Abstract
Small, non-coding strands of RNA have been identified as a significant player in the pathology of cancer. One of the first miRNAs to be shown as having aberrant expression in cancer was miR-10b. Since the inaugural study on miR-10b, its role as a metastasis promoting factor has been extensively validated. To date, more than 100 studies have been completed on miR-10b and metastasis across 18 cancer types. This immense set of information holds possibilities for novel methods to improve the lives of many. This review outlines what is currently understood of miR-10b’s clinical significance, its molecular regulation, and the possible diagnostic and therapeutic methods leveraging miR-10b as a fundamental target in metastatic cancer. Such methods would move us closer to developing a truly individualized therapeutic strategy against cancer and will likely provide unique information about cancer staging, disease outcome, metastatic potential, and ultimately survival.
Keywords: Metastasis, microRNA-10b, cancer, epithelial-to-mesenchymal transition
Introduction
Cancer is a complex disease that is the second-leading cause of death in the United States. Understanding of cancer as a genetic disease has greatly progressed over the past decade. Small, non-coding strands of RNA have been identified as a significant player in the pathology of cancer. These microRNAs (miRNAs) have been shown to be differentially expressed across different cancers and tissues.
MiRNAs exert their effect by working with the RNA-induced Silencing Complex (RISC) to pair with target messenger RNA (mRNA) sequences and post-transcriptionally regulate gene expression. It is this effect that allows miRNAs to either promote or suppress the formation and progression of cancerous tumors. The study of miRNA has blossomed in recent years allowing for many new candidates to be identified for further research in hopes of elucidating new methods for diagnosis or therapy.
One of the first miRNAs to be shown as having aberrant expression in cancer was miR-10b in the 2007 study by Ma L, et al. [1]. Since the inaugural study of miR-10b, its role as a metastasis promoting factor has been uncovered. To date, more than 100 studies have been completed on miR-10b and metastasis across 18 cancer types. This immense set of information holds possibilities for novel methods to improve the lives of many. The following review will outline the current stance of the literature on the clinical significance of miR-10b in each type of metastatic cancer, the pathway in which miR-10b exerts its effects, and the possible diagnostic and therapeutic applications.
Pre-clinical and clinical evidence of miR-10b’s role in cancer
MiR-10b is correlated with disease progression in most of the 18 types of cancer studied [1-28]. One of these cancers, glioblastoma, is well investigated but is not highlighted in this review, because it is rarely metastatic.
MiR-10b has been studied to the greatest degree in breast cancer relative to other types of cancer. The increased expression of miR-10b has been associated with multiple outcomes, including increased metastasis [1,6-14], increased invasive potential in vitro [6,29] and in vivo [1,12,30], increased migration [29,30], increased epithelial-mesenchymal transition [31], angiogenesis [1,32,33], and increased proliferation [8,30]. These changes result in worse clinical outcomes, including increased tumor size [8,11,29,34], advanced clinical stage [1,8], and short, relapse-free survival [13,35,36].
MiR-10b is also associated with the expression of already known biomarkers for breast cancer. Regardless of metastatic status, miR-10b expression is positively correlated with HER2 positivity [8,37] and negatively correlated with estrogen and progesterone receptor positivity [8,11]. This association further supports the link between miR-10b and breast cancer metastatic potential, given that HER2 positivity and hormone receptor negativity are known predictors of tumor aggressiveness.
Additional evidence of the pleiotropic effects of miR-10b as a driver of breast cancer invasiveness and metastasis is provided by the proven positive correlation between miR-10b expression and stemness or self-renewal in breast cancer stem cells [31]. Specifically, the authors found that stable overexpression of miR-10b in MCF-7 cells resulted in higher self-renewal and expression of genes that promote stemness, and epithelial-mesenchymal transition. Use of synthetic antagomirs against miR-10b resulted in decreased stem cell self-renewal [31].
miR-10b is also a well-studied biomarker in colorectal cancer. In the predominant number of investigations, miR-10b expression has been correlated to multiple outcomes that reflect its role as an oncogene. It has been repeatedly shown that miR-10b expression is associated with increased invasion [2,4,5,38] and increased metastasis [2-5,39]. Beyond its role in the spread of tumors, miR-10b has been associated with increased tumor size [2] and non-differentiated tumors [2,4], which suggests a role in proliferation as shown for other cancers. These characteristics influenced by miR-10b expression have resulted in associated clinical effects. Increased miR-10b expression is correlated with advanced stage [4,5] and poorer survival [4,38] in colorectal cancer.
In contrast to the above, a study found a decreased level of expression of miR-10b in the paired liver metastasis in comparison to the primary colorectal cancer tumors [40]. In a similar study of primary tumors with paired liver metastases, miR-10b expression was unchanged between controls, disease free, and metastatic tissues [41]. However, both of these studies were based on very small patient cohorts (20 or less) and likely did not have sufficient power to detect any effects.
A deep sequencing study on colorectal cancer tumors and their associated metastases yielded no significant clinicopathological outcomes that were associated with miR-10b except increased expression in tumors of the right colon compared to those of the left colon or rectum [42]. Still, this finding is significant because it is known that patients with a primary tumor on the right side of the colon (cecum, ascending colon, hepatic flexure, and transverse colon), have shorter overall survival (OS) compared with patients whose primary tumors are found on the left side of the colon (splenic flexure, descending colon, sigmoid, and rectum).
Other gastrointestinal malignancies that are linked to miRNA-10b include gastric, hepatocellular, and pancreatic cancer. With respect to these cancers, there is overall consensus in the literature regarding the role of miRNA-10b as an oncogene and driver of metastasis. In gastric cancer, miR-10b was found to be over expressed in metastasis-positive vs. metastasis-negative tumors [25,43]. The greater metastatic ability appears to be through enhanced invasion [25,43,44]. MiR-10b overexpression is also correlated with increased proliferation, migration, size of tumor, and metastasis [43,44]. In terms of clinical outcomes, the expression of miR-10b was correlated with prognosis and advanced clinical stage [43,44].
In hepatocellular carcinoma (HCC), miR-10b was shown to be upregulated in metastatic tissue [20,45]. Increased levels of miR-10b were associated with poorer overall survival and served as an independent prognostic factor [20]. Inhibition of miR-10b resulted in decreased migration and invasion [20], whereas the overexpression of miR-10b induced increased migration [20,45], invasion [20,45], and proliferation [45].
The role of miR-10b in pancreatic cancer is especially pronounced. MiR-10b expression has been shown to be elevated in pancreatic ductal adenocarcinoma (PDAC) in comparison to normal tissue [46,47]. While miR-10b expression levels do not differentiate between stages of PDAC [46], miR-10b is associated with EMT, proliferation, invasion, migration, and tumor growth [47], indicating a pleiotropic effect on disease aggressiveness.
Because of this strong biological association, miR-10b has been established as a significant prognostic biomarker. In EUS-FNA samples, miR-10b expression has been found to be higher in cancerous tissues compared to CK-19 positive benign tissues [18]. As a prognostic factor, higher miR-10b levels at EUS-FNA biopsy predicted shorter metastasis-free survival. Higher miR-10b levels at stages I and II of disease had a three-fold increase in risk of developing metastasis [46]. In a less invasive method than EUS-FNA biopsy, miR-10b plasma levels were increased in PDAC patients in comparison to healthy controls, suggesting that it could be used instead as the diagnostic measure [47,48].
In agreement with the above studies, it was also found that increased miR-10b expression in a common hepatic artery lymph node of patients being resected was correlated with increased disease recurrence and shorter relapse-free survival [49]. The increase of miR-10b expression was found in 14 of 30 studied periampullary carcinomas [49]. Further evidence derives from studies, which found that decreased miR-10b expression is associated with improved response to neoadjuvant therapy [18,46], increased survival [18], delayed time to metastasis [18], and improved likelihood of surgical resection [18,46]. Combined, these studies provide incontrovertible evidence that miR-10b could serve not only as a useful predictive biomarker but also as a potent therapeutic target in malignancies of gastrointestinal origin. The impact of such interventions would be especially high in pancreatic cancer, which is the only cancer with a survival rate of less than 10%. Exocrine pancreatic adenocarcinoma, in particular, is a devastating diagnosis defined by a mere 1% 5-year survival when diagnosed at an advanced inoperable stage, which defines 80% of the cases. Despite overall progress in research, the prognosis for people with this malignancy has not improved in over 40 years. There are currently no treatments found to be effective in the long term for patients with advanced disease who are ineligible for surgery, a prognosis representing the majority of pancreatic cancer diagnoses.
Another aggressive cancer, associated with low survival rates for which miR-10b could represent a useful diagnostic and therapeutic target, is lung cancer. Multiple studies have shown the relationship between increased miR-10b expression and increased metastasis in nonsmall-cell lung cancer (NSCLC) [21-24]. Upon investigation of peripheral blood mononuclear cells (PBMCs), miR-10b expression levels were significantly higher in NSCLC patients as compared to healthy controls. Higher miR-10b expression in NSCLC was correlated with lymph node and distant metastasis [22-24,50]. In terms of clinical outcomes, the higher miR-10b expression was correlated with a worsened 5-year survival rate and poorer prognosis [23,24]. In studying the underlying biology of miR-10b in NSCLC tumors, miR-10b overexpression was correlated to enhanced invasion and migration, contributing to greater metastatic potential [21,51]. Additionally, miR-10b expression was correlated with increased proliferation [51], linking miR-10b to not only metastatic potential but also tumor growth. In lung squamous cell carcinoma, it was found that miR-10b expression correlates with the presence of metastasis but not cancer recurrence, indicating an effect on migration and invasion but a lack of effect on stemness [50].
There is also strong evidence of association between miR-10b expression and disease progression in melanoma. In vitro studies revealed that miR-10b is upregulated in melanoma cells; suggesting that in this cancer, miR-10b may be involved in cancerogenesis [52]. In animal models, miR-10b was identified and verified as a marker of metastasis, showing a 3.7-fold increase between non-metastatic and metastatic primary tumors [17]. Clinical studies revealed that the levels of miR-10b in serum were higher and allowed discrimination between healthy volunteers and melanoma patients as well as between varying clinical stages of melanoma patients [52]. miR-10b expression was positively correlated with increased lymph node metastasis, advanced clinical stage, and shortened survival rate [52]. These results are important because they reveal a dual role of the miRNA in carcinogenesis and disease progression in some of the most common cancer types.
miR-10b has also been implicated in the progression of rare malignancies. An example is presented by nasopharyngeal carcinoma (NPC). NPC is commonly found in southeast Asia and Northern Africa and is closely linked to infection with Epstein-Barr virus (EBV) [53]. In one study, expression of miR-10b for EBV-positive NPC was not associated with metastases at the lymph nodes or at extra-nodal sites. However, miR-10b was associated with advanced T3-T4 clinical stage which suggests miR-10b could enhance proliferation and not migration or invasion for NPC [54]. Conversely, in other studies, miR-10b enhanced metastasis of EBV-positive NPC, but it did not initiate the process in non-metastatic cells [15]. Additionally, it was found that miR-10b does not promote growth of the tumor, but is associated with the mobility of cancer cells [55]. Further study confirmed miR-10b’s role in motility showing its effect in promoting epithelial-mesenchymal transition (EMT) [56].
Additional malignancies for which miR-10b appears to play a pro-tumorigenic, pro-metastatic role include endometrial cancer [57], osteosarcoma [58], esophageal cancer [59], laryngeal cancer [60], retinoic acid induced neuroblastoma [26], and thyroid cancer [19,61]. In all of these cancers, preclinical studies have linked the function of miR-10b to distinct molecular phenotypes including decreased apoptosis and increased proliferation and migration in endometrial cancer [57], increased proliferation, migration, and invasion as well as decreased apoptosis in osteosarcoma [58], increased motility and invasion without an associated effect on metastasis in esophageal cancer [59], increased migration, invasion, and metastasis as well as poorer differentiation in neuroblastoma [26], and increased migration, invasion, and EMT without an effect on proliferation in laryngeal carcinoma [60].
Thyroid cancer represents a distinct area of interest because in that malignancy, there is clear evidence that miR-10b acts as a driver of metastasis. Although minimally invasive follicular thyroid carcinoma (MI-FTC) is usually noninvasive, miR-10b was one of three miRNAs to be upregulated in the cases that did become metastatic [19]. Widely invasive FTC (WI-FTC), which commonly spreads, showed increased expression of miR-10b as well [19].
Contrary to the cancers highlighted above, there are malignancies that are either not strongly associated with miR-10b or in which miR-10b plays a tumor-suppressive role. One example is bladder cancer. Pre-clinical studies in cell lines and animal models showed that miR-10b is overexpressed with metastasis and its expression is associated with increased migration, invasion, and metastasis [28]. Conversely, in clinical studies, miR-10b was found to be downregulated in tumor tissue in comparison to adjacent normal tissue [62]. This study did not find miR-10b to correlate with any clinical parameters, including prognosis and survival [62].
Another example is presented by cervical cancer. The single study examining miR-10b and cervical cancer metastasis included 44 patients with small cell cervical cancer (SCCC) [63]. MiR-10b was found to be down-regulated in advanced-stage SCCC tissues in comparison to early-stage SCCC tissues [63]. However, miR-10b was not associated with metastasis or survival [63].
The most convincing evidence of a tumor-suppressive role of miR-10b is with relevance to clear cell renal cell carcinoma (ccRCC). In ccRCC, miR-10b alone was found to not be associated with any clinical parameters. However, the miR(21/10b) ratio was associated with disease severity and survival [64]. An increased value for the ratio, meaning decreased expression of miR-10b, was correlated with poor prognosis and was an independent prognostic factor for metastasis-free survival [64]. In further study, miR-10b expression was found to decrease in a step-wise fashion from healthy kidney tissue to primary ccRCC tissue to metastatic ccRCC tissue [65-69]. Increased miR-10b expression was also correlated with longer disease-free survival [65,67,68] and, in tumor sizes greater than 4 cm, overall survival [65].
These associations between miR-10b expression and clinical parameters as well as molecular phenotype are summarized in Table 1. They provide a clear record of the global and fundamental role played by miR-10b in human cancer. To a varying degree, depending on cancer type and methodological nuance, miR-10b has been implicated as a driver of tumor cell migration, invasion, differentiation, proliferation and apoptosis. In patients, these effects have translated into increased incidence of metastasis, greater disease aggressiveness, and shorter survival rates. However, while mostly oncogenic, miR-10b could also be associated with tumor-suppressive properties, particularly in renal carcinoma, calling for care in designing therapeutic interventions targeted at this miRNA.
Table 1.
miR-10b influences multiple clinical and molecular parameters across cancer types
| MiR-10b | Tumor size | Metastasis | Advanced stage | Overall survival | EMT | Invasion | Migration | Proliferation | Apoptosis |
|---|---|---|---|---|---|---|---|---|---|
| Colorectal cancer | ↑ | ↑ | ↑ | ↑ | |||||
| Breast cancer | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ||
| Nasopharyngeal carcinoma | ↑ | ↑ | ↓ | ↑ | ↑ | ↑ | |||
| Melanoma | ↑ | ↑ | ↓ | ||||||
| Endometrial cancer | ↑ | ↑ | ↑ | ↓ | |||||
| Pancreatic cancer | ↑ | ↓ | ↑ | ↑ | ↑ | ||||
| Renal cell cancer | ↑ | ↑ | |||||||
| Thyroid cancer | ↑ | ||||||||
| Hepatic cancer | ↑ | ↓ | ↑ | ↑ | |||||
| Lung cancer | ↑ | ↓ | ↑ | ↑ | ↑ | ||||
| Gastric cancer | ↑ | ↑ | ↑ | ↓ | ↑ | ||||
| Retinoic acid-induced neuroblastoma | ↑ | ↑ | ↑ | ||||||
| Esophageal cancer | ↑ | ||||||||
| Osteosarcoma | ↑ | ↑ | ↑ | ↓ | |||||
| Bladder cancer | ↑ | ↑ | ↑ | ||||||
| Laryngeal cancer | ↑ | ↑ | ↑ |
The miR-10b pathway
The molecular pathways in which miR-10b is known to participate are summarized in Figure 1. Overall, there is limited knowledge about upstream effectors of miR-10b expression or function. Here we would like to focus on a well-established upstream regulator of miR-10b that provides a direct link between miR-10b expression and the biological effects that it exerts on cancer progression and metastasis, such as EMT and tumor cell invasion. Namely, we would like to focus on the TWIST-1 member of the TWIST family of transcriptional factors.
Figure 1.
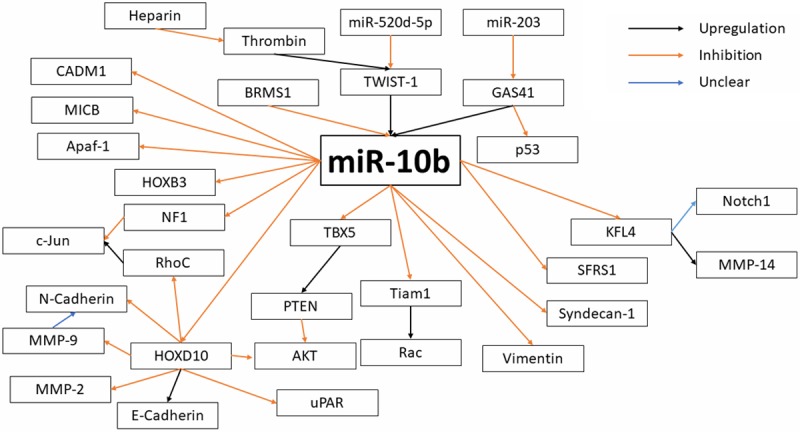
Molecular pathways of miR-10b regulation.
It has been shown across multiple studies that upregulation of TWIST-1 results in an increased expression of miR-10b [1,2,15,54,70,71]. One of the pathways by which TWIST-1 promotes miR-10b expression involves matrix hyaluronan (HA), an extracellular matrix component that serves as the ligand for CD44 [72]. CD44 is a protein implicated in cell-cell interactions, namely cell adhesion and migration. The HA-CD44 complex activates c-SRC which then phosphorylates TWIST-1. When TWIST-1 is phosphorylated, it is in the activated form allowing for promotion of miR-10b expression [72]. With this, tumor cell migration could be promoted.
A second upstream regulator of miR-10b expression is Epstein-Barr virus latent membrane protein 1 (LMP1). In addition to inducing increased expression of TWIST-1 [15,54], LMP1 is linked to mechanisms involving NF-kB [73], a demonstrated promoter of miR-10b, which is critical for EMT [74]. Additionally, LMP1 is linked to inducing EGFR expression, increasing activation of the P13K/Akt pathway resulting in prevention of apoptosis, downregulation of E-Cadherin, increased expression of the MMP family, and the transition from E- to N-Cadherin expression [73]. These processes provide an indirect connection between miR-10b and its role in cell death and migration/invasion.
A less well-known regulator of miR-10b expression is breast cancer metastasis suppressor 1 (BRMS1), a transcription factor that has long been associated with decreased metastasis and improved clinical outcomes [75]. However, the BRMS1 mechanism of action is still not fully understood with a variety of targets but no indication of direct or indirect control [76]. Additionally, the identified targets do not suffice for the complete anti-metastatic effects of BRMS1 [76]. BRMS1 has an inhibitory effect on miR-10b expression, a finding that helps to more completely explain its phenotypic effects [77,78].
Our understanding of the pathway miR-10b uses to exert its effects is constantly evolving. However, the currently known downstream effects of miR-10b can be divided into six pathways: promotion of migration and invasion, promotion of EMT, inhibition of apoptosis, promotion of proliferation, induction of angiogenesis, and disabling of immune checkpoints.
Migration and invasion are the hallmark of metastatic potential. As shown in Table 1 miR-10b is associated with migration and invasion for many of the researched metastatic cancers. Homeobox D10 (HOXD10), a transcription factor, has been shown to be a target of miR-10b, resulting in decreased HOXD10 expression in the presence of increased miR-10b expression [1,5,12,25,28,29,43,45]. The reduced HOXD10 expression was correlated with upregulation of RhoC [1,5,12,25,29,43,45], a Rho GTPase with an essential role in migration and invasion [79]; uPAR [45], a proteinase receptor with a role in migration [80]; MMP-2 [45], a metalloproteinase implicated in migration and invasion [81]; and MMP-9 [45], a metalloproteinase with wide-ranging effects, including being required for invasion by docking to CD44, promoting angiogenesis, and decreasing apoptosis [81]. Reduced HOXD10 expression is also associated with inhibition of E-Cadherin [28], an essential protein for cell adhesion and is correlated with the transition from a benign state to an invasive/metastatic state [82]. Thus, HOXD10 appears to be an essential part of miR-10b promoting invasion and migration.
A recent publication also identified the proto-oncogene c-Jun as a key target at the receiving end of the miR-10b pathway. c-Jun is a transcription factor that plays a critical role in stimulation of cell proliferation and tumor progression. Interestingly, c-Jun is translationally activated by loss of cell contacts or restructuring of the cytoskeleton-a process specific to the metastatic tumor cell and characterized by loss of E-cadherin expression [83]. This accumulation of c-Jun protein is not associated with an increase of c-Jun mRNA or increased MAPK activity [83]. Instead, the effect is mediated by RhoC and NF1, two downstream targets of miR-10b [83]. This is another essential part of the metastasis-promoting effects of miR-10b.
Some studies have identified downstream effects without including the complete pathway elucidation. Increased miR-10b expression results in decreased E-Cadherin expression [2,8,24,55,56,60,71,84], an effect related to reduced HOXD10 expression. E-Cadherin was found to concurrently decrease while N-Cadherin expression increases [60]. N-Cadherin is a protein that promotes migration and invasion [85]. This results in the acquisition of mesenchymal spindle-like morphology.
Increased miR-10b expression is also correlated with decreased Homeobox 3 (HOXB3) expression [57]. HOXB3 is a transcription factor that has been implicated in colorectal, prostate, and lung cancers with possible effects on migration [86]. The overexpression of miR-10b also results in decreased expression of vimentin [56], an intermediate filament protein associated with organizing critical proteins for attachment, migration, and cell signaling [87]. Additionally, miR-10b binds to the 3’-UTR of CDM1 mRNA to repress translation [20]. This relationship was further confirmed by CADM1 being inhibited by miR-10b overexpression [20]. Silencing of CADM1 resulted in a similar increase of migration and invasion to the overexpression of miR-10b [20].
KLF4 is another transcription factor that has been shown to be a target of miR-10b [28,51,56,58,59]. The decreased expression of KLF4 induced by miR-10b has been correlated with inhibition of MMP14 [28], a metallo-proteinase implicated in migration and invasion [81]. Finally, MiR-10b targets T Lymphoma invasion and metastasis-inducing protein 1 (Tiam1), which is a mediator of migration, invasion, and Rac activation, with unclear effects [88,89].
Epithelial-to-mesenchymal transition is an essential part of the metastatic phenotype. As shown in Table I, about a quarter of examined metastatic cancers show a relationship between miR-10b and EMT. As mentioned above, KLF4 is a target of miR-10b [28,51,56,58,59]. The associated inhibition of MMP14 [28] is not only implicated with migration and invasion but EMT as well [81]. In addition, the modulation of KLF4 expression by miR-10b overexpression is also correlated with an increased expression of Notch1, which was found to be a factor in the activation or deactivation of the EMT program [56]. HOXD10 also has a role in EMT through its alteration of RhoC expression. RhoC plays an essential role in metastasis through promotion of EMT in addition to migration and invasion [79].
Although miR-10b does not have a widespread role in apoptosis for metastatic cancers, as shown in Table I, there are aspects of the pathway that have elucidated how this effect is exerted. The overexpression of miR-10b results in decreased expression of Apaf-1 [84], a central scaffold protein of the apoptosome which is an essential part of the apoptosis-induction pathway [90], In addition, MMP-9, a target of HOXD10, has a demonstrated inhibitory role for apoptosis [81]. This link to apoptosis, as well as the association with proliferation, could explain the role of miR-10b in secondary tumor growth, as shown for some cancers. Proliferation is an important part of new colonization by metastasizing tumor cells. As seen in Table I, increased proliferation has been observed as an effect of increased miR-10b expression in metastatic cancers. MiR-10b is shown to inhibit phosphatase and tensin homolog (PTEN), which results in maintained AKT activation [31,84], a Ser/Thr kinase associated with proliferation, apoptosis, and growth [91]. This effect on the PI3K/AKT pathway allows for the improved self-renewal found in cancer stem cells highly expressing miR-10b.
Another study found that miR-10b inhibits TBX5, which, in turn, inhibits PTEN and DYRK1A, proposing that the inhibition of PTEN occurs through TBX5 [30]. This effect of AKT phosphorylation is one that was found to also be correlated with HOXD10 expression suggesting a possible overlap in the proposed pathways [25,43]. Thus, a pathway could include HOXD10 modulating TBX5 which then alters PTEN and subsequently AKT activation.
An additional pathway of increased proliferation through HOXD10 is through uPAR. uPAR has been demonstrated to promote proliferation in addition to its role in migration [80]. Finally, HOXB3 has been implicated with increased proliferation as well as its role in migration as described before [86].
miR-10b’s role in angiogenesis has only been examined in breast cancer. However, there have been several implicated pathways. Heparin has a demonstrated ability to inhibit tumor-associated angiogenesis, and thrombin has been shown to promote angiogenesis [33]. These effects were discovered to be exerted through modulation of miR-10b expression [33]. Thrombin upregulates miR-10b and upstream transcription factor TWIST-1 to result in inhibited HOXD10 expression [33]. Increased HOXD10 expression was shown to arrest angiogenesis [33]. Heparin binds to thrombin to inhibit the observed pro-angiogenic effects from thrombin [33]. Heparin is not able to inhibit miR-10b if thrombin is silenced by its siRNA [33].
A second target of miR-10b to promote angiogenesis is syndecan-1 [32]. Syndecan-1 expression was inversely correlated with miR-10b expression and found to be a predicted target of miR-10b [32]. Syndecan-1 has been demonstrated to modulate tumor angiogenesis [92].
Interestingly, miR-10b was shown to have a role in immune-checkpoint regulation by targeting MICB, a ligand of NKG2D, resulting in worsened elimination of tumor cells by NK cells [93]. Introduction of antistamiR-10b showed greater NKG2D-mediated killing of tumor cells in vitro and greater clearance of tumor in vivo [93]. This is a somewhat novel and unexpected effect of miR-10b that warrants further investigation in the context of immunotherapy.
Possible targets have been identified in other studies as suggestions for future research. A study using a Bayesian Network statistical model along with multibody simulation to identify miRNA-mRNA interactions with clinical features found an interaction model between miR-10b and the genes FOXO1, SATB1, SERPINB5, and STARD10 [94]. SATB1 is a promoter of EMT in breast cancer [95]. FOXO1, SERPINB5, and STARD10 are tumor suppressors for breast cancer [96-98].
With particular relevance to the role of miR-10b in ccRCC, miR-10b can take on a tumor suppressive role. This effect has not been as extensively studied, but the additional potential targets of miR-10b, as a tumor suppressor, are PDGFB [65], ETS1 [65], GRB2 [65], PIK3CA [65,66], PIK3R3 [65], CRK [65,66], BCL2 [65], MDM2 [65], and PAK7 [66].
This evidence provides a glimpse into the complex signaling networks that involve miR-10b as a key player in cancer progression and metastasis. However, complete pathway elucidation is far from completed. Nevertheless, if miR-10b is to be explored as a diagnostic and therapeutic target in cancer, a more complete picture of the molecular function of miR-10b within the tumor cell context is necessary, in order to not only design more efficient interventions but also to be able to predict any off-target effects resulting from these interventions.
Diagnostic applications of miR-10b
The staple of reducing mortality due to cancer has been early detection. One of the most promising features of miR-10b is the ability to use it as a predictive biomarker for cancer. First steps in this direction have already been made. Cerebrospinal fluid (CSF) was studied in patients, and miR-10b was found to be upregulated in the CSF of patients with glioblastoma or brain metastases from breast or lung cancer in comparison to the CSF of patients with tumors in remission or other non-neoplastic conditions [27]. Using miR-10b as part of a seven-miRNA signature, the model differentially detected glioblastoma and metastatic brain cancers with accuracy between 91 and 99% [27].
miR-10b levels could also be measured in the blood plasma. In a study of 4T1 injected BALB/c mice, miR-10b expression reached its peak in week six, the final week [99]. Following surgery at week six, miR-10b levels greatly decreased in plasma suggesting that the tumor released miR-10b into the circulation [99].
MiR-10b plasma levels are also associated with PDAC [47,48]. Patients with pancreatitis are at increased risk for PDAC, and the symptoms often overlap making a dignosis difficult. In the highlighted study, miR-10b plasma levels, measured retrospectively, could predict the presence of PDAC when 19-9 and carcinoembyonic antigen levels, two commonly used cancer biomarkers, were normal [48]. In NSCLC, miR-10b expression in peripheral blood mononuclear cells (PBMCs) was able to differentiate between healthy controls and NSCLC patients with an 86.5% sensitivity and a 76.9% specificity [23].
Using biopsies, miR-10b was used in a model to predict aggressiveness in ccRCCs which had a sensitivity of 93.8% and a specificity of 83.3% [67]. MiR-10b was also used to differentiate between benign and ccRCC tissues with a sensitivity of 86.7% and a specificity of 92.9% [67]. Further development of analysis models for biopsy samples will allow the identification of patients that are at increased risk of progression, an ability not currently available.
A 4-miRNA signature in tumor tissue, that includes miR-10b, created a risk status that was associated with metastasis in clear cell renal carcinoma with an OR of 5.5 [CI: 1.23-24.51, P<0.05] and survival with a relative risk of 12.68 [CI: 2.97-54.13, P<0.0001] for high risk versus low risk classifications by the signature [69]. The success of these models shows the possibilities of using miR-10b as a prognostic factor in the analysis of the existing tumor. Being able to stratify tumors based on aggressiveness will naturally better inform the need for more aggressive treatment and/or the need for increased surveillance of the patient.
In addition to serving as a predictive biomarker for disease progression, miR-10b could also be useful as a diagnostic biomarker. The miR-10a/10b ratio was predictive of the primary site of the tumor in head and neck and lung squamous cell carcinomas with a ROC of 0.922 to 0.982 [100]. This allows for better discrimination between two cancers that arise in similar locations.
The expression of miR-10b has also been correlated with a tumor’s sensitivity to treatments. Measuring the miR-10b levels of the tumor before beginning treatment could better inform therapeutic decisions. MiR-10b expression is negatively correlated to sensitivity to 5-fluorouracil (5-FU)-based therapies [38]. The pathway of effect was found to be miR-10b directly inhibiting pro-apoptotic BIM in vitro, which is a factor targeted by 5-FU and Cisplatin [101,102]. Resistance to tamoxifen, the most frequently used drug for estrogen receptor-positive breast cancer, is also related to miR-10b. MiR-10b expression induced greater tamoxifen resistance. The mechanism was determined to be miR-10b inhibiting histone deacetylase 4 (HDAC4) [103].
These examples illustrate the relevance of miR-10b as a diagnostic biomarker in cancer. This is a highly clinically relevant application, given the lower threshold for FDA approval, as compared to therapeutic interventions. By far the most valuable advancement using these miR-10b targeted diagnostic methods would be the ability to discriminate between high-risk and low-risk disease and the capacity to identify the presence of metastasis. Such knowledge would be instrumental in guiding treatment choice as part of a rational individualized therapeutic approach.
Therapeutic applications of miR-10b
Currently, there are a number of approaches that could be used to affect miRNA expression for therapy. Two general methods that are clinically relevant involve the local or systemic administration of antagomirs/mimics or small molecule miRNA inhibitors. One of the most notable therapeutic studies on the use of miR-10b antagomirs addressed metastatic breast cancer [10]. The results of this study showed a significant decrease in miR-10b expression and increased expression of HOXD10 [10]. Additionally, the treatment did not have negative effects on healthy cells. The treatment was highly specific and did not alter expression of other miRNAs, including miR-10a which shares the same stem loop sequence [10].
Inhibition of miR-10b using antisense technology has shown promise in other preclinical models. Reduced tumor growth was observed following treatment with miR-10b-antisense oligos in triple-negative breast cancer [104] and glioblastoma [84]. Additionally, introduction of miR-10b-antisense inhibitors to breast cancer stem cells resulted in a decrease in stem-cell self renewal [31]. With respect to its role in immune cell regulation, miR-10b inhibition showed greater NKG2D-mediated killing of tumor cells in vitro and greater clearance of tumor in vivo [93]. From these examples, it becomes clear that as a therapeutic target miR-10b has a diverse set of possible effects which all lend to improved clinical outcomes. This is a promising route of treatment. However, it cannot be indiscrimitely applied, as evidenced by the fact that miR-10b inhibition in LMP1-positive NPC did not affect invasiveness and motility, despite the fact that LMP1 is associated with inducing TWIST-1 expression, a promoter of miR-10b [15].
Taking advantage of the mechanism by which miR-10b exerts its promotion of tamoxifen-resistance, miR-10b inhibiting HDAC4, could allow for successful therapy [103]. This approach warrants further investigation as an alternative to using the modification of the HDAC family as a part of solving drug-resistance in ER-positive breast cancer. MiR-10b was found to be one of two miRNAs to be differentially expressed across each examined cisplatin-resistant germ cell tumor [105]. Therefore, further study is warranted for determining the association between miR-10b and the mechanism of cisplatin-resistance in hopes of elucidating a method to overcome the resistance.
Our own work focused on miR-10b as a therapeutic target in breast cancer based on the finding that miRNA-10b acts as a master regulator of the viability of metastatic tumor cells [106-108]. We developed a therapeutic strategy based on miR-10b inhibition, which relied on LNA antagomirs delivered to metastatic cells by dextran-coated iron oxide nanoparticles (termed MN-anti-miR10b). We demonstrated that MN-anti-miR10b prevented the genesis of new metastases, following intravenous injection [106]. When combined with low-dose chemotherapy, MN-anti-miR10b triggered durable regression of local lymph node metastases in a murine breast cancer model [107]. In a model of Stage IV metastatic breast cancer, we demonstrated that combination therapy with MN-anti-miR10b and low-dose doxorubicin led to regression of pre-existing distant metastases in 65% of the animals and inhibition of the formation of multiple organ metastases in 94% of the animals [108].
Evidence in favor of the choice of miR-10b as a therapeutic target comes from a pivotal study, which explored the effects of complete knock-out of miR-10b in murine models of breast cancer [30]. As it is essential to minimize off-target effects, miR-10b was shown to be dispensable for normal development but essential for tumorigenesis in miR-10b-deficient mice [30]. These mice showed delayed tumorigenesis and suppressed EMT, intravasation, and metastasis [30]. This demonstrates the value of miR-10b as a therapeutic target with potentially minimal off-target effects.
While not comprehensive, this list of studies illustrates first steps into the uncharted territory of miR-10b targeted cancer therapy. Given the lack of therapeutic approaches specifically directed towards unique properties of the metastatic tumor cell, such endeavors could yield significant and transformative outcomes against metastatic cancer, a distinct disease that has largely evaded attempts at curative therapeutic intervention.
Conclusion
As evidenced by this overview, the potential of miR-10b as a therapeutic and diagnostic target in cancer is highly significant. miR-10b has been linked to metastatic potential, disease progression, and outcome in multiple clinical studies spanning a wide range of cancers. This observation suggests that miR-10b plays a fundamental role in human malignancy and underscores the anticipated impact that could be made by therapeutic approaches centered around miR-10b. While still vague, the pathways by which miR-10b exerts its effects, especially with regard to metastasis, are beginning to emerge. As we gain a more thorough understanding of these networks, we would be able to more accurately predict the phenotypic effects exerted by miR-10b-centered interventions with the goal of stratifying patients based on disease outlook, anticipated treatment outcome, and off-target toxicity. Such an approach would move us closer to developing a truly individualized therapeutic strategy against cancer, despite its focus on a broadly-based fundamental molecular regulator of metastasis, such as miR-10b. With regard to miR-10b’s value in clinical diagnostics, there is already ample evidence, as detailed in this review, that it is feasible to develop minimally invasive or noninvasive methods that are centered around miR-10b as a biomarker. More importantly, such methods will likely provide unique information about cancer staging, disease outcome, metastatic potential, and ultimately survival, and have an extraordinary impact as guides to therapy.
Acknowledgements
This work was supported in part by R01CA135650 and R01CA16346101A1 from the National Cancer Institute to ZM.
Disclosure of conflict of interest
None.
References
- 1.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 2.Abdelmaksoud-Dammak R, Chamtouri N, Triki M, Saadallah-Kallel A, Ayadi W, Charfi S, Khabir A, Ayadi L, Sallemi-Boudawara T, Mokdad-Gargouri R. Overexpression of miR-10b in colorectal cancer patients: correlation with TWIST-1 and E-cadherin expression. Tumour Biol. 2017;39:1010428317695916. doi: 10.1177/1010428317695916. [DOI] [PubMed] [Google Scholar]
- 3.Hur K, Toiyama Y, Schetter AJ, Okugawa Y, Harris CC, Boland CR, Goel A. Identification of a metastasis-specific MicroRNA signature in human colorectal cancer. J Natl Cancer Inst. 2015:107. doi: 10.1093/jnci/dju492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang H, Liu J, Chen Y, Ma C, Li B, Hao T. Up-regulation of mir-10b predicate advanced clinicopathological features and liver metastasis in colorectal cancer. Cancer Med. 2016;5:2932–2941. doi: 10.1002/cam4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang YF, Li Z, Zhao XH, Zuo XM, Zhang Y, Xiao YH, Li J, Peng ZH. MicroRNA-10b is upregulated and has an invasive role in colorectal cancer through enhanced Rhoc expression. Oncol Rep. 2015;33:1275–1283. doi: 10.3892/or.2015.3737. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad A, Sethi S, Chen W, Ali-Fehmi R, Mittal S, Sarkar FH. Up-regulation of microRNA-10b is associated with the development of breast cancer brain metastasis. Am J Transl Res. 2014;6:384–390. [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W, Cai F, Zhang B, Barekati Z, Zhong XY. The level of circulating miRNA-10b and miRNA-373 in detecting lymph node metastasis of breast cancer: potential biomarkers. Tumour Biol. 2013;34:455–462. doi: 10.1007/s13277-012-0570-5. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Zhao J, Zhang PY, Zhang Y, Sun SY, Yu SY, Xi QS. MicroRNA-10b targets E-cadherin and modulates breast cancer metastasis. Med Sci Monit. 2012;18:BR299–308. doi: 10.12659/MSM.883262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma L. Role of miR-10b in breast cancer metastasis. Breast Cancer Res. 2010;12:210. doi: 10.1186/bcr2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma L, Reinhardt F, Pan E, Soutschek J, Bhat B, Marcusson EG, Teruya-Feldstein J, Bell GW, Weinberg RA. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol. 2010;28:341–347. doi: 10.1038/nbt.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Min W, Wang B, Li J, Han J, Zhao Y, Su W, Dai Z, Wang X, Ma Q. The expression and significance of five types of miRNAs in breast cancer. Med Sci Monit Basic Res. 2014;20:97–104. doi: 10.12659/MSMBR.891246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Negrini M, Calin GA. Breast cancer metastasis: a microRNA story. Breast Cancer Res. 2008;10:203. doi: 10.1186/bcr1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parrella P, Barbano R, Pasculli B, Fontana A, Copetti M, Valori VM, Poeta ML, Perrone G, Righi D, Castelvetere M, Coco M, Balsamo T, Morritti M, Pellegrini F, Onetti-Muda A, Maiello E, Murgo R, Fazio VM. Evaluation of microRNA-10b prognostic significance in a prospective cohort of breast cancer patients. Mol Cancer. 2014;13:142. doi: 10.1186/1476-4598-13-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao FL, Hu GD, Wang XF, Zhang XH, Zhang YK, Yu ZS. Serum overexpression of microRNA-10b in patients with bone metastatic primary breast cancer. J Int Med Res. 2012;40:859–866. doi: 10.1177/147323001204000304. [DOI] [PubMed] [Google Scholar]
- 15.Li G, Wu Z, Peng Y, Liu X, Lu J, Wang L, Pan Q, He ML, Li XP. MicroRNA-10b induced by Epstein-Barr virus-encoded latent membrane protein-1 promotes the metastasis of human nasopharyngeal carcinoma cells. Cancer Lett. 2010;299:29–36. doi: 10.1016/j.canlet.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Baffa R, Fassan M, Volinia S, O’Hara B, Liu CG, Palazzo JP, Gardiman M, Rugge M, Gomella LG, Croce CM, Rosenberg A. MicroRNA expression profiling of human metastatic cancers identifies cancer gene targets. J Pathol. 2009;219:214–221. doi: 10.1002/path.2586. [DOI] [PubMed] [Google Scholar]
- 17.Saldanha G, Elshaw S, Sachs P, Alharbi H, Shah P, Jothi A, Pringle JH. microRNA-10b is a prognostic biomarker for melanoma. Mod Pathol. 2016;29:112–121. doi: 10.1038/modpathol.2015.149. [DOI] [PubMed] [Google Scholar]
- 18.Preis M, Gardner TB, Gordon SR, Pipas JM, Mackenzie TA, Klein EE, Longnecker DS, Gutmann EJ, Sempere LF, Korc M. MicroRNA-10b expression correlates with response to neoadjuvant therapy and survival in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2011;17:5812–5821. doi: 10.1158/1078-0432.CCR-11-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jikuzono T, Kawamoto M, Yoshitake H, Kikuchi K, Akasu H, Ishikawa H, Hirokawa M, Miyauchi A, Tsuchiya S, Shimizu K, Takizawa T. The miR-221/222 cluster, miR-10b and miR-92a are highly upregulated in metastatic minimally invasive follicular thyroid carcinoma. Int J Oncol. 2013;42:1858–1868. doi: 10.3892/ijo.2013.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li QJ, Zhou L, Yang F, Wang GX, Zheng H, Wang DS, He Y, Dou KF. MicroRNA-10b promotes migration and invasion through CADM1 in human hepatocellular carcinoma cells. Tumour Biol. 2012;33:1455–1465. doi: 10.1007/s13277-012-0396-1. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Liu J, Fan Y, Li X, Dong M, Liu H, Chen J. Expression levels of microRNA-145 and microRNA-10b are associated with metastasis in non-small cell lung cancer. Cancer Biol Ther. 2016;17:272–279. doi: 10.1080/15384047.2016.1139242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang R, Chen XF, Shu YQ. Prediction of non-small cell lung cancer metastasis-associated microRNAs using bioinformatics. Am J Cancer Res. 2015;5:32–51. [PMC free article] [PubMed] [Google Scholar]
- 23.Yang YL, Xu LP, Zhuo FL, Wang TY. Prognostic value of microRNA-10b overexpression in peripheral blood mononuclear cells of nonsmall-cell lung cancer patients. Tumour Biol. 2015;36:7069–7075. doi: 10.1007/s13277-015-3366-6. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Xu L, Yang Z, Lu H, Hu D, Li W, Zhang Z, Liu B, Ma S. MicroRNA-10b indicates a poor prognosis of non-small cell lung cancer and targets E-cadherin. Clin Transl Oncol. 2015;17:209–214. doi: 10.1007/s12094-014-1213-7. [DOI] [PubMed] [Google Scholar]
- 25.Liu Z, Zhu J, Cao H, Ren H, Fang X. miR-10b promotes cell invasion through RhoC-AKT signaling pathway by targeting HOXD10 in gastric cancer. Int J Oncol. 2012;40:1553–1560. doi: 10.3892/ijo.2012.1342. [DOI] [PubMed] [Google Scholar]
- 26.Meseguer S, Mudduluru G, Escamilla JM, Allgayer H, Barettino D. MicroRNAs-10a and-10b contribute to retinoic acid-induced differentiation of neuroblastoma cells and target the alternative splicing regulatory factor SFRS1 (SF2/ASF) J Biol Chem. 2011;286:4150–4164. doi: 10.1074/jbc.M110.167817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teplyuk NM, Mollenhauer B, Gabriely G, Giese A, Kim E, Smolsky M, Kim RY, Saria MG, Pastorino S, Kesari S, Krichevsky AM. MicroRNAs in cerebrospinal fluid identify glioblastoma and metastatic brain cancers and reflect disease activity. Neuro Oncol. 2012;14:689–700. doi: 10.1093/neuonc/nos074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao H, Li H, Yu G, Xiao W, Hu J, Tang K, Zeng J, He W, Zeng G, Ye Z, Xu H. MicroRNA-10b promotes migration and invasion through KLF4 and HOXD10 in human bladder cancer. Oncol Rep. 2014;31:1832–1838. doi: 10.3892/or.2014.3048. [DOI] [PubMed] [Google Scholar]
- 29.Liang AL, Zhang TT, Zhou N, Wu CY, Lin MH, Liu YJ. MiRNA-10b sponge: an anti-breast cancer study in vitro. Oncol Rep. 2016;35:1950–1958. doi: 10.3892/or.2016.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J, Siverly AN, Chen D, Wang M, Yuan Y, Wang Y, Lee H, Zhang J, Muller WJ, Liang H, Gan B, Yang X, Sun Y, You MJ, Ma L. Ablation of miR-10b suppresses oncogene-induced mammary tumorigenesis and metastasis and reactivates tumor-suppressive pathways. Cancer Res. 2016;76:6424–6435. doi: 10.1158/0008-5472.CAN-16-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bahena-Ocampo I, Espinosa M, Ceballos-Cancino G, Lizarraga F, Campos-Arroyo D, Schwarz A, Maldonado V, Melendez-Zajgla J, Garcia-Lopez P. miR-10b expression in breast cancer stem cells supports self-renewal through negative PTEN regulation and sustained AKT activation. EMBO Rep. 2016;17:648–658. doi: 10.15252/embr.201540678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ibrahim SA, Yip GW, Stock C, Pan JW, Neubauer C, Poeter M, Pupjalis D, Koo CY, Kelsch R, Schüle R, Rescher U, Kiesel L, Götte M. Targeting of syndecan-1 by microRNA miR-10b promotes breast cancer cell motility and invasiveness via a Rho-GTPase- and E-cadherindependent mechanism. Int J Cancer. 2012;131:E884–E896. doi: 10.1002/ijc.27629. [DOI] [PubMed] [Google Scholar]
- 33.Shen X, Fang J, Lv X, Pei Z, Wang Y, Jiang S, Ding K. Heparin impairs angiogenesis through inhibition of MicroRNA-10b. J Biol Chem. 2011;286:26616–26627. doi: 10.1074/jbc.M111.224212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hafez MM, Hassan ZK, Zekri AR, Gaber AA, Al Rejaie SS, Sayed-Ahmed MM, Al Shabanah O. MicroRNAs and metastasis-related gene expression in Egyptian breast cancer patients. Asian Pac J Cancer Prev. 2012;13:591–598. doi: 10.7314/apjcp.2012.13.2.591. [DOI] [PubMed] [Google Scholar]
- 35.Chang CH, Fan TC, Yu JC, Liao GS, Lin YC, Shih AC, Li WH, Yu AL. The prognostic significance of RUNX2 and miR-10a/10b and their inter-relationship in breast cancer. J Transl Med. 2014;12:257. doi: 10.1186/s12967-014-0257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eissa S, Matboli M, Shehata HH, Essawy NO. MicroRNA-10b and minichromosome maintenance complex component 5 gene as prognostic biomarkers in breast cancer. Tumour Biol. 2015;36:4487–4494. doi: 10.1007/s13277-015-3090-2. [DOI] [PubMed] [Google Scholar]
- 37.Anfossi S, Giordano A, Gao H, Cohen EN, Tin S, Wu Q, Garza RJ, Debeb BG, Alvarez RH, Valero V, Hortobagyi GN, Calin GA, Ueno NT, Woodward WA, Reuben JM. High serum miR-19a levels are associated with inflammatory breast cancer and are predictive of favorable clinical outcome in patients with metastatic HER2+inflammatory breast cancer. PLoS One. 2014;9:e83113. doi: 10.1371/journal.pone.0083113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishida N, Yamashita S, Mimori K, Sudo T, Tanaka F, Shibata K, Yamamoto H, Ishii H, Doki Y, Mori M. MicroRNA-10b is a prognostic indicator in colorectal cancer and confers resistance to the chemotherapeutic agent 5-fluorouracil in colorectal cancer cells. Ann Surg Oncol. 2012;19:3065–3071. doi: 10.1245/s10434-012-2246-1. [DOI] [PubMed] [Google Scholar]
- 39.Li Z, Gu X, Fang Y, Xiang J, Chen Z. microRNA expression profiles in human colorectal cancers with brain metastases. Oncol Lett. 2012;3:346–350. doi: 10.3892/ol.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vychytilova-Faltejskova P, Pesta M, Radova L, Liska V, Daum O, Kala Z, Svoboda M, Kiss I, Slaby O. Genome-wide microRNA expression profiling in primary tumors and matched liver metastasis of patients with colorectal cancer. Cancer Genomics Proteomics. 2016;13:311–316. [PubMed] [Google Scholar]
- 41.Vickers MM, Bar J, Gorn-Hondermann I, Yarom N, Daneshmand M, Hanson JE, Addison CL, Asmis TR, Jonker DJ, Maroun J, Lorimer IA, Goss GD, Dimitroulakos J. Stage-dependent differential expression of microRNAs in colorectal cancer: potential role as markers of metastatic disease. Clin Exp Metastasis. 2012;29:123–132. doi: 10.1007/s10585-011-9435-3. [DOI] [PubMed] [Google Scholar]
- 42.Schee K, Lorenz S, Worren MM, Günther CC, Holden M, Hovig E, Fodstad O, Meza-Zepeda LA, Flatmark K. Deep sequencing the MicroRNA transcriptome in colorectal cancer. PLoS One. 2013;8:e66165. doi: 10.1371/journal.pone.0066165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang YY, Li L, Ye ZY, Zhao ZS, Yan ZL. MicroRNA-10b promotes migration and invasion through Hoxd10 in human gastric cancer. World J Surg Oncol. 2015;13:259. doi: 10.1186/s12957-015-0673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang YY, Ye ZY, Zhao ZS, Li L, Wang YX, Tao HQ, Wang HJ, He XJ. Clinicopathologic significance of miR-10b expression in gastric carcinoma. Hum Pathol. 2013;44:1278–1285. doi: 10.1016/j.humpath.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 45.Liao CG, Kong LM, Zhou P, Yang XL, Huang JG, Zhang HL, Lu N. miR-10b is overexpressed in hepatocellular carcinoma and promotes cell proliferation, migration and invasion through RhoC, uPAR and MMPs. J Transl Med. 2014;12:234. doi: 10.1186/s12967-014-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frampton AE, Krell J, Jacob J, Stebbing J, Jiao LR, Castellano L. microRNAs as markers of survival and chemoresistance in pancreatic ductal adenocarcinoma. Expert Rev Anticancer Ther. 2011;11:1837–1842. doi: 10.1586/era.11.184. [DOI] [PubMed] [Google Scholar]
- 47.Ouyang H, Gore J, Deitz S, Korc M. microRNA-10b enhances pancreatic cancer cell invasion by suppressing TIP30 expression and promoting EGF and TGF-β actions. Oncogene. 2014;33:4664–4674. doi: 10.1038/onc.2013.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muratore S, Zeng X, Korc M, McElyea S, Wilhelm J, Bellin M, Beilman G. Metastatic pancreatic adenocarcinoma after total pancreatectomy islet autotransplantation for chronic pancreatitis. Am J Transplant. 2016;16:2747–2752. doi: 10.1111/ajt.13851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen HV, Gore J, Zhong X, Savant SS, Deitz-McElyea S, Schmidt CM, House MG, Korc M. MicroRNA expression in a readily accessible common hepatic artery lymph node predicts time to pancreatic cancer recurrence postresection. J Gastrointest Surg. 2016;20:1699–1706. doi: 10.1007/s11605-016-3208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skrzypski M, Czapiewski P, Goryca K, Jassem E, Wyrwicz L, Pawłowski R, Rzyman W, Biernat W, Jassem J. Prognostic value of microRNA expression in operable non-small cell lung cancer patients. Br J Cancer. 2014;110:991–1000. doi: 10.1038/bjc.2013.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su QL, Li SQ, Wang DN, Liu F, Yuan B. Effects of MicroRNA-10b on lung cancer cell proliferation and invasive metastasis and the underlying mechanism. Asian Pac J Trop Med. 2014;7:364–367. doi: 10.1016/S1995-7645(14)60056-0. [DOI] [PubMed] [Google Scholar]
- 52.Bai M, Zhang H, Si L, Yu N, Zeng A, Zhao R. Upregulation of serum miR-10b is associated with poor prognosis in patients with melanoma. J Cancer. 2017;8:2487–2491. doi: 10.7150/jca.18824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hildesheim A, Levine PH. Etiology of nasopharyngeal carcinoma: a review. Epidemiol Rev. 1993;15:466–485. doi: 10.1093/oxfordjournals.epirev.a036130. [DOI] [PubMed] [Google Scholar]
- 54.Allaya N, Khabir A, Sallemi-Boudawara T, Sellami N, Daoud J, Ghorbel A, Frikha M, Gargouri A, Mokdad-Gargouri R, Ayadi W. Over-expression of miR-10b in NPC patients: correlation with LMP1 and Twist1. Tumour Biol. 2015;36:3807–3814. doi: 10.1007/s13277-014-3022-6. [DOI] [PubMed] [Google Scholar]
- 55.Sun XJ, Liu H, Zhang P, Zhang XD, Jiang ZW, Jiang CC. miR-10b promotes migration and invasion in nasopharyngeal carcinoma cells. Asian Pac J Cancer Prev. 2013;14:5533–5537. doi: 10.7314/apjcp.2013.14.9.5533. [DOI] [PubMed] [Google Scholar]
- 56.Zhang P, Hong H, Sun X, Jiang H, Ma S, Zhao S, Zhang M, Wang Z, Jiang C, Liu H. MicroRNA-10b regulates epithelial-mesenchymal transition by modulating KLF4/Notch1/E-cadherin in cisplatin-resistant nasopharyngeal carcinoma cells. Am J Cancer Res. 2016;6:141–156. [PMC free article] [PubMed] [Google Scholar]
- 57.Chen H, Fan Y, Xu W, Chen J, Xu C, Wei X, Fang D, Feng Y. miR-10b inhibits apoptosis and promotes proliferation and invasion of endometrial cancer cells via targeting HOXB3. Cancer Biother Radiopharm. 2016;31:225–231. doi: 10.1089/cbr.2016.1998. [DOI] [PubMed] [Google Scholar]
- 58.Wang J, Wang B, Chen LQ, Yang J, Gong ZQ, Zhao XL, Zhang CQ, Du KL. miR-10b promotes invasion by targeting KLF4 in osteosarcoma cells. Biomed Pharmacother. 2016;84:947–953. doi: 10.1016/j.biopha.2016.09.108. [DOI] [PubMed] [Google Scholar]
- 59.Tian Y, Luo A, Cai Y, Su Q, Ding F, Chen H, Liu Z. MicroRNA-10b promotes migration and invasion through KLF4 in human esophageal cancer cell lines. J Biol Chem. 2010;285:7986–7994. doi: 10.1074/jbc.M109.062877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang L, Sun J, Wang B, Ren JC, Su W, Zhang T. MicroRNA-10b triggers the epithelialmesenchymal transition (EMT) of laryngeal carcinoma Hep-2 cells by directly targeting the e-cadherin. Appl Biochem Biotechnol. 2015;176:33–44. doi: 10.1007/s12010-015-1505-6. [DOI] [PubMed] [Google Scholar]
- 61.Sondermann A, Andreghetto FM, Moulatlet AC, da Silva Victor E, de Castro MG, Nunes FD, Brandão LG, Severino P. MiR-9 and miR-21 as prognostic biomarkers for recurrence in papillary thyroid cancer. Clin Exp Metastasis. 2015;32:521–530. doi: 10.1007/s10585-015-9724-3. [DOI] [PubMed] [Google Scholar]
- 62.Zaravinos A, Radojicic J, Lambrou GI, Volanis D, Delakas D, Stathopoulos EN, Spandidos DA. Expression of miRNAs involved in angiogenesis, tumor cell proliferation, tumor suppressor inhibition, epithelial-mesenchymal transition and activation of metastasis in bladder cancer. J Urol. 2012;188:615–623. doi: 10.1016/j.juro.2012.03.122. [DOI] [PubMed] [Google Scholar]
- 63.Huang L, Lin JX, Yu YH, Zhang MY, Wang HY, Zheng M. Downregulation of six microRNAs is associated with advanced stage, lymph node metastasis and poor prognosis in small cell carcinoma of the cervix. PLoS One. 2012;7:e33762. doi: 10.1371/journal.pone.0033762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fritz HK, Lindgren D, Ljungberg B, Axelson H, Dahlbäck B. The miR(21/10b) ratio as a prognostic marker in clear cell renal cell carcinoma. Eur J Cancer. 2014;50:1758–1765. doi: 10.1016/j.ejca.2014.03.281. [DOI] [PubMed] [Google Scholar]
- 65.Khella HWZ, Daniel N, Youssef L, Scorilas A, Nofech-Mozes R, Mirham L, Krylov SN, Liandeau E, Krizova A, Finelli A, Cheng Y, Yousef GM. miR-10b is a prognostic marker in clear cell renal cell carcinoma. J Clin Pathol. 2017;70:854–859. doi: 10.1136/jclinpath-2017-204341. [DOI] [PubMed] [Google Scholar]
- 66.Wotschofsky Z, Liep J, Meyer HA, Jung M, Wagner I, Disch AC, Schaser KD, Melcher I, Kilic E, Busch J, Weikert S, Miller K, Erbersdobler A, Mollenkopf HJ, Jung K. Identification of metastamirs as metastasis-associated microRNAs in clear cell renal cell carcinomas. Int J Biol Sci. 2012;8:1363–1374. doi: 10.7150/ijbs.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kowalik CG, Palmer DA, Sullivan TB, Teebagy PA, Dugan JM, Libertino JA, Burks EJ, Canes D, Rieger-Christ KM. Profiling microRNA from nephrectomy and biopsy specimens: predictors of progression and survival in clear cell renal cell carcinoma. BJU Int. 2017;120:428–440. doi: 10.1111/bju.13886. [DOI] [PubMed] [Google Scholar]
- 68.Heinzelmann J, Henning B, Sanjmyatav J, Posorski N, Steiner T, Wunderlich H, Gajda MR, Junker K. Specific miRNA signatures are associated with metastasis and poor prognosis in clear cell renal cell carcinoma. World J Urol. 2011;29:367–373. doi: 10.1007/s00345-010-0633-4. [DOI] [PubMed] [Google Scholar]
- 69.Wu X, Weng L, Li X, Guo C, Pal SK, Jin JM, Li Y, Nelson RA, Mu B, Onami SH, Wu JJ, Ruel NH, Wilczynski SP, Gao H, Covarrubias M, Figlin RA, Weiss LM, Wu H. Identification of a 4-microRNA signature for clear cell renal cell carcinoma metastasis and prognosis. PLoS One. 2012;7:e35661. doi: 10.1371/journal.pone.0035661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Croset M, Goehrig D, Frackowiak A, Bonnelye E, Ansieau S, Puisieux A, Clézardin P. TWIST1 expression in breast cancer cells facilitates bone metastasis formation. J Bone Miner Res. 2014;29:1886–1899. doi: 10.1002/jbmr.2215. [DOI] [PubMed] [Google Scholar]
- 71.Tsukerman P, Yamin R, Seidel E, Khawaled S, Schmiedel D, Bar-Mag T, Mandelboim O. MiR-520d-5p directly targets TWIST1 and downregulates the metastamiR miR-10b. Oncotarget. 2014;5:12141–12150. doi: 10.18632/oncotarget.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bourguignon LY. Matrix hyaluronan promotes specific MicroRNA upregulation leading to drug resistance and tumor progression. Int J Mol Sci. 2016;17:517. doi: 10.3390/ijms17040517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dawson CW, Port RJ, Young LS. The role of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the pathogenesis of nasopharyngeal carcinoma (NPC) Semin Cancer Biol. 2012;22:144–153. doi: 10.1016/j.semcancer.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 74.Li X, Gao L, Cui Q, Gary BD, Dyess DL, Taylor W, Shevde LA, Samant RS, Dean-Colomb W, Piazza GA, Xi Y. Sulindac inhibits tumor cell invasion by suppressing NF-κB-mediated transcription of microRNAs. Oncogene. 2012;31:4979–4986. doi: 10.1038/onc.2011.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hurst DR, Welch DR. Unraveling the enigmatic complexities of BRMS1-mediated metastasis suppression. FEBS Lett. 2011;585:3185–3190. doi: 10.1016/j.febslet.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hurst DR, Welch DR. Metastasis suppressor genes at the interface between the environment and tumor cell growth. Int Rev Cell Mol Biol. 2011;286:107–180. doi: 10.1016/B978-0-12-385859-7.00003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Edmonds MD, Hurst DR, Vaidya KS, Stafford LJ, Chen D, Welch DR. Breast cancer metastasis suppressor 1 coordinately regulates metastasis-associated microRNA expression. Int J Cancer. 2009;125:1778–1785. doi: 10.1002/ijc.24616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hurst DR, Xie Y, Thomas JW, Liu J, Edmonds MD, Stewart MD, Welch DR. The C-terminal putative nuclear localization sequence of breast cancer metastasis suppressor 1, BRMS1, is necessary for metastasis suppression. PLoS One. 2013;8:e55966. doi: 10.1371/journal.pone.0055966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vega Francisco M, Ridley Anne J. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582:2093–2101. doi: 10.1016/j.febslet.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 80.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 81.Mikala E, Zena W. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 82.Pecina-Slaus N. Tumor suppressor gene E-cadherin and its role in normal and malignant cells. Cancer Cell Int. 2003;3:17. doi: 10.1186/1475-2867-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Knirsh R, Ben-Dror I, Modai S, Shomron N, Vardimon L. MicroRNA 10b promotes abnormal expression of the proto-oncogene c-Jun in metastatic breast cancer cells. Oncotarget. 2016;7:59932–59944. doi: 10.18632/oncotarget.11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma C, Wei F, Xia H, Liu H, Dong X, Zhang Y, Luo Q, Liu Y, Li Y. MicroRNA-10b mediates TGF-β1-regulated glioblastoma proliferation, migration and epithelial-mesenchymal transition. Int J Oncol. 2017;50:1739–1748. doi: 10.3892/ijo.2017.3947. [DOI] [PubMed] [Google Scholar]
- 85.Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA. Exogenous expression of n-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol. 2000;148:779–790. doi: 10.1083/jcb.148.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bhatlekar S, Fields JZ, Boman BM. HOX genes and their role in the development of human cancers. J Mol Med (Berl) 2014;92:811–823. doi: 10.1007/s00109-014-1181-y. [DOI] [PubMed] [Google Scholar]
- 87.Ivaska J, Pallari HM, Nevo J, Eriksson JE. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp Cell Res. 2007;313:2050–2062. doi: 10.1016/j.yexcr.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 88.Li Z, Lei H, Luo M, Wang Y, Dong L, Ma Y, Liu C, Song W, Wang F, Zhang J, Shen J, Yu J. DNA methylation downregulated mir-10b acts as a tumor suppressor in gastric cancer. Gastric Cancer. 2015;18:43–54. doi: 10.1007/s10120-014-0340-8. [DOI] [PubMed] [Google Scholar]
- 89.Moriarty CH, Pursell B, Mercurio AM. miR-10b targets Tiam1: implications for Rac activation and carcinoma migration. J Biol Chem. 2010;285:20541–20546. doi: 10.1074/jbc.M110.121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cain K, Bratton SB, Cohen GM. The Apaf-1 apoptosome: a large caspase-activating complex. Biochimie. 2002;84:203–214. doi: 10.1016/s0300-9084(02)01376-7. [DOI] [PubMed] [Google Scholar]
- 91.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 92.Beauvais DM, Ell BJ, McWhorter AR, Rapraeger AC. Syndecan-1 regulates α(v)β(3) and α(v)β(5) integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. J Exp Med. 2009;206:691–705. doi: 10.1084/jem.20081278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tsukerman P, Stern-Ginossar N, Gur C, Glasner A, Nachmani D, Bauman Y, Yamin R, Vitenshtein A, Stanietsky N, Bar-Mag T, Lankry D, Mandelboim O. MiR-10b downregulates the stress-induced cell surface molecule MICB, a critical ligand for cancer cell recognition by natural killer cells. Cancer Res. 2012;72:5463–5472. doi: 10.1158/0008-5472.CAN-11-2671. [DOI] [PubMed] [Google Scholar]
- 94.Lee S, Jiang X. Modeling miRNA-mRNA interactions that cause phenotypic abnormality in breast cancer patients. PLoS One. 2017;12:e0182666. doi: 10.1371/journal.pone.0182666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li QQ, Chen ZQ, Cao XX, Xu JD, Xu JW, Chen YY, Wang WJ, Chen Q, Tang F, Liu XP, Xu ZD. Involvement of NF-kappaB/miR-448 regulatory feedback loop in chemotherapy-induced epithelial-mesenchymal transition of breast cancer cells. Cell Death Differ. 2011;18:16–25. doi: 10.1038/cdd.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guttilla IK, White BA. Coordinate regulation of FOXO1 by miR-27a, miR-96, and miR-182 in breast cancer cells. J Biol Chem. 2009;284:23204–23216. doi: 10.1074/jbc.M109.031427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Walter BA, Gomez-Macias G, Valera VA, Sobel M, Merino MJ. miR-21 expression in pregnancy-associated breast cancer: a possible marker of poor prognosis. J Cancer. 2011;2:67–75. doi: 10.7150/jca.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vetter G, Saumet A, Moes M, Vallar L, Le Bechec A, Laurini C, Sabbah M, Arar K, Theillet C, Lecellier CH, Friederich E. miR-661 expression in SNAI1-induced epithelial to mesenchymal transition contributes to breast cancer cell invasion by targeting Nectin-1 and StarD10 messengers. Oncogene. 2010;29:4436–4448. doi: 10.1038/onc.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 99.Farsinejad S, Rahaie M, Alizadeh AM, Mir-Derikvand M, Gheisary Z, Nosrati H, Khalighfard S. Expression of the circulating and the tissue microRNAs after surgery, chemotherapy, and radiotherapy in mice mammary tumor. Tumour Biol. 2016;37:14225–14234. doi: 10.1007/s13277-016-5292-7. [DOI] [PubMed] [Google Scholar]
- 100.Muñoz-Largacha JA, Gower AC, Sridhar P, Deshpande A, O’Hara CJ, Yamada E, Godfrey TE, Fernando HC, Litle VR. miRNA profiling of primary lung and head and neck squamous cell carcinomas: addressing a diagnostic dilemma. J Thorac Cardiovasc Surg. 2017;154:714–727. doi: 10.1016/j.jtcvs.2017.02.071. [DOI] [PubMed] [Google Scholar]
- 101.Wang J, Zhou JY, Wu GS. Bim protein degradation contributes to cisplatin resistance. J Biol Chem. 2011;286:22384–22392. doi: 10.1074/jbc.M111.239566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sinicrope FA, Rego RL, Okumura K, Foster NR, O’Connell MJ, Sargent DJ, Windschitl HE. Prognostic impact of bim, puma, and noxa expression in human colon carcinomas. Clin Cancer Res. 2008;14:5810–5818. doi: 10.1158/1078-0432.CCR-07-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ahmad A, Ginnebaugh KR, Yin S, Bollig-Fischer A, Reddy KB, Sarkar FH. Functional role of miR-10b in tamoxifen resistance of ER-positive breast cancer cells through down-regulation of HDAC4. BMC Cancer. 2015;15:540. doi: 10.1186/s12885-015-1561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Devulapally R, Sekar NM, Sekar TV, Foygel K, Massoud TF, Willmann JK, Paulmurugan R. Polymer nanoparticles mediated codelivery of antimiR-10b and antimiR-21 for achieving triple negative breast cancer therapy. ACS Nano. 2015;9:2290–2302. doi: 10.1021/nn507465d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Port M, Glaesener S, Ruf C, Riecke A, Bokemeyer C, Meineke V, Honecker F, Abend M. Micro-RNA expression in cisplatin resistant germ cell tumor cell lines. Mol Cancer. 2011;10:52. doi: 10.1186/1476-4598-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yigit M, Ghosh S, Kumar M, Petkova V, Kavishwar A, Moore A, Medarova Z. Context-dependent differences in miR-10b breast oncogenesis can be targeted for the prevention and arrest of lymph node metastasis. Oncogene. 2013;32:1530. doi: 10.1038/onc.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yoo B, Kavishwar A, Ross A, Wang P, Tabassum DP, Polyak K, Barteneva N, Petkova V, Pantazopoulos P, Tena A. Combining miR-10b-targeted nanotherapy with low-dose doxorubicin elicits durable regressions of metastatic breast cancer. Cancer Res. 2015;75:4407–4415. doi: 10.1158/0008-5472.CAN-15-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yoo B, Kavishwar A, Wang P, Ross A, Pantazopoulos P, Dudley M, Moore A, Medarova Z. Therapy targeted to the metastatic niche is effective in a model of stage IV breast cancer. Sci Rep. 2017;7:45060. doi: 10.1038/srep45060. [DOI] [PMC free article] [PubMed] [Google Scholar]


