Abstract
Traditional thiazolidinediones (TZDs), such as rosiglitazone, are peroxisome proliferator-activated receptor γ (PPARγ) potent agonists that can be used to treat type 2 diabetes but carry unwanted effects, including increased risk for fracture. The present work aimed to compare the insulin-sensitizing efficacies and bone-loss side effects of CMHX008, a novel TZDs-like PPARγ partial agonist, with those of rosiglitazone. A TR-FRET PPARγ competitive binding assay was used to compare the binding affinity between CMHX008 and rosiglitazone. Mice were administered vehicle, CMHX008 or rosiglitazone for 16 weeks. Mesenchymal stem cells (MSCs) were used to examine differences in differentiation into osteoblasts after compounds treatment. TR-FRET showed lower affinity to PPARγ by CMHX008 compared with rosiglitazone. Mice treated with CMHX008 showed insulin sensitization similar to that of mice treated with rosiglitazone, which was related to the significant inhibition of PPARγ Ser273 phosphorylation and improved insulin sensitivity by facilitating the phosphorylation of insulin receptor and Akt in adipose tissues. Micro-CT and histomorphometric analyses demonstrated that the degree of trabecular bone loss after treatment with CMHX008 was weaker than that observed with rosiglitazone, as evidenced by consistent changes in BV/TV, Tb.N, Tb.Th, Tb.Sp, and the mineral apposition rate. MSCs treated with CMHX008 showed higher ALP activity and mRNA levels of bone formation markers than did cells treated with rosiglitazone in the osteoblast differentiation test. Thus, CMHX008 showed insulin-sensitizing effects similar to those of rosiglitazone with a lower risk of bone loss, suggesting that PPARγ sparing eliminates the skeletal side effects of TZDs while maintaining their insulin-sensitizing properties.
Keywords: Osteoblasts, Peroxisome proliferator-activated receptor γ, Thiazolidinediones, TR-FRET, Type 2 diabetes mellitus
Introduction
Type 2 diabetes mellitus (T2DM) has become a global epidemic metabolic disease over the last several decades.1, 2 One important characteristic of T2DM is resistance to insulin or reduced insulin secretion. Thiazolidinedioes (TZDs) improve whole-body insulin sensitivity and reduce the blood glucose levels in patients with T2DM as an activator of peroxisome proliferator-activated receptor γ (PPARγ).3 However, there are great concerns about classical TZDs, such as rosiglitazone, due to their risk of fluid retention, weight gain, bone loss and congestive heart failure.4, 5 A recent safety evaluation demonstrated that rosiglitazone showed no increased risk of heart attack compared to the standard T2DM medicines metformin and sulfonylurea.6 Another critical review performed on TZDs in the treatment of T2DM considered that TZDs should appropriately continue use after evaluating the positive and adverse effects.7
Bone loss and skeletal fragility, especially in female patients, are common undesirable effects after the long-term usage of classical TZDs.8, 9, 10 Physiologically, bone is constantly remodeled with bone resorption by osteoclasts, followed by bone formation by osteoblasts, depending on the subtle coordination between these two types of cells. Osteoblasts originate from mesenchymal progenitors, while osteoclast cells arise from hematopoietic progenitors of the monocyte/macrophage lineage. The concerted coupling of bone resorption and formation are accurately controlled by a variety of neuronal and hormonal factors, including nuclear receptor signaling.11 PPARγ is a nuclear receptor that is highly expressed in white and brown adipose tissue (WAT and BAT), which plays an essential role in adipocyte differentiation, glucose homeostasis and inflammation,12, 13 and also regulates the bone remodeling by increasing the abundance of osteoblasts without affection neither the differentiation nor the function of osteoclasts.14 Although PPARγ activators have been demonstrated as effective in the treatment of T2DM, other evidence also showed that modulating or even inhibiting PPARγ activity might be the preferred therapeutic strategy to improve glucose homeostasis while preventing adipogenesis.15, 16, 17 The attainment of insulin sensitizing properties does not require maximal PPARγ activation, which is one of the reasons why full and weak PPARγ agonists may provide comparable insulin sensitization and rationale to design partial PPARγ agonists.18 High-fat-diet-fed obese mice exhibit activated cyclin-dependent protein kinase-5 (CDK5) within adipose tissues, and rosiglitazone blocks the CDK5-mediated phosphorylation of PPARγ at serine 273.19 Selective PPARγ modulators (SPPARMs), due to their PPARγ partial agonistic behavior, improve glucose homeostasis but present few of the aforementioned side effects.18, 20, 21, 22 These studies provide insight into the mechanism underlying PPARγ-mediated insulin sensitization and its associated side effects and provide a potential strategy to develop safer PPARγ-specific drugs that reduce the adverse effects associated with TZDs while preserving insulin-sensitizing potential.
We recently identified CMHX008, a TZDs analog, chemically known as 3-tert-butoxycarbonylmethyl-5-[4-[2-[N-methyl-N-(2-pyridyl)amino]-ethoxy]benzyl]thiazolidine-2,4-dione (Fig. 1A), as a PPARγ partial agonist with a hypoglycemic effect comparable to that of rosiglitazone but with lower adipogenic capacity.23 To further characterize the pharmacological features of CMHX008, we performed a time-resolved fluorescence resonance energy transfer (TR-FRET) assay to determine the competitive binding affinity of CMHX008 with PPARγ compared to that with rosiglitazone. The therapeutic and side effects, especially on bone mineral metabolism, were evaluated in mice after relatively long (16 weeks) treatment. Furthermore, differences in differentiation into osteoblasts from mesenchymal stem cells (MSCs) after treatment with CMHX008 and rosiglitazone were investigated.
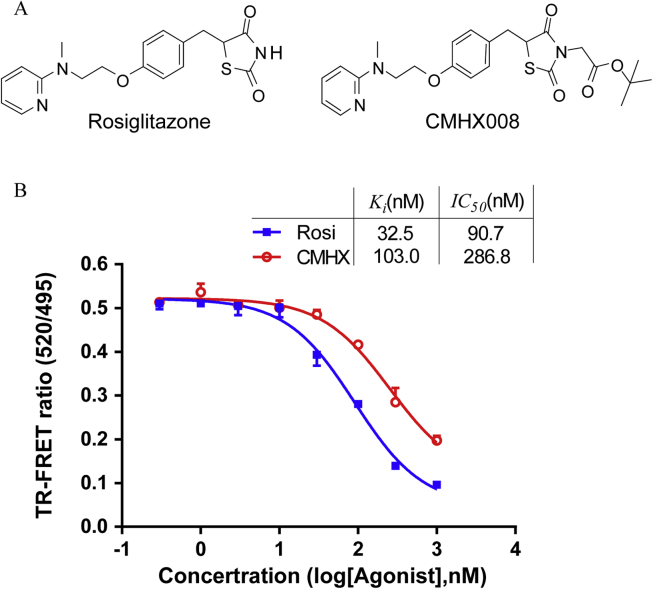
Figure 1.
The binding affinity of rosiglitazone (Rosi) and CMHX008 (CMHX) on PPARγ. (A) Chemical structure of Rosi and CMHX. (B) TR-FRET competitive binding assay was used to examine the binding affinity of the human PPARγ in response to rosiglitazone or CMHX008 at concentrations ranging from 0.3 to 1000 nM (n = 3).
Materials and methods
Animals and diets
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Chongqing Medical University. Male C57BL/6 mice (6 weeks old) were obtained from the Animal Laboratory of Chongqing Medical University. Leptin defect ob/ob mice were generated through breeding heterozygous ob ± littermates. All mice were housed under standard conditions with a 12 h/12 h light-dark cycle and constant temperature. C57BL/6 mice were fed either a low-fat diet (LFD, D12450B, 10% Kcal from fat, Research Diets, New Brunswick, USA) or high-fat diet (HFD, D12492, 60% Kcal from fat, Research Diets, New Brunswick, USA). After 12 weeks, HFD-fed mice with a body weight higher than the average plus 3 standard deviations of the LFD mice were considered diet-induced obese mice.6 HFD fed obese mice were randomly separated into seven groups: CMHX008 1 mg/kg (n = 7), 3 mg/kg (n = 7) or 10 mg/kg (n = 13), rosiglitazone (Taiji Group Ltd, Chongqing, China) 1 mg/kg (n = 7), 3 mg/kg (n = 7) or 10 mg/kg (n = 13), and vehicle (n = 13). The chemicals and vehicle were administered once daily by intragastric gavage for an additional 16 weeks. LFD and ob/ob mice were treated with 10 mg/kg/day of CMHX008 or rosiglitazone (LFD, n = 8/group; ob/ob, n = 4–6/group). At the end of the study, all mice were euthanatized by CO2 inhalation and sacrificed in a non-fasting state. Trunk blood, epididymal white adipose tissues (WAT), tibia, femur, and other tissues were collected.
PPARγ competitive ligand binding analysis
Serial concentrations of compounds were incubated with GST-fused human PPARγ-LBD, terbium-labeled anti-GST antibody, and PPAR ligand within TR-FRET PPARγ Competitive Binding Assay Kit (A15144, Invitrogen, Carlsbad, USA) in the dark at room temperature. The FRET signal was measured by excitation at 340 nm and emission at 520 nm for fluorescein and 495 nm for terbium. The signal may decrease when the test compounds binding to PPARγ-LBD competed with and replaced Pan-PPAR Green. The binding to the PPARγ-LBD was analyzed by the 520 nm/495 nm ratio.
Intraperitoneal glucose tolerance test (iGTT)
Intraperitoneal glucose tolerance test (iGTT) was performed 10 weeks after compounds or vehicle treatment. Mice were fasted for 12 h with free access to water, 2 g/kg of glucose was intraperitoneally injected and blood glucose was measured at 0, 15, 30, 60, 120 min after injection.24
Indirect calorimetry analysis
Mice were individually housed in metabolic chambers (TSE PhenoMaster/LabMaster Systems, Berlin, Germany) to acclimate for 4 d before indirect calorimetry studies. The system was used to measure the oxygen consumption (VO2), carbon dioxide production (VCO2), ambulatory activity, and food intake of the individual mice, as previously described.25 Data were collected and averaged from 3-day tests.
RNA isolation and quantitative real-time RT-PCR analysis
Total RNAs were isolated and purified using the Total RNA Extraction Kit (RP1202, Bioteke, Beijing, China). One milligram of total RNA was reverse transcribed on Thermal Cycler (Veriti, Applied Biosystems, Singapore). Real-time PCR was performed using SYBR and gene-specific primers and quantitated on the RT-qPCR detection system (CFX96, BIO-RAD, Singapore). All reactions were performed in triplicate, and relative mRNA levels were calculated by the comparative threshold cycle method by using GAPDH as an internal control. The primer sequences are provided as follows: Gapdh (forward – GACATCAAGAAGGTGGTGAAGC; reverse – GAAGGTGGAAGAGTGGGAGTT); osteocalcin (Ocn) (forward – CAAGCAGGGTTAAGCTCACA; reverse – GGTAGTGAACAGACTCCGGC); Runx2 (forward – GAAGCUUGAUGACUCUAAATTA; reverse - UUUAGAGUCAUCAAGCUUCTTC).
Western blotting analysis
WAT or cells were homogenized in buffer with protease and phosphatase inhibitors. Total protein was extracted with TRlzol Reagent (Sigma). Protein content was determined with a protein assay kit, and samples were then aliquoted and stored at −80 °C. The protein was separated by SDS-PAGE and transferred to nitrocellulose membranes, followed by blocking for 2 h and incubation for 12 h at 4 °C with primary antibodies. Anti-Insulin Receptor β (#3025), anti-Phospho-insulin receptor β (#3024), Phospho-Akt (Ser473) (#4060), and anti-Akt (#4691) antibodies were obtained from Cell Signaling Technology (Danvers, Massachusetts, USA); anti-phospho-PPARγ (ser273) (bs-4888R) was purchased from Bioss Inc. (Woburn, MA, USA); and anti-PPAR gamma (ab59256), anti-Osteocalcin (ab93876) and anti-Runx2 (ab23981) were obtained from Abcam (Cambridge, UK). The membrane was then incubated with the secondary antibodies for 1 h at room temperature. The protein bands were visualized with an enhanced chemiluminiscence (ECL) detection system. The band intensity was quantified with Fusion FX (Vliber Lourmat, Australia) software and all quantitative analyses were normalized to β-actin or GAPDH.
Micro-computed tomography (μCT) analysis
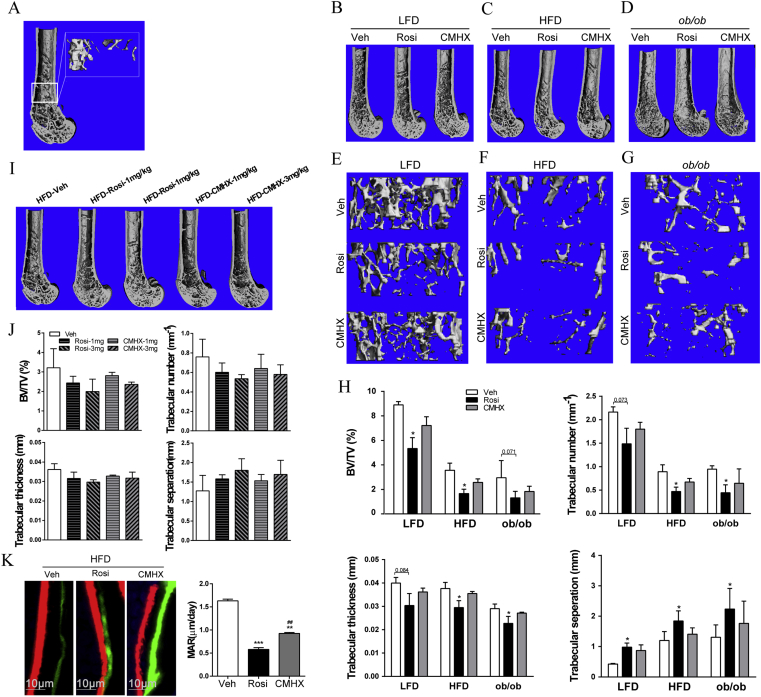
The right femur was measured by using a μCT scanner (VivaCT 40, Scanco Medical AG, Switzerland) after the removal of soft tissues and overnight fixation in 4% paraformaldehyde. The bone trabecular morphometry of distal metaphysis was performed at 100 kV and 98 μA between frames. The two-dimensional images were used to generate three-dimensional reconstructions to obtain quantitative data with the 3D Creator software supplied with the instrument. One millimeter of trabecular bone (96 slices) was analyzed (Fig. 4A), beginning with an image at 1 mm from the epiphyseal line.
Figure 4.
Effect of rosiglitazone (Rosi) or CMHX008 (CMHX) on bone formation. Mice were treated with vehicle (Veh), 1 mg/kg/day, 3 mg/kg/day or 10 mg/kg/day of rosiglitazone (Rosi) or CMHX008 (CMHX) for 16 weeks. (A) Three-dimensional μCT images of the longitudinal section of the distal femur selected based on median value of bone volume/tissue volume (BV/TV), and the rectangle shows the location of trabecular bone. Representative μCT images of longitudinal section of distal femur and trabecular bone from LFD (B and E), HFD (C and F) and ob/ob (D and G) mice treated with 10 mg/kg/day, respectively. The quantified μCT images of BV/TV, trabecular number, trabecular thickness, and trabecular separation of LFD, HFD and ob/ob mice treated at 10 mg/kg/day (H) and HFD mice treated at 3 mg/kg/day (J). (I) Representative μCT images of longitudinal section of distal femur of HFD mice treated at 1 and 3 mg/kg/day. (K) Representative mineral apposition rate (MAR) of HFD mice treated at 10 mg/kg/day. All data are expressed as the means ± SEM. N = 6 per group in HFD mice and 4–5 per group in LFD and ob/ob mice. *P < 0.05, **P < 0.01, ***P < 0.001 versus Veh, ##P < 0.01 versus Rosi.
Histomorphometrical analysis of bone
Calcein (C0875, Sigma, USA) and alizarin red (A5533, Sigma, USA) were used in double labeling test to evaluate the bone mineral apposition rate (MAR). MAR, calculated as the interlabel width/labeling period, is indicative of the amount of newly formed mineralized osteoid per day. Briefly, calcein (20 mg/kg) and alizarin red (30 mg/kg) were subcutaneously injected in HFD mice treatment at a dose of 10 mg/kg/day at 14 and 4 days prior to sacrifice. After removing the soft tissues, the right femurs were fixed in 75% ethanol and embedded in methylmethacrylate. Longitudinal sections of 80 μm thickness were cut by using a polycut-E microtome (LEICA1600, Germany) and ground to a final thickness of ∼30 μm, followed by imaging using a fluorescence microscope (FV1000, OLYMPUS, Japan).
Induction of osteoblastic differentiation
MSCs (C3H/10T1/2, ATCC, Manassas, VA, USA) were cultured in DMEM/F12 medium (HyClone, Utah, USA) containing 10% FBS (PAN, Adenbach, German). After growing to ∼80% confluency, the cells were switched into osteoblast differentiation medium (DMEM/F12 plus 10 mM β-glycerophosphate, 50 μg/ml ascorbic acid, 0.1 μM dexamethasone).26 The cells were treated with vehicle, rosiglitazone (3 μM), or CMHX008 (3 μM) at the start of differentiation. After differentiation for 7 days, the cell lysates were collected for western blotting. On day 14, alkaline phosphatase (ALP) staining was performed in mature osteoblasts and transcriptional levels of OCN and Runx2 were examined. The formation of mineralized matrix nodules was determined by alizarin red staining at day 21.
Staining and quantification for alkaline phosphatase and Alizarin red
Differentiated cells were rinsed and fixed. For ALP staining, fixed cells were stained with BCIP/NBT ALP staining solution (C3206, Beyotime, Beijing, China), and observed under Nikon microscope. For ALP quantification, differentiated cell lysates were homogenized on ice, and the supernatants were collected and mixed with ALP assay (P0321, Beyotime, Beijing, China), and ALP activity was determined by measuring absorbance at 405 nm. For Alizarin red staining (ARS), the fixed cells were stained with alizarin red (G8550, Solarbio, Beijing, China). The calcium deposition bounded with alizarin red was observed and photographed with a microscope. ARS quantification was performed by solubilizing the dye in cetylpyridinium chloride and the solution was detected at the absorbance of 570 nm.
Statistical analysis
IC50 value was analyzed through nonlin fit of GraphPad Prism. Other data were presented as means ± standard error of mean. The statistical significance of animal types and drug treatment effects was assessed by applying a one-way analysis of variance (ANOVA) in the SPSS 21 statistical package (SPSS Inc., USA). In all experiments, a value of P < 0.05 was considered significant.
Results
CMHX008 was identified as a selective PPARγ partial agonist
Our previous luciferase reporter assay and molecular docking studies indicated that CMHX008 is a SPPARM.23 To further confirm the efficiency of CMHX008 bound to PPARγ, we performed a competitive TR-FRET ligand-binding assay. Fig. 1B shows that CMHX008 directly bound to purified human PPARγ-LBD with a half-inhibitory concentration (IC50) of 286.8 nM (95% confidence intervals, 221.6–371.1 nM) and inhibition constant (Ki) of 103.0 nM. The IC50 and Ki values for rosiglitazone were 90.7 nM (95% confidence intervals, 70.96–115.9 nM) and 32.5 nM, respectively, suggesting that CMHX008 was weaker in binding to PPARγ compared with rosiglitazone.
CMHX008 treatment showed insulin sensitization comparable to that of rosiglitazone
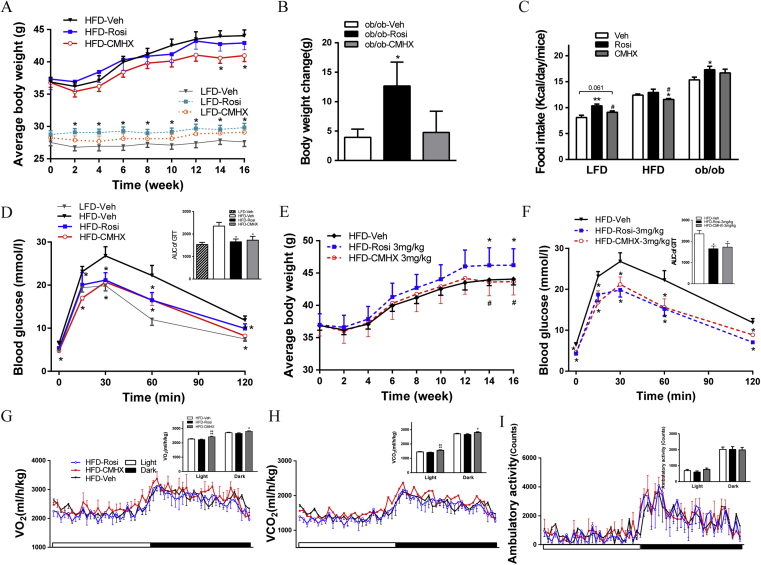
The body weight of HFD mice treated with 10 mg/kg/day of CMHX008 (HFD-CMHX) was significantly reduced, while HFD mice treated with the same dose of rosiglitazone (HFD-Rosi) showed no significant difference compared with that of HFD mice treated with vehicle (HFD-Veh) (Fig. 2A). The body weight of HFD-Rosi mice treated at a dose of 3 mg/kg/day (HFD-Rosi-3 mg) (Fig. 2E) was significantly increased compared with HFD-Veh and HFD-CMHX-3 mg mice, but no difference was observed at a dose of 1 mg/kg/day (data not shown). LFD mice treated with rosiglitazone (LFD-Rosi) showed increased body weights (Fig. 2A). The body weight change in ob/ob mice showed a significant increase after treatment with rosiglitazone (ob/ob-Rosi) compared with that after treatment with vehicle (ob/ob-Veh) (Fig. 2B). Accordingly, the food intake of LFD-Rosi or ob/ob-Rosi mice showed a significant increase or trend to increase compared with their vehicle-treated counterparts (Fig. 2C). HFD-CMHX mice exhibited a significant reduction in food intake compared with HFD-Veh and HFD-Rosi mice at a dose of 10 mg/kg/day (Fig. 2C). Low dosage treatment showed no difference in food intake (data not shown). After intraperitoneal loading glucose, both HFD-CMHX and HFD-Rosi mice showed a similar decrease in blood glucose levels at all tested time points compared with that of HFD-Veh (Fig. 2D). The same trend was found after treatment with a low dosage of 3 mg/kg/day (Fig. 2F). HFD-CMHX mice showed higher VO2 and VCO2 during both light and dark phases of a 24-h monitoring period compared with HFD-Veh and HFD-Rosi mice at a dose of 10 mg/kg/day, but there was no difference in ambulatory activity (Fig. 2G–I).
Figure 2.
Effects of rosiglitazone (Rosi) or CMHX008 (CMHX) on body weight (BW), food intake (FI), glucose homeostasis, and metabolic rate. (A) BW of HFD and LFD mice treated with 10 mg/kg/day of Rosi or CMHX for 16 weeks (n = 10–13/group). (B) BW changes in ob/ob mice treated with 10 mg/kg/day of Rosi or CMHX for 16 weeks (n = 4–6/group). (C) Daily FI of mice treated with 10 mg/kg/day measured two weeks before sacrifice (n = 4–6/group). (D) Glucose tolerance test (GTT) of HFD mice treated with 10 mg/kg/day performed at 10 weeks after treatment with the chemicals and area under the curve (AUC) of GTT (n = 5–10/group). (E) BW of HFD mice treated with 3 mg/kg/day of Rosi or CMHX for 16 weeks (n = 5/group). (F) GTT of HFD mice treated with 3 mg/kg/day performed at 10 weeks after treatment with the chemicals and AUC of GTT (n = 7/group). (G–I) Oxygen consumption (VO2), carbon dioxide production (VCO2) and ambulatory activity were measured for HFD mice treated with 10 mg/kg/day. *P < 0.05, **P < 0.01, versus Veh, #P < 0.05, ##P < 0.01 versus Rosi.
CMHX008 improves insulin sensitivity and inhibits PPARγ phosphorylation in HFD mice
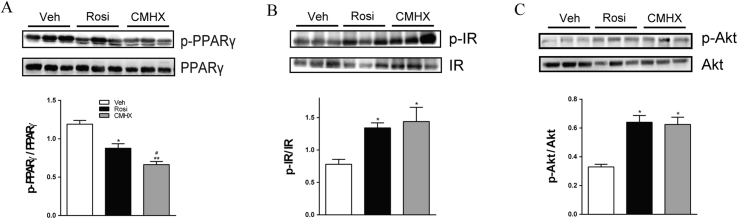
In WAT of HFD mice, the protein expression of PPARγ phosphorylation (p-PPARγ), total PPARγ, insulin receptor β phosphorylation (p-IR), total IR, Akt phosphorylation (p-Akt) and total Akt were measured by western blotting. The phosphorylation of PPARγ at serine 273 stimulates the expression of adipose tissues genes related to insulin resistance and diabetes.19, 27 As shown in Fig. 3A, at a dose of 10 mg/kg/day, both HFD-Rosi and HFD-CMHX mice displayed significantly inhibited PPARγ phosphorylation, and HFD-CMHX mice showed stronger inhibition than that of HFD-Rosi mice. HFD mice treated with rosiglitazone or CMHX008 showed increased protein levels of p-IR (Fig. 3B) and p-Akt (Fig. 3C).
Figure 3.
Effects of rosiglitazone (Rosi) or CMHX008 (CMHX) treatment on phosphorylation of PPARγ (p-PPARγ), insulin receptor (p-IR) and Akt (p-Akt). Representative Western blots of protein phosphorylation (A, B, C) in white adipose tissue. Bar graphs show the p-PPARγ, p-IR and p-Akt levels normalized to total appropriate protein levels.*P < 0.05, **P < 0.01 versus Veh, #P < 0.05 versus Rosi (n = 7/group).
CMHX008 treatment results in the minor bone loss
Micro-CT image of an undecalcified distal femur showed evidence of the overall loss of trabecular bone in HFD and ob/ob mice compared with that of LFD mice without any drug treatment. Treatment with either rosiglitazone or CMHX008 at the dose of 10 mg/kg/day in each type of mice displayed further loss of trabecular bone compared with vehicle treatment (Fig. 4B–D). The images of trabecular bone fragments further confirmed that rosiglitazone or CMHX008 treatment resulted in bone loss compared with vehicle treatment in LFD, HFD and ob/ob mice and, more importantly, that mice treated with rosiglitazone had a more severe decline than did mice treated with CMHX008 (Fig. 4E–G). Quantitative μCT data of rosiglitazone-treated HFD mice indicated a significant decrease in the bone volume over tissue volume ratio (BV/TV), trabecular number (Tb.N) and trabecular thickness (Tb.Th) in the region of interest, which was consistent with the increase in trabecular separation (Tb.Sp), while CMHX008 treatment did not alter the above skeletal markers (Fig. 4H). A similar change was also observed in LFD and ob/ob mice, although some trabecular bone parameters of LFD and ob/ob mice induced by rosiglitazone did not reach statistical significance (Fig. 4H). Furthermore, HFD mice treated with a low dosage of the two compounds showed a similar trend but with no difference in bone trabecular morphometry (Fig. 4I and J). In addition, dynamic histomorphometry indicated that both HFD-Rosi and HFD-CMHX mice treated with 10 mg/kg/day showed significantly decreased MAR in trabecular bone compared with that of HFD-Veh and that a lower MAR was observed in HFD-Rosi mice than in HFD-CMHX mice (Fig. 4K).
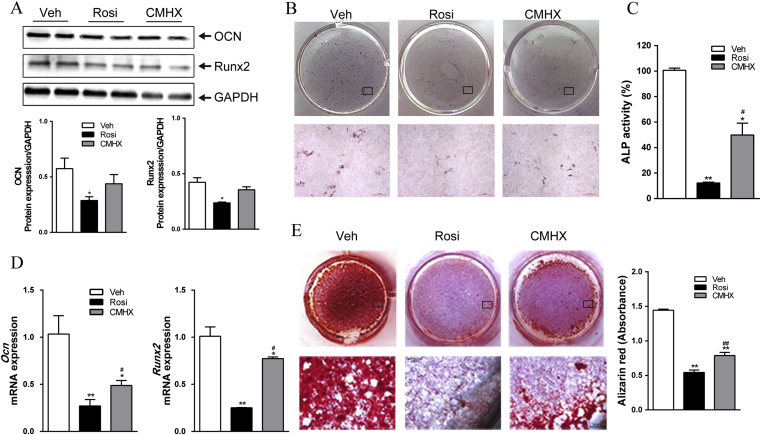
Inhibition of osteoblast differentiation by CMHX008 was weaker than rosiglitazone
At day 7 of osteoblast differentiation, rosiglitazone treatment significantly downregulated the protein levels of OCN, and Runx2 protein showed decreased trend. However, CMHX008 treatment did not alter OCN and Runx2 protein levels (Fig. 5A). On day 14, the cells were stained for ALP (Fig. 5B), and the ALP activities (Fig. 5C) and mRNA levels of Ocn and Runx2 (Fig. 5D) were measured. Both rosiglitazone and CMHX008 treated cells displayed a reduced number of osteoblast compared with vehicle treatment. However, rosiglitazone treatment showed a more significant reduction in the number of osteoblast cells compared with that of CMHX008 treatment. These changes were correlated with decreased ALP activities (Fig. 5C) and reduced mRNA levels of expression of Ocn and Runx2 (Fig. 5D), suggesting that the capacity of bone formation was more robustly inhibited by rosiglitazone. Furthermore, on day 21, ARS, an indicator of calcium deposition, was performed to quantify osteoblast differentiation. Both rosiglitazone and CMHX008-treated cells produced significantly fewer mineralized matrix compared with vehicle-treated cells (Fig. 5E). CMHX008 treatment displayed a more mineralized matrix than rosiglitazone treatment (Fig. 5E).
Figure 5.
Effect of rosiglitazone (Rosi) or CMHX008 (CMHX) on the differentiation of mesenchymal stem cells (MSCs) into osteoblasts. (A) Protein levels of the osteogenic marker gene OCN and Runx2 in MSCs after treatment with vehicle (Veh), rosiglitazone (Rosi) or CMHX008 (CMHX) for 7 days. (B) Representative ALP staining of osteoblasts after treatment for 14 days. Scale bar = 50 μm. (C) Quantitative ALP concentration after treatment for 14 days. (D) Expression of osteogenic marker genes by RT-PCR after treatment for 14 days. (E) Alizarin red staining of mineralized nodules after treatment for 21 days. *P < 0.05, **P < 0.01 versus Veh, #P < 0.05, ##P < 0.01 versus Rosi.
Discussion
Classical TZDs carry great concerns due to their increased risk of fluid retention, weight gain and bone loss, even though they are efficacious in the treatment of T2DM.6 PPARγ partial agonists maintain the antidiabetic properties of TZDs but present minimal negative effects.20, 21 The present study aimed to compare the insulin sensitizing effects and bone loss side effects of CMHX008 with those of the classical PPARγ agonist, rosiglitazone. Recent studies provided direct evidence that CMHX008 binds to the LBD of PPARγ with lower affinity than rosiglitazone via TR-FRET ligand-binding assay. Compared with a previous short-term (5 weeks) administration protocol,23 the presented sub-chronic treatment (16 weeks) of CMHX008 displayed similar antidiabetic properties, as did rosiglitazone treatment based on the improved effects on metabolic and glucose homeostasis. Notably, CMHX008 caused a lower degree of weight gain and bone loss compared with that of rosiglitazone. Mechanically, inhibition of phosphorylation of PPARγ at serine 273 and the regulation of osteoblast differentiation from mesenchymal stem cells, might account for the advantageous pharmacological profiles of CMHX008.
TR-FRET ligand-binding assay is a sensitive and reliable method widely used for the determination of the PPARγ binding affinity.28, 29, 30 The current results showed that CMHX008 directly bound to PPARγ-LBD with IC50 and Ki values almost 3-fold higher than those of rosiglitazone did, indicating that CMHX008 exerted a lower binding affinity to PPARγ. The binding affinity of CMHX008 was the same as those of the previously reported partial agonists GQ-1631 and amorfrutins17 but was weaker than that of honokiol29 according to Ki values. Cell-based reporter assays showed that CMHX008 was a selective activator of PPARγ without the activation of either PPARα or PPARδ.23 Accordingly, recent data confirmed that CMHX008 was a selective PPARγ ligand with weaker agonistic activity. The pharmacological profile may contribute to the recruitment of coactivators and may activate the transcriptional activity of PPARγ in a weaker way than typical full agonist rosiglitazone does, which has been observed for other partial agonists.30
Several natural or synthetic PPARγ partial agonists retained similar antidiabetic effects as rosiglitazone but showed minimized side effects, such as weight gain.22, 30, 32, 33, 34 Consistent with these findings, CMHX008 showed a similar efficacy as rosiglitazone in ameliorating glucose homeostasis in HFD or ob/ob mice in the absence of weight gain. We also observed increased oxygen consumption and carbon dioxide production as measured by indirect calorimetry, suggesting that CMHX008, similar to other PPARγ partial agonists, such as GQ-16, shared similar improving effects on the metabolic rate with rosiglitazone.35 The comparable hypoglycemic effect and weaker agonistic behavior of the receptor between CMHX008 and rosiglitazone suggested a potential PPARγ-independent mechanism. As the inhibition of PPARγ phosphorylation at serine 273 by rosiglitazone is an important pharmacological feature19 that contributes to its anti-diabetic effects, we examined the changes in PPARγ phosphorylation after CMHX008 treatment. Similar to the other natural or synthetic PPARγ partial agonists, such as amorfrutins,17 chelerythrine,22 F12016,30 and dihydromyricetin,36 CMHX008 induced the obvious inhibition of PPARγ phosphorylation, suggesting that this compound exerts antidiabetic effects via the inhibition of PPARγ phosphorylation, without activating the full range of PPARγ targets, and thereby avoiding known side effects, such as weight gain.
Another negative effect of the long-term administration of rosiglitazone is the impairment of osteoblastogenesis, which leads to osteoporosis and an increased risk of bone fractures.9, 10, 37 The fact that ligands with lower agonistic effects on PPARγ or even PPARγ suppressors exhibit beneficial influences on skeleton homeostasis30, 38, 39, 40, 41 prompted us to determine the effect of CMHX008 on bone density and bone turnover parameters. The micro-CT-determined skeletal mass and parameters of trabecular bone architecture displayed a decreased number of trabecular bone in HFD and ob/ob mice, suggesting that obesity is one of the risk factors in inducing osteoporosis42 and rosiglitazone or CMHX008 administration displayed a further loss of trabecular bone. However, mice treated with rosiglitazone had more severe decline in bone density than did those treated with CMHX008, suggesting that CMHX008 will cause less osteoporosis compared with the PPARγ agonist rosiglitazone. The activation of PPARγ inhibited osteoblast differentiation and promoted osteoclast differentiation, resulting in bone loss via the concerted inhibition of bone formation and stimulation of bone resorption.43 In addition, osteoblasts and adipocytes share a common progenitor, MSCs, which are associated with a reciprocal decrease of osteogenesis and an increase of adipogenesis. Under conditions of osteoblast differentiation, MSCs treated with rosiglitazone exhibited more significant inhibition of calcium deposition and osteogenesis compared with CMHX008 treatment, which was consistent with the changes in osteogenic markers OCN, and Runx2 at mRNA and protein levels. Accordingly, dynamic histomorphometry showed that HFD-fed mice treated with rosiglitazone showed a significantly lower bone MAR and lower bone mass in trabecular bone than did CMHX008 treatment, suggesting that the capacity of bone formation was less considerably inhibited by CMHX008. The lower inhibition of osteoblastogenesis by CMHX008 observed in the current studies was also consistent with that of other PPARγ partial agonists.30, 38, 41
In conclusion, our present study provides new evidence of ligand-dependent regulation on adipogenesis and osteoblastogenesis through PPARγ. CMHX008 displays a lower PPARγ activity, attenuates the metabolic defects in obese mice, does not carry side effects of weight gain, and shows minor osteoporotic complications compared with rosiglitazone. These pharmacological profiles are correlated with the ability of this compound to block the phosphorylation of PPARγ at serine 273 and reduce the agonistic properties of PPARγ, which subsequently favors osteoblastogenesis over adipogenesis. Our study also suggests that these activities can be pharmacologically separated and individually harnessed by specifically designed selective PPARγ agonists to retain insulin sensitization without the classical lipogenic and osteoporotic side effects.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (81270947 and 81570763, to XX), the National Program on Key Basic Research Project of China (973 Program, 2012CB517505, to XX), the Fundamental Science and Advanced Technology Research of Chongqing (Major Project, CSTC2015jcyjB0146), and Chongqing Graduate Student Research Innovation Fund (CYB15095, to HY). We are grateful to Prof. Xiaochao Yang from School of Biomedical Engineering, the Third Military Medical University, for the critical comments on the manuscript.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Jaacks L.M., Siegel K.R., Gujral U.P., Narayan K.M. Type 2 diabetes: a 21st century epidemic. Best Pract Res Clin Endocrinol Metab. 2016;30(3):331–343. doi: 10.1016/j.beem.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Zimmet P., Alberti K.G., Magliano D.J., Bennett P.H. Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat Rev Endocrinol. 2016;12(10):616–622. doi: 10.1038/nrendo.2016.105. [DOI] [PubMed] [Google Scholar]
- 3.Spiegelman B.M. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47(4):507–514. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- 4.Kong A.P., Yamasaki A., Ozaki R. A randomized-controlled trial to investigate the effects of rivoglitazone, a novel PPAR gamma agonist on glucose-lipid control in type 2 diabetes. Diabetes Obes Metab. 2011;13(9):806–813. doi: 10.1111/j.1463-1326.2011.01411.x. [DOI] [PubMed] [Google Scholar]
- 5.Nissen S.E., Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 6.Hiatt W.R., Kaul S., Smith R.J. The cardiovascular safety of diabetes drugs — insights from the rosiglitazone experience. N Engl J Med. 2013;369(14):1285–1287. doi: 10.1056/NEJMp1309610. [DOI] [PubMed] [Google Scholar]
- 7.Consoli A., Formoso G. Do thiazolidinediones still have a role in treatment of type 2 diabetes mellitus? Diabetes Obes Metab. 2013;15(11):967–977. doi: 10.1111/dom.12101. [DOI] [PubMed] [Google Scholar]
- 8.Lecka-Czernik B. Diabetes, bone and glucose-lowering agents: basic biology. Diabetologia. 2017;60(7):1163–1169. doi: 10.1007/s00125-017-4269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Billington E.O., Grey A., Bolland M.J. The effect of thiazolidinediones on bone mineral density and bone turnover: systematic review and meta-analysis. Diabetologia. 2015;58(10):2238–2246. doi: 10.1007/s00125-015-3660-2. [DOI] [PubMed] [Google Scholar]
- 10.Napoli N., Chandran M., Pierroz D.D., Abrahamsen B., Schwartz A.V., Ferrari S.L. Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017;13(4):208–219. doi: 10.1038/nrendo.2016.153. [DOI] [PubMed] [Google Scholar]
- 11.Watkins M.P., Norris J.Y., Grimston S.K. Bisphosphonates improve trabecular bone mass and normalize cortical thickness in ovariectomized, osteoblast Connexin43 deficient mice. Bone. 2012;51(4):787–794. doi: 10.1016/j.bone.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scotti E., Tontonoz P. Peroxisome proliferator-activated receptor gamma dances with different partners in macrophage and adipocytes. Mol Cell Biol. 2010;30(9):2076–2077. doi: 10.1128/MCB.00171-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tontonoz P., Spiegelman B.M. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 14.Cao J., Ou G., Yang N. Impact of targeted PPARgamma disruption on bone remodeling. Mol Cell Endocrinol. 2015;410:27–34. doi: 10.1016/j.mce.2015.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cock T.A., Houten S.M., Auwerx J. Peroxisome proliferator-activated receptor-gamma: too much of a good thing causes harm. EMBO Rep. 2004;5(2):142–147. doi: 10.1038/sj.embor.7400082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi S.S., Kim E.S., Jung J.E. PPARgamma antagonist gleevec improves insulin sensitivity and promotes the browning of white adipose tissue. Diabetes. 2016;65(4):829–839. doi: 10.2337/db15-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weidner C., de Groot J.C., Prasad A. Amorfrutins are potent antidiabetic dietary natural products. Proc Natl Acad Sci USA. 2012;109(19):7257–7262. doi: 10.1073/pnas.1116971109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins L.S., Depaoli A.M. Selective peroxisome proliferator-activated receptor gamma (PPARgamma) modulation as a strategy for safer therapeutic PPARgamma activation. Am J Clin Nutr. 2010;91(1):267S–272S. doi: 10.3945/ajcn.2009.28449E. [DOI] [PubMed] [Google Scholar]
- 19.Choi J.H., Banks A.S., Estall J.L. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466(7305):451–456. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DePaoli A.M., Higgins L.S., Henry R.R., Mantzoros C., Dunn F.L. Can a selective PPARgamma modulator improve glycemic control in patients with type 2 diabetes with fewer side effects compared with pioglitazone? Diabetes Care. 2014;37(7):1918–1923. doi: 10.2337/dc13-2480. [DOI] [PubMed] [Google Scholar]
- 21.Dunn F.L., Higgins L.S., Fredrickson J., DePaoli A.M. Selective modulation of PPARgamma activity can lower plasma glucose without typical thiazolidinedione side-effects in patients with Type 2 diabetes. J Diabet Complicat. 2011;25(3):151–158. doi: 10.1016/j.jdiacomp.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Zheng W., Qiu L., Wang R. Selective targeting of PPARgamma by the natural product chelerythrine with a unique binding mode and improved antidiabetic potency. Sci Rep. 2015;5:12222. doi: 10.1038/srep12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ming Y., Hu X., Song Y. CMHX008, a novel peroxisome proliferator-activated receptor gamma partial agonist, enhances insulin sensitivity in vitro and in vivo. PLoS One. 2014;9(7):e102102. doi: 10.1371/journal.pone.0102102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song Y., Li J., Zhao Y. Severe maternal hyperglycemia exacerbates the development of insulin resistance and fatty liver in the offspring on high fat diet. Exp Diabetes Res. 2012;2012:254976. doi: 10.1155/2012/254976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z., Lim C.Y., Su M.Y. Neonatal overnutrition in mice exacerbates high-fat diet-induced metabolic perturbations. J Endocrinol. 2013;219(2):131–143. doi: 10.1530/JOE-13-0111. [DOI] [PubMed] [Google Scholar]
- 26.Yuasa M., Yamada T., Taniyama T. Dexamethasone enhances osteogenic differentiation of bone marrow- and muscle-derived stromal cells and augments ectopic bone formation induced by bone morphogenetic protein-2. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0116462. e0116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banks A.S., McAllister F.E., Camporez J.P. An ERK/Cdk5 axis controls the diabetogenic actions of PPARgamma. Nature. 2015;517(7534):391–395. doi: 10.1038/nature13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stechschulte L.A., Czernik P.J., Rotter Z.C. PPARG post-translational modifications regulate bone formation and bone resorption. EBioMedicine. 2016;10:174–184. doi: 10.1016/j.ebiom.2016.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atanasov A.G., Wang J.N., Gu S.P. Honokiol: a non-adipogenic PPARγ agonist from nature. Biochim Biophys Acta Gen Subj. 2013;1830(10):4813–4819. doi: 10.1016/j.bbagen.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu C., Feng T., Zhu N. Identification of a novel selective agonist of PPARgamma with no promotion of adipogenesis and less inhibition of osteoblastogenesis. Sci Rep. 2015;5:9530. doi: 10.1038/srep09530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amato A.A., Rajagopalan S., Lin J.Z. GQ-16, a novel peroxisome proliferator-activated receptor gamma (PPARgamma) ligand, promotes insulin sensitization without weight gain. J Biol Chem. 2012;287(33):28169–28179. doi: 10.1074/jbc.M111.332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z., Vigueira P.A., Chambers K.T. Insulin resistance and metabolic derangements in obese mice are ameliorated by a novel peroxisome proliferator-activated receptor gamma-sparing thiazolidinedione. J Biol Chem. 2012;287(28):23537–23548. doi: 10.1074/jbc.M112.363960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X., Liu H., Sun B. Novel podophyllotoxin derivatives as partial PPARgamma agonists and their effects on insulin resistance and type 2 diabetes. Sci Rep. 2016;6:37323. doi: 10.1038/srep37323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atanasov A.G., Blunder M., Fakhrudin N. Polyacetylenes from notopterygium incisum–new selective partial agonists of peroxisome proliferator-activated receptor-gamma. PLoS One. 2013;8(4):e61755. doi: 10.1371/journal.pone.0061755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coelho M.S., de Lima C.L., Royer C. GQ-16, a TZD-derived partial PPARgamma agonist, induces the expression of thermogenesis-related genes in Brown fat and visceral white fat and decreases visceral adiposity in obese and hyperglycemic mice. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0154310. e0154310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L., Wan J., Lang H. Dihydromyricetin delays the onset of hyperglycemia and ameliorates insulin resistance without excessive weight gain in Zucker diabetic fatty rats. Mol Cell Endocrinol. 2017;439:105–115. doi: 10.1016/j.mce.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz A.V., Chen H., Ambrosius W.T. Effects of TZD use and discontinuation on fracture rates in ACCORD bone study. J Clin Endocrinol Metab. 2015;100(11):4059–4066. doi: 10.1210/jc.2015-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kolli V., Stechschulte L.A., Dowling A.R., Rahman S., Czernik P.J., Lecka-Czernik B. Partial agonist, telmisartan, maintains PPARgamma serine 112 phosphorylation, and does not affect osteoblast differentiation and bone mass. PLoS One. 2014;9(5):e96323. doi: 10.1371/journal.pone.0096323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marciano D.P., Kuruvilla D.S., Boregowda S.V. Pharmacological repression of PPARγ promotes osteogenesis. Nat Commun. 2015;6:7443. doi: 10.1038/ncomms8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva J.C., Cesar F.A., de Oliveira E.M. New PPARgamma partial agonist improves obesity-induced metabolic alterations and atherosclerosis in LDLr(-/-) mice. Pharmacol Res. 2016;104:49–60. doi: 10.1016/j.phrs.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 41.Fukunaga T., Zou W., Rohatgi N., Colca J.R., Teitelbaum S.L. An insulin-sensitizing thiazolidinedione, which minimally activates PPARγ, does not cause bone loss. J Bone Miner Res. 2015;30(3):481–488. doi: 10.1002/jbmr.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neglia C., Argentiero A., Chitano G. Diabetes and obesity as independent risk factors for osteoporosis: updated results from the ROIS/EMEROS registry in a population of five thousand post-menopausal women living in a region chnaracterized by heavy environmental pressure. Int J Environ Res Publ Health. 2016;13(11) doi: 10.3390/ijerph13111067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wan Y. PPARγ in bone homeostasis. Trends Endocrinol Metab. 2010;21(12):722–728. doi: 10.1016/j.tem.2010.08.006. [DOI] [PubMed] [Google Scholar]