Abstract
Ischemic stroke is a common disease with high mortality and morbidity worldwide. One of the important pathophysiological effects of ischemic stroke is apoptosis. A neuroprotective effect is defined as the inhibition of neuronal apoptosis to rescue or delay the infarction in the surviving ischemic penumbra. Resveratrol is a natural polyphenol that reportedly prevents cerebral ischemia injury by regulating the expression of PI3K/AKT/mTOR. Therefore, this study aimed to elucidate the neuroprotective effect of resveratrol on cerebral ischemia/reperfusion injury and to investigate the signaling pathways and mechanisms through which resveratrol regulates apoptosis in the ischemic penumbra. Rats were subjected to middle cerebral artery occlusion for 2 h followed by 24 h reperfusion. Cerebral infarct volume was measured using 2% TTC staining. TUNEL staining was conducted to evaluate neuronal apoptosis. Western blotting and immunohistochemistry were used to detect the proteins involved in the JAK2/STAT3/PI3K/AKT/mTOR pathway. The results suggested that resveratrol significantly improved neurological function, reduced cerebral infarct volume, decreased neuronal damage, and markedly attenuated neuronal apoptosis; these effects were attenuated by the inhibition of PI3K/AKT with LY294002 and JAK2/STAT3 with AG490. We also found that resveratrol significantly upregulated the expression of p-JAK2, p-STAT3, p-AKT, p-mTOR, and BCL-2 and downregulated expression of cleaved caspase-3 and BAX, which was partially reversed by LY294002 and AG490. These results suggested that resveratrol provides a neuroprotective effect against cerebral ischemia/reperfusion injury, which is partially mediated by the activation of JAK2/STAT3 and PI3K/AKT/mTOR. Resveratrol may indirectly upregulate the PI3K/AKT/mTOR pathway by activating JAK2/STAT3.
Keywords: AKT, Ischemic penumbra, mTOR, Resveratrol, STAT3, Stroke
Introduction
Stroke is a worldwide disease. Hemorrhagic strokes account for around 13% of strokes, and ischemic strokes account for around 87%.1 Despite extensive research and development work, there is no effective treatment for this widespread disorder.2 Cerebral ischemia leads to many injuries, including energy failure, intracellular calcium overload, and cell death (necrosis and apoptosis).3 In order to reduce these injuries, enough attention must be paid to apoptosis. The overall evidence suggests that anti-apoptotic factors are important for the protection of neurons from cerebral ischemia.4 Ischemic stroke core cells die within minutes, and cells in the surrounding area (ischemic penumbra) continue to die several hours or even several days after injury. The main form of cell death is apoptosis.5 Since the brain tissue damage in the ischemic penumbra develops more slowly, there is enough time for neuroprotective treatment.6 Thus, developing therapeutic agents that can inhibit neuronal apoptosis in the ischemic penumbra has become an important task in this field.
Resveratrol (3,4,5-trihydroxystilbene, Res), is a phenolic product found in Polygonum cuspidatum and also found abundantly in red wine and the skin of red grapes.7 It has been studied widely for its anti-apoptosis effects.3 Numerous studies have revealed that mitochondrial damage is a central step in stroke.8 Recent research shows that resveratrol can protect hippocampal neurons from damage caused by transient cerebral ischemia.9 However, the evidence revealing that resveratrol exerts neuroprotection in cerebral ischemia injury is not fully understood.
In recent years, some studies have shown that PI3K/AKT/mTOR signaling is an important pathway mediating cell survival and differentiation, proliferation, apoptosis, and metastasis.10 One study showed that the proliferation of hepatocellular carcinoma cells could be inhibited by downregulating the PI3K/AKT/mTOR pathway with certain anticancer drugs.11 Further evidence has shown that resveratrol-induced neuroprotection can be mediated through the activation of the PI3K/AKT signaling pathway, thereby leading to the prevention of neuronal death after brain ischemia in rats.12 Emerging evidence has also shown that blocking the PI3K/AKT/mTOR signaling pathway may be the key pathway for induction of apoptosis and inhibition of proliferation.13, 14
Studies have shown that the JAK/STAT signaling pathway can regulate the biological characteristics of cancer cells, such as proliferation, growth, differentiation, migration, and invasion.15 The JAK/STAT pathway is a major broad cytokine and growth factor signaling mechanism that mediates the constitutive JAK and STAT PI3K/AKT signal transduction reporter kinase.16, 17 AKT and STAT3 can induce the expression of Bcl-XL and the expression of BAX-binding molecule and inhibit the formation of BAX homodimers.18
In this study, we focused on investigating the mechanisms through which resveratrol exerts neuroprotection and identifying the relationship between JAK2/STAT3 and PI3K/AKT/mTOR. Our results suggested that resveratrol can induce the activation of JAK2/STAT3 and PI3K/AKT/mTOR, and resveratrol may indirectly upregulate the PI3K/AKT/mTOR pathway through the activation of JAK2/STAT3.
Methods and materials
Animals and study design
A total of 125 adult male Sprague–Dawley rats weighing 230–270 g (Experimental Animal Research Center, Chongqing Medical University, China) were used in this experiment. All of the animals were kept in a standard environment (25 ± 2 °C) with a 12:12 h light-dark cycle. Prior to operation, all rats were fasted for 12 h. The rats were randomly divided into five groups: the sham group (Sham, n = 25), the vehicle middle cerebral artery occlusion (MCAO) group (Veh, n = 25), the resveratrol MCAO group (Res, n = 25), the LY294002 (PI3K inhibitor) MCAO group (Res + LY294002, n = 25), and the AG490 (JAK2 inhibitor) MCAO group (Res + AG490, n = 25). The Sham group was subjected to the same operation steps, but the nylon filament was not inserted. Resveratrol (Solarbio, Beijing, China) was dissolved in 4% dimethyl sulfoxide (DMSO). Prior to MCAO surgery, the resveratrol, Res30 + LY294002, and Res30 + AG490 groups received an intraperitoneal injection of 30 mg/kg resveratrol once daily for 7 days and once again prior to operation. The vehicle group received the same volume of DMSO without resveratrol. The resveratrol and dose were chosen according to previous studies.19
Intracerebral ventricular injection
To Figureure out the role of the PI3K pathway following cerebral I/R, rats in the Res30 + LY294002 group were pretreated with LY294002 (Selleckchem, Houston, USA), an effective inhibitor of PI3K, as previously described.20 Prior to surgery, dimethyl sulfoxide (DMSO) and ethanol (ETOH) were used as solvents for LY294002, dissolved to a concentration of 20 mM. Animals were anesthetized (7% chloral hydrate, 350 mg/kg, IP) and fixed on a stereotaxic apparatus. The skull was exposed as follows: anteroposterior (AP), 0.8 mm posterior to bregma; mediolateral (ML), 1.4 mm away from midline on the right side; dorsoventral (DV), 3.6 mm deep into the skull surface. The preparation of LY294002 and the vehicle was performed by the same researcher who was responsible for the drug administration. At 30 min before surgery, intracerebroventricular injection of 5 μl LY294002 solution or vehicle (DMAO + ETOH) into the ischemic side was performed.21 We examined the effects of low (2 μl, 20 nM/ml), medium (4 μl, 20 nM/ml), and high (6 μl, 20 nM/ml) dosages of AG490 (JAK2 inhibitor; Selleckchem, Houston, USA) on cerebral I/R injury to identify the optimal dosage (6 μl, 20 nM/ml) for maximizing the inhibiting effects. The Res30 + AG490 group was subjected to the same procedure as the Res30 + LY294002 group.
Middle cerebral artery occlusion model
MCAO was used to induce ischemic brain injury in rats as described previously.22 In short, a 2 cm longitudinal incision was made on the right side of the neck, and the right internal carotid artery and external carotid artery were isolated and exposed. A standard 4–0 nylon filament with a heat-blunted tip was inserted into the internal carotid artery from the external carotid artery to block the middle cerebral artery for 2 h. Sham rats received the same operation except that the nylon filament was not inserted. After 2 h of ischemia, the rats were reperfused by removing the nylon filament. At 24 h after MCAO, a researcher blinded to the entire study assessed the extent of neurological deficits. Neurological deficit assessment was conducted using a 5-point system22: (0) no significant neurological deficits; (1) failure to completely extend the contralateral forepaws; (2) circling to the opposite side; (3) falling to the contralateral side; (4) unable to walk.
Infarct volume measurement
The infarct volume was assessed 24 h after MCAO. Brain tissue was removed and frozen at −20 °C for 30 min and then cut into 2 mm-thick coronal sections (6 slices) and incubated in 2% TTC (Sigma–Aldrich, Saint Louis, MO, USA) at 37 °C for 30 min. Each section was soaked in 4% paraformaldehyde for 24 h, and then a picture was taken. ImageJ software was used to analyze the infarct area.23
Hematoxylin and eosin (H&E) staining
All rats were treated with 4% paraformaldehyde (PFA), which was perfused through the heart. Their brains were then removed and post-fixed for 48 h. A cryostat vibratome (UltraPro 5000, USA) was used to cut brain sections coronally into a thickness of 15 μm. The sections were stained with H&E. Finally, a blinded investigator used a microscope to take images.24
Western blot analysis
Total protein from the ipsilateral side of the cerebral cortex was extracted using RIPA lysis buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS) with PMSF and a phosphatase inhibitor. A protein concentration assay kit (Beyotime Biotechnology, Beijing, China) was used to determine the protein content. SDS-PAGE was used to separate the protein. The anti-JAK2 (CST, MA, USA), anti-STAT3 (CST, MA, USA), anti-AKT (CST, MA, USA), anti-mTOR (CST, MA, USA), anti-phospho-JAK2 (Y1007 + 1008) (Abcam, CA, USA), anti-phospho-STAT3 (Tyr705) (CST, MA, USA), anti-phospho-AKT (Ser473) (CST, MA, USA), anti-phospho-mTOR (Ser2448) (CST, MA, USA), anti-BCL-2(CST, MA, USA), anti-BAX (CST, MA, USA), anti-cleaved caspase-3 (CST, MA, USA), and anti-GAPDH (CST, MA, USA) antibodies were incubated with the protein. The protein was visualized with SuperSignal West Dura Extended Duration Substrate (Thermo Fisher Scientific, MA, USA).3
Real-time quantitative PCR (RT-qPCR)
Total RNA was isolated using RNA Plus (Takara, Otsu, Shiga, Japan). Equal RNA samples (1000 ng) were reverse-transcribed to cDNA using the PrimeScript RT gDNA Eraser kit (Takara, Otsu, Shiga, Japan). Primer sets were as follows:
β-actin, forward: 5-AGATGTGGATCAGCAAGCA-3, reverse, 5-GCGCAAGTTAGGTTTTGTCA-3;
BCL-2, forward: 5- AGGATTGTGGCCTTCTTTGA-3, reverse: 5-CAGATGCCGGTTCAGGTACT-3;
BAX, forward: 5-GCTGGACACTGGACTTCCTC-3, reverse: 5-ACTCCAGCCACAAAGATGGT-3;
caspase-3, forward: 5-TGCCAGAAGATACCAGTGGA-3, reverse: 5-TGACTGGATGAACCATGACC-3.
The mRNA expression of BCL-2, BAX, and caspase-3 was normalized to the internal control, β-actin. Quantitative PCR was performed with SYBR Premix ExTaq TMII (Takara, Otsu, Shiga, Japan).
Immunohistochemistry
The fixed tissues were cut coronally into 5 μm-thick serial sections, and then immunohistochemistry was performed following the immunohistochemical kit (Boster Biological Technology, Wuhan, China).25 The BCL-2 (1:300; Cst, MA, USA), BAX (1:300; Cst, MA, USA), and cleaved caspase-3 (1:300; Cst, MA, USA) antibodies were used in this procedure. A blinded investigator used a microscope to take images and chose images randomly for each section. Analysis of BCL-2, BAX, and cleaved caspase-3 expression was conducted using Image-Pro Plus software.
TUNEL staining
TdT-mediated dUTP nick-end labeling (TUNEL) staining (Beyotime Biotechnology, Beijing, China)was performed to detect the apoptotic rate. Briefly, sections were permeabilized by proteinase K solution (20 μg/ml) at 37 °C for 30 min, then washed with PBS three times for 10 min each time. Then, the terminal deoxynucleotidyl transferase (TdT) and fluorescein were added to the section and incubated in a humidified box at 37 °C for 1 h. A blinded investigator used a microscope to take images and chose them randomly for each section.26 Image-Pro Plus 6.0 was used to quantify the number of TUNEL-positive neurons.
Data analysis and statistics
GraphPad Prism 5.0 and SPSS 19.0 were used to analyze all the data. All data are shown as mean ± SEM. One-way analysis of variance (ANOVA) was used for multiple comparison of the vehicle and treatment groups. A P value less than 0.05 was considered statistically significant.
Results
Resveratrol provides neuroprotection in MCAO rats
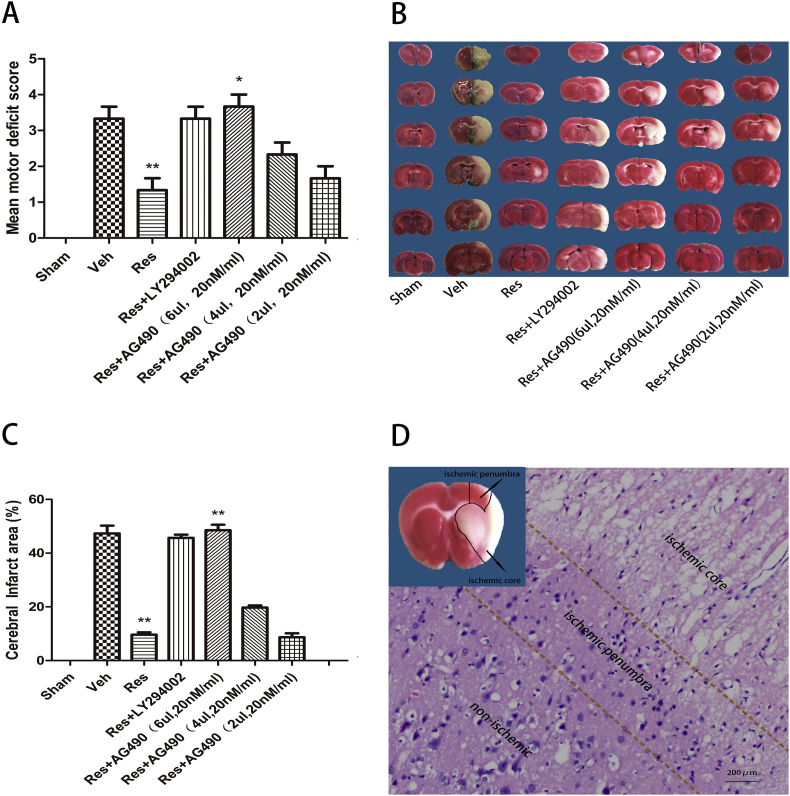
After 24 h of cerebral I/R, the neurological deficit scores and ischemia infarct areas were evaluated (Fig. 1). The sham group had lower neurological deficit scores, while the vehicle group had higher neurological deficit scores (Fig. 1A). The average neurological deficit score for both the vehicle group and the Res + LY294002 group was 3.33, which was much greater than the average of 1.33 in the resveratrol group (Fig. 1A; **P < 0.01, Veh and Res + LY294002 vs. Res). The average neurological deficit score in the Res + AG490 group was 3.67, which was higher compared with the resveratrol group and decreased with decreased dosage (Fig. 1A; **P < 0.01; Res + AG490 [6 μl, 20 nM/ml] vs. Res). TTC staining indicated that the cerebral infarct volumes in the vehicle group and Res + LY294002 group were 47.33% and 45.67%, respectively, which was higher compared with the resveratrol group (Fig. 1B,C; **P < 0.01; Veh and Res + LY294002 vs. Res). The average cerebral infarct volume in the Res + AG490 group was 48.5%, which was greater compared with the resveratrol group and decreased with decreased dosage (Fig. 1B,C; **P < 0.01; Res + AG490 [6 μl, 20 nM/ml] vs. Res). Since a significant inhibiting effect was observed with AG490 at 6 μl, 20 nM/ml, this dose was used in the rest of the study. In the non-ischemia area, H&E revealed that the cells were regular in shape and abundant in cytoplasm and had an intact cell nucleus. In the ischemic core region, the cell membrane was shrunken, and the nucleus was condensed and fragmented. In the ischemic penumbra zone, the cells had a regular shape, and a few cells were degenerated and necrotic compared with the ischemic core region. Taken together, these findings indicated that resveratrol may exert a neuroprotective effect in MCAO rats.
Figure 1.
Resveratrol exerts a neuroprotective effect 24 h after I/R. (A) The neurological deficit score in the resveratrol group was significantly decreased compared with the vehicle group. However, it was significantly increased after administration of 6 μl (20 nM/ml) AG490 in the Res + AG490 group compared with the Res group. (B–C) Infarct volumes in the resveratrol group were significantly decreased compared with the vehicle group. The infarct volumes were significantly increased after administration of 6 μl (20 nM/ml) AG490 in the Res + AG490 group compared with the Res group. Red represents normal tissue and white represents infarct tissue. (D) Morphology and structure of brain cells in Veh group rats. Data are presented as mean ± SEM. (*P < 0.05 and **P < 0.01; n = 5 in each group).
Resveratrol mediated the expression of proteins in the PI3K/AKT/mTOR pathway
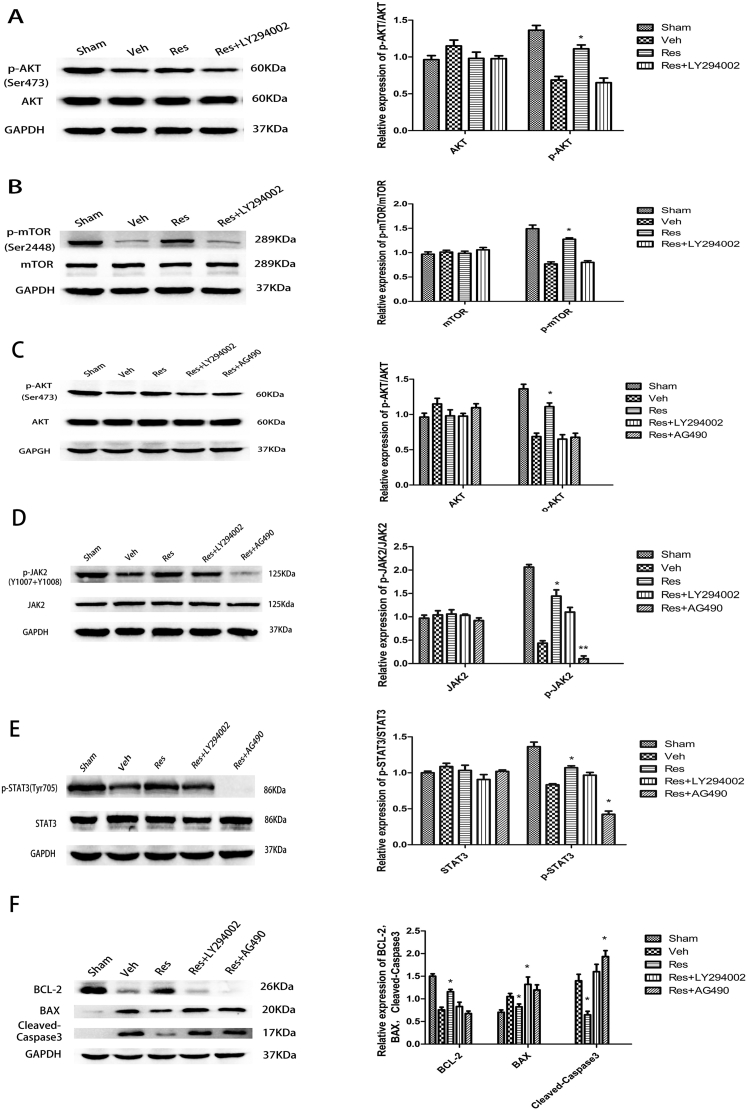
In order to investigate whether the PI3K/AKT/mTOR pathway is involved in the neuroprotective effects of resveratrol, the protein expression involved in the PI3K/AKT/mTOR pathway was carefully tested by Western blots (Fig. 2A,B). The results showed that the protein expression of p-AKT (Ser473) and p-mTOR (Ser2448) in the resveratrol group was increased 1.66-fold compared to the Veh group after 24 h of cerebral I/R (Fig. 2A,B; *P < 0.05; Res vs. Veh). In addition, LY294002 (PI3K inhibitor) significantly attenuated resveratrol-induced protein expression of p-AKT (Ser473) and p-mTOR (Ser2448) to 0.52% and 0.63%, respectively (Fig. 2A,B; *P < 0.05; Res + LY294002 vs. Res). The total protein expression of AKT and mTOR remained the same. These observations supported the hypothesis that resveratrol could provide a neuroprotective effect through activating the PI3K/AKT/mTOR pathway.
Figure 2.
Effects of resveratrol on protein expression of JAK2, STAT3, AKT, mTOR, p-JAK2, p-STAT3, p-AKT, p-mTOR, BCL-2, BAX, and cleaved caspase-3 at 24 h after reperfusion. (A) The protein expression of p-AKT was increased 1.66-fold in the resveratrol group compared to the vehicle group, which was partially reversed by LY294002 (PI3K inhibitor). (B) The protein expression of p-mTOR was increased 1.66-fold in the resveratrol group compared to the vehicle group, which was also partially reversed by LY294002. (C) JAK2 inhibitor AG490 significantly decreased the protein expression of p-AKT compared with the resveratrol group. (D) The effect of PI3K inhibitor LY294002 on p-JAK2 after resveratrol treatment was not significant. (E) JAK2 inhibitor AG490 significantly decreased the protein expression of p-STAT3 compared with the resveratrol group. (F) Western blot indicated significantly higher BLC-2 and lower BAX and cleaved caspase-3 in the resveratrol group than in the vehicle group. These effects were partially reversed by LY294002 and AG490. Data are presented as mean ± SEM. (*P < 0.05; n = 5 in each group).
The relationship between JAK2/STAT3 and PI3K/AKT/mTOR in the resveratrol-mediated signaling pathway
Western blotting indicated that the protein expression of p-JAK2 (Y1007 + Y1008) and p-STAT3 (Tyr705) in the resveratrol group increased 3.35-fold (Fig. 2D; **P < 0.01; Res vs. Veh) and 1.29-fold (Fig. 2E; *P < 0.05; Res vs. Veh), respectively, compared to the Veh group at 24 h after reperfusion. AG490 (JAK2 inhibitor) significantly attenuated resveratrol-induced p-JAK2 (Y1007 + Y1008), p-STAT3 (Tyr705), and p-AKT (Ser473) protein expression to 7% (Fig. 2D; **P < 0.01; Res + AG490 vs. Res), 33% (Fig. 2E; *P < 0.05; Res + AG490 vs. Res), and 61% (Fig. 2C; *P < 0.05; Res + AG490 vs. Res), respectively. The effect of LY294002 (PI3K inhibitor) on p-JAK2 and p-STAT3 was not significant (Fig. 2D,E; P > 0.05), and the total protein expression of JAK2 and STAT3 remained unchanged. These observations supported the hypothesis that resveratrol could exert neuroprotective effects through activating the JAK2/STAT3 pathway, and resveratrol might indirectly upregulate the PI3K/AKT/mTOR pathway by activating JAK2/STAT3.
Resveratrol mediated the protein and mRNA expression of BCL-2, BAX, and cleaved caspase-3 through JAK2/STAT3/PI3K/AKT/mTOR
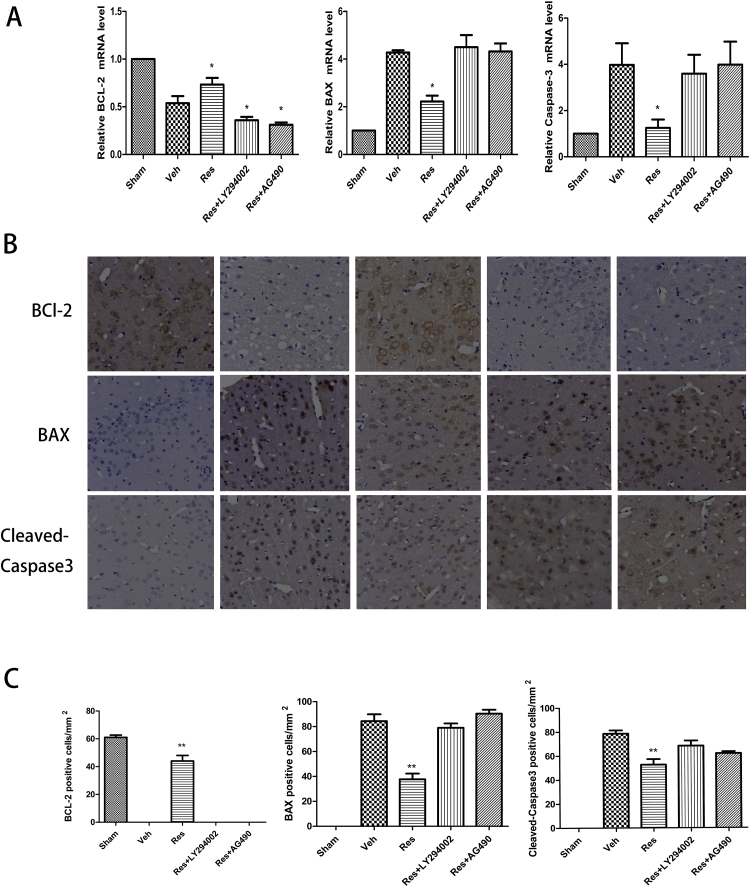
Using Western blotting, we found that the expression levels of BCL-2 in the resveratrol group increased 1.53-fold (Fig. 2F; *P < 0.05; Res vs. Veh) compared to the Veh group after 24 h of cerebral I/R, which was partially reversed by LY294002 and AG490. The BAX and cleaved caspase-3 expression in the resveratrol group decreased significantly to 78% (Fig. 2F; *P < 0.05; Res vs. Veh) and 46% (Fig. 2F; *P < 0.05; Res vs. Veh), respectively, compared to the Veh group, which was partially reversed by LY294002 and AG490. In the Res + LY294002 group, BAX increased significantly compared to the Veh group and Res + AG490 group (Fig. 2F; *P < 0.05; Res + LY294002 vs. Veh and Res + AG490). In the Res + AG490 group, cleaved caspase-3 increased significantly compared to the Veh group and Res + LY294002 group (Fig. 2F; *P < 0.05; Res + AG490 vs. Veh and Res + AG490). RT-qPCR analysis showed that BCL-2 levels were higher in the Res group compared to the Veh group. However, the BAX and cleaved caspase-3 levels were lower in the Res group compared to the Veh group, which was partially reversed by LY294002 and AG490, and the levels of BCL-2 were significantly lower in the Res + LY294002 group and Res + AG490 group compared to the Veh group (Fig. 3A). These results showed that resveratrol could inhibit cell apoptosis by downregulating the expression of pro-apoptotic BAX and cleaved caspase-3 and upregulating the expression of anti-apoptotic BCL-2 protein.
Figure 3.
Effects of resveratrol on mRNA and protein expression of BCL-2, BAX, and cleaved caspase-3 after 24 h of cerebral I/R. (A) RT-qPCR analysis showed significantly higher BLC-2 and lower BAX and cleaved caspase-3 in the resveratrol group than in the vehicle group. These effects were partially reversed by LY294002 and AG490. (B) Typical immunohistochemical photographs of BCL-2, BAX, and caspase-3. (C) Immunohistochemical staining showed more BLC-2-positive and fewer BAX- and cleaved caspase-3-positive cells in the resveratrol group compared to the vehicle group. These effects were partially reversed by LY294002 and AG490. Data are presented as the mean ± SEM. (*P < 0.05; n = 5 in each group).
Resveratrol treatment regulates the expression of apoptosis-related proteins
To clarify the effect of resveratrol on neuronal apoptosis in the ischemic penumbra, we detected the BCL-2, BAX, and cleaved caspase-3 protein expression in the ischemic penumbra after 24 h of cerebral I/R. Immunohistochemistry results indicated that BCL-2 protein expression was significantly increased, while the protein expression of BAX and cleaved caspase-3 was decreased in the resveratrol group compared to the Veh group (Fig. 3B,C; **P < 0.01; Res vs. Veh). These effects were partially reversed by LY294002 and AG490.
Resveratrol treatment decreases neuronal apoptosis in the ischemic penumbra
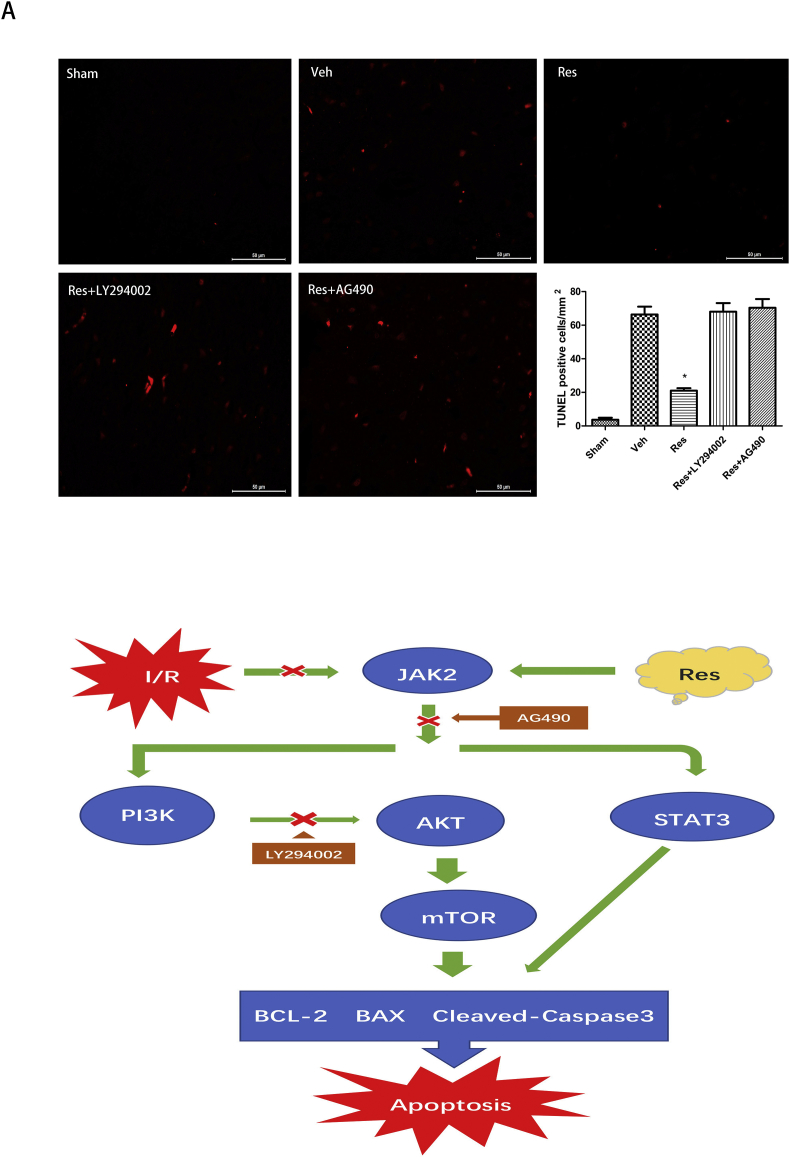
In order to observe the apoptosis of neurons in the ischemic penumbra, TUNEL was conducted after 24 h of cerebral I/R. There were many TUNEL-positive neurons in the ischemic penumbra of ischemic rats in the Veh group and fewer TUNEL-positive neurons in the sham group. There were fewer TUNEL-positive neurons in the resveratrol group than in the vehicle group (Fig. 4A; *P < 0.05; Res vs. Veh), which was partially reversed by LY294002 and AG490. These findings supported the hypothesis that resveratrol inhibited neuron apoptosis by upregulating the JAK2/STAT3/PI3K/AKT/mTOR pathway after stroke in rats.
Figure 4.
Effects of resveratrol on inhibition of neuronal apoptosis in the ischemic penumbra after 24 h of cerebral I/R and the mode of action for resveratrol's neuroprotection role in ischemic stroke. (A) TUNEL results showed that the TUNEL-positive cells in the resveratrol group were decreased compared to the vehicle group. These effects were partially reversed by LY294002 and AG490. Data are presented as mean ± SD (*P < 0.05; n = 5 in each group). (B) The diagram of the JAK2/STAT3 and PI3K/AKT signaling pathway, which was involved in the neuroprotective effect of resveratrol against I/R injury. Resveratrol activated the JAK2/STAT3 and then activated the PI3K/AKT/mTOR signaling pathway, which in turn led to increased BLC-2 and decreased BAX and cleaved caspase-3, resulting in inhibition of apoptosis; this was reversed by the PI3K inhibitor LY294002 and JAK2 inhibitor AG490. However, reperfusion after ischemic stroke prevented these effects to induce apoptosis.
Discussion
In the present study, we investigated resveratrol-induced neuroprotection, which attenuates neuronal apoptosis and ischemia/reperfusion injury, as well as its mechanisms and signaling pathways. Our results suggested that resveratrol-induced neuroprotection was partially due to inhibition of neuronal apoptosis in the ischemic penumbra. In addition, we identified the optimal dosage (6 μl, 20 nM/ml) for maximizing the inhibiting effects. The resveratrol-mediated relationship between the PI3K/AKT and JAK2/STAT3 signaling pathways was also investigated; we found that both JAK2/STAT3 and PI3K/AKT/mTOR increased BCL-2 expression but decreased BAX and cleaved caspase-3 expression in the ischemia/reperfusion. We believe that resveratrol-induced neuroprotection can be attributed to the upregulation of the PI3K/AKT/mTOR pathway by activating JAK2/STAT3 in the ischemic penumbra, thereby conferring cerebral ischemic tolerance.
In this study, resveratrol, administered on seven consecutive days before MCAO, significantly improved neurological function after 24 h of cerebral I/R. The mechanism through which resveratrol provides its neuroprotection through neuronal anti-apoptosis in cerebral I/R injury requires further exploration. Ischemic stroke-caused cerebral damage can be classified into necrotic cell death and penumbral cell death. The definition of the ischemic penumbra was based on animal experiments showing dysfunction and electrophysiological disorders, with reduced blood flow to the brain below a specific limit (functional threshold) and irreversible tissue damage with further-reduced blood supply (infarctional threshold). The perfusion range between these thresholds was called the “penumbra”.27 In the penumbra area, the limited blood supply conserves the energy metabolism. It has been reported that ischemic penumbra damage is reversible, whereas necrosis of the ischemic core is irreversible. Several studies have revealed that apoptosis is activated in the penumbra.28 Consistent with previous studies, our results indicated that I/R increased the numbers of TUNEL-positive cells and BAX-positive cells after 24 h of cerebral I/R in the penumbra (Fig. 4A), which partially indicated that apoptosis was activated in the penumbra. Furthermore, our results suggested that resveratrol can downregulate caspase-3 and BAX expression and decrease the number of TUNEL-positive cells, which indicates that resveratrol provides neuroprotection by inhibiting apoptosis in the penumbra area.
The PI3K/AKT pathway has been extensively studied for its neuroprotective effect in cerebral ischemia. Numerous studies have suggested that AKT activation plays an essential role in neuronal survival after cerebral I/R injury.29 In other studies, the neuroprotection of resveratrol was partially attributed to the activation of PI3K/AKT in cerebral I/R injury. Research has demonstrated that the investigated combined anti-apoptotic effects occur through the PI3K/AKT/caspase-3 pathway of resveratrol.30 Studies have also reported that polydatin can inhibit the proliferation of HeLa cells and induce apoptosis, and the PI3K/AKT/mTOR signaling pathway is involved in this process.31 It has been suggested that the proteins caspase, BCL-2, and BAX play key roles in the apoptotic process.17 Numerous studies have revealed that intrinsic or extrinsic pathways can induce apoptosis. Caspase is only activated when caspase is cleaved and initiator caspases, such as caspase-3, are activated.10 The BCL-2 family balances the mitochondrial potential of anti-apoptotic proteins (BCL-xL, BCL-2) and proapoptotic proteins (BAX) between protein upregulation and downregulation, determining whether cells undergo apoptosis or survive.32 Therefore, we tried to investigate the PI3K/AKT/mTOR pathway and cleaved caspase-3, BAX, and BCL-2, and further identified the underlying mechanisms.21
The PI3K/AKT/mTOR signaling pathway has many subtypes of each molecule, each of which has a different mode of action, as well as many phosphorylation sites, which control the anti-apoptosis properties of cells.33, 34, 35 To clarify whether the PI3K/AKT/mTOR pathway is involved, in this study, we chose LY294002 to inhibit the function of PI3K. Evaluation of the apoptosis, neuronal injury, and the cerebral infarct area suggested that PI3K is involved in the neuroprotection of resveratrol in cerebral I/R. Furthermore, Western blot results suggested that the p-AKT and p-mTOR protein expression was reduced in the vehicle group after MCAO, while resveratrol increased p-AKT and p-mTOR protein expression after 24 h of cerebral I/R (Fig. 2A,B). Western blot showed significantly higher BLC-2 and lower BAX and cleaved caspase-3 in the resveratrol group than in the vehicle group (Fig. 2F). These effects were largely reversed by LY294002. These findings suggested that the PI3K/AKT/mTOR pathway plays an important role in the neuroprotective and anti-apoptotic effects of resveratrol on cerebral I/R injury. Furthermore, our findings indicated that resveratrol could inhibit cell apoptosis by downregulating the BAX and cleaved caspase-3 proteins and upregulating the BCL-2 protein through activation of the PI3K/AKT/m-TOR pathway.
STAT proteins were also involved in cell apoptosis, proliferation, and differentiation.36 Specifically, JAK2 is important for cytokine receptor signaling. Upon activation, JAK2 kinase phosphorylates STAT3. Once activated, it then phosphorylates Y705.37, 38 Inhibiting the phosphorylation of STAT3 itself or blocking the upstream activators of STAT3 has been considered as a potential anticancer strategy. Many natural products have been widely reported to inhibit the activity of STAT3 and induce apoptosis in various tumor cell lines.37 In particular, there is growing evidence that salvianolic acid (Sal) significantly increases the phosphorylation of JAK2 and STAT3, and inhibition of JAK2 completely eliminates the beneficial effect of Sal on brain function recovery.39 The neuroprotective effect of curcumin is also associated with JAK2/STAT3 signaling activation. In one study, the administration of AG490 abolished the protective effect of curcumin, indicating that the phosphorylation of JAK2/STAT3 activation by curcumin and the neuroprotective signaling pathway are closely related.40
Lost regulation of apoptosis is involved in cancer development. The JAK/STAT and PI3K/AKT/mTOR signaling pathways is involved in cell apoptosis.17 However, how resveratrol regulates the JAK2/STAT3 and PI3K/AKT/mTOR signaling pathway and the molecular mechanisms contributing to cerebral I/R apoptosis are unknown. In this study, we found that p-mTOR and p-JAK2, p-STAT3, and p-AKT protein expression was increased, whereas the expression of total mTOR and JAK2/STAT3/AKT was not changed significantly (Fig. 2A–E). This suggested that resveratrol could activate the phosphorylation of key proteins in these pathways. Our findings also suggested that resveratrol inhibits apoptosis in cerebral I/R via the concomitant upregulation of the JAK2/STAT3 and PI3K/AKT/mTOR pathways, since AG490 significantly attenuated resveratrol-induced p-JAK2, p-STAT3, and p-AKT protein expression. However, the effect of LY294002 on JAK2 and STAT3 phosphorylation levels was not significant (Fig. 2C–E), which was consistent with previous research indicating that JAK2 plays an important role in mediating the activation of constitutive STAT3 and PI3K/AKT/mTOR signaling. The findings of the present study lead us to conclude that the resveratrol-mediated upregulation of BCL-2 expression, downregulation of BAX and cleaved caspase-3 expression, and deregulation of downstream gene (BCL-2 family, caspase family) expression contribute to anti-apoptosis progression in cerebral I/R. These processes are mediated by the activation of the JAK2/STAT3/PI3K/AKT/mTOR pathway, and resveratrol may indirectly upregulate the PI3K/AKT/mTOR pathway through the activation of JAK2/STAT3.
In many cancer tissues and cells, overactivation of the epidermal growth factor receptor (EGFR) can reduce apoptosis and promote cell proliferation. In gastric cancer cells, PKGII plays a pro-apoptotic role through inhibition of the EGF/EGFR-induced PI3K/AKT signaling pathway.41 EGF/EGFR signaling also initiates signal transduction of the JAK/STAT-mediated pathway.42 Afatinib combined with dasatinib can exert a pro-apoptotic effect in cancer cells by blocking EGFRs and their downstream signaling pathways, including PI3K/AKT and JAK/STAT.43 In non-small cell lung cancer (NSCLC), EGFR mutations promote proliferation, inhibit apoptosis and migration, and then promote tumor progression by activating the PI3K/AKT and JAK/STAT signaling pathways.44 Therefore, EGFR and the JAK/STAT and PI3K/Akt signaling pathways are closely related. Whether resveratrol exerts a neuroprotective effect through upregulation of EGFR and the JAK/STAT and PI3K/Akt signaling pathways requires further research.
In conclusion, the findings of this study suggest that resveratrol provides neuroprotective effects against cerebral I/R injury, which is partially mediated by activation of the JAK2/STAT3/PI3K/AKT/mTOR pathway. In addition, resveratrol may indirectly upregulate the PI3K/AKT/mTOR pathway by activating JAK2/STAT3. Our findings regarding the resveratrol-mediated molecular mechanisms and signaling pathways provides a new insight into and therapeutic target for cerebral ischemia. Future studies should focus on other possible relationships between JAK2/STAT3 and PI3K/AKT/mTOR in the action of resveratrol in cerebral ischemia.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgement
This project was supported by the Foundation for Science and Technology Research Project of Chongqing (cstc2012ggB1002).
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Gouriou Y., Demaurex N., Bijlenga P., De Marchi U. Mitochondrial calcium handling during ischemia-induced cell death in neurons. Biochimie. Dec 2011;93(12):2060–2067. doi: 10.1016/j.biochi.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Zhu H., Zou L., Tian J., Du G., Gao Y. SMND-309, a novel derivative of salvianolic acid B, protects rat brains ischemia and reperfusion injury by targeting the JAK2/STAT3 pathway. Eur J Pharmacol. Aug 15 2013;714(1–3):23–31. doi: 10.1016/j.ejphar.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 3.Wan D., Zhou Y., Wang K., Hou Y., Hou R., Ye X. Resveratrol provides neuroprotection by inhibiting phosphodiesterases and regulating the cAMP/AMPK/SIRT1 pathway after stroke in rats. Brain Res Bull. Mar 2016;121:255–262. doi: 10.1016/j.brainresbull.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Liu X., Zhang X., Zhang J. Diosmin protects against cerebral ischemia/reperfusion injury through activating JAK2/STAT3 signal pathway in mice. Neuroscience. May 30 2014;268:318–327. doi: 10.1016/j.neuroscience.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 5.Deng Y.H., He H.Y., Yang L.Q., Zhang P.Y. Dynamic changes in neuronal autophagy and apoptosis in the ischemic penumbra following permanent ischemic stroke. Neural Regen Res. Jul 2016;11(7):1108–1114. doi: 10.4103/1673-5374.187045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demyanenko S., Uzdensky A. Profiling of signaling proteins in penumbra after focal photothrombotic infarct in the rat brain cortex. Mol Neurobiol. Oct 22 2016 doi: 10.1007/s12035-016-0191-x. [DOI] [PubMed] [Google Scholar]
- 7.Liu S., Li T., Liu H. Resveratrol exerts antidepressant properties in the chronic unpredictable mild stress model through the regulation of oxidative stress and mTOR pathway in the rat hippocampus and prefrontal cortex. Behav Brain Res. Apr 01 2016;302:191–199. doi: 10.1016/j.bbr.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 8.Wang R., Liu Y.Y., Liu X.Y. Resveratrol protects neurons and the myocardium by reducing oxidative stress and ameliorating mitochondria damage in a cerebral ischemia rat model. Cell Physiol Biochem. 2014;34(3):854–864. doi: 10.1159/000366304. [DOI] [PubMed] [Google Scholar]
- 9.Hong J.H., Lee H., Lee S.R. Protective effect of resveratrol against neuronal damage following transient global cerebral ischemia in mice. J Nutr Biochem. Jan 2016;27:146–152. doi: 10.1016/j.jnutbio.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y.J., Wong B.S., Yea S.H., Lu C.I., Weng S.H. Sinularin induces apoptosis through mitochondria dysfunction and inactivation of the pI3K/Akt/mTOR pathway in gastric carcinoma cells. Mar Drugs. Jul 27 2016;14(8) doi: 10.3390/md14080142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou F., Li Y.H., Wang J.J., Pan J., Lu H. Endoplasmic reticulum stress could induce autophagy and apoptosis and enhance chemotherapy sensitivity in human esophageal cancer EC9706 cells by mediating PI3K/Akt/mTOR signaling pathway. Tumour Biol. Jun 2017;39(6) doi: 10.1177/1010428317705748. 1010428317705748. [DOI] [PubMed] [Google Scholar]
- 12.Simao F., Matte A., Pagnussat A.S., Netto C.A., Salbego C.G. Resveratrol prevents CA1 neurons against ischemic injury by parallel modulation of both GSK-3beta and CREB through PI3-K/Akt pathways. Eur J Neurosci. Oct 2012;36(7):2899–2905. doi: 10.1111/j.1460-9568.2012.08229.x. [DOI] [PubMed] [Google Scholar]
- 13.Wang L., Wang C., Jia Y., Liu Z., Shu X., Liu K. Resveratrol increases anti-proliferative activity of Bestatin through downregulating P-Glycoprotein expression via inhibiting PI3K/Akt/mTOR pathway in K562/ADR cells. J Cell Biochem. May 2016;117(5):1233–1239. doi: 10.1002/jcb.25407. [DOI] [PubMed] [Google Scholar]
- 14.Sui T., Ma L., Bai X., Li Q., Xu X. Resveratrol inhibits the phosphatidylinositide 3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway in the human chronic myeloid leukemia K562 cell line. Oncol Lett. Jun 2014;7(6):2093–2098. doi: 10.3892/ol.2014.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouyang J., Pan X., Lin H., Hu Z., Xiao P., Hu H. GKN2 increases apoptosis, reduces the proliferation and invasion ability of gastric cancer cells through down-regulating the JAK/STAT signaling pathway. Am J Transl Res. 2017;9(2):803–811. [PMC free article] [PubMed] [Google Scholar]
- 16.Xu W., Chen G.S., Shao Y. Gastrin acting on the cholecystokinin2 receptor induces cyclooxygenase-2 expression through JAK2/STAT3/PI3K/Akt pathway in human gastric cancer cells. Cancer Lett. May 10 2013;332(1):11–18. doi: 10.1016/j.canlet.2012.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Ke F., Wang Z., Song X. Cryptotanshinone induces cell cycle arrest and apoptosis through the JAK2/STAT3 and PI3K/Akt/NFkappaB pathways in cholangiocarcinoma cells. Drug Des Devel Ther. 2017;11:1753–1766. doi: 10.2147/DDDT.S132488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Y., Zhou J., Xu C. JAK/STAT and PI3K/AKT pathways form a mutual transactivation loop and afford resistance to oxidative stress-induced apoptosis in cardiomyocytes. Cell Physiol Biochem. 2008;21(4):305–314. doi: 10.1159/000129389. [DOI] [PubMed] [Google Scholar]
- 19.Simao F., Matte A., Breier A.C. Resveratrol prevents global cerebral ischemia-induced decrease in lipid content. Neurol Res. Jan 2013;35(1):59–64. doi: 10.1179/1743132812Y.0000000116. [DOI] [PubMed] [Google Scholar]
- 20.Gao G.S., Li Y., Zhai H. Humanin analogue, S14G-humanin, has neuroprotective effects against oxygen glucose deprivation/reoxygenation by reactivating Jak2/Stat3 signaling through the PI3K/AKT pathway. Exp Ther Med. Oct 2017;14(4):3926–3934. doi: 10.3892/etm.2017.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Z.H., Cai M., Xiang J. PI3K/Akt pathway contributes to neuroprotective effect of Tongxinluo against focal cerebral ischemia and reperfusion injury in rats. J Ethnopharmacol. Apr 02 2016;181:8–19. doi: 10.1016/j.jep.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 22.Longa E.Z., Weinstein P.R., Carlson S., Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. Jan 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 23.Aspey B.S., Taylor F.L., Terruli M., Harrison M.J. Temporary middle cerebral artery occlusion in the rat: consistent protocol for a model of stroke and reperfusion. Neuropathol Appl Neurobiol. Jun 2000;26(3):232–242. doi: 10.1046/j.1365-2990.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang R., Li J., Duan Y., Tao Z., Zhao H., Luo Y. Effects of erythropoietin on gliogenesis during cerebral ischemic/reperfusion recovery in adult mice. Aging Dis. Jul 2017;8(4):410–419. doi: 10.14336/AD.2016.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhenye L., Chuzhong L., Youtu W. The expression of TGF-beta1, Smad3, phospho-Smad3 and Smad7 is correlated with the development and invasion of nonfunctioning pituitary adenomas. J Transl Med. Mar 18 2014;12:71. doi: 10.1186/1479-5876-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y., Liang G., Wang S., Meng Q., Wang Q., Wei H. Effects of fetal exposure to isoflurane on postnatal memory and learning in rats. Neuropharmacology. Dec 2007;53(8):942–950. doi: 10.1016/j.neuropharm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heiss W.D., Zaro Weber O. Validation of MRI determination of the penumbra by PET measurements in ischemic stroke. J Nucl Med. Feb 2017;58(2):187–193. doi: 10.2967/jnumed.116.185975. [DOI] [PubMed] [Google Scholar]
- 28.Zhao G., Zhang W., Li L., Wu S., Du G. Pinocembrin protects the brain against ischemia-reperfusion injury and reverses the autophagy dysfunction in the penumbra area. Molecules. Sep 30 2014;19(10):15786–15798. doi: 10.3390/molecules191015786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu X., Chua C.C., Gao J. Neuroprotective effect of humanin on cerebral ischemia/reperfusion injury is mediated by a PI3K/Akt pathway. Brain Res. Aug 28 2008;1227:12–18. doi: 10.1016/j.brainres.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang S.D., Ma L., Yang D.L., Ding W.Y. Combined effect of 17beta-estradiol and resveratrol against apoptosis induced by interleukin-1beta in rat nucleus pulposus cells via PI3K/Akt/caspase-3 pathway. PeerJ. 2016;4:e1640. doi: 10.7717/peerj.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan J.H., Wang H.B., Du X.F., Liu J.Y., Zhang D.J. Polydatin induces human cervical cancer cell apoptosis via PI3K/AKT/mTOR signaling pathway. Zhongguo Zhong Yao Za Zhi. Jun 2017;42(12):2345–2349. doi: 10.19540/j.cnki.cjcmm.2017.0111. [DOI] [PubMed] [Google Scholar]
- 32.Lin K.H., Kuo W.W., Jiang A.Z. Tetramethylpyrazine Ameliorated hypoxia-induced myocardial cell apoptosis via HIF-1alpha/JNK/p38 and IGFBP3/BNIP3 inhibition to upregulate PI3K/Akt survival signaling. Cell Physiol Biochem. 2015;36(1):334–344. doi: 10.1159/000374076. [DOI] [PubMed] [Google Scholar]
- 33.Zhao L., Vogt P.K. Class I PI3K in oncogenic cellular transformation. Oncogene. Sep 18 2008;27(41):5486–5496. doi: 10.1038/onc.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janku F. Phosphoinositide 3-kinase (PI3K) pathway inhibitors in solid tumors: from laboratory to patients. Cancer Treat Rev. Sep 2017;59:93–101. doi: 10.1016/j.ctrv.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Paplomata E., O'Regan R. The PI3K/AKT/mTOR pathway in breast cancer: targets, trials and biomarkers. Ther Adv Med Oncol. Jul 2014;6(4):154–166. doi: 10.1177/1758834014530023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahn K.S., Sethi G., Sung B., Goel A., Ralhan R., Aggarwal B.B. Guggulsterone, a farnesoid X receptor antagonist, inhibits constitutive and inducible STAT3 activation through induction of a protein tyrosine phosphatase SHP-1. Cancer Res. Jun 01 2008;68(11):4406–4415. doi: 10.1158/0008-5472.CAN-07-6696. [DOI] [PubMed] [Google Scholar]
- 37.Wu K.J., Huang J.M., Zhong H.J. A natural product-like JAK2/STAT3 inhibitor induces apoptosis of malignant melanoma cells. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0177123. e0177123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim M.J., Nam H.J., Kim H.P. OPB-31121, a novel small molecular inhibitor, disrupts the JAK2/STAT3 pathway and exhibits an antitumor activity in gastric cancer cells. Cancer Lett. Jul 10 2013;335(1):145–152. doi: 10.1016/j.canlet.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Li Y., Zhang X., Cui L. Salvianolic acids enhance cerebral angiogenesis and neurological recovery by activating JAK2/STAT3 signaling pathway after ischemic stroke in mice. J Neurochem. Oct 2017;143(1):87–99. doi: 10.1111/jnc.14140. [DOI] [PubMed] [Google Scholar]
- 40.Li L., Li H., Li M. Curcumin protects against cerebral ischemia-reperfusion injury by activating JAK2/STAT3 signaling pathway in rats. Int J Clin Exp Med. 2015;8(9):14985–14991. [PMC free article] [PubMed] [Google Scholar]
- 41.Wu M., Chen Y., Jiang L. Type II cGMP-dependent protein kinase inhibits epidermal growth factor-induced phosphatidylinositol-3-kinase/Akt signal transduction in gastric cancer cells. Oncol Lett. Dec 2013;6(6):1723–1728. doi: 10.3892/ol.2013.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu M., Wu Y., Lan T., Jiang L., Qian H., Chen Y. Type II cGMPdependent protein kinase inhibits EGFinduced JAK/STAT signaling in gastric cancer cells. Mol Med Rep. Aug 2016;14(2):1849–1856. doi: 10.3892/mmr.2016.5452. [DOI] [PubMed] [Google Scholar]
- 43.Wang M., Yuang-Chi Chang A. Molecular mechanism of action and potential biomarkers of growth inhibition of synergistic combination of afatinib and dasatinib against gefitinib-resistant non-small cell lung cancer cells. Oncotarget. Mar 27 2018;9(23):16533–16546. doi: 10.18632/oncotarget.24814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baumgartner U., Berger F., Hashemi Gheinani A., Burgener S.S., Monastyrskaya K., Vassella E. miR-19b enhances proliferation and apoptosis resistance via the EGFR signaling pathway by targeting PP2A and BIM in non-small cell lung cancer. Mol Cancer. Feb 19 2018;17(1):44. doi: 10.1186/s12943-018-0781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]