Abstract
This phase I clinical trial tested the hypothesis that circulatory CD34+ cell therapy might be safe for old ischemic stroke (IS) (defined as IS>6 months) patients and also to evaluate the neurological function after the therapy. Nine old IS patients (with mean IS interval: 8.6 ± 6.4 years) were consecutively enrolled and received intra-carotid artery transfusion of circulatory-derived autologous CD34+ cells (3.0×107 cells/patient) into the ipsilateral brain infarct area at catheterization room by Catheter Looping Technique, after subcutaneous G-CSF injection (5 μg/kg twice a day for 4 days). The results showed that procedural safety was 100% with all patients uneventfully discharged. The circulating number of EPCs and angiogenesis (i.e., by Matrigel assay) were significantly higher at post than at prior to G-CSF treatment (all P<0.001). Time courses (0/5/10/30 minutes) of blood samplings from right-internal jugular vein exhibited significantly increased in levels of SDF-1α and EPCs numbers in time points of 5/10/30 minutes than in the baseline (0 minute) (all P<0.05). Barthel index was increased (defined as ≥5 scores) in 44.4% (4/9) and CASI score was notably improved (all P<0.01) at 6-month follow-up after the cell therapy as compared to the baseline. No recurrent IS or any tumorigenesis was found in these patients with a mean follow-up time interval of 16.5 ± 6.2 months. All of these patients remain survive and are followed up at outpatient department. In conclusion, CD34+ cell therapy is safe and might offer some benefit to old IS patients.
Keywords: Old ischemic stroke, CD34+ cell therapy, angiogenesis, neurological function, neuro-psychological assessment [clinical trial No.: ISRCTN14654908]
Introduction
Stroke, a growing epidemic, remains the second leading cause of death and the third cause of disability and death worldwide [1-4]. Although divergent etiologies have been clearly identified to cause stroke, the atherosclerotic intracranial arterial stenosis not only has been recognized as one of the common causes of stroke worldwide [5-8], but is also associated with a high risk of recurrent stroke even undergoing well medical treatment [9]. Intriguingly, despite of the epidemiology, etiologies, underlying mechanisms, classification, and clinical outcomes of ischemic stroke (IS) have been keenly surveyed for several decades [10-14], a safe and efficacious treatment for patients after IS has not been developed for universal application.
Growing data has shown that thrombolysis with tissue plasminogen activator (tPA) and endovascular intracranial treatment are two emerging modalities for acute IS with bright results in specific patient subgroups. However, stringent enrollment criteria and innumerable contraindications restrain their scope in daily clinical practice [15-21]. Besides, tPA therapy has been revealed to be associated with a relatively high incidence of intracranial bleeding complications [15,17-21] and poor patency rate in large-vessel occlusion, and yet catheter-based intracranial treatment is restricted to those of acute IS patients with large-intracranial vessel occlusion [16]. Accordingly, the majority of acute IS patients still lack an effective and safe treatment, suggesting that rehabilitation remains the only method for patients after IS. An alternative treatment needs to be developed for patients after IS, particularly for those who are not candidates for thrombolysis or endovascular intracranial treatment.
It is well recognized that coronary artery disease (CAD) and cerebrovascular disease (CVD), which comprise the majority of the same causal etiologies resulting in endothelial damage and arteriosclerosis, constitute arterial obstructive syndromes. Thus, CAD and CVD are two sides of the same coin. Our previous studies [22,23] have demonstrated that acute IS enhanced endothelial progenitor cells (EPC) mobilization into the circulation. Additionally, an increase in circulating level of EPCs was strongly associated with favorable clinical outcomes after IS [22,23]. Furthermore, growing data [24,25], including our previous [26] and recent [27] reports, have shown that EPC therapy not only was safe but also significantly improved angina, heart failure and ischemia-related LV dysfunction. We, therefore, performed a phase I clinical trial to test the hypothesis that circulatory CD34+ cell therapy might be safe for chronic IS patients and also to evaluate the neurological function after CD34+ cell therapy.
Materials and methods
Study design
This phase I clinical trial was approved by the Ministry of Health and Welfare, Taiwan, Republic of China (IRB No.: 102IND01014) and the Institutional Review Committee on Human Research at Chang Gung Memorial Hospital (IRB No.: 101-1240A) in 2011 and conducted at Kaohsiung Chang Gung Memorial Hospital, a tertiary referral center. This study was funded by a program grant from the Ministry of Science and Technology, Taiwan, Republic of China (MOST 102-2314-B-182A-054-MY3). This was a prospective phase I clinical trial to test the safety and evaluate the neurological function in old IS patients with circulation-derived CD34+ cell therapy at a single medical center. This study was designed to consecutively enroll 10 patients who had history of old IS [i.e., by history, chart recording and brain magnetic resonance imaging (MRI) or brain computerized tomography (CT) scan findings] willing to participate the study.
Inclusion and exclusion criteria
Inclusion criteria included patients (>20 yrs. old and <80 yrs. old) who had history of ischemic stroke more than 6 months and history of National Institutes of Health Stroke Scale (NIHSS) to be recognized ≥8 during hospitalization for his or her acute IS (referred to Table 2).
Table 2.
Clinical outcomes of 9 patients after circulatory autologous CD34+ cell therapy
| Patient list (No) | NIHSS | MRS | Barthel index | Clinical F/UM † | CASI score |
|---|---|---|---|---|---|
| No. 1 | 8‡ | x | x | 24 (S)* | |
| Cell therapy0D | 3 | 4 | 50 | 66.3 | |
| Cell therapy6M | 3 | 3 | 65 | 78.5 | |
| No. 2 | 10‡ | x | x | 24 (S)* | |
| Cell therapy0D | 6 | 2 | 95 | 82.9 | |
| Cell therapy6M | 6 | 2 | 100 | 90.7 | |
| No. 3 | 13‡ | x | x | 21 (S)* | |
| Cell therapy0D | 5 | 3 | 35 | 72.2 | |
| Cell therapy6M | 5 | 4 | 45 | 74.4 | |
| No. 4 | 10‡ | x | x | 21 (S)* | |
| Cell therapy0D | 3 | 2 | 100 | 86.2 | |
| Cell therapy6M | 3 | 2 | 100 | 97.5 | |
| No. 5 | 9‡ | x | x | 18 (S)* | |
| Cell therapy0D | 6 | 2 | 95 | 91 | |
| Cell therapy6M | 6 | 2 | 100 | 96.1 | |
| No. 6 | 9‡ | x | x | 15 (S)* | |
| Cell therapy0D | 0 | 2 | 100 | 96 | |
| Cell therapy6M | 1 | 2 | 100 | 89 | |
| No. 7 | 9‡ | x | x | 10 (S)* | |
| Cell therapy0D | 5 | 4 | 35 | 76.8 | |
| Cell therapy6M | 5 | 4 | 35 | 61.3 | |
| No. 8 | 10‡ | x | x | 9 (S)* | |
| Cell therapy0D | 9 | 4 | 25 | 87.4 | |
| Cell therapy6M | 9 | 4 | 25 | ||
| No. 9 | 9‡ | x | x | 7 (S)* | |
| Cell therapy0D | 5 | 1 | 100 | 83.3 | |
| Cell therapy6M | 4 | 1 | 100 |
NIHSS = National Institutes of Health Stroke Scale; mRS = modified Rankin Scale. D = day; M = month;
(S) indicates the patient is still survival and is followed up (F/U) at outpatient department.
indicates F/UM time (i.e., M = month) after CD34+ cell therapy.
indicates the history of NIHSS of each patient at the first time of acute ischemic stroke to be recognized during the hospitalization.
Those patients with the following conditions were excluded from the study: Hepatitis B or C carrier, surgery, trauma, or myocardial infarction within the preceding 3 months, liver cirrhosis, hematology disorders, renal insufficiency (defined as creatinine clearance <20 mL/min), malignancy, febrile disorders, acute or chronic inflammatory disease at study entry, severe mitral or aortic regurgitation, congestive heart failure (NYHA Fc 4), expected life expectancy <2.0 yrs., age <20 yrs. or ≥80 yrs., or pregnant women.
The investigators, including the neurologist responsible for neurological examination, radiologist and nuclear medicine physician for imaging interpretation, hematologist for CD34+ cell isolation and evaluation of the cell quantity and quality, cardiologists for catheter-based intra-carotid arterial transfusion of circulatory derived CD34+ cells, technicians, clinical nurses, and physicians who took care of the patients in out-patient clinics, participated in this phase I clinical trial.
Procedure and protocol for isolation of autologous CD34+ cells and percutaneous transcatheter intra-carotid artery transfusion [26, 28]
The procedure and protocol have been described in our previous report [26]. Briefly, prior to isolation of circulation-derived CD34+ cells, granulocyte-colony stimulating factor (G-CSF) (5 μg/kg, q12h for 8 doses) was subcutaneously given to each patient to increase the number of circulating CD34+ cells for subsequent collection via leukapheresis. After the last dose of G-CSF, the mononuclear cell preparation isolated during leukapheresis was enriched for CD34+ cells by using a commercially available device [COBE Spetra 6.1 (Terumo BCT, INC.)] at 8:00 a.m. through a double lumen catheter inserted into the right femoral vein. About four hours later, adequate numbers of circulation-derived CD34+ cells were isolated and ready for intra-carotid transfusion in cardiac catheterization room.
Catheter Looping and Retrograde Engagement Technique (CLARET) using a 6-French Kimny Miniradial guiding catheter (Boston Scientific, Scimed, Inc. Maple Grove, MN.) to engage the common carotid artery/internal carotid artery was utilized for each patient. The procedure and protocol of this catheter looping technique has been described in details in our previous report [28]. To avoid microembolization, the CD34+ cells were first filtered with embolic protection filter, followed by slowly transfused into the ipsilateral internal carotid artery and finally into the infarct site/area (i.e., the old infarct area) via the catheter.
Laboratory assessment of levels of EPCs in circulation and right internal jugular vein (RIJV) by flow cytometry and soluble angiogenesis factors by ELISA [26]
EPCs (CD34+KDR+CD45dim, CD34+CD133+CD-45dim, CD31+CD133+CD45dim, CD34+CD133+KDR+, CD34+ and CD133+ surface markers) in peripheral and RIJV blood were identified by flow cytometry using double staining as depicted in our recent report [26] through a fluorescence-activated cell sorter (FACSCaliburTM system; Beckman Coulter Inc, Brea, CA). Each analysis included 300,000 cells per sample. The assays for circulatory and RIJV EPCs in each blood sample were performed in duplicate and mean levels were reported. Intra-assay variability based on repeated measurement of the same blood sample was low with a mean coefficient of variance <3.9% study subjects.
One blood sample was drawn at 8:00 a.m. prior to G-CSF treatment and the other was collected following the last G-CSF treatment for flow cytometric analysis. In addition, to elucidate the serial changes in the levels of EPCs and stromal cell-derived growth factor (SDF)-1α in RIJV, time courses of blood samples were drawn from the RIJV at 0 minute prior to CD34+ cell transfusion and at 5, 10, and 30 minutes after CD34+ cell transfusion for flow cytometric and ELISA analyses, respectively.
Circulating levels of vascular endothelial growth factor (VEGF), epithelial growth factor (EGF), Fibroblast growth factor (FGF), hepatocyte growth factor (HGF), transforming growth factor (TGF)-β, and stromal cell-derived growth factor (SDF)-1α, five indicators of soluble angiogenesis biomarkers, were measured by duplicated determination with a commercially available ELISA method (R&D Systems, Minneapolis, MN, USA). Intra-observer variability of the measurements was also assessed and the mean intra-assay coefficients of variance were all <4.0%.
Other laboratory parameters were measured by following standard procedures in the Department of Clinical Biochemistry and Pathology of our hospital.
Collection of circulatory blood, EPC culture, and angiogenesis assessment by Matrigel assay
The procedure and protocol were based on our recent report [29]. In details, for measurement of angiogenesis ability, peripheral blood (10 cc) was drawn in each IS patient prior to and after G-CSF administration, respectively. The isolated mononuclear cells from peripheral blood were cultured in a 100 mm diameter dish with 10 mL DMEM culture medium containing 10% FBS. By 21-day culturing, abundant cobblestone-like cells, a typical feature of EPCs, were obtained from each patient. Flow cytometric analysis was performed for identification of cellular characteristics (i.e., EPC surface markers) with appropriate antibodies on day 21 of cell cultivation.
To evaluate whether there was different angiogenesis capacity between prior to and after GCSF treatment, peripheral blood-derived EPCs (1.0×104 cells) (n=9 per group) were incubated on 96-well plates at 1.0×104 cells/well in 150 µL serum-free M199 culture medium mixed with 50 µL cold Matrigel (Chemicon international) for 3-hour incubation at 37°C in 5% CO2. Three random microscopic images (200×) were taken at each well for counting cluster, tube, and network formations with the mean values derived (referred to Figure 2).
Figure 2.
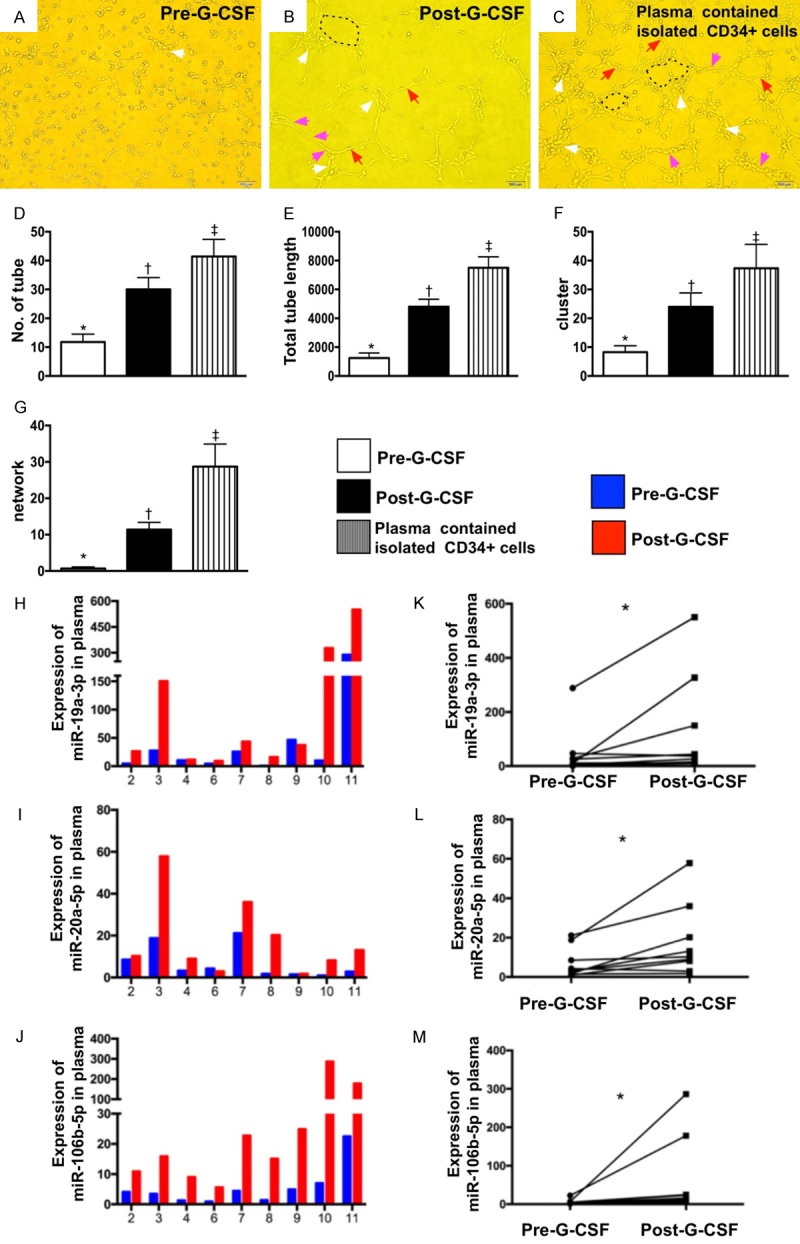
Matrigel assay for assessment of EPC angiogenesis ability and comparison of circulating microRNA expressions in plasma between prior to and after G-CSF treatment. (A-C) Microscopic findings (100×) Matrigel assay showing the angiogenesis results of tubular length (pink arrows) and number of tubular (red arrows), cluster (white arrows) and network (black dotted line) formation among three groups, i.e., (A) pre-G-CSF treatment, (B) post-G-CSF treatment and (C) plasma concentration containing the isolated CD34+ cells. (D) Analytical result of number of tubular formation, *vs. other groups with different symbols (†, ‡), P<0.0005. (E) Analytical result of total tubular length, *vs. other groups with different symbols (†, ‡), P<0.0001. (F) Analytical result of number of cluster formation, *vs. other groups with different symbols (†, ‡), P<0.005. (G) Analytical result of network formation, *vs. other groups with different symbols (†, ‡), p<0.0001. (H-J) Illustrating the expressions of circulating levels of five miRNAs: miR-19a-3p, miR-106b-5p, miR-26b-5p in individual patient prior to and after receiving G-CSF treatment. (K) Analytical result of miR-19a-3p level, *indicated pre- vs. post-G-CSF, P<0.001. (L) Analytical result of miR-20a-5p level, *indicated pre- vs. post-G-CSF, P<0.001. (M) Analytical result of miR-106b-5p level, *indicated pre- vs. post-G-CSF, P<0.001. All statistical analyses were performed by Friedman ANOVA, followed by post hoc analysis with Wilcoxon signed rank test (n=9 for each group). Symbols (*, †, ‡) indicate significance (at 0.05 level). HPF = high-power field; G-CSF = granulocyte-colony stimulating factor; EPC = endothelial progenitor cell.
MicroRNAs extraction and quantitative analysis for determining the integrity of endothelial function [29]
The procedure and protocol were identical to our previous report [29]. In details, total RNA extracted from plasma and cells using the miRNeasy kit (Qiagen) was based on the protocol of the manufacturer. C. elegans miR-39 (cel-miR-39-3p) mimics were loaded in plasma as Spike-in control. For mature miRNA quantification, cDNA was generated with reverse transcription using miScript II RT kit (Qiagen) and Cdna, which was further utilized as a template for real-time PCR. Expressions of human miR-19a-3p, miR-106b-5p and miR-20a-5p were then quantified by miScript SYBR Green PCR assay (Qiagen), and finally were normalized by cel-miR-39-3p in plasma and RNU6 in cells (Qiagen), respectively. Triplicate assays were performed for each sample on Step One-Plus machine (ABI).
Neuro-psychological tests by cognitive ability screening instrument (CASI) and mini-mental state examination (MMSE) prior to and at 6-month after CD34+ cell therapy
All neuropsychological tests were performed by a clinical neurologist. The Mini-Mental State Examination (MMSE) was used as a screen for the general cognitive condition [30]. The Cognitive Abilities Screening Instrument (CASI), a score range of 0 to 100, provided a quantitative assessment of attention, concentration, orientation, short-term memory, long-term memory, language abilities, visual construction, list-generating fluency, abstraction, and judgment [31,32]. In consideration of small sample size (i.e., n=9) that could distort the statistical significance, accordingly, those of patients with P<0.2 were defined as great response to CD34+ cell therapy.
Medications
Heparin (3000 IU) was intra-arterially given to each patient at the beginning of the procedure and its effect was immediately reversed by intra-venous 20 mg of protamine after the CD34+ cell transfusion. Aspirin was the first choice for the study patients unless they were allergic or intolerant to aspirin, including a history of peptic ulcer or upper gastrointestinal tract bleeding during aspirin therapy. Alternatively, clopidogrel was used for those patients who did not tolerate to aspirin therapy. Other commonly used drugs included statins, angiotensin converting enzyme inhibitors (ACEIs)/angiotensin II type I receptor blockers (ARB), calcium channel blocker and beta blocker agent.
Clinical follow-up
In addition to regular follow-up of each patient at our outpatient clinic, a case report form that recorded all clinical information of the patient including the presence or absence of acute or sub-acute events was designed and completed by a research nurse regularly after each visit and on readmission as well as through telephone interviews on an irregular basis.
Statistical analysis
All values are expressed as the mean ± SD, number, or percentage, as appropriate. We compared continuous variables between two groups with Mann-Whitney U test and categorical variables with Chi-square test or Fisher’s exact test. We also compared paired non-parametric samples before and after CD34+ cell therapy with Wilcoxon’s signed-rank test. Furthermore, non-parametric continuous variables at different time points were analyzed in advance using post hoc Wilcoxon’s signed-rank test for multiple comparisons after Friedman test. Statistical analysis was performed through Statistical Package for Social Sciences (SPSS) software package (version 17, SPSS Inc. Chicago, IL, USA). A value of P<0.05 was considered as statistically significant.
Results
The baseline characteristics of 9 study patients (Table 1)
Table 1.
Baseline characteristics of 9 old ischemic stroke patients
| Variables | % (n) or mean ± SD |
|---|---|
| Age (yrs.) (mean ± SD) | 62.8 ± 10.6 |
| Male gender (%) | 88.9% (8) |
| Current smoking (%) | 33.3% (3) |
| Hypertension (%) | 100% (9) |
| Dyslipidemia (%) | 33.3% (3) |
| Diabetes mellitus (%) | 22.2% (2) |
| Mean ischemic stroke period prior to cell therapy (yrs.) | 8.6 ± 6.4 |
| History of old myocardial infarction (%) | 0% (0) |
| Body mass index (mean ± SD) | 23.7 ± 2.3 |
| Atrial fibrillation (%) | 11.1% (1) |
| Obstructive coronary artery disease (≥50% stenosis) (%) | 88.9% (8) |
| History of coronary artery intervention (%) | 88.9% (8) |
| Medication (%) | |
| Antiplatelet agent (%) | 100% (9) |
| ACEI/ARB (%) | 33.3% (3) |
| Beta-blocker agent | 44.4% (4) |
| Statin (%) | 77.8% (7) |
| New oral coagulant agent (%) | 11.1% (1) |
| Calcium channel blocker (%) | 11.1% (1) |
| Laboratory parameters | |
| Red blood cell count (×106) | 4.53 ± 0.65 |
| White blood cell count (×103) | 6.6 ± 1.5 |
| Platelet count (×103) | 214.5 ± 48.8 |
| Hemoglobin (g/dL) | 13.8 ± 1.6 |
| Creatinine (mg/dL) | 1.31 ± 0.51 |
| Creatinine clearance rate (CCr) (ml/min) | 63.3 ± 24.5 |
ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin II type I receptor blocker.
The mean age of the study patients was about 62 years old and majority of these patients were male. All study patients had hypertension and more than 88% of them had received percutaneous coronary intervention.
The mean IS time interval prior to CD34+ cell therapy was 8.6 ± 6.4 years, suggesting that these patients have very long history of IS. Therefore, they were at a situation of very old IS time point. All of these patients had received regularly antiplatelet agents and only one patient had received additionally new oral anti-coagulant agent due to atrial fibrillation. Additionally, all patients received anti-hypertension drugs for controlling the blood pressure and more than 77% patients were treated by statins due to hypercholesterolemia.
Flow cytometric analysis for measuring the circulatory EPCs and hematopoietic stem cell (HSC) (Figure 1)
Figure 1.
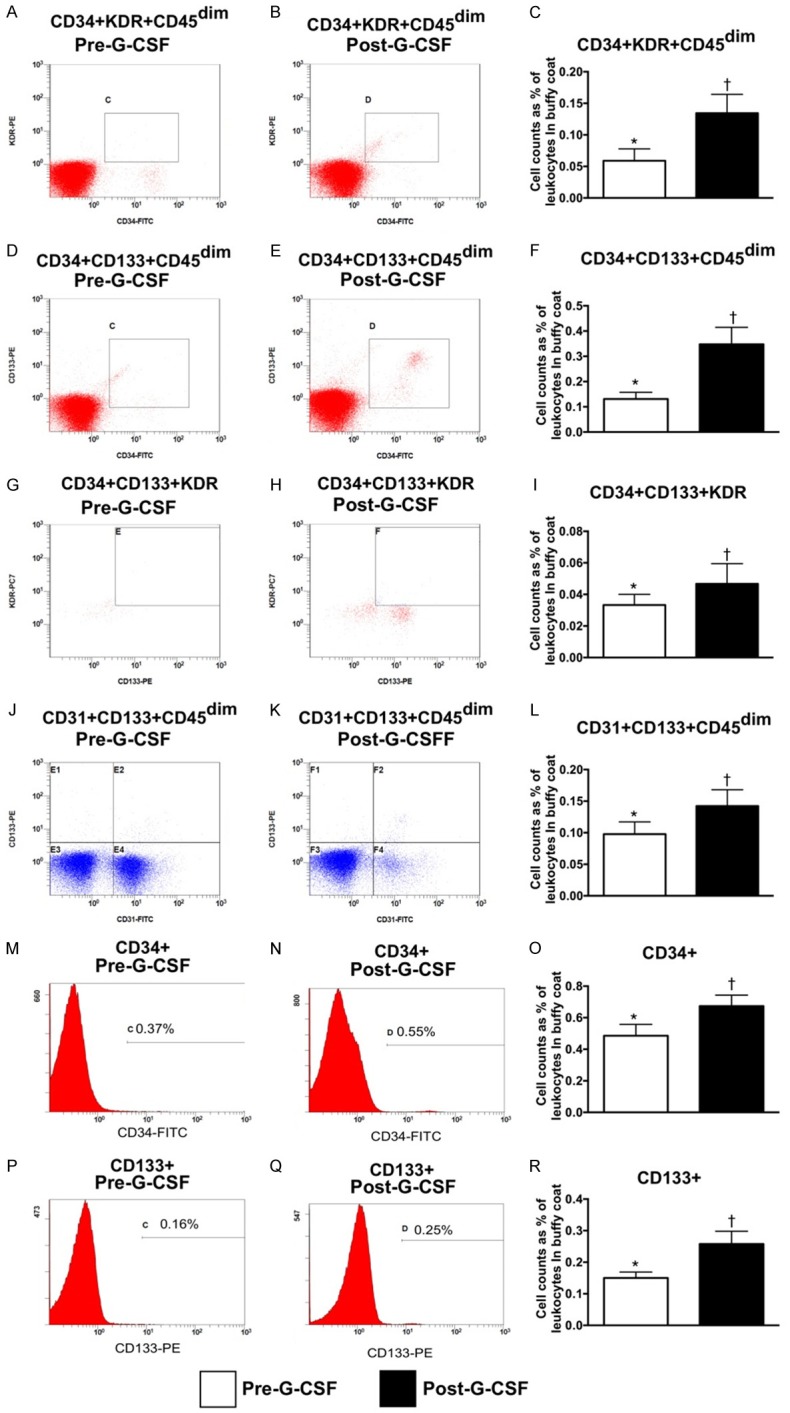
Flow cytometric analysis for measuring the circulatory endothelial progenitor cells and hematopoietic stem cells (n=9). (A, B) Illustrating the circulating level of CD34+KDR+CD45dim cells prior to (A) and after (B) G-CSF treatment. (C) Analytical result, * vs. †, P<0.001. (D, E) Illustrating the circulating level of CD34+CD133+CD45dim cells prior to (D) and after (E) G-CSF treatment. (F) Analytical result, * vs. †, P<0.001. (G, H) Illustrating the circulating level of CD34+CD133+KDR+ cells prior to (G) and after (H) G-CSF treatment. (I) Analytical result, * vs. †, P<0.01. (J, K) Illustrating the circulating level of CD31+CD133+CD45dim cells prior to (J) and after (K) G-CSF treatment. (L) Analytical result, * vs. †, P<0.01. (M, N) Illustrating the circulating level of CD34+ cells prior to (M) and after (N) G-CSF treatment. (O) Analytical result, * vs. †, P<0.01. (P, Q) Illustrating the circulating level of CD133+ cells prior (P) and after (Q) G-CSF treatment. (R) Analytical result, * vs. †, P<0.001. G-CSF = granulocyte colony stimulating factor.
As compared to baseline, the circulating numbers of EPCs (i.e., CD34+KDR+CD45dim, CD-34+CD133+CD45dim, CD31+CD133+CD45dim, CD34+CD133+KDR+ and CD133+ surface markers) and hematopoietic stem cell (HSC) (CD34+) were significantly increased among these 9 patients after receiving G-CSF treatment.
Matrigel assay for determinant of EPC angiogenesis ability and expressions of miR-19a, miR-20a, and miR-106b in plasma of 9 study patients prior to and after G-CSF treatment (Figure 2)
To elucidate the impact of G-CSF therapy on angiogenesis, the Matrigel assay was utilized in the present study. As expected, the results of Matrigel assay showed that the expressions of number of tubule, tubular length, cluster formation and network formation, four indicators of angiogenesis, were significantly increased after G-CSF treatment among the 9 study patients. Additionally, these parameters were notably further increased in concentrated plasma containing the isolated CD34+ cells.
In the present study, blood samplings were also utilized for assessment of circulating micro-RNAs of miR-19a-3p (with biological processes of angiogenesis, glycogenesis and anti-apoptosis), miR-106b-5p (with biological processes of proliferation, migration and anti-apoptosis) and miR-26b-5p (with biological processes of anti-autophagy, migration and anti-apoptosis) which have been identified to be strongly associated with chronic kidney disease (CKD) by our previous study [29]. The results showed that the levels of these three miRNAs were found to be significantly increased after receiving G-CSF treatment.
Time courses of RIJV levels of stromal cell-derived factor (SDF)-1α and EPCs/HSC, and circulatory soluble angiogenesis factors in 9 study patients (Figure 3)
Figure 3.
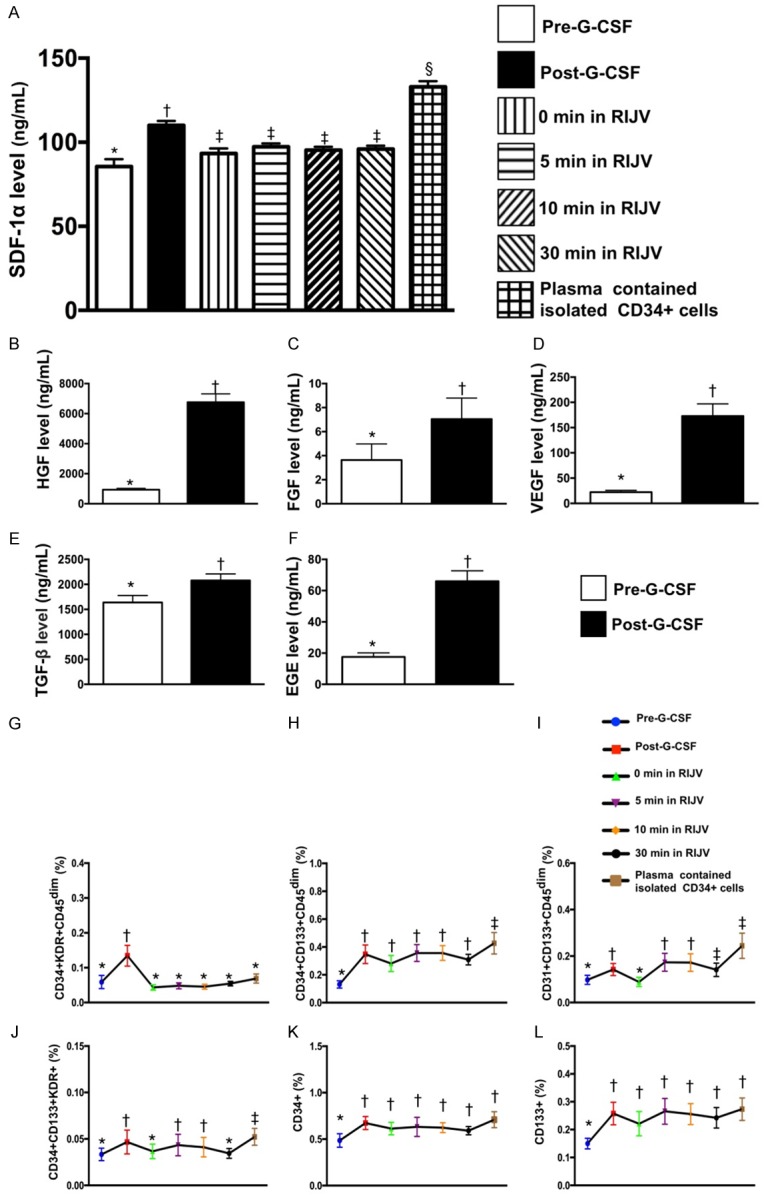
Time courses of RIJV levels of stromal cell-derived factor (SDF)-1α, EPCs and hematopoietic stem cell (HSC). (A) Illustrating the serum levels of stromal cell-derived factor (SDF)-1α by ELISA measurement; analytical results of SDF-1α concentration in different situations, *vs. other groups with different symbols (†, ‡, §), p<0.0001. (B-F) Circulating levels of hepatocyte growth factor (HGF) (B), fibroblast growth factor (FGF) (C), vascular endothelial growth factor (VEGF) (D), transforming growth factor (TGF)-β (E) and epidermal growth factor (EGF) (F), analytical results of pre- vs. post-G-CSF treatment, * vs. †, all p values <0.01. (G-L) The flow cytometric analysis showed that the EPCs (i.e., CD34+KDR+CD45dim, CD34+CD133+CD45dim, CD31+CD133+CD45dim, CD34+CD133+KDR+ and CD133+ surface markers) and HSC (CD34+) were continuously shedding (from 0, 5, 10 to 30 minutes) from RIJV into circulation. Analytical results: (1) for CD34+KDR+CD45dim, * vs. †, P<0.001; for (2) CD34+CD133+CD45dim, *vs. other groups with different symbols (†, ‡), P<0.001; (3) for CD31+CD133+CD45dim, *vs. other groups with different symbols (†, ‡), P<0.001; (4) for CD34+CD133+KDR+, *vs. other groups with different symbols (†, ‡), P<0.001; (5) CD34+, * vs. †, P<0.01; (6) CD133+, * vs. †, P<0.01. All statistical analyses were performed by Friedman ANOVA, followed by post hoc analysis with Wilcoxon signed rank test (n=9 for each group). Symbols (*, †, ‡, §) indicate significance (at 0.05 level). EPC = endothelial progenitor cell.
To elucidate the level of SDF-1α at different time points in cerebral circulation after CD34+ intra-carotid artery transfusion, we drew the blood samples from RIJV and measured this soluble angiogenesis factor at baseline (i.e., 0 minute) and at 5, 10, and 30 minutes after CD34+ cell transfusion. The baseline level of SDF-1α was significantly lower than that at the time intervals of 5, 10 and 30 minutes, suggesting the EPCs, such as CXCR4+ cells were trapped in blood vessels/capillary networks. Additionally, the SDF-1α level was significantly higher in concentrated plasma containing the isolated CD34+ cells and in circulation at the time after completing G-CSF treatment than in RIJV at the time of 5, 10, and 30 minutes post-CD34+ cell treatment. However, the SDF-1α level did not differ among the time points of 5, 10, and 30 minutes.
Additionally, ELISA results showed that except for fibroblast growth factor, the vascular endothelial growth factor, epithelial growth factor, hepatocyte growth factor and transforming growth factor, four indicators of soluble angiogenesis biomarkers, were significantly higher after G-CSF treatment as compared with the time interval prior to G-CSF treatment among the 9 study patients.
To elucidate the shedding rate of EPCs from RIJV after CD34+ cell intra-carotid artery administration, time courses of EPC and HSC measurement were performed by flow cytometry. The time courses of EPCs (i.e., CD34+KDR+CD45dim, CD34+CD133+CD45dim, CD31+CD-133+CD45dim, CD34+CD133+KDR+ and CD-133+ surface markers) and HSC (CD34+) were identified to be continuously drained from RIJV to circulation at time points of 5, 10, and 30 minutes after intra-carotid artery transfusion of CD34+ cells. Additionally, these parameters were significantly higher in the three time intervals as compared to the 0 minute prior to CD-34+ cell administration. Furthermore, among these patients, the circulating levels of EPCs and HSC were notably higher after G-CSF treatment than those prior to G-CSF treatment. This finding suggests that G-CSF treatment allowed the EPC and HSC homing from bone marrow to circulation.
Clinical outcomes of 9 patients after circulatory autologous CD34+ cell therapy (Table 2)
The Table 2 illustrates the clinical outcome of 9 patients after receiving CD34+ cell treatment. The NIHSS and modified Rankin Scale (mRS), two neurological functional indices, did not differ between baseline (i.e., prior to CD34+ cell therapy) and at post 6-month CD34+ cell therapy among the study patients. Surprisingly, the Barthel index was found to be notably improved (i.e., increased up to 5 scores) in 4 (44.4%) of 9 patients. Of importance was that the therapy was 100% safe and all patients were uneventfully discharged. Additionally, neither recurrent IS nor any tumorigenesis was found in these patients with a mean follow-up time interval of 16.5 ± 6.2 months. Furthermore, all of these patients still survive and receive regular follow-up at outpatient department.
The mean value of MMSE before and after CD34+ cell therapy was 25.5 ± 3.59 and 26.5 ± 3.59 (P=0.37), respectively. Additionally, the mean value of CASI before and after CD34+ cell therapy was 82.35 ± 9.95 and 83.61 ± 12.2 (P=0.73), respectively.
Comparison of standard deviation of Tc-99m ECD brain perfusion SPECT study before (baseline) and after (at six-month) CD34+ cell therapy in 9 study patients (Table 3)
Table 3.
Comparison of standard deviation of Tc-99m ECD brain perfusion SPECT study before (baseline) and after (at six-month) CD34+ cell therapy in 9 study patients
| Variables | Median SD (Q1, Q3) (day 0) | Median SD (Q1, Q3) (6 M) | Median difference (Q1, Q3) | P* |
|---|---|---|---|---|
| Basal ganglia (L) | 0.5 (-2.1, 1.7) | 0.5 (-2.2, 1.8) | 0 (-0.1, 0.1) | 0.766 |
| Basal ganglia (R) | 0 (-2.5, 0.4) | 0.1 (-2.2, 1.3) | -0.3 (-0.7, 0.2) | 0.172† |
| Central region (L) | -0.5 (-2.0, 2.2) | -0.5 (-1.2, 2.6) | -0.2 (-0.8, 0) | 0.188† |
| Central region (R) | -0.8 (-2.3, 0.3) | -0.5 (-2.2, 1.1) | -0.2 (-0.7, 0.4) | 0.406 |
| Cingulate & paracingulate gyri (L) | -0.1 (-1.4, 1.2) | 0.1 (-0.8, 1.5) | -0.3 (-0.5, 0.3) | 0.438 |
| Cingulate & paracingulate gyri (R) | 0.4 (-2.2, 0.8) | -1.6 (-2.5, 0.4) | -0.3 (-0.7, 0) | 0.250 |
| Frontal lobe (L) | -0.6 (-1.4, 2.8) | 0.4 (-0.9, 2.5) | -0.9 (-1.0, -0.5) | 0.008† |
| Frontal lobe (R) | -1.3 (-1.7, 0.3) | -0.8 (-1.2, 1.0) | -0.5 (-0.7, -0.3) | 0.094† |
| Occipital lobe (L) | 0.7 (-0.8, 1.9) | 1.1 (-0.1, 2.0) | -0.5 (-0.7, -0.63) | 0.109† |
| Occipital lobe (R) | 0.3 (0, 1.8) | 1.0 (0.6, 1.9) | -0.6 (-0.7, -0.5) | 0.078† |
| Parietal lobe (L) | -0.4 (-0.9, 2.0) | 0.1 (-0.2, 1.5) | 0 (-0.2, 0.3) | 0.945 |
| Parietal lobe (R) | -0.1 (-0.6, 1.4) | 0.6 (-0.5, 2.0) | -0.2 (-1.2, 0.1) | 0.313 |
| Temporal lobe (L) | 0.1 (-0.5, 1.8) | 0.2 (0, 1.4) | -0.3 (-0.8, 0.4) | 0.590 |
| Temporal lobe (R) | -0.4 (-0.4, 1.5) | 0.9 (-0.1, 1.5) | -0.3 (-1.3, 0.3) | 0.320 |
by Wilcoxon Sign-rank test for paired data.
L = left; R = right, M = month; SD = standard deviation.
Great response: defined as the P<0.2.
Intriguingly, 6 of 14 (42.9%) of different points of brain areas were identified to have great response for increasing brain perfusion, suggesting that G-CSF-assisted CD34+ cell therapy may restore some of blood perfusion in cerebral circulation.
Discussion
This phase I clinical trial which investigated the safe of intra-carotid transfusion of circulatory-derived autologous CD34+ cells yielded several striking clinical implications. First, the therapy was 100% safe and all of our study patients survive without recurrent IS and are still followed up at outpatient department. Second, the 6-month neurological function (i.e., Barthel index) was notably improved up to more than 40% of the study patients after receiving CD34+ cell therapy. Third, consistent with neurological function test, the 6-month neuro-psychological test (i.e., CASI) was also notably improved among these patients with the cell therapy.
Up to day, there is still lacking effective treatment for the old IS. Accordingly, majority of the patients in this setting would undoubtedly have progressive loss of memory, intelligence and neurological function. These raise the need for development of a new innovative and safe strategy for these patients. To the best of our knowledge, this is the first clinical trial worldwide to test the safety of intra-carotid artery administration of autologous-derived circulating CD34+ cells for patients with old IS. Of the importance is that this therapy was 100% safe and the survival rate at intermediate-term follow-up (i.e., 14 ± 12 months) was 100% without any recurrent stroke found among these study patients.
An unexpected finding in the present study was that more than 44.0% patients had notable improvement of Barthel index after receiving CD34+ cell therapy. Additionally, another unanticipated finding in the present study was greater than 44.0% of our patients had acceptable improvement of CASI score. Furthermore, the other unpredicted finding in the present study was that more than 42% of our patient had great response of brain reperfusion that was examined by Tc-99m ECD brain perfusion SPECT. Based on this phase I experience, a phase II clinical trial of intra-carotid transfusion of circulatory-derived autologous CD34+ cells for acute IS setting with a primary focus on safety and secondary outcomes including biological, imaging, neurological, recurrent stroke and clinical endpoints is ongoing in our institute.
An essential finding in the present study was that all of the investigated angiogenesis factors were identified to be markedly increased after G-CSF treatment. Additionally, the angiogenesis capacity of EPCs was markedly increased in post than in prior to G-CSF treatment. Furthermore, the circulating numbers of EPCs were significantly increased in post than in prior to G-CSF treatment and furthermore increased in automatic machine (i.e., COBE Spetra 6.1) isolated EPCs. Our findings may, at least in part, explain for why near half of our patients had great improvement of the brain perfusion (i.e., assessed by Tc-99m ECD brain perfusion SPECT) after CD34+ cell therapy. Intriguingly, previous studies have shown that soluble angiogenesis factors and EPCs always play a crucial role for development of angiogenesis/neovascularization and collaterals in ischemic organs, especially in setting of coronary artery obstruction for restoring the blood flow in ischemia [24-27,33,34]. Accordingly, our result is supported by the findings of previous studies [24-27,33,34].
Interestingly, our clinical trial has previously shown that erythropoietin (EPO) therapy enhanced circulating level of EPCs and a favorable clinical outcome was strongly and independently associated with an increase in circulating level of EPCs in patient after IS [23]. Additionally, our recent clinical trials [26,27] have revealed that intra-coronary administration of autologous circulatory derived CD34+ cells significantly improved heart failure, angina, heart function and long-term outcome in diffuse coronary artery disease patients that is mainly through CD34+ cell treatment enhanced angiogenesis/vasculogenesis and neovascularization. Furthermore, our previous study has revealed that circulating level of EPCs was a powerfully independent predictor of prognostic outcome in patients after acute IS stroke [22]. Moreover, our recent experimental study has demonstrated that intra-carotid artery administration of autologous EPCs improved neurological outcomes in rat after acute IS mostly through augmenting molecular-cellular levels of angiogenesis factors [35]. In this way, our findings, in addition to being supported by the results from our previous and recent studies [22,23,26,27], could partially explain for why the improvements of brain perfusion, Barthel index and CASI score were identified to be present in more than 40% of our patients, suggesting that these improvements resulted in the synergic effects of G-CSF, increased in soluble angiogenesis factors and intra-carotid artery administration of CD34+ cells.
One intriguing finding in the present study is that flow cytometric analysis showed that EPC levels were not only higher in RIJV than in circulation but were also found to be continuously drained from the RIJV to circulation. Additionally, an essential finding in the present study was that as compared with prior to CD34+ cell administration, the SDF-1α level was significantly higher at three time intervals of 5, 10 and 30 minutes after CD34+ cell transfusion in RIJV and further more in the plasma which contained the isolated CD34+ cells for ready transfusion. These findings imply that the level of EPCs was maintained at a higher level inside cerebral arteries/capillaries than that in RIJV for endothelial cell repair, angiogenesis and neovascularization. Interestingly, a concentration gradient of SDF-1α between bone marrow (lower level) and circulation (higher level) has been shown to play a crucial role in promoting EPC mobilization from bone marrow to circulation and EPC homing to ischemic area for angiogenesis [36,37]. Our findings are supported by those of previous studies [36,37]. We propose that trapping of EPCs in cerebral circulation would have happened and could be due to not only the higher level of SDF-1α in endothelial cells of cerebral arterial trees, but also the irregularity of endothelial layer (i.e., uneven surface) of the diffusely atherosclerotic cerebral artery that helps in cell retention.
Study limitation
This study has limitations. First, the sample size is small (because it is phase I clinical trial). Accordingly, the results of statistical analysis could be distorted. Second, without placebo-controlled group, the interpretation of the improved effect after cell therapy should be treated with reserve.
Conclusion
The results of this phase I clinical trial showed that intra-carotid artery administration of circulatory derived CD34+ cells is safe and may offer some potential benefit for old IS patients.
Acknowledgements
This study was supported by a program grant from National Science Council, Taiwan (MOST 102-2314-B-182A-054-MY3).
Disclosure of conflict of interest
None.
Abbreviations
- CASI
Cognitive Ability Screening Instrument
- CAD
coronary artery disease
- CKD
chronic kidney disease
- CVD
cerebrovascular disease
- EPC
endothelial progenitor cells
- IS
ischemic stroke
- HSC
hematopoietic stem cell
- MMSE
Mini-Mental State Examination
- NIHSS
National Institutes of Health Stroke Scale
- Tpa
tissue plasminogen activator
References
- 1.Kuklina EV, Tong X, George MG, Bansil P. Epidemiology and prevention of stroke: a worldwide perspective. Expert Rev Neurother. 2012;12:199–208. doi: 10.1586/ern.11.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Johnson W, Onuma O, Owolabi M, Sachdev S. Stroke: a global response is needed. Bull World Health Organ. 2016;94:634–634A. doi: 10.2471/BLT.16.181636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao-Melacini P, Zhang X, Pais P, Agapay S, Lopez-Jaramillo P, Damasceno A, Langhorne P, McQueen MJ, Rosengren A, Dehghan M, Hankey GJ, Dans AL, Elsayed A, Avezum A, Mondo C, Diener HC, Ryglewicz D, Czlonkowska A, Pogosova N, Weimar C, Iqbal R, Diaz R, Yusoff K, Yusufali A, Oguz A, Wang X, Penaherrera E, Lanas F, Ogah OS, Ogunniyi A, Iversen HK, Malaga G, Rumboldt Z, Oveisgharan S, Al Hussain F, Magazi D, Nilanont Y, Ferguson J, Pare G, Yusuf S. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761–775. doi: 10.1016/S0140-6736(16)30506-2. [DOI] [PubMed] [Google Scholar]
- 5.Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Wolf ME, Hennerici MG. The ASCOD phenotyping of ischemic stroke (updated ASCO phenotyping) Cerebrovasc Dis. 2013;36:1–5. doi: 10.1159/000352050. [DOI] [PubMed] [Google Scholar]
- 6.Ay H. Advances in the diagnosis of etiologic subtypes of ischemic stroke. Curr Neurol Neurosci Rep. 2010;10:14–20. doi: 10.1007/s11910-009-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radu RA, Terecoasa EO, Bajenaru OA, Tiu C. Etiologic classification of ischemic stroke: where do we stand? Clin Neurol Neurosurg. 2017;159:93–106. doi: 10.1016/j.clineuro.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Mohr JP, Gautier JC, Pessin MS. Stroke: pathophysiology, diagnosis, and management. 3rd edition. Churchill Livingston; 1998. Internal carotid artery disease; p. 1459. [Google Scholar]
- 9.Zaidat OO, Klucznik R, Alexander MJ, Chaloupka J, Lutsep H, Barnwell S, Mawad M, Lane B, Lynn MJ, Chimowitz M. The NIH registry on use of the Wingspan stent for symptomatic 70-99% intracranial arterial stenosis. Neurology. 2008;70:1518–1524. doi: 10.1212/01.wnl.0000306308.08229.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 11.Chen ZM, Sandercock P, Pan HC, Counsell C, Collins R, Liu LS, Xie JX, Warlow C, Peto R. Indications for early aspirin use in acute ischemic stroke: a combined analysis of 40,000 randomized patients from the chinese acute stroke trial and the international stroke trial. On behalf of the CAST and IST collaborative groups. Stroke. 2000;31:1240–1249. doi: 10.1161/01.str.31.6.1240. [DOI] [PubMed] [Google Scholar]
- 12.Grau AJ, Weimar C, Buggle F, Heinrich A, Goertler M, Neumaier S, Glahn J, Brandt T, Hacke W, Diener HC. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke. 2001;32:2559–2566. doi: 10.1161/hs1101.098524. [DOI] [PubMed] [Google Scholar]
- 13.Perin EC, Silva GV, Assad JA, Vela D, Buja LM, Sousa AL, Litovsky S, Lin J, Vaughn WK, Coulter S, Fernandes MR, Willerson JT. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J Mol Cell Cardiol. 2008;44:486–495. doi: 10.1016/j.yjmcc.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Yip HK, Liou CW, Chang HW, Lan MY, Liu JS, Chen MC. Link between platelet activity and outcomes after an ischemic stroke. Cerebrovasc Dis. 2005;20:120–128. doi: 10.1159/000086802. [DOI] [PubMed] [Google Scholar]
- 15.Adams HP Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EF American Heart Association; American Stroke Association Stroke Council; Clinical Cardiology Council; Cardiovascular Radiology and Intervention Council; Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 16.Minnerup J, Wersching H, Teuber A, Wellmann J, Eyding J, Weber R, Reimann G, Weber W, Krause LU, Kurth T, Berger K REVASK Investigators. Outcome after thrombectomy and intravenous thrombolysis in patients with acute ischemic stroke: a prospective observational study. Stroke. 2016;47:1584–1592. doi: 10.1161/STROKEAHA.116.012619. [DOI] [PubMed] [Google Scholar]
- 17.Pan Y, Wang A, Liu G, Zhao X, Meng X, Zhao K, Liu L, Wang C, Johnston SC, Wang Y, Wang Y. Cost-effectiveness of clopidogrel-aspirin versus aspirin alone for acute transient ischemic attack and minor stroke. J Am Heart Assoc. 2014;3:e000912. doi: 10.1161/JAHA.114.000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rangel-Castilla L, Snyder KV, Siddiqui AH, Levy EI, Hopkins NL. Endovascular intracranial treatment of acute ischemic strokes. J Cardiovasc Surg (Torino) 2016;57:36–47. [PubMed] [Google Scholar]
- 19.Stecksen A, Asplund K, Appelros P, Glader EL, Norrving B, Eriksson M, Riks-Stroke C. Thrombolytic therapy rates and stroke severity: an analysis of data from the Swedish stroke register (riks-stroke) 2007-2010. Stroke. 2012;43:536–538. doi: 10.1161/STROKEAHA.111.630590. [DOI] [PubMed] [Google Scholar]
- 20.Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Kuelkens S, Larrue V, Lees KR, Roine RO, Soinne L, Toni D, Vanhooren G SITS-MOST investigators. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369:275–282. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 21.Yeo LL, Paliwal P, Teoh HL, Seet RC, Chan BP, Liang S, Venketasubramanian N, Rathakrishnan R, Ahmad A, Ng KW, Loh PK, Ong JJ, Wakerley BR, Chong VF, Bathla G, Sharma VK. Timing of recanalization after intravenous thrombolysis and functional outcomes after acute ischemic stroke. JAMA Neurol. 2013;70:353–358. doi: 10.1001/2013.jamaneurol.547. [DOI] [PubMed] [Google Scholar]
- 22.Yip HK, Chang LT, Chang WN, Lu CH, Liou CW, Lan MY, Liu JS, Youssef AA, Chang HW. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke. 2008;39:69–74. doi: 10.1161/STROKEAHA.107.489401. [DOI] [PubMed] [Google Scholar]
- 23.Yip HK, Tsai TH, Lin HS, Chen SF, Sun CK, Leu S, Yuen CM, Tan TY, Lan MY, Liou CW, Lu CH, Chang WN. Effect of erythropoietin on level of circulating endothelial progenitor cells and outcome in patients after acute ischemic stroke. Crit Care. 2011;15:R40. doi: 10.1186/cc10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henry TD, Losordo DW, Traverse JH, Schatz RA, Jolicoeur EM, Schaer GL, Clare R, Chiswell K, White CJ, Fortuin FD, Kereiakes DJ, Zeiher AM, Sherman W, Hunt AS, Povsic TJ. Autologous CD34+ cell therapy improves exercise capacity, angina frequency and reduces mortality in no-option refractory angina: a patient-level pooled analysis of randomized double-blinded trials. Eur Heart J. 2018;39:2208–2216. doi: 10.1093/eurheartj/ehx764. [DOI] [PubMed] [Google Scholar]
- 25.Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, Traverse JH, Amrani D, Ewenstein BM, Riedel N, Story K, Barker K, Povsic TJ, Harrington RA, Schatz RA. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109:428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee FY, Chen YL, Sung PH, Ma MC, Pei SN, Wu CJ, Yang CH, Fu M, Ko SF, Leu S, Yip HK. Intracoronary transfusion of circulation-derived CD34+ cells improves left ventricular function in patients with end-stage diffuse coronary artery disease unsuitable for coronary intervention. Crit Care Med. 2015;43:2117–2132. doi: 10.1097/CCM.0000000000001138. [DOI] [PubMed] [Google Scholar]
- 27.Sung PH, Lee FY, Tong MS, Chiang JY, Pei SN, Ma MC, Li YC, Chen YL, Wu CJ, Sheu JJ, Lee MS, Yip HK. The five-year clinical and angiographic follow-up outcomes of intracoronary transfusion of circulation-derived CD34+ cells for patients with end-stage diffuse coronary artery disease unsuitable for coronary intervention-phase I clinical trial. Crit Care Med. 2018;46:e411–e418. doi: 10.1097/CCM.0000000000003051. [DOI] [PubMed] [Google Scholar]
- 28.Yip HK, Sung PH, Wu CJ, Yu CM. Carotid stenting and endarterectomy. Int J Cardiol. 2016;214:166–174. doi: 10.1016/j.ijcard.2016.03.172. [DOI] [PubMed] [Google Scholar]
- 29.Lee MS, Lee FY, Chen YL, Sung PH, Chiang HJ, Chen KH, Huang TH, Chen YL, Chiang JY, Yin TC, Chang HW, Yip HK. Investigated the safety of intra-renal arterial transfusion of autologous CD34+ cells and time courses of creatinine levels, endothelial dysfunction biomarkers and micro-RNAs in chronic kidney disease patients-phase I clinical trial. Oncotarget. 2017;8:17750–17762. doi: 10.18632/oncotarget.14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Lin KN, Wang PN, Liu CY, Chen WT, Lee YC, Liu HC. Cutoff scores of the cognitive abilities screening instrument, Chinese version in screening of dementia. Dement Geriatr Cogn Disord. 2002;14:176–182. doi: 10.1159/000066024. [DOI] [PubMed] [Google Scholar]
- 32.Teng EL, Hasegawa K, Homma A, Imai Y, Larson E, Graves A, Sugimoto K, Yamaguchi T, Sasaki H, Chiu D, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6:45–58. doi: 10.1017/s1041610294001602. discussion 62. [DOI] [PubMed] [Google Scholar]
- 33.Fujita M, Sasayama S. Coronary collateral growth and its therapeutic application to coronary artery disease. Circ J. 2010;74:1283–1289. doi: 10.1253/circj.cj-10-0376. [DOI] [PubMed] [Google Scholar]
- 34.Seiler C. The human coronary collateral circulation. Heart. 2003;89:1352–1357. doi: 10.1136/heart.89.11.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen YL, Tsai TH, Wallace CG, Chen YL, Huang TH, Sung PH, Yuen CM, Sun CK, Lin KC, Chai HT, Sheu JJ, Lee FY, Yip HK. Intra-carotid arterial administration of autologous peripheral blood-derived endothelial progenitor cells improves acute ischemic stroke neurological outcomes in rats. Int J Cardiol. 2015;201:668–683. doi: 10.1016/j.ijcard.2015.03.137. [DOI] [PubMed] [Google Scholar]
- 36.Wang CH, Verma S, Hsieh IC, Chen YJ, Kuo LT, Yang NI, Wang SY, Wu MY, Hsu CM, Cheng CW, Cherng WJ. Enalapril increases ischemiainduced endothelial progenitor cell mobilization through manipulation of the CD26 system. J Mol Cell Cardiol. 2006;41:34–43. doi: 10.1016/j.yjmcc.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Yip HK, Sun CK, Tsai TH, Sheu JJ, Kao YH, Lin YC, Shiue YL, Chen YL, Chai HT, Chua S, Ko SF, Leu S. Tissue plasminogen activator enhances mobilization of endothelial progenitor cells and angiogenesis in murine limb ischemia. Int J Cardiol. 2013;168:226–236. doi: 10.1016/j.ijcard.2012.09.090. [DOI] [PubMed] [Google Scholar]


