Abstract
Low-intensity pulsed ultrasound (LIPUS), which is a noninvasive form of mechanical energy, has been utilized as a clinical therapy for bone fracture healing. However, the mechanism how LIPUS affects osteoclast formation and osteoclast activity, has not been fully detailed. Here we found that LIPUS inhibited RANKL-induced osteoclast differentiation in vitro, characterized by decreased number and area of tartrate-resistant acid phosphatase (TRAP) positive cells. Moreover, the expression levels of osteoclast-specific gene were also suppressed by LIPUS treatment. Interestingly, F-actin staining and resorption pit assay showed that LIPUS did not affect the bone resorptive activity of mature osteoclasts. Mechanistically, LIPUS achieved these inhibitory effects by disrupting the phosphorylation of ERK and subsequent activation of the osteoclastic transcription factors, c-Fos and NFATc1. Collectively, our results demonstrated that LIPUS effectively suppresses osteoclast differentiation and osteoclast-specific gene expression through the inhibition of ERK-c-Fos-NFATC1 cascades.
Keywords: LIPUS, osteoclast, ERK, c-Fos, NFATc1
Introduction
Bone remodeling is a crucial metabolic process regulating bone structure and function. This process is continuously active in skeletal tissue through all stages of life and dyn amically balanced by osteoclastic bone resorption and osteoblastic bone formation [1,2]. Excess osteoclast activity leads to an imbalance between bone resorption and bone formation during bone remodeling, which underlies the pathogenesis of many skeletal diseases, including osteoporosis, rheumatoid arthritis, periodontal disease and metastatic cancers [3]. Therefore, modulating aberrant osteoclast activity might be the promising therapy for such diseases.
Osteoclasts, which are multinucleated giant cells originating from hematopoietic monocyte/macrophage precursors, are the only cells capable of bone resorption in human body. It is now known that receptor activator for NF-κB-ligand (RANKL), a cytokine released by activated osteoblasts, is indispensable for osteoclast differentiation [4]. Binding of RANKL to its receptor RANK promotes recruitment of adaptor protein TRAF6, which subsequently activates downstream signaling pathways, including nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) [5]. These signaling cascades lead to the upregulation of c-Fos and nuclear factor of activated T cells c1 (NFATc1), which are the key transcription factors for osteoclast differentiation [6,7]. Then mature osteoclasts are polarized and undergo structural changes to form a sealing zone. Secretion of the lytic enzymes, tartrate-resistant acid phosphatase (TRAP) and cathepsin K (CTSK), into the sealing zone results in the dissolution and degradation of the underlying bone [8].
Low-intensity pulsed ultrasound (LIPUS), which is a noninvasive form of mechanical energy, has been utilized as a clinical therapy for bone fracture healing [9,10]. Although numerous animal studies have shown that LIPUS is able to prevent many skeletal diseases, such as osteoporosis, periprosthetic osteolysis and bone nonunion [11-13], the mechanism how it works during bone remodeling is uncertain. Most studies of LIPUS have focused on osteoblastic growth and differentiation [14-16], however, studies regarding the effect of LIPUS on osteoclast activity are scant. Recently, some researchers reported that LIPUS can suppress osteoclast activity by regulating OPG and RANKL expression in osteoblasts [17,18]. As the molecular communication between osteoblasts and osteoclasts exists extensively in vivo, it is necessary to in investigate the direct effect of LIPUS on osteoclast formation and activity without the presence of osteoblasts in vitro. In this study, we aimed to investigate the effects of LIPUS on RANKL-induced osteoclast formation and bone resorption activity in vitro, and further figure out the underlying mechanisms.
Materials and methods
Media and reagents
Alpha modification of Eagle’s medium (α-MEM), Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and penicillin/streptomycin were purchased from Gibco-BRL (Gaithersburg, MD, USA). Recombinant mouse macrophage colony-stimulating factor (M-CSF) and RANKL were obtained from R&D Systems (Minneapolis, MN, USA). Specific antibodies against p38 (#9212), phospho-p38 (Thr180/Tyr182) (#4511), extracellular signal-regulated kinase (ERK) (#4695), phospho-ERK (Thr202/Tyr204) (#4370), c-Jun N-terminal kinase (JNK) 1/2 (#9252), phospho-JNK (Thr183/Tyr185) (#4668), inhibitor of nuclear factor kappa-B kinase subunit alpha (IκBα) (#4814), phospho-IκBα (Ser32) (#2859), p65 (#8242), phospho-p65 (Ser536) (#3033), c-Fos (#2250), nuclear factor of activated T cells c1 (NFATc1) (#8032), and β-tubulin (#2146) were obtained from Cell Signaling Technology (Danvers, MA, USA). Tartrate-resistant acid phosphatase (TRAP) staining kit, and other reagents were purchased from Sigma-Aldrich unless otherwise noted.
Cell culture
Monocyte/macrophage precursors were obtained from femur and tibia bone marrow of 6-week-old male C57BL/6 mice as described previously [19], and then differentiated into bone marrow macrophages (BMMs) in complete α-MEM (10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin) supplemented with 30 ng/mL M-CSF for 3 days. Mouse macrophage cell line RAW264.7 was obtained from the American Type Culture Collection (Rockville, MD, USA) and maintained in complete DMEM (10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin). All cell cultures were maintained in a humidified environment of 5% CO2 at 37°C.
Ultrasound application
Cells were stimulated using a LIPUS-generating system (Teijin Pharma Ltd., Tokyo, Japan), which was described previously [20]. The average intensity was 30 milliwatts/cm2, the pulsed frequency was 1.5 MHz, the repetition rate was 1 kHz, and the pulse lasted 200 μs. The culture plate was placed above these transducers with a thin layer of coupling gel. The pattern and intensity of the LIPUS signal used in this study were essentially the same as that used in clinical practice and in animal model experiments.
In vitro osteoclast differentiation
BMMs were seeded in 96-well plates at a density of 8×103 cells/well, in triplicate, in complete α-MEM supplemented with 30 ng/mL M-CSF and 50 ng/mL RANKL. Culture medium was replaced every 2 days. During osteoclastogenesis, LIPUS was conducted to treat cells for 20 min per day. After 3 or 5 days of culture, cells were washed twice with PBS, fixed with 4% paraformaldehyde (PFA) for 15 min, and stained for TRAP activity. TRAP-positive multinucleated cells with more than three nuclei were counted under a light microscope (Olympus BX51, Tokyo, Japan). Similarly, the effect of LIPUS on osteoclast differentiation was also tested on the RAW264.7 cell line.
F-actin ring immunofluorescence and resorption pit assay
To investigate the effect of LIPUS on F-actin ring formation, BMMs were treated with 30 ng/mL M-CSF and 50 ng/mL RANKL for 4 days. An equal number of BMM-derived osteoclasts were seeded into Osteo Assay Surface Multiple Well Plate (Corning, MA, USA) coated with hydroxyapatite, and allowed to adhere overnight. Cells were then treated with LIPUS for another 2 days. Next, cells were fixed with 4% PFA for 15 min, permeabilized for 5 min with 0.5% Triton X-100, and stained with rhodamine-conjugated phalloidin (1:200; Invitrogen Life Technologies, Carlsbad, CA, USA) diluted in 0.2% bovine serum albumin (BSA)-PBS for 1 h. Fluorescent images were captured with a fluorescence microscope (EU5888, Leica, Wetzlar, Germany) and analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA). Then, these plates were washed twice with PBS and incubated with 5% NaClO for 10 min to remove cells adhered to the plates. Resorption pit images were captured using a light microscope (Olympus) and analyzed using ImageJ software.
RNA extraction and quantitative PCR assay
Total RNA from cultured cells was extracted using the Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA, USA) following the manufacturer’s protocols. Complementary DNA (cDNA) was synthesized from 1 μg of total RNA using reverse transcriptase (TaKaRa Biotechnology, Otsu, Japan). Quantitative real-time PCR was performed using the SYBR Premix Ex Tag Kit (TaKaRa Biotechnology) and the ABI 7500 Sequencing Detection System (Applied Biosystems, Foster City, CA, USA). Each reaction was run for 40 cycles at the following conditions: 95°C for 5 s, 60°C for 20 s, and 72°C for 20 s. GAPDH was used as a housekeeping gene. The mouse primer sequences are shown in Table 1.
Table 1.
Primers used for quantitative real-time PCR
| Gene | Forward (F) and reverse (R) primer sequence (5’-3’) |
|---|---|
| GAPDH | F: ACCCAGAAGACTGTGGATGG |
| R: CACATTGGGGGTAGGAACAC | |
| CTSK | F: CTTCCAATACGTGCAGCAGA |
| R: TCTTCAGGGCTTTCTCGTTC | |
| TRAP | F: CTGGAGTGCACGATGCCAGCGACA |
| R: TCCGTGCTCGGCGATGGACCAGA | |
| DC-STAMP | F: AAAACCCTTGGGCTGTTCTT |
| R: AATCATGGACGACTCCTTGG | |
| c-Fos | F: CCAGTCAAGAGCATCAGCAA |
| R: AAGTAGTGCAGCCCGGAGTA | |
| NFATc1 | F: CCGTTGCTTCCAGAAAATAACA |
| R: TGTGGGATGTGAACTCGGAA | |
| CTR | F: TGGTTGAGGTTGTGCCCA |
| R: CTCGTGGGTTTGCCTCATC |
Western blotting
Western blotting was used to determine the main signaling pathways affected by LIPUS. BMMs or RAW 264.7 cells were seeded in 6-well plates at a density of 8×105 cells/well. After pretreatment with or without LIPUS for 20 min, cells were stimulated with 50 ng/mL RANKL for 0, 5, 15, 30 min. To examine the effects of LIPUS on c-Fos and NFATc1, BMMs were plated in 6-well plates at a density of 1×105 cells/well and cultured with 30 ng/mL M-CSF and 50 ng/mL RANKL in the presence or absence of LIPUS for 0, 1, 3, or 5 days. Total protein was extracted from cultured cells using radioimmunoprecipitation assay (RIPA) lysis buffer (Sigma-Aldrich). Each protein lysate containing 30 μg protein was separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). After non-specific blocking for 1 h, membranes were incubated with primary antibodies at 4°C overnight. After three washes with TBS-Tween, we subsequently incubated membranes with the appropriate secondary antibodies at 4°C for 2 h. The signals were detected by exposure in a Bio-Rad XRS chemiluminescence detection system (Hercules, CA, USA).
Statistical analysis
All data are expressed as mean ± SEM. Each experiment was repeated at least three times separately and the results were analyzed with Prism 6.01 (GraphPad Software, La Jolla, CA, USA). A two-tailed, unpaired Student’s t-test was used for the comparisons between two groups. One-way ANOVA with post hoc Newman-Keuls test was used to analyze differences in multiple comparisons. Values of P < 0.05 were considered statistically significantly different.
Results
LIPUS inhibits RANKL-induced osteoclast formation in vitro
To investigate the effect of LIPUS on osteoclast formation, BMMs were treated with M-CSF and RANKL, in the presence or absence of LIPUS for 5 days. As shown in Figure 1A, TRAP-positive cells appeared after 3 days of RANKL stimulation and differentiated into more numerous and mature osteoclasts in another 2 days. However, osteoclast formation was markedly inhibited by LIPUS treatment. Furthermore, the area and number of osteoclasts were significantly decreased by treatment with LIPUS (Figure 1B, 1C). Meanwhile, the inhibitory effect of LIPUS on osteoclast formation further confirmed in RAW264.7 cell line (Figure 1D-F).
Figure 1.
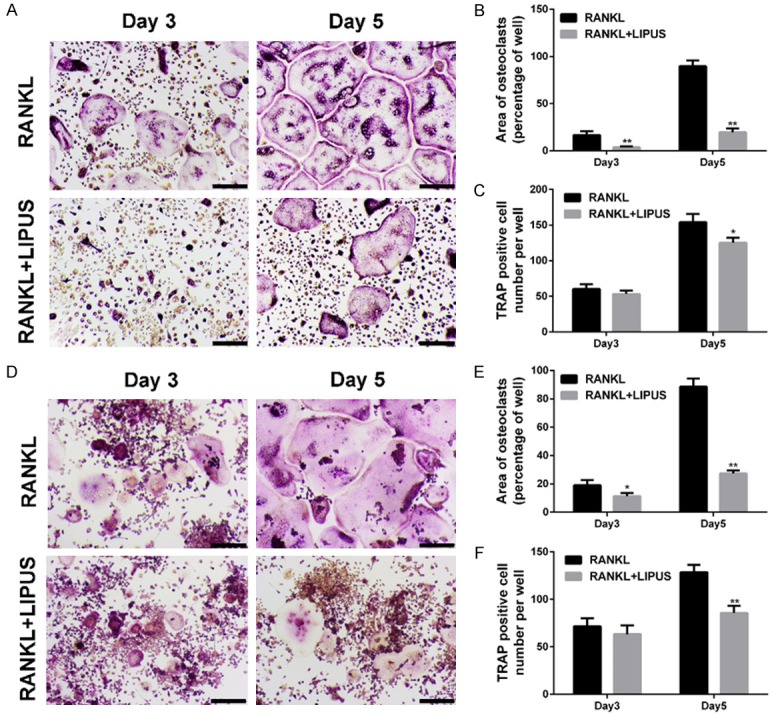
LIPUS suppresses RANKL-induced osteoclast formation in vitro. A. BMMs were stimulated with M-CSF (30 ng/ml) and RANKL (50 ng/ml) in the presence or absence of LIPUS for 3 or 5 days. Cells were then fixed and stained for TRAP activity. B. The area of TRAP positive cells was analyzed. C. The number of TRAP positive cells were analyzed. D. RAW264.7 cells were stimulated with RANKL (50 ng/ml) in the presence or absence of LIPUS for 3 or 5 days. Cells were then fixed and stained for TRAP activity. E. The area of TRAP positive cells was analyzed. F. The number of TRAP positive cells were counted. (Scale bars = 200 μm; *P < 0.05, **P < 0.01).
LIPUS suppresses RANKL-induced osteoclast-specific gene expression in vitro
With the stimulation of RANKL, osteoclast differentiation is related to the upregulation of several specific genes [21]. Therefore, we used quantitative real-time PCR to examine the inhibitory effect of LIPUS on RANKL-induced mRNA expression of these genes. As expected, RANKL dramatically induced the expression of all evaluated genes, including CTSK, TRAP, calcitonin receptor (CTR), DC-STAMP, c-Fos and NFATc1. However, LIPUS significantly inhibited the expression of these osteoclast-related genes in a time-dependent manner (Figure 2).
Figure 2.
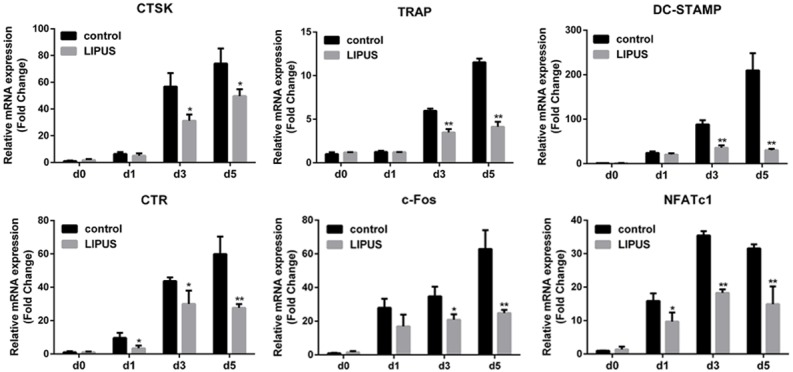
LIPUS inhibits RANKL-induce expression of osteoclast-specific genes. BMMs were cultured with M-CSF (30 ng/mL) and RANKL (50 ng/mL) with or without LIPUS treatment for 0, 1, 3, or 5 days. The mRNA expression of osteoclast-specific genes (CTSK, TRAP, DC-STAMP, CTR, c-Fos, NFATc1) was measured using quantitative real-time PCR. Results were normalized to the expression of GAPDH. (*P < 0.05, **P < 0.01).
LIPUS does not affect osteoclastic bone resorption activity
Since LIPUS obviously suppressed osteoclast formation, we further examine whether LIPUS could affect osteoclastic bone resorption activity. Mature osteoclasts were plated onto the Osteo Assay Plate in the presence of osteoclastogenic medium and treated with LIPUS for another 2 days. Optical images showed that osteoclasts extensively resorbed the bone surface and no significant difference in resorption activity was observed after LIPUS treatment (Figure 3A, 3B).
Figure 3.
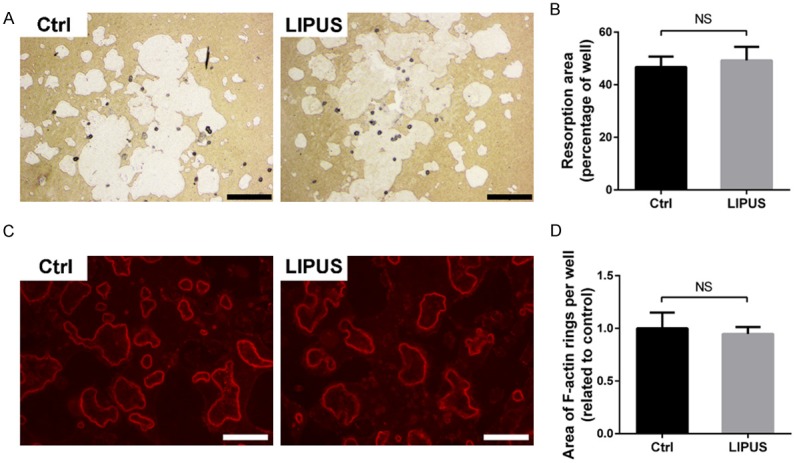
LIPUS does not affect osteoclastic bone resorption activity of BMMs in vitro. An equal number of BMM-derived osteoclasts were seeded into Osteo Assay Surface Multiple Well Plate in the presence or absence of LIPUS for 2 days. A. Representative light microscope images of resorption pits were shown. B. The area of resorption pits was quantified using ImageJ software. C. Representative fluorescence images of F-actin staining with rhodamine-phalloidin. D. The area of F-actin rings was measured using ImageJ software. (Black scale bars = 500 μm; white scale bars = 100 μm; NS, not significant).
A well-polarized F-actin ring is an observable characteristic of mature osteoclasts and an essential prerequisite for osteoclastic bone resorption [22]. Hence, we tested the effect of LIPUS on F-actin ring formation. Under fluorescence microscope, the characteristic F-actin ring structures were observed in the control group and these structures were not impaired by LIPUS treatment (Figure 3C). Quantitative analysis also confirmed our observation (Figure 3D). Collectively, our findings suggested that LIPUS did not affect osteoclastic bone resorption and F-actin ring formation in vitro.
LIPUS suppresses RANKL-induce activation of ERK signaling pathway in vitro
To elucidate the underlying mechanisms through which LIPUS inhibited osteoclast formation, we investigated the main signaling pathway involved in osteoclast differentiation. Previous studies revealed that the activation of NF-κB and MAPK signaling pathways are crucial for osteoclast formation [6,23]. As shown in Figure 4A, the phosphorylation of ERK was obviously activated in BMMs by RANKL stimulation, while LIPUS pretreatment significantly attenuated the RANKL-induced ERK phosphorylation. Interestingly, LIPUS had no obvious inhibitory effect on the phosphorylation of JNK, p38, p65. Similar results were observed in mouse macrophage cell line, RAW264.7, which further confirmed our findings (Figure 4B). Our data suggested that LIPUS impaired RANKL-induced osteoclast differentiation via the suppression of ERK signaling pathway.
Figure 4.
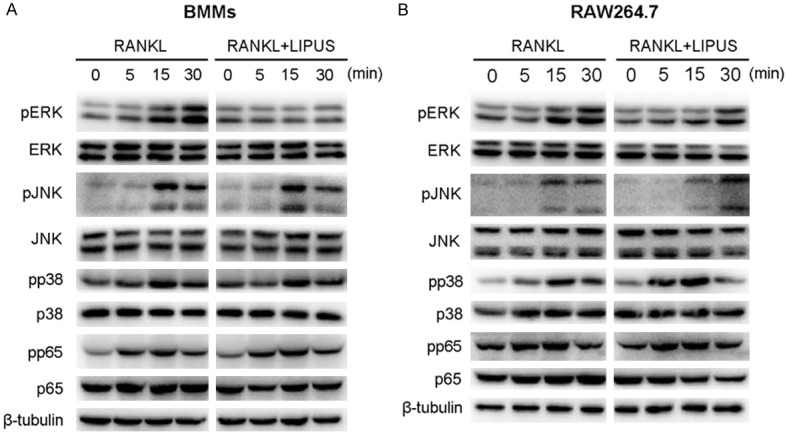
LIPUS inhibits osteoclast formation by specifically impairing RANKL-induced ERK signaling pathway. (A) BMMs or (B) RAW264.7 were pretreated with or without LIPUS for 20 min and then stimulated with RANKL (50 ng/mL) for the indicated periods. Cell lysates were analyzed with the indicated antibodies by Western blotting.
LIPUS inhibits RANKL-induced c-Fos and NFATc1 expression in vitro
c-Fos and NFATc1 are considered as the long-term regulators during osteoclastogenesis [23]. Therefore, we investigated the effects of LIPUS on RANKL-induced c-Fos and NFATc1 expression. BMMs were cultured with M-CSF and RANKL for 0, 1, 3, and 5 days, in the presence or absence of LIPUS. As expected, the protein levels of c-Fos and NFATc1 increased when the cells were stimulated with RANKL in the control group. In contrast, RANKL-induced activation and accumulation of NFATc1 and c-Fos were significantly suppressed by the treatment of LIPUS (Figure 5A). Quantitative analysis also confirmed our observation (Figure 5B). Taken together, these results suggested that LIPUS suppressed osteoclast differentiation and osteoclast-specific gene expression via the inhibition of ERK/c-Fos/NFATc1 cascades (Figure 5C).
Figure 5.
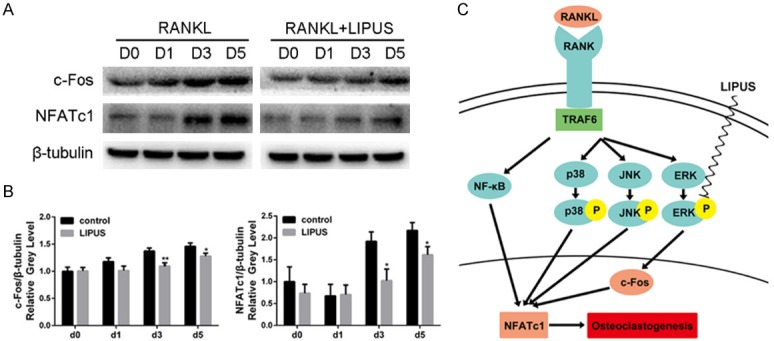
LIPUS suppresses RANKL-induce expression of c-Fos and NFATc1. BMMs were stimulated with M-CSF (30 ng/ml) and RANKL (50 ng/ml) in the presence or absence of LIPUS for 0, 1, 3 or 5 days. A. The protein expression levels of c-Fos and NFATc1 were analyzed using Western blotting. B. The grey levels of c-Fos and NFATc1 were quantified and normalized relative to β-tubulin by Image J. C. Schematic presentation of the inhibitory effects of LIPUS on RANKL signaling. LIPUS inhibited RANKL-induced osteoclast formation through disturbing ERK-c-Fos-NFATc1 signaling cascades. (*P < 0.05, **P < 0.01).
Discussion
Although LIPUS has been approved to be an effective form of physiotherapy in clinical application, the mechanism how it works during bone remodeling is uncertain. As both osteoblasts and osteoclasts have been reported to be responsible for bone remodeling, it is important to know the effects of LIPUS on them. A variety of researches have shown that LIPUS could enhance bone formation by promoting osteogenesis in vitro and in vivo [14,24]. However, the effects of LIPUS on osteoclast formation and activity is still controversial [17,25-27]. In this study, we have demonstrated that LIPUS suppressed RANKL-induced osteoclast formation and osteoclastic related gene expression via inhibiting the ERK-c-Fos-NFATc1 signaling cascades in vitro, further indicating that osteoclasts may be responsible for the effectiveness of LIPUS.
Previous studies have demonstrated that LIPUS is potential to be a noninvasive mechanical treatment for local bone diseases by modulating various cells in vivo to improve bone repairing and bone remodeling [13]. LIPUS, which is regarded as an acoustic form of mechanical energy, exerts its positive effects by transmitting the energy through and into living cells and tissues as a pressure wave with compression [28]. Several sensing receptors have been identified to response to mechanical stress and subsequently activate the following intracellular signaling cascades for the induction of cell proliferation, apoptosis, differentiation and functions [29-31]. Meanwhile, LIPUS has been verified to exert different effects on different cells. A positive effect of LIPUS has been observed on the osteogenic differentiation of osteoblasts and bone marrow stem cells (BMSCs) [14,32]. Some studies also have exhibited that LIPUS exerts an inhibitory effect on adipogenesis and a facilitated effect on angiogenesis [14,33]. As previous studies shown, LIPUS prevents osteolysis by suppressing osteoclast formation and activity in vivo [12,17]. In this study, we observed that LIPUS significantly inhibited RANKL-induced osteoclast formation using primary BMMs, which is consistent with previous in vivo studies. Some recent studies showed that different intensities of LIPUS exerts opposite effects on osteoclasts. The application of 100 mW/cm2 and 125 mW/cm2 decreased osteoclast activities [34,35], but 30 mW/cm2 LIPUS for 20 minute stimulation showed a promotive effect on osteoclastic resorptive activity of RAW264.7 cells [26]. However, in our study, no difference on osteoclastic resorptive activity was observed with or without the application of 30 mW/cm2 LIPUS. This might be due to the difference between primary macrophages and cell line.
The phosphorylation of ERK is a crucial signaling event which controls the differentiation of multiple cell types, including RANKL-induced osteoclastogenesis [36,37]. LIPUS has been reported to inhibit adipogenic differentiation via the suppression of ERK activation and promote osteogenesis via the enhancement of ERK activation, which indicated that LIPUS could regulate ERK signaling in different cell types [14]. In this study, LIPUS treatment showed a significant inhibitory effect on RANKL-induced ERK phosphorylation. In osteoclastogenesis, the binding of RANKL to RANK results in the phosphorylation of the MAPK signaling pathway, including ERK, JNK and p38 [38]. Subsequently, phosphorylated ERK promotes the activation of c-Fos, helping the formation of AP-1, an essential translation factor for osteoclast formation [39]. Additionally, c-Fos also showed a critical effect on the induction and translocation of NFATc1. NFATc1 is confirmed to be a master transcription factor for the formation and activities of osteoclasts [38]. Our results demonstrated that LIPUS markedly inhibited the mRNA and protein levels of NFATc1 and c-Fos, leading to the suppression of osteoclast-related genes expression, including TRAP, CTSK, CTR and DC-STAMP. Hence, it can be deduced that LIPUS suppressed osteoclast formation by the inhibition of the ERK-c-Fos-NFATc1 signaling pathway (Figure 5C), but it did not show any effects on mature osteoclast function.
In summary, our study has presented that LIPUS exerts an inhibitory effect on osteoclast formation by the inhibition of the ERK-c-Fos-NFATc1 signaling pathway, but no effect on mature osteoclast function. Our results reveal the exact effect and relative mechanism of LIPUS on osteoclasts, further helping the application of LIPUS clinically.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81772360, No. 31771106 and No. 81472113). The authors have no conflicts of interest to declare.
Disclosure of conflict of interest
None.
References
- 1.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 2.Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349–355. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- 3.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 4.Takayanagi H, Kim S, Matsuo K, Suzuki H, Suzuki T, Sato K, Yokochi T, Oda H, Nakamura K, Ida N, Wagner EF, Taniguchi T. RANKL maintains bone homeostasis through c-Fosdependent induction of interferon-beta. Nature. 2002;416:744–749. doi: 10.1038/416744a. [DOI] [PubMed] [Google Scholar]
- 5.Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251–264. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Feng X. RANKing intracellular signaling in osteoclasts. IUBMB Life. 2005;57:389–395. doi: 10.1080/15216540500137669. [DOI] [PubMed] [Google Scholar]
- 7.Huang H, Chang EJ, Ryu J, Lee ZH, Lee Y, Kim HH. Induction of c-Fos and NFATc1 during RANKL-stimulated osteoclast differentiation is mediated by the p38 signaling pathway. Biochem Biophys Res Commun. 2006;351:99–105. doi: 10.1016/j.bbrc.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Jurdic P, Saltel F, Chabadel A, Destaing O. Podosome and sealing zone: specificity of the osteoclast model. Eur J Cell Biol. 2006;85:195–202. doi: 10.1016/j.ejcb.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Jingushi S, Mizuno K, Matsushita T, Itoman M. Low-intensity pulsed ultrasound treatment for postoperative delayed union or nonunion of long bone fractures. J Orthop Sci. 2007;12:35–41. doi: 10.1007/s00776-006-1080-3. [DOI] [PubMed] [Google Scholar]
- 10.Wang SJ, Lewallen DG, Bolander ME, Chao EY, Ilstrup DM, Greenleaf JF. Low intensity ultrasound treatment increases strength in a rat femoral fracture model. J Orthop Res. 1994;12:40–47. doi: 10.1002/jor.1100120106. [DOI] [PubMed] [Google Scholar]
- 11.Wu S, Kawahara Y, Manabe T, Ogawa K, Matsumoto M, Sasaki A, Yuge L. Low-intensity pulsed ultrasound accelerates osteoblast differentiation and promotes bone formation in an osteoporosis rat model. Pathobiology. 2009;76:99–107. doi: 10.1159/000209387. [DOI] [PubMed] [Google Scholar]
- 12.Zhao X, Cai XZ, Shi ZL, Zhu FB, Zhao GS, Yan SG. Low-intensity pulsed ultrasound (LIPUS) may prevent polyethylene induced periprosthetic osteolysis in vivo. Ultrasound Med Biol. 2012;38:238–246. doi: 10.1016/j.ultrasmedbio.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Romano CL, Romano D, Logoluso N. Lowintensity pulsed ultrasound for the treatment of bone delayed union or nonunion: a review. Ultrasound Med Biol. 2009;35:529–536. doi: 10.1016/j.ultrasmedbio.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 14.Kusuyama J, Bandow K, Shamoto M, Kakimoto K, Ohnishi T, Matsuguchi T. Low intensity pulsed ultrasound (LIPUS) influences the multilineage differentiation of mesenchymal stem and progenitor cell lines through ROCKCot/Tpl2-MEK-ERK signaling pathway. J Biol Chem. 2014;289:10330–10344. doi: 10.1074/jbc.M113.546382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uddin SM, Qin YX. Enhancement of osteogenic differentiation and proliferation in human mesenchymal stem cells by a modified low intensity ultrasound stimulation under simulated microgravity. PLoS One. 2013;8:e73914. doi: 10.1371/journal.pone.0073914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naruse K, Miyauchi A, Itoman M, Mikuni-Takagaki Y. Distinct anabolic response of osteoblast to low-intensity pulsed ultrasound. J Bone Miner Res. 2003;18:360–369. doi: 10.1359/jbmr.2003.18.2.360. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z, Xu J, E L, Wang D. Ultrasound enhances the healing of orthodontically induced root resorption in rats. Angle Orthod. 2012;82:48–55. doi: 10.2319/030711-164.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki N, Hanmoto T, Yano S, Furusawa Y, Ikegame M, Tabuchi Y, Kondo T, Kitamura K, Endo M, Yamamoto T, Sekiguchi T, Urata M, Mikuni-Takagaki Y, Hattori A. Low-intensity pulsed ultrasound induces apoptosis in osteoclasts: fish scales are a suitable model for the analysis of bone metabolism by ultrasound. Comp Biochem Physiol A Mol Integr Physiol. 2016;195:26–31. doi: 10.1016/j.cbpa.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Chiou WF, Liao JF, Huang CY, Chen CC. 2-Methoxystypandrone represses RANKL-mediated osteoclastogenesis by down-regulating formation of TRAF6-TAK1 signalling complexes. Br J Pharmacol. 2010;161:321–335. doi: 10.1111/j.1476-5381.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakao J, Fujii Y, Kusuyama J, Bandow K, Kakimoto K, Ohnishi T, Matsuguchi T. Low-intensity pulsed ultrasound (LIPUS) inhibits LPS-induced inflammatory responses of osteoblasts through TLR4-MyD88 dissociation. Bone. 2014;58:17–25. doi: 10.1016/j.bone.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Shi Z, Silveira A, Liu J, Sawadogo M, Yang H, Feng X. Involvement of upstream stimulatory factors 1 and 2 in RANKL-induced transcription of tartrate-resistant acid phosphatase gene during osteoclast differentiation. J Biol Chem. 2003;278:20603–20611. doi: 10.1074/jbc.M212093200. [DOI] [PubMed] [Google Scholar]
- 22.Akisaka T, Yoshida H, Inoue S, Shimizu K. Organization of cytoskeletal F-actin, G-actin, and gelsolin in the adhesion structures in cultured osteoclast. J Bone Miner Res. 2001;16:1248–1255. doi: 10.1359/jbmr.2001.16.7.1248. [DOI] [PubMed] [Google Scholar]
- 23.Yamashita T, Yao Z, Li F, Zhang Q, Badell IR, Schwarz EM, Takeshita S, Wagner EF, Noda M, Matsuo K, Xing L, Boyce BF. NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J Biol Chem. 2007;282:18245–18253. doi: 10.1074/jbc.M610701200. [DOI] [PubMed] [Google Scholar]
- 24.Cheung WH, Chow SK, Sun MH, Qin L, Leung KS. Low-intensity pulsed ultrasound accelerated callus formation, angiogenesis and callus remodeling in osteoporotic fracture healing. Ultrasound Med Biol. 2011;37:231–238. doi: 10.1016/j.ultrasmedbio.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Inubushi T, Tanaka E, Rego EB, Ohtani J, Kawazoe A, Tanne K, Miyauchi M, Takata T. Ultrasound stimulation attenuates resorption of tooth root induced by experimental force application. Bone. 2013;53:497–506. doi: 10.1016/j.bone.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Feres MFN, Kucharski C, Diar-Bakirly S, El-Bialy T. Effect of low-intensity pulsed ultrasound on the activity of osteoclasts: an in vitro study. Arch Oral Biol. 2016;70:73–78. doi: 10.1016/j.archoralbio.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Hanmoto T, Tabuchi Y, Ikegame M, Kondo T, Kitamura KI, Endo M, Kobayashi I, Mishima H, Sekiguchi T, Urata M, Seki A, Yano S, Hattori A, Suzuki N. Effects of low-intensity pulsed ultrasound on osteoclasts: analysis with goldfish scales as a model of bone. Biomed Res. 2017;38:71–77. doi: 10.2220/biomedres.38.71. [DOI] [PubMed] [Google Scholar]
- 28.Costa V, Carina V, Fontana S, De Luca A, Monteleone F, Pagani S, Sartori M, Setti S, Faldini C, Alessandro R, Fini M, Giavaresi G. Osteogenic commitment and differentiation of human mesenchymal stem cells by low-intensity pulsed ultrasound stimulation. J Cell Physiol. 2018;233:1558–1573. doi: 10.1002/jcp.26058. [DOI] [PubMed] [Google Scholar]
- 29.Bandow K, Nishikawa Y, Ohnishi T, Kakimoto K, Soejima K, Iwabuchi S, Kuroe K, Matsuguchi T. Low-intensity pulsed ultrasound (LIPUS) induces RANKL, MCP-1, and MIP-1beta expression in osteoblasts through the angiotensin II type 1 receptor. J Cell Physiol. 2007;211:392–398. doi: 10.1002/jcp.20944. [DOI] [PubMed] [Google Scholar]
- 30.Manaka S, Tanabe N, Kariya T, Naito M, Takayama T, Nagao M, Liu D, Ito K, Maeno M, Suzuki N, Miyazaki M. Low-intensity pulsed ultrasound-induced ATP increases bone formation via the P2X7 receptor in osteoblastlike MC3T3-E1 cells. FEBS Lett. 2015;589:310–318. doi: 10.1016/j.febslet.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 31.Coskun ME, Coskun KA, Tutar Y. Determination of optimum operation parameters for low-intensity pulsed ultrasound and low-level laser based treatment to induce proliferation of osteoblast and fibroblast cells. Photomed Laser Surg. 2018;36:246–252. doi: 10.1089/pho.2017.4354. [DOI] [PubMed] [Google Scholar]
- 32.Unsworth J, Kaneez S, Harris S, Ridgway J, Fenwick S, Chenery D, Harrison A. Pulsed low intensity ultrasound enhances mineralisation in preosteoblast cells. Ultrasound Med Biol. 2007;33:1468–1474. doi: 10.1016/j.ultrasmedbio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Zhu H, Cai X, Lin T, Shi Z, Yan S. Low-intensity pulsed ultrasound enhances bone repair in a rabbit model of steroid-associated osteonecrosis. Clin Orthop Relat Res. 2015;473:1830–1839. doi: 10.1007/s11999-015-4154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monici M, Bernabei PA, Basile V, Romano G, Conti A, Breschi L, Masotti L, Cogoli A. Can ultrasound counteract bone loss? Effect of low-intensity ultrasound stimulation on a model of osteoclastic precursor. Acta Astronautica. 2007;60:383–390. [Google Scholar]
- 35.Chen SH, Wu CC, Wang SH, Li WT. The inhibition effect of low-intensity pulsed ultrasound on osteoclasts progenitor cells. 2012 Ieee International Ultrasonics Symposium (Ius) 2012:607–610. [Google Scholar]
- 36.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 37.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 38.Stevenson DA, Schwarz EL, Carey JC, Viskochil DH, Hanson H, Bauer S, Weng HY, Greene T, Reinker K, Swensen J, Chan RJ, Yang FC, Senbanjo L, Yang Z, Mao R, Pasquali M. Bone resorption in syndromes of the Ras/MAPK pathway. Clin Genet. 2011;80:566–573. doi: 10.1111/j.1399-0004.2010.01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu X, Ping Z, Gan M, Tao Y, Wang L, Shi J, Wu X, Zhang W, Yang H, Xu Y, Wang Z, Geng D. Theaflavin-3,3’-digallate represses osteoclastogenesis and prevents wear debris-induced osteolysis via suppression of ERK pathway. Acta Biomater. 2017;48:479–488. doi: 10.1016/j.actbio.2016.11.022. [DOI] [PubMed] [Google Scholar]


