Abstract
Emerging evidence suggests that the clinical success of conventional chemotherapy is not solely attributed to tumor cell toxicity, but also results from the restoration of immunosurveillance, which has been largely neglected in the past preclinical and clinical research. Antitumor immune response can be primed by immunogenic cell death (ICD), a type of cell death characterized by cell-surface translocation of calreticulin (CRT), extracellular release of ATP and high mobility group box 1 (HMGB1), and stimulation of type I interferon (IFN) responses. Here we summarize recent studies showing conventional chemotherapeutics as ICD inducers, which are capable of modulating tumor infiltrating lymphocytes (TILs) and reactivating antitumor immunity within an immuno-suppressive microenvironment. Such immunological effects of conventional chemotherapy are likely critical for better prognosis of cancer patients. Furthermore, combination of ICD-inducing chemotherapeutics with immunotherapy is a promising approach for improving the clinical outcomes of cancer patients.
Keywords: Antitumor immunity, Autophagy, Conventional chemotherapy, ER stress, Immunogenic cell death, Immunosurveillance
Introduction
Cancer was previously thought to be a cell-autonomous disease. This cancer cell-centric perspective has been significantly modified recently by incorporation of the concept of immunosurveillance, largely due to the recent success of immunotherapy with immune checkpoint blockers (ICBs).1, 2, 3, 4 It is now clear that naïve tumor cells can be effectively eliminated by the immune system except for those that successfully dodge the immune attack and establish an immunosuppressive microenvironment.1, 2, 5, 6 During this process, tumor cells initiate pathological manifestations and eventually become malignant.1, 2 Such an evolutional process has been delineated by studies of immuno-competent syngeneic tumor models, in which the immune system protects the host from oncogenesis and shapes the immunogenicity of progressive tumors.1 The highly dynamic immunosurveillance process comprises three phases: (1) removal of emerging tumor cells by the immune system (elimination); (2) failure of the elimination phase, leading to tumor dormancy (equilibrium) and the development of immunogenic stress that shapes genetically vulnerable tumors (editing); and (3) selection of tumor cell variants that cannot be recognized or eliminated by the immune system (escape).
Compromised immunosurveillance in the tumor microenvironment
Tumor development is not only driven by activation of oncogenes and inactivation of tumor suppressors, but also by alterations in the tumor microenvironment (TME), as indicated by altered density and composition of immune infiltrates in tumors.7 The elimination and equilibrium phases of immunosurveillance are mediated by cytotoxic T lymphocytes (CTLs), type I helper (Th1) CD4+ T lymphocytes, and natural killer (NK) cells. The escape phase is characterized by diminishing of immune infiltrates and accumulation of cells that suppress anticancer immunity, such as regulatory T cells (Tregs) and immunosuppressive myeloid cells.8 These alterations reflect the ability of tumors to generate immunosuppressive signals and foster immunosuppressive cells in the TME.
In anticancer immune response, CTLs utilize T cell receptor (TCR) and CD8 to recognize antigens presented by major histocompatibility complex (MHC) class I molecules on the plasma membrane of tumor cells, leading to release of perforin-1 (PRF1) and granzyme B to induce cytotoxicity.9 CTLs also suppress tumor growth by releasing interferon γ (IFNγ), an immuno-stimulatory cytokine.10 Th1 CD4+ T lymphocytes recognize antigens presented by MHC class II molecules on the plasma membrane of target cells through TCR and CD4, and release a variety of immuno-stimulatory cytokines such as IFNγ and interleukin-2 (IL-2).11 Upon the activation of NK cells, cancer cells lose inhibitory signals and present ligands to NK cell-activating receptors including CD226 and KLRK1 (killer cell lectin-like receptor subfamily K, member 1).12 CTLs and Th1 CD4+ T lymphocytes are actively involved in local immunosurveillance, while NK cells primarily defend against tumor metastasis.12 Under the immunological stress from CTLs, target cells lose the expression of MHC class I molecules and become vulnerable to NK cells. An optimal TME is infiltrated by a mixture of CTLs, Th1 CD4+ T lymphocytes and NK cells.
An effective antitumor immune response is often triggered by a combination of lymphocytes and a subset of dendritic cells (DCs).13, 14 CD8α+CD134 + DCs first engulf fractions of cancer cells, process them, and then present tumor-associated antigens to CTLs, leading to cross-priming of CD8+ T cells and anticancer immunity.15 In addition, DCs have a crucial immuno-modulatory effect on Th1 CD4+ T lymphocytes and NK cells.16 Accumulating evidence suggests that reactivation of immunosurveillance is critical for better prognosis and improved patient survival, which can be achieved by using agents that induce immunogenic cell death in tumor cells.
Immunogenic cell death in cancer cells
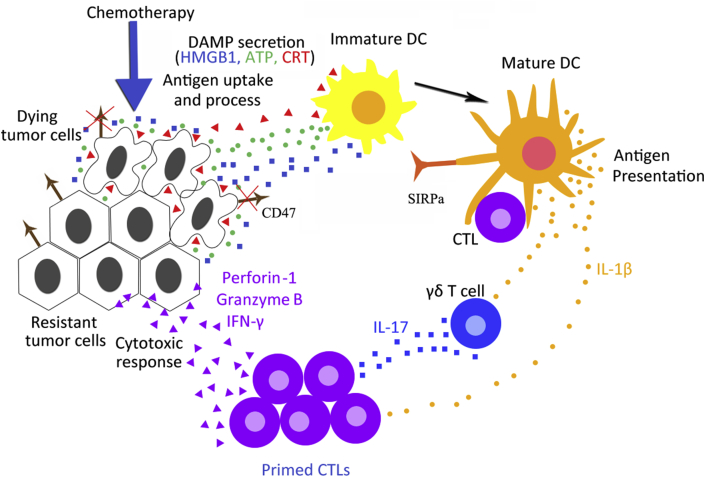
Immunogenic cell death (ICD) is a type of tumor cell death which primes an anticancer immune response.17 A variety of chemotherapeutic agents can induce ICD, as indicated by the alterations in TIL abundance and composition, which is a marker of favorable prognosis.7 For example, in response to anthracyclines or oxaliplatin treatment, breast and colorectal cancer patients have increased numbers of TILs and higher ratio of CD8+ CTLs vs. FOXP3+ Tregs, leading to favorable therapeutic response.18, 19, 20, 21, 22 ICD is defined by two criteria. First, tumor cells succumbing to ICD in vitro without any adjuvant can trigger antitumor immunity that protects mice against a subsequent challenge with live tumor cells of the same type.17 Second, ICD induced in vivo can stimulate local antitumor immunity, which is characterized by attracting immune effector cells into the TME, leading to tumor suppression that at least partially relies on the immune system.23, 24 In response to ICD-inducing chemotherapeutics, tumor cells expose CRT on cell surface prior to death, and release damage-associated molecular pattern (DAMP) molecules such as ATP during apoptosis or HMGB1 upon secondary necrosis. These DAMPs stimulate the recruitment of DCs into the tumor bed, the uptake and processing of tumor antigens, and the optimal antigen presentation to T cells. Cross-priming of CD8+ CTLs is triggered by mature DCs and γδ T cells in an IL-1β- and IL-17-dependent manner. Primed CTLs then elicit a direct cytotoxic response to kill remaining tumor cells through the generation of IFN-γ, perforin-1 and granzyme B (Fig. 1). Therefore, the hallmarks of ICD include calreticulin (CRT) plamsa-membrane translocation, extracellular ATP and/or HMGB1 release, and stimulation of type I interferon (IFN) responses.25, 26, 27, 28
Figure 1.
Mechanism of immunogenic cell death (ICD). In response to ICD-inducing chemotherapeutics, tumor cells expose CRT on their surface at a pre-apoptotic stage, secrete ATP during apoptosis, and release HMGB1 during secondary necrosis. These damage-associated molecular pattern (DAMP) molecules liberated from dying tumor cells stimulate the recruitment of DCs into the tumor bed, the uptake and processing of tumor antigens, and the optimal antigen presentation to T cells. However, the binding of cell-surface CD47 to SIRP on DCs inhibits their phagocytic function. Phagocytosis of tumor cells requires both the activation of pro-phagocytic signals as well as simultaneous disruption of the anti-phagocytic signal CD47. Cross-priming of CD8+ CTLs is triggered by mature DCs and γδ T cells in an IL-1β- and IL-17-dependent manner. Primed CTLs elicit direct cytotoxic response and kill remaining tumor cells through the generation of IFN-γ, perforin-1 and granzyme B.
Cell-surface CRT as a key pro-phagocytic signal
ICD induced by chemotherapeutics often involves endoplasmic reticulum (ER) stress, which promotes translocation of CRT from ER lumen to the outer leaflet of plasma membrane.29, 30 This occurs prior to membrane exposure of phosphatidylserine (PS), a marker of apoptosis.30 Upon treatment with the ICD-inducing chemotherapeutics such as anthracyclines or oxaliplatin, ER stress is initiated through phosphorylation of eukaryotic translation initiation factor 2α (eIF2α) by PKR-like ER kinase (PERK). This leads to subsequent signaling events including caspase-8-dependent proteolysis of the ER protein BAP31, activation of pro-apoptotic proteins BAX and BAK, translocation of CRT from the ER to Golgi apparatus, and exocytosis of CRT-containing vesicles, eventually leading to SNARE-mediated relocation of CRT onto cell surface.30 Production of reactive oxygen species (ROS) and nitric oxide facilitates the anthracycline-induced CRT exposure.30, 31
CRT exposure is essential as an ER immunogenic signal for the induction of tumor-associated immune responses.28 Pharmacological or genetic inhibition of the ER stress response pathway abrogates CRT translocation and attenuates the immunological effects of cytotoxic chemotherapeutics.30 Suppression of CRT exposure and ICD also abolishes the activity of dying tumor cells as a vaccine to trigger an anticancer immune response.29, 30, 32 Conversely, administration of recombinant CRT to the plasma membrane of cells treated with a non-ICD inducer (e.g., cisplatin) could reinstate ICD.30, 32 CRT is functionally considered as an “eat-me” signal to the immune system. Cells with CRT expression on their surface can be recognized and engulfed by CD91 + cells (e.g., DCs and macrophages), unless they simultaneously express a “don't-eat-me” signal (e.g., CD47) on the plasma membrane.33
CRT acts on target DCs via CD91 expressed on their surface to promote the release of pro-inflammatory cytokines (e.g., TNF-α and IL-6) and modulate the activity of type 17 helper T (Th17) cells in an immunosuppressive tumor bed. The binding of CRT to CD91 also facilitates the recruitment of antigen-presenting cells (e.g., DCs) into the tumor bed, engulfment of tumor cells by DCs, and optimal antigen presentation to T cells, eventually leading to activation of the immune system.34 Clinical evidence suggests a correlation between disease outcomes and CRT cell-surface translocation and CD47 surface expression in cancer cells. For example, deficient CRT exposure in colorectal tumor cells is associated with loss of TILs and poor prognosis.35 CRT expression and lack of CD47 expression on the surface of acute myeloid leukemia (AML) cells are associated with favorable anticancer immune response and higher survival rate in AML patients.36 Furthermore, CRT expression accompanied by CD47 exposure on the membrane of cancer cells is correlated with poor prognosis of neuroblastoma, bladder cancer and mantle cell lymphoma patients.37 Collectively, these findings indicate that CRT cell-surface translocation is a key event in ICD-mediated activation of the immune response.
ATP release
ATP secretion from tumor cells can also trigger an immune response, and is often associated with autophagy, a normal physiological process in response to stress or nutrients deprivation.23, 38 During autophagy, cytoplasmic content is sequestered into double-membraned organelles through a series of ordered events, including formation of autophagosomes, lysosomal fusion, and cargo digestion, which allow recycling of building blocks of cells into energy metabolic/anabolic reactions.38 Blocking autophagy by a pharmacological or genetic approach limited extracellular release of ATP without affecting either CRT translocation or HMGB1 secretion, and resulted in poor recruitment of macrophages and DCs, compromised anticancer immunity and unfavorable antitumor response.23 Autophagy has an antitumor effect and is often suppressed in tumor cells.39, 40 Autophagy-deficient tumors exhibited reduced ATP secretion and TILs in response to chemotherapy, further suggesting that autophagy suppression can be utilized by malignant cells to evade immunosurveillance.23
The level of extracellular ATP in response to chemotherapy is affected not only by autophagy, but also by ectonucleotidases (e.g., CD39 and CD73). CD39 catalyzes ATP hydrolysis, and the resulting products (e.g., ADP and AMP) are further hydrolyzed to immunosuppressive adenosine. This resulted in an extremely low level of ATP in the extracellular milieu.41, 42 TILs expressing CD39 (e.g., Treg and Th17 cells) have been shown to blunt immune response and promote tumor growth.43, 44 Interestingly, CD39/CD73-transfected cancer cells were refractory to chemotherapy or immunotherapy, which can be overcame by CD73-blocking antibodies that restore the activity of CD8+ T lymphocytes.42, 45
Extracellular ATP liberated from dying tumor cells generates a strong “find-me” signal for DCs and macrophages, upon its binding to P2Y2 receptors expressed on the surface of the target cells.46 In line with this notion, administration of ATPγS, an ATP analog that cannot be metabolized by CD39 and CD73, leads to the recruitment of DCs in a P2Y2-dependent manner.47 Extracellular ATP not only attracts immune cells into the tumor bed, but also modulates their activity. For example, ATP can induce the maturation of myeloid-derived DCs, which is accompanied by increased expression of CD40, CD80, CD83, and CD86, and also promote macrophage expansion through formation of lamellipodial membrane protrusions.46
HMGB1 release
HMGB1 is released from dying cells that are undergoing necrosis including programmed necrosis (necroptosis), as well as from immune cells that recognize pathogens.48 Upon its release, HMGB1 triggers a strong inflammatory response, and inhibition of HMGB1 using blocking antibodies or siRNAs suppressed anthracycline-induced anticancer immunity.25 In this context, HMGB1 activates DCs and stimulates an optimal presentation of tumor-associated antigens to T cells, upon its binding to TLR4. TLR4 is associated with ICD signaling, and inactivation of TLR4 diminished the DC-based cross-presentation of cancer-associated antigens and caused immune deficiency.25 RAGE (receptor for advanced glycation endproducts) is another important receptor for HMGB1. Binding of HMGB1 to RAGE promotes DCs' maturation and migration through activation of MAPKs (p38 and ERK1/2) and NF-κB.49
Negative regulation of ICD by the anti-phagocytic signal and oncogenes
Recent studies have identified CD47, a cell-surface glycoprotein and essential anti-phagocytic signal, as a potential negative regulator of ICD.50 The inhibitory effect of CD47 on phagocytosis is mediated by its binding to signal-regulatory protein α (SIRPα), which is expressed on macrophages and DCs. CD47 is expressed in all human solid tumor cells and protects them from being recognized and cleared by innate immunosurveillance. High CD47 mRNA expression is associated with poor survival of cancer patients.51 CD47 blockade by antibodies has been explored in various preclinical models, and is also being tested in multiple ongoing clinical trials.37, 52 Recent findings indicate that the CD47-SIRPα signaling pathway determines the clearance of cytosolic DNA in DCs, and anti-CD47 antibodies suppress cytosolic DNA degradation, leading to activation of the cGAS-STING-IRF3 pathway and adaptive antitumor immunity.53
Recent studies have revealed the mechanisms and upstream regulators that contribute to upregulation of CD47 in cancer cells. It was shown that the TNF/NF-κB1 pathway directly regulates CD47 in breast cancer cells through a CD47-associated super-enhancer.54 CD47 could also be upregulated by hypoxia-inducible factor 1 (HIF-1) in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells.55 c-Myc oncoprotein was found to upregulate CD47 and PD-L1 expression by directly binding to their promoters, and c-Myc depletion decreased the expression of CD47 in a variety of tumor cell lines.56 In addition, c-Myc can mediate immune escape by directly binding to the STAT1 promoter to suppress the type I and type II IFN response, as well as by indirectly reducing the secretion of IFNβ.57 Inactivation of oncogenes such as c-Myc often induces tumor cell death with features of ICD, and represents a promising strategy to elicit antitumor immunity.58, 59 A number of chemotherapeutic agents can downregulate c-Myc, which may restore antitumor immune response and promote remodeling of the TME.
Induction of ICD by cytotoxic chemotherapeutics
Conventional cytotoxic chemotherapeutics are generally classified according to their mechanisms of action: (1) alkylating agents that elicit inter- and intra-strand DNA crosslinks that destabilize DNA during replication (e.g., cyclophosphamide and oxaliplatin); (2) antimetabolites that disrupt DNA and/or RNA synthesis (e.g., 5-fluorouracil, gemcitabine, and mitoxantrone); (3) topoisomerase inhibitors that interfere with the DNA unwinding process during DNA replication and transcription (e.g., irinotecan); (4) microtubule poisons that inhibit tubulin polymerization/depolymerization and cause mitotic arrest (e.g., paclitaxel); (5) cytotoxic antibiotics that kill cancer cells via excessive production of ROS and DNA intercalation (e.g., anthracyclines and bleomycin). The immunogenic effects of these agents were largely neglected in previous studies that mostly utilized cell culture and immune-deficient animal models. In the past, promising anticancer agents were often moved forward into clinical trials without a thorough analysis of their immune modulatory effects. Most attention was paid to the common side effects of myelosuppression and lymphopenia of conventional chemotherapy, rather than chemotherapy-induced antitumor immune response.
Accumulating evidence indicates that the clinical success of chemotherapy is not solely attributed to tumor cell toxicity, but also the restoration of immunosurveillance (Table 1). It is now clear that a subset of chemotherapeutics can elicit the immunogenicity of tumor cells and modulate the density, composition, distribution and function of TILs, to influcence prognosis and differential therapeutic responses in cancer patients.60, 61, 62, 63, 64 These agents can induce ICD to stimulate local antitumor immune response and facilitate immune infiltration through various mechanisms.65, 66 For example, chemotherapy-induced phosphorylation of p53 upregulates the NKG2D ligands presented on the membrane of target tumor cells, improving their recognition by NKG2D + NK cells.67 Chemotherapy-induced membrane expression of heat shock proteins (e.g., HSP70 and HSP90) also contributes to immune stimulation.68, 69 The ICD-inducing chemotherapeutics are compatible with other immune stimulatory agents. For instance, DNA-, peptide-, or DC-based vaccines trigger anticancer immunological effects in patients administered with conventional chemotherapeutics.70 Although the majority of conventional chemotherapeutic agents are incapable of inducing ICD, their efficacy can be significantly improved by the co-administration of chemicals that produce ICD-associated DAMPs. Therefore, the effects on antitumor immune response need to be taken into consideration to improve chemotherapy regimens.
Table 1.
Antitumor immunological effects of conventional chemotherapy.
| Conventional chemotherapeutics | Major targets | ICD-associated DAMPs | Clinical use | Evidence of antitumor immunity |
|---|---|---|---|---|
| Cylophosphamide | DNA and RNA | Cell-surface CRT, | Lymphomas, brain cancer, leukemia, | Deplete Treg cells, favor the expansion of |
| HMGB1 and ATP secretion | and some solid tumors | NK cells and stimulate DCs to produce IL-12 | ||
| Oxaliplatin | DNA and RNA synthesis | Cell-surface CRT, | Colorectal cancer | Increase the CTL/Treg cell ratio, deplete MDSCs, |
| HMGB1 and ATP secretion | and improve the activity of neutrophils and macrophages | |||
| 5-FU | Thymidylate synthase inhibitor | HMGB1 and ATP secretion | Colorectal, breast cancer, GIST | Increase the frequency of tumor-infiltrating CTLs, |
| DNA replication | and deplete circulating MDSCs | |||
| Gemcitabine | DNA replication | HMGB1 and ATP secretion | NSCLC, pancreatic, bladder, | Stimulate cross-priming of CTLs, increase the number of |
| breast cancer | immunostimulatory TAMs, and deplete circulating MDSCs | |||
| Mitoxantrone | DNA and RNA synthesis, | Cell-surface CRT and ERp57, | Leukemias, Hodgkin's lymphoma | Increase the CTL/Treg cell ratio, |
| and anthracyclines | topoisomerase II, ROS | HMGB1 and ATP secretion | breast, colon, lung cancer and so on | and deplete circulating Treg cells |
| Bleomycin | DNA strand breaks, ROS | Cell-surface CRT and ERp57, | Testicular cancer, ovarian cancer, | Stimulate ICD |
| Bortezomib | 26S proteasome | Cell-surface HSP90 | Multiple melanoma, mantle cell lymphoma | Stimulate ICD |
Abbreviations: ICD, immunogenic cell death; DAMPs, damage-associated molecular patterns; ROS, radical oxygen species; CRT, calreticulin; CTL, cytotoxic T lymphocytes; Treg, regulatory T cells; NK, natural killer; DCs, dendritic cells; IL-12, interleukin-12; HMGB1, high-mobility group protein B1; ATP, adenosine triphosphate; HSP, heat-shock protein; MDSCs, myeloid-derived suppressor cells; TAMs, tumor-associated macrophages.
Alkylating agents
Alkylating agents can elicit an antitumor immune response by directly affecting the immune cells. For example, cyclophosphamide reinstated the activity of T and NK cells by ablating Tregs in TME.71 The gram-positive bacteria of the intestinal microbiota contribute to the cyclophosphamide-induced immune stimulation in mice.72 Cyclophosphamide treatment also promoted translocation of gram-positive bacteria to secondary lymphoid organs via gap junctions in the intestinal epithelium, which stimulates the production of Th17 cells and secretion of IL-17 and IFNγ, resulting in potent antitumor immunity.72 A variety of transplantable tumors and carcinogen-induced mouse tumors were more sensitive to oxaliplatin/anthracycline treatment when growing in a syngeneic immuno-competent host, compared to an immuno-deficient host.23, 25, 73, 74 Interestingly, agents with similar chemical structures may have very different immunological consequences. For example, oxaliplatin, but not cisplatin, is a potent inducer of ICD, and can trigger ER stress and antitumor immunity against both transplantable and oncogene-driven murine tumors.75, 76 A combination of cisplatin and chemicals that cause ER stress can induce ICD.77, 78
Antimetabolites
Antimetabolites such as Mitoxantrone were found to facilitate tumor infiltration of immune cells in mouse models. Their therapeutic efficacy can be attenuated by: (1) depletion of CTLs and γδ T cells, but without an association with diminished NK cells and B lymphocytes24, 73, 79; (2) repression of tumor-infiltrating DCs by an anti-CD11b antibody or P2RY2 inhibitors;80, 81 (3) genetic or pharmacological inhibition of immuno-stimulatory cytokines (e.g., type I IFN, IFNγ, IL-1β, and IL-17) or their receptors24, 25, 26, 82; or (4) blockade of DAMP secretion from dying tumor cells.83 Gemcitabine, a nucleoside analog widely used for treating breast cancer, non-small cell lung carcinoma (NSCLC), and pancreatic cancer, is capable of stimulating cross-priming of CD8+ T cells, increasing the number of immuno-stimulatory tumor-associated macrophages (TAMs), and depleting circulating myeloid-derived suppressor cells (MDSCs) in mouse models.84, 85, 86 5-Fluorouracil (5-FU) can also deplete MDSCs in mouse models.87 A positive association was found between tumor-infiltrating CTLs and clinical outcomes of colorectal cancer patients treated with 5-FU-based chemotherapy.88 In combination with radiotherapy, 5-FU enhances the tumor-infiltrating CTLs in locally advanced rectal cancer, leading to a favorable therapeutic response of patients.89
Cytotoxic antibiotics
Doxorubicin markedly changes the immune infiltrate of breast tumors,90 and high infiltration of CTLs in breast tumors at diagnosis predicts favorable therapeutic response.19, 91 The immuno-stimulatory effect of anthracyclines has been shown to result from their cytotoxicity to cancer cells through a multipronged adaptive stress response, involving autophagy, ER stress response, and type I IFN pathways. Autophagy is obligatory for dying tumor cells to release ATP that works both as a chemotactic (via binding to P2RY2) and as an immuno-stimulatory (via binding to P2RX2) factor for DCs.23 Cell-surface CRT elicited by ER stress stimulates the recognition of tumor-associated antigens and engulfment of tumor cells by DCs upon binding to CD91.28 In anthracycline-treated tumor cells, autocrine/paracrine type I IFN response is involved in the secretion of CXCL10, which is chemoattractant to lymphocytes.82 Upon binding to TLR4 and RAGE, HMGB1 released from dying tumor cells boosts the activation and maturation of DCs.25
Bleomycin is a cytotoxic antibiotic glycopeptide that causes DNA damage by over-generation of ROS.92 It is clinically administered to patients with testicular cancer and Hodgkin's lymphoma.93 Similar to anthracyclines and oxaliplatin, bleomycin is capable of eliciting ICD via ER stress response and ROS generation, which lead to membrane translocation of ER proteins (e.g., CRT and ERp57). Bleomycin-induced autophagy stimulated the release of HMGB1 and ATP from dying tumor cells. ICD induction by bleomycin reactivated the antitumor immunity mediated by CD8+ T cells.94 In addition, bleomycin treatment facilitates the release of transforming growth factor β (TGFβ) from dying tumor cells, leading to the spreading of FOXP3+ Tregs. Interestingly, the therapeutic efficacy of bleomycin was greatly improved by the ablation of TGFβ and/or Tregs.83
Other drugs
Bortezomib is a specific inhibitor of the 26S proteasome subunit that has exhibited favorable therapeutic efficacy in several types of cancer. Bortezomib-induced apoptosis in cancer cells generally results from the suppression of NF-κB and/or induction of unfolded protein response.95 The restoration of immunogenicity of bortezomib-treated dying cancer cells was initiated from the phagocytosis process between DCs and dying cancer cells, and was regulated by bortezomib-induced upregulation of HSP90 on the plasma membrane of dying cancer cells.68 DCs are primarily involved in the uptake and presentation of antigens from dying cells. Expansion of antitumor T cells is modulated through the cross-presentation of tumor antigens by DCs. Interestingly, addition of an HSP90 inhibitor significantly promoted bortezomib-induced apoptosis in cancer cells, but abolished immunogenicity.68 Therefore, HSP90 cell-surface exposure might be another important "eat me" signal of ICD in addition to CRT exposure.
Combinations of chemotherapy and immunotherapy
Understanding the immunological effects of chemotherapy has helped design a variety of combination regimens with potentially improved efficacy. For example, CTLA4 (cytotoxic T lymphocyte-associated protein 4) neutralizing antibodies can be used to increase the immuno-stimulatory effect of gemcitabine by ablating tumor-infiltrating Tregs.96 Such combinations can also be used to mitigate immunosuppressive effects of certain chemotherapies. For example, bleomycin induces CTL-dependent antitumor immunity, but also boosts TGFβ1-dependent spreading of immunosuppressive Tregs, which can be suppressed by co-administration of a TGFβ1 antibody.94 Anti-CD47 antibodies have shown promises in preclinical studies on brain, breast, colon, and ovary tumors, but are not sufficiently effective for eradicating these tumors.51 A combination of CD47 blockade with other therapeutic agents can enhance cell-surface CRT and/or eliminate Tregs and MDSCs.52 Numerous clinical trials have demonstrated the additive/synergistic effects of chemotherapy in combination with ICB-based immunotherapy.97 For example, the therapeutic efficacy of carboplatin or paclitaxel can be significantly increased by the co-administration of the anti-CTLA4 antibody ipilimumab in small cell lung carcinoma and NSCLC.98, 99 We expect that the results of these trials will provide invaluable information on how to better combine chemotherapy and immunotherapy to improve the clinical management of cancer.
Perspectives and conclusions
Molecular mechanisms of ICD remain to be further delineated. For example, it is still unclear why p53-dependent apoptosis mediated through the intrinsic pathway is less immunogenic than that involving ER stress. Necroptosis is a highly immunogenic form of cell death.100 However, the functional role of necroptosis in chemotherapy has not been well defined.101 The interplays and crosstalk of different cell death pathways, such as apoptosis, necroptosis, ferroptosis, pyroptosis, and autophagy, in determining the immunological consequences of chemotherapy remain to be elucidated. In addition to c-Myc, the effect of other cancer drivers on ICD and the immunological effects of chemotherapy also need to be established. ICD can be induced in response to other pathological conditions, and is involved in tissue injury and damage repair. It is important to understand the differences between the chemotherapy-induced ICD in tumor cells and that involved in normal physiological process and other pathological conditions, in order to more specifically target tumor cells.
Several strategies have been explored to harness the immunological effects of chemotherapy. The immunological markers such as alterations of TIL density, composition, function and localization as the result of treatment have been increasingly recognized as a valuable predictor for clinical outcomes. The ICD hallmarks such as cell-surface CRT exposure, ATP secretion and HMGB1 release in tumor tissues have been analyzed and potentially useful as biomarkers for predicting favorable prognosis and survival of cancer patients.102 Combinations of markers from tumor cells and local and systematic immune response remain to be explored, and may help to improve the predictive power of applying these markers. Further defining the molecular mechanisms and biomarkers of ICD will undoubtedly help rational design of new combination regimens for improving therapeutic efficacy.
In conclusion, emerging preclinical and clinical evidence supports the concept that conventional chemotherapeutics can engage the immune system to act beyond tumor cells, which can be manipulated and will hopefully provide lasting benefits to cancer patients via enhanced and long-term immunosurveillance of minimum residual disease. It might be very useful to test promising anticancer drugs or combinations for their capability to induce ICD in immuno-competent models to boost antitumor immunity and improve therapeutic efficacy.
Conflicts of interest
There is no conflict of interest.
Acknowledgements
We apologize for not being able to cite many excellent original articles by our colleagues due to space limitation. We thank our lab members for critical reading. Research in the authors’ labs is supported U.S. National Institutes of Health grants (R01CA172136, R01CA203028 and R01CA201741 to L.Z.; U19AI068021 and R01CA215481 to J.Y.; and P30CA047904 to the UPMC Hillman Cancer Center).
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Schreiber R.D., Old L.J., Smyth M.J. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 2.Zitvogel L., Tesniere A., Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 3.Sharma P., Allison J.P. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 4.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Shankaran V., Ikeda H., Bruce A.T. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 7.Galon J., Costes A., Sanchez-Cabo F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 8.Finn O.J. Cancer immunology. N Engl J Med. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 9.Anthony D.A., Andrews D.M., Watt S.V., Trapani J.A., Smyth M.J. Functional dissection of the granzyme family: cell death and inflammation. Immunol Rev. 2010;235:73–92. doi: 10.1111/j.0105-2896.2010.00907.x. [DOI] [PubMed] [Google Scholar]
- 10.Vesely M.D., Kershaw M.H., Schreiber R.D., Smyth M.J. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 11.Fridman W.H., Pages F., Sautes-Fridman C., Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 12.Vivier E., Raulet D.H., Moretta A. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broz M.L., Binnewies M., Boldajipour B. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26:638–652. doi: 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruffell B., Chang-Strachan D., Chan V. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26:623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Y., Aymeric L., Locher C., Kroemer G., Zitvogel L. The dendritic cell-tumor cross-talk in cancer. Curr Opin Immunol. 2011;23:146–152. doi: 10.1016/j.coi.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Ferlazzo G., Morandi B. Cross-talks between natural killer cells and distinct subsets of dendritic cells. Front Immunol. 2014;5:159. doi: 10.3389/fimmu.2014.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green D.R., Ferguson T., Zitvogel L., Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeNardo D.G., Brennan D.J., Rexhepaj E. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denkert C., Loibl S., Noske A. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 20.Halama N., Michel S., Kloor M. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011;71:5670–5677. doi: 10.1158/0008-5472.CAN-11-0268. [DOI] [PubMed] [Google Scholar]
- 21.Ladoire S., Mignot G., Dabakuyo S. In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival. J Pathol. 2011;224:389–400. doi: 10.1002/path.2866. [DOI] [PubMed] [Google Scholar]
- 22.West N.R., Milne K., Truong P.T., Macpherson N., Nelson B.H., Watson P.H. Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res. 2011;13:R126. doi: 10.1186/bcr3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michaud M., Martins I., Sukkurwala A.Q. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 24.Ma Y., Aymeric L., Locher C. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J Exp Med. 2011;208:491–503. doi: 10.1084/jem.20100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apetoh L., Ghiringhelli F., Tesniere A. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 26.Ghiringhelli F., Apetoh L., Tesniere A. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 27.Tesniere A., Schlemmer F., Boige V. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482–491. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- 28.Obeid M., Tesniere A., Ghiringhelli F. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 29.Garg A.D., Krysko D.V., Verfaillie T. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 2012;31:1062–1079. doi: 10.1038/emboj.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panaretakis T., Kepp O., Brockmeier U. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009;28:578–590. doi: 10.1038/emboj.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Boo S., Kopecka J., Brusa D. iNOS activity is necessary for the cytotoxic and immunogenic effects of doxorubicin in human colon cancer cells. Mol Cancer. 2009;8:108. doi: 10.1186/1476-4598-8-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panaretakis T., Joza N., Modjtahedi N. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ. 2008;15:1499–1509. doi: 10.1038/cdd.2008.67. [DOI] [PubMed] [Google Scholar]
- 33.Gardai S.J., McPhillips K.A., Frasch S.C. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 34.Pawaria S., Binder R.J. CD91-dependent programming of T-helper cell responses following heat shock protein immunization. Nat Commun. 2011;2:521. doi: 10.1038/ncomms1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng R.Q., Chen Y.B., Ding Y. Expression of calreticulin is associated with infiltration of T-cells in stage IIIB colon cancer. World J Gastroenterol. 2010;16:2428–2434. doi: 10.3748/wjg.v16.i19.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wemeau M., Kepp O., Tesniere A. Calreticulin exposure on malignant blasts predicts a cellular anticancer immune response in patients with acute myeloid leukemia. Cell Death Dis. 2010;1:e104. doi: 10.1038/cddis.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chao M.P., Jaiswal S., Weissman-Tsukamoto R. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010;2:63ra94. doi: 10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kroemer G., Marino G., Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karantza-Wadsworth V., Patel S., Kravchuk O. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21:1621–1635. doi: 10.1101/gad.1565707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maiuri M.C., Tasdemir E., Criollo A. Control of autophagy by oncogenes and tumor suppressor genes. Cell Death Differ. 2009;16:87–93. doi: 10.1038/cdd.2008.131. [DOI] [PubMed] [Google Scholar]
- 41.Ohta A., Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 42.Stagg J., Beavis P.A., Divisekera U. CD73-deficient mice are resistant to carcinogenesis. Cancer Res. 2012;72:2190–2196. doi: 10.1158/0008-5472.CAN-12-0420. [DOI] [PubMed] [Google Scholar]
- 43.Chalmin F., Mignot G., Bruchard M. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity. 2012;36:362–373. doi: 10.1016/j.immuni.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 44.Sun X., Wu Y., Gao W. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology. 2010;139:1030–1040. doi: 10.1053/j.gastro.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stagg J., Smyth M.J. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29:5346–5358. doi: 10.1038/onc.2010.292. [DOI] [PubMed] [Google Scholar]
- 46.Kronlage M., Song J., Sorokin L. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci Signal. 2010;3:ra55. doi: 10.1126/scisignal.2000588. [DOI] [PubMed] [Google Scholar]
- 47.Muller T., Robaye B., Vieira R.P. The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy. 2010;65:1545–1553. doi: 10.1111/j.1398-9995.2010.02426.x. [DOI] [PubMed] [Google Scholar]
- 48.Andersson U., Tracey K.J. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dumitriu I.E., Baruah P., Valentinis B. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol. 2005;174:7506–7515. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- 50.McCracken M.N., Cha A.C., Weissman I.L. Molecular pathways: activating T cells after cancer cell phagocytosis from blockade of CD47 “Don't eat me” signals. Clin Cancer Res. 2015;21:3597–3601. doi: 10.1158/1078-0432.CCR-14-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Willingham S.B., Volkmer J.P., Gentles A.J. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gholamin S., Mitra S.S., Feroze A.H. Disrupting the CD47-SIRPalpha anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci Transl Med. 2017:9. doi: 10.1126/scitranslmed.aaf2968. [DOI] [PubMed] [Google Scholar]
- 53.Xu M.M., Pu Y., Han D. Dendritic cells but not macrophages sense tumor mitochondrial DNA for cross-priming through signal regulatory protein alpha signaling. Immunity. 2017;47:363–373. doi: 10.1016/j.immuni.2017.07.016. e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Betancur P.A., Abraham B.J., Yiu Y.Y. A CD47-associated super-enhancer links pro-inflammatory signalling to CD47 upregulation in breast cancer. Nat Commun. 2017;8:14802. doi: 10.1038/ncomms14802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang H., Lu H., Xiang L. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc Natl Acad Sci USA. 2015;112:E6215–E6223. doi: 10.1073/pnas.1520032112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Casey S.C., Tong L., Li Y. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352:227–231. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schlee M., Holzel M., Bernard S. C-myc activation impairs the NF-kappaB and the interferon response: implications for the pathogenesis of Burkitt's lymphoma. Int J Cancer. 2007;120:1387–1395. doi: 10.1002/ijc.22372. [DOI] [PubMed] [Google Scholar]
- 58.Dranoff G. Experimental mouse tumour models: what can be learnt about human cancer immunology? Nat Rev Immunol. 2011;12:61–66. doi: 10.1038/nri3129. [DOI] [PubMed] [Google Scholar]
- 59.Mittal D., Gubin M.M., Schreiber R.D., Smyth M.J. New insights into cancer immunoediting and its three component phases–elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16–25. doi: 10.1016/j.coi.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anitei M.G., Zeitoun G., Mlecnik B. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res. 2014;20:1891–1899. doi: 10.1158/1078-0432.CCR-13-2830. [DOI] [PubMed] [Google Scholar]
- 61.Ascierto P.A., Capone M., Urba W.J. The additional facet of immunoscore: immunoprofiling as a possible predictive tool for cancer treatment. J Transl Med. 2013;11:54. doi: 10.1186/1479-5876-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Remark R., Becker C., Gomez J.E. The non-small cell lung cancer immune contexture. A major determinant of tumor characteristics and patient outcome. Am J Respir Crit Care Med. 2015;191:377–390. doi: 10.1164/rccm.201409-1671PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nelson B.H. New insights into tumor immunity revealed by the unique genetic and genomic aspects of ovarian cancer. Curr Opin Immunol. 2015;33:93–100. doi: 10.1016/j.coi.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 64.Ness N., Andersen S., Valkov A. Infiltration of CD8+ lymphocytes is an independent prognostic factor of biochemical failure-free survival in prostate cancer. Prostate. 2014;74:1452–1461. doi: 10.1002/pros.22862. [DOI] [PubMed] [Google Scholar]
- 65.Bracci L., Schiavoni G., Sistigu A., Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21:15–25. doi: 10.1038/cdd.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romero A.I., Chaput N., Poirier-Colame V. Regulation of CD4(+)NKG2D(+) Th1 cells in patients with metastatic melanoma treated with sorafenib: role of IL-15Ralpha and NKG2D triggering. Cancer Res. 2014;74:68–80. doi: 10.1158/0008-5472.CAN-13-1186. [DOI] [PubMed] [Google Scholar]
- 67.Raulet D.H., Guerra N. Oncogenic stress sensed by the immune system: role of natural killer cell receptors. Nat Rev Immunol. 2009;9:568–580. doi: 10.1038/nri2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spisek R., Charalambous A., Mazumder A., Vesole D.H., Jagannath S., Dhodapkar M.V. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood. 2007;109:4839–4845. doi: 10.1182/blood-2006-10-054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fucikova J., Kralikova P., Fialova A. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res. 2011;71:4821–4833. doi: 10.1158/0008-5472.CAN-11-0950. [DOI] [PubMed] [Google Scholar]
- 70.Bloy N., Pol J., Aranda F. Trial watch: dendritic cell-based anticancer therapy. OncoImmunology. 2014;3:e963424. doi: 10.4161/21624011.2014.963424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghiringhelli F., Menard C., Puig P.E. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Viaud S., Saccheri F., Mignot G. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Casares N., Pequignot M.O., Tesniere A. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mattarollo S.R., Loi S., Duret H., Ma Y., Zitvogel L., Smyth M.J. Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res. 2011;71:4809–4820. doi: 10.1158/0008-5472.CAN-11-0753. [DOI] [PubMed] [Google Scholar]
- 75.Shalapour S., Font-Burgada J., Di Caro G. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature. 2015;521:94–98. doi: 10.1038/nature14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martins I., Kepp O., Schlemmer F. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene. 2011;30:1147–1158. doi: 10.1038/onc.2010.500. [DOI] [PubMed] [Google Scholar]
- 77.Menger L., Vacchelli E., Adjemian S. Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci Transl Med. 2012;4:143ra199. doi: 10.1126/scitranslmed.3003807. [DOI] [PubMed] [Google Scholar]
- 78.Aranda F., Bloy N., Pesquet J. Immune-dependent antineoplastic effects of cisplatin plus pyridoxine in non-small-cell lung cancer. Oncogene. 2015;34:3053–3062. doi: 10.1038/onc.2014.234. [DOI] [PubMed] [Google Scholar]
- 79.Yamazaki T., Hannani D., Poirier-Colame V. Defective immunogenic cell death of HMGB1-deficient tumors: compensatory therapy with TLR4 agonists. Cell Death Differ. 2014;21:69–78. doi: 10.1038/cdd.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma Y., Adjemian S., Mattarollo S.R. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38:729–741. doi: 10.1016/j.immuni.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 81.Ma Y., Mattarollo S.R., Adjemian S. CCL2/CCR2-dependent recruitment of functional antigen-presenting cells into tumors upon chemotherapy. Cancer Res. 2014;74:436–445. doi: 10.1158/0008-5472.CAN-13-1265. [DOI] [PubMed] [Google Scholar]
- 82.Sistigu A., Yamazaki T., Vacchelli E. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20:1301–1309. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- 83.Kroemer G., Galluzzi L., Kepp O., Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 84.Di Caro G., Cortese N., Castino G.F. Dual prognostic significance of tumour-associated macrophages in human pancreatic adenocarcinoma treated or untreated with chemotherapy. Gut. 2016;65:1710–1720. doi: 10.1136/gutjnl-2015-309193. [DOI] [PubMed] [Google Scholar]
- 85.Nowak A.K., Lake R.A., Marzo A.L. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170:4905–4913. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 86.Suzuki E., Kapoor V., Jassar A.S., Kaiser L.R., Albelda S.M. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 87.Vincent J., Mignot G., Chalmin F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitum or immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 88.Emile J.F., Julie C., Le Malicot K. Prospective validation of a lymphocyte infiltration prognostic test in stage III colon cancer patients treated with adjuvant FOLFOX. Eur J Cancer. 2017;82:16–24. doi: 10.1016/j.ejca.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 89.Lim S.H., Chua W., Cheng C. Effect of neoadjuvant chemoradiation on tumor-infiltrating/associated lymphocytes in locally advanced rectal cancers. Anticancer Res. 2014;34:6505–6513. [PubMed] [Google Scholar]
- 90.Ruffell B., Au A., Rugo H.S., Esserman L.J., Hwang E.S., Coussens L.M. Leukocyte composition of human breast cancer. Proc Natl Acad Sci USA. 2012;109:2796–2801. doi: 10.1073/pnas.1104303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Denkert C., von Minckwitz G., Brase J.C. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33:983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 92.Dresp J., Schmid E., Bauchinger M. The cytogenetic effect of bleomycin on human peripheral lymphocytes in vitro and in vivo. Mutat Res. 1978;56:341–353. doi: 10.1016/0027-5107(78)90203-8. [DOI] [PubMed] [Google Scholar]
- 93.Williams S.D., Birch R., Einhorn L.H., Irwin L., Greco F.A., Loehrer P.J. Treatment of disseminated germ-cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N Engl J Med. 1987;316:1435–1440. doi: 10.1056/NEJM198706043162302. [DOI] [PubMed] [Google Scholar]
- 94.Bugaut H., Bruchard M., Berger H. Bleomycin exerts ambivalent antitumor immune effect by triggering both immunogenic cell death and proliferation of regulatory T cells. PLoS One. 2013;8:e65181. doi: 10.1371/journal.pone.0065181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4:349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- 96.Lesterhuis W.J., Salmons J., Nowak A.K. Synergistic effect of CTLA-4 blockade and cancer chemotherapy in the induction of anti-tumor immunity. PLoS One. 2013;8:e61895. doi: 10.1371/journal.pone.0061895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vacchelli E., Aranda F., Eggermont A. Trial Watch: tumor-targeting monoclonal antibodies in cancer therapy. OncoImmunology. 2014;3:e27048. doi: 10.4161/onci.27048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lynch T.J., Bondarenko I., Luft A. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 99.Reck M., Bondarenko I., Luft A. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24:75–83. doi: 10.1093/annonc/mds213. [DOI] [PubMed] [Google Scholar]
- 100.Yatim N., Jusforgues-Saklani H., Orozco S. RIPK1 and NF-kappaB signaling in dying cells determines cross-priming of CD8(+) T cells. Science. 2015;350:328–334. doi: 10.1126/science.aad0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen D., Yu J., Zhang L. Necroptosis: an alternative cell death program defending against cancer. Biochim Biophys Acta. 2016;1865:228–236. doi: 10.1016/j.bbcan.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Galluzzi L., Buque A., Kepp O., Zitvogel L., Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28:690–714. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]