Abstract
This study aimed to investigate the effects of microglial activation on the onset of epilepsy. Microglias cultured in vitro were stimulated with different concentrations of coriaria lactone (CL), and the effects on cell cycle and apoptosis were examined using flow cytometry. Then microglia were stimulated with 5×10-5 mol/L CL, and levels of cyclin D1 and interleukin-1β (IL-1β) mRNA were measured by fluorescence quantitative PCR; tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) in supernatant were detected by radioimmunoassay. Finally, microglia-conditioned medium (MCM) obtained after various durations of CL treatment was infused into rat lateral ventricle, and rat behavior was observed and cortical electroencephalograms (EEGs) were recorded. Immunofluorescence staining was used to measure glutamate content in the rat cerebral cortex and hippocampus. Compared with the cell cycle phase distribution in the control group, the percentage of CL-treated cultured microglia in G0/G1 phase was significantly lower, while the percentages of microglia in S phase and G2/M phases were significantly higher. CL increased the gene expression of cyclin D1 and the secretion of TNF-α and IL-1β. Epileptic seizures were induced in rats after intraventricular injection with MCM from CL-treated cells, with animals showing bilateral beard shaking and forelimb tremor. EEGs from these animals exhibited epileptiform waveforms (such as spike waves, sharp waves and spike-slow waves), and glutamate content in the cerebral cortex and hippocampus was significantly increased. CL may therefore activate microglia by promoting proliferation and upregulation of cell factor (e.g., cyclin D1, TNF-α and IL-1β) expression. We have shown that CL-treated MCM can induce the onset of epilepsy in rats in vivo, and its mechanism of action may involve the upregulation of glutamate expression. In summary, microglial activation is an important link in the pathogenesis of the onset of epilepsy.
Keywords: Microglial activation, coriaria lactone, epilepsy, TNF-α, IL-1β
Introduction
Epilepsy is a common clinical syndrome of the central nervous system. Patients suffer recurrent seizures, which are a result of aberrant, excessive and synchronous firing of groups of neurons within the brain. Epilepsy pathogenesis is associated with regulatory imbalance of the neuro-immune-endocrine network [1]. Studies of the relationship between the immune system and epilepsy have made some progress in recent years. Children with epilepsy often have impaired immune function [2]. Microglias are immune effector cells located in the central nervous system, and they play an important role in the initiation, development and prognosis of immune inflammatory reactions in the brain [3]. Under normal physiological conditions, microglia in a quiescent state can monitor immune function. Under conditions of central nervous system damage and various types of immune stimulation, microglia are rapidly activated. Therefore, microglias are also called “central pathological receptors” [4,5]. To understand the role of activated microglia in the pathogenesis of epilepsy, in this study microglia were activated by the chemical epileptogenic agent coriaria lactone (CL). Then the activated microglia-conditioned medium (MCM) was infused into rat lateral ventricle. Rat behavior and electroencephalogram (EEG) recordings were observed to explore the role of microglial activation in epileptic seizures.
Materials and methods
Experimental animals
Newborn Sprague Dawley rats (within 24 hours) and healthy adult Sprague Dawley rats (male, 170-210 g) were provided by the Experimental Animal Center of Tongji Medical College of Huazhong University of Science and Technology in China. This study was approved by the Ethical Committee of Tongji Medical College, Huazhong University of Science and Technology.
Purification, isolation and culture of microglia
In accordance with the modified method of McCarthy [6], the cerebral cortex of newborn Sprague Dawley rats was fully minced, digested with 0.125% trypsin at 37°C for 5 min, filtered with 200-mesh nylon mesh, and centrifuged at 1,000 rpm for 10 min. The supernatant was discarded. Cell density was adjusted to 5×105/cm2 by adding Dulbecco’s Modified Eagle Medium (DMEM)/F12 medium containing 10% fetal bovine serum and 10% calf serum. The cells were incubated in polylysine-coated culture flasks at 37°C with 5% CO2. At 7-9 d of primary culture, cells grew to more than one layer. After removal of the medium, lidocaine hydrochloride (12 mmol/L) was added, and then the culture flask was placed in an incubator at 37°C for 3-5 min without stirring. The culture flask was shaken for approximately 2 min, and cells were observed under a microscope. The cell suspension was collected and centrifuged at 1,000 rpm for 5 min. Cells were harvested and incubated in a culture plate for 30 min. The medium was replaced with fresh medium and non-adherent cells were removed. Purified microglias at a density of 4×104/cm2 were further cultured in the culture flask. One third of the medium was replaced every two to three d. Static cells were identified according to Tanaka’s definition [3]. That is, (1) cells have at least two processes extending from the cell body; the length of the processes is at least half the diameter of the cell body (usually 15-20 µm), where cell body diameter is the average of the long and short axes; (2) if cells have only two processes extending from the cell body, one of the processes must have a branch.
Flow cytometry
Coriaria lactone (CL) was diluted with PBS to different concentrations (10-3, 10-4, 10-5 or 10-6 mol/L) and used to stimulate microglia. Then 5×10-5 mol/L CL was chosen to stimulate microglia for different lengths of time (0, 1, 2, 4, 8 or 24 h). Indicators of microglial cell cycle phases and apoptosis were detected by flow cytometry. Purified microglias were cultured for 5-7 d until cell bodies were retracted and processes extended. At 1, 2, 4, 8, 12 and 24 hours after stimulation, cells were digested with 0.125% trypsin, centrifuged at 1,000 rpm for 5 min and washed twice with PBS. Cells were shaken and fixed with 75% alcohol (-20°C) at 4°C overnight. After centrifugation and two washes with PBS, cells were stained with propidium iodide in the dark for 30 min. Cell cycle phase and apoptotic status were determined with FACScalibur flow cytometry.
Real time fluorescence quantitative polymerase chain reaction (PCR)
In accordance with a previous study [7], real time fluorescence quantitative PCR was performed with SYBR Green I using an Mx3000PT detection system. The reaction solution (25 μl) consisted of 150 ng cDNA reverse transcribed from total RNA, 1 μl Taq DNA polymerase (1 unit/μl), 1X PCR reaction buffer, 3 mmol/L MgCl2, 2 mmol/L dNTPs, SYBR Green I (20X) 0.25 μl, 20 pmol/L cyclin D1 (or IL-1β or β-actin) upstream and downstream primers. Reaction conditions were as follows: pre-denaturation at 95°C for 5 min, denaturation at 95°C for 30 s, annealing at 62°C for 40 s, extension at 72°C for 30 s, 85°C for 8 s, for a total of 35-40 cycles. Fluorescence intensity was detected in each cycle at 85°C (cyclin D1 or β-actin) or 83°C (IL-1β). Primers were designed and synthesized by Beijing Ao Ke Co., Beijing, China (Table 1).
Table 1.
Primers and sequences for SYBR Green 1 fluorescent quantitative PCR
| Gene | Primer | Sequence (5’ to 3’) | Product size (bp) |
|---|---|---|---|
| cyclin D1 | Forward | CGCGTACCCTGACACCAATC | 293 |
| Reverse | TCTTAGAGGCCACGAACATGC | ||
| IL-1β | Forward | CTCCATGAGCTTTGTACAAGG | 245 |
| Reverse | TGCTGATGTACCAGTTGGGG | ||
| β-Actin | Forward | TCCTCCCTGGAGAAGAGCTA | 302 |
| Reverse | TCAGGAGGAGCAATGATCTTG |
Intraventricular injection with MCM, EEG recording and behavioral observations
Microglia-conditioned medium (MCM) was extracted in accordance with Kim’s method [8]. Purified microglias seeded on 24-well plates were incubated with DMEM containing 5×10-5 mol/L CL. At 0, 1, 2, 4, 8 and 24 h, microglias were washed three times with D-hanks. 400 μl of serum-free DMEM was added overnight. The supernatant was collected and centrifuged at 1,000 rpm for 5 min. MCM collected at various time points (designated MCM0, MCM2, MCM4, MCM8 or MCM24 according to the stimulation time with coriaria lactone) was stored at -20°C until further use.
Forty-two healthy adult male Sprague Dawley rats weighing 170-210 g were randomly assigned to six groups of equal size (MCM0, MCM2, MCM4, MCM8, MCM24 and control). The control group comprised rats treated with physiological saline. The rats were anesthetized via intraperitoneal injection and fixed on a stereotaxic apparatus in a prone position. A screw electrode was put in each side of the head (6.0 mm posterior to bregma, 4.3 mm lateral to the midlin), to record cortical potential, while a reference electrode was placed 7.0 mm anterior to the bregma, to transfer the signal to the RM6240 multi-channel physiological signal acquisition system. In each group, 4 μl of MCM was injected in the lateral ventricle, 0.8 mm posterior to the anterior fontanelle, 1.5 mm right of the median line, and 3.8 mm below the dura mater. Baseline EEG was observed for 10 min. After injection, EEG was monitored and recorded for 2 hours. Three rats from each group were selected for behavioral observation and EEG recording, and three for glutamate immunohistochemistry. Rat behaviors were classified according to Diehl’s levels [5]: 0, no reaction; I, beard moving, chewing; II, head and facial twitching; III, forelimb or hindlimb twitching; IV, rhythmic twitching of limbs; V, tonic convulsions of limbs, with swinging tail or rolling.
Immunofluorescence staining
At 45 min after injection, rats were rapidly decapitated. The brain was placed on ice, embedded with optimal cutting temperature solution, and placed in -70°C acetone for 1 minute. The brain tissue was then sliced into sections. The sections were fixed with 4°C acetone for 15 min, washed with PBS and blocked with PBS supplemented with 5% bovine serum albumin for 1 hour. The sections were then incubated with rabbit polyclonal anti-Glu antibody (1:200, Boster Biological Technology Co. Ltd) at 37°C for 1 hour and Cy2-labeled goat anti-rabbit IgG (1:1,000) at 37°C for 45 min. For negative controls, sections were incubated with PBS instead of primary antibody.
Image analysis and statistical analysis
Five sections from each rat were examined. Images were analyzed with Image pro plus 5.0 software. Fluorescence intensity was obtained by measuring the mean gray value of the images. Data were processed with the SPSS 19.0 software package. All data were expressed as the mean ± SD. Analysis of variance was used to calculate P values. P < 0.05 was considered statistically significant.
Results
Microglial culture
Most microglia were adherent after 15-30 min. These microglias were round (Figure 1A). After two d of culture, cell bodies were retracted. A few cell bodies extended processes, and most cells were unipolar (Figure 1B). Three to five d later, approximately half of the cells were altered from the active state to the stationary state. Cell bodies were elongated, and asymmetrical branches were visible (Figure 1C, 1D). Positive staining with CD11b/c antibody is shown in Figure 1E. Positive cells were of ≥98% purity. Immunocytochemical staining revealed that NF-κB p65 expression was present in the cytoplasm (Figure 1F).
Figure 1.
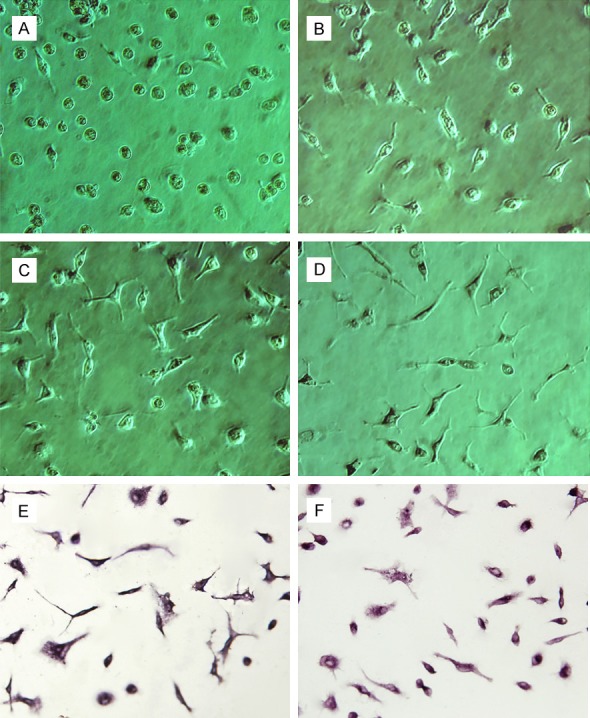
Purified cultures of microglia (phase contrast microscope, ×250). (A) Microglia were round and irregular at day 1; (B) A few microglia extended protuberances at day 2; (C, D) Cell bodies were elongated, and asymmetrical branches were visible at day 4 (C) and day 5 (D); (E) On day 5, microglia were positive for CD11b/c; (F) On day 5, microglia were positive for NF-кB p65 in the cytoplasm.
Activated microglia after stimulation with coriaria lactone
Cell cycle and apoptosis detected by flow cytometry
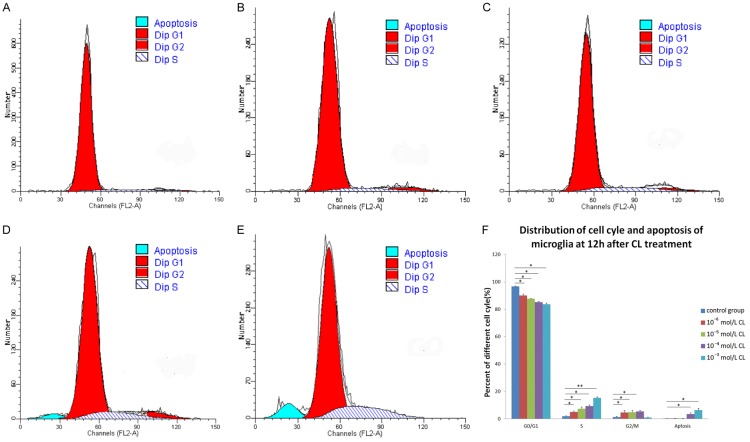
After 12 h of stimulation with coriaria lactone (CL), in vitro purified microglia in the different groups were mainly in the G0/G1 phase, and seldom in S or G2/M phases (Figure 2). Compared with the control group, the percentage of microglia in the stimulated groups in G0/G1 phase was significantly higher (P < 0.05), whereas the percentage of cells in S phase was significantly lower (P < 0.05); both of these effects were dose-dependent. The percentage of microglia in G2/M phases was significantly higher (P < 0.05) than control in each concentration stimulation group except for 10-3 mol/L CL. After stimulation with 10-4 mol/L and 10-3 mol/L CL, the percentage of apoptotic cells was higher than those of the control group and lower concentrations of CL treatment. S-phase arrest was visible during treatment with 10-3 mol/L CL (Figure 2).
Figure 2.
Microglial cell cycle phase and apoptosis 12 h after stimulation with coriaria lactone (CL) as detected by flow cytometry. (A) control group; (B) 10-6 mol/L CL group; (C) 10-5 mol/L CL group; (D) 10-4 mol/L CL group; (E) 10-3 mol/L CL group; (F) Distribution of microglial cell cycle phase and apoptosis in each group 12 h after stimulation with CL. Compared with the control group, the percentage of microglia in G0/G1 phase was significantly lower (P < 0.05), but the percentage of microglia in S phase was significantly higher (P < 0.05) in each concentration stimulation group. The percentage of microglia in G2/M phases was significantly higher (P < 0.05) in each concentration stimulation group except for the 10-3 mol/L CL group. The percentages of apoptotic cells in 10-4 mol/L and 10-3 mol/L CL groups were higher compared with that of the control group (P < 0.05).
Microglial cell cycle phase was detected by flow cytometry at different time points (0, 1, 2, 4, 8 and 24 h) after stimulation with 5×10-5 mol/L CL. Compared with the control group, the percentage of cells in G0/G1 phase was significantly lower in each group (P < 0.01), whereas the percentage of cells in S phase was significantly higher (P < 0.01), especially after 1 h of treatment. The percentage of cells in G2/M phase was significantly higher (P < 0.01) compared with that of the control group, especially after 2 h of treatment (Figure 3).
Figure 3.
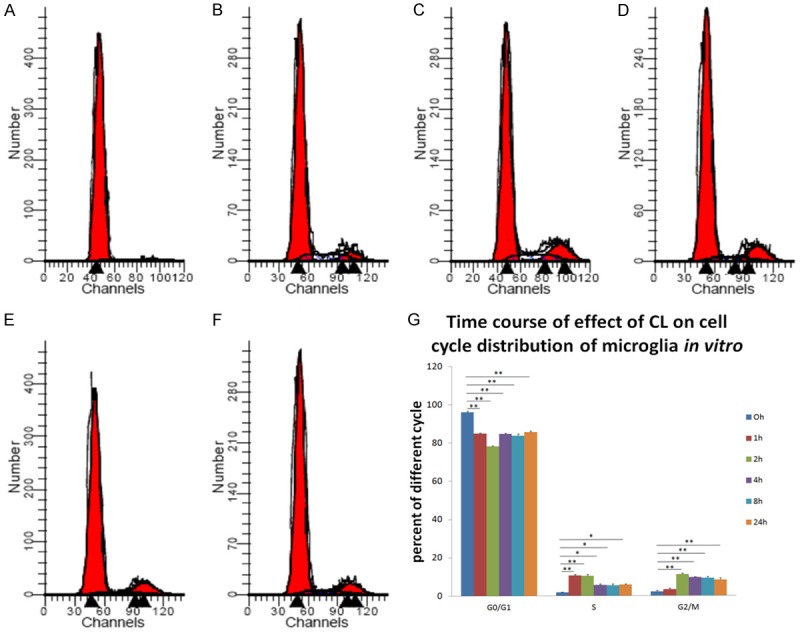
Effects of 5×10-5 mol/L coriaria lactone (CL) on cell cycle of microglia cultured in vitro at different time points as measured by flow cytometry. (A) 0 h (control); (B) 1 h; (C) 2 h; (D) 4 h; (E) 8 h; (F) 24 h; (G) Effects of 5×10-5 mol/L CL on cell cycle of microglia purified and cultured in vitro at different time points. Compared with the control group, the percentage of cells in G0/G1 phase was significantly lower in each group (P < 0.01), but the percentage of cells in S phase was significantly higher (P < 0.05), especially at 1 h of treatment. The percentage of cells in G2/M phase was significantly higher than that in the control group (P < 0.01), especially at 2 h of treatment.
Morphological observation under fluorescence microscope
After 1 h of CL stimulation (5×10-5 mol/L), the microglial cells showed stronger CD11b/c expression, with enlarged cell bodies. The morphological changes reached a peak after 2 h of culture. After 4 h of culture, most of the cells were round and phagocytic and rarely displayed branching. After 10-3 mol/L and 10-4 mol/L coriaria lactone stimulation, a small number of cells had irregular edges (Figure 4B).
Figure 4.
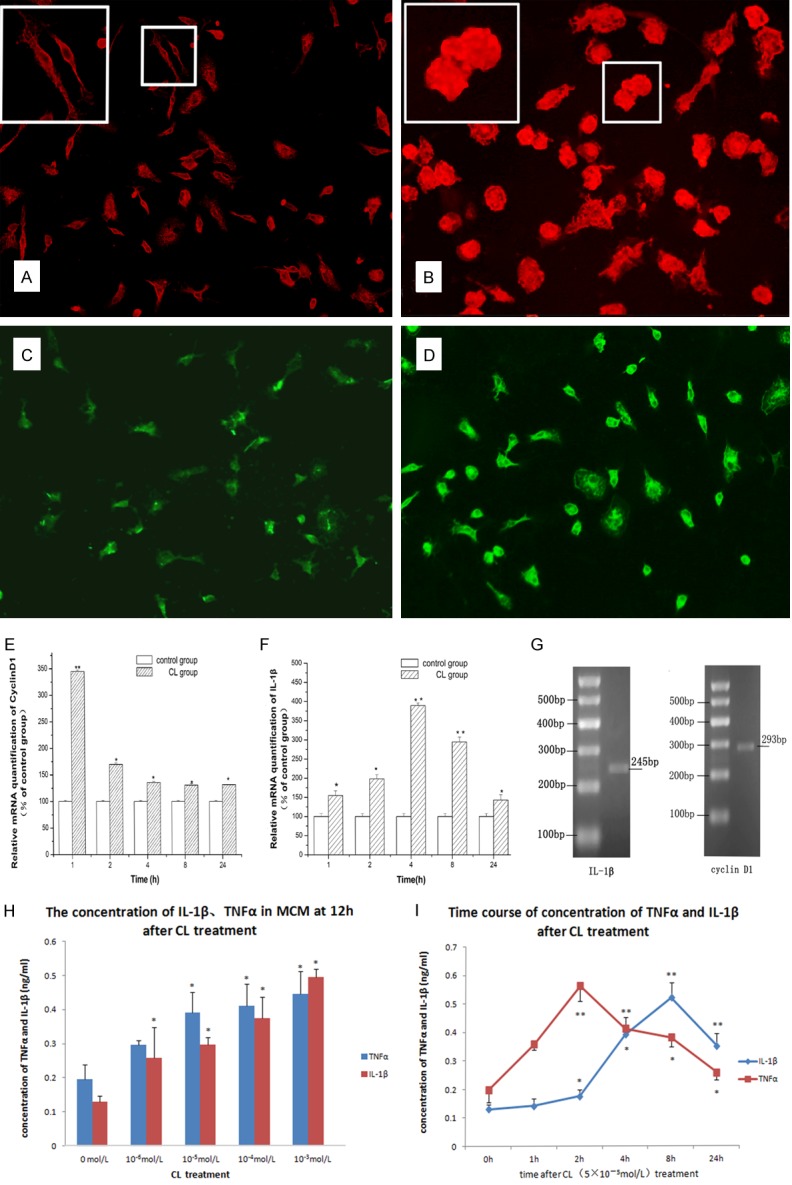
Activation of microglia by coriaria lactone (CL) (5×10-5 mol/L). A-D. Immunofluorescence staining revealed that CD11b/c and IL-1β expression levels in microglia cultured in vitro were significantly enhanced after treatment with 5×10-5 mol/L CL, compared with those of the control group. A. Expression of CD11b/c in microglia in control group, ×200; B. Expression of CD11b/c in microglia 2 d after CL (5×10-5 mol/L) treatment, ×400. C. IL-1β expression in microglia in control group, ×200; D. IL-1β expression in microglia 2 h after treatment with CL, ×200; E. Fluorescence quantitative PCR results demonstrated that cyclin D1 mRNA expression in microglia cultured in vitro was significantly higher compared with that of the control group, especially at 1 h after treatment with CL (P < 0.01). F. Fluorescence quantitative PCR results showed that IL-1β mRNA expression was significantly higher compared with that of the control group, especially at 4 h after treatment with CL (P < 0.01). G. Agarose gel electrophoresis revealed RT-PCR amplification products of cyclin D1 and IL-1β; H. Radioimmunoassay showed that TNF-α and IL-1β content in supernatant of culture medium of microglia increased after different concentrations of CL treatment at 12 h, and the effect was dose-dependent; I. TNF-α and IL-1β content in supernatant of culture medium of microglia increased at various time points after treatment with CL.
Effects of 5×10-5 mol/L coriaria lactone on cyclin D1, TNF-α and IL-1β expression in microglia
Immunofluorescence staining revealed that IL-1β expression in microglia cultured in vitro was significantly enhanced after treatment with 5×10-5 mol/L CL compared with that of the control group, especially after 2 h (Figure 4C, 4D). Fluorescence quantitative PCR results demonstrated that compared with the control group, cyclin D1 mRNA expression in microglia cultured in vitro was significantly higher at 1 h after treatment with CL (P < 0.01). From then on, cyclin D1 mRNA expression gradually declined. However, cyclin D1 mRNA expression was still higher than that of the control group at various time points (P < 0.05) (Figure 4E); IL-1β mRNA expression was significantly higher at 1 h (P < 0.05), peaked at 4 h (P < 0.01), then gradually declined, and was still higher than that of the control group at 24 h (Figure 4F).
Radioimmunoassay was used to measure TNF-α and IL-1β levels in supernatant. CL treatment resulted in release of TNF-α and IL-1β from microglia, and the effect was dose-dependent (Figure 4G). The increase in TNF-α levels appeared earlier, and became significant at 1 h after treatment with 5×10-5 mol/L CL (P < 0.05). TNF-α levels peaked at approximately 2 h (P < 0.05), and then gradually decreased, but were still high at 24 h (P < 0.05). IL-1β levels were increased 2 h after treatment with CL (P < 0.05). After this time, IL-1β levels rapidly increased and peaked at 8 h (P < 0.01). IL-1β levels were still significantly higher than in the control group at 24 h of treatment (P < 0.01; Figure 4I).
Intraventricular injection of MCM
EEG recording
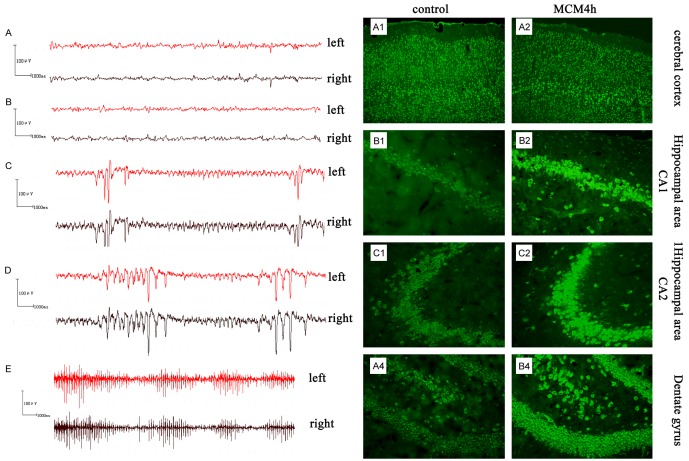
No obvious abnormal behavior was observed in the control group after injection, with all animals scoring 0 on the Diehl scale. Normal brain waves were observed in EEG (Figure 5A); these were mainly α waves (frequency 8-13 Hz). Cortical potential amplitude was 12.39 ± 5.09 μV. Except for the MCM2 and MCM4 groups, no abnormal behavior was observed in the MCM groups, with all animals scoring 0 on the Diehl scale. No significant difference in potential waveform or amplitude between the control group and MCM groups was detected (Figure 5B). In the MCM2 group, approximately 45 min after injection, beard shaking and forelimb twitching were seen for approximately 20 min, resulting in a score of III. Simultaneously, the EEG showed epileptiform waveforms, i.e., spike wave, sharp wave and spike-slow wave. The epileptiform wave amplitude was 62.17 ± 12.24 μV (Figure 5C). In the MCM4 group, 45 min after injection, beard shaking and forelimb twitching were observed, resulting in a score of III. Epileptiform wave amplitude was 60.96 ± 8.30 μV. The EEG revealed spike wave, sharp wave and spike-slow wave (Figure 5D). No further behavioral changes were observed. At 1 h after injection, the EEG showed enhanced amplitude (95.29 ± 17.52 μV), and mainly sharp waves were seen (Figure 5E). 1.5 h after injection, rat behavior and cortical potential returned to normal.
Figure 5.
EEG recording and glutamate (Glu) expression in brain tissue after intraventricular injection with microglia-conditioned medium (MCM). (A-E) EEG showed epileptiform waveforms after MCM injection. (A) EEG from the control group. (B-D) EEG at 45 min after intraventricular injection in the MCM0 (B), MCM2 (C) or MCM4 (D) group. No significant difference in potential waveform or amplitude between the control group and MCM0 groups was detected. Epileptiform waveforms (e.g., spike wave, sharp wave and spike-slow wave) were seen in the MCM2 and MCM4 groups. (E) EEG at 1 h after intraventricular injection in the MCM4 group displayed enhanced amplitude and sharp waves. (A, B) Expression of Glu in rat cerebral cortex and hippocampus, determined by immunofluorescence. Glutamate content in was increased to different degrees in all areas compared with the control group 45 min after injection with MCM4. (A1-4) control group; (B1-4) MCM4 group. (B1) Cerebral cortex, ×100; (B2) Hippocampal CA1 region, ×200; (B3) Hippocampal CA3 region, ×200; (B4) Dentate gyrus, ×200.
Glutamate content in brain tissue
Glutamate is mainly expressed in the cytoplasm in the cerebral cortex (mainly pyramidal layer) and hippocampus (CA1 and CA3). 45 min after injection of MCM, the glutamate content in all areas examined was higher (to different degrees) than that of the control group; no significant difference was observed between the MMC0 group and control group (Figure 5B; Table 2).
Table 2.
MGV of Glu immunofluorescence staining of rat cortex, hippocampus and dentate gyrus (45 min) (mean ± SD)
| Group | Cerebral cortex | CA1 | CA3 | Dentate Gyrus |
|---|---|---|---|---|
| Control | 34.23 ± 1.25 | 27.55 ± 2.37 | 26.32 ± 0.96 | 26.18 ± 2.58 |
| MMC0 | 35.25 ± 1.86 | 27.49 ± 0.38 | 28.56 ± 1.74 | 28.29 ± 2.13 |
| MMC1 | 44.15 ± 0.72* | 33.25 ± 1.67* | 32.58 ± 1.35* | 31.28 ± 1.47* |
| MMC2 | 49.33 ± 3.15* | 37.63 ± 2.18* | 39.22 ± 2.15** | 38.97 ± 1.95* |
| MMC4 | 57.26 ± 2.53** | 44.65 ± 1.14** | 47.73 ± 1.97** | 47.66 ± 2.36** |
| MMC8 | 42.36 ± 1.36* | 35.21 ± 1.49* | 31.56 ± 1.56* | 34.26 ± 1.21* |
| MMC24 | 41.39 ± 2.33* | 34.41 ± 2.37* | 32.38 ± 2.63* | 31.38 ± 1.67* |
P < 0.05, compared with control;
P < 0.01, compared with control.
Discussion
Microglias are one of the three major glial cell types in the central nervous system, accounting for 10-15% of all cells found within the brain. Recent studies have shown that microglia, as immunocompetent cells and macrophage cells in the central nervous system, play a very important role in diseases of the central nervous system [9]. Microglial activation is a common pathological phenomenon in central nervous system diseases. The effect of activated microglia is still controversial. On one hand, activated microglias are important for defense and removal of external pathogenic factors in the central nervous system; activated microglias are actively involved in the repair of damage. On the other hand, activated microglia and microglia-mediated inflammatory reactions promote the occurrence and persistence of various diseases [10-12].
Approximately 50 million people have epilepsy, making it the most common chronic and severe neurological disease worldwide, with increased risk of mortality and psychological and socioeconomic consequences impairing quality of life. More than 30% of patients with epilepsy have inadequate control of their seizures with drug therapy. However, the progression of seizure activity and the development of drug resistance remain difficult to predict, irrespective of the underlying epileptogenic condition [13]. Previous studies of epilepsy have focused on neurons and astrocytes, while paying less attention to other important components of the central nervous system, such as microglia. Najjar et al. hypothesized that microglial activation and proliferation initiate a cycle of inflammation-induced seizures and seizure-induced inflammation [14]. Moreover, microglia-driven epilepsy may be the primary pathogenic process in a small number of cases, as suggested by the pathology and therapeutic response in patients, but may contribute to epileptogenesis in many more [15].
Effect of coriaria lactone on activating microglia cultured in vitro
The currently available animal models of epilepsy are most often induced by chemical substances, e.g., kainic acid and pilocarpine, or electrical methods [16,17]. Coriaria lactone (CL) consists of the active components of the plant Loranthus that grows on Coriaria sinica Maxim, a medicinal herb. CL is the main active ingredient from Coriaria sinica, and when used in the treatment of schizophrenia it can eliminate mental symptoms, hallucinations and delusions, and control excitement, and increase activity in patients with less movement [18]. When CL was used as a folk remedy for schizophrenia treatment in China, epileptiform seizures were observed in humans [19]. This phenomenon suggested that CL could be a convulsive agent in primates, with the advantage that a CL-induced animal model should be relevant to human disease, given that it has been shown to induce epileptiform seizures in humans [20]. An animal study confirmed that CL induces convulsions and epilepsy [21]. Therefore, we used CL to activate microglia in this study.
Cyclin D1 is a key protein in the transition of cells from G1 to S phase. The cyclin D1- cdk4/6 complex is activated by cdk-activating kinase, and promotes passage through the restriction point and transit into S phase [22]. Our results demonstrate that cyclin D1 synthesis and expression is increased in microglia treated with CL. Microglia switched from the quiescent state to the active state after treatment with CL, characterized by branch retraction and increased cell body size. Furthermore, the percentage of microglia in S and G2/M phases was markedly increased in each group after treatment with CL. These findings indicate that CL affects the distribution of microglia cell cycle by promoting the transition from G0/G1 phase to S phase, and ultimately contributes to the activation and proliferation of microglia.
TNF-α and IL-1β secretion in activated microglia
Microglial activation is accompanied by changes in the secretion of various factors, including cytokines, reactive oxygen species and nitric oxide. A variety of substances act on their own or other cell receptors of the central nervous system by autocrine and paracrine mechanisms, and form cascade networks [23]. TNF-α and IL-1β, which are important pro-inflammatory cytokines, play an important role in regulating the secretion of bioactive substances, and are important upstream promoters of cascade networks, often being located at or near the top of a cascade network [24,25]. Human and animal data have identified a pivotal role for IL-1β and the persistent brain inflammation triggered by status epilepticus in the mechanisms of epileptogenesis [26]. The long-term increase in seizure susceptibility produced by inflammation in the immature brain may also be produced by TNF-α-dependent mechanisms, independent of IL-1β [27]. Somera-Molina et al. showed that a ‘second-hit’ in adulthood seizure model produces greater cytokine release, microglial activation and recruitment, leading to increased susceptibility to seizures, enhanced neuronal injury and neurobehavioral impairment [27]. As a result, excessive microglial activation and the associated overproduction of proinflammatory cytokines have been suggested to play a pivotal role in mechanisms of epileptogenesis and post-seizure injury in both experimental animal models and human epilepsy [28]. In this study, we found that CL increased TNF-α and IL-1β levels, indicating that CL activated microglia and promoted the secretion of bioactive substances.
Activated MCM induces the onset of epilepsy
Epilepsy is accompanied by microglial activation [29,30]. Our results demonstrate that CL induces microglial proliferation and activation, indicating that microglial activation actively participates in the epileptogenic effect of CL. In this experiment, MCM from different cell activation states was injected into the ventricles of the brain to observe the effects on epileptic seizure. The results showed that: (1) activated MCM has an epileptogenic effect. Supersynchronous discharge of neurons is a prominent feature of epilepsy [31]. In normal brain, the discharge of single neurons is spontaneous. During epileptic seizures, epileptiform discharges are observed in some neurons, and their membrane currents form an electric field. The excitability and discharge rhythm of neighboring cells are altered by electrotonus, resulting in supersynchronous discharge [8,32]. Cortical potential and behavior are the main indicators used to evaluate epileptogenic effects in experimental models and to diagnose epilepsy in the clinic [33]. This study verified that MCM has remarkable epileptogenic effects 2 and 4 h after stimulation with CL, using behavioral observations and cortical potential monitoring. (2) The epileptogenic effect of MCM is associated with cell activation levels. The results demonstrated that cell activation levels were significantly higher at 2 and 4 h post-stimulation than other time points; i.e., cell activation peaked at 2 and 4 h of treatment with CL.
It is well established that metabolism plays an important role in neuronal network stability and that metabolic dysfunction is a strong influence in certain epilepsy syndromes. Glycolysis feeds into the citric acid cycle to ultimately produce glutamate and other important neurotransmitters. Glutamate is not only the main excitatory neurotransmitter in the brain, but also a potential neurotoxin leading to neuronal cell death [34]. To maintain the sensitivity of synaptic transmission, glutamate must be removed promptly. In the brain, uptake by astrocytes is the most important route to maintain the extracellular glutamate concentration. Alterations in the way neurons metabolize glucose and harvest this energy could result in the large changes in gene expression that lead to the sudden, spontaneous electrical firings seen with epileptic seizures. The absolute or relative increase of excitatory transmitter in the internal environment is the main mechanism responsible for epilepsy [35]. Meidenbauer et al. demonstrated that reduced glucose utilization is necessary to confer seizure protection under long-term calorie restriction in EL mice [36]. Balosso et al. showed that inflammatory mediators including TNF-α modulate glutamatergic excitatory neurotransmission, and suggest that glutamate interactions may play a role in pathological conditions (e.g., seizures, neurodegeneration) characterized by the activation of both systems [37]. In the present study, glutamate content was enhanced in cerebral cortex and hippocampus, suggesting that MCM including TNF-α could increase glutamate content through regulating the activity of astrocytes [16], which may be associated with the epileptogenic mechanism of activated microglia.
Conclusion
We have shown that coriaria lactone (an epileptogenic agent) causes microglial activation including changes in cell shape and proliferation, and that activated microglia can secrete various cytokines (e.g., TNF-α and IL-1β). In addition, microglia-conditioned medium can induce the onset of epilepsy in rats in vivo, and its mechanism may be associated with upregulation of glutamate expression. Therefore, the activation of microglia may be an important process for the onset of epileptic seizures. It is important to note that in addition to release of cytokines, activated microglia can exert effects through migration, contact, superoxide anion and nitric oxide in an in vivo environment.
Acknowledgements
This work was supported by a grant from the research fund of the Tongji Medical College, Huazhong University of Science and Technology (NO 0118519028) and the Key Project of National Natural Science Foundation of China (NO 30230140).
Disclosure of conflict of interest
None.
References
- 1.Eyo UB, Murugan M, Wu LJ. Microglia-neuron communication in epilepsy. Glia. 2017;65:5–18. doi: 10.1002/glia.23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suleiman J, Dale RC. The recognition and treatment of autoimmune epilepsy in children. Dev Med Child Neurol. 2015;57:431–440. doi: 10.1111/dmcn.12647. [DOI] [PubMed] [Google Scholar]
- 3.Beggs S, Salter MW. Snapshot: microglia in disease. Cell. 2016;165:1294–1294. doi: 10.1016/j.cell.2016.05.036. [DOI] [PubMed] [Google Scholar]
- 4.Lui H, Zhang J, Makinson SR, Cahill MK, Kelley KW, Huang HY, Shang Y, Oldham MC, Martens LH, Gao F, Coppola G, Sloan SA, Hsieh CL, Kim CC, Bigio EH, Weintraub S, Mesulam MM, Rademakers R, Mackenzie IR, Seeley WW, Karydas A, Miller BL, Borroni B, Ghidoni R, Farese RV Jr, Paz JT, Barres BA, Huang EJ. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell. 2016;165:921–935. doi: 10.1016/j.cell.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 6.McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia H, Chen Y, Wu KJ, Zhao H, Xiong CL, Huang DH. Role of C-type natriuretic peptide in the function of normal human sperm. Asian J Androl. 2016;18:80–84. doi: 10.4103/1008-682X.150254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YJ, Hwang SY, Oh ES, Oh S, Han IO. IL-1beta, an immediate early protein secreted by activated microglia, induces iNOS/NO in C6 astrocytoma cells through p38 MAPK and NF-kappaB pathways. J Neurosci Res. 2006;84:1037–1046. doi: 10.1002/jnr.21011. [DOI] [PubMed] [Google Scholar]
- 9.Fu R, Shen Q, Xu P, Luo JJ, Tang Y. Phagocytosis of microglia in the central nervous system diseases. Mol Neurobiol. 2014;49:1422–1434. doi: 10.1007/s12035-013-8620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stirling DP, Cummins K, Mishra M, Teo W, Yong VW, Stys P. Toll-like receptor 2-mediated alternative activation of microglia is protective after spinal cord injury. Brain. 2014;137:707–723. doi: 10.1093/brain/awt341. [DOI] [PubMed] [Google Scholar]
- 11.Wang HL, Liu H, Xue ZG, Liao QW, Fang H. Minocycline attenuates post-operative cognitive impairment in aged mice by inhibiting microglia activation. J Cell Mol Med. 2016;20:1632–1639. doi: 10.1111/jcmm.12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma AK, Ghosh S, Pradhan S, Basu A. Microglial activation induces neuronal death in Chandipura virus infection. Sci Rep. 2016;6:22544. doi: 10.1038/srep22544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobow K, Blümcke I. Epigenetics in epilepsy. Neurosci Lett. 2018;667:40–46. doi: 10.1016/j.neulet.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Najjar S, Pearlman D, Miller DC, Devinsky O. Refractory epilepsy associated with microglial activation. Neurologist. 2011;17:249–254. doi: 10.1097/NRL.0b013e31822aad04. [DOI] [PubMed] [Google Scholar]
- 15.Sakurai M, Morita T, Takeuchi T, Shimada A. Relationship of angiogenesis and microglial activation to seizure-induced neuronal death in the cerebral cortex of shetland sheepdogs with familial epilepsy. Am J Vet Res. 2013;74:763–770. doi: 10.2460/ajvr.74.5.763. [DOI] [PubMed] [Google Scholar]
- 16.Balosso S, Ravizza T, Aronica E, Vezzani A. The dual role of TNF-α and its receptors in seizures. Exp Neurol. 2013;247:267–271. doi: 10.1016/j.expneurol.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Buckmaster PS. Laboratory animal models of temporal lobe epilepsy. Comp Med. 2004;54:473–485. [PubMed] [Google Scholar]
- 18.Hong Z, Yang TH, Tang MH, Zhang H, Li HX, Chen L, Chen Q, Zhou D. A novel kindling model of temporal lobe epilepsy in rhesus monkeys induced by coriaria lactone. Epilepsy Behav. 2013;29:457–465. doi: 10.1016/j.yebeh.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 19.Stables JP, Bertram EH, White HS, Coulter DA, Dichter MA, Jacobs MP, Loscher W, Lowenstein DH, Moshe SL, Noebels JL, Davis M. Models for epilepsy and epileptogenesis: report from the NIH workshop, Bethesda, Maryland. Epilepsia. 2002;43:1410–1420. doi: 10.1046/j.1528-1157.2002.06702.x. [DOI] [PubMed] [Google Scholar]
- 20.Hu GC. Anticonvulsants used for controlling induced seizures during the treatment of schizophrenia with lactoni Coriariae. Zhong Xi Yi Jie He Za Zhi. 1984;4:675–578. [PubMed] [Google Scholar]
- 21.Cheng L, Lei S, Chen SH, Hong Z, Yang TH, Li L, Chen F, Li HX, Zhou D, Li JM. Pretreatment with intravenous levetiracetam in the rhesus monkey Coriaria lactone-induced status epilepticus model. J Neurol Sci. 2015;348:111–120. doi: 10.1016/j.jns.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 22.Shimura T, Fukumoto M, Kunugita N. The role of cyclin D1 in response to long-term exposure to ionizing radiation. Cell Cycle. 2013;12:2738–2743. doi: 10.4161/cc.25746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chuang DY, Simonyi A, Kotzbauer PT, Gu Z, Sun GY. Cytosolic phospholipase A2 plays a crucial role in ROS/NO signaling during microglial activation through the lipoxygenase pathway. J Neuroinflammation. 2015;12:199. doi: 10.1186/s12974-015-0419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aguilar Del Rey FJ, García Portales R, Haro Liger M, Rodríguez Andreu J, Casals Sánchez JL, Pérez González R. Effect of tumour necrosis factor α blockade on bone metabolism in chronic inflammatory joint diseases. Med Clin (Barc) 2016;147:56–62. doi: 10.1016/j.medcli.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 25.Gren ST, Janciauskiene S, Sandeep S, Jonigk D, Kvist PH, Gerwien JG, Håkansson K, Grip O. The protease inhibitor cystatin C down-regulates the release of IL-β and TNF-α in lipopolysaccharide activated monocytes. J Leukoc Biol. 2016;100:811–822. doi: 10.1189/jlb.5A0415-174R. [DOI] [PubMed] [Google Scholar]
- 26.Zheng H, Zhu W, Zhao H, Wang X, Wang W, Li Z. Kainic acid-activated microglia mediate increased excitability of rat hippocampal neurons in vitro and in vivo: crucial role of interleukin-1beta. Neuroimmunomodulation. 2010;17:31–38. doi: 10.1159/000243083. [DOI] [PubMed] [Google Scholar]
- 27.Somera-Molina KC, Nair S, Van Eldik LJ, Watterson DM, Wainwright MS. Enhanced microglial activation and proinflammatory cytokine upregulation are linked to increased susceptibility to seizures and neurologic injury in a ‘two-hit’ seizure model. Brain Res. 2009;1282:162–172. doi: 10.1016/j.brainres.2009.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravizza T, Gagliardi B, Noé F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008;29:142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Galic MA, Riazi K, Heida JG, Mouihate A, Fournier NM, Spencer SJ, Kalynchuk LE, Teskey GC, Pittman QJ. Postnatal inflammation increases seizure susceptibility in adult rats. J Neurosci. 2008;28:6904–6913. doi: 10.1523/JNEUROSCI.1901-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gui WS, Wei X, Mai CL, Murugan M, Wu LJ, Xin WJ, Zhou LJ, Liu XG. Interleukin-1β overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents. Mol Pain. 2016:12. doi: 10.1177/1744806916646784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SK, Kim JE, Kim YJ, Kim MJ, Kang TC. Hyperforin attenuates microglia activation and inhibits p65-Ser276 NFκB phosphorylation in the rat piriform cortex following status epilepticus. Neurosci Res. 2014;85:39–50. doi: 10.1016/j.neures.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Edakawa K, Yanagisawa T, Kishima H, Fukuma R, Oshino S, Khoo HM, Kobayashi M, Tanaka M, Yoshimine T. Detection of epileptic seizures using phase-amplitude coupling in intracranial electroencephalography. Sci Rep. 2016;6:25422. doi: 10.1038/srep25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouma HK, Labos C, Gore GC, Wolfson C, Keezer MR. The diagnostic accuracy of routine electroencephalography after a first unprovoked seizure. Eur J Neurol. 2016;23:455–463. doi: 10.1111/ene.12739. [DOI] [PubMed] [Google Scholar]
- 34.Citraro R, Leo A, De Fazio P, De Sarro G, Russo E. Antidepressants but not antipsychotics have antiepileptogenic effects with limited effects on comorbid depressive-like behaviour in the WAG/Rij rat model of absence epilepsy. Br J Pharmacol. 2015;172:3177–3188. doi: 10.1111/bph.13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vishnoi S, Raisuddin S, Parvez S. Glutamate excitotoxicity and oxidative stress in epilepsy: modulatory role of melatonin. J Environ Pathol Toxicol Oncol. 2016;35:365–374. doi: 10.1615/JEnvironPatholToxicolOncol.2016016399. [DOI] [PubMed] [Google Scholar]
- 36.Sen S, Keough K, Gibson J. Clinical reasoning: novel GLUT1-DS mutation: refractory seizures and ataxia. Neurology. 2015;84:e111–e114. doi: 10.1212/WNL.0000000000001467. [DOI] [PubMed] [Google Scholar]
- 37.Balosso S, Ravizza T, Pierucci M, Calcagno E, Invernizzi R, Di Giovanni G, Esposito E, Vezzani A. Molecular and functional interactions between tumor necrosis factor-alpha receptors and the glutamatergic system in the mouse hippocampus: implications for seizure susceptibility. Neuroscience. 2009;161:293–300. doi: 10.1016/j.neuroscience.2009.03.005. [DOI] [PubMed] [Google Scholar]