Abstract
Previous studies have reported the upregulation of stem cell biomarkers that are associated with tumorigenesis, in particular with cancer infiltration, recurrence and metastasis. Infection by human papilloma virus (HPV) is the main etiopathological factor of cervical carcinogenesis, but the expression of stem cell markers in cervical carcinoma and HPV infection have yet to be investigated so far. A total of 94 cases of fresh cervical tissues, 116 cases of paraffin-embedded cervical specimens and 72 cases of peripheral blood samples were collected from Uighur women who were either diagnosed with cervical squamous cell carcinoma (SCC) or cervical intraepithelial neoplasia (CIN) II–III, or from healthy subjects (negative controls, NC). HPV infection was detected in tissue DNA by polymerase chain reaction (PCR) with a HPV genotyping kit. The mRNA expression levels of aldehyde dehydrogenase 1 family member A1 (ALDH1A1), nanog homeobox (NANOG), POU class 5 homeobox 1 (OCT4), SRY-box 2 (SOX2) and twist family BHLH transcription factor 1 (Twist1) were determined using reverse transcription-quantitative PCR (RT-qPCR). Histological analysis was performed in order to examine the protein expression of ALDH1A1 and OCT4 in paraffin-embedded tissue specimens by immunohistochemical staining and the plasma levels of those two proteins was measured by ELISA. RT-qPCR analysis indicated a significant increase in the mRNA expression of ALDH1A1 and OCT4 in CIN II–III and SCC tissue specimens compared with NC (P<0.05). Although the expression levels of NANOG, SOX2 and Twist1 were significantly higher in SCC compared with NC (P<0.05), no significant difference was revealed in CIN II–III tissues compared with SCC or NC (P>0.05). Subsequent analysis by immunohistochemistry staining confirmed that the upregulation of ALDH1A1 and OCT4 was also significantly increased in SCC and CIN II–III compared with controls at the protein level. Notably, ELISA analysis detected significantly higher levels of ALDH1A1 and OCT4 in the peripheral blood (plasma) of patients with SCC compared with healthy subjects. The upregulation of stem cell markers ALDH1A1 and OCT4 in cervical carcinoma and its precursor lesions, in particular in the peripheral blood, indicates that ALDH1A1 and OCT4 may serve as biomarkers for the early detection of cervical carcinoma or for the monitoring of treatment of patients.
Keywords: cervical carcinoma, human papilloma virus infection, stem cell marker, aldehyde dehydrogenase 1 family member A1, POU class 5 homeobox 1
Introduction
A model that explains the phenotypic and functional heterogeneity of cancer cells within the same tumor is the cancer stem cell (CSC) model. According to the CSC model, a tumor has a hierarchical cellular structure, where a small population of tumorigenic CSCs differentiates into non-stem cancer cells or non-tumorigenic progeny (1). Cancer stem cells are thought to possess stem-like properties of self-renewal, and initiate and drive tumor growth and metastasis, in addition to therapy resistance and recurrence following conventional therapy (2). As genital infection with human papillomaviruses (HPVs), in particular the persistent infection of high-risk species (including HPV16 and 18), promotes disease progression from cervical intraepithelial neoplasia (CIN) to malignancy, the virus may interact with CSCs in the epithelium of the uterine cervix (3,4).
In general, the cervix is composed of hard squamous cells in the ectocervix, soft columnar cells in the endocervix and metaplastic cells in the squamocolumnar junction, also known as the transformation zone (5). Squamous and columnar epithelial cells are regenerated by the active division of stem-like reserve cells in the transformation zone, where cervical cancer is hypothesized to originate (6,7). It has been suggested that HPV enters the cell by distinct pathways, including clathrin- or caveolar-mediated endocytosis, or alternative routes in which tetraspanin-enriched microdomains are involved (8). The dependence of HPV infection on the activity of cyclin dependent kinases and microtubule reorganization, suggests that the incorporation of the viral genome into the nucleus of reserve cells may require the cell to enter into mitosis (9). HPV may program the cell to undergo symmetric division, by the disturbance of asymmetric division, therefore allowing the cells to proliferate and produce viral particles, but preventing them from differentiation (10,11). Accordingly, reserve cells may be converted to cervical CSCs by the interplay with high risk-HPV viral oncogenes and cellular alterations during cervical carcinogenesis (11). In fact, the presence of CSCs in cervical carcinoma is detectable by using stem cell markers. Early studies suggested that transcription factor tumor protein p63 (p63) and cytokeratin 17 (KRT17) are markers for reserve cells and reserve cell hyperplasia in the epithelium of cervical lesions and are upregulated during HPV infection of epithelial cells (10,12,13). Nanog homeobox (NANOG), POU class 5 homeobox 1 (OCT4) and SRY-box 2 (SOX2) are key regulators of embryonic stem cells and incorporate primarily into the regulatory network responsible for self-renewal and pluripotency (14,15). NANOG is expressed in cervical lesions but may not associate with the prognosis of cervical carcinoma (16). Different isoforms of OCT4 are expressed in cultured cells of cervical carcinoma, with the highest level in HPV-positive cells (17,18). Aldehyde dehydrogenase (ALDH), a stem cell marker for early stem-cell differentiation, is detectable in cervical epithelial lesions and carcinoma in addition to stem-like cells that are isolated from cervical carcinoma cells (19–21). Transcription factor twist family BHLH transcription factor 1 (Twist1) may serve a function in the disruption of E-cadherin-mediated cell-cell adhesion and the induction of epithelial-mesenchymal transition in cervical cancer stem-like cells (20–23). These previous studies suggest that cervical carcinoma or cell lines harbor a small population of CSCs that are characterized by the expression of stem cell markers; however, to date, the expression pattern of stem cell markers in cervical carcinoma and precursor lesions have yet to be well-characterized.
In the present study, the transcription and protein expression of ALDH family member A1 (ALDH1A1), NANOG, OCT4, SOX2 and Twist1 in cervical lesions and blood plasma from patients with squamous cell carcinoma (SCC) and its precursor lesion, CIN, were detected using reverse transcription-quantitative polymerase chain reaction (RT-qPCR), immunohistochemistry (IHC) and ELISA. The results of the present study may contribute to current knowledge on the association of cervical carcinogenesis with the regulation of stem cell marker expression, and provide potential biomarkers for the early prognosis, diagnosis and monitoring of cancer treatment.
Materials and methods
Tissue specimens
The present study was approved and monitored by the Ethics Committee of Xinjiang Medical University (Xinjiang, China). All procedures performed in the present study were in accordance with the Helsinki Declaration of 1975, as revised in 2000. Written informed consent was obtained from all patients and healthy individuals prior to the study, and all data were analyzed anonymously. Patients diagnosed with cervical SCC, CIN and subjects (negative control, NC) with a normal cervix were enrolled in the present study, according to the diagnostic criteria of the World Health Organization and the Chinese Medical Association as described elsewhere (24). A total of 94 fresh tissue samples were sourced from patients and healthy controls by routine biopsies and surgical operations at the Department of Gynecology at the First Affiliated Hospital and The Third Affiliated Cancer Hospital of Xinjiang Medical University from March 2013 to June 2014. The specimens were pathologically classified, and included 32 cases of cervical SCC, 30 cases of CIN stages II–III and 32 cases of normal controls. CIN II and III are classified as a high-grade squamous intraepithelial lesion, which is considered a significant precancerous lesion, whereas CIN I, as a low-grade squamous intraepithelial lesion, is considered a much more benign lesion since most of these lesions regress; those diagnosed at this stage (CIN I) were excluded from the present study (24). Fresh tissue specimens were frozen quickly in liquid nitrogen following resection and stored at −80°C for six months or in liquid nitrogen storage for up to two years.
For immunohistochemical (IHC) analysis, 116 cases of paraffin-embedded cervical specimens (3-µm thickness) were obtained from the specimen bank in the Pathology Department of the First Affiliated Hospital of Xinjiang Medical University. These specimens were selected following a case review by two experienced pathologists, and these included 47 cases of SCC, 37 cases of CIN stages II–III and 32 cases of NC.
For ELISA analysis, plasma samples from 85 Uighur women were collected, including 40 cases of SCC, 28 cases of CIN II–III and 17 cases of NC. A total of 3 ml blood samples were obtained from each donor by venipuncture into evacuated blood collection tubes that contained EDTA as an anticoagulant, and the plasma was preserved at −80°C for further use following centrifugation at 800 × g and 4°C for 10 min. The median age of patients was 53 years (range 25–65 years), and normal controls were age-matched to patients.
The clinical staging of patients was based on guidelines established by the International Federation of Gynecology and Obstetrics of 1994–1997, as revised in 1999 by Pecorelli et al (25). Tumor specimens were collected from patients with cancer who underwent radical surgery for cervical SCC at clinical stages I–IIa. Precancerous lesions were collected from patients with CIN stages II–III as biopsies or from cervical conization. Normal controls were obtained from patients without a history of cervical lesions or any form of cancer who underwent a hysterectomy for nonmalignant reasons during the same time period. Indications for hysterectomy were fibroids, prolapsed uterus or adenomyosis, and primarily a combination of fibroids with prolapse.
DNA extraction and HPV detection
Genomic DNA was extracted from fresh cervical specimens using a QIAamp DNA Mini kit for tissue DNA (cat no. 51306; Qiagen, Inc., Valencia, CA, USA). Concentration and purity of extracted DNA were measured using a spectrophotometer (NanoDrop 2000; Thermo Fisher Scientific, Inc., Waltham, MA, USA). HPV infection of 8 high-risk HPV genotypes, including HPV 16, 18, 45, 31, 33, 52, 58 and 67, was determined by multi-fluorescent PCR assay using the Real Quality RQ-HPV HR kit (cat no. RQ-22-120A; AB Analitica SRL, Padua, Italy) containing specific primers for HPV16, HPV18/45 and HPV31, and general primers for HPV33/45/52/58/67. Following the activation of the polymerase at 95°C for 1 min, 40 cycles of the denaturation at 95°C for 15 sec, annealing at 57°C for 30 sec and elongation at 72°C for 30 sec, were performed on the ABI-7500 PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The human β-actin gene was used as an internal control for DNA quality.
RNA extraction and RT-qPCR analysis
Fresh frozen tissues (~50 mg) were packed in aluminum foil and pulverized by grinding under liquid nitrogen. Total RNA was isolated from the powder by dissolving with TRIzol® lysis buffer (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) followed by phenol/chloroform extraction and ethanol precipitation. cDNA was synthesized from 1 µg of total mRNA by RT using a RevertAid kit (Fermentas; Thermo Fisher Scientific, Inc.) at 42°C for 60 min. For RT-qPCR analysis, primer pairs specific to the mRNA sequences of target genes and suitable for use were designed and synthesized by Takara Bio (Takara Biotechnology Co., Ltd., Dalian, China; Table I). Each cDNA sample (20 ng) was analyzed in a 25 µl by RT-qPCR using SYBR Premix Ex Taq™ kit (TIi RNase H Plus; Takara Biotechnology Co., Ltd.) on an iQ5™ PCR system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and. The thermocycling conditions were as follows: Initial denaturation at 95°C for 30 sec, followed by 40 cycles of denaturation at 95°C for 5 sec and synthesis at 60°C for 30 sec and a final incubation at 4°C for 10 min. Expression levels of target genes were quantified using the 2−ΔΔCq method (26) using the software iQ5 Standard Edition (Version 1.028; Bio-Rad Laboratories, Inc.), setting β-actin as an internal control. Experiments were repeated in triplicate for each sample.
Table I.
List of primers used in reverse transcription-quantitative polymerase chain reaction analysis.
| Gene | Accession number | Forward primer (5′-3′) | Reverse primer (5′-3′) | Product (bp) |
|---|---|---|---|---|
| ALDH1A1 | NM_000689 | TTGTCCAGCCCACAGTGTTCTC | TGTCTTTGGTAAACACTCCTGCTGA | 168 |
| OCT4 | NM_002701.4 | GTGCCGTGAAGCTGGAGAA | TGGTCGTTTGGCTGAATACCTT | 192 |
| NANOG | NM_024865 | CCTGTGATTTGTGGGCCTGA | CTCTGCAGAAGTGGGTTGTTTG | 168 |
| SOX2 | NM_003106 | GTGAGCGCCCTGCAGTACAA | GCGAGTAGGACATGCTGTAGGTG | 82 |
| TWIST1 | NM_000474 | CAGCTACGCCTTCTCGGTCT | CTGTCCATTTTCTCCTTCTCTGG | 138 |
| β-actin | NM_001101.3 | CATCCGTAAAGACCTCTATGCCAAC | ATGGAGCCACCGATCCACA | 171 |
ALDH1A1, aldehyde dehydrogenase 1 family member A1; NANOG, nanog homeobox; OCT4, POU class 5 homeobox 1; SOX2, SRY-box 2; Twist1, twist family BHLH transcription factor 1.
IHC analysis
The paraffin-embedded tissues were sectioned into 3-µm slices. The IHC staining was performed with the streptavidin peroxidase-conjugated method using primary rabbit polyclonal antibodies recognizing target proteins ALDH1 (cat. no. ab23375; Abcam, Cambridge, UK) and OCT3/4 (cat. no. PA5-27438; Thermo Fisher Scientific Inc.) at 1:200 dilutions, and IHC kits containing biotin-labeled goat anti-rabbit secondary antibody (cat. no. ZB-2010; Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd.; OriGene Technologies, Beijing, China) at the 1:300 dilution according to titration experiments and the manufacturer's protocol. Briefly, tissue sections were dewaxed in 100% xylene and rehydrated in ethanol at 100, 85 and 75% gradients and each for 5 min at room temperature, and washed with distilled water at room temperature followed by antigen retrieval with heating in the microwave oven for 15 min at 95°C in EDTA buffer (pH 8.0). Subsequent to cooling and rinsing in distilled water, endogenous peroxidase activity was blocked by incubating sections for 15 min in 3% H2O2 at room temperature followed by rinsing in 0.01 M PBS (pH 7.4) for 10 min. Following treatment at room temperature with the protein blocking solution provided by the IHC kits for 10 min, the sections were incubated with the primary antibodies in a humid chamber overnight at 4°C. Then, the sections were washed with PBS three times and incubated with the secondary antibody for 15 min at room temperature. The staining was visualized with the DAB Kit (Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd.; OriGene Technologies). Negative controls were treated with PBS instead of primary antibodies. All tissue sections were counterstained using 100% hematoxylin at room temperature for up to 30 sec, in accordance with the preliminary experiments. PBS was used in place of the primary antibody as a negative control. For evaluation, stained tissue sections were scored under a light microscope (magnifications, ×20 and ×40) by two experienced pathologists. The results were scored on a scale of 0 to 3 by an estimation of the percentage and intensity of positive staining in 5 fields each time using a previously described method for evaluation and interpretation of IHC staining (27). A mean percentage of positive staining was calculated according to the number of positively stained cells in each field and time and scored as 0 for negativity, 1 for 0 to 30% positivity, 2 for >30 to 60% positivity and 3 for >60% positivity. Positive staining intensity was scored as 0 for a negative signal, 1 for weak signal intensity, 2 for moderate signal intensity and 3 for high signal intensity. A consensus number between the two investigators was reached for each tissue slice. An overall score was calculated by the addition of the two scores and the overall scores were classified according to the following categories: i) 0–2, a loss of expression or weak expression; ii) 3–4, moderate expression and iii) 5–6, strong expression.
ELISA
Plasma levels of ALDH1 and OCT4 were determined in blood samples from patients and controls by 96-well plate sandwich ELISA using commercially available kits for ALDH1 (cat. no. SEE824Hu) and OCT4 (cat. no. SEA424Hu; both Uscn Life Sciences, Inc., Wuhan, China) according to the manufacturer's protocol. The quantification of the relative plasma content was based on the standard curves derived from standard substances (proteins) of ALDH1 and OCT4 supplied with the indicated kits, in which the absolute concentration of each protein was known.
Statistical analysis
Statistical analysis was performed using SPSS (version 17.0; SPSS Inc., Chicago, IL, USA). All P-values were two-sided, and P<0.05 was considered to indicate a statistically significant difference. All data was presented as the mean ± standard deviation. Data derived from the results of RT-qPCR and ELISA tests were compared for statistical differences. Between and within group analysis were carried out using one-way ANOVA followed by Dunnett's post-hoc test. Continuous data derived from IHC scoring was analyzed using Mann-Whitney U test.
Results
Transcription of stem cell markers in cervical carcinoma and its precursor lesions
The present study focused on five stem cell markers, including ALDH1A1, OCT4, NANOG, SOX2 and Twist1, which are considered to be potential biomarkers for cervical carcinoma as previously reported (16–23). The transcription of genes encoding these proteins was analyzed in tissue specimens from patients with SCC, CIN II–III and NC using RT-qPCR using primer pairs specific for mRNA sequences. The data indicated an increase in the transcription of these genes with the progression of cervical cancer (Fig. 1, Table II). Statistical analysis confirmed significant differences in the expression of all five genes in SCC tissues compared with NC tissues (P<0.05). In addition, significant differences were detected in the expression of ALDH1A1, OCT4 and NANOG in CIN II–III tissues compared with NC tissues (P<0.05). There were also significant differences detected in ALDH1A1 and OCT4 expression in SCC tissues compared with CIN II–III tissues (P<0.05). These findings indicate that cervical carcinogenesis may be characterized by the upregulation of stem cell markers, in particular the differential expression of ALDH1A1 and OCT4 in SCC tissues compared with its precursor lesions and normal cervical tissue.
Figure 1.
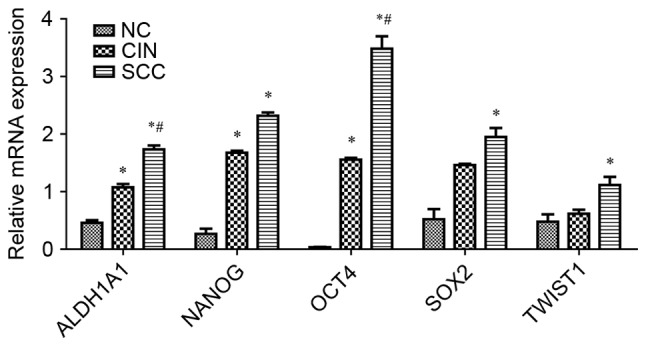
mRNA expression patterns of five genes, including ALDH1A1, NANOG, OCT4, SOX2 and Twist1 in SCC, CIN II–III and NC tissues analyzed by reverse transcription-quantitative polymerase chain reaction. *P<0.05 vs. NC; #P<0.05 vs. CIN. ALDH1A1, aldehyde dehydrogenase 1 family member A1; NANOG, nanog homeobox; OCT4, POU class 5 homeobox 1; SOX2, SRY-box 2; Twist1, twist family BHLH transcription factor 1; SCC, cervical squamous cell carcinoma; CIN, cervical intraepithelial neoplasia; NC, negative controls.
Table II.
mRNA expression levels of five stem cell markers in cervical lesions as determined by reverse transcription-quantitative polymerase chain reaction.
| Relative mRNA expression level (mean ± standard deviation) | One-way analysis of variance (P-value) | |||||||
|---|---|---|---|---|---|---|---|---|
| Markers | NC (n=32) | CIN II–III (n=30) | SCC (n=32) | F-value | P-value | SCC vs. NC | CIN vs. NC | SCC vs. CIN |
| ALDH1A1 | 0.458±0.047 | 1.076±0.055 | 1.733±0.068 | 10.943 | <0.0001 | <0.0001 | 0.031 | 0.022 |
| NANOG | 0.267±0.090 | 1.676±0.036 | 2.318±0.058 | 6.825 | 0.003 | 0.001 | 0.019 | 0.272 |
| OCT4 | 0.034±0.006 | 1.555±0.031 | 3.482±0.216 | 19.723 | <0.0001 | <0.0001 | 0.009 | 0.001 |
| SOX2 | 0.521±0.176 | 1.461±0.022 | 1.949±0.156 | 4.281 | 0.020 | 0.006 | 0.069 | 0.338 |
| TWIST1 | 0.476±0.130 | 0.615±0.072 | 1.114±0.142 | 3.384 | 0.043 | 0.017 | 0.613 | 0.061 |
ALDH1A1, aldehyde dehydrogenase 1 family member A1; NANOG, nanog homeobox; OCT4, POU class 5 homeobox 1; SOX2, SRY-box 2; Twist1, twist family BHLH transcription factor 1; SCC, cervical squamous cell carcinoma; CIN, cervical intraepithelial neoplasia; NC, negative controls.
Among the 94 cases of fresh tissue specimens, HPV infection was detected in 64 cases with qPCR using probes for HPV16, 31, 18/45, and 33/52/58/67 (Table III). Of these 64 cases, 32 cases of SCC, 24 cases of CIN II–III and 8 cases of normal controls (NC) were positive for HPV16 infection, including several cases co-infected by HPV16 and other HPV species. Due to high HPV positivity, in particular the predominance of HPV16 infection in SCC (32/32) and CIN II–III (24/30) and the HPV negativity of NC (24/32), the data of HPV detection was not suitable for analyses for the association of gene expression with HPV16 infection. Nevertheless, due to the contrast between HPV16 positive cervical lesions and HPV-negative normal controls, it is hypothesized that a regulation of stem cell markers independent of HPV16 infection was integrated into the result of RT-qPCR (Table II).
Table III.
HPV genotyping of cervical specimens.
| Samples | HPV16 | HPV16/18/45 | HPV16/31 | HPV16/33/52/58/67 |
|---|---|---|---|---|
| NC (n=32) | 6 | 2 | n.d. | n.d. |
| CIN II–III (n=30) | 20 | 4 | n.d. | n.d. |
| SCC (n=32) | 20 | 3 | 3 | 6 |
SCC, cervical squamous cell carcinoma; CIN, cervical intraepithelial neoplasia; NC, negative controls; n.d., not detected; HPV, human papilloma virus.
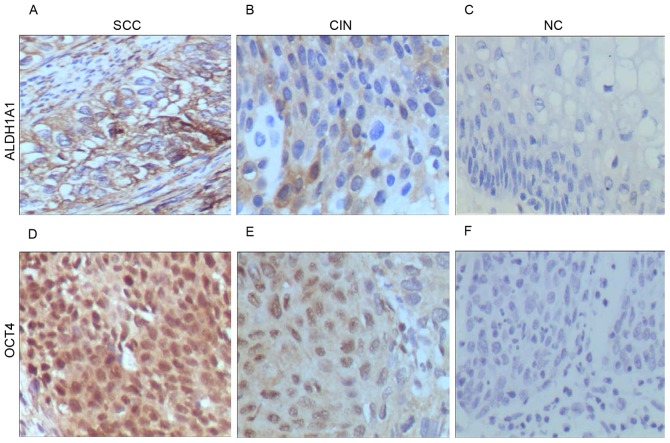
Verification of ALDH1A1 and OCT4 expression by immunohistochemistry
To validate the results of the RT-qPCR analysis, the protein expression of ALDH1A1 and OCT4 in 116 cases of formalin-fixed and paraffin-embedded tissue specimens of SCC, CIN II–III and NC were analyzed by immunohistochemical analysis. Positive staining for ALDH1A1 and OCT4 was localized in the cytoplasm of cervical epithelial or carcinoma cells (Fig. 2). Statistical analysis revealed that the strong and moderate positive staining of ALDH1A1 was significantly increased in CIN II–III and SCC compared with NC and from CIN II–III to SCC (P<0.05; Table IV). Although a weak staining of OCT4 was observed in a number of the specimens from SCC, CIN II–III and NC, moderate staining of OCT4 was significantly increased in SCC compared with CIN II–III and NC (P<0.05). These results indicated that the transcription and protein expression of ALDH1A1 and OCT4 is increased during the development of cervical carcinoma and its precursor lesions compared with normal tissues.
Figure 2.
Expression pattern of ALDH1 and OCT4 proteins in cervical lesions as detected by immunohistochemical staining. Expression of ALDH1A1 in (A) SCC, (B) CIN and (C) NC tissues. Expression of OCT4 in (D) SCC, (E) CIN and (F) NC tissues. Magnification, ×400. ALDH1A1, aldehyde dehydrogenase 1 family member A1; OCT4, POU class 5 homeobox 1; SCC, cervical squamous cell carcinoma; CIN, cervical intraepithelial neoplasia; NC, negative controls.
Table IV.
Immunohistochemical staining analyses of ALDH1A1 and OCT4 expression in cervical lesions.
| A, ALDH1A1 | ||||||
|---|---|---|---|---|---|---|
| Samples | − | + | ++ | +++ | P-value | |
| NC (n=33) | 8 | 18 | 7 | 0 | <0.0001a | |
| CIN II–III (n=37) | 11 | 4 | 19 | 3 | 0.028b | |
| SCC (n=47) | 5 | 0 | 25 | 17 | <0.0001b | <0.0001c |
| B, OCT4 | ||||||
| Samples | − | + | ++ | +++ | P-value | |
| NC (n=33) | 20 | 12 | 1 | 0 | 0.001a | |
| CIN II–III (n=37) | 19 | 13 | 5 | 0 | 0.138b | |
| SCC (n=47) | 16 | 14 | 17 | 0 | <0.0001b | 0.029c |
-, negative expression; +, weak positive expression; ++, moderate positive expression; +++, strong positive expression
Comparison between all three groups
Comparison with NC
Comparison with CIN II–III. ALDH1A1, aldehyde dehydrogenase 1 family member A1; OCT4, POU class 5 homeobox 1; SCC, cervical squamous cell carcinoma; CIN, cervical intraepithelial neoplasia; NC, negative controls.
Upregulation of ALDH1A1 and OCT4 in circulating blood plasma with the development of cervical carcinoma
To evaluate the potential of ALDH1A1 and OCT4 as biomarkers for non-invasive diagnosis, the levels of these proteins in 85 cases of plasma samples from patients with SCC and CIN and healthy subjects were detected (Table V). The ELISA analysis indicated significantly higher levels of ALDH1A1 and OCT4 in the plasma of patients with SCC compared with patients with CIN II–III and healthy controls (P<0.05), but there was no significant difference between patients with CIN II–III and healthy controls (P>0.05; Fig. 3). Therefore, the quantitative increase of these proteins in plasma may serve as biomarkers for cervical carcinoma.
Table V.
Plasma levels of ALDH1A1 and OCT4 in healthy controls and patients with cervical lesions as analyzed using ELISA.
| Plasma protein level (ng/ml) | One-way analysis of variance (P-value) | |||||||
|---|---|---|---|---|---|---|---|---|
| Markers | NC (n=17) | CIN II–III (n=28) | SCC (n=40) | F-value | P-value | SCC vs. NC | CIN vs. NC | SCC vs. CIN II–III |
| ALDH1A1 | 9.923±0.585 | 11.432±0.818 | 14.287±0.069 | 3.449 | 0.034 | 0.034 | 0.759 | 0.034 |
| OCT4 | 3.170±0.808 | 3.885±0.033 | 5.143±0.914 | 5.946 | 0.004 | 0.032 | 0.553 | 0.002 |
ALDH1A1, aldehyde dehydrogenase 1 family member A1; OCT4, POU class 5 homeobox 1; SCC, cervical squamous cell carcinoma; CIN, cervical intraepithelial neoplasia; NC, negative controls.
Figure 3.
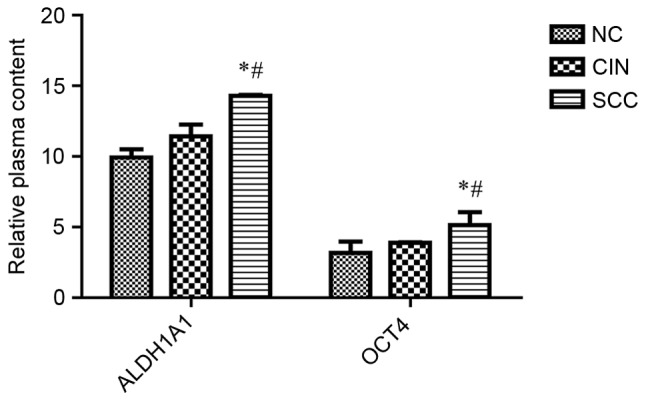
Plasma levels of ALDH1 and OCT4 in controls and patients with cervical lesions as determined by ELISA. *P<0.05 vs. NC; #P<0.05 vs. CIN. ALDH1A1, aldehyde dehydrogenase 1 family member A1; OCT4, POU class 5 homeobox 1; SCC, cervical squamous cell carcinoma; CIN, cervical intraepithelial neoplasia; NC, negative controls.
Discussion
The concept of cancer stem cells in human tumors opens up novel research directions on how cancer is initiated and how cancer cells are capable of switching from dormancy to malignancy, and challenges previous understanding of tumor recurrence, angiogenesis, metastasis and drug resistance (28–30). Accordingly, the studies on stem cell markers have notably shifted from focusing on the function of the markers in embryonic development to their involvement in tumorigenesis (31–33). In previous studies, cervical CSCs were most frequently identified and characterized in cell lines of cervical carcinoma by detection of stem cell markers, including p63, KRT17, ALDH1, OCT4, NANOG, SOX2 and Twist1 (12,16,17,21,22). However, the expression profile of these stem cell markers in cervical lesions has yet to be intensively studied (5). Furthermore, the application of stem cell markers for clinical diagnosis is limited due to the lack of sufficient validation studies. In the present study, it was revealed that the transcription and protein expression of ALDH1A1 and OCT4 were significantly upregulated in cervical lesions from patients with CIN II–III and SCC compared with normal controls, and also significantly higher in the plasma of patients with SCC compared with healthy controls. In the case of NANOG, SOX2 and Twist1, the transcription of these genes in SCC tissues was significantly higher compared with NC, but no significant difference was identified in CIN II–III compared with SCC or NC.
As ALDH1 and OCT 4 are considered as biomarkers for embryonic and/or cancer stem cells (14,20), the results of the present study indicate that the deregulation of these two proteins in cervical lesions reflects the function of CSCs or cancer cells with stem-like properties during cervical carcinogenesis (14,21). However, the outcome of the association analysis between the gene expression and HPV infection may be limited due to the high HPV16 positivity of SCC and CIN specimens in comparison with normal controls negative for HPV infection. Nevertheless, as the expression levels of stem cell markers were analyzed in SCC and CIN compared with the normal controls in the present study, it was suggested that the expression of stem cell markers potentially regulated by HPV16 infection may be reflected in the RT-qPCR results, and presumably in IHC analyses of tissue specimens that were not tested for HPV infection. Therefore, independent analyses, in particularly in vitro studies, associated with HPV16 infection are required to validate the outcome of the present study.
The ALDH family has 19 different isoforms that are localized in the cytoplasm, mitochondria or nucleus, and serve crucial functions in cellular protection by the oxidation of intracellular aldehydes, which induces resistance to a number of alkylation agents used in cancer therapy (34). Among them, ALDH1A1, ALDH1A2, ALDH1A3 and ALDH8A1 participate in the oxidation of retinol to retinoic acid in the cytoplasm, which in turn translocate into the nucleus and initiates the transcription of genes involved in early stem-cell differentiation (35,36). In addition, ALDH1A1 may contribute to the majority of ALDH activity in CSCs (37). The predominant expression of ALDH1A1 has been associated with ALDH activity in prostate and thyroid cancer (38,39). In the case of 6 ALDH1 isoenzymes, ALDH1A1 may be a major contributor of ALDH1 activity and a biomarker for predicting the poor survival of patients with breast cancer (40). High levels of ALDH1A1 and ALDH1A3 expression are associated with malignant transformation to lung adenocarcinoma (41). Little is known about the regulation of ALDH1A1 expression in cervical lesions, but the function of ALDH in cervical CSCs or cervical carcinoma has been described in a number of studies: CSCs that are isolated and enriched from cervical carcinoma cell lines with high ALDH activity or ALDH1 positivity exhibit enhanced stem-like properties of self-renewal, high tumorigenicity and resistance to cisplatin treatment compared with those with low ALDH activity or negative for ALDH1 expression (20,21). ALDH-positivity and expression levels are increased in tumor specimens of the uterine cervix, and therefore ALDH may serve as a predictive marker for poor prognosis, poor clinical outcome and resistance to chemotherapy in patients with cervical carcinoma (42–44). Corresponding with these results, the results of the present study also revealed that the upregulation of ALDH1A1 in the progression of precancerous lesions (CIN II–III) to cervical carcinoma. This indicates that ALDH1A1 may be a potential biomarker for the early diagnosis of cervical carcinoma.
OCT4 is a well-established stem cell factor that cooperates with SOX2 in maintaining the self-renewal and pluripotency of human and mouse embryonic stem cells (45,46). The loss of OCT4 expression or downregulation is associated with stem cell differentiation (47). OCT4 is detected in tumor-initiating cells or stem-like cancer cells enriched from tumor tissues or cell lines, indicating its function in tumorigenesis, metastasis and resistance to anticancer therapies (48). The co-expression of OCT4 with SOX2 has been detected in CIN but not in SCC, and the elevated expression of OCT4 in the absence of SOX2 is associated with the poor prognosis of patients with cervical carcinoma (49). However, the upregulation of both OCT4 and SOX2 in cervical carcinoma and its precursor lesions was detected in the present study by RT-qPCR. Consistent with the findings in the present study, a number of previous studies suggest that SOX2 functions cooperatively with other dosage-sensitive transcription factors, including OCT4 and NANOG, in order to maintain the regulatory networks responsible for self-renewal and to repress differentiation programs in embryonic stem cells (50,51). These studies indicate that the expression profile of OCT4 and SOX2 and NANOG may serve as biomarkers for the prognosis of cervical carcinoma.
Genital infection by HPVs, in particular by oncogenic high-risk HPV types (including HPV 16 and 18), is generally known to be the primary etiological factor in cervical carcinogenesis (52). However, the effects of HPV detection in cervical specimens are constantly debated in studies on cervical carcinoma, as the majority of HPV infections are transient and harmless or eliminated by the host immunity (4). Even persistent infections have a relatively long latent period prior to the induction of cancer, indicating that HPV testing alone is not sufficient in the prognosis of cervical carcinoma (3). Therefore, HPV-based cancer prognoses may require the use of auxiliary diagnostic biomarkers that are present during HPV-induced carcinogenesis (53). However, based on the results of HPV genotyping performed in the present study, it was difficult to define the association between the expression of target genes and HPV infection as the majority of cases of SCC and CIN II–III were positive for HPV and most of the normal controls were HPV-negative. Nevertheless, the potential effects of HPV infection on the expression of stem cell markers have been described in previous studies. In cervical cancer cells, HPV16 may activate OCT4 expression, which in turn induces the expression of miR-125b that targets BCL2 antagonist/killer 1 and consequently leads to the suppression of apoptosis (54,55). Stem-like cancer cells isolated from primary cervical tumors or cell lines express OCT4 together with NANOG and SOX2 and have sphere-forming and self-renewal abilities, which are abolished by the suppression of HPV-coding E6 protein (56). This suggests that independent analyses are required in order to reveal the potential mechanism of the regulation of stem cell markers by HPV infection.
In biomarker discovery, the sensitivity and specificity as well as the convenience of diagnosis for physicians should be taken into account. Due to its invasive and metastatic potential, CSCs may be able to spread from primary tumors and survive in the circulating blood by evading immune surveillance, and subsequently form metastases (57). Accordingly, the determination of a stem cell marker that is released from circulating CSCs may become a fast and direct approach to plasma-based diagnosis of cancer.
A previous study reported that ALDH1 is elevated in the blood of patients with non-small-cell lung cancer prior to surgery but is not detectable in post-operative samples (58). The detection of serum ALDH1A1 has been suggested to have a predictive value in monitoring the chemotherapy of patients with primary and metastatic breast cancer (59). Additionally, high levels of serum OCT4 and NANOG are detectable in patients with hepatocellular carcinoma and hepatitis B virus infection (60).
It is also important for the detection of stem cell markers with a tumor origin to take into account the complexity of stem cells, multipotent stem-like cells or progenitor cells in the blood circulation. Notably, the blood contains highly heterogenic populations of vascular-resident stem or progenitor cells with proliferative capacity and clonogenicity, including mesenchymal stem cells, pericytes, endothelial progenitor cells and smooth muscle progenitor cells, which serve important functions in vascular homeostasis (61). These cells are either derived from the bone marrow and other sources or frequently released from the vascular endothelium to the blood by hemodynamic forces, including fluid shear stress and cyclic strain or pathologic processes during heart and vascular diseases (62,63). In addition, mesenchymal stem cells are multipotent and are able to differentiate into vascular smooth muscle cells and endothelial cells (64). High ALDH activity may be detected in human bone marrow-derived mesenchymal stromal cells with multipotent stromal and pro-vascular regenerative functions (65). Consistent with these findings, the results of the present study indicate that the increased levels of ALDH1A1 in the blood may, to a high extent, contribute to cervical carcinogenesis, and therefore ALDH1A1 may be used as a marker to distinguish patients with cervical carcinoma from normal controls.
To the best of our knowledge, the number of previous studies on the detection of OCT4 in the blood of patients with cancer is limited. In the present study, there were no significant differences between the plasma levels of ALDH1 and OCT4 in patients with CIN II–III and normal controls. However, the plasma levels of ALDH1 and OCT4 significantly increased in patients with cervical carcinoma compared with CIN II–III and controls. These results were consistent with the findings from IHC, which revealed that in specimens from normal subjects, the expression of ALDH1 and OCT4 proteins was negative, whereas the expression of these proteins was moderate or strongly positive in cervical carcinoma. Additionally, the differences in expression of ALDH1 and OCT4 proteins between specimens from normal subjects and patients with cervical carcinoma were statistically significant. Therefore, OCT4 may additionally contribute to the diagnosis and prognosis of cervical carcinoma, when its detection is combined with the detection of ALDH1 and other biomarkers.
NANOG expression is detectable in embryonic stem cells, embryonic limb bud cells and embryonal carcinoma cells (66,67). The expression of NANOG is elevated at advanced clinical stages of breast cancer, glioma and bladder cancer, which may be associated with poor prognosis (68). The results of the present study revealed that NANOG transcription was significantly increased in tissues from patients with SCC or CIN compared with controls, but the expression was not significantly different between specimens from patients with SCC and CIN. In addition, a previous study also indicated that the high expression of NANOG in cervical lesions may not be associated with the prognosis of cervical carcinoma (16). Accordingly, NANOG expression was not additionally analyzed by IHC and plasma-based ELISA experiments. However, the combined detection of ALDH, OCT4 and NANOG markers may improve the accuracy and specificity of the biomarker profile for diagnosis of cervical carcinoma.
In conclusion, it was demonstrated that ALDH1A1 and OCT4 were upregulated in cervical carcinoma tissues and its precursor lesions at the level of mRNA and protein, as well as in blood plasma, which represents the protein levels in the whole body. Notably, the samples subjected to analysis by RT-qPCR, IHC and ELISA were obtained from three independent populations of patients and controls, which may increase the feasibility and accuracy of quantitative verification. Despite the limited prognostic potential of ALDH1A1 and OCT4 in the blood-based detection, it may be suggested that the combined detection of these proteins at the level of transcription, protein expression and in the blood circulation may provide an auxiliary profile for the prognosis, diagnosis and monitoring of the treatment of cervical carcinoma.
Cervical cancer is a challenge for the Uighur female population in Xinjiang, China, where there is a high incidence rate (490–560/100,000) accompanied with the prevalence of HPV infection (69,70). In accordance with preliminary data, the case detection rate of cervical carcinoma accounts for ~20% of all patients diagnosed with cancer in Xinjiang (71). Therefore, further validation of this profiling, and further in-depth studies on the association of the altered expression of stem cell markers with HPV infection by other approaches, will contribute to establishing an auxiliary diagnostic profile for HPV-based cancer prognosis and greatly benefit the women in areas with a high prevalence, including Uighur women in China.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the Natural Science Foundation of China (grant no. 81360321) and the Natural Science Fund for Young Scholars of Xinjiang Uighur Autonomous Region China (grant. no. 2017D01C139). The funders had no role in the study design, data collection, analysis, decision to publish or preparation of the manuscript.
Availability of data and materials
The datasets generated and/or analysed during this study are available from the corresponding author on reasonable request.
Authors' contributions
AA designed the research. WT, RY and LS performed the research. MR and RY were responsible for clinical diagnosis and sample collection. DL contributed to data collection and statistical analysis. AA and WT wrote the manuscript. All the authors read and approved the final manuscript.
Ethics approval and consent to participate
Te present study was approved and monitored by the Ethics Committee of Xinjiang Medical University (Urumqi, China). All procedures performed in this study were followed in accordance with the Helsinki Declaration of 1975, as revised in 2000 (5). Written informed consent was obtained from all patients and healthy individuals prior to the study, and all data were analyzed anonymously.
Patient consent for publication
Written informed consent was obtained from all individual participants included in the present study.
Competing interests
All authors declare that they have no competing interest.
References
- 1.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Islam F, Gopalan V, Smith RA, Lam AK. Translational potential of cancer stem cells: A review of the detection of cancer stem cells and their roles in cancer recurrence and cancer treatment. Exp Cell Res. 2015;335:135–147. doi: 10.1016/j.yexcr.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 3.Ferenczy A, Franco E. Persistent human papillomavirus infection and cervical neoplasia. Lancet Oncol. 2002;3:11–16. doi: 10.1016/S1470-2045(01)00617-9. [DOI] [PubMed] [Google Scholar]
- 4.Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer. 2002;2:59–65. doi: 10.1038/nrc700. [DOI] [PubMed] [Google Scholar]
- 5.Chhabra R. Cervical cancer stem cells: Opportunities and challenges. J Cancer Res Clin Oncol. 2015;141:1889–1897. doi: 10.1007/s00432-014-1905-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.López J, Valdez-Morales FJ, Benitez-Bribiesca L, Cerbón M, Carrancá AG. Normal and cancer stem cells of the human female reproductive system. Reprod Biol Endocrinol. 2013;11:53. doi: 10.1186/1477-7827-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herfs M, Yamamoto Y, Laury A, Wang X, Nucci MR, McLaughlin-Drubin ME, Münger K, Feldman S, McKeon FD, Xian W, Crum CP. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc Natl Acad Sci USA. 2012;109:10516–10521. doi: 10.1073/pnas.1202684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letian T, Tianyu Z. Cellular receptor binding and entry of human papillomavirus. Virol J. 2010;7:2. doi: 10.1186/1743-422X-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pyeon D, Pearce SM, Lank SM, Ahlquist P, Lambert PF. Establishment of human papillomavirus infection requires cell cycle progression. PLoS Pathog. 2009;5:e1000318. doi: 10.1371/journal.ppat.1000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quade BJ, Yang A, Wang Y, Sun D, Park J, Sheets EE, Cviko A, Federschneider JM, Peters R, McKeon FD, Crum CP. Expression of the p53 homologue p63 in early cervical neoplasia. Gynecol Oncol. 2001;80:24–29. doi: 10.1006/gyno.2000.5953. [DOI] [PubMed] [Google Scholar]
- 11.Sell S. On the stem cell origin of cancer. Am J Pathol. 2010;176:2584–2494. doi: 10.2353/ajpath.2010.091064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martens JE, Arends J, Van der Linden PJ, De Boer BA, Helmerhorst TJ. Cytokeratin 17 and p63 are markers of the HPV target cell, the cervical stem cell. Anticancer Res. 2004;24:771–775. [PubMed] [Google Scholar]
- 13.Mighty KK, Laimins LA. p63 is necessary for the activation of human papillomavirus late viral functions upon epithelial differentiation. J Virol. 2011;85:8863–8869. doi: 10.1128/JVI.00750-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Oron E, Nelson B, Razis S, Ivanova N. Distinct lineage specification roles for NANOG, OCT4 and SOX2 in human embryonic stem cells. Cell Stem Cell. 2012;10:440–454. doi: 10.1016/j.stem.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Hattori N, Imao Y, Nishino K, Hattori N, Ohgane J, Yagi S, Tanaka S, Shiota K. Epigenetic regulation of Nanog gene in embryonic stem and trophoblast stem cells. Genes Cells. 2007;12:387–396. doi: 10.1111/j.1365-2443.2007.01058.x. [DOI] [PubMed] [Google Scholar]
- 16.Ye F, Zhou C, Cheng Q, Shen J, Chen H. Stem-cell-abundant proteins Nanog, Nucleostemin and Musashi1 are highly expressed in malignant cervical epithelial cells. BMC cancer. 2008;8:108. doi: 10.1186/1471-2407-8-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu D, Zhou P, Zhang L, Wu G, Zheng Y, He F. Differential expression of Oct4 in HPV-positive and HPV-negative cervical cancer cells is not regulated by DNA methyltransferase 3A. Tumour Biol. 2011;32:941–950. doi: 10.1007/s13277-011-0196-z. [DOI] [PubMed] [Google Scholar]
- 18.Li SW, Wu XL, Dong CL, Xie XY, Wu JF, Zhang X. The differential expression of OCT4 isoforms in cervical carcinoma. PLoS One. 2015;10:e0118033. doi: 10.1371/journal.pone.0118033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao T, Chen Q, Zhang B, Zhou H, Lin Z. The expression of ALDH1 in cervical carcinoma. Med Sci Monit. 2011;17:HY21–HY26. doi: 10.12659/MSM.881886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao QX, Yao TT, Zhang BZ, Lin RC, Chen ZL, Zhou H, Wang LJ, Lu HW, Chen Q, Di N, Lin ZQ. Expression and functional role of ALDH1 in cervical carcinoma cells. Asian Pac J Cancer Prev. 2012;13:1325–1331. doi: 10.7314/APJCP.2012.13.4.1325. [DOI] [PubMed] [Google Scholar]
- 21.Liu SY, Zheng PS. High aldehyde dehydrogenase activity identifies cancer stem cells in human cervical cancer. Oncotarget. 2013;4:2462–2475. doi: 10.18632/oncotarget.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Zhou BP. Activation of beta-catenin and Akt pathways by Twist are critical for the maintenance of EMT associated cancer stem cell-like characters. BMC Cancer. 2011;11:49. doi: 10.1186/1471-2407-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin J, Liu X, Ding D. Evidence for epithelial-mesenchymal transition in cancer stem-like cells derived from carcinoma cell lines of the cervix uteri. Int J Clin Exp Pathol. 2015;8:847–855. [PMC free article] [PubMed] [Google Scholar]
- 24.Saslow D, Runowicz CD, Solomon D, Moscicki AB, Smith RA, Eyre HJ, Cohen C. American Cancer, Society: American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52:342–362. doi: 10.3322/canjclin.52.6.342. [DOI] [PubMed] [Google Scholar]
- 25.Pecorelli S, Benedet JL, Creasman WT, Shepherd JH. FIGO staging of gynecologic cancer. 1994–1997 FIGO Committee on Gynecologic Oncology. International Federation of Gynecology and Obstetrics. Int J Gynaecol Obstet. 1999;65:243–249. doi: 10.1016/S0020-7292(99)00070-3. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Mason DY. Oxford Textbook of Pathology. Vol. 2. Oxford University Press; Oxford: 1992. Immunocytochemical analysis of human tissue; pp. 2275–2284. [Google Scholar]
- 28.Ping YF, Bian XW. Consice review: Contribution of cancer stem cells to neovascularization. Stem Cells. 2011;29:888–894. doi: 10.1002/stem.650. [DOI] [PubMed] [Google Scholar]
- 29.Facompre N, Nakagawa H, Herlyn M, Basu D. Stem-like cells and therapy resistance in squamous cell carcinomas. Adv Pharmacol. 2012;65:235–265. doi: 10.1016/B978-0-12-397927-8.00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel P, Chen EI. Cancer stem cells, tumor dormancy, and metastasis. Front Endocrinol (Lausanne) 2012;3:125. doi: 10.3389/fendo.2012.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrews TE, Wang D, Harki DA. Cell surface markers of cancer stem cells: Diagnostic macromolecules and targets for drug delivery. Drug Deliv Transl Res. 2013;3:121–142. doi: 10.1007/s13346-012-0075-1. [DOI] [PubMed] [Google Scholar]
- 32.Xia P. Surface markers of cancer stem cells in solid tumors. Curr Stem Cell Res Ther. 2014;9:102–111. doi: 10.2174/1574888X09666131217003709. [DOI] [PubMed] [Google Scholar]
- 33.Allegra A, Alonci A, Penna G, Innao V, Gerace D, Rotondo F, Musolino C. The cancer stem cell hypothesis: A guide to potential molecular targets. Cancer Invest. 2014;32:470–495. doi: 10.3109/07357907.2014.958231. [DOI] [PubMed] [Google Scholar]
- 34.Sladek NE. Human aldehyde dehydrogenases: Potential pathological, pharmacological, and toxicological impact. J Biochem Mol Toxicol. 2003;17:7–23. doi: 10.1002/jbt.10057. [DOI] [PubMed] [Google Scholar]
- 35.Chute JP, Muramoto GG, Whitesides J, Colvin M, Safi R, Chao NJ, McDonnell DP. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc Natl Acad Sci USA. 2006;103:11707–11712. doi: 10.1073/pnas.0603806103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcato P, Dean CA, Giacomantonio CA, Lee PW. Aldehyde dehydrogenase: Its role as a cancer stem cell marker comes down to the specific isoform. Cell Cycle. 2011;10:1378–1384. doi: 10.4161/cc.10.9.15486. [DOI] [PubMed] [Google Scholar]
- 37.Tomita H, Tanaka K, Tanaka T, Hara A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget. 2016;7:11018–11032. doi: 10.18632/oncotarget.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Magnen C, Bubendorf L, Rentsch CA, Mengus C, Gsponer J, Zellweger T, Rieken M, Thalmann GN, Cecchini MG, Germann M, et al. Characterization and clinical relevance of ALDHbright populations in prostate cancer. Clin Cancer Res. 2013;19:5361–5371. doi: 10.1158/1078-0432.CCR-12-2857. [DOI] [PubMed] [Google Scholar]
- 39.Kurashige T, Shimamura M, Yasui K, Mitsutake N, Matsuse M, Nakashima M, Minami S, Eguchi S, Nagayama Y. Studies on the expression of aldehyde dehydrogenase in normal and cancerous tissues of thyroids. Horm Metab Res. 2015;47:194–199. doi: 10.1055/s-0034-1387770. [DOI] [PubMed] [Google Scholar]
- 40.Wu S, Xue W, Huang X, Yu X, Luo M, Huang Y, Liu Y, Bi Z, Qiu X, Bai S. Distinct prognostic values of ALDH1 isoenzymes in breast cancer. Tumour Biol. 2015;36:2421–2426. doi: 10.1007/s13277-014-2852-6. [DOI] [PubMed] [Google Scholar]
- 41.Patel M, Lu L, Zander DS, Sreerama L, Coco D, Moreb JS. ALDH1A1 and ALDH3A1 expression in lung cancers: Correlation with histologic type and potential precursors. Lung Cancer. 2008;59:340–349. doi: 10.1016/j.lungcan.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 42.Hou T, Zhang W, Tong C, Kazobinka G, Huang X, Huang Y, Zhang Y. Putative stem cell markers in cervical squamous cell carcinoma are correlated with poor clinical outcome. BMC Cancer. 2015;15:785. doi: 10.1186/s12885-015-1826-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao T, Wu Z, Liu Y, Rao Q, Lin Z. Aldehyde dehydrogenase 1 (ALDH1) positivity correlates with poor prognosis in cervical cancer. J Int Med Res. 2014;42:1038–1042. doi: 10.1177/0300060514527060. [DOI] [PubMed] [Google Scholar]
- 44.Xie Q, Liang J, Rao Q, Xie X, Li R, Liu Y, Zhou H, Han J, Yao T, Lin Z. Aldehyde dehydrogenase 1 expression predicts chemoresistance and poor clinical outcomes in patients with locally advanced cervical cancer treated with neoadjuvant chemotherapy prior to radical hysterectomy. Ann Surg Oncol. 2016;23:163–170. doi: 10.1245/s10434-015-4555-7. [DOI] [PubMed] [Google Scholar]
- 45.Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 46.Kim J, Chu J, Shen X, Wang J, Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeineddine D, Hammoud AA, Mortada M, Boeuf H. The Oct4 protein: More than a magic stemness marker. Am J Stem Cells. 2014;3:74–82. [PMC free article] [PubMed] [Google Scholar]
- 48.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rizzino A. Concise review: The Sox2-Oct4 connection: Critical players in a much larger interdependent network integrated at multiple levels. Stem Cells. 2013;31:1033–1039. doi: 10.1002/stem.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liedtke S, Stephan M, Kögler G. Oct4 expression revisited: Potential pitfalls for data misinterpretation in stem cell research. Biol Chem. 2008;389:845–850. doi: 10.1515/BC.2008.098. [DOI] [PubMed] [Google Scholar]
- 51.Wang YJ, Herlyn M. The emerging roles of Oct4 in tumor-initiating cells. Am J Physiol Cell Physiol. 2015;309:C709–C718. doi: 10.1152/ajpcell.00212.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang YD, Cai N, Wu XL, Cao HZ, Xie LL, Zheng PS. OCT4 promotes tumorigenesis and inhibits apoptosis of cervical cancer cells by miR-125b/BAK1 pathway. Cell Death Dis. 2013;4:e760. doi: 10.1038/cddis.2013.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Echelman D, Feldman S. Management of cervical precancers: a global perspective. Hematol Oncol Clin North Am. 2012;26:31–44. doi: 10.1016/j.hoc.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 54.Kim BW, Cho H, Choi CH, Ylaya K, Chung JY, Kim JH, Hewitt SM. Clinical significance of OCT4 and SOX2 protein expression in cervical cancer. BMC Cancer. 2015;15:1015. doi: 10.1186/s12885-015-2015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu D, Zhou P, Zhang L, Zheng Y, He F. HPV16 activates the promoter of Oct4 gene by sequestering HDAC1 from repressor complex to target it to proteasomal degradation. Med Hypotheses. 2012;79:531–534. doi: 10.1016/j.mehy.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 56.Tyagi A, Vishnoi K, Mahata S, Verma G, Srivastava Y, Masaldan S, Roy BG, Bharti AC, Das BC. Cervical cancer stem cells selectively overexpress HPV oncoprotein E6 that controls stemness and self-renewal through upregulation of HES1. Clin Cancer Res. 2016;22:4170–4184. doi: 10.1158/1078-0432.CCR-15-2574. [DOI] [PubMed] [Google Scholar]
- 57.Yang MH, Imrali A, Heeschen C. Circulating cancer stem cells: The importance to select. Clin Cancer Res. 2015;27:437–449. doi: 10.3978/j.issn.1000-9604.2015.04.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao YT, Li JH, Wang YT, Fu YW, Xu J. Serum ALDH1A1 is a tumor marker for the diagnosis of non-small cell lung cancer. Tumori. 2014;100:214–218. doi: 10.1177/030089161410000216. [DOI] [PubMed] [Google Scholar]
- 59.Sladek NE, Kollander R, Sreerama L, Kiang DT. Cellular levels of aldehyde dehydrogenases (ALDH1A1 and ALDH3A1) as predictors of therapeutic responses to cyclophosphamide-based chemotherapy of breast cancer: A retrospective study. Rational individualization of oxazaphosphorine-based cancer chemotherapeutic regimens. Cancer Chemother Pharmacol. 2002;49:309–321. doi: 10.1007/s00280-001-0412-4. [DOI] [PubMed] [Google Scholar]
- 60.Chang TS, Wu YC, Chi CC, Su WC, Chang PJ, Lee KF, Tung TH, Wang J, Liu JJ, Tung SY, et al. Activation of IL6/IGFIR confers poor prognosis of HBV-related hepatocellular carcinoma through induction of OCT4/NANOG expression. Clin Cancer Res. 2015;21:201–210. doi: 10.1158/1078-0432.CCR-13-3274. [DOI] [PubMed] [Google Scholar]
- 61.Bobryshev YV, Orekhov AN, Chistiakov DA. Vascular stem/progenitor cells: Current status of the problem. Cell Tissue Res. 2015;362:1–7. doi: 10.1007/s00441-015-2231-7. [DOI] [PubMed] [Google Scholar]
- 62.Krenning G, Barauna VG, Krieger JE, Harmsen MC, Moonen JR. Endothelial plasticity: Shifting phenotypes through force feedback. Stem Cells Int. 2016;2016:9762959. doi: 10.1155/2016/9762959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Minhajat R, Nilasari D, Bakri S. The role of endothelial progenitor cell in cardiovascular disease risk factors. Acta Med Indones. 2015;47:340–347. [PubMed] [Google Scholar]
- 64.Gu W, Hong X, Potter C, Qu A, Xu Q. Mesenchymal stem cells and vascular regeneration. Microcirculation. 2017;24 doi: 10.1111/micc.12324. doi: 10.1111/micc.12324. [DOI] [PubMed] [Google Scholar]
- 65.Sherman SE, Kuljanin M, Cooper TT, Putman DM, Lajoie GA, Hess DA. High aldehyde dehydrogenase activity identifies a subset of human mesenchymal stromal cells with vascular regenerative potential. Stem Cells. 2017;35:1542–1553. doi: 10.1002/stem.2612. [DOI] [PubMed] [Google Scholar]
- 66.Constantinescu S. Stemness, fusion and renewal of hematopoietic and embryonic stem cells. J Cell Mol Med. 2003;7:103–112. doi: 10.1111/j.1582-4934.2003.tb00209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hattori N, Imao Y, Nishino K, Hattori N, Ohgane J, Yagi S, Tanaka S, Shiota K. Epigenetic regulation of Nanog gene in embryonic stem and trophoblast stem cells. Genes Cells. 2007;12:387–396. doi: 10.1111/j.1365-2443.2007.01058.x. [DOI] [PubMed] [Google Scholar]
- 68.Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suzuke L, Peng YH, Zhou K, et al. The analysis of pathogenetic tendency of cervical cancer in various ethnic women in Xinjiang. J Xinjiang Med Univ. 2006;29:569–571. (In Chinese) [Google Scholar]
- 70.Abudoukadeer A, Niyazi M, Aikula A, Kamilijian M, Sulaiman X, Mutailipu A, Abudula A. Association of EBV and HPV co-infection with the development of cervical cancer in ethnic Uyghur women. Eur J Gynaecol Oncol. 2015;36:546–550. [PubMed] [Google Scholar]
- 71.Zhang GQ, LIU KJ, Lai XJ, et al. Distribution of malignant tumor patients in hospital from 1989 to 2002 in the Affiliated Tumor Hospital of Xinjiang Medical University. J Xinjiang Med Univ. 2003;26:393–395. (In Chinese) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during this study are available from the corresponding author on reasonable request.