-dependent manner, and wild-type p53 is critical for their effects. More importantly, concerns regarding the drug resistance and side effects of these synthetic inhibitors have been raised.114 Natural products targeting the p53-MDM2 pathway have recently gained momentum in their development as cancer chemopreventive and chemotherapeutic agents due to their lower toxicity compared to synthetic compounds. As reviewed above, a number of natural products (e.g., flavonoids, isoflavonoids, ginsenosides, terpenoids, alkaloids, curcumin, and peptides) have been identified to target MDM2, p53 or the p53-MDM2 pathway, and many of these are under development as cancer chemopreventive and chemotherapeutic agents.
Although these natural products have shown significant anticancer activity in various in vitro models, many of them have not been evaluated for in vivo efficacy yet. Further studies must be performed to examine the efficacy of these natural products in clinically-relevant cancer models, e.g., orthotopic models, metastasis models, transgenic mouse models, and patient-derived cancer models. These natural products often have poor bioavailability due to their high molecular weight, poor water solubility, and low dissolution rate, making it difficult to test them in preclinical and clinical studies. Nanotechnology holds promise in improving the bioavailability of these natural products through novel nano-delivery methods. Structural modification of these natural products is another way to enhance their bioavailability and increase their efficacy.
In addition, while most of these natural product inhibitors have been observed to decrease the expression levels of MDM2 and/or mutant p53, or to restore normal p53 activity, the detailed molecular mechanisms are still unclear. Having a thorough understanding of these natural products, especially their in vivo efficacy, physicochemical properties, bioavailability, and mechanisms of action, will lead to better translation of their cancer preventive and therapeutic activity to the preclinical and clinical settings.
Conclusion
Targeting the p53-MDM2 pathway can be a promising approach to develop compounds for cancer treatment and prevention. A number of natural products have been developed to target the p53-MDM2 pathway by 1) inhibiting MDM2 expression or protein stability, 2) inhibiting the p53-MDM2 interaction, 3) inhibiting the E3 ligase activity of MDM2, 4) reactivating or reinstating the wild-type functions of mutant p53, and/or 5) inhibiting the expression or protein stability of mutant p53. These natural products have shown potent chemopreventive and chemotherapeutic activity in various preclinical cancer models. Based on our experience, the natural products falling into category 1 may prove to be most potent, because they directly target MDM2 and exert activities against cancers regardless of the p53 status (wild-type, mutant, or null). Natural product MDM2 inhibitors in categories 2 and 3 selectively target cancers harboring wild-type p53, while the natural products in categories 4 and 5 specifically inhibit cancers containing mutant p53.
Overall, there has been progress in the development of natural products targeting the p53-MDM2 pathway for cancer therapy and prevention, but there are various issues that still need to be addressed. In particular, more detailed investigations of the in vivo efficacy, toxicity, bioavailability, and mechanisms of action are needed to hasten the development of effective and safe cancer preventive and therapeutic drugs for clinical use.
Conflicts of interest
These authors have no conflicts of interest to declare.
Acknowledgements
We thank the current and former members of our laboratory and our collaborators for their contributions to the publications cited in this review article. The development of natural products for cancer is a rapidly growing field, and we apologize for not being able to cite all of the recent publications due to the limited space allotted for this review.
This work was supported by National Institutes of Health (NIH)/National Cancer Institute (NCI) grants (R01 CA186662 and R01 CA214019 to RZ). The content is solely the responsibility of the authors, and does not necessarily represent the official views of the National Institutes of Health. W.W. was also supported by American Cancer Society (ACS) grant RSG-15-009-01-CDD. R.Z. was also supported by funds for the Robert L. Boblitt Endowed Professor in Drug Discovery and research funds from the College of Pharmacy and the University of Houston. H.W. was supported by grants from the National Nature Science Foundation (81630086, 81427805, 81672763, 31401611, and 81502122), the Key Research Program (ZDRW-ZS-2017-1), the Strategic Priority Research Program (XDA12020319) of the Chinese Academy of Sciences, and the Science and Technology Commission of Shanghai Municipality (16391903700 and 14391901800).
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Lee E.Y., Muller W.J. Oncogenes and tumor suppressor genes. Cold Spring Harb Perspect Biol. 2010;2(10):a003236. doi: 10.1101/cshperspect.a003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu K., Liu Q., Zhou Y. Oncogenes and tumor suppressor genes: comparative genomics and network perspectives. BMC Genom. 2015;16(suppl 7):S8. doi: 10.1186/1471-2164-16-S7-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger T., Saunders M.E., Mak T.W. Beyond the oncogene revolution: four new ways to combat cancer. Cold Spring Harb Symp Quant Biol. 2016;81:85–92. doi: 10.1101/sqb.2016.81.031161. [DOI] [PubMed] [Google Scholar]
- 4.Sabapathy K. The contrived mutant p53 oncogene - beyond loss of functions. Front Oncol. 2015;5:276. doi: 10.3389/fonc.2015.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fritsche M.K., Knopf A. The tumor suppressor p53 in mucosal melanoma of the head and neck. Genes. 2017;8(12):384. doi: 10.3390/genes8120384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Velez-Cruz R., Johnson D.G. The retinoblastoma (RB) tumor suppressor: pushing back against genome instability on multiple fronts. Int J Mol Sci. 2017;18(8):1776. doi: 10.3390/ijms18081776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bishayee A., Sethi G. Bioactive natural products in cancer prevention and therapy: progress and promise. Semin Cancer Biol. 2016;40–41:1–3. doi: 10.1016/j.semcancer.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Qin J., Wang W., Zhang R. Novel natural product therapeutics targeting both inflammation and cancer. Chin J Nat Med. 2017;15(6):401–416. doi: 10.1016/S1875-5364(17)30062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen D., Liao W., Zeng S.X. Reviving the guardian of the genome: small molecule activators of p53. Pharmacol Ther. 2017;178:92–108. doi: 10.1016/j.pharmthera.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nag S., Qin J., Srivenugopal K.S. The MDM2-p53 pathway revisited. J Biomed Res. 2013;27(4):254–271. doi: 10.7555/JBR.27.20130030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin J.J., Nag S., Voruganti S. Natural product MDM2 inhibitors: anticancer activity and mechanisms of action. Curr Med Chem. 2012;19(33):5705–5725. doi: 10.2174/092986712803988910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ware P.L., Snow A.N., Gvalani M. MDM2 copy numbers in well-differentiated and dedifferentiated liposarcoma: characterizing progression to high-grade tumors. Am J Clin Pathol. 2014;141(3):334–341. doi: 10.1309/AJCPLYU89XHSNHQO. [DOI] [PubMed] [Google Scholar]
- 13.Momand J., Jung D., Wilczynski S. The MDM2 gene amplification database. Nucleic Acids Res. 1998;26(15):3453–3459. doi: 10.1093/nar/26.15.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onel K., Cordon-Cardo C. MDM2 and prognosis. Mol Cancer Res. 2004;2(1):1–8. [PubMed] [Google Scholar]
- 15.Yu Q., Li Y., Mu K. Amplification of Mdmx and overexpression of MDM2 contribute to mammary carcinogenesis by substituting for p53 mutations. Diagn Pathol. 2014;9:71. doi: 10.1186/1746-1596-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nag S., Zhang X., Srivenugopal K.S. Targeting MDM2-p53 interaction for cancer therapy: are we there yet? Curr Med Chem. 2014;21(5):553–574. doi: 10.2174/09298673113206660325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karni-Schmidt O., Lokshin M., Prives C. The roles of MDM2 and MDMX in cancer. Annu Rev Pathol. 2016;11:617–644. doi: 10.1146/annurev-pathol-012414-040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barak Y., Gottlieb E., Juven-Gershon T. Regulation of mdm2 expression by p53: alternative promoters produce transcripts with nonidentical translation potential. Genes Dev. 1994;8(15):1739–1749. doi: 10.1101/gad.8.15.1739. [DOI] [PubMed] [Google Scholar]
- 19.Oliner J.D., Pietenpol J.A., Thiagalingam S. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362(6423):857–860. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- 20.Haupt Y., Maya R., Kazaz A. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387(6630):296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 21.Kubbutat M.H., Jones S.N., Vousden K.H. Regulation of p53 stability by Mdm2. Nature. 1997;387(6630):299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 22.Kulikov R., Letienne J., Kaur M. Mdm2 facilitates the association of p53 with the proteasome. Proc Natl Acad Sci U S A. 2010;107(22):10038–10043. doi: 10.1073/pnas.0911716107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carter S., Bischof O., Dejean A. C-terminal modifications regulate MDM2 dissociation and nuclear export of p53. Nat Cell Biol. 2007;9(4):428–435. doi: 10.1038/ncb1562. [DOI] [PubMed] [Google Scholar]
- 24.Geyer R.K., Yu Z.K., Maki C.G. The MDM2 RING-finger domain is required to promote p53 nuclear export. Nat Cell Biol. 2000;2(9):569–573. doi: 10.1038/35023507. [DOI] [PubMed] [Google Scholar]
- 25.Qin J.J., Wang W., Zhang R. Experimental therapy of advanced breast cancer: targeting NFAT1-MDM2-p53 pathway. Prog Mol Biol Transl Sci. 2017;151:195–216. doi: 10.1016/bs.pmbts.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouska A., Eischen C.M. Murine double minute 2: p53-independent roads lead to genome instability or death. Trends Biochem Sci. 2009;34(6):279–286. doi: 10.1016/j.tibs.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Bohlman S., Manfredi J.J. p53-independent effects of Mdm2. Subcell Biochem. 2014;85:235–246. doi: 10.1007/978-94-017-9211-0_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rayburn E.R., Ezell S.J., Zhang R. Recent advances in validating MDM2 as a cancer target. Anticancer Agents Med Chem. 2009;9(8):882–903. doi: 10.2174/187152009789124628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z., Wang H., Li M. Novel MDM2 p53-independent functions identified through RNA silencing technologies. Ann N Y Acad Sci. 2005;1058:205–214. doi: 10.1196/annals.1359.030. [DOI] [PubMed] [Google Scholar]
- 30.Turner N., Moretti E., Siclari O. Targeting triple negative breast cancer: is p53 the answer? Cancer Treat Rev. 2013;39(5):541–550. doi: 10.1016/j.ctrv.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Haupt S., Vijayakumaran R., Panimaya J. The role of MDM2 and MDM4 in breast cancer development and prevention. J Mol Cell Biol. 2017;9(1):53–61. doi: 10.1093/jmcb/mjx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z., Zhang R. p53-independent activities of MDM2 and their relevance to cancer therapy. Curr Cancer Drug Targets. 2005;5(1):9–20. doi: 10.2174/1568009053332618. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z., Li M., Wang H. Antisense therapy targeting MDM2 oncogene in prostate cancer: effects on proliferation, apoptosis, multiple gene expression, and chemotherapy. Proc Natl Acad Sci U S A. 2003;100(20):11636–11641. doi: 10.1073/pnas.1934692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z., Wang H., Li M. MDM2 is a negative regulator of p21WAF1/CIP1, independent of p53. J Biol Chem. 2004;279(16):16000–16006. doi: 10.1074/jbc.M312264200. [DOI] [PubMed] [Google Scholar]
- 35.Jin Y., Lee H., Zeng S.X. MDM2 promotes p21waf1/cip1 proteasomal turnover independently of ubiquitylation. EMBO J. 2003;22(23):6365–6377. doi: 10.1093/emboj/cdg600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu H., Zhang Z., Li M. MDM2 promotes proteasomal degradation of p21Waf1 via a conformation change. J Biol Chem. 2010;285(24):18407–18414. doi: 10.1074/jbc.M109.059568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z., Wang H., Li M. Stabilization of E2F1 protein by MDM2 through the E2F1 ubiquitination pathway. Oncogene. 2005;24(48):7238–7247. doi: 10.1038/sj.onc.1208814. [DOI] [PubMed] [Google Scholar]
- 38.Ohtani K., DeGregori J., Nevins J.R. Regulation of the cyclin E gene by transcription factor E2F1. Proc Natl Acad Sci U S A. 1995;92(26):12146–12150. doi: 10.1073/pnas.92.26.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uchida C., Miwa S., Kitagawa K. Enhanced Mdm2 activity inhibits pRB function via ubiquitin-dependent degradation. EMBO J. 2005;24(1):160–169. doi: 10.1038/sj.emboj.7600486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sdek P., Ying H., Chang D.L. MDM2 promotes proteasome-dependent ubiquitin-independent degradation of retinoblastoma protein. Mol Cell. 2005;20(5):699–708. doi: 10.1016/j.molcel.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 41.Yang J.Y., Zong C.S., Xia W. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008;10(2):138–148. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brenkman A.B., de Keizer P.L., van den Broek N.J. Mdm2 induces mono-ubiquitination of FOXO4. PLoS One. 2008;3(7):e2819. doi: 10.1371/journal.pone.0002819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang J.Y., Zong C.S., Xia W. MDM2 promotes cell motility and invasiveness by regulating E-cadherin degradation. Mol Cell Biol. 2006;26(19):7269–7282. doi: 10.1128/MCB.00172-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li M., Zhang Z., Hill D.L. Genistein, a dietary isoflavone, down-regulates the MDM2 oncogene at both transcriptional and posttranslational levels. Cancer Res. 2005;65(18):8200–8208. doi: 10.1158/0008-5472.CAN-05-1302. [DOI] [PubMed] [Google Scholar]
- 45.Fang J., Xia C., Cao Z. Apigenin inhibits VEGF and HIF-1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways. FASEB J. 2005;19(3):342–353. doi: 10.1096/fj.04-2175com. [DOI] [PubMed] [Google Scholar]
- 46.Mu R., Qi Q., Gu H. Involvement of p53 in oroxylin A-induced apoptosis in cancer cells. Mol Carcinog. 2009;48(12):1159–1169. doi: 10.1002/mc.20570. [DOI] [PubMed] [Google Scholar]
- 47.Alonso M., Tamasdan C., Miller D.C. Flavopiridol induces apoptosis in glioma cell lines independent of retinoblastoma and p53 tumor suppressor pathway alterations by a caspase-independent pathway. Mol Cancer Ther. 2003;2(2):139–150. [PubMed] [Google Scholar]
- 48.Demidenko Z.N., Blagosklonny M.V. Flavopiridol induces p53 via initial inhibition of Mdm2 and p21 and, independently of p53, sensitizes apoptosis-reluctant cells to tumor necrosis factor. Cancer Res. 2004;64(10):3653–3660. doi: 10.1158/0008-5472.CAN-04-0204. [DOI] [PubMed] [Google Scholar]
- 49.Wang W., Wang H., Rayburn E.R. 20(S)-25-methoxyl-dammarane-3beta, 12beta, 20-triol, a novel natural product for prostate cancer therapy: activity in vitro and in vivo and mechanisms of action. Br J Cancer. 2008;98(4):792–802. doi: 10.1038/sj.bjc.6604227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W., Rayburn E.R., Zhao Y. Novel ginsenosides 25-OH-PPD and 25-OCH3-PPD as experimental therapy for pancreatic cancer: anticancer activity and mechanisms of action. Cancer Lett. 2009;278(2):241–248. doi: 10.1016/j.canlet.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang W., Rayburn E.R., Hang J. Anti-lung cancer effects of novel ginsenoside 25-OCH(3)-PPD. Lung Cancer. 2009;65(3):306–311. doi: 10.1016/j.lungcan.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang W., Rayburn E.R., Hao M. Experimental therapy of prostate cancer with novel natural product anti-cancer ginsenosides. Prostate. 2008;68(8):809–819. doi: 10.1002/pros.20742. [DOI] [PubMed] [Google Scholar]
- 53.Wang W., Zhang X., Qin J.J. Natural product ginsenoside 25-OCH3-PPD inhibits breast cancer growth and metastasis through down-regulating MDM2. PLoS One. 2012;7(7):e41586. doi: 10.1371/journal.pone.0041586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang F., Li M., Wu X. 20(S)-ginsenoside Rg3 promotes senescence and apoptosis in gallbladder cancer cells via the p53 pathway. Drug Des Devel Ther. 2015;9:3969–3987. doi: 10.2147/DDDT.S84527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu X.H., Yao S., Levine A.C. Nandrolone, an anabolic steroid, stabilizes Numb protein through inhibition of mdm2 in C2C12 myoblasts. J Androl. 2012;33(6):1216–1223. doi: 10.2164/jandrol.112.016428. [DOI] [PubMed] [Google Scholar]
- 56.Kong Y., Lu Z.L., Wang J.J. Platycodin D, a metabolite of Platycodin grandiflorum, inhibits highly metastatic MDA-MB-231 breast cancer growth in vitro and in vivo by targeting the MDM2 oncogene. Oncol Rep. 2016;36(3):1447–1456. doi: 10.3892/or.2016.4935. [DOI] [PubMed] [Google Scholar]
- 57.Zhang X., Gu L., Li J. Degradation of MDM2 by the interaction between berberine and DAXX leads to potent apoptosis in MDM2-overexpressing cancer cells. Cancer Res. 2010;70(23):9895–9904. doi: 10.1158/0008-5472.CAN-10-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu J., Zhang X., Liu A. Berberine induces apoptosis in p53-null leukemia cells by down-regulating XIAP at the post-transcriptional level. Cell Physiol Biochem. 2013;32(5):1213–1224. doi: 10.1159/000354520. [DOI] [PubMed] [Google Scholar]
- 59.Wang W., Nijampatnam B., Velu S.E. Discovery and development of synthetic tricyclic pyrroloquinone (TPQ) alkaloid analogs for human cancer therapy. Front Chem Sci Eng. 2016;10(1):1–15. [Google Scholar]
- 60.Zhou N., Li J., Li T. Matrineinduced apoptosis in Hep3B cells via the inhibition of MDM2. Mol Med Rep. 2017;15(1):442–450. doi: 10.3892/mmr.2016.5999. [DOI] [PubMed] [Google Scholar]
- 61.Proietti S., Cucina A., Dobrowolny G. Melatonin down-regulates MDM2 gene expression and enhances p53 acetylation in MCF-7 cells. J Pineal Res. 2014;57(1):120–129. doi: 10.1111/jpi.12150. [DOI] [PubMed] [Google Scholar]
- 62.Rong J.J., Hu R., Qi Q. Gambogic acid down-regulates MDM2 oncogene and induces p21(Waf1/CIP1) expression independent of p53. Cancer Lett. 2009;284(1):102–112. doi: 10.1016/j.canlet.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 63.Tian L., Yin D., Ren Y. Plumbagin induces apoptosis via the p53 pathway and generation of reactive oxygen species in human osteosarcoma cells. Mol Med Rep. 2012;5(1):126–132. doi: 10.3892/mmr.2011.624. [DOI] [PubMed] [Google Scholar]
- 64.Xiong J., Li J., Yang Q. Gossypol has anti-cancer effects by dual-targeting MDM2 and VEGF in human breast cancer. Breast Cancer Res. 2017;19(1):27. doi: 10.1186/s13058-017-0818-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang M., Zhang H., Liu T. Triptolide inhibits MDM2 and induces apoptosis in acute lymphoblastic leukemia cells through a p53-independent pathway. Mol Cancer Ther. 2013;12(2):184–194. doi: 10.1158/1535-7163.MCT-12-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang B.Y., Cao J., Chen J.W. Triptolide induces apoptosis of gastric cancer cells via inhibiting the overexpression of MDM2. Med Oncol. 2014;31(11):270. doi: 10.1007/s12032-014-0270-7. [DOI] [PubMed] [Google Scholar]
- 67.Gopal Y.N., Chanchorn E., Van Dyke M.W. Parthenolide promotes the ubiquitination of MDM2 and activates p53 cellular functions. Mol Cancer Ther. 2009;8(3):552–562. doi: 10.1158/1535-7163.MCT-08-0661. [DOI] [PubMed] [Google Scholar]
- 68.Qin J.J., Wang W., Voruganti S. Identification of a new class of natural product MDM2 inhibitor: In vitro and in vivo anti-breast cancer activities and target validation. Oncotarget. 2015;6(5):2623–2640. doi: 10.18632/oncotarget.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qin J.J., Wang W., Voruganti S. Inhibiting NFAT1 for breast cancer therapy: new insights into the mechanism of action of MDM2 inhibitor JapA. Oncotarget. 2015;6(32):33106–33119. doi: 10.18632/oncotarget.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qin J.J., Wang W., Sarkar S. Inulanolide A as a new dual inhibitor of NFAT1-MDM2 pathway for breast cancer therapy. Oncotarget. 2016;7(22):32566–32578. doi: 10.18632/oncotarget.8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qin J.J., Li X., Wang W. Targeting the NFAT1-MDM2-MDMX network inhibits the proliferation and invasion of prostate cancer cells, independent of p53 and androgen. Front Pharmacol. 2017;8:917. doi: 10.3389/fphar.2017.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qin J.J., Sarkar S., Voruganti S. Identification of lineariifolianoid A as a novel dual NFAT1 and MDM2 inhibitor for human cancer therapy. J Biomed Res. 2016;30(4):322–333. doi: 10.7555/JBR.30.20160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li M., Zhang Z., Hill D.L. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007;67(5):1988–1996. doi: 10.1158/0008-5472.CAN-06-3066. [DOI] [PubMed] [Google Scholar]
- 74.Tian Z., An N., Zhou B. Cytotoxic diarylheptanoid induces cell cycle arrest and apoptosis via increasing ATF3 and stabilizing p53 in SH-SY5Y cells. Cancer Chemother Pharmacol. 2009;63(6):1131–1139. doi: 10.1007/s00280-008-0832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu T.L., Huang G.J., Wang H.J. Hispolon promotes MDM2 downregulation through chaperone-mediated autophagy. Biochem Biophys Res Commun. 2010;398(1):26–31. doi: 10.1016/j.bbrc.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 76.Loch-Neckel G., Bicca M.A., Leal P.C. In vitro and in vivo anti-glioma activity of a chalcone-quinoxaline hybrid. Eur J Med Chem. 2015;90:93–100. doi: 10.1016/j.ejmech.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 77.Stoll R., Renner C., Hansen S. Chalcone derivatives antagonize interactions between the human oncoprotein MDM2 and p53. Biochemistry. 2001;40(2):336–344. doi: 10.1021/bi000930v. [DOI] [PubMed] [Google Scholar]
- 78.Tsukamoto S., Yoshida T., Hosono H. Hexylitaconic acid: a new inhibitor of p53-HDM2 interaction isolated from a marine-derived fungus, Arthrinium sp. Bioorg Med Chem Lett. 2006;16(1):69–71. doi: 10.1016/j.bmcl.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 79.Duncan S.J., Gruschow S., Williams D.H. Isolation and structure elucidation of Chlorofusin, a novel p53-MDM2 antagonist from a Fusarium sp. J Am Chem Soc. 2001;123(4):554–560. doi: 10.1021/ja002940p. [DOI] [PubMed] [Google Scholar]
- 80.Malloy K.L., Choi H., Fiorilla C. Hoiamide D, a marine cyanobacteria-derived inhibitor of p53/MDM2 interaction. Bioorg Med Chem Lett. 2012;22(1):683–688. doi: 10.1016/j.bmcl.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cominetti M.M., Goffin S.A., Raffel E. Identification of a new p53/MDM2 inhibitor motif inspired by studies of chlorofusin. Bioorg Med Chem Lett. 2015;25(21):4878–4880. doi: 10.1016/j.bmcl.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 82.Hsu Y.L., Uen Y.H., Chen Y. Tricetin, a dietary flavonoid, inhibits proliferation of human breast adenocarcinoma mcf-7 cells by blocking cell cycle progression and inducing apoptosis. J Agric Food Chem. 2009;57(18):8688–8695. doi: 10.1021/jf901053x. [DOI] [PubMed] [Google Scholar]
- 83.Brew C.T., Aronchik I., Hsu J.C. Indole-3-carbinol activates the ATM signaling pathway independent of DNA damage to stabilize p53 and induce G1 arrest of human mammary epithelial cells. Int J Cancer. 2006;118(4):857–868. doi: 10.1002/ijc.21445. [DOI] [PubMed] [Google Scholar]
- 84.Patil S.P., Pacitti M.F., Gilroy K.S. Identification of antipsychotic drug fluspirilene as a potential p53-MDM2 inhibitor: a combined computational and experimental study. J Comput Aided Mol Des. 2015;29(2):155–163. doi: 10.1007/s10822-014-9811-6. [DOI] [PubMed] [Google Scholar]
- 85.Vogel S.M., Bauer M.R., Joerger A.C. Lithocholic acid is an endogenous inhibitor of MDM4 and MDM2. Proc Natl Acad Sci U S A. 2012;109(42):16906–16910. doi: 10.1073/pnas.1215060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen C.Y., Hsu Y.L., Chen Y.Y. Isokotomolide A, a new butanolide extracted from the leaves of Cinnamomum kotoense, arrests cell cycle progression and induces apoptosis through the induction of p53/p21 and the initiation of mitochondrial system in human non-small cell lung cancer A549 cells. Eur J Pharmacol. 2007;574(2–3):94–102. doi: 10.1016/j.ejphar.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 87.Menendez S., Higgins M., Berkson R.G. Nuclear export inhibitor leptomycin B induces the appearance of novel forms of human Mdm2 protein. Br J Cancer. 2003;88(4):636–643. doi: 10.1038/sj.bjc.6600752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang W., Rayburn E.R., Velu S.E. In vitro and in vivo anticancer activity of novel synthetic makaluvamine analogues. Clin Cancer Res. 2009;15(10):3511–3518. doi: 10.1158/1078-0432.CCR-08-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang F., Ezell S.J., Zhang Y. FBA-TPQ, a novel marine-derived compound as experimental therapy for prostate cancer. Invest New Drugs. 2010;28(3):234–241. doi: 10.1007/s10637-009-9232-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen T., Xu Y., Guo H. Experimental therapy of ovarian cancer with synthetic makaluvamine analog: in vitro and in vivo anticancer activity and molecular mechanisms of action. PLoS One. 2011;6(6):e20729. doi: 10.1371/journal.pone.0020729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang X., Xu H., Zhang X. Preclinical evaluation of anticancer efficacy and pharmacological properties of FBA-TPQ, a novel synthetic makaluvamine analog. Mar Drugs. 2012;10(5):1138–1155. doi: 10.3390/md10051138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang W., Rayburn E.R., Velu S.E. A novel synthetic iminoquinone, BA-TPQ, as an anti-breast cancer agent: in vitro and in vivo activity and mechanisms of action. Breast Cancer Res Treat. 2010;123(2):321–331. doi: 10.1007/s10549-009-0638-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nadkarni D.H., Wang F., Wang W. Synthesis and in vitro anti-lung cancer activity of novel 1, 3, 4, 8-tetrahydropyrrolo [4, 3, 2-de]quinolin-8(1H)-one alkaloid analogs. Med Chem. 2009;5(3):227–236. doi: 10.2174/157340609788185873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sasiela C.A., Stewart D.H., Kitagaki J. Identification of inhibitors for MDM2 ubiquitin ligase activity from natural product extracts by a novel high-throughput electrochemiluminescent screen. J Biomol Screen. 2008;13(3):229–237. doi: 10.1177/1087057108315038. [DOI] [PubMed] [Google Scholar]
- 95.Clement J.A., Kitagaki J., Yang Y. Discovery of new pyridoacridine alkaloids from Lissoclinum cf. badium that inhibit the ubiquitin ligase activity of Hdm2 and stabilize p53. Bioorg Med Chem. 2008;16(23):10022–10028. doi: 10.1016/j.bmc.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kandoth C., McLellan M.D., Vandin F. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502(7471):333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dong P., Tada M., Hamada J. p53 dominant-negative mutant R273H promotes invasion and migration of human endometrial cancer HHUA cells. Clin Exp Metastasis. 2007;24(6):471–483. doi: 10.1007/s10585-007-9084-8. [DOI] [PubMed] [Google Scholar]
- 98.Mantovani F., Walerych D., Sal G.D. Targeting mutant p53 in cancer: a long road to precision therapy. FEBS J. 2017;284(6):837–850. doi: 10.1111/febs.13948. [DOI] [PubMed] [Google Scholar]
- 99.Bykov V.J.N., Eriksson S.E., Bianchi J. Targeting mutant p53 for efficient cancer therapy. Nat Rev Cancer. 2018;18(2):89–102. doi: 10.1038/nrc.2017.109. [DOI] [PubMed] [Google Scholar]
- 100.Itabashi M., Segawa K., Ikeda Y. A new bioactive steroidal saponin, furcreastatin, from the plant Furcraea foetida. Carbohydr Res. 2000;323(1–4):57–62. doi: 10.1016/s0008-6215(99)00255-4. [DOI] [PubMed] [Google Scholar]
- 101.Lu Y.P., Lou Y.R., Liao J. Administration of green tea or caffeine enhances the disappearance of UVB-induced patches of mutant p53 positive epidermal cells in SKH-1 mice. Carcinogenesis. 2005;26(8):1465–1472. doi: 10.1093/carcin/bgi086. [DOI] [PubMed] [Google Scholar]
- 102.Kramata P., Lu Y.P., Lou Y.R. Effect of administration of caffeine or green tea on the mutation profile in the p53 gene in early mutant p53-positive patches of epidermal cells induced by chronic UVB-irradiation of hairless SKH-1 mice. Carcinogenesis. 2005;26(11):1965–1974. doi: 10.1093/carcin/bgi162. [DOI] [PubMed] [Google Scholar]
- 103.Liu J., Jiang Z., Xiao J. Effects of triptolide from Tripterygium wilfordii on ERalpha and p53 expression in two human breast cancer cell lines. Phytomedicine. 2009;16(11):1006–1013. doi: 10.1016/j.phymed.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 104.Karimi M., Conserva F., Mahmoudi S. Extract from Asteraceae Brachylaena ramiflora induces apoptosis preferentially in mutant p53-expressing human tumor cells. Carcinogenesis. 2010;31(6):1045–1053. doi: 10.1093/carcin/bgq084. [DOI] [PubMed] [Google Scholar]
- 105.Cho J.H., Lee J.G., Yang Y.I. Eupatilin, a dietary flavonoid, induces G2/M cell cycle arrest in human endometrial cancer cells. Food Chem Toxicol. 2011;49(8):1737–1744. doi: 10.1016/j.fct.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 106.Nian Y., Zhu H., Tang W.R. Triterpenes from the aerial parts of Cimicifuga yunnanensis and their antiproliferative effects on p53(N236S) mouse embryonic fibroblasts. J Nat Prod. 2013;76(5):896–902. doi: 10.1021/np4000262. [DOI] [PubMed] [Google Scholar]
- 107.Al Dhaheri Y., Eid A., AbuQamar S. Mitotic arrest and apoptosis in breast cancer cells induced by Origanum majorana extract: upregulation of TNF-alpha and downregulation of survivin and mutant p53. PLoS One. 2013;8(2):e56649. doi: 10.1371/journal.pone.0056649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ding X., Zhu F., Yang Y. Purification, antitumor activity in vitro of steroidal glycoalkaloids from black nightshade (Solanum nigrum L.) Food Chem. 2013;141(2):1181–1186. doi: 10.1016/j.foodchem.2013.03.062. [DOI] [PubMed] [Google Scholar]
- 109.Wang L., Zeng Y., Liu Y. Fucoxanthin induces growth arrest and apoptosis in human bladder cancer T24 cells by up-regulation of p21 and down-regulation of mortalin. Acta Biochim Biophys Sin. 2014;46(10):877–884. doi: 10.1093/abbs/gmu080. [DOI] [PubMed] [Google Scholar]
- 110.Thongrakard V., Titone R., Follo C. Turmeric toxicity in A431 epidermoid cancer cells associates with autophagy degradation of anti-apoptotic and anti-autophagic p53 mutant. Phytother Res. 2014;28(12):1761–1769. doi: 10.1002/ptr.5196. [DOI] [PubMed] [Google Scholar]
- 111.Hiraki M., Hwang S.Y., Cao S. Small-molecule reactivation of mutant p53 to wild-type-like p53 through the p53-Hsp40 regulatory axis. Chem Biol. 2015;22(9):1206–1216. doi: 10.1016/j.chembiol.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nayak S.K., Khatik G.L., Narang R. p53-Mdm2 interaction inhibitors as novel nongenotoxic anticancer agents. Curr Cancer Drug Targets. 2017 doi: 10.2174/1568009617666170623111953. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 113.Wang S., Zhao Y., Aguilar A. Targeting the MDM2-p53 protein-protein interaction for new cancer therapy: progress and challenges. Cold Spring Harb Perspect Med. 2017;7(5) doi: 10.1101/cshperspect.a026245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tisato V., Voltan R., Gonelli A. MDM2/X inhibitors under clinical evaluation: perspectives for the management of hematological malignancies and pediatric cancer. J Hematol Oncol. 2017;10(1):133. doi: 10.1186/s13045-017-0500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]