Abstract
Laryngeal squamous cell carcinoma (LSCC) is the second highest incidence among the head and neck malignancies. Long noncoding RNA (lncRNA) small nucleolar RNA host gene 1 (SNHG1) functions as an oncogene in various human cancers. However, the biological functions and molecular mechanisms of SNHG1 in LSCC have not been reported. In this study, we found that lncRNA SNHG1 is significantly upregulated in LSCC and associated with prognosis of LSCC patients. Knockdown of SNHG1 inhibited cell proliferation, migration and invasion and induced cell apoptosis. In addition, knockdown of SNHG1 inhibits LSCC growth and metastasis in vivo. Mechanistically, SNHG1 promotes YAP1 expression and Hippo signaling activity by competitively sponging miR-375. Moreover, YAP1 could occupy the SNHG1 promoter to enhance its transcription, suggesting that there exists a positive feedback regulation between YAP1 and SNHG1. Collectively, our study first elucidates the mechanism of SNHG1-mediated malignant phenotypes through evoking the miR-375/YAP1/Hippo signalling axis, which provides a novel target for LSCC treatment.
Keywords: YAP1, Hippo pathway, miR-345
Introduction
About 160,000 new cases of laryngeal cancer are diagnosed annually [1]. Laryngeal squamous cell carcinoma (LSCC), the most common laryngeal cancer, is the second highest incidence among the head and neck malignancies, and has a poor prognosis due to its uncontrolled invasion and metastasis under the presently available treatments, including surgery and chemotherapy. Importantly, the incidence of LSCC has been recently increasing [2]. In order to develop effective therapy and improve the LSCC patient’s prognosis and long-term quality of life, the efforts towards understanding the underlying pathological mechanisms of LSCC have been intensified recently.
Long noncoding RNAs (lncRNAs) are defined as RNA transcripts larger than 200 nucleotides without protein-coding potential. Increasing evidence indicates that lncRNAs play a critical role in cellular function and disease processes, including transcription, mRNA stability, epigenetic alteration, alternative splicing, translation, protein-protein interactions and protein stability. lncRNAs can interact with DNA, RNA, or protein molecules to regulate gene expression and then affect cellular processes [3-5]. Recently, it has been demonstrated that lncRNAs can act as oncogenes or tumor suppressor genes in initiation and progression of cancers. Moreover, lncRNAs may serve as potential biomarkers for early diagnosis, prognosis, and therapeutic targets in various cancers [6-8].
lncRNA small nucleolar RNA host gene 1 (SNHG1) functions as an oncogene in various human cancers. For example, upregulation of SNHG1 was found in osteosarcoma tissues and correlated with poor overall survival. SNHG1 promoted growth and metastasis of osteosarcoma cells through sponging miR-326 as a competing endogenous RNA (ceRNA) of NOB1 [9]. Li et al. demonstrated that SNHG1 was aberrantly upregulated in prostate carcinoma tissues. SNHG1 increased CDK7 expression by competitively binding miR-199a-3p, and then promoted cell proliferation and cell cycle progression in prostate cancer [10]. However, the biological functions and molecular mechanisms of SNHG1 in LSCC have not been reported. In this study, we investigated the biological functions of SNHG1 in LSCC, especially to explore its role in growth and metastasis.
Materials and methods
Cell culture
AMC-HN-8 and Hep-2 cells of human LSCC were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (Gibco; Life Technologies), 100 U/ml penicillin and 100 mg/ml streptomycin in humidified air at 37°C with 5% CO2.
Tissues sample collection
Sixty-five matched cancerous and noncancerous tissues were obtained from patients who had undergone surgery at The First Affiliated Hospital of Zhengzhou University Hospital, between 2011 and 2016 and who were diagnosed with LSCC based on histopathological evaluation. All the tissue samples were snap-frozen and stored at -80°C. The study was approved by the Research Ethics Committee of The First Affiliated Hospital of Zhengzhou University Hospital. Informed consent was obtained from all patients.
Lentivirus production and construction of stable cells with overexpression or knockdown of SNHG1
To obtain cell lines stably expressing SNHG1 or SNHG1-mut, AMC-HN-8 and Hep-2 cells were transfected with the plasmid pcDNA3.1-SNHG1 or pcDNA3.1-SNHG1-mut, and selected with neomycin (800 μg/ml) for four weeks.
shRNA targeting SNHG1 were subcloned into a lentiviral vector pLKO.1. The target sequences of SNHG1 shRNAs were shown as follows: sh1: GCTGAAGTTACAGGTCTGA, sh2: GACCTAGCTTGTTGCCAAT. We used a scramble shRNA as the negative control. The shSNHG1 or negative control lentiviral was packaged and harvested from 293 T cells, and then infected AMC-HN-8 and Hep-2 cells in the presence of 8 μg/ml polybrene. The cells were selected with puromycin (1 μg/ml) for one week.
Transient transfection
Transfections were performed using the Lipofectamine 3000 (Invitrogen) according to the manufacturer’s instructions. The siRNAs or microRNA mimics and their respective negative control RNAs (GenePharma) were introduced into cells at a final concentration of 50 nM. The cells were harvested at 48 hour after transfection.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from cells or tissues by the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer’s instructions. Total RNA was quantified using the NanoDrop 1000 (NanoDrop Technologies, Rockland, DE, USA), and the RNA integrity was assessed using standard denaturing agarose gel electrophoresis. The GoScriptTM Reverse Transcription System kit (Promega, Madison, WI, USA) was used to reverse-transcribe total RNA into cDNA. cDNA was used as a template with the GoTaq® qPCR Master Mix kit (Promega) and the reaction was monitored in an StepOne Plus System (ABI, USA) to detect RNA expression levels. The expression of mRNA and miRNA and lncRNA was normalized to GAPDH or U6. The relative expression was calculated using the 2-ΔΔCt. Primer sequences were shown as follows: SNHG1-forward: CCAAACTCAGGCACTGTATAGAT, SNHG1-reverse: ACAGACACGAAGTGGAGTTATG; GAPDH-forward: CAAGAGCACAAGAGGAAGAGAG, GAPDH-reverse: CTACATGGCAACTGTGAGGAG; YAP1-forward: CCTGAACAGTGTGGATGAGATG, YAP1-reverse: GGAATGGCTTCAAGGTAGTCTG.
Western blot analysis
Cells lysates were subjected to western blot analysis as previously described [11]. Antibodies employed in the analysis were as follows: anti-YAP1 (Santa Cruz Biotechnology), anti-β-actin (Cell Signaling) and secondary antibodies (Jackson).
Microarray analysis
The total RNA was extracted from above mentioned control and SNHG1 knockdown Hep-2 cells, amplified and transcribed into fluorescent cRNA using the Quick Amp Labeling kit (Agilent Technologies, Palo Alto, CA). The labeled cRNA was then hybridized onto the Agilent SurePrint G3 Human Gene Expression version 2 arrays (Agilent Technologies), and after the washing steps, the arrays were scanned by the Agilent Scanner G2505B. Agilent Feature Extraction software was used to analyze acquired array images. Quantile normalization and subsequent data processing were performed using the GeneSpring GX v11.5.1 software package (Agilent Technologies). The differentially expressed mRNAs with statistical significance were identified using volcano plot filtering. The threshold we used to screen upregulated or downregulated mRNAs is fold change >2 and a p-value <0.05. Gene expression profiles of the Hep-2 cells with or without SNHG1 knockdown were determined using Phalanx human One-Array microarrays (HOA 6.1) following the manufacturer’s instructions.
RNA pull-down assay
SNHG1 or SNHG1-mut were in vitro transcribed respectively from vector pSPT19-SNHG1 or pSPT19-SNHG1-mut, and biotin-labeled with the Biotin RNA Labeling Mix (Roche) and T7 RNA polymerase (Roche), treated with RNase-free DNase I (Roche), and purified with an RNeasy Mini Kit (Qiagen). One milligram of whole-cell lysates from Hep-2 cells were incubated with 3 ug of purified biotinylated transcripts for 1 hr at 25°C; complexes were isolated with streptavidin agarose beads (Invitrogen). The RNA present in the pull-down material was detected by qRT-PCR analysis.
RNA immunoprecipitation (RIP) assay
LSCC cells were co-transfected with pcDNA3.1-MS2, pcDNA3.1-MS2-SNHG1 or pcDNA3.1-MS2-SNHG1 and pMS2-GFP (Addgene). After 48 hrs, cells were used to perform RNA immunoprecipitation (RIP) experiments using a GFP antibody (Abcam) and the Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Millipore) according to the manufacturer’s instructions.
Proliferation and colony formation assays
Cell proliferation was assessed using CCK-8 Assay (Dojindo) according to manufacturer’s instruction. For colony formation assays, cells were cultured for 10 days, fixed with 70% ethanol for 15 min and stained with 2% crystal violet. Colonies with more than 50 cells per colony were counted.
Cell apoptosis assays
LSCC cells were harvested by trypsinization. After double staining with fluorescein isothiocyanate (FITC)-Annexin V and propidium iodide, the cells were analyzed by flow cytometry (FAC-Scan; BD Biosciences) equipped with CellQuest software (BD Biosciences).
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay
TUNEL assay was performed by TUNEL Apoptosis Assay Kit (Sango Biocompany) according to the manufacturer’s instruction.
Migration and invasion assays
The cell invasion assays were conducted using Biocoat Matrigel Invasion Chambers (BD Biosciences) according to manufacturer’s instruction. The cell migration assays were performed by Transwell Polycarbonate Membrane Inserts (Corning). Cells that passed through the gel and adhered to the bottom side of the chambers were counted. All the cell functional experiments were performed three times independently.
Construction of luciferase reporter plasmids and luciferase reporter assay
The SNHG1 or SNHG1-mut was amplified using PCR and subcloned into the pmirGLO vector (Promega) for Luciferase reporter assay using the one step directed cloning kit (Novoprotein). The 3’ untranslated regions (3’-UTR) of YAP1 mRNA containing the intact miR-375 recognition sequences were PCR-amplified and subcloned into pmirGLO vector. pmirGLO, pmirGLO-SNHG1 or pmirGLO-SNHG1-mut was cotransfected with miR-375 mimics or negative control miR-NC into Hep-2 cells by Lipofectamine 3000 (Invitrogen). pmirGLO or pmirGLO-YAP1 was transfected into different cell clones by Lipofectamine 3000 (Invitrogen). Each experiment was done in triplicate. The relative luciferase activity was normalized to Renilla luciferase activity 48 hr after transfection.
To assess changes of Hippo signaling, we performed a luciferase assay according to the manufacturer’s protocol (Promega, USA). Cells were transfected with the TEAD luciferase reporter plasmid (YouBio, Changsha, China). Two days after transfection, the cells were analyzed for luciferase activities.
Chromatin immunoprecipitation assay (ChIP)
ChIP assays were performed by using the EZ Magna ChIP Kit (Millipore) according to manufacturer’s instruction. Briefly, cells were cross-linked and then sonicated into DNA fragments. Anti-YAP1 antibody (Abcam) and negative control normal IgG (Millipore) were used for each immunoprecipitation. Immunoprecipitated DNAs were subjected to qRT-PCR analysis.
In vivo tumorigenicity and metastasis study
To detect the tumor growth, 107 Hep-2 stable cells were injected subcutaneously into the right flanks of 4-week old Balb/c nude mice. Tumor growth was measured every 3 days. Tumor weights were calculated 30 days after inoculation. To detect the tumor metastasis, the stable Hep-2 cells were intravenously injected (2×106 cells per mouse) into the tail vein of mice. After 50 days of inoculation, the mice were sacrificed and then their lung tissues were obtained and fixated in Bolin’s fluid. Then the tissues were histologically analyzed with H&E staining for the presence of micrometastases. All animal handling and experimental procedures were approved by the Animal Experimentation Ethics Committee of The First Affiliated Hospital of Zhengzhou University Hospital.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism Software (GraphPad Software). Pearson chi-square test was used to analyze the correlation between SNHG1 expression and clinical features of LSCC patients. The Kaplan-Meier method was used to estimate the survival rate for each parameter. The equivalences of the survival curves were tested by log-rank statistics. Student’s t test or one-way ANOVA were used for comparison between different groups. A two-tailed P-value of less than 0.05 was considered statistically significant.
Results
Upregulation of SNHG1 is correlated with poor prognosis in LSCC
Firstly, qRT-PCR analysis was performed to determine the expression level of SNHG1 in 80 pairs of human primary LSCC tissues and adjacent nontumourous samples. We found that the expression of SNHG1 in LSCC tissues was markedly higher than that observed in pair-matched adjacent nontumourous tissues (Figure 1A). Moreover, the SNHG1 expression was much higher in patients with advanced stage LSCC than early stage LSCC (Figure 1B). In addition, to understand the prognostic significance of SNHG1 upregulation in LSCC, we analyzed the relationship between SNHG1 expression in LSCC and patients’ survival. It was found that high-level SNHG1 expression was significantly associated with a poor 5-year overall survival rate in our LSCC cohort (Figure 1C). Collectively, these results suggest that increased level of SNHG1 may be a feature involved in development and progression and prognosis of human LSCC.
Figure 1.
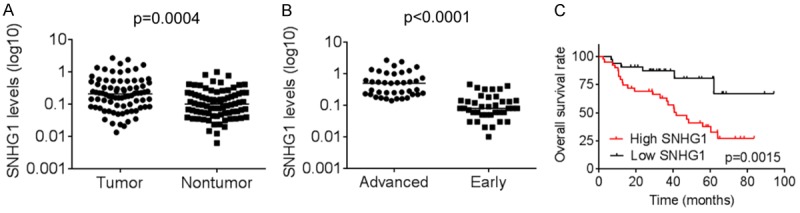
Upregulation of SNHG1 is correlated with poor prognosis in LSCC. A. The SNHG1 expression levels in 80 pairs of human primary LSCC tissues and adjacent nontumourous samples were examined by qRT-PCR. B. The SNHG1 expression levels of 80 LSCC tissues in early and advanced stage of LSCC were detected by qRT-PCR. C. Kaplan-Meier curves depicting overall survival of LSCC patients. Patients were grouped according to SNHG1 expression in HCC tissues. The median expression level was used as the cutoff.
Knockdown of SNHG1 inhibits cell proliferation and induces apoptosis
To further investigate the function of SNHG1 in LSCC, we established stable AMC-HN-8 and Hep-2 cells with SNHG1 silence by using lentiviral infection, and the knockdown of SNHG1 was confirmed by qRT-PCR (Figure 2A). The CCK-8 assays showed that the proliferative abilities of AMC-HN-8 and Hep-2 cells expressing SNHG1 shRNAs was significantly inhibited compared with the control cells (Figure 2B). To further testify the anti-proliferative effect of SNHG1 silence on LSCC cells, colony formation assay was performed. As shown in Figure 2C, the colony numbers of AMC-HN-8 and Hep-2 cells expressing SNHG1 shRNAs were significantly lower than those cells expressing control shRNAs. Thus, the results of colony formation assay were consistent with those of CCK-8 assay and further indicated that knockdown of SNHG1 could inhibit proliferation of LSCC cells. Because the above results indicate that SNHG1 exerts an oncogene in LSCC cells, we then investigated whether SNHG1 is involved in regulating cell apoptosis using flow cytometry and TUNEL analyses. Compared with the control cells, knockdown of SNHG1 significantly increased the LSCC cell apoptotic rate (Figure 2D and 2E).
Figure 2.
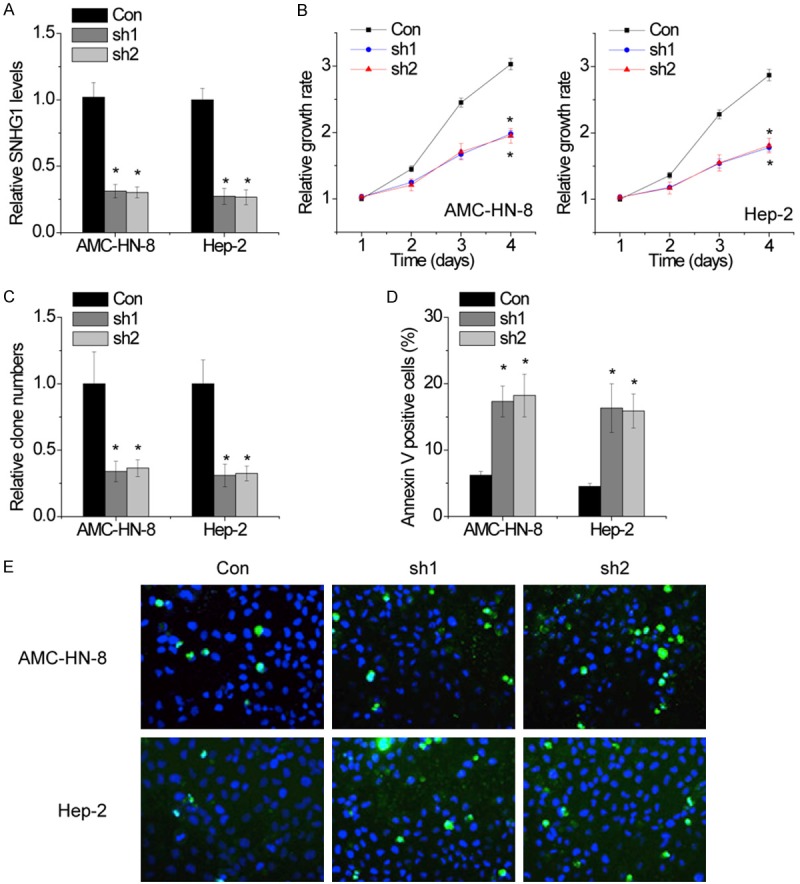
Knockdown of SNHG1 inhibits cell proliferation and induces apoptosis. A. Stable AMC-HN-8 and Hep-2 cells with SNHG1 silence were established by using lentiviral infection, and the knockdown of SNHG1 was confirmed by qRT-PCR. B. Proliferation of LSCC cells assessed by CCK8 assays. SNHG1 interference suppressed both AMC-HN-8 and Hep-2 cells proliferation. C. Clone formation assay of differently treated LSCC cells. The data graphs depict the count number from three independent experiments. D. AMC-HN-8 and Hep-2 cell apoptosis after transfection with SNHG1 shRNA or the negative control was evaluated by flow cytometry by measuring the percentage of Annexin V-stained cells. E. AMC-HN-8 and Hep-2 apoptosis after transfection with SNHG1 shRNA or the negative control was evaluated by TUNEL staining assays. Dead cells were labeled with TUNEL (green); nuclear fractions were labeled with DAPI (blue). The mean values and SEs were calculated from triplicates of a representative experiment. *P<0.05.
SNHG1 promotes cell migration and invasion in LSCC cells
To evaluate the effect of SNHG1 on the progression of LSCC, we performed transwell chamber assays to detect the migration and invasion of AMC-HN-8 and Hep-2 cells. We found that the migratory and invasive ability of the LSCC cells was dramatically impaired following deletion of SNHG1 (Figure 3A and 3B).
Figure 3.
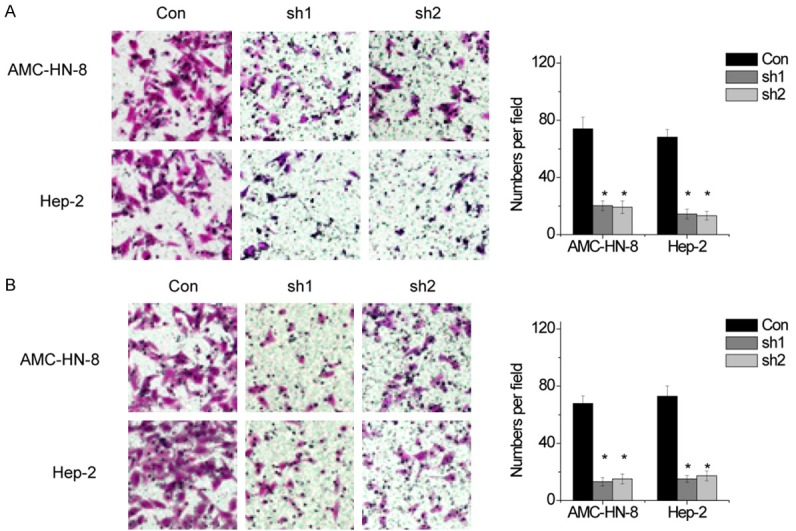
SNHG1 promotes cell migration and invasion in LSCC cells. (A and B) The migration (A) and invasive ability (B) after knockdown of SNHG1 in AMC-HN-8 and Hep-2 cells was assessed using transwell assays. The mean values and SEs were calculated from triplicates of a representative experiment. *P<0.05.
Knockdown of SNHG1 inhibits tumor growth and metastasis in vivo
To determine whether SNHG1 could influence the tumorigenesis of LSCC cells in vivo, the SNHG1 stable knockdown of Hep-2 cells or control cells were injected into nude mice. The results showed that tumors grown from SNHG1 stable knockdown Hep-2 cells were smaller than tumors grown from the control cells (Figure 4A). The tumor weight of the shSNHG1 group was also significantly lower than that of the control group (Figure 4B).
Figure 4.
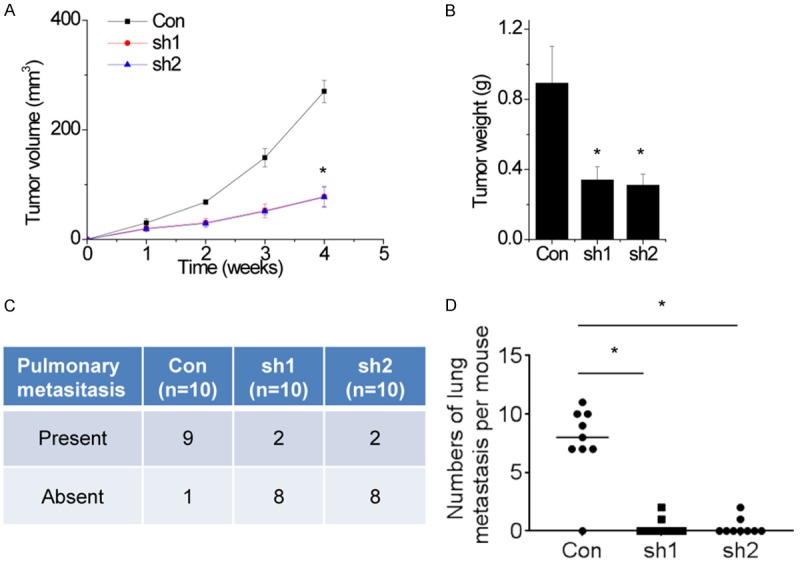
Knockdown of SNHG1 inhibits tumor growth and metastasis in vivo. A. Hep-2 cells stably expressing SNHG1 shRNAs or the negative control were used for in vivo tumorigenesis. Tumor growth curves after subcutaneous injection of Hep-2 cells containing two stable knockdown of SNHG1 or the negative control are shown. The tumor volumes were measured every 1 week after inoculation. B. Tumor weights are represented. C. Incidence of lung metastasis in each group of nude mice. D. Number of metastatic lung foci observed in each group. The mean values and SEs were calculated from triplicates of a representative experiment. *P<0.05.
On the basis of the above findings that SNHG1 promotes migration and invasion in LSCC cells, we next investigated the effects of SNHG1 on cancer metastatic ability in vivo. To establish a metastatic cancer model in vivo, control and SNHG1 knockdown Hep-2 cells were injected into tail vein of nude mice. As shown in Figure 4C, the incidence of lung metastasis in the shSNHG1 groups were significantly decreased, compared with the control group. In addition, downregulation of SNHG1 resulted in a reduction in metastatic nodules on the mice lungs when compared with those in the control group (Figure 4D).
SNHG1 is physically associated with miR-375
Increasing evidence demonstrated that many lncRNAs act as competing endogenous RNAs (ceRNAs) by competitively binding miRNAs [12,13]. To reveal the underlying mechanism of SNHG1 in LSCC progression, we tested whether miRNAs are involved in the process. Bioinformatics analysis showed that SNHG1 contains a binding site of miR-375 (Figure 5A). miR-375 is frequently downregulated in several cancers and acts as a tumor suppressor [14-16]. To validate the direct interaction between SNHG1 and miR-375, we performed an RIP assay with MS2-binding protein (MS2bp) which specifically binds target RNA containing MS2-binding sequences (MS2bs). We generated a construct containing SNHG1 transcripts combined with MS2bs elements and cotransfected into LSCC cells with a construct containing GFP-MS2bp (Figure 5B). The immunoprecipitation was then carried out using anti-GFP antibody (IgG was used as a negative control), and miR-375 level was analyzed using qRT-PCR. Intriguingly, as shown in Figure 5C, SNHG1 was able to significantly enrich miR-375 compared with the empty vector (MS2) and SNHG1 with mutations in miR-375 targeting sites (SNHG1-mut). The specific binding between SNHG1 and miR-375 was further validated by RNA pull-down assay using a biotin-labeled-specific SNHG1 probe (Figure 5D).
Figure 5.
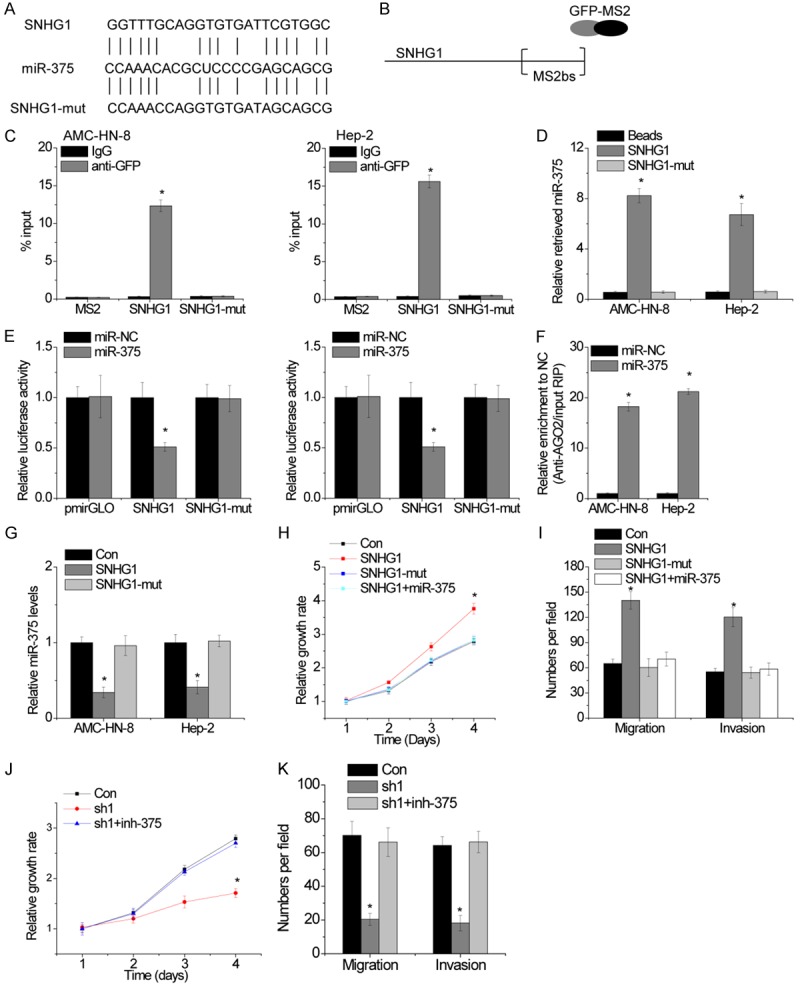
SNHG1 is physically associated with miR-375. A. Schematic outlining the predicted binding sites of miR-375 on SNHG1 or SNHG1-mut. B. Schematic diagram of MS2-RIP assay. C. RIP followed by qRT-PCR to analyze endogenous miR-375 interacted with SNHG1. D. LSCC cell lysates were incubated with biotin-labeled SNHG1 or SNHG1-mut. After pull-down, miR-375 was assessed by qRT-PCR. E. Luciferase activity in LSCC cells co-transfected with miR-NC or miR-375 and luciferase reporters containing wild-type or mutant SNHG1. F.Anti-AGO2 RIP was performed in Hela cells with overexpression of miR-NC or miR-375 and followed by qRT-PCR to detect SNHG1 pulled down by AGO2. G. The relative RNA levels of miR-375 in SNHG1 or SNHG1-mut overexpressed LSCC cells. H. The cell proliferation was determined by CCK-8 assay in the indicated LSCC cells. I. The cell migration and invasion was determined by Transwell assay in the indicated LSCC cells. J. Cell proliferation of LSCC cells treated with the indicated treatments. K. Migration and invasion assay of LSCC cells treated with the indicated treatments. The mean values and SEs were calculated from triplicates of a representative experiment. *P<0.05.
We then constructed a luciferase construct containing SNHG1 or SNHG1-mut. Luciferase assay showed that miR-375 significantly inhibited the luciferase activity of SNHG1, but it has no effect on the SNHG1-mut (Figure 5E). miRNAs bind their target genes and cause posttranscriptional repression in an AGO2-dependent manner. To assay whether SNHG1 was regulated by miR-375 in such a manner, we performed anti-AGO2 RIP in LSCC cells transiently transfected with miR-375. Endogenous SNHG1 was specifically enriched in miR-375-transfected LSCC cells (Figure 5F), supporting that miR-375 is a bona fide SNHG1-targeting miRNA. In addition, ectopically expressed SNHG1, but not the SNHG1, reduced miR-375 expression (Figure 5G). Taken together, these data demonstrate that SNHG1 physically binds with miR-375 and may function as a ceRNA.
We then tested whether SNHG1 promotes LSCC progression in a miR-375-dependent manner. Overexpression of SNHG1, but not the mutant, increases Hep-2 cells proliferation, migration and invasion, whereas transfection of miR-375 mimics abolished this increase (Figure 5H and 5I). Reciprocally, the depletion of SNHG1 inhibited Hep-2 cells proliferation, migration and invasion, which were rescued by inhibition of miR-375 (Figure 5J and 5K). There results suggest that SNHG1 exerts oncogenic effects in a miR-375-dependent manner.
SNHG1 increases YAP1 expression and activates Hippo pathway
As described above, SNHG1 could inhibit miR-375 expression and function in LSCC cells. We hypothesized that suppression of miR-375 might decrease repression to its mRNA targets, thereby further promoted the progression of LSCC. Consequently, by performing a computational screen for genes with complementary sites of miR-375 in their 3’-UTR using online softwares including TargetScan and miRBase, we found that YAP1 was a putative target of miR-375 (Figure 6A). A previous also demonstrated that YAP1 was targeted by miR-375 [14]. YAP1 were upregulated at both the mRNA and protein levels after SNHG1 overexpression, while SNHG1-mut did not have an effect on YAP1 expression (Figure 6B and 6C). Simultane-ous ectopic expression of miR-374a abrogated this increase. In contrast, YAP1 mRNA and protein levels decreased after SNHG1 knockdown. These effects were reversed by inhibition of miR-374a (Figure 6D and 6E). To further determine whether the SNHG1-mediated YAP1 up-regulation depends on regulation of the YAP1 3’UTR, luciferase reporters containing YAP1 3’UTR was constructed. As shown in Figure 6F, overexpression of SNHG1, but not the SNHG1-mut, increased the luciferase activity of YAP1 3’UTR. Ectopic expression of miR-375 abolished this increase. In contrast, the downregulation of SNHG1 suppressed the luciferase activity of YAP1 3’UTR, which were rescued by inhibition of miR-375 with antisense oligonucleotides inhibitors (Figure 6G).
Figure 6.
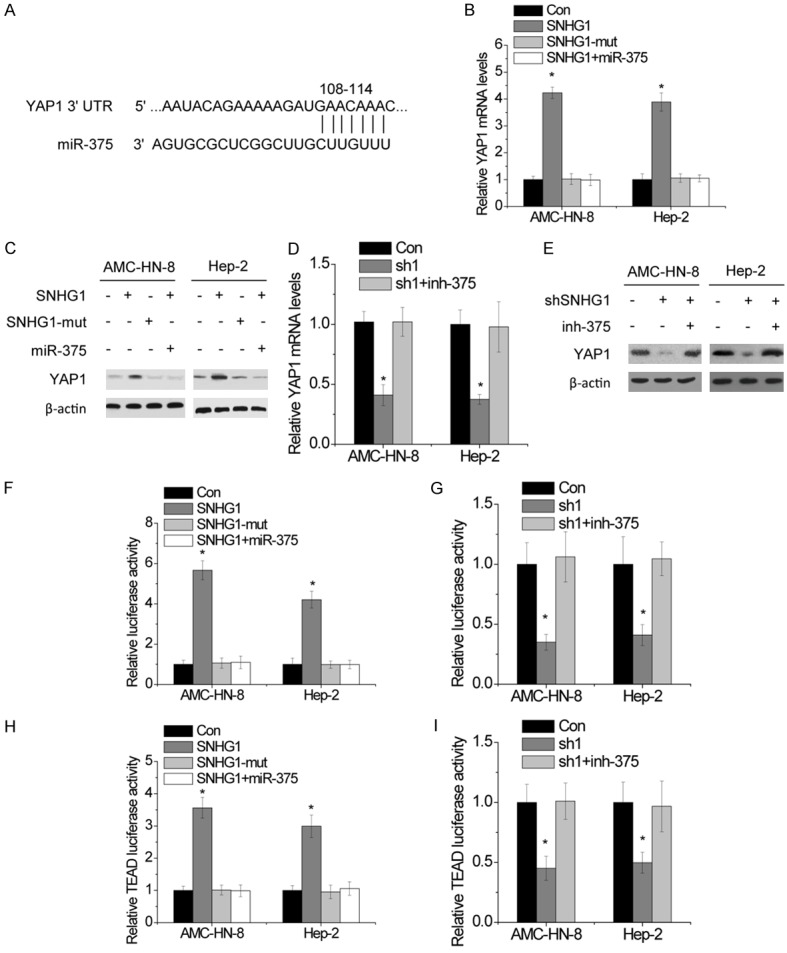
SNHG1 increases YAP1 expression and activates Hippo pathway. A. Schematic outlining the predicted binding sites of miR-375 on YAP1 3’UTR. B. The relative mRNA levels of YAP1 in LSCC cells with indicated treatment. C. The protein levels of YAP1 in LSCC cells with indicated treatment. D. The changes in mRNA levels of YAP1 in the indicated LSCC cells. E. The changes in protein levels of YAP1 in the indicated LSCC cells. F. Luciferase activity of YAP1 3’UTR in LSCC cells cotransfected with miR-375 or non-target microRNAs and SNHG1 or mutant transcript. G. Luciferase activity of YAP1 3’UTR in LSCC cells cotransfected with miR-375 inhibitor or non-target microRNAs inhibitor and SNHG1 shRNA. H. TEAD luciferase activity in LSCC cells cotransfected with miR-375 or non-target microRNAs and SNHG1 or mutant transcript. I. TEAD luciferase activity in LSCC cells cotransfected with miR-375 inhibitor or non-target microRNAs inhibitor and SNHG1 shRNA. The mean values and SEs were calculated from triplicates of a representative experiment. *P<0.05.
As YAP1 is the major effector of the Hippo pathway, we then investigated the effect of SNHG1 on Hippo signaling activity. The activity of the TEAD luciferase reporter indicates the transcriptional activity of YAP1 and the activity of Hippo signaling. As expected, overexpression of SNHG1, but not SNHG1-mut, markedly increased the TEAD luciferase activity. miR-375 mimics transfection reversed this increase (Figure 6H). Conversely, the transactivating activity of YAP1 was decreased by SNHG1 knockdown, which was reversed when miR-375 was inhibited by antisense inhibitors (Figure 6I). These data indicate that SNHG1 alters YAP1 expression and Hippo signaling activity through miR-375.
YAP1 activates SNHG1 transcription
Of note, the JASPAR online database predited that YAP1 may bound to the SNHG1 promoter region (Figure 7A). LSCC cells were transfected with YAP1 siRNAs, and the qRT-PCR results showed that SNHG1 expression was decreased after knockdown of YAP1 (Figure 7B). Moreover, we designed two primers that covered the SNHG1 binding sites and performed chromatin immunoprecipitation (ChIP) assays to validate whether YAP1 could bind to these sites. The ChIP results showed that YAP1 could bind to both sites (Figure 7C).
Figure 7.
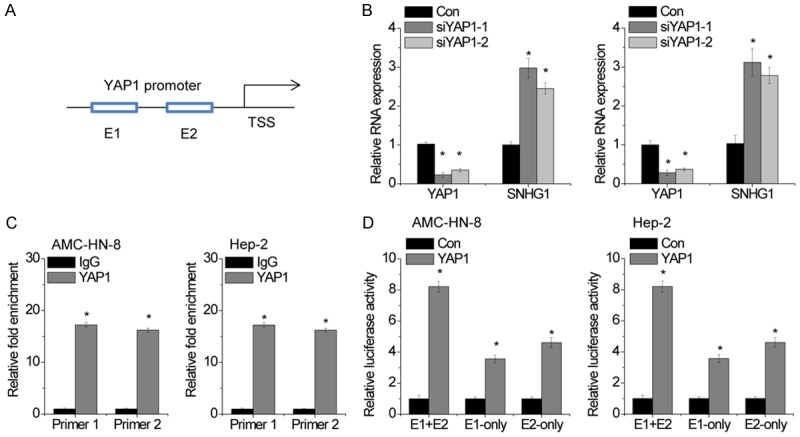
YAP1 activates SNHG1 transcription. A. The Schematic diagram of the YAP1-binding sites in SNHG1 promoter. B. The LSCC cells were transfected with two different siRNAs targeting YAP1. After 48 hours, the YAP1 and SNHG1 expression levels were detected by qRT-PCR. C. The ChIP assay was used to detect the binding of YAP1 on SNHG1 promoter region. IgG was taken as a negative control. D. Two predicted YAP1-binding sites of the SNHG1 promoter was individually deleted. Luciferase assay was used to examine transcriptional activities of the two SNHG1 promoter deletion mutants when YAP1 was overexpressed in LSCC cells. The mean values and SEs were calculated from triplicates of a representative experiment. *P<0.05.
To clarify which element was necessary for YAP1-mediated SNHG1 expression, the two predicted YAP1-binding sites were individually deleted. Dual luciferase reporter assays showed that YAP1 could bind to both the E1 and E2 elements and activate luciferase, and the luciferase reporter containing both E1 and E2 had higher luciferase activity than that containing only E1 or E2 (Figure 7D). These findings suggest that there is a regulatory feed-back loop between SNHG1 and YAP1, which may synergistically exert their oncogenic activities.
Discussion
In this study, we provided several new insights into the function of lncRNA SNHG1 in LSCC growth and metastasis. Our results clearly showed a positive feedback mechanism involving SNHG1 and YAP1, thus promoting the understanding of deregulated Hippo signaling in LSCC. More importantly, SNHG1 conferred clinical significance and potential therapeutic target of novel treatment for LSCC.
LncRNA SNHG1 has been reported to be upregulated in many cancers [17,18]. And we also found that SNHG1 expression was significantly increased in LSCC tissues compared with adjacent normal tissues. Recent evidence has identified elevated SNHG1 as a poor prognostic factor for many cancers [19,20]. Consistent with these studies, high-level SNHG1 expression was also correlated with poor prognosis of patients with LSCC. Knockdown of SNHG1 induced markedly suppression of cell proliferation, migration and invasion ability, whereas promotion of apoptosis in LSCC both in vitro and in vivo. These data demonstrated that SNHG1 functions as an oncogene and plays a critical role in LSCC progression.
miRNAs are approximately 20 nucleotides in length and regulate gene expression by induction of mRNA degradation or inhibition of mRNA translation [21]. Abnormal expression of miRNAs have observed in cancers and closely related to tumorigenesis and tumor metastasis [22]. Recent studies have demonstrated that miR-375 is frequently downregulated in multiple types of cancer and functions as an important tumor suppressor by inhibiting malignant properties of cancer cells [23]. Lin et al. found that miR-375 inhibited cell proliferation, autophagy, clonogenicity, migration, and invasion and also induces G1 arrest and apoptosis by targeting AEG-1 and ATG7 [24,25]. Moreover, Liu et al.6 reported that overexpression of miR-375 decreased liver cancer cell proliferation and by invasion targeting oncogene YAP1 [26]. Recently, a new posttranscriptional regulatory mechanism that lncRNAs act as a natural miRNA sponge has been identified [27]. In this case, lncRNAs interact with miRNAs to induce the derepression of miRNAs targets. In our present study, RIP and RNA pull-down assays demonstrated that SNHG1 could specifically associate with miR-375 in LSCC cells. Previous studies also showed that SNHG1 sponges different miRNAs to regulate cancer progression, such as miR-145, miR-15b, miR-326, and miR-199a [9,10,18,28]. Taken together, these data revealed that SNHG1 could function as an effective miRNAs sponge. Apart from interaction with miRNA, lncRNAs also act as protein scaffolds for bound proteins [33]. It was reported that SNHG1 directly interacted with FUBP1 and antagonized the binding of FBP-interacting repressor to FUBP1, thereby coordinating the expression of the oncogene MYC [29]. More functions and mechanisms of SNHG1 in LSCC development and progression are still need to be investigated.
Hippo signaling pathway is an emerging kinase cascade in tumorigenesis and tumor metastasis [30]. As the key downstream mediator of Hippo pathway, the oncogenic role of YAP1 has been extensively investigated [31]. YAP1 is activated in multiple cancer types and functions as a driver oncogene, even bypassing oncogenic RAS signaling [32]. Additionally, YAP1 functions as a transcription co-activator and TEAD transcription factors are the main binding partner for YAP1, together they exert oncogenic roles in tumorigenesis [33]. However, to date, the functional role of SNHG1 in Hippo signaling remains unknown. Here, for the first time, we demonstrated that SNHG1 increased YAP1 expression and activated Hippo signaling pathway through suppression miR-375. Intriguingly, our findings also showed that YAP1 directly bound to SNHG1 promoter region and activated its transcription. Taken together, our data indicated that YAP1 increased the expression level of SNHG1, and SNHG1 promoted YAP1 expression via sponging miR-375, which constitutes a feedback loop among SNHG1/miR-375/YAP1.
Collectively, we identify the positive feed-forward loop between SNHG1 and YAP1 that involves in the regulation of malignant property of LSCC. Targeting the SNHG1/miR-375/YAP1 axis might be an useful therapeutic strategy for clinical transformation. Taken together, the discovery of SNHG1 and its interaction with Hippo signaling may provide a promising option for facilitating the investigation of LSCC development and progression.
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Rothenberg SM, Ellisen LW. The molecular pathogenesis of head and neck squamous cell carcinoma. J Clin Invest. 2012;122:1951–1957. doi: 10.1172/JCI59889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Z, Lin Z, Pang X, Tariq MA, Ao X, Li P, Wang J. Epigenetic regulation of long non-coding RNAs in gastric cancer. Oncotarget. 2018;9:19443–19458. doi: 10.18632/oncotarget.23821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu G, Niu F, Humburg BA, Liao K, Bendi S, Callen S, Fox HS, Buch S. Molecular mechanisms of long noncoding RNAs and their role in disease pathogenesis. Oncotarget. 2018;9:18648–18663. doi: 10.18632/oncotarget.24307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang Y, He Y, Zhang P, Wang J, Fan C, Yang L, Xiong F, Zhang S, Gong Z, Nie S, Liao Q, Li X, Li X, Li Y, Li G, Zeng Z, Xiong W, Guo C. LncRNAs regulate the cytoskeleton and related Rho/ROCK signaling in cancer metastasis. Mol Cancer. 2018;17:77. doi: 10.1186/s12943-018-0825-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z, Shen J, Chan MTV, Wu WKK. The long non-coding RNA SPRY4-IT1: An emerging player in tumorigenesis and osteosarcoma. Cell Prolif. 2018 doi: 10.1111/cpr.12446. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng Z, Liu C, Wu M. New insights into long noncoding RNAs and their roles in glioma. Mol Cancer. 2018;17:61. doi: 10.1186/s12943-018-0812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang L, Tang Y, Xiong F, He Y, Wei F, Zhang S, Guo C, Xiang B, Zhou M, Xie N, Li X, Li Y, Li G, Xiong W, Zeng Z. LncRNAs regulate cancer metastasis via binding to functional proteins. Oncotarget. 2018;9:1426–1443. doi: 10.18632/oncotarget.22840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Cao L, Wu J, Wang Q. Long non-coding RNA SNHG1 regulates NOB1 expression by sponging miR-326 and promotes tumorigenesis in osteosarcoma. Int J Oncol. 2018;52:77–88. doi: 10.3892/ijo.2017.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Zhang Z, Xiong L, Guo C, Jiang T, Zeng L, Li G, Wang J. SNHG1 lncRNA negatively regulates miR-199a-3p to enhance CDK7 expression and promote cell proliferation in prostate cancer. Biochem Biophys Res Commun. 2017;487:146–152. doi: 10.1016/j.bbrc.2017.03.169. [DOI] [PubMed] [Google Scholar]
- 11.Wang B, Ma A, Zhang L, Jin WL, Qian Y, Xu G, Qiu B, Yang Z, Liu Y, Xia Q, Liu Y. POH1 deubiquitylates and stabilizes E2F1 to promote tumour formation. Nat Commun. 2015;6:8704. doi: 10.1038/ncomms9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang FQ, Zhang JQ, Jin JJ, Yang CY, Zhang WJ, Zhang HM, Zheng JH, Weng ZM. HOXA11-AS promotes the growth and invasion of renal cancer by sponging miR-146b-5p to upregulate MMP16 expression. J Cell Physiol. 2018 doi: 10.1002/jcp.26864. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Shen Q, Jiang Y. LncRNA NNT-AS1 promotes the proliferation, and invasion of lung cancer cells via regulating miR-129-5p expression. Biomed Pharmacother. 2018;105:176–181. doi: 10.1016/j.biopha.2018.05.123. [DOI] [PubMed] [Google Scholar]
- 14.Kang W, Huang T, Zhou Y, Zhang J, Lung RWM, Tong JHM, Chan AWH, Zhang B, Wong CC, Wu F, Dong Y, Wang S, Yang W, Pan Y, Chak WP, Cheung AHK, Pang JCS, Yu J, Cheng ASL, To KF. miR-375 is involved in Hippo pathway by targeting YAP1/TEAD4-CTGF axis in gastric carcinogenesis. Cell Death Dis. 2018;9:92. doi: 10.1038/s41419-017-0134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou Q, Yi W, Huang J, Fu F, Chen G, Zhong D. MicroRNA-375 targets PAX6 and inhibits the viability, migration and invasion of human breast cancer MCF-7 cells. Exp Ther Med. 2017;14:1198–1204. doi: 10.3892/etm.2017.4593. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Zhang B, Li Y, Hou D, Shi Q, Yang S, Li Q. MicroRNA-375 inhibits growth and enhances radiosensitivity in oral squamous cell carcinoma by targeting insulin like growth factor 1 receptor. Cell Physiol Biochem. 2017;42:2105–2117. doi: 10.1159/000479913. [DOI] [PubMed] [Google Scholar]
- 17.Zhao S, Wang Y, Luo M, Cui W, Zhou X, Miao L. Long noncoding RNA small nucleolar RNA host gene 1 (SNHG1) promotes renal cell carcinoma progression and metastasis by negatively regulating miR-137. Med Sci Monit. 2018;24:3824–3831. doi: 10.12659/MSM.910866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Q, Shan S, Li Y, Zhu D, Jin W, Ren T. Long noncoding RNA SNHG1 promotes non-small cell lung cancer progression by up-regulating MTDH via sponging miR-145-5p. FASEB J. 2018;32:3957–3967. doi: 10.1096/fj.201701237RR. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Y, Li B, Liu Z, Jiang L, Wang G, Lv M, Li D. Up-regulation of lncRNA SNHG1 indicates poor prognosis and promotes cell proliferation and metastasis of colorectal cancer by activation of the Wnt/beta-catenin signaling pathway. Oncotarget. 2017;8:111715–111727. doi: 10.18632/oncotarget.22903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q, Li Q, Zhou P, Deng D, Xue L, Shao N, Peng Y, Zhi F. Upregulation of the long non-coding RNA SNHG1 predicts poor prognosis, promotes cell proliferation and invasion, and reduces apoptosis in glioma. Biomed Pharmacother. 2017;91:906–911. doi: 10.1016/j.biopha.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Hayes EL, Lewis-Wambi JS. Mechanisms of endocrine resistance in breast cancer: an overview of the proposed roles of noncoding RNA. Breast Cancer Res. 2015;17:40. doi: 10.1186/s13058-015-0542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duchaine TF, Fabian MR. Mechanistic insights into MicroRNA-Mediated gene silencing. Cold Spring Harb Perspect Biol. 2018 doi: 10.1101/cshperspect.a032771. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan JW, Lin JS, He XX. The emerging role of miR-375 in cancer. Int J Cancer. 2014;135:1011–1018. doi: 10.1002/ijc.28563. [DOI] [PubMed] [Google Scholar]
- 24.He XX, Chang Y, Meng FY, Wang MY, Xie QH, Tang F, Li PY, Song YH, Lin JS. MicroRNA-375 targets AEG-1 in hepatocellular carcinoma and suppresses liver cancer cell growth in vitro and in vivo. Oncogene. 2012;31:3357–3369. doi: 10.1038/onc.2011.500. [DOI] [PubMed] [Google Scholar]
- 25.Chang Y, Yan W, He X, Zhang L, Li C, Huang H, Nace G, Geller DA, Lin J, Tsung A. miR-375 inhibits autophagy and reduces viability of hepatocellular carcinoma cells under hypoxic conditions. Gastroenterology. 2012;143:177–187. e178. doi: 10.1053/j.gastro.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Liu AM, Poon RT, Luk JM. MicroRNA-375 targets Hippo-signaling effector YAP in liver cancer and inhibits tumor properties. Biochem Biophys Res Commun. 2010;394:623–627. doi: 10.1016/j.bbrc.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 27.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Lian YJ, Ma YQ, Wu CJ, Zheng YK, Xie NC. LncRNA SNHG1 promotes alpha-synuclein aggregation and toxicity by targeting miR-15b-5p to activate SIAH1 in human neuroblastoma SH-SY5Y cells. Neurotoxicology. 2017 doi: 10.1016/j.neuro.2017.12.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Sun Y, Wei G, Luo H, Wu W, Skogerbo G, Luo J, Chen R. The long noncoding RNA SNHG1 promotes tumor growth through regulating transcription of both local and distal genes. Oncogene. 2017;36:6774–6783. doi: 10.1038/onc.2017.286. [DOI] [PubMed] [Google Scholar]
- 30.Kang W, Cheng AS, Yu J, To KF. Emerging role of Hippo pathway in gastric and other gastrointestinal cancers. World J Gastroenterol. 2016;22:1279–1288. doi: 10.3748/wjg.v22.i3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang W, Tong JH, Chan AW, Lee TL, Lung RW, Leung PP, So KK, Wu K, Fan D, Yu J, Sung JJ, To KF. Yes-associated protein 1 exhibits oncogenic property in gastric cancer and its nuclear accumulation associates with poor prognosis. Clin Cancer Res. 2011;17:2130–2139. doi: 10.1158/1078-0432.CCR-10-2467. [DOI] [PubMed] [Google Scholar]
- 32.Kapoor A, Yao W, Ying H, Hua S, Liewen A, Wang Q, Zhong Y, Wu CJ, Sadanandam A, Hu B, Chang Q, Chu GC, Al-Khalil R, Jiang S, Xia H, Fletcher-Sananikone E, Lim C, Horwitz GI, Viale A, Pettazzoni P, Sanchez N, Wang H, Protopopov A, Zhang J, Heffernan T, Johnson RL, Chin L, Wang YA, Draetta G, DePinho RA. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell. 2014;158:185–197. doi: 10.1016/j.cell.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai ZC, Guan KL. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]


