Abstract
Uric acid is the final product of purine metabolism. Hyperuricemia is defined as a condition where the level of uric acid exceeds the normal range. The most well-known disease induced by hyperuricemia is gout. However, many studies have reported that hyperuricemia also plays important roles in cardiac-kidney-vascular system diseases and metabolic syndrome. Although hyperuricemia has been known for a long time, its pathophysiology remains poorly understood. In this review, we highlight studies on advanced pathological mechanisms for injuries induced by hyperuricemia, summarize epidemiological studies on hyperuricemia and its associated diseases, and take a brief look at hyperuricemia prevention.
Keywords: Hyperuricemia, uric acid, metabolic syndrome, cardiac-kidney-vascular system diseases, pathological mechanisms
Introduction
Uric acid is the final oxidation product of purine degradation. In humans, uric acid is mainly derived from endogenous production and food intake, with 70% being excreted by the kidneys and the remainder being primarily eliminated by the intestine [1]. Hyperuricemia is defined as a condition where the serum uric acid (SUA) level exceeds 339 μmol/L for premenopausal women and 416 μmol/L for men and postmenopausal women [2]. Previous studies have suggested that uric acid is a metabolic waste product that forms crystalline deposits in multiple organs or tissues, causing damage such as kidney stones and gout arthritis. With deeper research, it has been recognized that uric acid exerts a strong antioxidant effect that can remove oxygen free radicals generated by oxidative stress and avoid oxidative damage [1]. Consequently, uric acid may play a preventive role against the development of neurodegenerative processes leading to dementia [3], and exert a protective effect on bone metabolism to enhance bone mass, depress bone turnover, and reduce the prevalence of vertebral fractures [4] through its antioxidant characteristic. SUA levels may also be associated with cancer mortality. Specifically, a study from Holland found that SUA levels were associated with a lower risk of mortality from any type of cancer among males in a general population cohort followed up for 38 years, and this association was retained after adjustment for serum total cholesterol and triglyceride (TG) levels [5]. However, under hyperuricemic conditions, the beneficial effects of uric acid are replaced by deleterious effects. Hyperuricemia may be associated with metabolic syndrome (MetS) [6,7], and may also be related to vascular diseases, such as cardiovascular disease, and kidney disease. This article reviews the role of hyperuricemia in vascular diseases and MetS, and highlights the mechanisms underlying the effects of uric acid and its associated diseases.
Hyperuricemia and cardiovascular disease
Hyperuricemia is closely associated with cardiovascular diseases [8], including hypertension, coronary atherosclerotic heart disease, atrial fibrillation (AF), and heart failure (Figure 1).
Figure 1.
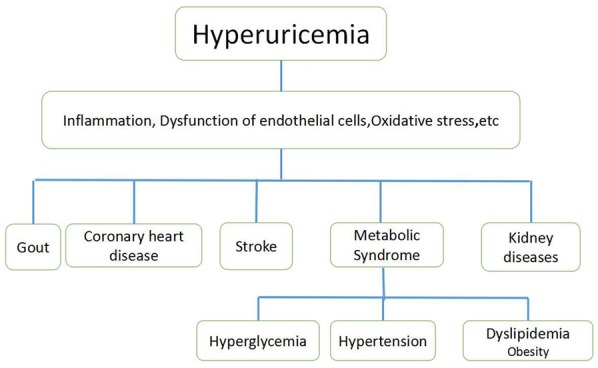
Hyperuricemia and its associated diseases.
Hyperuricemia and hypertension
Several studies have shown that hyperuricemia is an independent risk factor for hypertension [9,10]. Wang et al. [11] described that the odds ratio (OR) for prehypertension is 1.71 in subjects with UA ≥ 322.2 μmol/L compared with those with UA < 219.2 μmol/L after adjusting for many confounders among 1869 Chinese young adults from Shaanxi Province, China. Another study from Japan including a total of 2335 Japanese male workers without hypertension who ranged in age from 18 to 64 years with 6 years follow-up show that, compared with the lowest quartiles (UA ≤ 303.45 μmol/L), the highest serum UA quartiles (UA > 398.65 μmol/L) were 1.65 times greater multivariable-adjusted risk of incident hypertension [12]. In a study of the Multiple Risk factors Intervention in normotensive men, the presence of SUA levels greater than 7 mg/dl increased the risk of developing hypertension by 80% [13]. A randomized study showed that SUA lowering drugs were able to reduce blood pressure values in adolescent pre-hypertensive and hypertensive individuals. All these studies demonstrate that hypertension is related with high level of uric acid [14]. The mechanisms for hyperuricemia-induced hypertension may be as follows. (1) Hyperuricemia can lead to hypertension by blocking the production of nitric oxide (NO). NO can induce vasodilation to increase blood flow, reduce vascular smooth muscle cell (VSMC) proliferation, and modulate thrombosis, so it plays an important role in protecting the vasculature under physiological concentrations [15]. The decreases in NO induced by hyperuricemia are mediated by several signaling pathways and mechanisms as follows. (i) High levels of uric acid can lead to descending of NO by inducing reactive oxygen species (ROS). Li et al. [16] found that hyperuricemia can induce ROS accumulation in Human umbilical vein endothelial cells (HUVECs), thereby increasing phosphorylation of eNOS at Thr495 but not at Ser1177 (phosphorylation at Thr495 can reduce eNOS activity; on contrary, phosphorylation at Ser1177 can enhance eNOS activity), that eNOS phosphorylation at Thr495 is mediated by the PKC signaling pathway, and that inhibition of PKC activity can decrease uric acid-induced eNOS phosphorylation at Thr495, suggesting that uric acid induces phosphorylation of eNOS at Thr495 through a PKC-dependent pathway (Figure 2). Li et al. [16] also found that eNOS phosphorylation at Thr495 can reduce the interaction between eNOS and calmodulin, because eNOS activity is triggered by calmodulin binding [17,18] (Figure 2). In addition, the antioxidant can improve uric acid-induced eNOS inhibition and NO production. (ii) Hyperuricemia can reduce production of NO by impairing insulin receptor (IR) signaling pathway. As well-known, the binding of insulin and IR can activate two signaling pathway including the PI3K/Akt signaling pathway which promotes metabolic effects, and the MAPK-related signaling pathway which promotes cellular proliferation, differentiation, and gene expression. Choi et al. [19] revealed that uric acid can inhibit insulin-stimulated eNOS phosphorylation at Ser1177 in a time-dependent manner and reduced production of NO in endothelial cells mediated by the PI3K/Akt pathway. Afterwards Eliezer et al. [20] observed that high levels of uric acid can recruit ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1), a plasma membrane enzyme which is expressed in all major insulin target tissues to inhibit the function of insulin receptor thus impacting eNOS phosphorylation via PI3K/Akt signaling pathway (Figure 2). These results demonstrated that high uric acid level can decrease NO production independent of its ability to increase the oxidative stress burden at cellular level. Meanwhile, Chao et al. [21] found that uric acid can increase the level of ET-1 by stimulating ET-1 gene expression through insulin receptor signaling pathway which involves mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway (Figure 2). A balanced action of insulin for endothelial production of NO and endothelin (ET)-1 (one of the most powerful vasoconstrictor substances) is critical for maintaining hemodynamic homeostasis under healthy conditions [22]. High levels of uric acid can inhibit insulin-induced vasodilatation and promote insulin-induced vasoconstriction [20,21,23]. (iii) NO is primarily synthesized from L-arginine in endothelial cells by eNOS, and plasma L-arginine is transported in endothelial cells and stored in an arginine pool through the cation amino acid transporter (CAT) on the cell membrane, meaning that eNOS activity and L-arginine transmembrane transport are two rate-limiting steps for NO production (Figure 2). L-arginine is the only substrate for NO synthesis by eNOS, and is metabolized by several pathways including intracellular arginase, which is the final enzyme in the L-arginine-urea cycle. Some researchers found that high levels of uric acid can enhance L-arginine-arginase enzymatic activity by attenuating stimulated cGMP production in pulmonary arterial endothelial cells, leading to catabolism of arginine [17]. Schwartz et al. [24] found that hyperuricemia can reduce arginine uptake in endothelial cells, suggesting that uric acid can affect NO production through alterations in arginine isolated from eNOS activity (Figure 2). (2) Hyperuricemia-induced hypertension may be associated with the RAS. Zheng H et al. [25] carried out an experiment to investigate the relationship between hyperuricemia and atherosclerosis in an experimental rabbit model, they found that compared to the control, uric acid, plasma renin and plasma angiotensin II activities were enhanced (P < 0.001) in the hyperuricemia groups, smooth muscle cell (SMC) proliferating cell nuclear antigen expression increased strongly and intima thickness and intima areas elevated significantly, all these reactions can be blocked by losartan (a kind of angiotensin II receptor antagonist) at the dose of 30 mg/kg per day. Kirca et al. [26] observed that vascular smooth muscle cell (VSMCs) of rat aorta underwent proliferation in the condition of hyperuricemia which could be inhibited by losartan, and the proliferative pathways involved phosphorylation of p38 mitogen-activated protein kinase (p38 MAPK), p44/42 mitogen-activated protein kinase (p44/42 MAPK) and platelet-derived growth factor receptor β (PDGFRβ). Several observational studies in humans have investigated the positive correlation between uric acid levels and RAS activity in humans [27,28]. (3) As mentioned above, NO can relax vascular smooth muscle, and its effect is antagonistic to the sympathetic nervous system and the vasoconstrictor action of the vascular renin-angiotensin system (RAS) that together maintain the function of blood vessels. Excess uric acid reacts directly with NO to produce unstable nitrosouric acid and finally produces stable 6-aminouracil [2]; this weakens the role of NO in relaxing blood vessels and inhibiting proliferation of smooth muscle, causing the sympathetic nervous system and the RAS to act in a relatively hyperfunctional manner. (4) It is well known that the renal epithelial sodium channel (ENaC) is responsible for the rate-limiting step of sodium reabsorption and thus plays an important role in the maintenance of sodium balance, extracellular fluid volume, and blood pressure. Xu et al. demonstrated that hyperuricemia induces hypertension through activation of ENaC, and also found that the expressions of α-, β-, and γ-ENaC were significantly increased in hyperuricemic rats to enhance the absorption of sodium ions in the renal tubules, leading to an increase in blood volume that can cause hypertension [30]. (5) Uric acid crystals can be deposited in vascular endothelial cells to induce vascular endothelial damage directly, followed by lipid deposition from blood under the endothelium that damages endothelial cells, thereby inducing vascular endothelial injury and atherosclerosis. In addition, by acting as a barrier, endothelial cells have an anticoagulant effect, and damage to these cells not only weakens the anticoagulant effect, but also exposes subendothelial collagen to induce platelet aggregation and adhesion, thus promoting thrombus formation. Taken together, these two mechanisms can lead to the occurrence and development of hypertension. (6) Hyperuricemia may affect blood pressure by decreasing the production of adiponectin. Adiponectin, which is produced in adipose tissue, has many functions including anti-atherogenesis, insulin sensitization, lipid oxidation enhancement, regulation of platelet activation and vasodilatation [31,32]. Brzeska et al. [33] found that adiponectin was negatively correlated with uric acid. Nishizawa et al. [32] carried out a clinical trial to investigate the effect of febuxostat on circulating adiponectin in hyperuricemic patients, they found that plasma levels of adiponectin were lower in hyperuricemic patients than in normouricemic controls and the adiponectin increased significantly after only 6 months of febuxostat treatment. Other research has proven that high levels of uric acid can reduce adiponectin production both in vivo [34] and in vitro [35].
Figure 2.
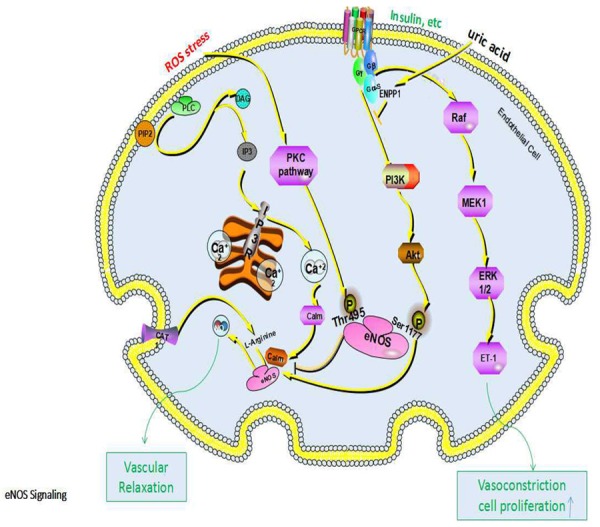
Mechanisms and pathways for the decrease in NO induced by uric acid.
Hyperuricemia and coronary atherosclerotic heart disease
There are some complex correlations between hyperuricemia and coronary heart disease (CHD). A study carried by Kim et al. [36] illustrates the relationship between uric acid and coronary artery calcification (CAC). They conducted a cross-sectional retrospective single-center study involving 4884 participants without overt coronary artery disease and obtained their CAC scores. The CAC score showed a significant positive association with the SUA level, with retention of statistical significance after adjusting for confounding factors. CAC score was reported to be useful for identifying individuals at high risk for CHD and a strong predictor for all-cause mortality [37,38]. Choi et al. [39] conducted the first study on the effects of SUA on arterial stiffness. For this, all of their participants underwent brachial-ankle pulse wave velocity (baPWV) assessment to detect arterial stiffness. They found that elevated SUA was independently associated with increased baPWV. A study from China involving 6347 middle-aged and elderly Chinese patients revealed that the 10-year CHD risk was increased by 2.76 times in patients with hyperuricemia compared with patients without hyperuricemia in the female population [40]. Hyperuricemia may promote the development and progression of CHD by forming uric acid crystals, inducing NO production, exerting oxidative stress, promoting inflammatory reactions, promoting oxidation modification of low-density lipoprotein-cholesterol (LDL-C), stimulating proliferation of VSMCs through the RAS [39,40], and reducing adiponectin production [41].
Hyperuricemia and atrial fibrillation
Atrial fibrillation (AF) is a common cardiac arrhythmia associated with the risks of heart failure and stroke, and can increase the risks of mortality and morbidity [42]. A large-scale epidemiological study involving 90,117 subjects carried by Kuwabara et al. [42] analyzed 49,292 subjects without competing risk factors (hypertension, type 2 diabetes mellitus, cardiovascular disease, chronic kidney disease, heart failure) and observed that hyperuricemia was an independent competing risk factor for AF in an apparently healthy general population. A meta-analysis of cohort studies produced a similar result [43]. The mechanism for hyperuricemia-induced AF may be as follows. (1) Hyperuricemia-induced AF may associate with increased calpain-1 expression and activation. Yan M et al. [44] described the expression and activation of calpain-1, which has been reported to participate in several pathological conditions affecting the cardiovascular system, was increased in the cardiac tissue of hyperuricemic rats inducing cardiomyocyte ER stress and subsequent apoptosis as well as interstitial fibrosis, and the allopurinol pretreatment mitigated all the above changes induced by hyperuricemia (Figure 3). (2) Hyperuricemia-induced AF may associate with inflammation. As mentioned above, high dose UA increased expression of inflammation cytokines [45]. Inflammatory cytokine such as Interleukin-6 (IL-6), Interleukin-8 (IL-8), Interleukin-10 (IL-10) and tumor necrosis factor (TNF-α) et al. can activate nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) signaling pathway causing extracellular matrix (ECM) proteins deposition, thus inducing fibrotic remodeling [46] (Figure 3). (2) The ROS generation induced by activation of xanthine oxidases and NADPH oxidases plays an important role in hyperuricemia-induced-atrial fibrillation. Korantzopoulos et al. [47] reported that increased SUA was accompanied by an increase in cardiac tissue xanthine oxidase activation. ROS production through activation of xanthine oxidase may contribute to the pathological consequences of AF such as thrombosis, inflammation, and tissue remodeling [48,49]. A series of researchers demonstrated that allopurinol therapy decreased atrial vulnerability by inhibiting atrial remodeling [49,50]. The activation of NADPH oxidases also exert similar effect on cardiac myocyte [51,52]. The ROS can shorten the atrial action potentials (APD) and promote delayed after depolarizations (DAD) by altering various ion channels such as L-type Ca2+-channel (LTCC) [53] and IK1 channel, both of which involved in nuclear factor of activated T cell (NFAT) signaling pathway and Kv1.5 channel, which involved in ERK1/2 signaling pathway [51] (Figure 3). (3) Hyperuricemia can activate the RAS system to increase the risk of AF. The RAS plays an significant role in the development of various cardiovascular diseases, including atrial fibrillation (AF). Just as mentioned above [25], hyperuricemia can increase the angiotensin II level. A recent study found that mice treated with angiotensin II showed increased neutrophil infiltration into their atrial tissue [54]. In clinical settings, the angiotensin receptor blocker losartan showed a 33% reduction in the incidence of new-onset AF, suggesting that losartan may decrease the risk of AF by abolishing the RAS system activation induced by hyperuricemia [55]. The arrhythmogenic effects of angiotensin II are associated with atrial fibrosis induced by increased production of the extracellular matrix (ECM) proteins which involved PKC pathway, ERK1/2 pathway and NF-κB pathway, and with the alteration of various ion channels induced by oxidative stress and inflammation [47,52,53], thus resulting in electrical and fibrotic remodeling (Figure 3). Taken together, hyperuricemia is involved in the pathogenesis of AF through electrical and structural remodeling.
Figure 3.
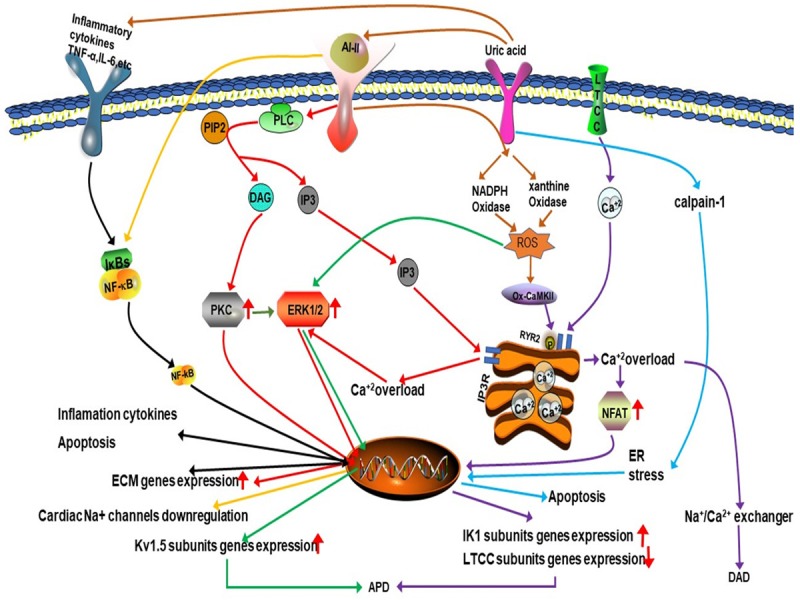
The mechanism of hyperuricemia-induced atrial fibrillation.
Hyperuricemia and hyperglycemia
Hyperuricemia is strongly associated with insulin resistance and abnormal glucose metabolism. Several studies reported that high uric acid level showed a negative effect on islet beta cells and glucose regulation [56-59], and allopurinol lowers uric acid and improves insulin resistance and systemic inflammation in asymptomatic hyperuricemia [60]. (1) Zhu et al. [61] found that increased ROS can be generated in an hyperuricemia mouse model, that the produced ROS can increase phosphorylation of insulin receptor substrate at a serine residue and decrease its phosphorylation at a tyrosine residue, and that two reactions block the phosphorylation of protein kinase B at a serine residue, leading to failure of downstream signaling and resulting in insulin resistance (Figure 4). (2) Hyperuricemia-induced-insulin resistance is associated with decrease of endothelial NO levels. As mentioned above, hyperuricemia can reduce the endothelial NO production. NO is a key regulator for insulin sensitivity of peripheral tissues for NO leads to increase blood flow and to enhance glucose uptake by cells [62] (Figure 4). (3) Hyperuricemia can induce insulin resistance and beta cell apoptosis through the activation of the NLRP3 pathway. High Uric acid level can cause lysosomal dysfunction and activate NLRP3 pathway producing inflammatory factors (IL-1β, IL-18), thus activating JNks pathway inducing insulin resistance and NF- kappa B pathway initiating the procedure of apoptosis in islet B cells [63,64] (Figure 4). (2) Hyperuricemia can set negative effect on pancreatic β-cell growth and insulin secretion through activation of adenosine monophosphate-activated protein kinase (AMPK) which could be induced by ROS and inflammation. Zhang et al. [65] revealed the presence of increased ROS at high levels in uric acid-treated β-cells. As a target for oxidative stress, it is known that adenosine monophosphate-activated protein kinase (AMPK) can be phosphorylated by the actions of ROS. In the cited study, the authors found the effects of high levels of uric acid on β-cells through oxidative damage and growth inhibition are mediated by activation of the AMPK and ERK signaling pathways, and found that ERK is a downstream target of AMPK in uric acid-treated β-cells. Other researchers also observed that the activation of AMPK caused by ROS and inflammation can induce β-cells injury and apoptosis [66-69]. As well known, AMPK contributes to the salutary effects of adipokines on fatty acid oxidation, glucose utilization and insulin sensitivity, relatively short-term activation of AMPK has been regarded as a therapeutic strategy for obesity and T2DM [67], however, sustained activation of the enzyme can lead to programmed cell death and this process may involve in various signaling pathway [66] (Figure 5). (3) The activation of nuclear factor (NF)-κB and subsequent production of NO by inducible nitric oxide synthase (iNOS) have been proven to be responsible for β-cell damage and death. In the study by Jia et al. [70], hyperuricemia was observed to cause pancreatic β-cell death and dysfunction through the NF-κB-iNOS-NO signaling axis. In addition, they found that high levels of uric acid enhanced the degradation of musculoaponeurotic fibrosarcoma oncogene homolog A (MafA) protein (one of the transcription factors for insulin synthesis), thereby reducing glucose-stimulated insulin synthesis and secretion (Figure 5). (4) Hyperuricemia can increase insulin resistance by increasing the production of monocyte chemoattractant protein (MCP)-1 and reducing adiponectin production. MCP-1, a chemotactic factor that causes aggregation of monocytes in tissues, has an important role in inflammatory processes. MCP-1 was reported to be significantly increased in patients with type 2 diabetes, and has been suggested as a possible molecular marker for this disease [70,71]. Yang L et al. described MCP-1 can decrease the function and quantity of islet β cells and induce insulin resistance by contributing to macrophage infiltration into islet β cells and the target tissue of insulin [72]. Another important factor affecting insulin resistance is adiponectin, which is mainly produced in adipose tissue [41] and controlled by peroxisome proliferator-activated receptor-γ (PPAR-γ) [73,74]. As adiponectin receptors are expressed in both fat and muscle tissues in humans, adiponectin can exert a potent insulin-sensitizing effect [75]. Baldwin et al. [35] observed a significant increase in the abundance of MCP-1 mRNA and a gradual decrease in adiponectin mRNA in dose-dependent and time-dependent manners in differentiated mouse 3T3-L1 adipocytes cultured with high levels of uric acid. They further found that the uric acid-induced increase in MCP-1 production could be inhibited by antioxidants and rosiglitazone (a PPAR-γ agonist), suggesting that activation of MCP-1 expression and secretion in adipocytes is mediated by superoxide-dependent ROS and a mechanism involving PPAR-γ. However, the effect of uric acid on adiponectin production was not prevented by antioxidants, but could be abrogated by rosiglitazone, suggesting that uric acid-induced inhibition of adiponectin production is not mediated by redox-dependent signaling, but rather by a mechanism involving PPAR-γ. They also found that uric acid could induce downregulation of PPAR-γ by affecting xanthine oxidoreductase (an enzyme producing uric acid that acts as a crucial upstream regulator of PPAR-γ activity) [35] via a negative feedback mechanism (Figure 6).
Figure 4.
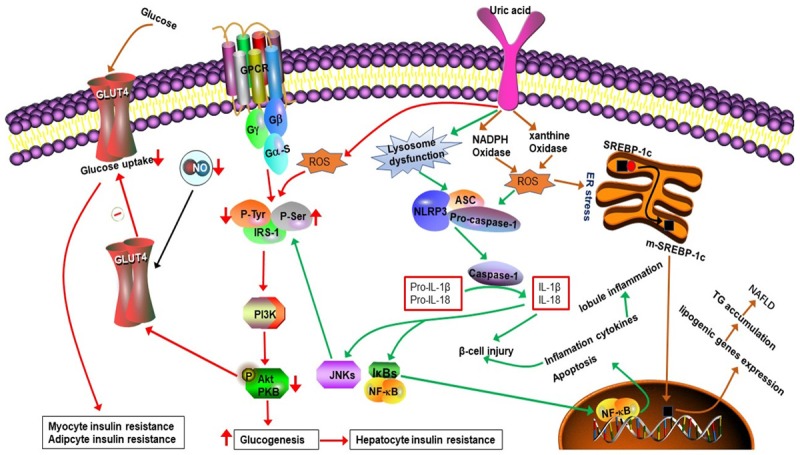
The mechanism of hyperuricemia-induced insulin resistance and Nonalcoholic fatty liver.
Figure 5.
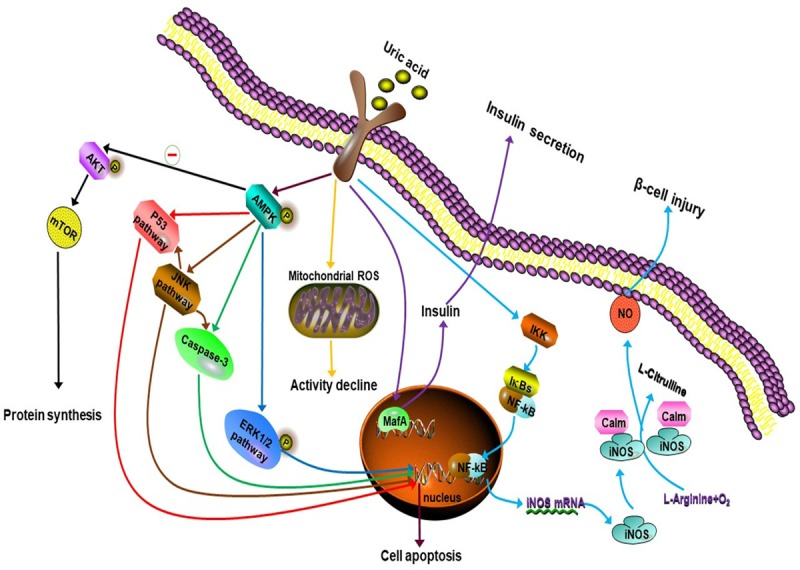
Mechanisms of hyperuricemia-induced β-Cell injury and dysfunction.
Figure 6.
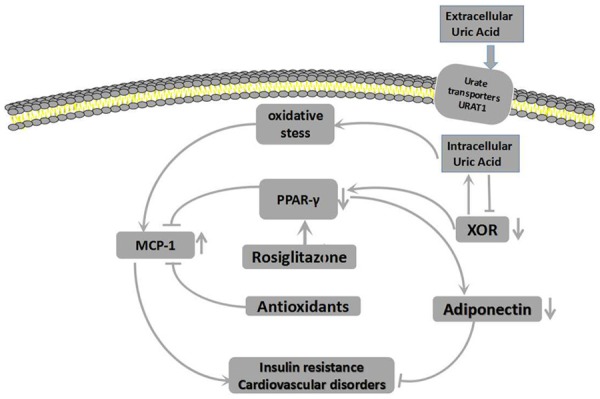
Mechanisms for the increase in MCP-1 and decrease in adiponectin induced by uric acid.
Hyperuricemia and obesity, NAFLD and dyslipidemia
A previous study showed that increased uric acid levels are associated with MetS components, such as hypertriglyceridemia, insulin resistance, elevated blood pressure, and low high-density lipoprotein-cholesterol (HDL-C), but the relationship between uric acid and hypertriglyceridemia was the strongest and most stable [76]. Zheng X et al. reported that elevated uric acid level shows independently positive associations with the prevalence of Non-alcoholic fatty liver disease (NAFLD) and the severity of fatty liver [77]. Sirota et al. [78] got the similar results and found that the positive association between SUA and NAFLD was independent of insulin resistance. Yang C et al. [79] observed that hyperuricemic subjects had significant higher TC and TG levels, compared to obese and non-obese normouricemic subjects. Kuwabara, et al. [80] reported that an elevated SUA increases the risk for developing high LDL cholesterol, as well as hypertriglyceridemia. The hyperuricemia-induced- abnormal storage of fat may be related to the following mechanisms. (1) High uric acid level can induce insulin resistance resulting hyperinsulinemia [61,72], high level of insulin promotes lipolysis in the adipocyte and increases free fatty acids (FFAs) delivered to the liver, where subsequent accumulation of the triglycerides may lead to hepatic steatosis. (2) SUA levels contribute to NAFLD pathogenesis through their association with ROS and subsequent ER stress [81,82]. High uric acid level activates NADPH Oxidase (NOX) after entering into hepatocytes and induces membranous NOX-dependent ROS production, which stimulates ER stress. ER stress stimulates sterol regulatory element-binding protein-1c (SREBP-1c) cleavage into mature form, which is translocated into nucleus and activates the transcription of lipogenic genes (lipogenic Enzymes). Metformin, an inhibitor of SREBP-1c, and antioxidants can blocked hepatic fat accumulation. NOX-dependent ROS further activates mitochondrial ROS production, which can also be activated by ER stress. Mitochondrial ROS is also reported to be an important mechanism of TG accumulation in hepatocyte [82] (Figure 4). (3) The prevalence rates of high NAFLD activity score (NAS) and lobule inflammation tended to increase with increases in the SUA level, which suggested that the inflammation of NAFLD tended to increase as the SUA level increased [83], the inflammation development of NAFLD may involve in NLRP3 inflammasome-dependent mechanism [63] (Figure 4). (4) Hyperuricemia-induced high TG levels may be associated with a decreased activity in glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a protein that is known to be one of the major targets of ROS [84]. Given that GAPDH is a key enzyme in the glycolysis pathway and the central link for glucose metabolism, a decrease in its activity will lead to inhibition of glycolysis, which can lead to accumulation of not only glucose, but also large amounts of glyceraldehyde 3-phosphate, thus promoting its isomerization or transformation of dihydroxyphosphate acetone into glycerol. As glycerol is an important material for TG synthesis, this can in turn lead to an increase in TG synthesis. (5) Hyperuricemia may affect lipid metabolism by reducing the activity of lipoprotein lipase through inflammation. Some researchers described that IL-1β, a product of NLRP3 inflammasome pathway, could inhibit lipoprotein lipase [64]. It is known that lipoprotein lipase can break down the core of lipoproteins (TG), and that a decrease in the expression of this enzyme can lead to a decrease in TG catabolism. (6) Hyperuricemia can induce dyslipidemia by reducing adiponectin production. Some researchers reported that adiponectin was positively associated with HDL-C and lipoprotein lipase and negatively associated with LDL-C and TG concentrations [85]. Similar correlations among adiponectin, HDL-C, and TG were also observed in postmenopausal women and female adolescents [41] and, as previously mentioned, hyperuricemia can decrease adiponectin production. (7) Obesity is associated with infiltration of monocytes into adipose tissue, which can lead to activation of inflammation pathways, reduction in lipid turnover, and deposition of fat in ectopic locations [86]. As mentioned above, hyperuricemia can lead to elevated MCP-1 levels in adipose tissue, which cause aggregation of monocytes in adipose tissues, and in turn, these accumulated monocytes in adipose tissues secrete inflammatory cytokines, such as TNF-α, IL-6, and MCP-1 [87]. These effects create a vicious circle that aggravates the metabolic disorder in adipose tissues.
Hyperuricemia and kidney diseases
Hyperuricemia is associated with chronic kidney disease (CKD). A prospective cohort study involving 18,778 men who participated in a health checkup program was carried out to test the relationship between elevated SUA levels and risk of developing CKD over a 4-year follow-up period. It was found that a high SUA level was significantly associated with an increased likelihood for development of CKD after adjusting for well-known independent risk factors for CKD development such as age, baseline estimated glomerular filtration rate (eGFR), systolic blood pressure, homeostasis model assessment of insulin resistance, TG, body mass index, alcohol intake, smoking status, regular exercise, hypertension, and diabetes mellitus [87]. Weiner et al. [88] revealed that the risk of developing CKD increased by 7-11% per 1 mg/dL uric acid.
Hyperuricemia may also increase mortality among CKD patients undergoing hemodialysis. In a study aiming to determine the impact of hyperuricemia on long-term (19.5 years) survival of CKD patients, it was observed that the mortality of normouricemic patients (50%) was lower than that of hyperuricemic patients (82.4%) [89].
Elevated SUA levels can delay renal recovery in living kidney donors. A study carried by Bravo et al. [90] analyzed the association between pre-donation SUA level in 291 live kidney donors and residual renal function at 6 months and 1 year after nephrectomy. They showed that females in the highest tertile (SUA > 4.5 mg/dL) had a significantly lower eGFR and a significantly higher percentage of donors with eGFR < 60 mL/min/1.73 m2 compared with those in the lower tertiles at 6 months and 1 year post-donation. Furthermore, a logistic regression showed that SUA was a significant independent predictor for development of eGFR < 60 mL/min/1.73 m2 at 1 year after donation in female donors. Other researchers reported that a 59.5 µmol/L increase in baseline SUA was associated with a 1.7-fold higher risk of a > 25% decrease in eGFR in women [91]. These findings suggest that pre-donation SUA is predictive of delayed renal recovery post-nephrectomy in female donors.
Hyperuricemia plays an important role in the development of all-cause (peritonitis, non-compliance, operational problem, ultrafiltration insufficiency, dialysis inadequacy, mechanical problems, structural defect, surgical procedure, tunnel infection, other reason, unknown) and peritonitis-related technique failure in patients starting continuous ambulatory peritoneal dialysis (CAPD) as renal replacement therapy. Hsieh et al. [92] conducted a retrospective study involving 371 participants on CAPD. During the study period, technique failure occurred in 34.4% patients in the hyperuricemia group compared with 19.4% in the normouricemia group. (5) Hyperuricemia is a major risk factor for contrast induced-acute kidney injury (CI-AKI). A meta-analysis including 18 relevant studies involving a total of 13,084 patients presented compelling evidence that higher SUA was associated with a 1.68-fold higher risk for CI-AKI compared with lower SUA [93]. It was also observed that patients with hyperuricemia had higher in-hospital mortality and higher risk for renal replacement therapy after coronary angiography and/or percutaneous coronary intervention. Another meta-analysis including 10 studies with 10,427 patients revealed that higher SUA conferred a 2-fold higher risk for CI-AKI compared with lower SUA, and that administration of allopurinol may help to reduce the risk of CI-AKI among patients with hyperuricemia undergoing coronary procedures [94]. As hyperuricemia is a major risk factor for CI-AKI, its levels can be measured to assess the risk of CI-AKI development and short-term clinical outcomes before cardiac procedures. The impact of hyperuricemia in CKD and acute kidney injury may be related to the following mechanisms. First, as mentioned above, a high level of uric acid can activate the RAAS, inducing strong vascular contraction and VSMC proliferation, and further diminish the renal blood flow as well as increase renal vascular resistance, especially in afferent arterioles [25,26]. Second, hyperuricemia can enhance oxidative stress induced by nicotinamide adenine dinucleotide phosphate (NADP) oxidase and xanthine oxidase [95-97] and reduce NO production and bioavailability, inducing further endothelial dysfunction [20]. Third, uric acid can increase the production of many inflammatory factors, including IL-1, IL-6 and C-reactive protein, thereby stimulating the inflammatory response and inducing tubular injury and leading to glomerular hypertrophy and renal tubular interstitial fibrosis [97,98]. Fourth, uric acid crystals can directly injure tubules.
Sufficient attention should be paid to the damage induced by hyperuricemia. The incidence of hyperuricemia can be reduced by eating less foods containing high purine levels, such as seafood, animal offal, and increasing intake of vegetables, fruits, milk, and eggs. Health education can be carried out to improve people’s awareness of hyperuricemia, which may contribute to a reduction or delay in the occurrence and development of hyperuricemia and its complications.
Acknowledgements
This study was supported by a research grant from Science and Technology Department of Jilin Province (20160101035JC, to WG).
Disclosure of conflict of interest
None.
References
- 1.Zou H, Wang H, Liu T, Li X, Zhu X, Wang Z. Protective role of α-lipoic acid in hyperuricemiainduced endothelial dysfunction. Exp Ther Med. 2017;13:3047–3054. doi: 10.3892/etm.2017.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen C, Lü JM, Yao Q. Hyperuricemia-related diseases and xanthine oxidoreductase (XOR) inhibitors: an overview. Med Sci Monit. 2016;22:2501–2512. doi: 10.12659/MSM.899852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuven B, Soysal P, Unutmaz G, Kaya D, Isik AT. Uric acid may be protective against cognitive impairment in older adults, but only in those without cardiovascular risk factors. Exp Gerontol. 2017;89:15–19. doi: 10.1016/j.exger.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Muka T, de Jonge EA, Kiefte-de Jong JC, Uitterlinden AG, Hofman A, Dehghan A, Zillikens MC, Franco OH, Rivadeneira F. The influence of serum uric acid on bone mineral density, hip geometry, and fracture risk: the rotterdam study. J Clin Endocrinol Metab. 2016;101:1113–1122. doi: 10.1210/jc.2015-2446. [DOI] [PubMed] [Google Scholar]
- 5.Taghizadeh N, Vonk JM, Boezen HM. Serum uric acid levels and cancer mortality risk among males in a large general population-based cohort study. Cancer Causes Control. 2014;25:1075–1080. doi: 10.1007/s10552-014-0408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbasian M, Ebrahimi H, Delvarianzadeh M, Norouzi P, Fazli M. Association between serum uric acid (SUA) levels and metabolic syndrome (MetS) components in personnel of Shahroud University of Medical Sciences. Diabetes Metab Syndr. 2016;10:132–136. doi: 10.1016/j.dsx.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Choi H, Kim HC, Song BM, Park JH, Lee JM, Yoon DL, Yoon YM, Rhee Y, Youm Y, Kim CO. Serum uric acid concentration and metabolic syndrome among elderly Koreans: the Korean urban rural elderly (KURE) study. Arch Gerontol Geriatr. 2016;64:51–58. doi: 10.1016/j.archger.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Safiri S, Qorbani M, Heshmat R, Tajbakhsh R, Eslami Shahr Babaki A, Djalalinia S, Motlagh ME, Tajadini MH, Asayesh H, Safari O, Kelishadi R. Association of serum uric acid with cardiometabolic risk factors and metabolic syndrome in iranian adolescents: the CASPIAN-III study. Iran J Kidney Dis. 2016;10:126–134. [PubMed] [Google Scholar]
- 9.Katsiki N, Doumas M, Athyros VG, Karagiannis A. Hyperuricemia as a risk factor for cardiovascular disease. Expert Rev Cardiovasc Ther. 2015;13:19–20. doi: 10.1586/14779072.2015.987129. [DOI] [PubMed] [Google Scholar]
- 10.Marotta T, Liccardo M, Schettini F, Verde F, Ferrara AL. Association of hyperuricemia with conventional cardiovascular risk factors in elderly patients. J Clin Hypertens (Greenwich) 2015;17:27–32. doi: 10.1111/jch.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Hu JW, Qu PF, Wang KK, Yan Y, Chu C, Zheng WL, Xu XJ, Lv YB, Ma Q, Gao K, Yuan Y, Li H, Yuan ZY, Mu JJ. Association between urinary sodium excretion and uric acid, and its interaction on the risk of prehypertension among Chinese young adults. Sci Rep. 2018;8:7749. doi: 10.1038/s41598-018-26148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kansui Y, Matsumura K, Morinaga Y, Inoue M, Kiyohara K, Ohta Y, Goto K, Ohtsubo T, Ooboshi H, Kitazono T. Impact of serum uric acid on incident hypertension in a worksite population of Japanese men. J Hypertens. 2018;36:1499–1505. doi: 10.1097/HJH.0000000000001743. [DOI] [PubMed] [Google Scholar]
- 13.Orlando A, Cazzaniga E, Giussani M, Palestini P, Genovesi S. Hypertension in children: role of obesity, simple carbohydrates, and uric acid. Front Public Health. 2018;6:129. doi: 10.3389/fpubh.2018.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMullan CJ, Borgi L, Fisher N, Curhan G, Forman J. Effect of uric acid lowering on renin-angiotensin-system activation and ambulatory bp: a randomized controlled trial. Clin J Am Soc Nephrol. 2017;12:807–816. doi: 10.2215/CJN.10771016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Förstermann U, Xia N, Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res. 2017;120:713–735. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 16.Li P, Zhang L, Zhang M, Zhou C, Lin N. Uric acid enhances PKC-dependent eNOS phosphorylation and mediates cellular ER stress: a mechanism for uric acid-induced endothelial dysfunction. Int J Mol Med. 2016;37:989–997. doi: 10.3892/ijmm.2016.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haque MM, Ray SS, Stuehr DJ. Phosphorylation controls endothelial nitric-oxide synthase by regulating its conformational dynamics. J Biol Chem. 2016;291:23047–23057. doi: 10.1074/jbc.M116.737361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JH, Jin YM, Hwang S, Cho DH, Kang DH, Jo I. Uric acid attenuates nitric oxide production by decreasing the interaction between endothelial nitric oxide synthase and calmodulin in human umbilical vein endothelial cells: a mechanism for uric acid-induced cardiovascular disease development. Nitric Oxide. 2013;32:36–42. doi: 10.1016/j.niox.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Tassone EJ, Cimellaro A, Perticone M, Hribal ML, Sciacqua A, Andreozzi F, Sesti G, Perticone F. Uric acid impairs insulin signaling by promoting Enpp1 binding to insulin receptor in human umbilical vein endothelial cells. Front Endocrinol (Lausanne) 2018;9:98. doi: 10.3389/fendo.2018.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi YJ, Yoon Y, Lee KY, Hien TT, Kang KW, Kim KC, Lee J, Lee MY, Lee SM, Kang DH, Lee BH. Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. FASEB J. 2014;28:3197–3204. doi: 10.1096/fj.13-247148. [DOI] [PubMed] [Google Scholar]
- 21.Chao HH, Liu JC, Lin JW, Chen CH, Wu CH, Cheng TH. Uric acid stimulates endothelin-1 gene expression associated with NADPH oxidase in human aortic smooth muscle cells. Acta Pharmacol Sin. 2008;29:1301–1312. doi: 10.1111/j.1745-7254.2008.00877.x. [DOI] [PubMed] [Google Scholar]
- 22.Dubó S, Gallegos D, Cabrera L, Sobrevia L, Zúñiga L, González M. Cardiovascular action of insulin in health and disease: endothelial l-arginine transport and cardiac voltage-dependent potassium channels. Front Physiol. 2016;7:74. doi: 10.3389/fphys.2016.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turaihi AH, Bakker W, van Hinsbergh VW, Serné EH, Smulders YM, Niessen HW, Eringa EC. Insulin receptor substrate 2 controls insulin-mediated vasoreactivity and perivascular adipose tissue function in muscle. Front Physiol. 2018;9:245. doi: 10.3389/fphys.2018.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz IF, Grupper A, Chernichovski T, Grupper A, Hillel O, Engel A, Schwartz D. Hyperuricemia attenuates aortic nitric oxide generation, through inhibition of arginine transport, in rats. J Vasc Res. 2011;48:252–260. doi: 10.1159/000320356. [DOI] [PubMed] [Google Scholar]
- 25.Zheng H, Li N, Ding Y, Miao P. Losartan alleviates hyperuricemia-induced atherosclerosis in a rabbit model. Int J Clin Exp Pathol. 2015;8:10428–35. [PMC free article] [PubMed] [Google Scholar]
- 26.Kırça M, Oğuz N, Çetin A, Uzuner F, Yeşilkaya A. Uric acid stimulates proliferative pathways in vascular smooth muscle cells through the activation of p38 MAPK, p44/42 MAPK and PDGFRβ. J Recept Signal Transduct Res. 2017;37:167–173. doi: 10.1080/10799893.2016.1203941. [DOI] [PubMed] [Google Scholar]
- 27.Assadi F. Allopurinol enhances the blood pressure lowering effect of enalapril in children with hyperuricemic essential hypertension. J Nephrol. 2014;27:51–56. doi: 10.1007/s40620-013-0009-0. [DOI] [PubMed] [Google Scholar]
- 28.Chida R, Hisauchi I, Toyoda S, Kikuchi M, Komatsu T, Hori Y, Nakahara S, Sakai Y, Inoue T, Taguchi I. Impact of irbesartan, an angiotensin receptor blocker, on uric acid level and oxidative stress in high-risk hypertension patients. Hypertens Res. 2015;38:765–769. doi: 10.1038/hr.2015.82. [DOI] [PubMed] [Google Scholar]
- 29.Tani S, Nagao K, Hirayama A. Effect of febuxostat, a xanthine oxidase inhibitor, on cardiovascular risk in hyperuricemic patients with hypertension: a prospective, open-label, pilot study. Clin Drug Investig. 2015;35:823–831. doi: 10.1007/s40261-015-0349-8. [DOI] [PubMed] [Google Scholar]
- 30.Xu W, Huang Y, Li L, Sun Z, Shen Y, Xing J, Li M, Su D, Liang X. Hyperuricemia induces hypertension through activation of renal epithelial sodium channel (ENaC) Metabolism. 2016;65:73–83. doi: 10.1016/j.metabol.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 31.Srikanthan K, Feyh A, Visweshwar H, Shapiro JI, Sodhi K. Systematic review of metabolic syndrome biomarkers: a panel for early detection, management, and risk stratification in the West Virginian population. Int J Med Sci. 2016;13:25–38. doi: 10.7150/ijms.13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishizawa T, Taniura T, Nomura S. Effects of febuxostat on platelet-derived microparticles and adiponectin in patients with hyperuricemia. Blood Coagul Fibrinolysis. 2015;26:887–892. doi: 10.1097/MBC.0000000000000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brzeska A, Sołtysiak M, Ziemak J, Miazgowski T, Widecka K. Plasma adiponectin in hypertensive patients with and without metabolic syndrome. Arterial Hypertension. 2018;22:29–36. [Google Scholar]
- 34.Tamba S, Nishizawa H, Funahashi T, Okauchi Y, Ogawa T, Noguchi M, Fujita K, Ryo M, Kihara S, Iwahashi H, Yamagata K, Nakamura T, Shimomura I, Matsuzawa Y. Relationship between the serum uric acid level, visceral fat accumulation and serum adiponectin concentration in Japanese men. Intern Med. 2008;47:1175–1180. doi: 10.2169/internalmedicine.47.0603. [DOI] [PubMed] [Google Scholar]
- 35.Baldwin W, McRae S, Marek G, Wymer D, Pannu V, Baylis C, Johnson RJ, Sautin YY. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes. 2011;60:1258–1269. doi: 10.2337/db10-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim H, Kim SH, Choi AR, Kim S, Choi HY, Kim HJ, Park HC. Asymptomatic hyperuricemia is independently associated with coronary artery calcification in the absence of overt coronary artery disease: a single-center cross-sectional study. Medicine (Baltimore) 2017;96:e6565. doi: 10.1097/MD.0000000000006565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valenti V, Ó Hartaigh B, Heo R, Cho I, Schulman-Marcus J, Gransar H, Truong QA, Shaw LJ, Knapper J, Kelkar AA, Sandesara P, Lin FY, Sciarretta S, Chang HJ, Callister TQ, Min JK. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. JACC Cardiovasc Imaging. 2015;8:900–909. doi: 10.1016/j.jcmg.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JH, Han D, Ó Hartaigh B, Rizvi A, Gransar H, Park HB, Park HE, Choi SY, Chun EJ, Sung J, Park SH, Han HW, Min JK, Chang HJ. Warranty period of zero coronary artery calcium score for predicting all-cause mortality according to cardiac risk burden in asymptomatic Korean adults. Circ J. 2016;80:2356–2361. doi: 10.1253/circj.CJ-16-0731. [DOI] [PubMed] [Google Scholar]
- 39.Choi HY, Kim SH, Choi AR, Kim SG, Kim H, Lee JE, Kim HJ, Park HC. Hyperuricemia and risk of increased arterial stiffness in healthy women based on health screening in Korean population. PLoS One. 2017;12:e0180406. doi: 10.1371/journal.pone.0180406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Y, Tian J, Zeng C, Wei J, Li LJ, Xie X, Yang T, Li H, Lei GH. Relationship between hyperuricemia and risk of coronary heart disease in a middle-aged and elderly Chinese population. J Int Med Res. 2017;45:254–260. doi: 10.1177/0300060516673923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katsiki N, Mantzoros C, Mikhailidis DP. Adiponectin, lipids and atherosclerosis. Curr Opin Lipidol. 2017;28:347–354. doi: 10.1097/MOL.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 42.Kuwabara M, Niwa K, Nishihara S, Nishi Y, Takahashi O, Kario K, Yamamoto K, Yamashita T, Hisatome I. Hyperuricemia is an independent competing risk factor for atrial fibrillation. Int J Cardiol. 2017;231:137–142. doi: 10.1016/j.ijcard.2016.11.268. [DOI] [PubMed] [Google Scholar]
- 43.Xu X, Du N, Wang R, Wang Y, Cai S. Hyperuricemia is independently associated with increased risk of atrial fibrillation: a meta-analysis of cohort studies. Int J Cardiol. 2015;184:699–702. doi: 10.1016/j.ijcard.2015.02.038. [DOI] [PubMed] [Google Scholar]
- 44.Yan M, Chen K, He L, Li S, Huang D, Li J. Uric acid induces cardiomyocyte apoptosis via activation of calpain-1 and endoplasmic reticulum stress. Cell Physiol Biochem. 2018;45:2122–2135. doi: 10.1159/000488048. [DOI] [PubMed] [Google Scholar]
- 45.Zhen H, Gui F. The role of hyperuricemia on vascular endothelium dysfunction. Biomed Rep. 2017;7:325–330. doi: 10.3892/br.2017.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J. 2015;79:495–502. doi: 10.1253/circj.CJ-15-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Korantzopoulos P, Letsas KP, Liu T. Xanthine oxidase and uric Acid in atrial fibrillation. Front Physiol. 2012;3:150. doi: 10.3389/fphys.2012.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liao HT, Liu FZ, Xue YM, Zhan XZ, Fang XH, Huang J, Wei W, Rao F, Deng H, Liu Y, Lin WD, Wu SL. Predictive value of serum uric acid on left atrial spontaneous echo contrast in nonvalvular atrial fibrillation patients. J Geriatr Cardiol. 2015;12:641–646. doi: 10.11909/j.issn.1671-5411.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y, Zhao J, Qiu J, Li J, Liang X, Zhang Z, Zhang X, Fu H, Korantzopoulos P, Letsas KP, Tse G, Li G, Liu T. Xanthine oxidase inhibitor allopurinol prevents oxidative stress-mediated atrial remodeling in alloxan-induced diabetes mellitus rabbits. J Am Heart Assoc. 2018:7. doi: 10.1161/JAHA.118.008807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakabe M, Fujiki A, Sakamoto T, Nakatani Y, Mizumaki K, Inoue H. oxidase inhibition prevents atrial fibrillation in a canine model of atrial pacing-induced left ventricular dysfunction. J Cardiovasc Electrophysiol. 2012;23:1130–1135. doi: 10.1111/j.1540-8167.2012.02356.x. [DOI] [PubMed] [Google Scholar]
- 51.Maharani N, Ting YK, Cheng J, Hasegawa A, Kurata Y, Li P, Nakayama Y, Ninomiya H, Ikeda N, Morikawa K, Yamamoto K, Makita N, Yamashita T, Shirayoshi Y, Hisatome I. Molecular mechanisms underlying urate-induced enhancement of Kv1.5 channel expression in HL-1 atrial myocytes. Circ J. 2015;79:2659–2668. doi: 10.1253/circj.CJ-15-0416. [DOI] [PubMed] [Google Scholar]
- 52.Youn JY, Zhang J, Zhang Y, Chen H, Liu D, Ping P, Weiss JN, Cai H. Oxidative stress in atrial fibrillation: an emerging role of NADPH oxidase. J Mol Cell Cardiol. 2013;62:72–9. doi: 10.1016/j.yjmcc.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nattel S, Harada M. Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. J Am Coll Cardiol. 2014;63:2335–2345. doi: 10.1016/j.jacc.2014.02.555. [DOI] [PubMed] [Google Scholar]
- 54.Friedrichs K, Adam M, Remane L, Mollenhauer M, Rudolph V, Rudolph TK, Andrié RP, Stöckigt F, Schrickel JW, Ravekes T, Deuschl F, Nickenig G, Willems S, Baldus S, Klinke A. Induction of atrial fibrillation by neutrophils critically depends on CD11b/CD18 integrins. PLoS One. 2014;9:e89307. doi: 10.1371/journal.pone.0089307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi EK. Angiotensin receptor blocker for stroke prevention in atrial fibrillation: beyond blood pressure lowering? Korean Circ J. 2016;46:307–308. doi: 10.4070/kcj.2016.46.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu YT, He H, Wang X, Zhang M, An ZM, Huang HJ. Serum uric acid and islet β-cell function in patients with pre-diabetes and type 2 diabetes mellitus. Sichuan Da Xue Xue Bao Yi Xue Ban. 2018;49:69–73. [PubMed] [Google Scholar]
- 57.Wang Y, Hu JW, Qu PF, Wang KK, Yan Y, Chu C, Zheng WL, Xu XJ, Lv YB, Ma Q, Gao K, Yuan Y, Li H, Yuan ZY, Mu JJ. Association between urinary sodium excretion and uric acid, and its interaction on the risk of prehypertension among Chinese young adults. Sci Rep. 2018;8:7749. doi: 10.1038/s41598-018-26148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anothaisintawee T, Lertrattananon D, Thamakaison S, Reutrakul S, Ongphiphadhanakul B, Thakkinstian A. Direct and indirect effects of serum uric acid on blood sugar levels in patients with prediabetes: a mediation analysis. J Diabetes Res. 2017;2017:6830671. doi: 10.1155/2017/6830671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang W, Fu Q, Zhang Q, Sun M, Gao Y, Liu X, Qian L, Shan S, Yang T. The association between serum uric acid and residual β -cell function in type 2 diabetes. J Diabetes Res. 2014;2014:709691. doi: 10.1155/2014/709691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takir M, Kostek O, Ozkok A, Elcioglu OC, Bakan A, Erek A, Mutlu HH, Telci O, Semerci A, Odabas AR, Afsar B, Smits G, ALanaspa M, Sharma S, Johnson RJ, Kanbay M. Lowering uric acid with allopurinol improves insulin resistance and systemic inflammation in asymptomatic hyperuricemia. J Investig Med. 2015;63:924–929. doi: 10.1097/JIM.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 61.Zhu Y, Hu Y, Huang T, Zhang Y, Li Z, Luo C, Luo Y, Yuan H, Hisatome I, Yamamoto T, Cheng J. High uric acid directly inhibits insulin signalling and induces insulin resistance. Biochem Biophys Res Commun. 2014;447:707–714. doi: 10.1016/j.bbrc.2014.04.080. [DOI] [PubMed] [Google Scholar]
- 62.Yerlikaya A, Dagel T, King C, Kuwabara M, Lanaspa MA, Andres-Hernando A, Covic A, Manitius J, Sag AA, Kanbay M. Dietary and commercialized fructose: Sweet or sour? Int Urol Nephrol. 2017;49:1611–1620. doi: 10.1007/s11255-017-1544-8. [DOI] [PubMed] [Google Scholar]
- 63.Wan X, Xu C, Lin Y, Lu C, Li D, Sang J, He H, Liu X, Li Y, Yu C. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism. J Hepatol. 2016;64:925–932. doi: 10.1016/j.jhep.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 64.Haneklaus M, O’Neill LA. NLRP3 at the interface of metabolism and inflammation. Immunol Rev. 2015;265:53–62. doi: 10.1111/imr.12285. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y, Yamamoto T, Hisatome I, Li Y, Cheng W, Sun N, Cai B, Huang T, Zhu Y, Li Z, Jing X, Zhou R, Cheng J. Uric acid induces oxidative stress and growth inhibition by activating adenosine monophosphate-activated protein kinase and extracellular signal-regulated kinase signal pathways in pancreatic β cells. Mol Cell Endocrinol. 2013;375:89–96. doi: 10.1016/j.mce.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 66.Chang TJ, Tseng HC, Liu MW, Chang YC, Hsieh ML, Chuang LM. Glucagon-like peptide-1 prevents methylglyoxal-induced apoptosis of beta cells through improving mitochondrial function and suppressing prolonged AMPK activation. Sci Rep. 2016;6:23403. doi: 10.1038/srep23403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yavari A, Stocker CJ, Ghaffari S, Wargent ET, Steeples V, Czibik G, Pinter K, Bellahcene M, Woods A, Martínez de Morentin PB, Cansell C, Lam BY, Chuster A, Petkevicius K, Nguyen-Tu MS, Martinez-Sanchez A, Pullen TJ, Oliver PL, Stockenhuber A, Nguyen C, Lazdam M, O’Dowd JF, Harikumar P, Tóth M, Beall C, Kyriakou T, Parnis J, Sarma D, Katritsis G, Wortmann DD, Harper AR, Brown LA, Willows R, Gandra S, Poncio V, de Oliveira Figueiredo MJ, Qi NR, Peirson SN, McCrimmon RJ, Gereben B, Tretter L, Fekete C, Redwood C, Yeo GS, Heisler LK, Rutter GA, Smith MA, Withers DJ, Carling D, Sternick EB, Arch JR, Cawthorne MA, Watkins H, Ashrafian H. Chronic activation of γ2 AMPK induces obesity and reduces β cell function. Cell Metab. 2016;23:821–836. doi: 10.1016/j.cmet.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riboulet-Chavey A, Diraison F, Siew LK, Wong FS, Rutter GA. Inhibition of AMP-activated protein kinase protects pancreatic beta-cells from cytokine-mediated apoptosis and CD8+ T-cell-induced cytotoxicity. Diabetes. 2008;57:415423. doi: 10.2337/db07-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ryu GR, Lee MK, Lee E, Ko SH, Ahn YB, Kim JW, Yoon KH, Song KH. Activation of AMP-activated protein kinase mediates acute and severe hypoxic injury to pancreatic beta cells. Biochem Biophys Res Commun. 2009;386:356–362. doi: 10.1016/j.bbrc.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 70.Jia L, Xing J, Ding Y, Shen Y, Shi X, Ren W, Wan M, Guo J, Zheng S, Liu Y, Liang X, Su D. Hyperuricemia causes pancreatic β-cell death and dysfunction through NF-κB signaling pathway. PLoS One. 2013;8:e78284. doi: 10.1371/journal.pone.0078284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Czemplik M, Kulma A, Wang YF, Szopa J. Therapeutic strategies of plant-derived compounds for diabetes via regulation of monocyte chemoattractant protein-1. Curr Med Chem. 2017;24:1453–1468. doi: 10.2174/0929867324666170303162935. [DOI] [PubMed] [Google Scholar]
- 72.Lin Y, Ye S, He Y, Li S, Chen Y, Zhai Z. Shortterm insulin intensive therapy decreases MCP-1 and NF-κB expression of peripheral blood monocyte and the serum MCP-1 concentration in newly diagnosed type 2 diabetics. Arch Endocrinol Metab. 2018;62:212–220. doi: 10.20945/2359-3997000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salma N, Song JS, Kawakami A, Devi SP, Khaled M, Cacicedo JM, Fisher DE. Tfe3 and Tfeb transcriptionally regulate peroxisome proliferator-activated receptor γ2 expression in adipocytes and mediate adiponectin and glucose levels in mice. Mol Cell Biol. 2017:37. doi: 10.1128/MCB.00608-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kadooka K, Sato M, Matsumoto T, Kuhara S, Katakura Y, Fujimura T. Pig testis extract augments adiponectin expression and secretion through the peroxisome proliferator-activated receptor signaling pathway in 3T3-L1 adipocytes. Cytotechnology. 2018;70:983–992. doi: 10.1007/s10616-018-0213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kou H, Deng J, Gao D, Song A, Han Z, Wei J, Jin X, Ma R, Zheng Q. Relationship among adiponectin, insulin resistance and atherosclerosis in non-diabetic hypertensive patients and healthy adults. Clin Exp Hypertens. 2018:1–8. doi: 10.1080/10641963.2018.1425414. [DOI] [PubMed] [Google Scholar]
- 76.Norvik JV, Storhaug HM, Ytrehus K, Jenssen TG, Zykova SN, Eriksen BO, Solbu MD. Overweight modifies the longitudinal association between uric acid and some components of the metabolic syndrome: the tromsø Study. BMC Cardiovasc Disord. 2016;16:85. doi: 10.1186/s12872-016-0265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng X, Gong L, Luo R, Chen H, Peng B, Ren W, Wang Y. Serum uric acid and non-alcoholic fatty liver disease in non-obesity Chinese adults. Lipids Health Dis. 2017;16:202. doi: 10.1186/s12944-017-0531-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sirota JC, McFann K, Targher G, Johnson RJ, Chonchol M, Jalal DI. Elevated serum uric acid levels are associated with non-alcoholic fatty liver disease independently of metabolic syndrome features in the United States: liver ultrasound data from the national health and nutrition examination survey. Metabolism. 2013;62:392–399. doi: 10.1016/j.metabol.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang C, Yang S, Feng C, Zhang C, Xu W, Zhang L, Yan Y, Deng J, Ohore OE, Li J. Associations of hyperuricemia and obesity with remission of nonalcoholic fatty liver disease among Chinese men: a retrospective cohort study. PLoS One. 2018;13:e0192396. doi: 10.1371/journal.pone.0192396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuwabara M, Borghi C, Cicero AF, Hisatome I, Niwa K, Ohno M, Johnson RJ, Lanaspa MA. Elevated serum uric acid increases risks for developing high LDL cholesterol and hypertriglyceridemia: a five-year cohort study in Japan. Int J Cardiol. 2018;261:183–188. doi: 10.1016/j.ijcard.2018.03.045. [DOI] [PubMed] [Google Scholar]
- 81.Yang Y, Zhou Y, Cheng S, Sun JL, Yao H, Ma L. Effect of uric acid on mitochondrial function and oxidative stress in hepatocytes. Genet Mol Res. 2016:15. doi: 10.4238/gmr.15028644. [DOI] [PubMed] [Google Scholar]
- 82.Choi YJ, Shin HS, Choi HS, Park JW, Jo I, Oh ES, Lee KY, Lee BH, Johnson RJ, Kang DH. Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP-1c activation in hepatocytes. Lab Invest. 2014;94:1114–1125. doi: 10.1038/labinvest.2014.98. [DOI] [PubMed] [Google Scholar]
- 83.Liu J, Xu C, Ying L, Zang S, Zhuang Z, Lv H, Yang W, Luo Y, Ma X, Wang L, Xun Y, Ye D, Shi J. Relationship of serum uric acid level with non-alcoholic fatty liver disease and its inflammation progression in non-obese adults. Hepatol Res. 2017;47:E104–E112. doi: 10.1111/hepr.12734. [DOI] [PubMed] [Google Scholar]
- 84.Rodacka A, Strumillo J, Serafin E, Puchala M. Effect of resveratrol and tiron on the inactivation of glyceraldehyde-3- phosphate dehydrogenase induced by superoxide anion radical. Curr Med Chem. 2014;21:1061–1069. doi: 10.2174/09298673113206660274. [DOI] [PubMed] [Google Scholar]
- 85.Abdel-Fadeil MR, Abedelhaffez AS, Makhlouf HA, Al Qirshi GA. Obstructive sleep apnea: Influence of hypertension on adiponectin, inflammatory markers and dyslipidemia. Pathophysiology. 2017;24:305–315. doi: 10.1016/j.pathophys.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 86.Sam S, Mazzone T. Adipose tissue changes in obesity and the impact on metabolic function. Transl Res. 2014;164:284–292. doi: 10.1016/j.trsl.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 87.Ryoo JH, Choi JM, Oh CM, Kim MG. The association between uric acid and chronic kidney disease in Korean men: a 4-year follow-up study. J Korean Med Sci. 2013;28:855–860. doi: 10.3346/jkms.2013.28.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS. Uric acid and incident kidney disease in the community. J Am Soc Nephrol. 2008;19:1204–1211. doi: 10.1681/ASN.2007101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Petreski T, Bevc S, Ekart R, Hojs R. Hyperuricemia and long-term survival in patients with chronic kidney disease undergoing hemodialysis. Clin Nephrol. 2017;88:69–72. doi: 10.5414/CNP88FX17. [DOI] [PubMed] [Google Scholar]
- 90.Bravo RC, Gamo MB, Lee HH, Yoon YE, Han WK. Investigating serum uric acid as a risk factor in the development of delayed renal recovery in living kidney donors. Transplant Proc. 2017;49:930–934. doi: 10.1016/j.transproceed.2017.03.034. [DOI] [PubMed] [Google Scholar]
- 91.Cho A, Lee JE, Jang HR, Huh W, Kim DJ, Oh HY, Kim YG. Association between pre-donation serum uric acid concentration and change in renal function after living kidney donation in women. Intern Med J. 2014;44:1217–1222. doi: 10.1111/imj.12591. [DOI] [PubMed] [Google Scholar]
- 92.Hsieh YP, Chang CC, Kor CT, Yang Y, Wen YK, Chiu PF, Lin CC. Relationship between uric acid and technique failure in patients on continuous ambulatory peritoneal dialysis: a long-term observational cohort study. BMJ Open. 2017;7:e010816. doi: 10.1136/bmjopen-2015-010816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zuo T, Jiang L, Mao S, Liu X, Yin X, Guo L. Hyperuricemia and contrast-induced acute kidney injury: a systematic review and meta-analysis. Int J Cardiol. 2016;224:286–294. doi: 10.1016/j.ijcard.2016.09.033. [DOI] [PubMed] [Google Scholar]
- 94.Kanbay M, Solak Y, Afsar B, Nistor I, Aslan G, Çağlayan OH, Aykanat A, Donciu MD, Lanaspa MA, Ejaz AA, Johnson RJ, Covic A. Serum uric acid and risk for acute kidney injury following contrast. Angiology. 2017;68:132–144. doi: 10.1177/0003319716644395. [DOI] [PubMed] [Google Scholar]
- 95.Li Z, Sheng Y, Liu C, Li K, Huang X, Huang J, Xu K. Nox4 has a crucial role in uric acid-induced oxidative stress and apoptosis in renal tubular cells. Mol Med Rep. 2016;13:4343–4348. doi: 10.3892/mmr.2016.5083. [DOI] [PubMed] [Google Scholar]
- 96.Bove M, Cicero AF, Borghi C. The effect of xanthine oxidase inhibitors on blood pressure and renal function. Curr Hypertens Rep. 2017;19:95. doi: 10.1007/s11906-017-0793-3. [DOI] [PubMed] [Google Scholar]
- 97.Liu H, Xiong J, He T, Xiao T, Li Y, Yu Y, Huang Y, Xu X, Huang Y, Zhang J, Zhang B, Zhao J. High uric acid-induced epithelial-mesenchymal transition of renal tubular epithelial cells via the TLR4/NF-kB signaling pathway. Am J Nephrol. 2017;46:333–342. doi: 10.1159/000481668. [DOI] [PubMed] [Google Scholar]
- 98.Romi MM, Arfian N, Tranggono U, Setyaningsih WA, Sari DC. Uric acid causes kidney injury through inducing fibroblast expansion, Endothelin-1 expression, and inflammation. BMC Nephrol. 2017;18:326. doi: 10.1186/s12882-017-0736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


