Abstract
Mutation profiles of advanced radioactive iodine (RAI)-refractory differentiated thyroid cancer have revealed the pathogenic roles of the established oncogenic mutations of BRAF and PI3KCA, but the involvement of other genes is presently unknown. In the present study, we performed whole-exome sequencing on 10 tissue samples of metastases of RAI-refractory differentiated thyroid cancers and identified a recurrent hot-spot mutation (c.1924G>T) in the RasGRP3 gene, which codes for Ras guanine nucleotide-releasing protein 3. This mutation was found to occur at a high frequency (20%) in samples of metastases of RAI-refractory differentiated thyroid cancers compared with other types of thyroid cancer. Overexpression of mutant RasGRP3 significantly promoted cell proliferation, migration, and invasiveness of 8505C and BHT101 cells compared with cells transfected with wild-type RasGRP3 or an empty vector. In addition, mutant RasGRP3 decreased the expression of sodium iodide symporter (NIS) and thyroid-stimulating hormone receptor (TSHR), reduced the iodine uptake ability, and increased Akt phosphorylation in thyroid cancer cells. Finally, we showed that LY294002, an inhibitor of PI3K/Akt signaling, attenuated the effects of mutant RasGRP3 on thyroid cancer cells. Thus, our study revealed that the c.1924G>T hot-spot mutation in RasGRP3 is a more frequent genetic alteration in metastases of RAI-refractory differentiated thyroid cancer. This mutant RasGRP3 activated the Akt pathway, promoted thyroid cancer cell proliferation and invasion, and reduced NIS expression and the iodine uptake ability.
Keywords: Whole-exome sequencing, RasGRP3, sodium-iodide symporter, thyroid cancer, Akt signaling pathway
Introduction
Differentiated thyroid carcinoma is one of the most common endocrine cancers, and the current preferred treatments of this cancer are usually a surgical intervention, radioactive iodine (RAI), and thyroid-stimulating hormone (TSH) suppression [1,2]. RAI therapy has proven to be an effective treatment of residual and recurrent thyroid cancers, and the overall survival rate of patients with non-radioiodine-avid differentiated thyroid carcinoma is significantly lower than that of the patients with iodine-avid lesions [3,4]. Nonetheless, distant metastases, encountered in fewer than 10% of patients with differentiated thyroid cancer, significantly decrease survival [5]. RAI therapy is the initial systemic treatment of choice, with 67% of patients demonstrating appreciable radioiodine accumulation in metastatic lesions [6]. Approximately 30% of patients with advanced metastatic differentiated thyroid carcinoma have radioiodine-refractory disease [7]. An enhanced ability to concentrate radioiodine in metastases of differentiated thyroid carcinoma may be associated with malignant transformation of more differentiated cell types. Thus, the molecular pathogenesis of differentiated thyroid cancer and targeting of genes in radioiodine-refractory disease need to be evaluated and detected.
Whole-exome sequencing of matched tumorous and normal tissue samples is a powerful method for identifying somatic mutations implicated in tumor initiation or progression [8]. Numerous genetic alterations that have a fundamental role in the tumorigenesis of various cancers have been identified. For example, Fewings et al. have reported that germline pathogenic variants of the E-cadherin gene (CDH1) are strongly associated with hereditary diffuse gastric cancer [9]. Philip et al. have shown that mutant IDH1 promotes glioma formation in vivo [10]. Pruszko et al. have found that mutant p53 and ID4 contribute to breast cancer angiogenesis in a zebrafish experimental model [11]. Some studies have shown that many molecular alterations represent novel diagnostic and prognostic molecular markers of (and therapeutic targets in) thyroid cancer and thereby provide unprecedented opportunities for further research and clinical development of novel treatment strategies against this cancer [12]. Nonetheless, these variants are not present in metastases of RAI-refractory differentiated thyroid cancers (RAIR-DTCs). We aimed to identify new candidate genes and analyzed their effects in the metastases of RAIR-DTC.
In this study, we performed next-generation whole-exome sequencing on a series of tissue samples of metastatic RAIR-DTC tissues to investigate the genetic alterations important for these tumors. We found that a substantial percentage of cases of these cancers involves a recurrent hot-spot mutation in RasGRP3, which participates in the regulation of thyroid cell proliferation, migration, invasion, sodium-iodide symporter (NIS) expression, and the iodine uptake ability.
Materials and methods
Subjects
We collected 10 paired fresh frozen metastatic RAIR-DTC samples (8 cases of lymph node metastasis and 2 cases of lung metastasis) and normal tissues for next-generation whole-exome sequencing. In addition, we analyzed an independent set of thyroid carcinoma samples (n = 185 in total) from metastases of 30 patients with RAI-refractory papillary thyroid carcinoma (M-RAIR-PTC) and from a primary tumor of 45 patients with primary RAI-refractory papillary thyroid carcinoma (P-RIAR-PTC), 60 patients with advanced stage radioiodide affinity papillary thyroid carcinoma (AS-RAIA-PTC), and 50 patients with papillary thyroid microcarcinoma (PTMC). All the patients provided written informed consent, and the study protocol was approved by the ethics committee of each participating institution.
Whole-exome sequencing
Genomic DNA from patients’ samples was randomly sheared by ultrasonication for ~3 min using a Bioruptor XL Sonication System to generate paired-end libraries with an average insert size of B300 bp. Whole-exome capture was performed with the SureSelect Human All Exon 50 Mb kit (Agilent Technologies, Santa Clara, CA). The captured template DNA fragments of the constructed libraries were hybridized to the surface of flow cells, were amplified to form clusters, and were sequenced on an Illumina HiSeq 2500 system, generating 100 bp paired-end reads. All the samples yielded enough high-quality sequencing data: 13.26 GB of raw data on average after Illumina pass filtering.
Validation of mutations by PCR and Sanger sequencing
Exons where the polymorphisms of interest are located were amplified on a Dual 96-well GeneAmp PCR System 9700 (Applied Biosystems, Courtaboeuf, France), by means of 20 ng of template DNA from each sample per reaction. The PCR products were sequenced on a 3730XL DNA Analyzer (Applied Biosystems, Courtaboeuf, France). All sequences were studied and processed in the Sequencing Analysis Software (version 5.2, Applied Biosystems, Courtaboeuf, France).
Cell lines
Human papillary thyroid carcinoma cell line BHT101 collected from the Chinese Academy of Sciences (Shanghai, China) and anaplastic thyroid carcinoma cell line 8505C were purchased from Jennio Biotech Co., Ltd. (Guangzhou, China). The cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% of fetal bovine serum and were incubated at 37°C in a humidified chamber supplemented with 5% CO2.
Plasmid construction and cell transfection
RasGRP3 mutation c.1924G>T was introduced using the Q5 Site-direct Mutagenesis kit. The complementary DNAs encoding RasGRP3 and mutant RasGRP3 were PCR-amplified and inserted into pcDNA3.1 (Invitrogen, Carlsbad, CA); the resultant plasmids were named RasGRP3 WT and RasGRP3 MUT, respectively. The pcDNA3.1 empty vector, RasGRP3 WT plasmid, or RasGRP3 MUT plasmid was transfected into cells using Lipofectamine 2000 (Invitrogen).
Western blot assay
Total-cellular-protein samples were extracted with cell lysis buffer and were quantified using a BCA protein assay kit. Proteins in the samples were next separated by sodium dodecyl sulfate polyacrylamide electrophoresis (SDS-PAGE) in 10% gels and then transferred to PVDF membranes. After blocking in a blocking solution (Western Breeze, Thermo Fisher Scientific), the membranes were incubated with primary antibodies. Antibodies against RasGRP3, p-Akt, Akt, NIS, and TSHR were purchased from Proteintech (Wuhan, China). The protein expression level of GAPDH served as a control. Membranes were washed three times in Tris-buffered saline containing 0.1% of Tween 20 and were incubated with a peroxidase-conjugated secondary antibody (Sigma-Aldrich, St. Louis, MO, USA) for 40 min at room temperature. The proteins were visualized with enhanced chemiluminescence reagents.
The MTT assay
For this assay, transfected 8505C or BHT101 cells were seeded in 96-well plates at 104 cells per well, and cell viability was measured at different time points (1, 2, 3, and 4 days after seeding). Briefly, the cells were stained with 20 μL of the MTT dye (0.5 mg/mL; Sigma), the medium was removed after 4 h, and then 100 μL of dimethyl sulfoxide (Sigma) was added into each well, and absorbance was measured at 570 nm on a spectrophotometric plate reader.
Transwell assay
Cell invasion assays were performed in 24-well Transwell plates (8-μm pore size; Minipore) precoated with Matrigel (BD Biosciences, Franklin Lakes, NJ). In total, 105 cells were resuspended in 100 μL of Dulbecco’s modified Eagle’s medium with 1% of fetal bovine serum and were added into the upper chamber; 600 μL of Dulbecco’s modified Eagle’s medium with 10% of fetal bovine serum was placed in the lower chamber. After 48 h of incubation, the Matrigel and the cells remaining in the upper chamber were removed by cotton swabs. Cells on the lower surface of the membrane were fixed, stained with 0.1% crystal violet, and counted under a light microscope. Cells in five visual fields were counted and photographed. The Transwell migration assay was performed in the same way as the invasion assay but without the Matrigel coating.
In vitro iodine uptake assay
Parental 8505C and BHT101 cells and transfected cell clones were plated in 24-well plates at a density of 5 × 104 per well. The cells were washed with Hank’s balanced salt solution (HBSS) twice and incubated at 37°C with 1 mL of HBSS containing 0.1 μCi of 125I and 1 μmol/L NaI. After 30 min, the cells were collected and washed with HBSS. Radioactivity was quantified in a γ-counter [13].
Statistical analysis
The GraphPad Prism 5.0 software was employed for statistical analysis. The data are presented as the mean ± SD of three independent experiments. The data were subjected to one-way analysis of variance or Student’s t test for comparison between groups. Data with P < 0.05 were considered significant.
Results
Identification of a hot-spot RasGRP3 mutation in thyroid cancer
Exome sequencing was performed on a library prepared from the genomic DNA extracted from the 10 thyroid cancer samples and paired normal samples, and single nucleotide variants and small insertions and deletions (indels) were identified. We found a total of 194 mutations from 184 genes (BioProject: PRJNA486135). Among them, 9 candidate somatic mutations with a high frequency of mutation, including 5 missense mutations, 2 nonsense mutations, 1 in-frame deletion, and 1 frameshift deletion were shown in Figure 1A. BRAF mutations were detected in papillary carcinomas (60%), a mutation frequently observed in other carcinomas. HRAS and AKT1 mutations were detected in two cases (20%), and TP53, PTEN, PIK3CA, MUC58, and LAMC2 mutations were all detected in one case (10%; Figure 1A). In particular, a hot-spot substitution (c.1924G>T) in RasGRP3 was found in three cases (30%; Figure 1A and 1B). On the basis of these initial results, we screened a large series of PTCs (n = 185) for the presence of the hot-spot RasGRP3 c.1924G>T mutation. These samples came from 30 M-RAIR-PTC patients, 45 P-RIAR-PTC patients, 60 AS-RAIA-PTC patients, and 50 PTMC patients. This RasGRP3 mutation was found in 6 of 30 (20%) M-RAIR-PTC samples, 3 of 45 (6.67%) P-RIAR-PTC samples, 2 of 60 (3.33%) AS-RAIA-PTC samples, and 1 of 50 (2%) PTMC samples. Figure 1C indicates that this RasGRP3 mutation was most prevalent in group M-RAIR-PTC. These results suggested that this RasGRP3 mutation may play a critical role in the pathogenesis of thyroid cancer.
Figure 1.
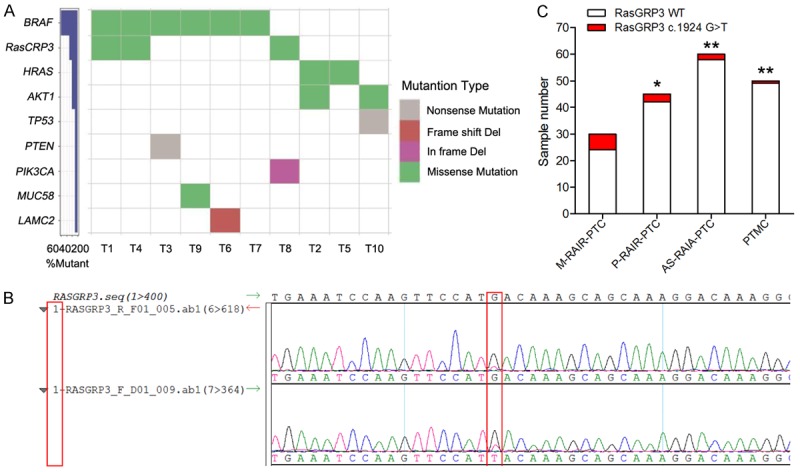
Identification of a hot-spot RasGRP3 mutation in thyroid cancer. A. Frequency and types of mutation in 9 genes identified by whole-exome sequencing. B. A sequence chromatogram of one thyroid cancer sample carrying the c.1924G>T mutation. C. Frequency of the RasGRP3 c.1924G>T mutation in different types of thyroid cancer. *P < 0.05 vs. the M-RAIR-PTC group. **P < 0.01 vs. M-RAIR-PTC group.
RasGRP3 mutation stimulated thyroid cancer cell metastasis
To test whether the RasGRP3 mutation affects RasGRP3 activity in thyroid cancer cells, 8505C and BHT101 cells were transfected with either a vector expressing wild-type RasGRP3 (RasGRP3-WT) or mutant RasGRP3 (RasGRP3-MUT). Western blot results showed that both RasGRP3-WT and -MUT were expressed in similar amounts in the transfected cells (Figure 2A). To explore the effect of the RasGRP3 mutation on cell proliferation and metastasis, a series of biological experiments were performed in vitro, including the MTT assay, migration assay and invasion assay. We first evaluated the effect of RasGRP3-MUT on cell proliferation, and the results revealed that overexpression of RasGRP3-MUT was associated with increased cell proliferation (Figure 2B). The Transwell assay indicated that the migratory and invasive abilities of 8505C and BHT101 cells transfected with RasGRP3-MUT were obviously higher than those of the cells transfected with RasGRP3-WT or the empty vector (Figure 2C and 2D). Therefore, our results did indeed reveal that the RasGRP3 mutation has a greater metastasis potential in thyroid cancer cells.
Figure 2.
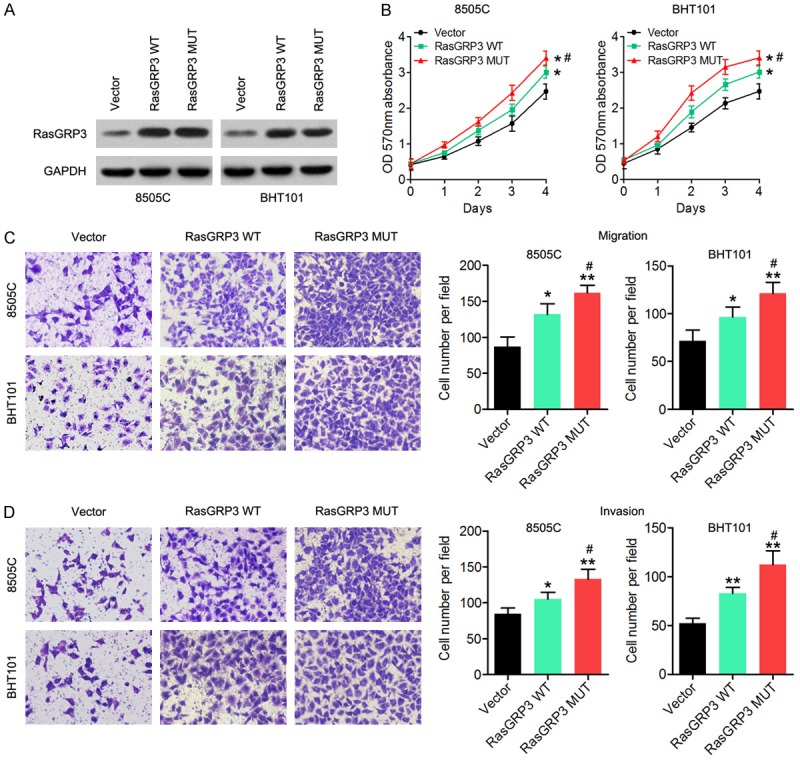
Functional characterization of the RasGRP3 mutant. A. The RasGRP3 levels were measured by a western blot assay in 8505C and BHT101 cells transfected with RasGRP3-WT, RasGRP3-MUT, or the empty vector. B. MTT assay results showed that RasGRP3-MUT promoted cell proliferation. C. The Transwell migration assay indicates that RasGRP3-MUT promoted the cell migration ability of thyroid cancer cells. D. The Transwell invasion assay suggests that RasGRP3-MUT enhanced cell invasive ability of thyroid cancer cells. GAPDH served as an internal control. *P < 0.05 vs. the empty-vector group. **P < 0.01 vs. the empty-vector group. #P < 0.05 vs. the RasGRP3 WT group.
The RasGRP3 mutation can regulate the expression of NIS and TSHR and alters the iodine uptake function of thyroid cancer
A selective increase of NIS-mediated active iodide uptake in thyroid cells allows for the use of radioiodine 131I for the diagnosis and targeted treatment of thyroid cancer [14]. Here, western blot results showed that RasGRP3-MUT decreased NIS and TSHR levels in 8505C and BHT101 cells compared with RasGRP3-WT-transfected or empty vector - transfected cells (Figure 3A). Furthermore, the iodine uptake ability of these two cell lines was detected, and the results indicated that RasGRP3-MUT significantly decreased the iodine uptake ability of 8505C and BHT101 cells compared with the cells transfected with RasGRP3-WT or the empty vector (Figure 3B). These results indicated that the RasGRP3 mutation is associated with the NIS expression and radioiodine uptake in thyroid cancer.
Figure 3.
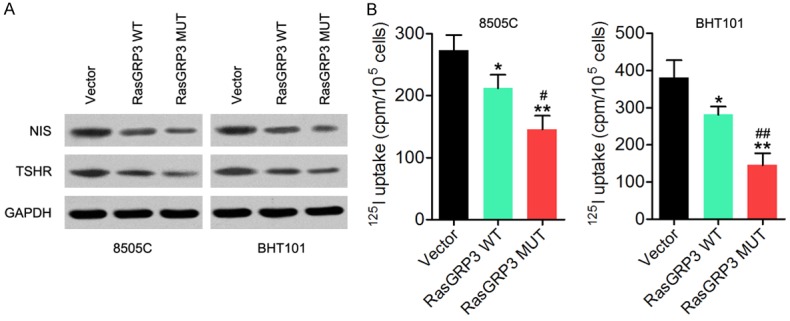
The RasGRP3 mutation can regulate the expression of NIS and TSHR and alter the iodine uptake ability in thyroid cancer cells. A. Expression of NIS and TSHR was detected in 8505C and BHT101 cells transfected with RasGRP3-MUT, RasGRP3-WT, or the empty vector in a western blot assay. B. The iodine uptake ability was measured in 8505C and BHT101 cells transfected with RasGRP3-MUT, RasGRP3-WT, or the empty vector. *P < 0.05 vs. vector group. **P < 0.01 vs. vector group. #P < 0.05 vs. RasGRP3 WT group. ##P < 0.01 vs. RasGRP3 WT group.
RasGRP3 mutant regulates PI3K/Akt pathway in thyroid cancer
Our previous study has shown that RasGRP3 controls cell proliferation and migration in papillary thyroid cancer by regulating the PI3K/Akt pathway, and Serrano-Nascimento et al. have reported that the PI3K/Akt cascade mediates the acute and rapid inhibitory effect of I (-) excess on NIS expression and activity. Therefore, we speculated that the effect of mutant RasGRP3 on cell proliferation, invasion, and NIS expression may be also be associated with the PI3K/Akt pathway. As presented in Figure 4A, the mutant RasGRP3 significantly increased the phospho-Akt (p-Akt) expression in 8505C and BHT101 cells compared with the cells transfected with RasGRP3-WT or the empty vector. Cells overexpressing RasGRP3-MUT were treated with LY294002, an inhibitor of PI3K/AKT signaling. As expected, expression of p-Akt was significantly reduced while RasGRP3-MUT expression stayed unchanged after treatment with LY294002 (Figure 4B). The MTT and Transwell invasion assays showed that cell viability and the invasive ability decreased after treatment with LY294002 (Figure 4C and 4D), suggesting that the Akt pathway participated in RasGRP3 mutant mediated cell proliferation and invasion of thyroid cancer cells. NIS and TSHR levels were significantly increased after treatment with LY294002 in cells overexpressing RasGRP3-MUT (Figure 4E). The iodine uptake ability were also enhanced strengthened after treatment with LY294002 in cells overexpressing RasGRP3-MUT (Figure 4F). Taken together, these results support a role of the RasGRP3 c.1924G>T mutation in the modulation of the tumorigenic potential via regulation of the Akt pathway.
Figure 4.
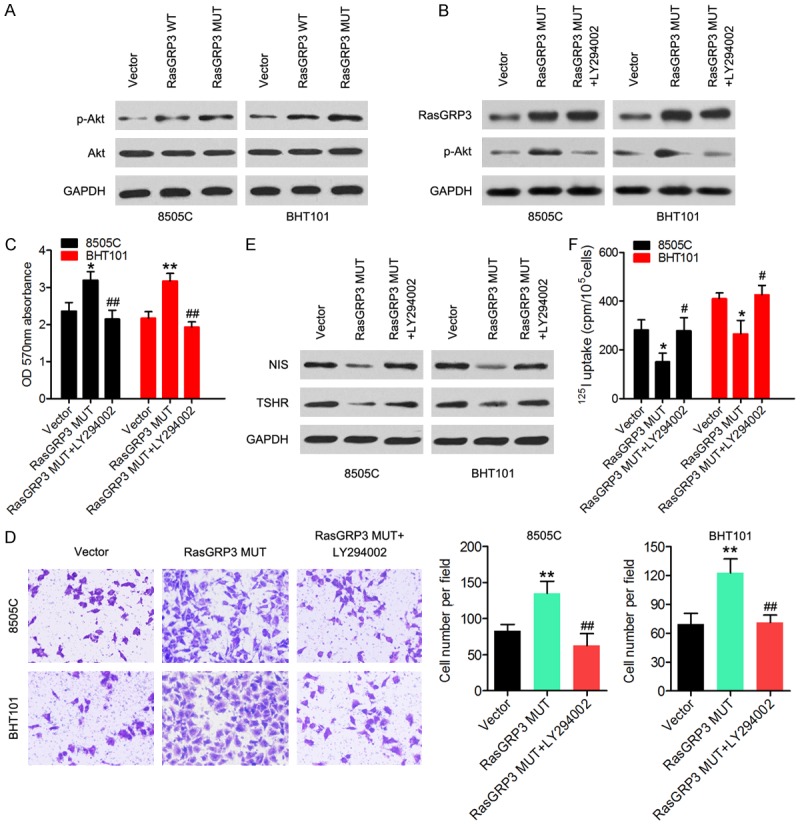
The mutant RasGRP3 regulates the PI3K/Akt pathway. (A) Western blot revealed that RasGRP3-MUT increased p-Akt amounts in 8505C and BHT101 cells. (B) Expression of RasGRP3-MUT and amounts of p-Akt were measured in cells overexpressing RasGRP3-MUT that were treated with LY294002. (C) Cell viability and (D) the invasive ability were measured by MTT and Transwell assays, respectively. (E) Western blotting was carried out to determine NIS and TSHR expression. (F) The iodine uptake ability was measured in cells overexpressing RasGRP3-MUT that were treated with LY294002. *P < 0.05 vs. the empty-vector group. **P < 0.01 vs. the empty-vector group. #P < 0.05 vs. the RasGRP3 MUT group. ##P < 0.01 vs. the RasGRP3 MUT group.
Discussion
Patients with radioiodine-refractory disease are not amenable to 131I therapy, which is the initial systemic treatment of choice for nonrefractory metastatic thyroid cancer. In our study, we performed whole-exome sequencing on the metastases of RAIR-DTCs and identified a hotspot substitution (c.1924G>T) in RasGRP3. In addition, we screened a large series of papillary thyroid carcinomas (n = 185) for the presence of the hot-spot RasGRP3 c.1924G>T mutation and found that the prevalence of this RasGRP3 mutation was higher in group M-RAIR-PTC than in group P-RAIR-PTC, AS-RAIA-PTC, or PTMC, respectively. Moreover, we found that mutant RasGRP3 significantly promoted cell proliferation, migration, and invasion by regulating the Akt pathway, while decreasing NIS and TSHR levels in 8505C and BHT101 cells as compared with RasGRP3-WT or the empty vector. Our results suggest that this RasGRP3 mutation may be crucial for the progression of thyroid cancer.
Numerous genetic alterations that play a fundamental role in the tumorigenesis of thyroid cancer have been identified [12]. A prominent example is the T1799A transverse point mutation of BRAF, which results in the expression of the BRAFV600E mutant protein, and the BRAFV600E mutation occurs in approximately 45% of papillary thyroid carcinoma cases [15]. The BRAFV600E mutant maintains proliferation, transformation, and tumorigenicity of BRAF-mutant papillary thyroid cancer cells [16]. Mutant MYO1F alters the mitochondrial network and induces tumor proliferation in thyroid cancer [17]. In addition, HRAS, KRAS, and NRAS are predominantly mutated in thyroid tumors [18]. Whole-exome sequencing of matched tumorous and normal tissue samples is a powerful method for identifying somatic mutations implicated in tumor progression or initiation. Here, we performed whole-exome sequencing on the thyroid cancer tissue samples and paired normal tissue samples to investigate the genetic alterations important for these tumors, and found that BRAF, RasGRP3, HRAS, AKT1, TP53, PTEN, PIK3CA, MUC58, and LAMC2 were mutated in thyroid cancers. Our previous study has revealed that RasGRP3 controls cell proliferation and migration in papillary thyroid carcinoma by regulating the Akt-MDM2 pathway [19]. Acquired resistance to BRAF inhibition induces EMT in BRAFV600E mutant thyroid cancer via c-Met-mediated AKT activation [20]. In the present study, we focused on one RasGRP3 mutation and found that the prevalence of this RasGRP3 mutation is significantly higher in the M-RIAR-PTC group than in group P-RIAR-PTC, AS-RAIA-PTC, or PTMC. Thus, we hypothesized that mutant RasGRP3 may participate in thyroid cancer metastases and affect iodide uptake. Here, we showed that the RasGRP3 mutant increased proliferation, migration, and invasiveness of 8505C and BHT101 cells compared with the cells transfected with RasGRP3-WT or the empty vector. Moreover, mutant RasGRP3 significantly reduced the p-Akt expression in cells, indicating that the Akt pathway may take part in RasGRP3 mutant mediated cell metastases in thyroid cancer.
NIS is a transmembrane glycoprotein that is expressed on the basolateral membrane of thyroid follicular cells [21]. The selective increase in NIS-mediated active iodide uptake in thyroid cells enables the use of radioiodine 131I for diagnosis and targeted treatment of thyroid cancers [14]. Approximately 30% of patients with advanced metastatic differentiated thyroid cancer have radioiodine-refractory disease, according to decreased expression of NIS, diminished membrane targeting of NIS, or both [7]. Xing et al. have demonstrated that the BRAFV600E mutation is associated with poor clinical outcomes of papillary thyroid carcinoma, including aggressive pathological features, increased recurrence, loss of radioiodine avidity, and treatment failures [22]. Excess iodide downregulates NIS gene transcription by activating the PI3K/Akt pathway [14,23]. In our study, we showed that the RasGRP3 mutant decreased NIS and TSHR levels and the iodine uptake ability in 8505C and BHT101 cells compared with RasGRP3-WT-transfected cells and empty vector-transfected cells. These findings mean that mutant RasGRP3 suppresses the NIS gene expression and radioiodine uptake function in thyroid cancer.
In conclusion, we performed whole-exome sequencing and selected RasGRP3-mutant for further research. We found significantly higher prevalence of the RasGRP3 c.1924G>T mutation in RAI refractory lymph node and lung metastases. We demonstrated that this RasGRP3 mutation is associated with cell migration, invasion, and the iodine uptake function of thyroid cancer cell lines by regulating the Akt pathway. These results may point to new strategies for the diagnosis and treatment of RAIR-DTC.
Acknowledgements
This project was supported by the National Natural Science Foundation of China (No. 81472499).
Disclosure of conflict of interest
None.
References
- 1.Clark OH. Total thyroidectomy: the treatment of choice for patients with differentiated thyroid cancer. Ann Surg. 1982;196:361–370. doi: 10.1097/00000658-198209000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin Y, Van Nostrand D, Cheng L, Liu M, Chen L. Radioiodine refractory differentiated thyroid cancer. Crit Rev Oncol Hematol. 2018;125:111–120. doi: 10.1016/j.critrevonc.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Schlumberger M, Tubiana M, De Vathaire F, Hill C, Gardet P, Travagli JP, Fragu P, Lumbroso J, Caillou B, Parmentier C. Long-term results of treatment of 283 patients with lung and bone metastases from differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1986;63:960–967. doi: 10.1210/jcem-63-4-960. [DOI] [PubMed] [Google Scholar]
- 4.Budiawan H, Salavati A, Kulkarni HR, Baum RP. Peptide receptor radionuclide therapy of treatment-refractory metastatic thyroid cancer using (90)Yttrium and (177)Lutetium labeled somatostatin analogs: toxicity, response and survival analysis. Am J Nucl Med Mol Imaging. 2013;4:39–52. [PMC free article] [PubMed] [Google Scholar]
- 5.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 6.Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire F, Schlumberger M. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–2899. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 7.Spitzweg C, Bible KC, Hofbauer LC, Morris JC. Advanced radioiodine-refractory differentiated thyroid cancer: the sodium iodide symporter and other emerging therapeutic targets. Lancet Diabetes Endocrinol. 2014;2:830–842. doi: 10.1016/S2213-8587(14)70051-8. [DOI] [PubMed] [Google Scholar]
- 8.Calebiro D, Grassi ES, Eszlinger M, Ronchi CL, Godbole A, Bathon K, Guizzardi F, de Filippis T, Krohn K, Jaeschke H, Schwarzmayr T, Bircan R, Gozu HI, Sancak S, Niedziela M, Strom TM, Fassnacht M, Persani L, Paschke R. Recurrent EZH1 mutations are a second hit in autonomous thyroid adenomas. J Clin Invest. 2016;126:3383–3388. doi: 10.1172/JCI84894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fewings E, Larionov A, Redman J, Goldgraben MA, Scarth J, Richardson S, Brewer C, Davidson R, Ellis I, Evans DG, Halliday D, Izatt L, Marks P, McConnell V, Verbist L, Mayes R, Clark GR, Hadfield J, Chin SF, Teixeira MR, Giger OT, Hardwick R, di Pietro M, O’Donovan M, Pharoah P, Caldas C, Fitzgerald RC, Tischkowitz M. Germline pathogenic variants in PALB2 and other cancer-predisposing genes in families with hereditary diffuse gastric cancer without CDH1 mutation: a whole-exome sequencing study. Lancet Gastroenterol Hepatol. 2018;3:489–498. doi: 10.1016/S2468-1253(18)30079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Philip B, Yu DX, Silvis MR, Shin CH, Robinson JP, Robinson GL, Welker AE, Angel SN, Tripp SR, Sonnen JA, VanBrocklin MW, Gibbons RJ, Looper RE, Colman H, Holmen SL. Mutant IDH1 promotes glioma formation in vivo. Cell Rep. 2018;23:1553–1564. doi: 10.1016/j.celrep.2018.03.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pruszko M, Milano E, Zylicz A, Zylicz M, Blandino G, Fontemaggi G. Zebrafish as experimental model to establish the contribution of mutant p53 and ID4 to breast cancer angiogenesis in vivo. J Thorac Dis. 2018;10:E231–E233. doi: 10.21037/jtd.2018.03.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–199. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen CT, Qiu ZL, Song HJ, Wei WJ, Luo QY. miRNA-106a directly targeting RARB associates with the expression of Na(+)/I(-) symporter in thyroid cancer by regulating MAPK signaling pathway. J Exp Clin Cancer Res. 2016;35:101. doi: 10.1186/s13046-016-0377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu YY, Zhang X, Ringel MD, Jhiang SM. Modulation of sodium iodide symporter expression and function by LY294002, Akti-1/2 and Rapamycin in thyroid cells. Endocr Relat Cancer. 2012;19:291–304. doi: 10.1530/ERC-11-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 16.Liu D, Liu Z, Condouris S, Xing M. BRAF V600E maintains proliferation, transformation, and tumorigenicity of BRAF-mutant papillary thyroid cancer cells. J Clin Endocrinol Metab. 2007;92:2264–2271. doi: 10.1210/jc.2006-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diquigiovanni C, Bergamini C, Evangelisti C, Isidori F, Vettori A, Tiso N, Argenton F, Costanzini A, Iommarini L, Anbunathan H, Pagotto U, Repaci A, Babbi G, Casadio R, Lenaz G, Rhoden KJ, Porcelli AM, Fato R, Bowcock A, Seri M, Romeo G, Bonora E. Mutant MYO1F alters the mitochondrial network and induces tumor proliferation in thyroid cancer. Int J Cancer. 2018 doi: 10.1002/ijc.31548. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Abubaker J, Jehan Z, Bavi P, Sultana M, Al-Harbi S, Ibrahim M, Al-Nuaim A, Ahmed M, Amin T, Al-Fehaily M, Al-Sanea O, Al-Dayel F, Uddin S, Al-Kuraya KS. Clinicopathological analysis of papillary thyroid cancer with PIK3CA alterations in a Middle Eastern population. J Clin Endocrinol Metab. 2008;93:611–618. doi: 10.1210/jc.2007-1717. [DOI] [PubMed] [Google Scholar]
- 19.Qiu W, Xia X, Qiu Z, Guo M, Yang Z. RasGRP3 controls cell proliferation and migration in papillary thyroid cancer by regulating the Akt-MDM2 pathway. Gene. 2017;633:35–41. doi: 10.1016/j.gene.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 20.Byeon HK, Na HJ, Yang YJ, Ko S, Yoon SO, Ku M, Yang J, Kim JW, Ban MJ, Kim JH, Kim DH, Kim JM, Choi EC, Kim CH, Yoon JH, Koh YW. Acquired resistance to BRAF inhibition induces epithelial-to-mesenchymal transition in BRAF (V600E) mutant thyroid cancer by c-Met-mediated AKT activation. Oncotarget. 2017;8:596–609. doi: 10.18632/oncotarget.13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castro MR, Bergert ER, Goellner JR, Hay ID, Morris JC. Immunohistochemical analysis of sodium iodide symporter expression in metastatic differentiated thyroid cancer: correlation with radioiodine uptake. J Clin Endocrinol Metab. 2001;86:5627–5632. doi: 10.1210/jcem.86.11.8048. [DOI] [PubMed] [Google Scholar]
- 22.Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Rhoden KJ, Carson KA, Vasko V, Larin A, Tallini G, Tolaney S, Holt EH, Hui P, Umbricht CB, Basaria S, Ewertz M, Tufaro AP, Califano JA, Ringel MD, Zeiger MA, Sidransky D, Ladenson PW. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–6379. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 23.Serrano-Nascimento C, Nicola JP, Teixeira Sda S, Poyares LL, Lellis-Santos C, Bordin S, Masini-Repiso AM, Nunes MT. Excess iodide downregulates Na(+)/I(-) symporter gene transcription through activation of PI3K/Akt pathway. Mol Cell Endocrinol. 2016;426:73–90. doi: 10.1016/j.mce.2016.02.006. [DOI] [PubMed] [Google Scholar]


