Abstract
At present there is no consensus on the treatment of classical Hodgkin's lymphoma (CHL) following relapse. The aim of the present study was to access the class I-selective histone deacetylase (HDAC) inhibitor (HDACI) MGCD0103 on the expression levels of Bcl-2, nuclear factor (NF)-κB and programmed death-ligand 1 (PD-L1) in CHL, to explore the possible therapeutic value of MGCD0103 in combined relative target drugs for patients with CHL. In L1236 and L428 cell lines, apoptosis and cell cycle stage were identified using flow cytometry, and the effects of HDACI on CHL were assessed in terms of Bcl-2, NF-κB and PD-L1 expression levels, which were detected by western blotting and co-focusing experiments. The results demonstrated that MGCD0103 could induce cell apoptosis and cell cycle arrest, down-regulate Bcl-2 and increase NF-κB and PD-L1 expression levels in L1236 and L428 cell lines. MGCD0103 decreases Bcl-2 levels and upregulates PD-L1, which indicates that the combined use of HDACIs and a PD-L1 inhibitor in theory may improve treatment outcomes in patients with CHL. MGCD0103 may also up-regulate NF-κB, which seems to induce resistance towards anti-apoptotic drugs. Clinical trials combining HDACIs with NF-κB and/or PD-L1 inhibitors should be designed to further improve treatment outcomes for patients with CHL.
Keywords: Hodgkin's lymphoma, histone deacetylase inhibitor, nuclear factor-κB, programmed death-ligand 1
Introduction
Hodgkin's lymphoma (HL), a B cell-derived lymphoma, is a potentially curable lymphoid malignancy (1). Classical HL (CHL), which accounts for 95% of all HL, is a lymphatic hematopoietic systemic disease characterized by the occurrence of Hodgkin-Reed-Sternberg (HRS) cells in affected reactive lymphadenopathy (2). CHL is classified into 4 types, including nodular sclerosis HL, lymphocyte-depleted classical HL, lymphocyte-rich HL and mixed cellularity HL (3). At present, the most popular therapeutic regimen consists of adriamycin, bleomycin, vinblastine and dacarbazine, and remains the first-line therapy for patients with CHL (4). CHL is curable in the majority of cases (70–80% all stages) treated by conventional chemotherapy and/or combined radiotherapy; peripheral stem-cell transplantation can improve the outcome of patients with relapse following first-line chemotherapy, but with an increased rate of inevitable risks of lung and heart disease, or even other secondary cancers (5). Therefore, novel therapeutics are required for patients with CHL.
Histone deacetylase (HDAC) is a protein deacetylase that causes genetic changes, altering chromatin structure and modulating transcriptional and translational processes (6,7). HDACs are expressed in various malignant tumors, downregulating relevant tumor suppressor genes (8). HDACs serve an important role in regulating carcinogenesis in various tumors, including CHL (9,10). HDAC inhibitors (HDACIs), a class of therapeutic anticancer drugs, have been widely investigated (7,11). HDACIs induce a series of changes, including chromatin remodeling, regulation of transcription factors, cell cycle arrest and apoptosis induction (12–14). The class I HDAC-selective inhibitor MGCD0103, a specific benzamide histone deacetylase inhibitor, has been effective in controlling a number of cancers such as follicular lymphoma, myelogenous leukemia and Hodgkin's lymphoma in clinical trials (15–17). Previous studies suggested that HDACIs are a target for specific epigenetic changes associated with cancer and other diseases, and many HDACIs have entered clinical studies (18). A better understanding of gene expression and phenotype, homeostasis and neoplastic development that is altered by HDACs would help gain more knowledge about CHL, and may represent efficient tools for enhancing treatment in patients with CHL.
Nuclear factor (NF)-κB has been recently investigated and demonstrated to have an important role in CHL (19,20). Programmed death-ligand 1 (PD-L1) inhibitors are used in treating patients with relapsed CHL (21,22). Academic researches indicated that PD-L1 inhibitors, including nivolumab and pembrolizumab demonstrate remarkable activity in relapsed CHL (23,24). MGCD0103 effects on B cell lymphoma-2 (Bcl-2), NF-κB and PD-L1 levels require further study. In the present study, the expression levels of HDAC1, 2, 3 and 11 in CHL tissues were examined, and the effects of MGCD0103 on NF-κB and PD-L1 levels in CHL were assessed, to explore the potential therapeutic value of the class-I HDAC inhibitor MGCD0103 in combined relative target drugs for patients with CHL.
Materials and methods
Reagents
The anchorage-dependent cell line L1236 and the suspension-cultured cell line L428 used in the present study were obtained from Chinese Academy of Sciences (Shanghai, China). The HDACI MGCD0103 was supplied from MethylGene, Inc. (Toronto, Canada). Antibodies against Bcl-2 (cat. no. ab32124) were provided by the Department of Pathology, Shanghai Cancer Center, Fudan University (Shanghai, China). Antibody against α-tubulin (cat. no. ab52866) was obtained from Abcam (Cambridge, MA, USA). Antibodies against NF-κB (cat. no. 8242) and PD-L1 (cat. nos. 13684 and 25048) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Horseradish peroxidase-labeled goat anti-rabbit immunoglobulin G (IgG) antibody (cat. no. 10285-1-AP) was acquired from ProteinTech Group, Inc. (Chicago, IL, USA). Annexin V FITC Apoptosis Detection kit was acquired from BD Biosciences (Franklin Lakes, NJ, USA). Fluorescent-dye conjugated secondary antibodies (Alexa Fluor® 488-conjugated; cat. no. ab150077) were obtained from Abcam (Cambridge, UK).
Cell culture and group design
The L1236 and L428 cell lines, maintained at an atmosphere of 5% CO2 and 37°C, were cultured in RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplied with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.). Cells in the exponential phase were harvested and used in subsequent experiments. Cells in the MGCD0103 0.5, 1 and 2 µM groups were treated for 24 h with HDACI MGCD0103 at 0.5, 1 and 2 µM, respectively. An equal volume of dimethyl sulfoxide (DMSO) was added in the control group.
Protein extraction and western blot analysis
Radioimmunoprecipitation assay extraction reagents with 1% phenylmethanesulfonyl fluoride and 1% DL-dithiothreitol were applied to extract the total protein of the L236 cells. L428 cells were harvested and dissolved in 9.8 M urea (S1961; Beyotime Institute of Biotechnology, Haimen, China), 15 mM EDTA (P1045; Beyotime Institute of Biotechnology) and 30 mM Tris medium (ST774; Beyotime Institute of Biotechnology), and treated with a cell disruption step using the ultrasonic technique. Disrupted cells were then centrifuged (1,000 × g; 5 min; 4°C), insoluble compounds were removed and the supernatant was collected. A bicinchoninic protein assay (Pierce; Thermo Fisher Scientific, Inc.) was employed to measure the concentrations of the lysate protein of the two cell types. Equal amounts of protein (20 µg) in each group were separated by 12% SDS-PAGE, and then the proteins were transferred onto polyvinylidene difluoride membranes (PVDF). Subsequently, 5% non-fat dry milk dissolved in TBST (20 mM Tris-HCl, 150 mM NaCl, 0.1% Tween 20; pH 7.40) was used to block non-specific antigens on the PVDF membranes at room temperature for 1 h. Subsequently, the membranes were incubated with primary antibodies at 4°C overnight (anti-Bcl-2, 1:1,000; anti-NF-κB, 1:1,000; anti-PD-L1, 1:1,000). Subsequently, the membranes were washed with TBST three times for 5 min and incubated with goat anti-rabbit IgG secondary antibody (1:1,000) at room temperature for 1 h. α-tubulin was used as a loading control (α-tubulin antibody, 1:1,000). The images of western blotting were captured using an Omega Lum G imaging system (Gel Company, Inc., San Francisco, CA, USA) and the intensity of bands was determined using AlphaEase FC software 4.1.0 (Alpha Innotech Corporation; ProteinSimple, San Jose, CA, USA).
Cell apoptosis and cycle analyzed by flow cytometry
According to the manufacturer's protocol, cell apoptosis and cycle analysis were measured using propidium iodide and Annexin-V staining. Initially, L1236 and L428 cells were treated with MGCD0103 or DMSO for 24 h at 37°C as described above. L1236 cells were seeded onto a 6-well plate with RPMI 1640 medium at a density of 1×106 cells/ml and L428 cells were seeded onto a 6-well plate and suspended at a density of 1×106 cells/ml in RPMI-1640 medium per well 4°C, following treatment. Subsequently, L1236 and L428 cells were harvested and washed with PBS twice. For the analysis of the cell cycle, cells were treated with RNase (Invitrogen; Thermo Fisher Scientific, Inc.) at a final concentration of 0.2 mg/ml, and stained with propidium iodide (FITC Annexin V Apoptosis Detection kit I; BD Biosciences, Franklin Lakes, NJ, USA) at a final concentration of 10 µg/ml in the dark at 4°C for 20 min, subsequently. Finally, cells were detected by Immunocytometer Systems (FACSCalibur; BD Biosciences) and data was analyzed using Flowing software (version 2.5.1; http://flowingsoftware.btk.fi/). For the analysis of cell apoptosis, both types of cells were suspended in binding buffer from the kit at a density of 1×105/well, respectively. Subsequently, the cells were stained with 5 µl propidium iodide and 5 µl Annexin V-fluorescein isothiocyanate for 30 min in darkness at 4°C, and then detected using Immunocytometer Systems.
Fluorescence staining and confocal laser scanning techniques
Coverslips were kept flat on the bottom of a 6-well plate following cleaning, disinfection and 24-h ultraviolet irradiation. Subsequently, the L1236 cells were seeded on coverslips at a density of 1×106/well and cultured in an incubator at 37°C for 12 h. Cells were treated with MGCD0103 (0.5, 1 and 2 µm) or with DMSO in the control cells for 24 h at 37°C. L1236 and L428 cells were rinsed with PBS three times for 5 min and fixed with 4% paraformaldehyde at 25°C for 15 min, followed by permeabilization of the cells in 0.2% Triton X-100 at 25°C for a further 20 min. Subsequently, the coverslips were rinsed with PBS again three times for 5 min and blocked by incubating the L1236-attached cells in 5% bovine serum albumin (BSA; A8010; Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) at 25°C for 60 min. L428 cells were suspended and blocked in 5% BSA at 25°C for 60 min. Cells were then incubated with rabbit anti-human Bcl-2 (1:100), NF-κB (1:400) and PD-L1 (1:50) antibodies for 12 h at 4°C. Following washing with PBS three times for 5 min, the cells were incubated with Alexa 488-coupled goat anti-rabbit IgG secondary antibodies (1:1,000) for 1 h at 4°C in the dark. Finally, DAPI was used as a counterstain to label the nuclei at 25°C for 15 min. The stained L1236-attached cells and L428-suspension cells were the acquired and images were captured under fluorescent and laser confocal microscopy (magnification, ×600; Lexel Laser, Fremont, CA, USA).
Statistical analysis
Data are presented as the mean + standard error of the mean, and experiments were performed and repeated three times independently. Statistical analysis data of the total Annexin-V positive cells (% DMSO), data of the cell cycle distribution (% of DMSO), and the data of expressions of Bcl-2, NF-κB and PD-L1 (integrated optical density at the wavelength of 520 nm/area; compared with DMSO) were analyzed for significant differences using Student's t-test. Bcl-2, NF-κB and PD-L1 protein expression (relevant to DMSO) were analyzed for significant differences using one-way analysis of variance and post hoc Turkey's tests. SPSS 20.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
MGCD0103 downregulates Bcl-2, and increases NF-κB and PD-L1 expression levels
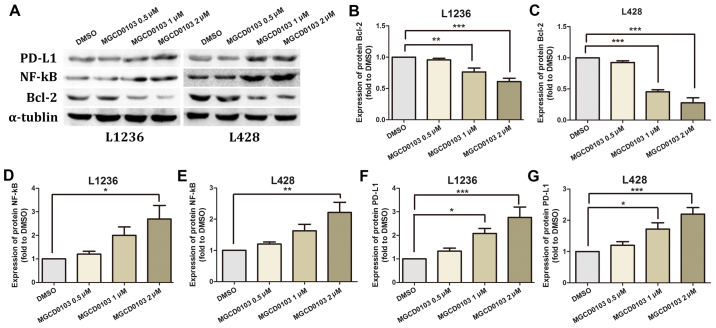
Two HL L1236 and L428 cell lines were treated with varying concentrations of MGCD0103 (0, 0.5, 1 or 2 µM) for 24 h, and protein levels of Bcl-2, NF-κB and PD-L1 were measured by western blotting (Fig. 1A). No statistically significant differences were identified in protein expression levels following treatment of the 2 cell lines with MGCD0103 at a concentration of 0.5 µM (Fig. 1B-G). In the L1236 cell line, MGCD0103 significantly inhibited Bcl-2 expression at a concentration of 1 (P<0.01) and 2 µM (P<0.001; Fig. 1B), and upregulated NF-κB at 2 µM (P<0.05, Fig. 1D) and PD-L1 at 1 (P<0.05) and 2 µM (P<0.001; Fig. 1F). Similarly, in the L428 cell line, MGCD0103 inhibited Bcl-2 expression at 1 (P<0.001) and 2 µM (P<0.001; Fig. 1C), and upregulated NF-κB at 2 µM (P<0.01; Fig. 1E) and PD-L1 at 1 (P<0.05) and 2 µM (P<0.001; Fig. 1G).
Figure 1.
(A) Western blotting was employed to assess Bcl-2, NF-κB and PD-L1 protein levels, with α-tubulin as a loading control. In MGCD0103 groups, cells were treated for 24 h with MGCD0103 at 0.5, 1 and 2 µM, with the DMSO group considered as control cells. (B and C) Bcl-2 levels decreased in a dose-dependent manner in the MGCD0103 groups compared with the control group. (D and E) NF-κB expression was higher in the MGCD0103 groups than in controls. (F and G) Similarly, PD-L1 expression was higher in the MGCD0103 group compared with control cells. Data are presented as the mean + the standard error of the mean. *P<0.05, **P<0.01, and ***P<0.001. Bcl-2, B cell lymphoma-2; NF, nuclear factor; PD-L1, programmed death-ligand 1; DMSO, dimethyl sulfoxide.
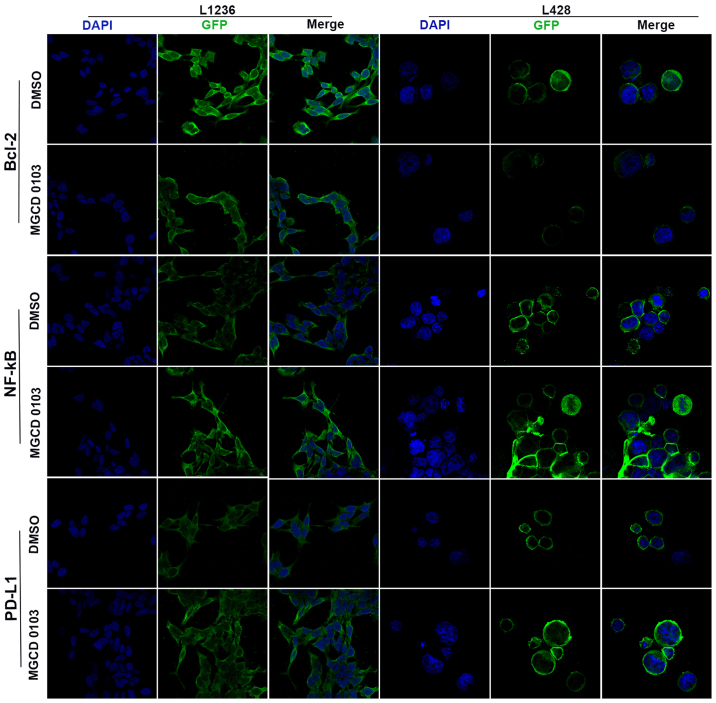
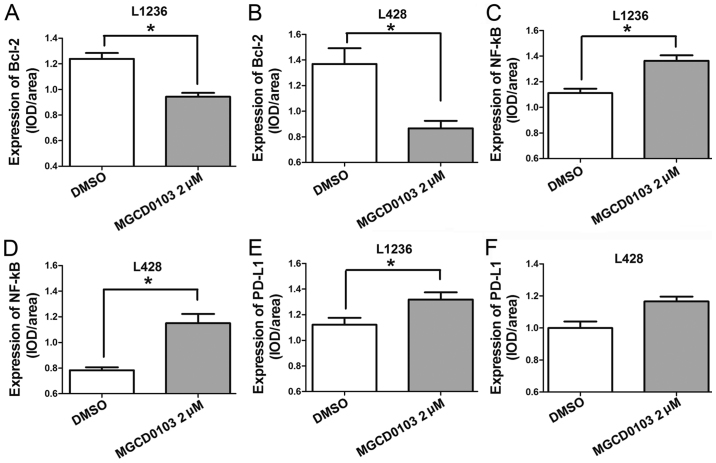
Laser confocal microscopy was also applied to examine the protein expression levels following treatment with MGCD0103 at 2 µM (Figs. 2 and 3). The expression levels of various proteins were assessed using the IOD/area ratio. These findings suggested that Bcl-2 expression was decreased whereas NF-κB and PD-L1 were upregulated in the MGCD0103 2 µM group compared with the DMSO group in the L1236 cell line; all differences were statistically significant (P<0.05; Fig. 3A, C and E). In the L428 cell line, Bcl-2 was also downregulated and NF-κB upregulated following treatment with 2 µM MGCD0103 (P<0.05; Fig. 3B and D); and although there was no significant difference, PD-L1 was markedly increased in the MGCD0103 2 µM group compared with the DMSO group (Fig. 3F).
Figure 2.
Bcl-2, NF-κB and PD-L1 protein levels were measured by representative confocal microscopic images (magnification, ×600). In the MGCD0103 2 µM group, cells were treated for 24 h with MGCD0103 at 2 µM, with DMSO considered as a control. Bcl-2, B cell lymphoma-2; NF, nuclear factor; PD-L1, programmed death-ligand 1; DMSO, dimethyl sulfoxide; GFP, green fluorescent protein.
Figure 3.
Representative confocal microscopic data for IOD/area detection. (A and B) The IOD/area ratio for Bcl-2 was lower in the MGCD0103 group than in control cells, in both cell lines. IOD/area ratios for (C and D) NF-κB and (E and F) PD-L1 were higher in the MGCD0103 group than in controls, in both cell lines. Data are presented as the mean + the standard error of the mean. *P<0.05. IOD, integrated optical density; Bcl-2, B cell lymphoma-2; NF, nuclear factor; PD-L1, programmed death-ligand 1; DMSO, dimethyl sulfoxide.
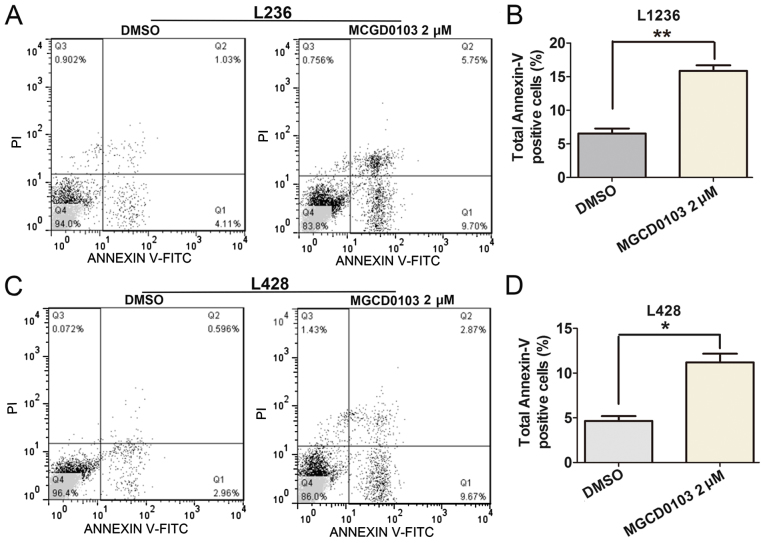
MGCD0103 induces cell apoptosis and cell cycle arrest in L1236 and L428 cells
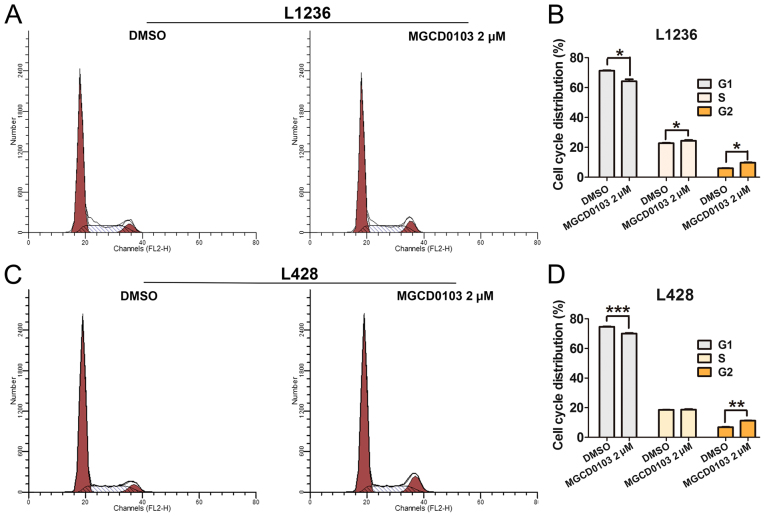
To explore the role of MGCD0103 on cell apoptosis, flow cytometry was applied (Fig. 4). Following treatment with MGCD0103 at a final concentration of 2 µM for 24 h, the apoptosis rate was significantly increased in the MGCD0103 group compared with the DMSO group, as revealed by the increased proportion of total Annexin-V positive-stained cells (Fig. 4A and C). Quantitative analysis of total Annexin-V positive-stained cells was performed, and total rates of Annexin-V positive cells were significantly increased following treatment with MGCD0103 in the L1236 (P<0.01; Fig. 4B) and L428 (P<0.05; Fig. 4D) cell lines. Furthermore, flow cytometry was employed to assess the effects of MGCD0103 on cell cycle in L1234 and L428 cells (Fig. 5). The results demonstrated that MGCD0103 treatment resulted in significantly decreased numbers of cells in the G1 phase in L1236 (P<0.05; Fig. 5A and B) and L428 (P<0.001; Fig. 5C and D) cells, with significantly increased cells in the G2 phase in L1236 (P<0.05; Fig. 5B) and L428 (P<0.01; Fig. 5D) cells compared with control groups. These findings demonstrated that MGCD0103 could induce cell apoptosis and cell cycle arrest.
Figure 4.
Apoptotic rates were determined by flow cytometry with Annexin-V/PI double-staining. In the MGCD0103 2 µM group, cells were treated with MGCD0103 at a concentration of 2 µM, with the DMSO group considered the control group; cells were treated for 24 h. Total percentages of Annexin-V-positive cells in DMSO and MGCD0103 groups were measured in (A and B) L1236 and (C and D) L428 cells. The percentage of Annexin-V-positive cells in the DMSO groups was lower than that of the MGCD0103 group, in both cell lines. Data are presented as the mean + the standard error of the mean. *P<0.05 and **P<0.01. PI, propidium iodide; DMSO, dimethyl sulfoxide; FITC, fluorescein isothiocyanate.
Figure 5.
Cell cycle distribution was detected by flow cytometry, and quantitative analysis of the different cell cycle phases was performed in (A and B) L1236 and (C and D) L428 cells. In the MGCD0103 2 µM group, cells were treated with MGCD0103 at a concentration of 2 µM for 24 h, with DMSO as control. Fewer cells in the G1 phase were found in the MGCD0103 group compared with the DMSO group, both in L1236 and L428 cells; whereas more cells in the G2 phase were detected in the MGCD0103 group compared with DMSO treated cells, both in L1236 and L428 cells. Cells in the S phase were significantly increased in the MGCD0103 group of L1236 cells. Data are presented as the mean + the standard error of the mean. *P<0.05, **P<0.01, and ***P<0.001. DMSO, dimethyl sulfoxide.
Discussion
No consensus is currently available regarding the treatment of CHL following relapse. Immune checkpoint inhibitors are a potential avenue for such patients; however, the complete remission rate is ~17–21% (25). A previous study demonstrated that expression levels of certain HDACs are associated with clinicopathological characteristics in CHL (26). The results suggested that HDAC1, 3 and 11 are expressed at increased levels in CHL, whereas HDAC2 is decreased (26). In addition, increased expression of HDAC1 predicts shorter progression-free and overall survival (OS), while an increased expression of HDAC11 predicts lower OS (26). The current findings provided insights into the effects on Bcl-2, NF-κB and PD-L1 levels by the treatment of the class I HDACI MGCD0103 in an experimental system; namely, that MGCD0103 enhanced the expression levels of PD-L1 and NF-κB, and reduced the expression of Bcl-2 in CHL.
Bcl-2, a regulatory protein of the Bcl-2 family, serves an important role in promoting cell survival and inhibiting pro-apoptotic proteins (27). It has been demonstrated that Bcl-2 overstimulation and overexpression, and upregulation of the oncogene myc may induce aggressive B-cell malignancies (28). The present findings demonstrated that MGCD0103 had a direct dose-dependent effect in inducing Bcl-2 expression, an apoptosis-related protein, and arresting cell cycle in CHL cell lines.
NF-κB is a protein complex associated with DNA transcription, cytokine regulation and cell survival in multiple cell types (29). As a nuclear transcription factor, NF-κB promotes cell proliferation in acute myelogenous leukemia cells (30). VvpE, an elastase mediated by NF-κB, is associated with cell death and the inflammatory response in human intestinal epithelial cells (31). It was demonstrated that NF-κB is widely expressed in malignant lymphoma, and activation of NF-κB subunits may be associated with the biological functions of HL (32). Buglio et al (33) identified that MGCD0103 is able to induce tumor necrosis factor-α expression and secretion, in association with NF-κB activation. They demonstrated that MGCD0103 may synergize with proteasome inhibitors by HDAC6-independent mechanisms, providing mechanistic rationale for exploring this potentially less-toxic combination for the treatment of lymphoma. Thus, HDACIs combined with NF-κB inhibitor may yield synergistic anti-tumor effects, in accordance with the present findings.
PD-L1, also known as B7 homolog 1 or cluster of differentiation 274 (CD274), is a transmembrane protein encoded by the CD274 gene. PD-L1 has been demonstrated to serve an important role in suppressing the immune system in multiple processes, including pregnancy, inflammation and autoimmune diseases (34–36). Notably, antibodies specifically targeting PD-L1 ligands have provided novel treatments of multiple types of cancer (37). In metastatic renal cell carcinoma, McDermott et al (38) demonstrated that immune-oncology monotherapy can be regarded as ideal second-in-line treatment option. Increased expression of PD-L1 predicts a poor prognosis in colon carcinoma and PD-L1 may describe a future treatment target (39). Previous studies further demonstrated the efficacy of PD-1-targeted therapy in patients with metastatic gastric cancer (40). Previous studies have indicated that PD-1 is associated with inducing T cell tolerance, and can limit T cell responses that may prevent immune-medicated tissue damage (41–43). PD-L1 is correlated with antitumor immunity (44). PD-L1 expressed on the cell surface may help identify immune checkpoint blockade therapies for patients with non-Hodgkin's lymphoma (45). It has been suggested that MGCD0103 may directly inhibit CHL cell growth and survival (46). The present study demonstrated that MGCD0103 may enhance the protein expression levels of NF-κB and PD-L1; these findings indicated that MGCD0103 may regulate cell-mediated immunity of CHL. To a certain extent, this effect of MCD0103 is detrimental to anti-tumor immune function in the microenvironment in which HRS cells reside. Therefore, whether MGCD0103 and PD-1 inhibitors have synergistic effects in the treatment of CHL requires further investigation.
Previous studies have indicated that HDACIs may regulate PD-L1 expression; however these findings have been inconsistent. Booth et al (47) recently demonstrated that HDACIs are capable of reducing HDAC protein expression levels as well as PD-L1 amounts in melanoma cells; meanwhile, Woods et al (48) revealed that class I HDACIs upregulate PD-L1 in melanoma. Therefore, these studies indicated that HDACs have dual-regulation functions and mechanisms in regulating multiple physiological and biochemical processes. The present findings indicated that HDACIs may upregulate PD-L1. This may depend on tumor type and specific molecular biological characteristics in the specific tumor microenvironment.
Briere et al (49) demonstrated that MGCD0103 upregulated PD-L1 and antigen presentation genes including class I and II human leukocyte antigen family members in a panel of non-small cell lung cancer cell lines in vitro. It was concluded that the combination of MGCD0103 and PD-L1 inhibitor demonstrated increased anti-tumor activity compared with either therapy alone in two syngeneic tumor models. In addition, MGCD0103 decreased T-regulatory cell numbers in the tumor microenvironment.
The present results demonstrate that the type I HDACI MGCD0103 decreases Bcl-2 levels and upregulates PD-L1, which indicates the decreased immune ability of CD4+ in the microenvironment of CHL. The combined use of HDACIs and a PD-L1 inhibitor theoretically may improve treatment outcome in patients with CHL. Furthermore, the type I HDACI MGCD0103 may also upregulate NF-κB, which seems to induce resistance towards anti-apoptotic drugs. It seems, therefore, necessary to use anti-NF-κB drugs in combination with HDACIs. Clinical trials combining HDACIs with NF-κB and/or PD-L1 inhibitors should be designed to further improve treatment outcomes for patients with CHL.
The present study had some limitations. The molecular mechanisms by which HDACIs affect CHL have not been deeply investigated in this primary study. A previous study demonstrated that blockage of PD-L1/PD-L2 on 9p24.1 may prolong progression-free survival in patients with CHL (50). However, the effects of HDACIs on 9p24.1 amplification in CHL have not yet been reported. Based on the present data, the effects of HDACIs on 9p24.1 amplification deserve further assessment. The current study focused on exploring the possibility of combining HDACIs and other targeted drugs such as NF-κB and/or PD-L1 inhibitors. Therefore, the effects of HDACIs on CHL were assessed in terms of Bcl-2, NF-κB and PD-L1 expression levels.
Acknowledgements
The authors would like to thank Professor Allen Cusack for his valuable suggestions and language editing services.
Funding
The present study was supported by the Science Foundation of Shanghai Municipal Commission of Science and Technology (grant no. 15ZR1437500) and Science Foundation of Xinjiang Commission of Science and Technology (grant no. 2018D01C243).
Availability of data and materials
All data generated or analyzed during the study are included in this article.
Authors' contributions
XL, SY, ASS and ZM conceived and designed the present study. RH and XZ performed the experiments. RH XZ, ASS, ZM and XL collected the data. RH, XZ, SY, XL, ASS and ZM performed the data analysis and interpretation. RH, XL, SY, ASS and ZM were responsible for literature search. RH was involved in the preparation of manuscript. All the authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Gobbi PG, Ferreri AJ, Ponzoni M, Levis A. Hodgkin lymphoma. Crit Rev Oncol Hematol. 2013;85:216–237. doi: 10.1016/j.critrevonc.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Venkataraman G, Mirza MK, Eichenauer DA, Diehl V. Current status of prognostication in classical Hodgkin lymphoma. Br J Haematol. 2014;165:287–299. doi: 10.1111/bjh.12759. [DOI] [PubMed] [Google Scholar]
- 3.Küppers R. The biology of Hodgkin's lymphoma. Nat Rev Cancer. 2009;9:15–27. doi: 10.1038/nrc2542. [DOI] [PubMed] [Google Scholar]
- 4.Jalali A, Ha FJ, Chong G, Grigg A, Mckendrick J, Schwarer AP, Doig R, Hamid A, Hawkes EA. Hodgkin lymphoma: An Australian experience of ABVD chemotherapy in the modern era. Ann Hematol. 2016;95:809–816. doi: 10.1007/s00277-016-2611-4. [DOI] [PubMed] [Google Scholar]
- 5.Meyer RM, Gospodarowicz MK, Connors JM, Pearcey RG, Wells WA, Winter JN, Horning SJ, Dar AR, Shustik C, Stewart DA, et al. ABVD alone versus radiation-based therapy in limited-stage Hodgkin's lymphoma. N Engl J Med. 2012;366:399–408. doi: 10.1056/NEJMoa1111961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morales CR, Li DL, Pedrozo Z, May HI, Jiang N, Kyrychenko V, Cho GW, Kim SY, Wang ZV, Rotter D, et al. Inhibition of class I histone deacetylases blunts cardiac hypertrophy through TSC2-dependent mTOR repression. Sci Signal. 2016;9:ra34. doi: 10.1126/scisignal.aad5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falkenberg KJ, Johnstone RW. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Discov. 2014;13:673–691. doi: 10.1038/nrd4360. [DOI] [PubMed] [Google Scholar]
- 8.Phelps MP, Bailey JN, Vleeshouwer-Neumann T, Chen EY. CRISPR screen identifies the NCOR/HDAC3 complex as a major suppressor of differentiation in rhabdomyosarcoma. Proc Natl Acad Sci USA. 2016;113:15090–15095. doi: 10.1073/pnas.1610270114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Locatelli SL, Cleris L, Stirparo GG, Tartari S, Saba E, Pierdominici M, Malorni W, Carbone A, Anichini A, Carlo-Stella C. BIM upregulation and ROS-dependent necroptosis mediate the antitumor effects of the HDACi Givinostat and Sorafenib in Hodgkin lymphoma cell line xenografts. Leukemia. 2014;28:1861–1871. doi: 10.1038/leu.2014.81. [DOI] [PubMed] [Google Scholar]
- 10.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 11.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 12.Zagni C, Floresta G, Monciino G, Rescifina A. The search for potent, small-molecule HDACIs in cancer treatment: A decade after vorinostat. Med Res Rev. 2017;37:1373–1428. doi: 10.1002/med.21437. [DOI] [PubMed] [Google Scholar]
- 13.Lin TY, Fenger J, Murahari S, Bear MD, Kulp SK, Wang D, Chen CS, Kisseberth WC, London CA. AR-42, a novel HDAC inhibitor, exhibits biologic activity against malignant mast cell lines via down-regulation of constitutively activated Kit. Blood. 2010;115:4217–4225. doi: 10.1182/blood-2009-07-231985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peart MJ, Smyth GK, van Laar RK, Bowtell DD, Richon VM, Marks PA, Holloway AJ, Johnstone RW. Identification and functional significance of genes regulated by structurally different histone deacetylase inhibitors. Proc Natl Acad Sci USA. 2005;102:3697–3702. doi: 10.1073/pnas.0500369102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batlevi CL, Crump M, Andreadis C, Rizzieri D, Assouline SE, Fox S, van der Jagt RHC, Copeland A, Potvin D, Chao R, Younes A. A phase 2 study of mocetinostat, a histone deacetylase inhibitor, in relapsed or refractory lymphoma. Br J Haematol. 2017;178:434–441. doi: 10.1111/bjh.14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jude JG, Spencer GJ, Huang X, Somerville TDD, Jones DR, Divecha N, Somervaille TCP. A targeted knockdown screen of genes coding for phosphoinositide modulators identifies PIP4K2A as required for acute myeloid leukemia cell proliferation and survival. Oncogene. 2015;34:1253–1262. doi: 10.1038/onc.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirschbaum MH. Histone deacetylase inhibitors and Hodgkin's lymphoma. Lancet Oncol. 2011;12:1178–1179. doi: 10.1016/S1470-2045(11)70327-8. [DOI] [PubMed] [Google Scholar]
- 18.Zagni C, Floresta G, Monciino G, Rescifina A. The search for potent, small-molecule HDACIs in cancer treatment: A decade after vorinostat. Med Res Rev. 2017;37:1373–1428. doi: 10.1002/med.21437. [DOI] [PubMed] [Google Scholar]
- 19.Rosenquist R, Stamatopoulos K. B-cell malignancies: All roads lead to NF-κB activation. Semin Cancer Biol. 2016;39:1–2. doi: 10.1016/j.semcancer.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Weniger MA, Küppers R. NF-κB deregulation in Hodgkin lymphoma. Semin Cancer Biol. 2016;39:32–39. doi: 10.1016/j.semcancer.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Roemer MGM, Redd R, Cader F. Major histocompatibility complex class II and programmed death ligand 1 expression predict outcome after programmed death 1 blockade in classic hodgkin lymphoma. J Clin Oncol. 2018;36:942–950. doi: 10.1200/JCO.2017.77.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka Y, Maeshima AM, Nomoto J, Makita S, Fukuhara S, Munakata W, Maruyama D, Tobinai K, Kobayashi Y. Expression pattern of PD-L1 and PD-L2 in classical Hodgkin lymphoma, primary mediastinal large B-cell lymphoma, and gray zone lymphoma. Eur J Haematol. 2018;100:511–517. doi: 10.1111/ejh.13033. [DOI] [PubMed] [Google Scholar]
- 23.Pianko MJ, Moskowitz AJ, Lesokhin AM. Immunotherapy of lymphoma and myeloma: Facts and hopes. Clin Cancer Res. 2018;24:1002–1010. doi: 10.1158/1078-0432.CCR-17-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alinari L, Blum K. How I treat relapsed classical Hodgkin lymphoma after autologous stem cell transplant. Blood. 2016;127:287–295. doi: 10.1182/blood-2015-10-671826. [DOI] [PubMed] [Google Scholar]
- 25.Villasboas JC, Ansell S. Checkpoint inhibition: programmed cell death 1 and programmed cell death 1 ligand inhibitors in Hodgkin lymphoma. Cancer J. 2016;22:17–22. doi: 10.1097/PPO.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 26.Huang R, Zhang X, Sophia S, Min Z, Liu X. Clinicopathological features and prediction values of HDAC1, HDAC2, HDAC3, and HDAC11 in classical Hodgkin lymphoma. Anticancer Drugs. 2018;29:364–370. doi: 10.1097/CAD.0000000000000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delbridge A, Grabow S, Strasser A, Vaux DL. Thirty years of BCL-2: Translating cell death discoveries into novel cancer therapies. Nat Rev Cancer. 2016;16:99–109. doi: 10.1038/nrc.2015.17. [DOI] [PubMed] [Google Scholar]
- 28.Karube K, Campo E. MYC alterations in diffuse large B-cell lymphomas. Semin Hematol. 2015;52:97–106. doi: 10.1053/j.seminhematol.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Richmond A. Nf-kappa B, chemokine gene transcription and tumour growth. Nat Rev Immunol. 2002;2:664–674. doi: 10.1038/nri887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Tang P, Chen Y, Chen J, Ma R, Sun L. Overexpression of microRNA-125b inhibits human acute myeloid leukemia cells invasion, proliferation and promotes cells apoptosis by targeting NF-κB signaling pathway. Biochem Biophys Res Commun. 2017;488:60–66. doi: 10.1016/j.bbrc.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Lee SJ, Jung YH, Song EJ, Jang KK, Choi SH, Han HJ. Vibrio vulnificus VvpE stimulates IL-1β production by the hypomethylation of the IL-1β promoter and NF-κB activation via lipid raft-dependent ANXA2 recruitment and reactive oxygen species signaling in intestinal epithelial cells. J Immunol. 2015;195:2282–2293. doi: 10.4049/jimmunol.1500951. [DOI] [PubMed] [Google Scholar]
- 32.de Oliveira KA, Kaergel E, Heinig M, Fontaine JF, Patone G, Muro EM, Mathas S, Hummel M, Andrade-Navarro MA, Hübner N, Scheidereit C. A roadmap of constitutive NF-κB activity in Hodgkin lymphoma: Dominant roles of p50 and p52 revealed by genome-wide analyses. Genome Med. 2016;8:28. doi: 10.1186/s13073-016-0280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buglio D, Mamidipudi V, Khaskhely NM, Brady H, Heise C, Besterman J, Martell RE, MacBeth K, Younes A. The class-I HDAC inhibitor MGCD0103 induces apoptosis in Hodgkin lymphoma cell lines and synergizes with proteasome inhibitors by an HDAC6-independent mechanism. Br J Haematol. 2010;151:387–396. doi: 10.1111/j.1365-2141.2010.08342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang YH, Tian M, Tang MX, Liu ZZ, Liao AH. Recent insight into the role of the PD-1/PD-L1 pathway in feto-maternal tolerance and pregnancy. Am J Reprod Immunol. 2015;74:201–208. doi: 10.1111/aji.12365. [DOI] [PubMed] [Google Scholar]
- 35.Le Burel S, Champiat S, Routier E, Aspeslagh S, Albiges L, Szwebel TA, Michot JM, Chretien P, Mariette X, Voisin AL, Lambotte O. Onset of connective tissue disease following anti-PD1/PD-L1 cancer immunotherapy. Ann Rheum Dis. 2018;77:468–470. doi: 10.1136/annrheumdis-2016-210820. [DOI] [PubMed] [Google Scholar]
- 36.Seko Y, Yagita H, Okumura K, Azuma M, Nagai R. Roles of programmed death-1 (PD-1)/PD-1 ligands pathway in the development of murine acute myocarditis caused by coxsackievirus B3. Cardiovasc Res. 2007;75:158–167. doi: 10.1016/j.cardiores.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, Fong L, Joseph RW, Pal SK, Reeves JA, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24:749–757. doi: 10.1038/s41591-018-0053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen XY, Zhang J, Hou LD, Zhang R, Chen W, Fan HN, Huang YX, Liu H, Zhu JS. Upregulation of PD-L1 predicts poor prognosis and is associated with miR-191-5p dysregulation in colon adenocarcinoma. Int J Immunopathol Pharmacol. 2018;32:2058738418790318. doi: 10.1177/2058738418790318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–726. doi: 10.1016/S1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 41.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ravi R, Noonan KA, Pham V, Bedi R, Zhavoronkov A, Ozerov IV, Makarev E, Artemov V A, Wysocki PT, Mehra R, et al. Bifunctional immune checkpoint-targeted antibody-ligand traps that simultaneously disable TGFβ enhance the efficacy of cancer immunotherapy. Nat Commun. 2018;9:741. doi: 10.1038/s41467-017-02696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lau J, Cheung J, Navarro A, Lianoglou S, Haley B, Totpal K, Sanders L, Koeppen H, Caplazi P, McBride J, et al. Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice. Nat Commun. 2017;8:14572. doi: 10.1038/ncomms14572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gravelle P, Burroni B, Péricart S, Rossi C, Bezombes C, Tosolini M, Damotte D, Brousset P, Fournié JJ, Laurent C. Mechanisms of PD-1/PD-L1 expression and prognostic relevance in non-Hodgkin lymphoma: A summary of immunohistochemical studies. Oncotarget. 2017;8:44960–44975. doi: 10.18632/oncotarget.16680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buglio D, Mamidipudi V, Khaskhely NM, Brady H, Heise C, Besterman J, Martell RE, MacBeth K, Younes A. The class-I HDAC inhibitor MGCD0103 induces apoptosis in Hodgkin lymphoma cell lines and synergizes with proteasome inhibitors by an HDAC6-independent mechanism. Br J Haematol. 2010;151:387–396. doi: 10.1111/j.1365-2141.2010.08342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Booth L, Roberts J, Poklepovic A, Kirkwood J, Dent P. HDAC inhibitors enhance the immunotherapy response of melanoma cells. Oncotarget. 2017;8:83155–83170. doi: 10.18632/oncotarget.17950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woods DM, Sodré AL, Villagra A, Sarnaik A, Sotomayor EM, Weber J. HDAC inhibition upregulates PD-1 ligands in melanoma and augments immunotherapy with PD-1 blockade. Cancer Immunol Res. 2015;3:1375–1385. doi: 10.1158/2326-6066.CIR-15-0077-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Briere D, Sudhakar N, Woods DM, Hallin J, Engstrom LD, Aranda R, Chiang H, Sodré AL, Olson P, Weber JS, Christensen JG. The class I/IV HDAC inhibitor mocetinostat increases tumor antigen presentation, decreases immune suppressive cell types and augments checkpoint inhibitor therapy. Cancer Immunol Immunother. 2018;67:381–392. doi: 10.1007/s00262-017-2091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roemer MG, Advani RH, Ligon AH, Natkunam Y, Redd RA, Homer H, Connelly CF, Sun HH, Daadi SE, Freeman GJ, et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol. 2016;34:2690–2697. doi: 10.1200/JCO.2016.66.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the study are included in this article.