Abstract
Idiopathic pulmonary fibrosis (IPF) is a lethal idiopathic interstitial pulmonary disease characterized by progressive deterioration in lung function that commonly affects eldly people. The pathogenesis of the disease is incompletely understood and therefore lacking effective therapy. Ouabain a digitalis has been reported to be able to suppress lung fibroblast activation via downregulating TGF-β-smad signal pathway in vitro. Here, we investigated the effects of ouabain in pulmonary fibrosis in vivo. Pulmonary fibrosis was induced in C57/BL6 mice by a intratracheal instillation of bleomycin (2.0 mg/kg), ouabain (0.6 mg/kg) was given daily via intraperitonealinjection for one week starting at 7 days after intratracheal instillation of bleomycin. Our study showed ouabain significantly reduce α-SMA, fibronectin and collagen I expression in lung fibrosis animal model. Further, ouabain inhibits cells proliferation and promotes apoptosis of lung fibroblasts in vitro. In conclusion, our results indicate ouabain a novel effective drug that inhibits lung fibrosis progression.
Keywords: Ouabain, bleomycin, pulmonary fibrosis
Introduction
Idiopathic interstitial pulmonary fibrosis (IPF) is a chronic, progressive fibrotic interstitial pneumonia occurring in adults and is characterized by the patterns of usual interstitial pneumonia in radiologic and/or histopathologic manifestation. The pathogenesis of IPF, is incompletely understood, and the effective drugs on IPF is lack. So, it is urgent to find new more effective therapeutic strategies for IPF [1,2]. Accumulation of activated fibroblasts/myofibroblasts and deposition of massive extracellular matrix are crucial processes for fibrotic lung remodeling in IPF. TGF-β, one of the major profibrotic cytokines in IPF, plays a pivotal role in activation of fibroblasts [2,3]. The levels of TGF-β were increased in the lungs of IPF patients and in bleomycin-induced pulmonary fibrosis animal model [2]. TGF-β signal pathway regulates epithelial cells injury and fibroblast proliferation and transdifferentiation into myofibroblasts. Thus the suppression of TGF-β signal pathway alleviates pulmonary fibrosis [4-6].
Ouabain, a digitalis mainly for heart failure, is an inhibitor of Na, K-ATPase. In addition, Ouabain, has multiply biological functions such as regulating cell proliferation and apoptosis, modulating inflammation, inducing autophagy, etc. [7-9]. Recently, some researches reveal that ouabain inhibits lung fibroblasts activation and epithelial mesenchymal plasticity in vitro by blocking TGF-β-smad signal pathway including downregulation smad2, TGFβRII transcription and smad2 phosphorylation [10,11]. However, the effects of ouabain on the progress of pulmonary fibrosis remains unknown, so we want to know if ouabain could be a new drug for IPF via bleomycin induced pulmonary fibrosis mice model.
In this study, we investigated the effects of ouabain on cell proliferation, apotosis in lung fibroblasts and evaluated whether ouabain has the anti-profibrotic effects in bleomycin-induced pulmonary fibrosis mouse model.
Materials and methods
Mouse model
C57BL/6N mice (male, 7~8 weeks old, weight 22~24 g) were purchased from Vital River Laboratory Animal Technology Co. Beijing, China. Animals were maintained at a controlled temperature of 24±1°C with a 12:12 h light-dark cycle, and were fed a standard diet. Water was freely available. A dose of 2 mg/Kg bleomycin (Nippon Kayaku Co., Tokyo, Japan) was intratracheally administered. Intraperitoneal dose (0.6 mg/kg) were given from day 7 to day 13. Body weight of the mice were assessed at daily following after ouabain or PBS treatment. Mice were euthanasia on day 14. The lungs were removed for hematoxylin & eosin (HE), Masson trichrome stain, immunohistochemistry stain and gene expression analysis.
Cell culture
MRC-5 human fetal lung fibroblasts were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) ,The cells were maintained in Minimum Essential Medium (MEM)-α (Invitrogen) with 10% FBS (Invitrogen), 100 U/ml penicillin (Hyclone), and 100 mg/ml streptomycin (Hyclone) in a humidified incubator (Thermo Scientific, Waltham, MA, USA) at 37°C in 95% air (21% O2) and 5% CO2.
Western blotting
Western blotting was performed as previously described [12,13]. The primary antibodies used were anti-mouse α-SMA (ab124964, Abcam), fibronectin (ab2413, Abcam), and β-actin (ab6267, Abcam). The denatured proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (Bio-Rad, Hercules, CA, USA) using a Mini-Protean electrophoresis module assembly (Bio-Rad) at 80 mV and transferred to nitrocellulose membranes (Millipore, Billerica, MA, USA) for 100~120 min using the Mini Trans-Blot electrophoresis transfer cell (Bio-Rad) at 300 mA accoding to the molecular weight. After incutating with the primary antibodies, the membranes were treated with IRDyeTM800 (green)- or IRDyeTM700 (red)-conjugated affinity purified anti-rabbit or anti-mouse IgG (LI-COR, Lincoln, NE, USA). Positive bands were visualized, and the intensity of the bands was evaluated using a LI-COR Odyssey infrareddouble-fluorescence imaging system (LI-COR).
Gene expression analysis
Total RNA of lung tissue was homogenized in liquid nitrogen and isolated using the TRIzol reagent (Invitrogen). Reverse transcription was performed on 1 μg total RNA with oligo (dT) primers in 25 μl reactions using the Omniscript RT kit (Tiangen Biotech, Beijing, China) following the manuals. The primers were purchased from Sangon Biotech, Shanghai, China, β-actin 5’-AGGCCAACCGTGAAAAGATG-3’ and 5’-AGAGCATAGCCCTCGTAGATGG-3’; Fn1 5’-GTGTAGCACAACTTCCAATTACGAA-3’ and 5’-GGAATTTCCGCCTCGAGTCT-3’; Col1a1 5’-CCAAGAAGACATCCCTGAAGTCA-3’ and 5’-TGCACGTCATCGCACACA-3’; α-SMA 5’-GCTGGTGATGATGCTCCCA-3’ and 5’-GCCCATTCCAACCATTACTCC-3’. Real-time RT-PCR was performed on an ABI PRISM® 7500 instrument (Applied Biosystems, Foster, CA, USA) as previously described [14].
Lung histology
The preparation of mouse lungs for histology was performed as previously described [15]. Briefly, the lung was dehydrated, paraffin-em-bedded, and cut into 4-μm sections. Lung sections were stained with H & E and Masson’s trichrome stain for assessment of pathological changes. For immunohistochemistry, the tissue sections were deparaffinized and rehydrated as described above. After a microwave treatment for 20 min in ethylenediaminetetraacetic acid (EDTA) buffer and subsequent cooling, the endogenous peroxidase activity was blocked by 0.3% hydrogen peroxide in methanol for 15 min in dark. After blocking in 5% goat serum for 20 min, sections were incubated with antibodies against α-SMA (ab124964, Abcam), fibronectin (ab2413, Abcam), collagen I overnight at 4°C as described previously [16].
Hydroxyproline assay
Total lung collagen was determined by analysis of hydroxyproline on day 14 after bleomycin instillation as previously described [19]. Lung homogenates in 1.5 ml HCl (2 mol/l) were incubated at 110°C overnight. Chloramine T solution (56 mmol/l chloramine T and 10% 1-propanol in citrate/acetate buffer) were added at room temperature for 20 min. The mixture was then incubated with Ehrlich’s solution at 65°C for 20 min. After cooling to room temperature, the absorbance was measured at 550 nm by a spectrophotometer and L-hydroxyproline content was determined using a standard curve as previously describled [17].
Cell proliferation assay
For the CCK-8 assay, cells were seeded in 96-well plates (2 × 103 cells/well), and 1% FBS starvation for 24 h. After treated with PBS or ouabain, cells were incubated at 37°C in CO2 incubator; Cell proliferation indices were measured at 24, 48 and 72 h using a CCK-8 kit (Dojindo Co., Ltd. Japan). The absorbance was measured at 450 nm by a spectrophotometer as previously described [18].
Cell apoptosis assay
Cell apoptosis assay was performed as previously described [19]. To evaluate the effect of ouabain on MRC-5 cells, annexin-V and propidium iodide (PI) (Apoptosis Detection Kit, annexin-V-FITC, BD, Biosciences Pharmingen, San Diego, CA) were used to distinguish apoptosis (annexin-V positive, PI negative) from necrosis (annexin-V positive, PI positive) cells. 2 × 105 cells under different treatment were resuspended in 100 μl binding buffer, and then incubated with 2 μl Annexin-V-FITC (20 μg/ml) for 15 minutes in dark at room tempreture. Each sample was then incubated with 1 μl PI (50 μg/ml) for 2 minutes, and were immediately analyzed by an Accuri C6 flow cytometer (BD Accuri Cytometers, MI). A minimum of 10,000 events was acquired for each sample.
Statistics analysis
Data are expressed as mean ± standard error of the mean (SEM). Two-tailed Student’s t-test was performed using two-way analysis of variance. Comparison of the difference among multiple groups were annalyzed two-way analysis of variance (ANOVA) with Sidak multiple comparisons test. Kaplan-Meier survival analysis with log-rank test was used to compare survival rates. Statistical analysis was performed using the Prism software (Graphpad Software, CA, USA). For all analyses, P values < 0.05 were considered as significant.
Results
Ouabain treatment rescued bleomycin-induced weight loss
As shown in Figure 1A, the body weight of the mice decreased significantly 5 days after the bleomycin treatment. In addition, compared with the bleomycin group, ouabain (0.5 mg/kg) administration reduced body weight loss from day 7 to day 14. (n = 10, P < 0.05, Figure 1B). As expected, ouabain alone did not change mouse body weight.
Figure 1.
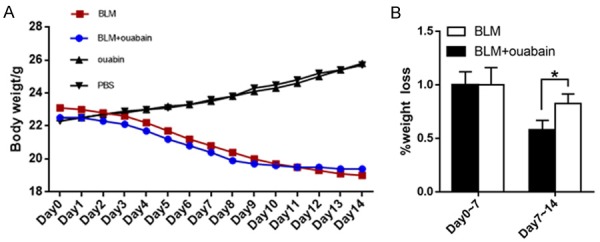
Ouabain treatment rescued BLM-induced weight loss. A. The average body weight of BLM group, BLM+ouabain group and ouabainn group from day 0 to day 14. B. The body weight loss has little difference between BLM group and BLM+ouabain group from day 0 to day 7. After ouabain treatment, body weight loss of ouabain group was significantly attenuated. Results are expressed as mean ± SEM, n = 10 mice per group, *, P < 0.05.
Ouabain alleviates pathological changes induced by bleomycin
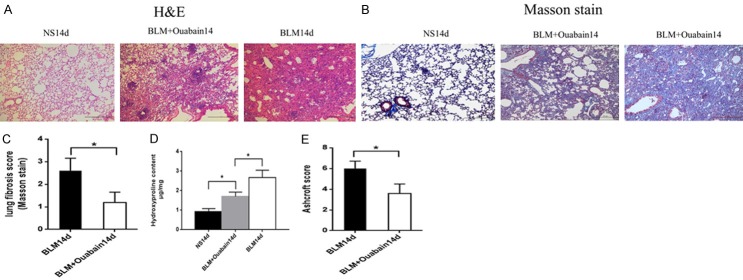
Bleomycin-induced pulmonary damage and fibrosis in mice was determined by histological analysis. The lungs of bleomycin treated mice have thickening of alveolar septa, collaps of alveolar spaces, loss of alveolar structure, over-proliferation of fibroblasts and infiltration of inflammatory cell infiltration by H & E staining (Figure 2A). Masson’s staining and hydroxyproline assay showed that bleomycin induced extensive collagen deposition in lung (Figure 2B). Ouabain treatment markedly ameliorated these injuries and evidently decreased collagen deposition (Figure 2C, 2D). More important, our data showed that ouabain treatment ameliorated bleomycin-induced pulmonary fibrosis as assessed by Ashcroft scoring (P < 0.05 Figure 2E).
Figure 2.
Ouabain alleviates pathological changes. Representative lung histology of (A) HE staining, original magnifications 100 ×, Scale bar = 20 μm. (B) Masson’s trichrome original magnifications 100 ×, Scale bar = 20 μm. (C) Fibrosis scoring of the Masson trichrome staining on day 14, BLM group (black bar), BLM+Ouabain group (white bar) ,results are expressed as mean ± SEM, n = 4-6 mice per group, *P < 0.05. (D) The hydroxyproline content in lung tissue was significantly higher in the mice treated with BLM (no ouabian) than those treated with BLM+ouabain. results are expressed as mean ± SEM, n = 4-6 mice per group, *P < 0.05. (E) Assessment of the severity of pulmonary fibrosis on Ashcroft scale on day 14, BLM group (black bar), BLM+ouabian group (white bar), results are expressed as mean ± SEM, n = 4-6 mice per group, *P < 0.0.
Ouabain treatment suppresses α-SMA, fibronectin, collagen expression in bleomycin-induced lung fibrosis
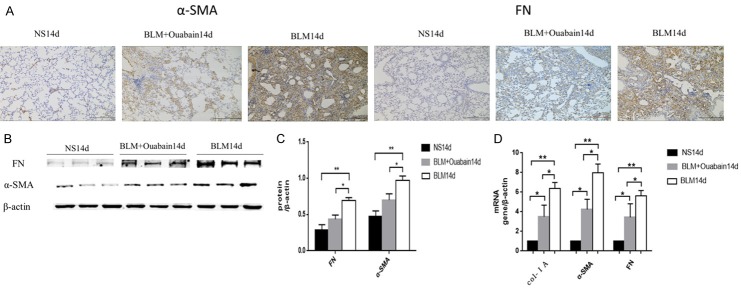
The expression of collagen I, α-SMA and fibronectin in the lung tissue from mice was assayed by immunohistochemistry, Western blotting and qPCR. The results demonstrated that the expression of collagen I, α-SMA, fibronectin were markedly increased following exposure to bleomycin (Figure 3). Treatment with ouabain significantly prevented bleomycin-induced changes the protein level of α-SMA, and fibronectin (Figure 3A-C). Moreover, ouabain treatment significantly decreased the mRNA level of collagen I a, α-SMA, and fibronectin in the lung tissues (Figure 3D).
Figure 3.
Ouabain suppresses profibrotic proteins expression. A. Representative lung histology immunohistochemistry of α-SMA and Fibronectin original magnifications 100 ×, Scale bar = 20 μm. B. Whole lung tissues western blotting of α-SMA and Fibronectin. C. Normal saline group (black bar), BLM group (gray bar), BLM+Ouabain group (white bar), results are expressed as mean ± SEM, n = 4-6 mice per group, *P < 0.05 **P < 0.01. D. Relative mRNA expression of collagen 1A, α-SMA and Fibronectin from lung tissues Normal saline group (black bar), BLM group (gray bar), BLM+Ouabain group (white bar), r esults are expressed as mean ± SEM, n = 4-6 mice per group, *P < 0.05 **P < 0.01.
Ouabain inhibits the proliferation of lung fibroblasts
The CCK-8 assay was used to assess the effects of ouabain on the proliferation of MRC-5 cells. 100 nM Ouabain treatment markedly reduced OD values compared with control group at 24 h, 48 h, 72 h (Figure 4A), These data indicated that ouabian inhibits lung fibroblasts proliferation.
Figure 4.
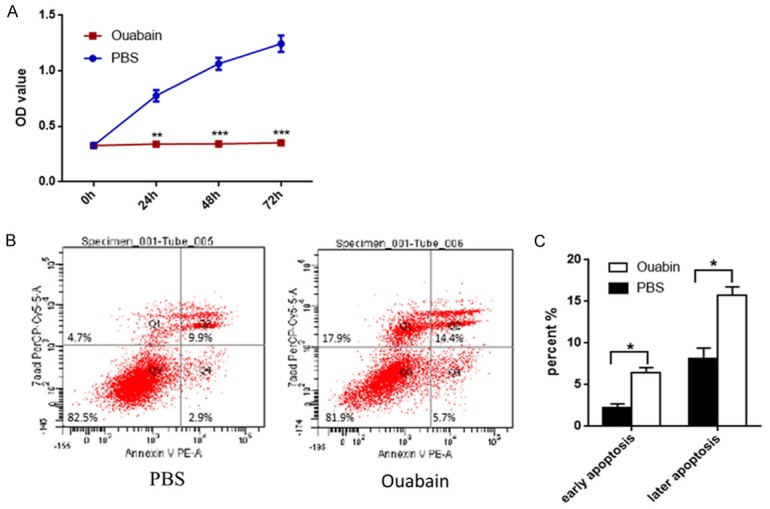
Ouabain inhibits proliferation and promotes apoptosis of lung fibroblasts. A. CCK-8 assay of MRC-5 cells treatment with ouabain or PBS, results are expressed as mean ± SEM, *P < 0.05 **P < 0.01. B. MCR-5 cells were treated with ouabain or PBS for 24 h in the indicated concentrations, and the apoptosis was detected by FCM Annexin V/7AAD staining. The proportions of Annexin V+/7AAD- and Annexin V+/7AAD+ cells indicated the early and later apoptosis, respectively. C. Ouabain group (black bar), PBS group (white bar), results are expressed as mean ± SEM, *P < 0.05.
Ouabain promotes apoptosis of lung fibroblasts
Cell apoptosis was detected by Annexin V and 7AAD staining. MRC-5 cells were exposed to 100 nM ouabain for 48 h. As shown in Figure 4B and 4C, ouabain markedly increased early apoptotic and late apoptotic cell populations as well as necrosis cell population compared to control group. These results suggest that ouabain can significantly increase lung fibroblasts apoptosis.
Discussion
IPF is a fatal interstitial lung disease characterized by alveolar epithelial cells apoptosis, fibroblasts proliferation, myofibroblasts differentiation and excessive ECM deposition. Since the pathogenesis mechanisms remain largely unknown, few drugs are effective for IPF. In this study, we found that ouabain, a digitalis, protects against bleomycin induced mouse pulmonary fibrosis by inhibiting proliferation and promoting apoptosis of lung fibroblasts. Thus, ouabian might be is a promising drug for pulmonary fibrosis.
TGF-β signaling plays a central role in pulmonary fibrosis [20,21]. Previous researches indicated that ouabain inhibits lung fibroblast activation and alveolar cell epithelium mesenchymal transition by suppressing smad2 and TGF-βRII expression and smad2 phosphorylation [10,11] in vitro. To examine the anti-fibrotic effect of ouabain in vivo, we used bleomycin-induced pulmonary fibrosis-mouse model. We found that and the expression of collagenI, α-SMA, fibronectin in the lungs of bleomycin-treated mice. ouabain ameliorates bleomycin induced pulmonary fibrosis.
The activating behaviors of lung fibroblasts from IPF patients including over-proliferation and resistance to apoptosis is involved in the development of IPF [22-25]. One of the therapeutic strategy for pulmonary fibrosis is to inhibit the proliferation and to promote apoptosis of lung fibroblasts. The effect of Ouabain on cell proliferation dependent on drug concentration, However, many studies report ouabain induces cell apoptosis [9,26-29]. We tested the effect of ouabain at 100 nM on lung fibroblasts proliferation by CCK-8 assay and apoptosis by flow cytometry. The results show ouabain significantly inhibits growth and promote apoptosis of MRC-5 lung fibroblasts. Several researches indicated the relationship between ouabain and autophagy [9,30], enhancing autophagy may be play an important role in anti-fibrosis.
As we all know, cardiac glycosides is used for heart failure with narrow therapic window, since the serious side effects including arrhythmia. According to introduction of ouabain, the LD50 is 1.213 mg/Kg in mouse by intraperitoneal injection. We used dose gradients and found that higher than 0.6 mg/kg dose intraperitoneal injection to mice caused a sudden death easily, moreover, lower than 0.6 mg/kg dose couldn’t get satisfied effect. So, we chose 0.6 mg/kg ouabain treatment for mice by intraperitoneal injection. Since the narrow therapic window, the dose of ouabain for cure IPF patients needs further explorations [31].
In summary, our data showed that ouabain ameliorates bleomycin-induced pulmonary fibrosis in mouse model by inhibiting proliferation and promoting apoptosis of lung fibroblasts. Therefore, ouabain might be a promising novel treatment for pulmonary fibrosis. Further investigation is needed to evaluate the anti-fibrotic mechanisms of ouabain in animal model and its clinical applications.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China [Nos. 81430001 and 81470258]. We thank Prof. Wen Ning, State Key Laboratory of Medicinal Chemical Biology, College of Life Sciences, Nankai University, Prof. Ying Li, Department of Medical Research, Beijing Chao-Yang Hospital, Capital Medical University and Prof. Yunchao Su, Department of Pharmacology & Toxicology, Charlie Norwood Veterans Affairs Medical Center for their excellent technical assistance.
Disclosure of conflict of interest
None.
References
- 1.Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, Brozek JL, Collard HR, Cunningham W, Homma S, Johkoh T, Martinez FJ, Myers J, Protzko SL, Richeldi L, Rind D, Selman M, Theodore A, Wells AU, Hoogsteden H, Schünemann HJ American Thoracic Society; European Respiratory Society; Japanese Respiratory Society; Latin American Thoracic Association. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3–19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 2.Luppi F, Spagnolo P, Cerri S, Richeldi L. The big clinical trials in idiopathic pulmonary fibrosis. Curr Opin Pulm Med. 2012;18:428–432. doi: 10.1097/MCP.0b013e3283567ff9. [DOI] [PubMed] [Google Scholar]
- 3.Araya J, Nishimura SL. Fibrogenic reactions in lung disease. Annu Rev Pathol. 2010;5:77–98. doi: 10.1146/annurev.pathol.4.110807.092217. [DOI] [PubMed] [Google Scholar]
- 4.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 5.Selman M, Pardo A, Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS Med. 2008;5:e62. doi: 10.1371/journal.pmed.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodwin A, Jenkins G. Role of integrin-mediated TGFbeta activation in the pathogenesis of pulmonary fibrosis. Biochem Soc Trans. 2009;37:849–854. doi: 10.1042/BST0370849. [DOI] [PubMed] [Google Scholar]
- 7.Liu L, Zhao X, Pierre SV, Askari A. Association of PI3K-Akt signaling pathway with digitalis-induced hypertrophy of cardiac myocytes. Am J Physiol Cell Physiol. 2007;293:C1489–1497. doi: 10.1152/ajpcell.00158.2007. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi M, Usui-Kawanishi F, Karasawa T, Kimura H, Watanabe S, Mise N, Kayama F, Kasahara T, Hasebe N, Takahashi M. The cardiac glycoside ouabain activates NLRP3 inflammasomes and promotes cardiac inflammation and dysfunction. PLoS One. 2017;12:e0176676. doi: 10.1371/journal.pone.0176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng L, Wen Y, Zhou M, Li J, Wang T, Xu P, Ouyang J. Ouabain induces apoptosis and autophagy in Burkitt’s lymphoma Raji cells. Biomed Pharmacother. 2016;84:1841–1848. doi: 10.1016/j.biopha.2016.10.114. [DOI] [PubMed] [Google Scholar]
- 10.La J, Reed EB, Koltsova S, Akimova O, Hamanaka RB, Mutlu GM, Orlov SN, Dulin NO. Regulation of myofibroblast differentiation by cardiac glycosides. Am J Physiol Lung Cell Mol Physiol. 2016;310:L815–823. doi: 10.1152/ajplung.00322.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.La J, Reed E, Chan L, Smolyaninova LV, Akomova OA, Mutlu GM, Orlov SN, Dulin NO. Downregulation of TGF-beta receptor-2 expression and signaling through inhibition of Na/KATPase. PLoS One. 2016;11:e0168363. doi: 10.1371/journal.pone.0168363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geng J, Huang X, Li Y, Xu X, Li S, Jiang D, Liang J, Jiang D, Wang C, Dai H. Down-regulation of USP13 mediates phenotype transformation of fibroblasts in idiopathic pulmonary fibrosis. Respir Res. 2015;16:124. doi: 10.1186/s12931-015-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu X, Wan X, Geng J, Li F, Yang T, Dai H. Rapamycin regulates connective tissue growth factor expression of lung epithelial cells via phosphoinositide 3-kinase. Exp Biol Med (Maywood) 2013;238:1082–1094. doi: 10.1177/1535370213498976. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Geng J, Xu X, Huang X, Leng D, Jiang D, Liang J, Wang C, Jiang D, Dai H. miR-130b-3p modulates epithelial-mesenchymal crosstalk in lung fibrosis by targeting IGF-1. PLoS One. 2016;11:e0150418. doi: 10.1371/journal.pone.0150418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong Y, Geng Y, Li L, Li X, Yan X, Fang Y, Li X, Dong S, Liu X, Li X, Yang X, Zheng X, Xie T, Liang J, Dai H, Liu X, Yin Z, Noble PW, Jiang D, Ning W. Blocking follistatin-like 1 attenuates bleomycin-induced pulmonary fibrosis in mice. J Exp Med. 2015;212:235–252. doi: 10.1084/jem.20121878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yogo Y, Fujishima S, Inoue T, Saito F, Shiomi T, Yamaguchi K, Ishizaka A. Macrophage derived chemokine (CCL22), thymus and activation-regulated chemokine (CCL17), and CCR4 in idiopathic pulmonary fibrosis. Respir Res. 2009;10:80. doi: 10.1186/1465-9921-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li XX, Jiang DY, Huang XX, Guo SL, Yuan W, Dai HP. Toll-like receptor 4 promotes fibrosis in bleomycin-induced lung injury in mice. Genet Mol Res. 2015;14:17391–17398. doi: 10.4238/2015.December.21.8. [DOI] [PubMed] [Google Scholar]
- 18.Chen Z, Zhu R, Zheng J, Chen C, Huang C, Ma J, Xu C, Zhai W, Zheng J. Cryptotanshinone inhibits proliferation yet induces apoptosis by suppressing STAT3 signals in renal cell carcinoma. Oncotarget. 2017;8:50023–50033. doi: 10.18632/oncotarget.18483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng QQ, Wang K, Cheng KJ, Yang HG, Qiu JG, Zhang WJ, Jiang QW, Yang Y, Zheng DW, Huang JR, Wei MN, Shi Z, Wang W. Caragaphenol a induces reactive oxygen species related apoptosis in human gastric cancer cells. Am J Transl Res. 2017;9:3804–3815. [PMC free article] [PubMed] [Google Scholar]
- 20.Palumbo-Zerr K, Zerr P, Distler A, Fliehr J, Mancuso R, Huang J, Mielenz D, Tomcik M, Furnrohr BG, Scholtysek C, Dees C, Beyer C, Kronke G, Metzger D, Distler O, Schett G, Distler JH. Orphan nuclear receptor NR4A1 regulates transforming growth factor-beta signaling and fibrosis. Nat Med. 2015;21:150–158. doi: 10.1038/nm.3777. [DOI] [PubMed] [Google Scholar]
- 21.Wei Y, Kim TJ, Peng DH, Duan D, Gibbons DL, Yamauchi M, Jackson JR, Le Saux CJ, Calhoun C, Peters J, Derynck R, Backes BJ, Chapman HA. Fibroblast-specific inhibition of TGF-beta1 signaling attenuates lung and tumor fibrosis. J Clin Invest. 2017;127:3675–3688. doi: 10.1172/JCI94624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramos C, Montano M, Garcia-Alvarez J, Ruiz V, Uhal BD, Selman M, Pardo A. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol. 2001;24:591–598. doi: 10.1165/ajrcmb.24.5.4333. [DOI] [PubMed] [Google Scholar]
- 23.Nho RS, Hergert P, Kahm J, Jessurun J, Henke C. Pathological alteration of FoxO3a activity promotes idiopathic pulmonary fibrosis fibroblast proliferation on type i collagen matrix. Am J Pathol. 2011;179:2420–2430. doi: 10.1016/j.ajpath.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang W, Wei K, Jacobs SS, Upadhyay D, Weill D, Rosen GD. SPARC suppresses apoptosis of idiopathic pulmonary fibrosis fibroblasts through constitutive activation of beta-catenin. J Biol Chem. 2010;285:8196–8206. doi: 10.1074/jbc.M109.025684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sisson TH, Maher TM, Ajayi IO, King JE, Higgins PD, Booth AJ, Sagana RL, Huang SK, White ES, Moore BB, Horowitz JC. Increased survivin expression contributes to apoptosis-resistance in IPF fibroblasts. Adv Biosci Biotechnol. 2012;3:657–664. doi: 10.4236/abb.2012.326085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren YP, Zhang MJ, Zhang T, Huang RW. Dual effects of ouabain on the regulation of proliferation and apoptosis in human umbilical vein endothelial cells: involvement of Na(+)-K(+)-ATPase alpha-subunits and NF-kappaB. Int J Clin Exp Med. 2014;7:1214–1222. [PMC free article] [PubMed] [Google Scholar]
- 27.Penniiainen VA, Kipenko AV, Lopatina EV, Krylov BV. [The influence of ouabagenin on the growth and proliferation of cells in the organotypical culture] . Ross Fiziol Zh Im I M Sechenova. 2014;100:1303–1309. [PubMed] [Google Scholar]
- 28.Venugopal J, Blanco G. Ouabain enhances ADPKD cell apoptosis via the intrinsic pathway. Front Physiol. 2016;7:107. doi: 10.3389/fphys.2016.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan X, Liang F, Li D, Zheng J. Ouabain elicits human glioblastoma cells apoptosis by generating reactive oxygen species in ERK-p66SHCdependent pathway. Mol Cell Biochem. 2015;398:95–104. doi: 10.1007/s11010-014-2208-y. [DOI] [PubMed] [Google Scholar]
- 30.Trenti A, Grumati P, Cusinato F, Orso G, Bonaldo P, Trevisi L. Cardiac glycoside ouabain induces autophagic cell death in non-small cell lung cancer cells via a JNK-dependent decrease of Bcl-2. Biochem Pharmacol. 2014;89:197–209. doi: 10.1016/j.bcp.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 31.Leite JA, Alves AK, Galvao JG, Teixeira MP, Cavalcante-Silva LH, Scavone C, Morrot A, Rumjanek VM, Rodrigues-Mascarenhas S. Ouabain modulates zymosan-induced peritonitis in mice. Mediators Inflamm. 2015;2015:265798. doi: 10.1155/2015/265798. [DOI] [PMC free article] [PubMed] [Google Scholar]