Abstract
We investigated the effect of eplerenone on myocardial infarcted diabetic rats via modulation of the PI3K/Akt pathway and its downstream target GSK-3β. Diabetes was induced by administration of a single dose of streptozotocin (55 mg/kg IP). Diabetic rats received either eplerenone or PI3k/Akt antagonist (wortmannin) or in combination for 14 days with concurrent administration of isoproterenol (100 mg/kg s.c) on 13th and 14th day. Isoproterenol prompted cardiotoxicity and was demonstrated by a decrease in the maximal positive rate of developed left ventricular pressure, the maximal negative rate of developed left ventricular pressure and an increase in left ventricular end-diastolic pressure along with oxidative stress. Myocardial infarcted diabetic rats exhibited increased myonecrosis, edema, and apoptotic cell death. Treatment with eplerenone significantly improved the redox status of the myocardium. Eplerenone markedly inhibited Bax expression, TUNEL-positive cells, and myonecrosis. On the other hand, the administration of eplerenone and wortmanin did not draw out the same effects, when administered concomitantly or individually. Moreover, the rats treated with eplerenone showed increased expression of PI3K/Akt and decreased its downstream target GSK-3β. The present study confirms the protective effects of eplerenone on myocardial infarction in diabetic rats via modulation of PI3K/Akt pathway and its downstream regulator GSK-3β.
Keywords: STZ, ISO, PI3K/Akt/GSK-3β, eplerenone, diabetes
Introduction
Myocardial infarction is extensively built up in patients with diabetes mellitus, foremost to augmented mortality and morbidity levels. Oxidative stress and cardiac apoptosis have been identified as root causes of diabetes-induced cardiovascular complications [1,2]. Whereas, Myocardial infarction in current scenario generates billions of dollars in healthcare costs globally and leads to fear-provoking round about all the countries [3]. It has been well proven that increased in the generation of superoxide anions and reactive oxygen species is in-line with diabetic complications arising in humans as well as in animals [4,5]. On the whole, the treatments accessible for ischemic injury, including myocardial infarction are focussed toward reinstatement of blood supply to ischemic tissue and preventing the damage inflicted at the injury [6].
Isoproterenol, a synthetic non-selective β-adrenoceptor agonist, has been recognized to provoke myocardial infarction in rats as a result of distressed physiological equilibrium between production of free radicals and antioxidative defense system [7]. The pathophysiological and morphological changes coupled with isoproterenol in rats are analogous to those observed for human myocardial infarction [8].
Recently, much progress has been made in elucidating the signal transduction pathways involve in the cardioprotection in relation to convey the extracellular signal initiated by the stimuli to the intracellular targets of cardioprotection, with many of these pathways involving the activation of a diverse array of survival protein kinase cascades. Among the known cell survival pathways, the PI-3-kinase (phosphatidylinositol 3-kinase)/Akt pathways are of considerable importance [9]. Activation of PI3K/Akt pathway prevents ROS-induced apoptosis in human umbilical vein endothelial cells [10]. In relation to the PI3K/Akt pathway, the glycogen synthase kinase-3β (GSK-3β) has been revealed to promote apoptosis and turn on apoptosis-related protein kinase, caspases proteins, and Bax gene responsible for apoptosis, led to produces apoptosis [11]. It has been already reported that GSK-3β is regulated by PI3K/Akt signal transduction pathway [12].
Eplerenone, a selective aldosterone antagonist, is a renowned remedy used to treat heart failure and related complications [13]. Though, the soundness of its beneficial effect in diabetic cardiac complications has not been explored yet. Pre-clinical evidences put forward that aldosterone can have an undesirable effect on the myocardium. Previous reports suggests that blockade of aldosterone shown to have reduced oxidative stress in atherosclerosis [14]. Moreover, recent studies revealed that eplerenone activates the PI3K/Akt signalling pathway [15]. Owing to this, activation of Akt leads to downstream the GSK-3β. Hence, in view of this fact, the present study was designed to explore, the effect of eplerenone via modulation of PI3K-Akt and phosphorylation of its downstream target GSK-3β pathway to affect cardiac function, cardiac injury markers, endogenous antioxidants, and tissue architecture in diabetic rats.
Materials and methods
Animals
The Male Wistar rats, (200-250 g) were kept at standard laboratory conditions under natural light and dark cycles, humidity (60±10%) and a constant room temperature (25±5°C). The study protocol was approved by the Institutional Animal Ethics Committee (Approval No. IAEC/CPCSEA/RCPIPER/2014-17) of R. C. Patel Institute of Pharmaceutical Education and Research, Shirpur, India and conforms to the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA, Reg. No. 651/02/c/CPCSEA).
Chemicals
Eplerenone was obtained as a gift sample from Glenmark laboratories, Nashik, India. Isoproterenol was Purchased from Sigma Aldrich, USA. The ABC staining kit and primary antibodies like Bax mouse monoclonal IgG2b, Bcl-2 mouse monoclonal IgGI, PI3K (SC-1637, LotK1910), AKT (SC-81434, Lot J3014) and GSK3 β (SC-81462 Lot-10511) were procured from Santa Cruz Biotechnology, USA.
Experimental protocol
Induction of diabetes
Diabetes was induced by a single intraperitoneal injection of streptozotocin [16] (STZ) (55 mg/kg body weight) dissolved in 0.1 M cold citrate buffer (pH 4.5). Diabetes was confirmed 3 days of post-injection of STZ by estimating the serum glucose level. Rats with blood glucose level higher than 250 mg/dl were considered diabetic and included in the study protocol.
Induction of myocardial infarction
On the days 13th and 14th of study protocol, isoproterenol (ISO) (100 mg/kg) was injected subcutaneously to rats with 24 h of interval to induce experimental myocardial infarction.
Experimental design
After confirming the onset of diabetes using serum glucose level, rats were randomly divided into five different groups (n=14).
Group I diabetic control
Rats were treated with distilled water (1 ml/kg/day p.o.) for 14 days and on the day 13th and 14th they were treated with 0.3 ml of saline at the interval of 24 h.
Group II diabetic isoproterenol
Rats were administered water orally for 14 days along with concurrent administration of ISO (100 mg/kg, s.c.at the interval of 24 h) on the day 13th and 14th.
Group III diabetic isoproterenol rats treated with eplerenone
Animals were treated with eplerenone (150 mg/kg/day) orally for a period of 14 days along with concurrent administration of ISO on the day 13th and 14th.
Group V diabetic isoproterenol rats treated with wortmanin
Animals were treated with wortmanin (1 mg/kg/day, i.p.) for a period of 14 days with concurrent administration of ISO on days 13th and 14th at the interval of 24 h.
Group IV diabetic isoproterenol rats treated with wortmanin and eplerenone
Animals were treated with wortmanin 15 min prior to administration of eplerenone for 14 days with concurrent administration of ISO on days 13th and 14th at the interval of 24 h.
The experimental animals were examined at regular intervals throughout the course of the study and any changes in body weight and/or food and water intake as well as mortality rate were recorded.
Evaluation of parameters
Surgical procedures for recording hemodynamic parameters
In brief, rats were anesthetized with an intraperitoneal injection containing pentobarbitone sodium (60 mg/kg) and atropine (0.1 mg/kg) to lessen the Bronchotracheal discharge as well as to sustain the heart rate, mainly throughout the phase of surgery in all experimental groups. The surgery for measurement of hemodynamic parameters and left ventricular assessment were performed according to the method of Reddy et al., (2015) [8].
Estimation of cardiac injury markers and oxidative stress
A 10% homogenate was used to estimate the amount of creatine kinase on the myocardial bundle (CK-MB) and Lactate dehydrogenase (LDH) [17]. The oxidative stress was estimated by analyzing the content of malondialdehyde (MDA) [18], reduced glutathione (GSH) [19], Catalase [19] and activity of superoxide dismutase (SOD) [7].
Determination of myocardial apoptosis
Immunohistostaining for the tracing of Bax and Bcl-2 proteins
Immunohistostaining for the identification of Bax and Bcl-2 proteins as an apoptotic markers was performed as previously described by Rani et al., (2016) [20].
TUNEL assay
TUNEL assay was performed using a cell death detection kit (Bio Vision, Inc, USA) as described by Kocak et al., (2016) [21] and according to the manufacturer’s instructions.
Western blot analysis
Myocardial tissue was homogenized in RIPA lysis buffer and protein was estimated. Western blot analysis was performed as described previously [22].
Light microscopic evaluation
Myocardial tissues were fixed in buffered formalin solution and embedded in paraffin. Serial sections (3 mm thick) were cut using microtome. Every section was stained with haematoxylin and eosin (H&E). Sections were examined under the light microscope (Nikon, Tokyo, Japan), and photographs were taken. The pathologist performing microscopy was blind to the treatment standing of the experimental subject.
Statistical analysis
The data are expressed as mean ± SEM, statistical significances were analyzed by one-way ANOVA (Analysis of variance) followed by Bonferroni’s post hoc test using a graph pad, prism software, version 6.0, USA. The value of P<0.05 was considered as a significant.
Results
Mortality
An on the whole mortality of 5% was observed throughout the study protocol. The animals were lost because of stern increase in diabetes, bleeding during surgery for recording of hemodynamic parameters.
General observations
Administration of STZ in animals increases blood glucose in entire experimental groups as compared to their basal readings. However, body weights, heart weight and heart to body weight ratio does not show any significant change in dissimilar experimental groups (Table 1).
Table 1.
Effect of eplerenone on changes in heart weight, blood glucose, body weight and heart weight to body weight ration
| Treatment groups (All diabetic) | Heart weight (g) | Blood glucose (mg/dl) | BW (g) | HW/BW ratio (mg/g) |
|---|---|---|---|---|
| Control | 0.69±0.04 | 490.8±53.6 | 245±3.9 | 2.816±0.0102 |
| ISO | 0.54±0.06# | 506.5±50.6## | 220±3.4# | 2.454±0.0176 |
| EPL | 0.61±0.07* | 490.5±86.7* | 244±2.7* | 2.4796±0.025 |
| Wort | 0.45±0.09 | 524.3±77.1 | 208±3.6 | 2.163±0.0241 |
| Wort + EPL | 0.58±0.04$ | 505.5±75.46 | 226±4.1 | 2.566±0.0251 |
Data were expressed as the mean ± S. E. M. Significance was determined by one-way ANOVA (Analysis of variance) followed by the Bonferroni’s post hoc test:
P<0.05 as compared to diabetic ISO;
P<0.05 as compared to diabetic control;
P<0.01 as compared to diabetic control;
P<0.001 as compared to eplerenone.
(BW: body weight, HW: heart weight. All groups were diabetic ISO: Isoproterenol; EPL: Eplerenone; Wort: wortmannin; EPL + Wort: Eplerenone + wortmanin).
Eplerenone normalised ECG waveforms in STZ-isoproterenol challenged rats
Diabetic rats treated with ISO showed alterations in ECG waveforms as a significant elevation (P<0.01) of the ST segment was observed as compared to diabetic control. While treatment with eplerenone at the dose of 150 mg/kg showed normal wave formations in the ECG and significantly normalised the ST-segment as compared with diabetic ISO treated rats (Figure 1). The diabetic rats treated with wortmanin showed significant increase in the ST segment as well as deformations in the ECG wave formations as compared with diabetic isoproterenol treated rats (Figure 1).
Figure 1.
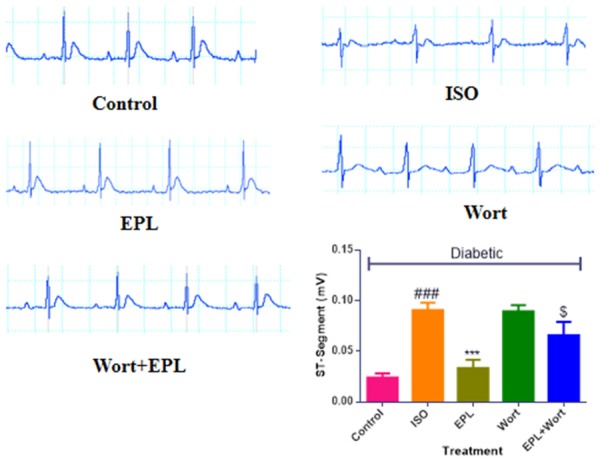
Effect of EPL on cardiac function and ECG wave forms in STZ-isoproterenol challenged rats. All groups are diabetic, where ISO: Isoproterenol; EPL: Eplerenone; Wort: wortmannin; Wort + EPL: wortmanin + Eplerenone. Data were expressed as the mean ± S.E.M. Significance was determined by one-way ANOVA (Analysis of variance) followed by the Bonferroni’s post hoc test: *P<0.05, **P<0.01 as compared to Diabetic ISO; ###P<0.001 as compared to diabetic control; $P<0.001 as compared to eplerenone.
Eplerenone improved hemodynamic and ventricular dysfunction in STZ-isoproterenol challenged rats
Diabetic ISO treated rats showed a significant decrease (P<0.001) in SAP, DAP and MAP as well as maximal positive and a negative rate of left ventricular pressure (±LVdP/dtmax), and increase in left ventricular end-diastolic pressure (LVEDP) as compared to the diabetic control rats. Treatment of eplerenone for fourteen days normalised hemodynamic parameters and left ventricular assessment functions with respect to the diabetic ISO rats (Figures 2 and 3). Administration of wortmanin to diabetic myocardial infarcted rats does not attenuated the SAP, DAP and MAP, confirms the antagonistic activity of PI3K.
Figure 2.
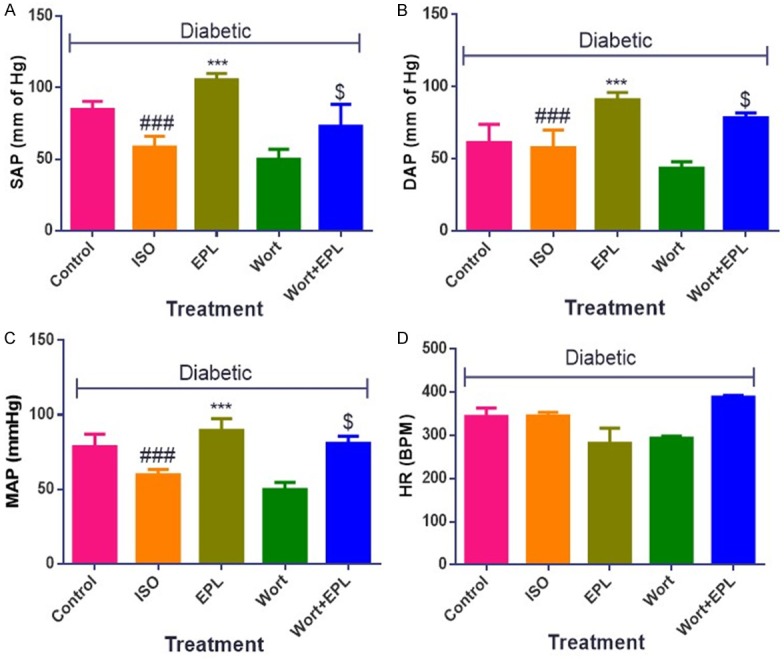
Effects of EPL on hemodynamic parameters in STZ-isoproterenol challenged rats. A. Systolic arterial pressure (SAP). B. Diastolic arterial pressure (DAP). C. Mean arterial pressure (MAP). D. Heart Rate. Data were expressed as the mean ± S.E.M. Significance was determined by one-way ANOVA (Analysis of variance) followed by the Bonferroni’s post hoc test: ***P<0.001 as compared to Diabetic ISO; ###P<0.001 as compared to diabetic control; $P<0.001 as compared to eplerenone. (All the animals from groups were diabetic, where ISO: Isoproterenol; EPL: Eplerenone; Wort: wortmannin; Wort + EPL: wortmanin + Eplerenone).
Figure 3.
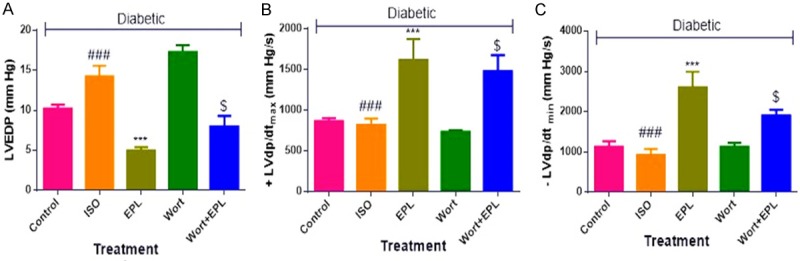
Effects of EPL on peak positive and negative pressure development in STZ-isoproterenol challenged rats. A. Left ventricular end diastolic pressure (LVEDP). B. Maximal positive rate of left ventricular pressure (+LVdP/dtmax). C. Maximal negative rate of left ventricular pressure (-LVdP/dtmin). Data were expressed as the mean ± S.E.M. Significance was determined by one-way ANOVA (Analysis of variance) followed by the Bonferroni’s post hoc test: ***P<0.001 as compared to Diabetic ISO; ###p<0.001 as compared to diabetic control; $P<0.001 as compared to eplerenone. (All the animals from groups were diabetic, where ISO: Isoproterenol; EPL: Eplerenone; Wort: wortmannin; Wort + EPL: wortmanin + Eplerenone).
Eplerenone restored the oxidative stress in STZ-isoproterenol challenged rats
Diabetic ISO challenged rats showed noteworthy boost in the oxidative stress as a significant increase (P<0.001) in malondialdehyde content as well as a decrease in the levels of GSH, SOD and catalase (P<0.001) in tissue homogenate. Treatment with eplerenone showed contrast results that obtained with diabetic ISO challenged rats as it normalised the level of MDA as well as significantly increase (P<0.001) the levels of endogenous antioxidants in respect to reduce the oxidative stress, while treatment with the wortmanin alone or in combination with eplerenone showed increased in the oxidative stress (Table 2).
Table 2.
Effect of eplerenone on oxidative stress in streptozotocin-isoproterenol challenged rats
| Treatment groups (All diabetic) | MDA (µg/mg of protein) | GSH (µg/mg of protein) | SOD (U/mg of protein) | Catalase (U/mg of protein) |
|---|---|---|---|---|
| Control | 92.90±12.89 | 0.999±0.013 | 22.23±1.23 | 28.63±0.36 |
| ISO | 267.1±14.15### | 0.373±0.153### | 3.62±0.98### | 2.65±0.03### |
| EPL | 144.4±15.34*** | 0.937±0.029*** | 18.36±1.26*** | 21.36±0.41*** |
| Wort | 253.2±10.23 | 0.350±0.035 | 5.32±0.35 | 6.36±0.09 |
| Wort + EPL | 105.3±21.33$ | 0.610±0.056$ | 12.36±1.03$ | 18.56±0.19$ |
Data were expressed as the mean ± S.E.M. Significance was determined by one-way ANOV (Analysis of variance) followed by the Bonferroni’s post hoc test:
p<0.001 as compared to Diabetic ISO;
p<0.001 as compared to diabetic control;
p<0.001 as compared to eplerenone.
(All groups were diabetic ISO: Isoproterenol; EPL: Eplerenone; Wort: wortmannin; EPL + Wort: Eplerenone + wortmanin).
Eplerenone recovered cardiac injury markers in STZ-isoproterenol challenged rats
Diabetic ISO challenged rats showed significant decreased (P<0.001) in the activities of CK-MB and LDH. Treatment with eplerenone showed a significant attenuation (P<0.001) of CK-MB and LDH showed positive effect and maintained the myocardial membrane integrity as compared with the diabetic ISO treated rats (Figure 4).
Figure 4.
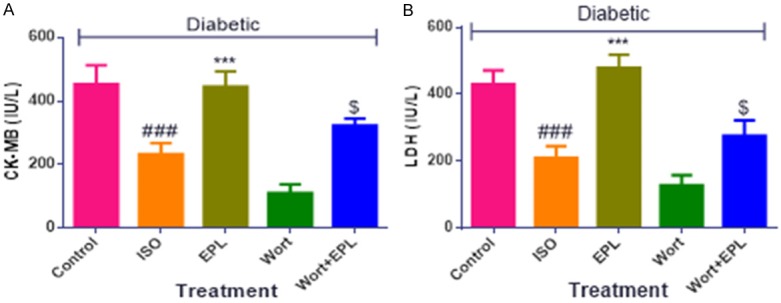
Effects of EPL on cardiac injury markers in STZ-isoproterenol challenged rats. A. Creatine kinase-MB (CK-MB), B. Lactate dehydrogenase (LDH). Data were expressed as the mean ± S.E.M. Significance was determined by one-way ANOVA (Analysis of variance) followed by the Bonferroni’s post hoc test: ***P<0.001 as compared to Diabetic ISO; ###P<0.001 as compared to diabetic control; $P<0.001 as compared to eplerenone. (All the animals from groups were diabetic, where ISO: Isoproterenol; EPL: Eplerenone; Wort: wortmannin; Wort + EPL: wortmanin + Eplerenone).
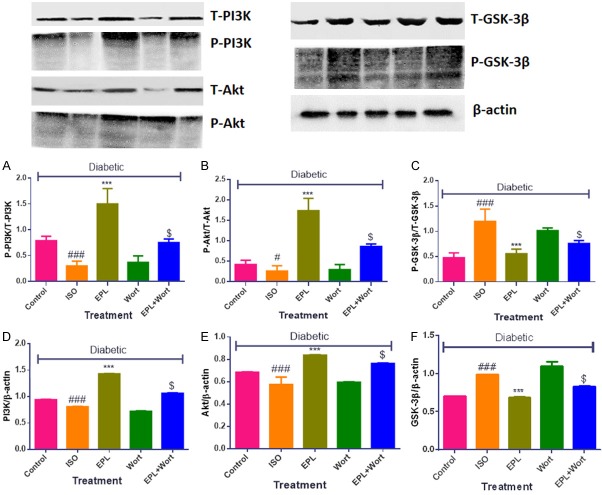
Eplerenone amplified PI3K, Akt expression while repressed the GSK-3β expression in STZ-isoproterenol challenged rats
The rats challenged with diabetic ISO showed significant decrease in the expression of PI3K and Akt (P<0.001) while amplify the expression of GSK-3β (P<0.001) in myocardial tissue homogenates as shown in (Figure 5). Fourteen days treatment with the eplerenone significantly modulates the protein expression and phosphorylates PI3K and Akt (P<0.001), however significantly depressed (P<0.001) GSK-3β expression. The diabetic ISO rats treated with wortmanin showed inhibition of PI3K/Akt, Thus, the results obtained confirms the PI3K modulatory and subsequent GSK-3β inhibitory activity of eplerenone in diabetic ISO challenged rats (Figure 5).
Figure 5.
Effects of EPL on PI3K, Akt and GSK-3β expression in STZ-isoproterenol challenged rats. (A) Ratio of P-PI3K to T-PI3K, (B) Ratio of P-Akt to T-Akt, (C) Ratio of P-GSK-3β to T-GSK-3β, (D) Ratio of PI3K to β-actin, (E) Ratio of Akt to β-actin, (F) Ratio of GSK-3β to β-actin. Data were expressed as the mean ± S.E.M. Significance was determined by one-way ANOVA (Analysis of variance) followed by the Bonferroni’s post hoc test: ***P<0.001 as compared to Diabetic ISO; ###P<0.001 as compared to diabetic control; $P<0.001 as compared to eplerenone. (All the animals from groups were diabetic, where ISO: Isoproterenol; EPL: Eplerenone; Wort: wortmannin; Wort + EPL: wortmanin + Eplerenone).
Eplerenone reduced Bax and increased Bcl-2 expression in STZ-isoproterenol challenged rats
In comparison to diabetic control rats, the rats challenged with ISO showed significant increase in the Bax and decreased Bcl2 expression showed augmented in the apoptosis, while treatment with the eplerenone showed decreased in the protein expression of Bax and increased Bcl2. The rat treated with wortmanin does not show any significant change in the expression of the Bax and Bcl2 (Figure 6A-J).
Figure 6.
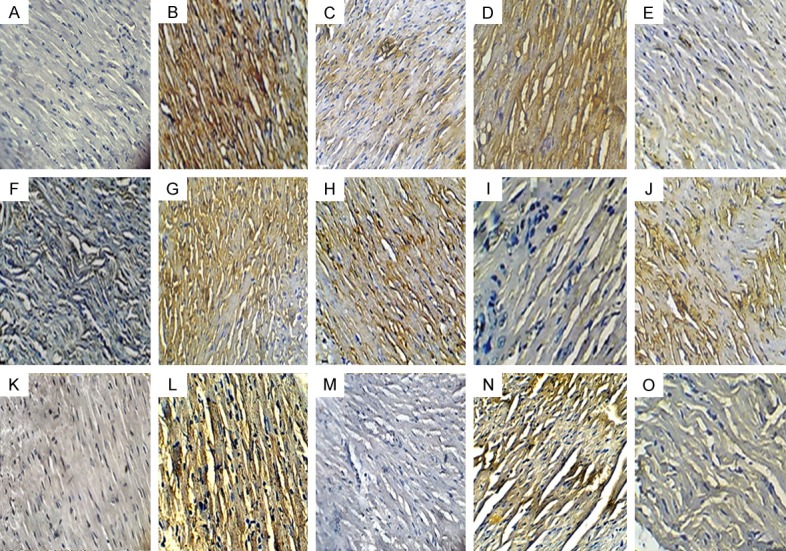
Effects of EPL on expression of Bcl-2, Bax proteins and TUNEL-positive cells in STZ-isoproterenol challenged rats. Immunohistochemical analysis of Bax (A-E) and Bcl-2 (F-J); TUNEL Positive cell (K-O; 20X; scale bar 100 µm) in various groups. (A, F, K) Diabetic control; (B, G, L) Diabetic + isoproterenol; (C, H, M): Diabetic Isoproterenol + Eplerenone; (D, I, N) Diabetic isoproterenol + wortmannin (E, J, O): Diabetic Isoproterenol + Eplerenone + wortmanin.
Further, to support the role of eplerenone on apoptosis, TUNEL assay was performed to detect DNA fragmentation in apoptotic nuclei. An increase in the number of TUNEL positive nuclei was observed in diabetic ISO challenged rats myocardium. On the contrary, few TUNEL positive apoptotic nuclei were seen in eplerenone treatment group (Figure 6K-O).
Eplerenone conserved myocardium arrangement in STZ-isoproterenol challenged rats
Diabetic control rats exhibited normal myocardial architecture with no evidence of inflammation and edemaa. Diabetic ISO injured myocardium displayed marked edema and membrane damage along with infiltration of inflammatory cells and higher histological score. Intriguingly, treatment with eplerenone improved myonecrosis, preserved myocardial architecture, and exhibited a low histological injury score. The rats treated with wortmanin alone or combination with eplerenone showed increased inflammation and increases the histological scores (Figure 7 and Table 3).
Figure 7.
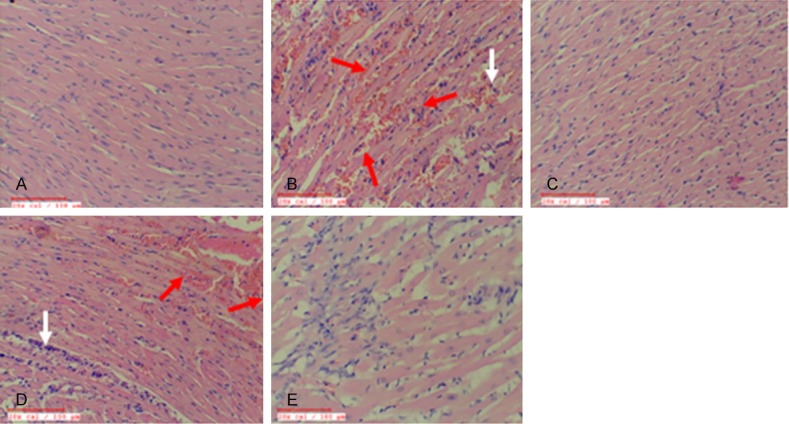
Effect of EPL on histopathology in STZ-isoproterenol challenged rats. A. Diabetic control. B. Diabetic Isoproterenol. C. Diabetic Isoproterenol + Eplerenone. D. Diabetic Isoproterenol + wortmannin. E. Diabetic Isoproterenol + Eplerenone + wortmanin. The red arrows indicate the myonecrosis and the white arrows indicates the appearance of automatic structures in the myocytes.
Table 3.
Effect of Eplerenone on histopathological changes in different experimental groups
| Treatment groups (All diabetic) | Myonecrosis | Inflammatory cells | Edema |
|---|---|---|---|
| Control | - | - | - |
| ISO | ++ | ++ | + |
| EPL | - | - | - |
| Wort | +++ | ++ | +++ |
| Wort + EPL | + | + | - |
Severe (+++), moderate (++), less (+), absent (-). All groups were diabetic ISO: Isoproterenol; EPL: Eplerenone; Wort: wortmannin; EPL+ Wort: Eplerenone + wortmanin.
Discussion
The present study for the first time evidenced the involvement of PI3K/Akt and there downstream GSK-3β pathway in the diabetic myocardial infarction. In this, streptozotocin was used for the orientation of diabetes in rats, seeing as it enters the β-cells of pancreas and alkylates DNA. This diminishes the activity of NAD+ as well as ATP and exaggerates the diabetic condition similar in the humans by activating poly ADP-ribosylation pathway [23,24].
Administration of subcutaneous injections of ISO causes imbalance between oxygen supply and demand by the myocytes from end to end increasing the chronotropism and inotropism important to explicit myocardial function and amplify in the calcium overload in the myocardium [7]. The present study deals to establish, the effects of eplerenone in treatment of diabetic cardiac complication in animals by exhibiting improved cardiac functionalities, boosting endogenous defense systems, reducing myocardial apoptosis, preserving myofibril structure and morphology through its modulatory effect on PI3K/Akt pathway and downstream target GSK-3β. In the present study, we employed wortmanin as PI3K/Akt antagonist to build the confirmation of modulatory activity of eplerenone.
Previously, it has been described that administration of isoproterenol in rats associated with the myocardial structural injury. Which eventually emerges in perturbed hemodynamic and contractile dysfunction [25], alteration in the hemodynamic parameters as evidenced by decrease in the systolic, diastolic and mean arterial blood pressure, also significantly impaired inotropic and lusitropic state (±LVdP/dtmax), and increased ventricular remodelling (preload LVEDP). While treatment with eplerenone improved LVEDP by escalating inotropic (+LVdP/dtmax, a marker of myocardial contraction) and lusitropic (-LVdP/dtmax, a marker of myocardial relaxation) circumstances of myocardium, PI3k/Akt antagonist wortmanin deteriorate the situation. Moreover, prior treatment of eplerenone than wortmanin also prevented the rats from further toxicity of the isoproterenol. Modulation of PI3K/Akt pathway is shown to have beneficial effects in the cardiac injury as observed by Hua et al., (2007) [26]. Thus, these observations propose the protection of myocardium because of eplerenone in diabetic myocardial infarcted rats by means of PI3K/Akt pathway and so as to its activation may augment these effects.
Administration of isoproterenol in diabetic rats produces a myocardial excited function due to augmented chronotropism, inotropism and led to deleterious oxidative stress. These observations are in accordance with the previous literature [27]. In the present study, administration of eplerenone in diabetic myocardial infarcted rats showed decrease in the oxidative stress as observed by decreasing the malondialdehyde content as well as increasing the levels of endogenous antioxidant enzymes, while administration of wortmanin worsen the condition as compared with the diabetic control rats. The creatinine kinase-MB isoenzyme and lactate dehydrogenase are present in the myocardium and have been dilapidated as a forecaster for pathology and internal changes occurs in the myocardium [28]. In the current study, we observed that these enzymes were decreased in the myocardium of diabetic ISO rats, which is in line with the previous study reported by Zaitone et al. (2016) [29]. Treatment with eplerenone prevent the loss of membrane bound enzymes in diabetic myocardial infarcted rats, while treatment with the wortmanin does not prevent the loss of enzymes from the myocardium and confirms the involvement of PI3K/Akt in the protection of myocardial infarction. The present study demonstrated that administration of eplerenone in diabetic myocardial infarcted rats showed increased expression of PI3k, and Akt protein, also reduced the expression of GSK-3β through western blot analysis, whereas the rats treated with wortmanin significantly decreased the concentration of PI3K, Akt protein expression on account of the opposed possessions of wortmanin, thus the observation confirms the role of PI3k/Akt mediated pathway in the diabetic cardiac complications. Various reports have demonstrated the role of PI3K and Akt in different pathological conditions [30,31] while, several additional confirmative studies may be required for further strengthening of this hypothesis. In addition to above parameters, myocardial apoptosis was also studied, which is recommended to be one of the chief pathological parameters getting worse in diabetic myocardial injury [32].
Chen et al. (2016) [33] reported that reducing the apoptosis signaling in the myocardium might be helpful to prevent the loss of contractile function of the myocardium, and decrease the chances of further damage to the myocardium. From the above report, we explored the fundamental mechanism responsible for the enhancement of myocardial utility following administration of eplerenone in diabetic cardiac complications, the expression of pro apoptotic protein Bax and anti-apoptotic protein Bcl-2 were performed using immunohistochemistry and TUNEL assay was executed, which could be employed for the detection of DNA fragmentation.
Diabetic myocardial infarcted rats significantly reduced Bcl-2 and increased Bax expression as well as TUNEL-positive cells. Furthermore, treatment with eplerenone in myocardial infarcted diabetic rats showed significant anti-apoptotic activity as represented by amplified Bcl-2 expression and dwindled expression of Bax also the TUNEL-positive cells. Though, administration of wortmanin deteriorates the situation in the myocardial infarcted diabetic rats. The involvement of PI3K/Akt pathway in the process of apoptosis is well established [34]. Thus, the reduction of eplerenone induced improvement in the diabetic myocardial infarction in presence of wortmanin confirms the involvement of PI3K/Akt pathway in the eplerenone mediated actions in diabetic myocardial infarcted rats. Further to confirm the salvaging effect of eplerenone in the myocardial infarcted diabetic rats light microscopy was performed, the rats subjected to diabetic myocardial infarction showed signs of infarction with Inflammatory cells, myonecrosis and edematic structure. Eplerenone treatment in myocardial infarcted diabetic rats showed the defense of the regular morphology of myocardium with no signs of infarction, the opposite results were observed in the rats treated with wortmannin as an increase in the myonecrosis oedema and increased inflammatory cells.
Conclusion
Taken together, all the studied parameters, it was concluded that eplerenone significantly attenuates streptozotocin-isoproterenol induced myocardial infarction and the mechanism for this favorable effect could be explained by PI3k/Akt activation and downstream of its GSK-3β. Therefore, we have provided direct evidence that the PI3K/Akt and GSK-3β signalling pathway plays a significant role in regulating oxidative stress and subsequent tissue injury. This is the first study ever conducted to describe the cardio protective effect of eplerenone through the PI3K/Akt and its downstream target GSK3-β in myocardial infarcted diabetic rats.
Acknowledgements
The authors gratefully acknowledge the financial support received under Extra Mural Research (File no. EMR/2016/005106) of Science and Engineering Research Board (SERB), Department of Science and Technology, New Delhi, India. The authors also acknowledge the financial support to Dr. Shreesh Ojha received from University Program for Advanced Research (UPAR), United Arab Emirates University, United Arab Emirates.
Disclosure of conflict of interest
None.
References
- 1.Caporali A, Miscianinov V, Saif J, Emanueli C. MicroRNAs transport in cardiovascular complication of diabetes. Biochim Biophys Acta. 2016;186:2111–2120. doi: 10.1016/j.bbalip.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Moon JY, Woo JS, Seo JW, Lee A, Kim DJ, Kim YG. The dose-dependent organ-pecific ffects of a dipeptidyl peptidase-4 inhibitor on cardiovascular complications in a model of type 2 diabetes. PLoS One. 2016;11:e0150745. doi: 10.1371/journal.pone.0150745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sloth AD, Schmidt MR, Munk K, Schmidt M, Pedersen L, Sørensen HT. Cost-effectiveness of remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2016:2048872615626657. doi: 10.1177/2048872615626657. [DOI] [PubMed] [Google Scholar]
- 4.Ni R, Zheng D, Xiong S, Hill DJ, Sun T, Gardiner RB. Mitochondrial calpain-1 disrupts ATP synthase and induces superoxide generation in type 1 diabetic hearts: a novel mechanism contributing to diabetic cardiomyopathy. Diabetes. 2016;65:255–268. doi: 10.2337/db15-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Cardiolipin alterations and mitochondrial dysfunction in heart ischemia/reperfusion injury. 2015;10:415–429. [Google Scholar]
- 6.Luis M, Stertzer SH, Argentieri J, Penaloza E, Altman PA. Treatment for chronic myocardial infarct. Google Patents. 2015 US8496926 B2. [Google Scholar]
- 7.Goyal SN, Sharma C, Mahajan UB, Patil CR, Agrawal YO, Kumari S. Protective effects of cardamom in isoproterenol-induced myocardial infarction in rats. Int J Mol Sci. 2015;16:27457–69. doi: 10.3390/ijms161126040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy NM, Mahajan UB, Patil CR, Agrawal YO, Ojha S, Goyal SN. Eplerenone attenuates cardiac dysfunction and oxidative stress in β-receptor stimulated myocardial infarcted rats. Am J Transl Res. 2015;7:1602. [PMC free article] [PubMed] [Google Scholar]
- 9.Fang J, Hu F, Ke D, Yan Y, Liao Z, Yuan X. N, N-dimethylsphingosine attenuates myocardial ischemia-reperfusion injury by recruiting regulatory T cells through PI3K/Akt pathway in mice. Basic Res Cardiol. 2016;111:1–15. doi: 10.1007/s00395-016-0548-3. [DOI] [PubMed] [Google Scholar]
- 10.Liu MH, Li GH, Peng LJ, Qu SL, Zhang Y, Peng J. PI3K/Akt/FoxO3a signaling mediates cardioprotection of FGF-2 against hydrogen peroxideinduced apoptosis in H9c2 cells. Mol Cell Biochem. 2016;414:57–66. doi: 10.1007/s11010-016-2658-5. [DOI] [PubMed] [Google Scholar]
- 11.Lin CF, Tsai CC, Huang WC, Wang YC, Tseng PC, Tsai TT. Glycogen synthase kinase-3β and caspase-2 mediate ceramide-and etoposide-induced apoptosis by regulating the lysosomal-mitochondrial axis. PLoS One. 2016;11:e0145460. doi: 10.1371/journal.pone.0145460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Wang Y, Wei C, Bai S, Zhao Y, Li H. Mediation of exogenous hydrogen sulfide in recovery of ischemic post-conditioning-induced cardioprotection via down-regulating oxidative stress and up-regulating PI3K/Akt/GSK-3β pathway in isolated aging rat hearts. Cell Biosci. 2015;5:1. doi: 10.1186/s13578-015-0003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chin KL, Collier TJ, Pitt B, McMurray JJ, Swedberg K, van Veldhuisen DJ. Aspirin does not reduce the clinical benefits of the mineralocorticoid receptor antagonist eplerenone in patients with systolic heart failure and mild symptoms: an analysis of the EMPHASIS-HF study. Eur J Heart Fail. 2016;18:1175–1181. doi: 10.1002/ejhf.485. [DOI] [PubMed] [Google Scholar]
- 14.Keidar S, Hayek T, Kaplan M, Pavlotzky E, Hamoud S, Coleman R. Effect of eplerenone, a selective aldosterone blocker, on blood pressure, serum and macrophage oxidative stress, and atherosclerosis in apolipoprotein Edeficient mice. J Cardiovasc Pharmacol Ther. 2003;41:955–63. doi: 10.1097/00005344-200306000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Nishida H, Sohara E, Nomura N, Chiga M, Alessi DR, Rai T. PI3K/Akt signaling pathway activates the WNK-OSR1/SPAK-NCC phosphorylation cascade in hyperinsulinemic db/db mice. Hypertens. 2012;60:981. doi: 10.1161/HYPERTENSIONAHA.112.201509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goyal S, Bharti S, Bhatia J, Nag T, Ray R, Arya D. Telmisartan, a dual ARB/partial PPAR-γ agonist, protects myocardium from ischaemic reperfusion injury in experimental diabetes. Diabetes Obes Metab. 2011;13:533–41. doi: 10.1111/j.1463-1326.2011.01377.x. [DOI] [PubMed] [Google Scholar]
- 17.Suchal K, Malik S, Gamad N, Malhotra RK, Goyal SN, Chaudhary U. Kaempferol attenuates myocardial ischemic injury via inhibition of MAPK signaling pathway in experimental model of myocardial ischemia-reperfusion injury. Oxid Med Cell Longev. 2016;2016:7580731. doi: 10.1155/2016/7580731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panda V, Laddha A, Nandave M, Srinath S. Dietary phenolic acids of macrotyloma uniflorum (Horse Gram) protect the ratheart against isoproterenol-induced myocardial infarction. Phytother Res. 2016;30:1146–1155. doi: 10.1002/ptr.5620. [DOI] [PubMed] [Google Scholar]
- 19.Kumar M, Kasala ER, Bodduluru LN, Dahiya V, Lahkar M. Baicalein protects isoproterenol induced myocardial ischemic injury in male Wistar rats by mitigating oxidative stress and inflammation. Inflamm Res. 2016:1–10. doi: 10.1007/s00011-016-0944-z. [DOI] [PubMed] [Google Scholar]
- 20.Rani N, Bharti S, Bhatia J, Nag T, Ray R, Arya DS. Chrysin, a PPAR-γ agonist improves myocardial injury in diabetic rats through inhibiting AGE-RAGE mediated oxidative stress and inflammation. Chem Biol Interact. 2016;250:59–67. doi: 10.1016/j.cbi.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 21.Kocak C, Kocak FE, Akcilar R, Isiklar OO, Kocak H, Bayat Z. Molecular and biochemical evidence on the protective effects of embelin and carnosic acid in isoproterenol-induced acute myocardial injury in rats. Life Sci. 2016;147:15–23. doi: 10.1016/j.lfs.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 22.Mao HP, Wang XY, Gao YH, Chang YX, Chen L, Niu ZC, Ai JQ, Gao XM. Danhong injection attenuates isoproterenol-induced cardiac hypertrophy by regulating p38 and NF-κb pathway. J Ethnopharmacol. 2016;186:20–29. doi: 10.1016/j.jep.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Agrawal YO, Sharma PK, Shrivastava B, Ojha S, Upadhya HM, Arya DS. Hesperidin produces cardioprotective activity via PPAR-γ pathway in ischemic heart disease model in diabetic rats. PLoS One. 2014;9:e111212. doi: 10.1371/journal.pone.0111212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang B, Cao F, Zhao H, Zhang J, Jiang B, Wu Q. Betanin ameliorates isoproterenol-induced acute myocardial infarction through iNOS, inflammation, oxidative stress-myeloperoxidase/low-density lipoprotein in rat. Int J Clin Exp Pathol. 2016;9:2777–2786. [Google Scholar]
- 25.Goyal S, Arora S, Sharma A, Joshi S, Ray R, Bhatia J. Preventive effect of crocin of crocus sativus on hemodynamic, biochemical, histopathological and ultrastuctural alterations in isoproterenol-induced cardiotoxicity in rats. Phytomedicine. 2010;17:227–232. doi: 10.1016/j.phymed.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Hua F, Ha T, Ma J, Li Y, Kelley J, Gao X, Browder IW, Kao RL, Williams DL, Li C. Protection against myocardial ischemia/reperfusion injury in TLR4-deficient mice is mediated through a phosphoinositide 3-kinase-dependent mechanism. J Immunol. 2007;178:7317–7324. doi: 10.4049/jimmunol.178.11.7317. [DOI] [PubMed] [Google Scholar]
- 27.Aragno M, Mastrocola R, Alloatti G, Vercellinatto I, Bardini P, Geuna S. Oxidative stress triggers cardiac fibrosis in the heart of diabetic rats. Endocrinology. 2008;149:380–388. doi: 10.1210/en.2007-0877. [DOI] [PubMed] [Google Scholar]
- 28.Dianita R, Jantan I, Amran AZ, Jalil J. Protective effects of labisia pumila var. alata on biochemical and histopathological alterations of cardiac muscle cells in isoproterenol-induced myocardial infarction rats. Molecules. 2015;20:4746–4763. doi: 10.3390/molecules20034746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaitone SA, Abo-Gresha NM. Rosuvastatin promotes angiogenesis and reverses isoproterenol-induced acute myocardial infarction in rats: role of iNOS and VEGF. Eur J Pharmacol. 2012;691:134–142. doi: 10.1016/j.ejphar.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 30.Follo MY, Manzoli L, Poli A, McCubrey JA, Cocco L. PLC and PI3K/Akt/mTOR signalling in disease and cancer. Adv Biol Regul. 2015;57:10–16. doi: 10.1016/j.jbior.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Ma XY, Feng YF, Ma ZS, Wang J, Ma TC. Osseointegration of chitosan coated porous titanium alloy implant by reactive oxygen species-mediated activation of the PI3K/AKT pathway under diabetic conditions. Biomaterials. 2015;36:44–54. doi: 10.1016/j.biomaterials.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Chen XG, Lv YX, Zhao D, Zhang L, Zheng F, Yang JY, Li XL, Wang L, Guo LY, Pan YM, Yan YW, Chen SY, Wang JN, Tang JM, Wan Y. Vascular endothelial growth factor-C protects heart from ischemia/reperfusion injury by inhibiting cardiomyocyte apoptosis. Mol Cell Biochem. 2016;413:9–23. doi: 10.1007/s11010-015-2622-9. [DOI] [PubMed] [Google Scholar]
- 33.Chen Z, Chua CC, Ho YS, Hamdy RC, Chua BH. Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2001;280:H2313–H2320. doi: 10.1152/ajpheart.2001.280.5.H2313. [DOI] [PubMed] [Google Scholar]
- 34.Seo BR, Min Kj, Cho IJ, Kim SC, Kwon TK. Correction: curcumin significantly enhances dual PI3K/Akt and mTOR inhibitor NVPBEZ235-induced apoptosis in human renal carcinoma caki cells through down-regulation of p53-dependent Bcl-2 expression and inhibition of Mcl-1 protein stability. PLoS One. 2016;11:e0151886. doi: 10.1371/journal.pone.0151886. [DOI] [PMC free article] [PubMed] [Google Scholar]