Abstract
Triple-negative breast cancer (TNBC), the most difficult-to-treat breast cancer subtype, lacks well-defined molecular targets. TNBC has increased programmed death-ligand 1 (PD-L1) expression, and its immunosuppressive nature makes it suitable for immune checkpoint blockade therapy. However, the response rate of TNBC to anti-PD-L1 or anti-programmed cell death protein 1 (PD-1) therapy remains unsatisfactory, as only 10-20% of TNBC patients have a partial response. Glycosylated PD-L1, the functional form of PD-L1, is required for PD-L1-PD-1 interaction. TNBC cells have significantly higher levels of glycosylated PD-L1 than non-TNBC cells do. In a screening of glucose analogs to block PD-L1 glycosylation, we found that 2-deoxyglucose (2-DG) can act as a glucose analog to decrease PD-L1 glycosylation. Because PARP inhibition upregulates PD-L1, 2-DG reduced PARP inhibition-mediated expression of glycosylated PD-L1. The combination of PARP inhibition and 2-DG had potent anti-tumor activity. Together, our results provide a strong rationale for investigating the targeting of PD-L1 glycosylation in TNBC further.
Keywords: 2-deoxyglucose, 2-DG, glycosylation, PD-L1, PD-1, immunosuppression, PARP inhibitor, triple-negative breast cancer
Introduction
Compared with other breast cancer subtypes, triple-negative breast cancer (TNBC), which lacks estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2) overexpression, is more aggressive and is associated with higher rates of relapse and mortality. TNBC’s heterogeneity and lack of well-defined molecular targets pose treatment challenges; in particular, TNBC has a short-lived response to systemic chemotherapy, the standard of care, and invariably develops resistance to such treatment. Thus, novel effective therapies for TNBC are urgently needed.
Immunotherapy is emerging as a promising cancer therapy including breast cancer, especially TNBC. TNBC has higher genomic instability and higher mutation rates, which can lead to the production of neoantigens and increased immunogenicity [1,2]. TNBC also has increased expression of the programmed death-ligand 1 (PD-L1) in the tumor microenvironment [3], making the disease an ideal candidate for immune checkpoint blockade therapy. The programmed cell death protein 1 (PD-1)/PD-L1 inhibitory pathway can silence the immune system by increasing the expression of PD-L1 on the tumor cell surface [4]. Anti-PD-L1 or anti-PD-1 antibodies can restore T-cell function and increase the efficacy of standard chemotherapy for TNBC. The impressive and durable clinical responses achieved with checkpoint blockade immunotherapy resulted in the U.S. Food and Drug Administration’s approval of ipilimumab, nivolumab, pembrolizumab, and atezolizumab for the treatment of multiple cancer types, including melanoma, Hodgkin lymphoma, lung cancer, and bladder cancer [5-8]. However, the therapeutic efficacy of anti-PD-L1 or anti-PD-1 antibody in TNBC was not satisfactory. Post-translational modifications such as glycosylation control the critical biological activity of proteins and occur on approximately two-thirds of all proteins [9]. Glycosylated PD-L1, the functional form of PD-L1, interacts with PD-1 to suppress effector T cell activity in the tumor microenvironment [10-12]. In TNBC, PD-L1 is heavily glycosylated, making glycosylated PD-L1 an ideal therapeutic target in TNBC [13].
Poly (ADP-ribose) polymerase (PARP) inhibition (PARPi), by inducing synthetic lethality, is an effective therapeutic strategy against tumors associated with germline mutations in double-strand DNA repair genes [14]. PARPi has achieved promising results in patients with BRCA-mutated breast cancer in clinical trials. Olaparib was the first PARP inhibitor to show a significant progression-free survival benefit over standard therapy in a randomized phase III trial in patients with BRCA-mutated, HER2-negative metastatic breast cancer [15]. Another PARP inhibitor, talazoparib, achieved similar results in the EMBRACA study, a large phase III randomized trial in patients with germline BRCA1 or BRCA2 mutation [16]. Thus far, combining PARPi with other targeted therapy, immunotherapy, or chemotherapy is a new strategy in clinical trials. In a previous study, we found that PARPi treatment upregulates the PD-L1 expression of TNBC cells [17], and thus might reduce the response rate of PARPi. The above results suggest that combining PARPi and immunotherapy would be effective against TNBC. Since TNBC has heavily glycosylated PD-L1, which is the functional form of PD-L1. The purpose of this study was to explore the deglycosylation agents which have the potential for combination with PARPi in the treatment of TNBC.
Materials and methods
Cell lines
All cell lines (MB468, BT549, HCC1806 and MB231) were obtained from ATCC (Manassas, VA) and were independently validated by short tandem repeat DNA fingerprinting at MD Anderson Cancer Center. Cells were grown in Dulbecco’s modified Eagle’s medium/F12 medium or RPMI 1640 medium supplemented with 10% fetal bovine serum. PD-L1-stable transfectants in MDA-MB-231 and BT549 cells were selected using puromycin (InvivoGen, San Diego, CA) and have been described previously [17].
Antibodies and agents
2-deoxyglucose (2-DG) was purchased from Sigma-Aldrich (St. Louis, MO; cat. #D8375). Biosciences (Lincoln, NE; cat. #926-08946). The PARPi (Olaparib and Talazoparib) and the anti-PD-L1 antibody (cat. #13684) were purchased from Cell Signaling Technology (Danvers, MA), and α-tubulin (cat. #B-5-1-2) was obtained from Sigma-Aldrich. Olaparib and talazoparib were purchased from Selleckchem (Houston, TX). Cycloheximide was purchased from Sigma-Aldrich. The human anti-CD274 (B7-H1, PD-L1) antibody for the T-cell killing assay was from BioLegend (San Diego, CA; cat. #329709).
Detection of cell-surface PD-L1
For the detection of cell-surface PD-L1, cells were suspended in 100 µL of cell staining buffer (BioLegend; cat. #420201) and incubated with the allophycocyanin (APC)-conjugated anti-human PD-L1 antibody (BioLegend; cat. #329708) at room temperature for 30 minutes. The stained cells were washed in the staining buffer and then analyzed using a flow cytometer (BD Biosciences, San Jose, CA).
PD-L1/PD-1 interaction assay
Cells were incubated with 5 mg/mL recombinant human PD-1 Fc chimera protein (R&D Systems, Minneapolis, MN; cat. #1086-PD-050) at room temperature for 60 minutes. The cells were washed with staining buffer and then incubated with anti-human Alexa Fluor 488-conjugated antibody (Thermo Fisher Scientific, Waltham, MA) at room temperature for 30 minutes. The cells were then washed in staining buffer and then analyzed by fluorescence-activated cell sorting (FACS). The FACS data were analyzed using the FlowJo software program (Tree Star, Inc., Ashland, OR); the cut-off value for the relative positive percentage was the median of the maximum signal. For monitoring dynamic PD-1 protein binding on the live cell surface, PD-L1-expressing BT549 cells were incubated with Alexa Fluor 488-conjugated PD-1 Fc protein and images of the cells were captured every hour using an IncuCyte ZOOM microscope (Essen BioScience, Ann Arbor, MI).
T cell-mediated tumor cell killing assay
MDA-MB-231 cells were seeded into a 24-well plate and NucLight Red (nuclear-labeled red fluorescent protein)-expressing MDA-MB-231 cells (Essen BioScience; cat. #4457) were seeded into a 96-well plate. Human peripheral blood mononuclear cells (PBMCs) obtained from Stemcell Technologies (Vancouver, Canada; cat. #70025) were activated with 100 ng/mL anti-CD3 antibody, 100 ng/mL anti-CD28 antibody, and 10 ng/mL interleukin 2 (BioLegend; cat. #317303, #302913, and #589102, respectively) and then cocultured with MDA-MB-231 cells or NucLight Red-expressing MDA-MB-231 cells at a 15:1 ratio with treatment of PARPi (olaparib or talazoparib) and/or 2-DG for 48 hours. The NucLight Red-expressing MDA-MB-231 cells were cocultured with fluorescence caspase-3/7 substrate (Essen BioScience; cat. #4440).
Statistical analysis
Statistical analyses were performed using the SPSS Statistics 20.0 software program (SPSS, Chicago, IL). The Student t-test was used for experimental data. P values <0.05 were considered statistically significant.
Results
2-DG deglycosylates PD-L1 protein
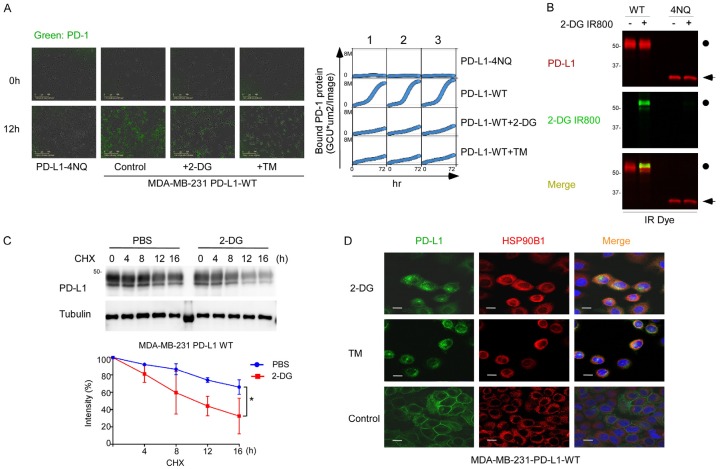
2-DG treatment led to a band shift of PD-L1 from ~45 kDa to 33 kDa (Figure 1A, lane 3; Figure 1B, lanes 2 vs 4), suggesting that 2-DG downregulates the glycosylation of PD-L1. While Lactose and N-acetylglucosamine didn’t observe the phenomenon. According to our previous experience [10], the 45-kDa PD-L1 is the glycosylated form of PD-L1 (Figure 1A, lane 1), and the 33-kDa PD-L1 is the non-glycosylated form of the protein (Figure 1A, lane 6; Figure 1B, lane 1, 5, 6). Similar effects were observed in other cell lines, including MB468, HCC1806, and BT549 (Figure 1C). To investigate whether 2-DG downregulates the glycosylation of PD-L1 induced by interferon-gamma (IFN-γ) or epithelial growth factor (EGF), we pretreated BT-549 and MB468 cells with IFN-γ and EGF before the addition of 2-DG. That IFN-γ mediates the transcriptional regulation of PD-L1 via STAT or NF-κB is well established. EGF did not influence PD-L1 mRNA expression but enhanced PD-L1 glycosylation through the inactivation of GSK3β, which mediated PD-L1 degradation [10]. Similarly, 2-DG reduced the PD-L1 glycosylation induced by IFN-γ and EGF (Figure 1E). Treating cells with the indicated concentrations of 2-DG reduced PD-L1 glycosylation in a dose-dependent manner (Figure 1D). PD-L1 expressed on the surface of cancer cells exerts immunosuppressive effects by binding with a PD-1 receptor on activated T cells. To determine whether 2-DG affects cell-surface PD-L1 expression levels, we treated IFN-γ-pretreated BT549 cells with 2-DG and tunicamycin. Cell-surface PD-L1 expression was assessed using an APC-conjugated PD-L1 antibody, and cells were analyzed with FACS. The cell-surface PD-L1 expression level was significantly reduced after treatment with 2-DG and tunicamycin (Figure 1F). To investigate the functional significance of PD-L1’s downregulation by 2-DG, we assessed whether 2-DG alters the interaction between PD-1 and PD-L1 and found that 2-DG significantly reduced PD-1 binding levels in BT549 cells (Figure 1F). Together, these results suggest that 2-DG downregulates the glycosylation of both endogenous and cytokine-induced PD-L1.
Figure 1.
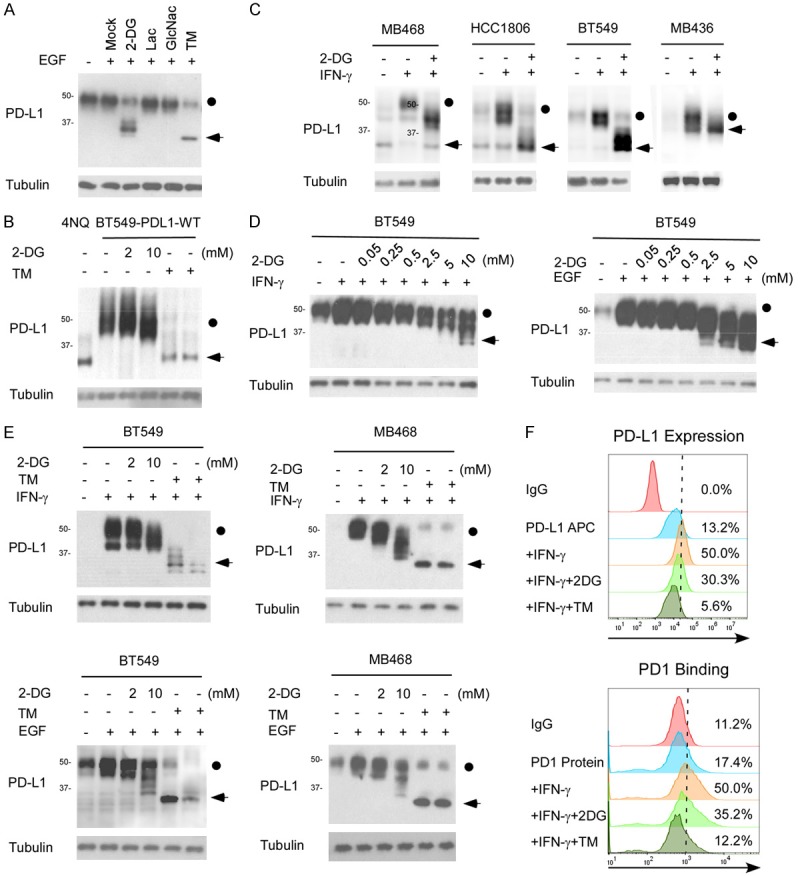
2-DG deglycosylates PD-L1 in TNBC. A. Western blot analysis of PD-L1 protein expression in MDA-MB-231 cells treated with 2-DG, lactacystin (Lac), N-acetylglucosamine (GlcNac), or tunicamycin (TM). The dot indicates the glycosylated PD-L1 and the arrow indicates the deglycosyalted PD-L1. B. PDL1-overexpressing BT549 cells were treated with 2 or 10 mmol/L 2DG or with 1 µgml-1 TM for 12 hours and then subjected to immunoblotting with antibodies against PD-L1. BT549-4NQ cells were included as a negative control. C. Western blot analysis of PD-L1 protein expression in MB468, HCC1806, and BT549 cells treated with IFN-γ with or without 2-DG. D. BT549 cells were treated with indicated concentrations of 2-DG and IFN-γ or EGF for 12 hours, and then PD-L1 protein expression was determined by Western blot analysis. E. Western blot analysis of PD-L1 protein expression in MB468 and BT549 cells treated with 2 or 10 mmol/L 2-DG in combination with IFN-γ or EGF. TM treatment was used as the positive deglycosylation control. F. PD-L1 expression and PD-1 binding on the surface of BT549 cells were analyzed with FACS. The cells were treated with 10 mmol/L 2-DG, 1 µgml-1 TM, or 1 µgml-1 IFN-γ for 12 hours.
2-DG deglycosylates PARPi-induced PD-L1 protein
Our western blot analysis revealed that PARPi consistently enhanced PD-L1 expression in MDA-MB-231 and BT549 cells which were consistent with our previous result [17]. To validate whether 2-DG reduces the PARPi-induced upregulation of PD-L1, we treated MDA-MB-231 and BT549 cells with 2-DG and/or PARP inhibitors. 2-DG significantly reduced not only PARPi-induced PD-L1 expression but also the basal level of PD-L1 (Figure 2A, 2B). Similarly, the cell-surface PD-L1 expression levels were significantly decreased after 2-DG treatment. As a result, 2-DG also reduced the binding of PD-1 to PD-L1 on the cell surface (Figure 2C). Together, these results indicate that 2-DG deglycosylates PARPi-induced PD-L1 protein in TNBC.
Figure 2.
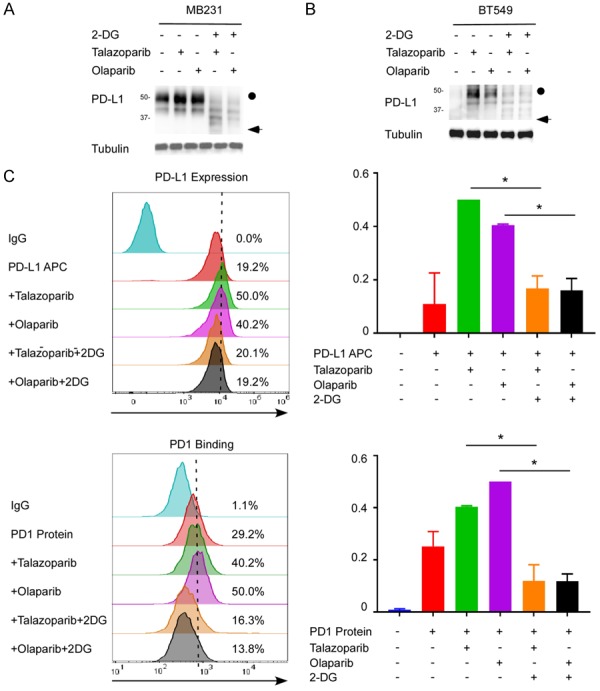
2-DG deglycosylates PARPi-induced PD-L1 protein in TNBC. A. Western blot analysis of the PD-L1 protein expression of MDA-MB-231 cells treated with 2-DG and/or PARPi (talazoparib or olaparib). B. Western blot analysis of the PD-L1 protein expression of BT549 cells treated with 2-DG and/or PARPi (talazoparib or olaparib). C. PD-L1 expression and PD-1 binding on the surface of MDA-MB-231 cells treated with 2-DG and/or PARPi were analyzed with FACS. *P<0.05.
2-DG downregulates PD-L1-PD-1 interaction and decreases PD-L1 translocation and stabilization
Because 2-DG suppressed cell-surface PD-L1 expression levels, we next sought to determine whether 2-DG affects PD-L1 stability. Time-lapse images showed that 2-DG significantly reduced the amount of PD-1 protein bound to cell-surface PD-L1 continuously (Figure 3A). 2-DG, as a glucose analog, was incorporated into the PD-L1 glycosylation process (Figure 3B). In the presence of the protein synthesis inhibitor cycloheximide, the turnover rate of PD-L1 with the treatment of 2-DG was faster than that in the control group (Figure 3C). In addition, 2-DG trapped PD-L1 inside the endoplasmic reticulum (Figure 3D). These results suggest that 2-DG reduces cell-surface PD-L1 expression levels by reducing the normal glycosylation of PD-L1.
Figure 3.
2-DG decreases PD-L1 translocation and stabilization. A. Left, The quantitative binding of PD-1 Fc protein on PD-L1-overexpressing MDA-MB-231 cells was assessed at the indicated times. Cells were treated with 10 mmol/L 2-DG, 1 µgml-1 tunicamycin (TM), or 10 µmol/L olaparib. Right, Images of PD1 Fc protein on PD-L1-overexpressing MDA-MB-231 cells from 0 to 72 hours. B. Western blot analysis of PD-L1 protein expression in PD-L1-overexpressing MDA-MB-231 cells and MDA-MB-231-4NQ cells treated with 2 mmol/L 2-DG IR800. C. Western blot analysis of PD-L1 protein expression in PD-L1-overexpressing MDA-MB-231 cells. Cells were treated with 20 mM cycloheximide (CHX) with or without 2 mmol/L 2-DG at the indicated times. The intensity of PD-L1 protein expression was quantified using a densitometer. *P<0.05. D. Confocal microscopy image showing HSP90B1 and PD-L1 expression in PD-L1-overexpressing MDA-MB-231 cells after treatment with 2-DG. Scale bar, 20 mm.
2-DG re-sensitizes PARPi-treated cancer cells to T-cell killing
To understand the functional significance of the downregulation of PD-L1 by 2-DG, we performed a T-cell-mediated killing assay by co-culturing activated human PBMCs with MDA-MB-231 cells treated with 2-DG and/or PAPRi with olaparib or talazoparib. To find the 2-DG concentration that does not significantly affect cell growth, we tested several 2-DG concentrations with or without PARPi with 10 µM olaparib or 10 nM talazoparib; we used 2 mM 2-DG as our final concentration (Figure S1A-C). PARPi-treated cells were strongly resistant to activated T-cell killing, which is consistent with the findings of our previous study [17]. 2-DG sensitized the PARPi-treated cells to T-cell killing, and the combination treatment group (2-DG+PARPi) had the best killing efficiency (Figure 4A-D). These results indicate that 2-DG re-sensitizes PARPi-treated MDA-MB-231 cells to activated T-cell killing.
Figure 4.
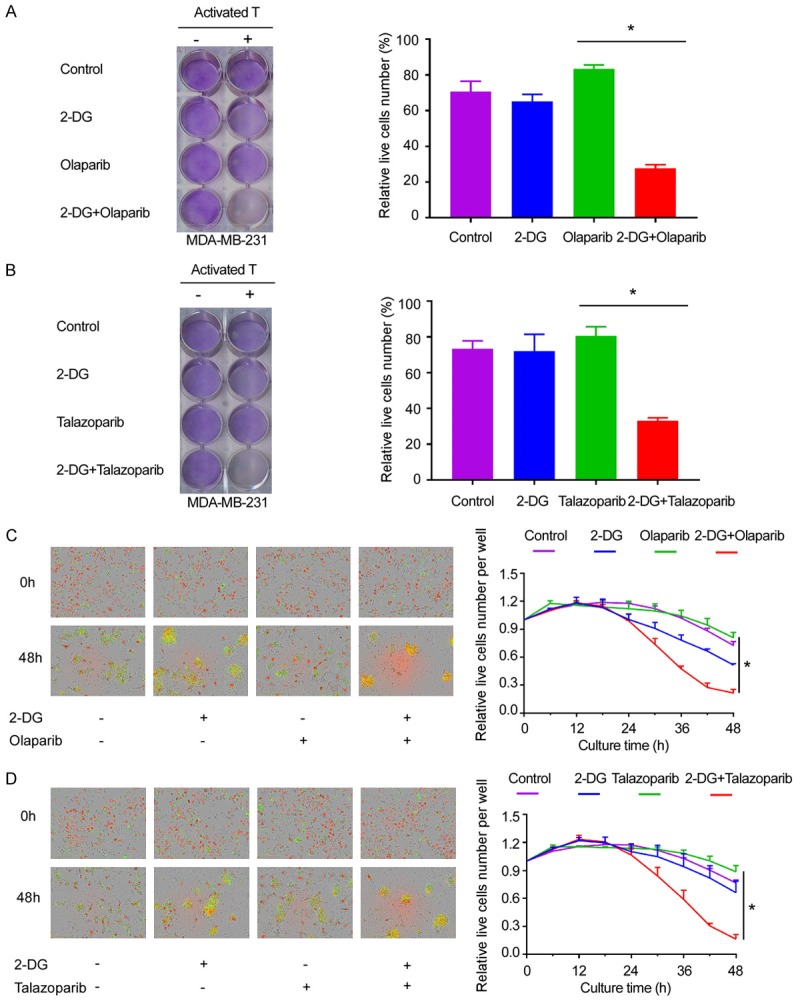
2-DG enhances T-cell killing efficiency. A. PBMCs were co-cultured with MDA-MB-231 cells at a 15:1 ratio for 48 hours and treated with 2 mmol/L 2-DG and/or 10 µmol/L olaparib. Left, the cells were stained with crystal violet. Right, the percentages of live MDA-MB-231 cells co-cultured with activated PBMCs (normalized to MDA-MB-231 cells cultured without PBMCs) at 48 hours. *P<0.05. B. MDA-MB-231 cells were cultured as those shown as in panel and cells were then treated with 2 mmol/L 2-DG and/or 10 nmol/L talazoparib. Left, the cells were stained with crystal violet. Right, the percentage of live MDA-MB-231 cells co-cultured with activated PBMCs (normalized to those cultured without PBMCs) at 48 hours. *P<0.05. C. MDA-MB-231 cells expressing nuclear red fluorescent protein (RFP) seeded in the 96-well plate were first treated as those shown in panel a. Left, representative merged images showing red fluorescent (nuclear-restricted RFP) and green fluorescent (caspase-3/7 substrate) objects. Images were captured using an IncuCyte Zoom microscope. Right, The number of live cells (red fluorescent objects) at 6 hours interval were counted and normalized to that at the zero time point. *P<0.05. D. MDA-MB-231 cells expressing nuclear RFP were first treated as those shown in panel b were. Left, Representative merged images showing red fluorescent (nuclear-restricted RFP) and green fluorescent (caspase-3/7 substrate) objects. Images were captured using an IncuCyte ZOOM microscope. Right, the number of live cells (red fluorescent objects) were counted at 6 hours interval were counted and normalized to that at the zero time point. *P<0.05.
Discussion
Although the metabolic effects of 2-DG have been well studied, the role of 2-DG in cancer-associated immunity is still largely unknown. The results of our study demonstrate that 2-DG reduces cell-surface PD-L1 expression primarily through the deglycosylation of PD-L1, which resensitized cancer cells to T-cell-mediated cytotoxicity. These data provide a strong rationale for using 2-DG in combination with PARP inhibitors that upregulate PD-L1 in TNBC.
2-DG is most frequently used to inhibit glucose metabolism. 2-DG is phosphorylated by hexokinase, resulting in the depletion of intracellular ATP and the induction of autophagy [18]. However, 2-DG’s high dose requirement as a single agent (65-100 mg/kg body weight) [19,20] limits its application in the clinic. Several recent studies have reported that 2-DG enhances the anticancer effects of other drugs in prostate, lung, and breast cancer [21-24]. Because glucose is also a key source of polysaccharide compositions on glycoproteins, the energy-independent function of glucose for glycoproteins in tumor progression is of interest. By depleting the cell of available glucose, 2-DG also inhibits protein glycosylation, trapping proteins in the endoplasmic reticulum and triggering the unfolded protein response, which can induce apoptosis [25,26]. 2-DG is a glucose analog that can be incorporated into cells via glucose transporters. In our study, we found that 2-DG could be incorporated into the glycoprotein (Figure 3A), which might change the glycosylation structure and thereby affect various biological functions related to glycoproteins.
Our previous study showed that, although glycosylation is involved in many co-inhibitory signaling interactions, co-stimulatory signaling does not require glycosylation [12]. 2-DG might change the glycan structure of the inhibitory interaction proteins. Thus, 2-DG might have a more substantial effect on relieving the immunosuppressive status of tumors by 1) restricting the energy for cancer cell metabolism, 2) deglycosylating PD-L1 for degradation, and 3) disrupting PD-L1-PD-1 interaction. Therefore, combination 2-DG could re-sensitize the drugs that induce PD-L1 expression, which might play an important role in the resistance.
Previous studies showed that immune checkpoint blockade has promise for the treatment of TNBC. However, antibodies targeting PD-1 or PD-L1 did not elicit a satisfactory response rate [27-29] and offered a survival benefit no better than that achieved with traditional treatment in patients with metastatic TNBC. Only a minority of patients benefit from the therapy, and the predictive biomarkers for patient selection are not clear. Improving the response rates, the durability of response and overall survival remain challenges in practice. Combination strategies with potential synergistic treatment, primarily with chemotherapy, radiotherapy, targeted therapies, as well as other immune therapies, to improve overall response rate and OS are actively studied in various clinical trials [30-34]. Our previous study showed that combination therapy with gefitinib and an anti-PD-1 antibody could enhance the treatment result [10].
In this study, we found that 2-DG reduced the PD-L1 expression on the cell surface, which induce PD-L1 trapped inside the endoplasmic reticulum. We demonstrated that 2-DG reverses the PARPi-induced upregulation of PD-L1 by deglycosylating PD-L1 in TNBC. Our results provide a strong scientific base for investigating the combination of 2-DG and PARPi in TNBC further.
Acknowledgements
This work was funded in part by the following: National Institutes of Health (CCSG CA016672; R01 CA211615, U01 CA201777); Cancer Prevention & Research Institutes of Texas (RP160710); National Breast Cancer Foundation, Inc.; Breast Cancer Research Foundation (BCRF-17-069); Patel Memorial Breast Cancer Endowment Fund; The University of Texas MD Anderson-China Medical University and Hospital Sister Institution Fund; NIH T32 Training Grant in Cancer Biology (5T32CA186892; to L.-C.C.).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Safonov A, Jiang T, Bianchini G, Győrffy B, Karn T, Hatzis C, Pusztai L. Immune gene expression is associated with genomic aberrations in breast cancer. Cancer Res. 2017;77:3317–24. doi: 10.1158/0008-5472.CAN-16-3478. [DOI] [PubMed] [Google Scholar]
- 2.Vonderheide RH, Domchek SM, Clark AS. Immunotherapy for breast cancer: what are we missing? Clin Cancer Res. 2017;23:2640–6. doi: 10.1158/1078-0432.CCR-16-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM, Akcakanat A, Chawla A, Curran M, Hwu P, Sharma P, Litton JK, Molldrem JJ, Alatrash G. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–70. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zielinski C, Knapp S, Mascaux C, Hirsch F. Rationale for targeting the immune system through checkpoint molecule blockade in the treatment of non-small-cell lung cancer. Ann Oncol. 2013;24:1170–9. doi: 10.1093/annonc/mds647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbé C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 7.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O’Donnell PH, Balmanoukian A, Loriot Y, Srinivas S, Retz MM, Grivas P, Joseph RW, Galsky MD, Fleming MT, Petrylak DP, Perez-Gracia JL, Burris HA, Castellano D, Canil C, Bellmunt J, Bajorin D, Nickles D, Bourgon R, Frampton GM, Cui N, Mariathasan S, Abidoye O, Fine GD, Dreicer R. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–20. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baccelli I, Stenzinger A, Vogel V, Pfitzner BM, Klein C, Wallwiener M, Scharpff M, Saini M, Holland-Letz T, Sinn HP, Schneeweiss A, Denkert C, Weichert W, Trumpp A. Co-expression of MET and CD47 is a novel prognosticator for survival of luminal breast cancer patients. Oncotarget. 2014;5:8147–60. doi: 10.18632/oncotarget.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li CW, Lim SO, Xia W, Lee HH, Chan LC, Kuo CW, Khoo KH, Chang SS, Cha JH, Kim T, Hsu JL, Wu Y, Hsu JM, Yamaguchi H, Ding Q, Wang Y, Yao J, Lee CC, Wu HJ, Sahin AA, Allison JP, Yu D, Hortobagyi GN, Hung MC. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun. 2016;7:12632. doi: 10.1038/ncomms12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim SO, Li CW, Xia W, Cha JH, Chan LC, Wu Y, Chang SS, Lin WC, Hsu JM, Hsu YH, Kim T, Chang WC, Hsu JL, Yamaguchi H, Ding Q, Wang Y, Yang Y, Chen CH, Sahin AA, Yu D, Hortobagyi GN, Hung MC. Deubiquitination and Stabilization of PD-L1 by CSN5. Cancer Cell. 2016;30:925–39. doi: 10.1016/j.ccell.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li CW, Lim SO, Chung EM, Kim YS, Park AH, Yao J, Cha JH, Xia W, Chan LC, Kim T, Chang SS, Lee HH, Chou CK, Liu YL, Yeh HC, Perillo EP, Dunn AK, Kuo CW, Khoo KH, Hsu JL, Wu Y, Hsu JM, Yamaguchi H, Huang TH, Sahin AA, Hortobagyi GN, Yoo SS, Hung MC. Eradication of triple-negative breast cancer cells by targeting glycosylated PD-L1. Cancer Cell. 2018;33:187–201. e10. doi: 10.1016/j.ccell.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung JC, Reithmeier RA. Scanning N-glycosylation mutagenesis of membrane proteins. Methods. 2007;41:451–9. doi: 10.1016/j.ymeth.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Sonnenblick A, de Azambuja E, Azim HA Jr, Piccart M. An update on PARP inhibitors--moving to the adjuvant setting. Nat Rev Clin Oncol. 2015;12:27–41. doi: 10.1038/nrclinonc.2014.163. [DOI] [PubMed] [Google Scholar]
- 15.Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A, Wu W, Goessl C, Runswick S, Conte P. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–33. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 16.Litton J, Rugo HS, Ettl J, et al. EMBRACA: a phase 3 trial comparing talazoparib, an oral PARP inhibitor, to physician’s choice of therapy in patients with advanced breast cancer and a germline BRCA mutation. Cancer Research. 2018:78. [Google Scholar]
- 17.Jiao S, Xia W, Yamaguchi H, Wei Y, Chen MK, Hsu JM, Hsu JL, Yu WH, Du Y, Lee HH, Li CW, Chou CK, Lim SO, Chang SS, Litton J, Arun B, Hortobagyi GN, Hung MC. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res. 2017;23:3711–20. doi: 10.1158/1078-0432.CCR-16-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–46. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 19.Raez LE, Papadopoulos K, Ricart AD, Chiorean EG, Dipaola RS, Stein MN, Rocha Lima CM, Schlesselman JJ, Tolba K, Langmuir VK, Kroll S, Jung DT, Kurtoglu M, Rosenblatt J, Lampidis TJ. A phase I dose-escalation trial of 2-deoxy-Dglucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2013;71:523–30. doi: 10.1007/s00280-012-2045-1. [DOI] [PubMed] [Google Scholar]
- 20.Stein M, Lin H, Jeyamohan C, Dvorzhinski D, Gounder M, Bray K, Eddy S, Goodin S, White E, Dipaola RS. Targeting tumor metabolism with 2-deoxyglucose in patients with castrate-resistant prostate cancer and advanced malignancies. Prostate. 2010;70:1388–94. doi: 10.1002/pros.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben Sahra I, Laurent K, Giuliano S, Larbret F, Ponzio G, Gounon P, Le Marchand-Brustel Y, Giorgetti-Peraldi S, Cormont M, Bertolotto C, Deckert M, Auberger P, Tanti JF, Bost F. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 2010;70:2465–75. doi: 10.1158/0008-5472.CAN-09-2782. [DOI] [PubMed] [Google Scholar]
- 22.Cheng G, Zielonka J, Dranka BP, McAllister D, Mackinnon AC Jr, Joseph J, Kalyanaraman B. Mitochondria-targeted drugs synergize with 2-deoxyglucose to trigger breast cancer cell death. Cancer Res. 2012;72:2634–44. doi: 10.1158/0008-5472.CAN-11-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao J, Cui S, Li S, Du C, Tian J, Wan S, Qian Z, Gu Y, Chen WR, Wang G. Targeted cancer therapy with a 2-deoxyglucose-based adriamycin complex. Cancer Res. 2013;73:1362–73. doi: 10.1158/0008-5472.CAN-12-2072. [DOI] [PubMed] [Google Scholar]
- 24.Maschek G, Savaraj N, Priebe W, Braunschweiger P, Hamilton K, Tidmarsh GF, De Young LR, Lampidis TJ. 2-deoxy-D-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res. 2004;64:31–4. doi: 10.1158/0008-5472.can-03-3294. [DOI] [PubMed] [Google Scholar]
- 25.Xi H, Kurtoglu M, Liu H, Wangpaichitr M, You M, Liu X, Savaraj N, Lampidis TJ. 2-Deoxy-D-glucose activates autophagy via endoplasmic reticulum stress rather than ATP depletion. Cancer Chemother Pharmacol. 2011;67:899–910. doi: 10.1007/s00280-010-1391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung HJ, Duran EM, Kurtoglu M, Andreansky S, Lampidis TJ, Mesri EA. Activation of the unfolded protein response by 2-deoxy-D-glucose inhibits Kaposi’s sarcoma-associated herpesvirus replication and gene expression. Antimicrob Agents Chemother. 2012;56:5794–803. doi: 10.1128/AAC.01126-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basile D, Pelizzari G, Vitale MG, Lisanti C, Cinausero M, Iacono D, Puglisi F. Atezolizumab for the treatment of Breast Cancer. Expert Opin Biol Ther. 2018;18:595–603. doi: 10.1080/14712598.2018.1469619. [DOI] [PubMed] [Google Scholar]
- 28.Adams S, Schmid P, Rugo HS, et al. Phase 2 study of pembrolizumab (pembro) monotherapy for previously treated metastatic triple-negative breast cancer (mTNBC): KEYNOTE-086 cohort A. J. Clin. Oncol. 2017:35. [Google Scholar]
- 29.Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, Pathiraja K, Aktan G, Cheng JD, Karantza V, Buisseret L. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J. Clin. Oncol. 2016;34:2460–7. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McArthur HL, Diab A, Page DB, Yuan J, Solomon SB, Sacchini V, Comstock C, Durack JC, Maybody M, Sung J, Ginsberg A, Wong P, Barlas A, Dong Z, Zhao C, Blum B, Patil S, Neville D, Comen EA, Morris EA, Kotin A, Brogi E, Wen YH, Morrow M, Lacouture ME, Sharma P, Allison JP, Hudis CA, Wolchok JD, Norton L. A pilot study of preoperative single-dose ipilimumab and/or cryoablation in women with early-stage breast cancer with comprehensive immune profiling. Clin Cancer Res. 2016;22:5729–37. doi: 10.1158/1078-0432.CCR-16-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, Minn AJ. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–7. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adams S, Loi S, Toppmeyer DL, et al. KEYNOTE-086 cohort B: pembrolizumab monotherapy for PD-L1-positive, previously untreated, metastatic triple-negative breast cancer (mTNBC) Cancer Res. 2018:78. doi: 10.1093/annonc/mdy518. [DOI] [PubMed] [Google Scholar]
- 34.Tolaney SM, Kalinsky K, Kaklamani V, et al. Phase 1b/2 study to evaluate eribulin mesylate in combination with pembrolizumab in patients with metastatic triple-negative breast cancer. Cancer Res. 2018:78. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.