Abstract
N6-methyladenosine (m6A) modification in RNA has been implicated in diverse biological processes including the maintenance of embryonic stem cells, early development and diseases. Although the m6A modification was discovered several decades ago, its biological function remained unclear. The recent discovery of enzymes responsible for ‘writing’ or ‘erasing’ the modification and single-nucleotide resolution mapping by next-generation sequencing technology have revealed its function in biological processes. Its enrichment pattern is conserved in mammalian transcriptomes, and the level of m6A is tightly regulated by methyltransferases (writers), demethylases (erasers) and binding proteins (readers). Furthermore, accumulating evidence suggests that the aberrant regulation of m6A turnover is associated with multiple types of cancer including acute myeloid leukemia, breast cancer, glioblastoma, lung cancer and liver cancer. Studies have demonstrated that factors involved in m6A metabolism serve either oncogenic or tumor-suppressor roles in different contexts. The previous studies of the role of m6A in cancer biology are discussed in the present review.
Keywords: cancer, RNA, N6-methyladenosine, methylation, epitranscriptomics, gene expression
1. Introduction
RNA is a central component of gene expression and is the link between genetic information (DNA) and proteins. Multiple mechanisms are involved in regulating gene expression. One such mechanism is mediated by chemical modification of RNA either during or after transcription. More than 100 post-transcriptional modifications have been found in all RNA species (1). The functions of these modifications are diverse and depend on the biological context. Among the modifications, recent studies revealed that N6-methyladenosine (m6A) modification in eukaryotic mRNAs plays significant roles in pleiotropic biological processes including stem cell biology, development, immunology, and cancer biology (2–5). m6A modification was discovered several decades ago in mouse and human transcriptomes (6–11). However, its role was unclear until recently. The discovery that fat mass and obesity-associated protein (FTO) is an enzyme that demethylates m6A prompted studies to evaluate the biological consequences of m6A (12). FTO protein was originally recognized as a regulator of lipid metabolism (13–15). The availability of m6A-specific antibodies and transcriptome-wide m6A mapping studies further revealed the unique pattern of m6A distribution and functions in the regulation of RNA biology (16,17). Studies demonstrated that aberrant regulation of m6A turnover is linked to pathophysiological disorders such as excessive fatty acid deposition, developmental retardation, type 2 diabetes mellitus, aberrant germ cell formation, circadian period elongation, and cancers (13–15,18–35). Among these, emerging evidence suggests that factors metabolizing m6A are involved in multiple forms of human cancer, including lung cancer, glioblastoma, breast cancer, and acute myeloid leukemia (AML). The review discusses recent progress in understanding m6A-related cancers (23–35).
2. m6A enrichment and its function
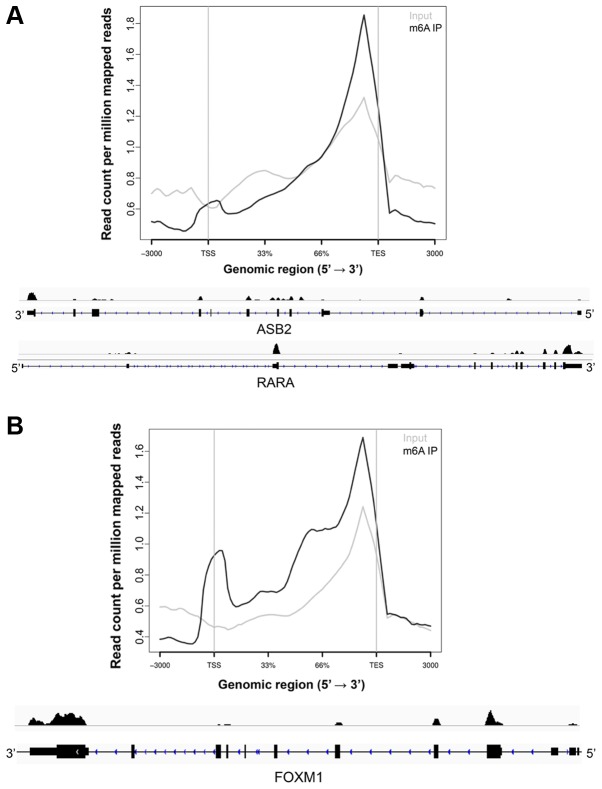
In the 1970s, several groups detected m6A in human and mouse cells. The early discovery of m6A modification was made in noncoding RNAs including snoRNAs, tRNAs, and rRNAs, and mRNAs (6–11). However, the function of the modification was unknown because of technical limitations in functional analysis. In 2012, two independent studies profiled the transcriptome-wide m6A distribution and found a conserved pattern of its enrichment in both the human and mouse transcriptome (16,17). Since then, multiple studies confirmed similar patterns of the transcriptome-wide distribution of m6A in some eukaryotes including cow, yeast, and plant (36–38). It is estimated that more than ≥7,600 coding and ≥300 noncoding RNAs contain m6A, and 0.1–0.4% of total adenines (As) were methylated in mammalian transcripts (11,16,17,39). In general, the studies revealed the following: i) There is a consensus sequence [(G/A/U)(G>A)m6AC(U>A>C)] marked by m6A (16,17,39–42). ii) m6A is preferentially enriched around the transcription end site (TES) in mRNAs or last exon in noncoding RNAs (16,17) (Fig. 1). Relatively less enriched m6A peaks are also observed at the transcription start site (TSS) (43) (Fig. 1). iii) Some m6A-marked genes are conserved in human and mouse cells (16). For example, ~46% of conserved m6A peaks between human and mouse ES cells were identified. The data suggest a conserved function of the modification in regulating stem cell biology. The unique pattern of m6A enrichment implicates its functions in translation efficiency, splicing, mRNA export, and alternative polyadenylation (16,40,44–46). In general, m6A accumulation accelerates transcript decay (45). In contrast, TSS m6A is known to accelerate the CAP-independent translation start by changing the 3D structure of mRNA (47). These structural changes recruit transcription initiation proteins such as eIF3 (47). Although early functional studies of m6A were shown to positively regulate splicing, a recent study demonstrated that m6A is observed in exons of newly synthesized transcripts. In contrast to previous hypotheses, m6As in exon are quite static, as m6A exists in the exons of chromatin-associated RNAs and the same pattern of m6A distribution is observed even after translocation into the cytoplasm (48). Therefore, the data suggest that m6A plays an independent role in splicing. The study confirmed that m6A methylation is negatively correlated with the half-life of the transcript. Ke et al (49) showed that genes with a long last exon have a higher density of m6A compared to genes with short last exons. Furthermore, the study suggested that m6A density affects utilization of the polyA site; if a gene more frequently utilizes a proximal polyA site, m6A density around the proximal polyA site is low, whereas if a gene less frequently utilizes a proximal polyA site, m6A density around the site is high (49). The idea of its role in controlling splicing was originally proposed based on the observation that m6A was more observed in intron compared to exon sequences. However, the study suggests that m6A-mediated regulation of splicing is less likely.
Figure 1.
Analysis of m6A enrichment in MON-MAC-6 cells (acute monocytic leukemia) (A) and glioblastoma stem-like cells (B) using publically available data sets (GSE87515 and GSE76414). The hg38 human genome assembly was used for the m6A-Seq analysis. m6A distribution in genebody is visualized using ngs.plot tool. Representative genes [(A) ASB2 and RARA genes in MON-MAC-6 cells and (B) FOXM1 gene in glioblastoma stem-like cells] for m6A distributions are presented using Integrative Genomics Viewer. Note that m6A preferentially is enriched around TSS and TES. FOXM1, forkhead box protein M1; TSS, transcription start sites; TES, transcription end site.
3. Factors metabolizing m6A
m6A methyltransferases (writer): METTL3, METTL14, and WTAP
Several enzymes catalyze m6A formation. The first protein identified to have this function is methyltransferase-like protein (METTL) 3, which forms a heterodimeric complex with another methyltransferase, METTL14 (50). Although both METTL3 and METTL14 contain conserved catalytic domains, only METTL3 appears to contain a methyl donor, S-adenosylmethionine, binding domain (50). METTL3 is highly conserved in most eukaryotes and broadly expressed, and its enriched subcellular localization is found in nuclear speckles (51). Genetic ablation of mouse METTL3 or METTL14 causes aberrant differentiation of embryonic stem cells, spermatogonial cells, and neuronal lineages with bulk reduction of m6A levels (2,52,53). Wilms's tumor protein 1 (WT1)-associated protein (WTAP), known as a splicing factor and binding partner of human WT1, has shown to be required for embryonic development and cell cycle progression (40). WTAP-depleted mice display lethal at peri-gastrula stages with failure in cell proliferation (54). Although WTAP does not possess methyltransferase activity, it appears to modulate m6A methylation by interacting with the METTL3-METTL14 complex.
M6A demethylases (eraser): FTO and ALKBH5
FTO protein belongs to a member of Fe(II)- and α-ketoglutarate-dependent AlkB dioxygenase family and is known to be linked to childhood and adult obesity (55). FTO was shown to demethylate m6A in vitro and its loss results in growth retardation and aberrant metabolism with increased m6A levels (12,56). A forced overexpression of FTO leads to decreased m6A levels in HeLa cells (12). FTO has been suggested as an important player in neuronal development and cancers in recent studies (26,28,32,57). ALKBH5 is also a member of the Fe(II)- and α-ketoglutarate-dependent AlkB dioxygenase family. ALKBH5-depleted male animals suffer from sterility with accumulation of polyA-tailed RNAs in the cytoplasm (58). As described above, iron as a cofactor in the biochemical reaction appears to be essential, as mutations of amino acid residues in the iron binding pocket lead to loss of catalytic activity (59).
m6A-interacting proteins (reader): YTHDF2 and YTHDF3
Recent studies demonstrated that m6A modifications can be recognized by at least three proteins. The first proteins identified in immunoprecipitation experiments were YT521-B homology domain-containing family protein (YTHDF)2, and YTHDF3 (60). The two proteins contain the conserved YTH domain, which is known to preferentially interact with single-stranded RNA compared to single-stranded DNA (60). In mouse, there are five known YTH domain-containing proteins including four cytoplasmic (YTHDF1-3 and YTHDC2) and one nuclear (YTH domain-containing 1) (61,62). YTHDF2 binds to m6A and accelerates degradation of m6A-containing RNAs (61). YTHDF2 depletion causes female-specific sterility (63). Furthermore, YTHDF2 was shown to play a critical role in maternal-to-zygotic transition by accelerating the decay of maternally inherited mRNAs in zebrafish (64). In contrast, YTHDF3 promotes mRNA translation (62). Together, the studies suggest that regulation of the half-life of RNAs by m6A addition and/or removal plays an important role in mammalian early development.
4. Role of m6A in cancer
Cancer types that are associated with proteins regulating m6A generation or removal are summarized in Table I.
Table I.
Role of factors regulating m6A in human cancer.
| Cancer type | Cell types examined | Associated proteins and expression patterns | Target genes (effects on their expression) | (Refs.) |
|---|---|---|---|---|
| Breast cancer | MDA-MB-231, MDA-MB-435, MCF-7, ZR75.1, T47D, BT-474, HCC-1954, SUM-149, SUM-159 | ALKBH5: Upregulated | NANOG (activated) | (23,24) |
| AML | WTAP: Peripheral blood mononuclear cells from AML patients | WTAP: CNV | WTAP-related: Not identified METTL3-related: PTEN, | (25–30) |
| METTL3: MOLM13, THP-1, MV4-11, | METTL3: Upregulated | c-MYC and BCL2 (activated) | ||
| NOMO-1, HL-60, KG-1, HEL, | ALKBH5: Loss of CNV | ALKBH5-related: Not identified | ||
| OCI-AML2, OCI-AML3, EOL-1 and AML patient samples | FTO: Upregulated | FTO-related: ASB2 and RARA (repressed) | ||
| ALKBH5: Database in TCGA Research Network FTO: Bone marrow cells isolated from primary MLL-rearranged AML patients | ||||
| Glioblastoma | Hs683, SW1783, U87MG, LN229, U251MG, GBM05 and patient-derived glioblastoma cells | ALKBH5: Upregulated | ALKBH5-related: FOXM1 (activated) | (31) |
| Lung cancer | A549, H1299 and H1792 | METTL3: Upregulated | Not identified | (34) |
| Liver cancer | Patient-derived HCC cells | METTL3: Upregulated | SOCS2 (repressed) | (35) |
m6A, N6-methyladenosine; WTAP, Wilms's tumor protein 1 (WT1)-associated protein; CNV, copy number variations; AML, acute myeloid leukemia; METTL3, methyltransferase-like protein 3; ALKBH5, alkylation repair homolog 5; FTO, fat mass and obesity-associated protein; PTEN, phosphatase and tensin homolog; Myc, myelocytomatosis oncogene; Bcl-2, B-cell lymphoma 2; TCGA, The Cancer Genome Atlas; ASB2, ankyrin repeat and SOCS box containing 2; RARA, retinoic acid receptor alpha; FOXM1, forkhead box protein M1; SOCS2, suppressor of cytokine signaling 2; HCC, hepatocellular carcinoma.
Cancer stem cell formation in breast cancer
Zhang et al (23) demonstrated that exposure to hypoxic conditions increased ALKBH5 expression in a subset of breast cancer cells. The increased ALKBH5 level is mediated by elevated expression of hypoxia-inducible factor (HIF)-1α and HIF-2α. Furthermore, ALKBH5 was shown to demethylate NANOG mRNA, a pluripotent factor in cancer stem cells. Depletion of ALKBH5 in MDAMB-231 human breast cancer cells reduces tumor initiation capacity and metastasis into the lungs by decreasing the number of breast stem cells (23). More recently, the same group showed that breast cancer cells under hypoxic conditions show increased expression of ZNF217, which is a known factor for sequestering METTL3 (24). ZNF217-mediated METTL3 sequestering leads to inhibition of m6A generation in NANOG, KLF4, and SOX2 mRNAs in embryonic stem cells. Similarly, hypoxia-dependent induction of ZNF217 in breast cancer cells inhibits m6A methylation in NANOG and KLF4 mRNAs (24). These studies revealed that elevated levels of HIFs and ALKBH5 by hypoxia enhance pluripotency factor expression and specification of breast cancer stem cells by negatively regulating m6A biogenesis.
AML
AML is a devastating disease characterized by defects in differentiation, increased proliferation, and inhibition of cell apoptosis (65). WTAP has been suggested to have an oncogenic role in AML (25). Approximately 32% of AML patients carrying NPM1 and/or FTL3-ITD mutation(s) exhibit aberrant overexpression of the WTAP gene (25). Genome-scale analysis revealed that 2–9% of AML patients carry copy number variations (CNV) in m6A regulatory genes including FTO, ALKBH5, YTHDF1, YTHDF2, METTL3, and METTL14 (26). Loss of CNV in the ALKBH5 gene is the most obviously observed in AML patients (26). In addition, METTL3 depletion in human hematopoietic progenitor cells and myeloid leukemia cell lines leads to accelerated cell differentiation with reduced cell proliferation, as well as delayed leukemic progression after transplantation into mice, respectively (27). m6A modification enhances the translation of PTEN, c-MYC, and BCL2 transcripts (28,29). In contrast, FTO is aberrantly upregulated in AMLs with t(15;17)/PML-RARA, t(11q23)/MLL, FLT3-ITD, and/or NPM1 mutations (30). FTO promotes cell transformation and leukemogenesis. Mechanistically, FTO-mediated m6A reduction directly inhibits all-trans-retinoic acid-induced AML cell differentiation by regulating the expression of ASB2 and RARA in vitro (30). The data suggests that fine-tuning of m6A formation closely controls differentiation, survival, and lineage commitment of hematopoietic cells.
Glioblastoma
Glioblastoma is a life-threatening brain tumor showing a median overall survival between 10 and 20 months. The incidence of glioblastoma is approximately 3–4/100,000, increases with age, and peaks in patients aged 50–60 years (66,67). Multiple genes have shown to be involved in causing glioblastoma (67). A high level of ALKBH5 expression is detected in glioblastoma stem-like cells (GSCs), and ALKBH5 silencing inhibits the proliferation of patient-derived GSCs (31). Mechanistically, ALKBH5 upregulates FOXM1 expression by direct m6A demethylation in the FOXM1 transcript (31). Depletion of ALKBH5 abolishes GSC tumorigenesis by reducing FOXM1 expression. Silencing of a methyltransferase, METTL14 or METTL3, promotes human GSC self-renewal, growth, and tumorigenesis, whereas overexpression of METTL3 inhibits GSC self-renewal and growth (32,33).
Other cancers
It has been shown that METTL3 is upregulated in lung adenocarcinoma and promotes the survival, growth, and invasion of human lung cancer cells by promoting the translation of genes related to cancer (34). More recently, METTL3 expression was found to be upregulated in human hepatocellular carcinoma (HHC) and its overexpression was related to poor prognosis in patients with HCC (35). Loss of METTL3 function leads to suppressed HCC tumorigenicity and lung metastasis in mice by increasing SOCS2 expression. The regulation of SOCS2 expression appears to be accelerated by binding of YTHDF2 to its mRNAs. Consistent with the notion, YTHDF2 depletion results in delayed degradation of the SOCS2 transcript (35).
5. Perspectives
Epitranscriptomics refers to regulation of gene expression by post-transcriptional covalent modification of the transcript (68,69). m6A is now known to be involved in one of the most important epitranscriptomic mechanisms, is readily detected transcriptome-wide and controls gene expression by modulating the biology of RNAs. m6A enrichment displays a unique pattern. As described above, studies have shown that regulation of m6A formation is linked to human cancers. Based on these findings, it is reasonable to speculate that there are unidentified cancer types caused by dysregulation of m6A modification. Furthermore, m6A and enzymes may be therapeutic targets for treatment of these identified cancers. Additionally, screening of chemicals that potentially regulate m6A formation or removal is an appropriate approach for therapeutic purposes.
Acknowledgements
The author would like to thank Ms. Hyunjin Yoo for editing the manuscript and Mr. Muhammad Arsalan Iqbal for helping with data analysis.
Glossary
Abbreviations
- m6A
N6-methyladenosine
- NGS
next-generation sequencing
- FTO
fat mass and obesity-associated protein
- AML
acute myeloid leukemia
- A
adenine
- TSS
transcription start site
- TES
transcription end site
- METTL3
methyltransferase-like protein 3
- WTAP
Wilms's tumor protein 1 (WT1)-associated protein
- ALKBH5
alkylation repair homolog 5
- YTHDF2
YT521-B homology (YTH) domain-containing family protein 2
- HIF-1α
hypoxia-inducible factor-1α
- CNV
copy number variation
- GSCs
glioblastoma stem-like cells
- HHC
human hepatocellular carcinoma
- PTEN
phosphatase and tensin homolog
- Myc
myelocytomatosis oncogene
- ASB2
ankyrin repeat and SOCS box containing 2
- RARA
retinoic acid receptor alpha
- FOXM1
forkhead box protein M1
- SOCS2
suppressor of cytokine signaling 2
Funding
The present study was supported by the National Research Foundation of Korea (grant no. NRF-2015M3A9C6028965).
Availability of data and materials
The datasets and codes used for computational analyses are available from corresponding author upon request.
Authors' contributions
KH designed and conceived the data analyses and wrote the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Machnicka MA, Milanowska K, Oglou Osman O, Purta E, Kurkowska M, Olchowik A, Januszewski W, Kalinowski S, Dunin-Horkawicz S, Rother KM, et al. MODOMICS: A database of RNA modification pathways-2013 update. Nucleic Acids Res. 2013;41:D262–D267. doi: 10.1093/nar/gks1007. (Database Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batista PJ, Molinie B, Wang J, Qu K, Zhang J, Li L, Bouley DM, Lujan E, Haddad B, Daneshvar K, et al. m(6)A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell. 2014;15:707–719. doi: 10.1016/j.stem.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geula S, Moshkovitz Moshitch S, Dominissini D, Mansour AA, Kol N, Divon Salmon M, Hershkovitz V, Peer E, Mor N, Manor YS, et al. Stem cells. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science. 2015;347:1002–1006. doi: 10.1126/science.1261417. [DOI] [PubMed] [Google Scholar]
- 4.O'Connell MA, Mannion NM, Keegan LP. The epitranscriptome and innate immunity. PLoS Genet. 2015;11:e1005687. doi: 10.1371/journal.pgen.1005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tajaddod M, Jantsch MF, Licht K. The dynamic epitranscriptome: A to I editing modulates genetic information. Chromosoma. 2016;125:51–63. doi: 10.1007/s00412-015-0526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger RNA from novikoff hepatoma cells. Proc Natl Acad Sci USA. 1974;71:3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perry RP, Kelley DE. Existence of methylated messenger RNA in mouse L cells. Cell. 1974;1:37–42. doi: 10.1016/0092-8674(74)90153-6. [DOI] [Google Scholar]
- 8.Adams JM, Cory S. Modified nucleosides and bizarre 5′-termini in mouse myeloma mRNA. Nature. 1975;255:28–33. doi: 10.1038/255028a0. [DOI] [PubMed] [Google Scholar]
- 9.Furuichi Y, Shatkin AJ, Stavnezer E, Bishop JM. Blocked, methylated 5′-terminal sequence in avian sarcoma virus RNA. Nature. 1975;257:618–620. doi: 10.1038/257618a0. [DOI] [PubMed] [Google Scholar]
- 10.Lavi S, Shatkin AJ. Methylated simian virus 40-specific RNA from nuclei and cytoplasm of infected BSC-1 cells. Proc Natl Acad Sci USA. 1975;72:2012–2016. doi: 10.1073/pnas.72.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei CM, Gershowitz A, Moss B. Methylated nucleotides block 5′ terminus of HeLa cell messenger RNA. Cell. 1975;4:379–386. doi: 10.1016/0092-8674(75)90158-0. [DOI] [PubMed] [Google Scholar]
- 12.Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG, He C. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scuteri A, Sanna S, Chen WM, Uda M, Albai G, Strait J, Najjar SS, Nagarajah R, Orrú M, Usala G, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity related traits. PLoS Genet. 2007;3:e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dina C, Meyre D, Gallina S, Durand E, Körner A, Jacobson P, Carlsson LM, Kiess W, Vatin V, Lecoeur C, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39:724–726. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- 15.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dominissini D, Moshkovitz Moshitch S, Schwartz S, Divon Salmon M, Ungar L, Osenberg S, Cesarkas K, Hirsch Jacob J, Amariglio N, Kupiec M, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 17.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen F, Huang W, Huang JT, Xiong J, Yang Y, Wu K, Jia GF, Chen J, Feng YQ, Yuan BF, Liu SM. Decreased N(6)-methyladenosine in peripheral blood RNA from diabetic patients is associated with FTO expression rather than ALKBH5. J Clin Endocrinol Metab. 2015;100:E148–E154. doi: 10.1210/jc.2014-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Huang W, Huang JT, Shen F, Xiong J, Yuan EF, Qin SS, Zhang M, Feng YQ, Yuan BF, Liu SM. Increased N6-methyladenosine in human sperm RNA as a risk factor for asthenozoospermia. Sci Rep. 2016;6:24345. doi: 10.1038/srep24345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fustin JM, Doi M, Yamaguchi Y, Hida H, Nishimura S, Yoshida M, Isagawa T, Morioka MS, Kakeya H, Manabe I, Okamura H. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell. 2013;155:793–806. doi: 10.1016/j.cell.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Daoud H, Zhang D, Mcmurray F, Yu A, Luco SM, Vanstone J, Jarinova O, Carson N, Wickens J, Shishodia S, et al. Identification of a pathogenic FTO mutation by next-generation sequencing in a newborn with growth retardation and developmental delay. J Med Genet. 2016;53:200–207. doi: 10.1136/jmedgenet-2015-103399. [DOI] [PubMed] [Google Scholar]
- 22.Davis W, van Rensburg SJ, Cronje FJ, Whati L, Fisher LR, van der Merwe L, Geiger D, Hassan MS, Matsha T, Erasmus RT, Kotze MJ. The fat mass and obesity-associated FTO rs9939609 polymorphism is associated with elevated homocysteine levels in patients with multiple sclerosis screened for vascular risk factors. Metab Brain Dis. 2014;29:409–419. doi: 10.1007/s11011-014-9486-7. [DOI] [PubMed] [Google Scholar]
- 23.Zhang C, Samanta D, Lu H, Bullen JW, Zhang H, Chen I, He X, Semenza GL. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m6A-demethylation of NANOG mRNA. Proc Natl Acad Sci USA. 2016;113:E2047–E2056. doi: 10.1073/pnas.1602883113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang C, Zhi WI, Lu H, Samanta D, Chen I, Gabrielson E, Semenza GL. Hypoxia-inducible factors regulate pluripotency factor expression by ZNF217- and ALKBH5-mediated modulation of RNA methylation in breast cancer cells. Oncotarget. 2016;7:64527–64542. doi: 10.18632/oncotarget.11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bansal H, Yihua Q, Iyer SP, Ganapathy S, Proia DA, Penalva LO, Uren PJ, Suresh U, Carew JS, Karnad AB, et al. WTAP is a novel oncogenic protein in acute myeloid leukemia. Leukemia. 2014;28:1171–1174. doi: 10.1038/leu.2014.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwok CT, Marshall AD, Rasko JE, Wong JJ. Genetic alterations of m6A regulators predict poorer survival in acute myeloid leukemia. J Hematol Oncol. 2017;10:39. doi: 10.1186/s13045-017-0419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang C, Chen Y, Sun B, Wang L, Yang Y, Ma D, Lv J, Heng J, Ding Y, Xue Y, et al. m6A modulates haematopoietic stem and progenitor cell specification. Nature. 2017;549:273–276. doi: 10.1038/nature23883. [DOI] [PubMed] [Google Scholar]
- 28.Barbieri I, Tzelepis K, Pandolfini L, Shi J, Millán-Zambrano G, Robson SC, Aspris D, Migliori V, Bannister AJ, Han N, et al. Promoter-bound METTL3 maintains myeloid leukaemia by m6A-dependent translation control. Nature. 2017;552:126–131. doi: 10.1038/nature24678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vu LP, Pickering BF, Cheng Y, Zaccara S, Nguyen D, Minuesa G, Chou T, Chow A, Saletore Y, MacKay M, et al. The N6-methyladenosine (m6A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med. 2017;23:1369–1376. doi: 10.1038/nm.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z, Weng H, Su R, Weng X, Zuo Z, Li C, Huang H, Nachtergaele S, Dong L, Hu C, et al. FTO plays an oncogenic role in acute myeloid leukemia as a N6-methyladenosine RNA demethylase. Cancer Cell. 2017;31:127–141. doi: 10.1016/j.ccell.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, Lu Z, Chen Y, Sulman EP, Xie K, Bögler O, et al. m6A demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell. 2017;31(591–606):e6. doi: 10.1186/s12935-016-0373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui Q, Shi H, Ye P, Li L, Qu Q, Sun G, Sun G, Lu Z, Huang Y, Yang CG, et al. m6A RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep. 2017;18:2622–2634. doi: 10.1016/j.celrep.2017.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin DI, Lee SW, Han ME, Kim HJ, Seo SA, Hur GY, Jung S, Kim BS, Oh SO. Expression and roles of Wilms' tumor 1-associating protein in glioblastoma. Cancer Sci. 2012;103:2102–2109. doi: 10.1111/cas.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin S, Choe J, Du P, Triboulet R, Gregory RI. The m(6)A methyltransferase METTL3 promotes translation in human cancer cells. Mol Cell. 2016;62:335–345. doi: 10.1016/j.molcel.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen M, Wei L, Law CT, Tsang FH, Shen J, Cheng CL, Tsang LH, Ho DW, Chiu DK, Lee JM, et al. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2 dependent posttranscriptional silencing of SOCS2. Hepatology. 2018;67:2254–2270. doi: 10.1002/hep.29683. [DOI] [PubMed] [Google Scholar]
- 36.Horowitz S, Horowitz A, Nilsen TW, Munns TW, Rottman FM. Mapping of N6-methyladenosine residues in bovine prolactin mRNA. Proc Natl Acad Sci USA. 1984;81:5667–5671. doi: 10.1073/pnas.81.18.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz S, Agarwala SD, Mumbach MR, Jovanovic M, Mertins P, Shishkin A, Tabach Y, Mikkelsen TS, Satija R, Ruvkun G, et al. High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell. 2013;155:1409–1421. doi: 10.1016/j.cell.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo GZ, MacQueen A, Zheng G, Duan H, Dore LC, Lu Z, Liu J, Chen K, Jia G, Bergelson J, He C. Unique features of the m6A methylome in Arabidopsis thaliana. Nat Commun. 2014;5:5630. doi: 10.1038/ncomms6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao X, Yang Y, Sun BF, Shi Y, Yang X, Xiao W, Hao YJ, Ping XL, Chen YS, Wang WJ, et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014;24:1403–1419. doi: 10.1038/cr.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, Adhikari S, Shi Y, Lv Y, Chen YS, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smemo S, Tena JJ, Kim KH, Gamazon ER, Sakabe NJ, Gómez-Marín C, Aneas I, Credidio FL, Sobreira DR, Wasserman NF, et al. Obesity-associated variants within FTO form long range functional connections with IRX3. Nature. 2014;507:371–375. doi: 10.1038/nature13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rottman F, Shatkin AJ, Perry RP. Sequences containing methylated nucleotides at the 5′ termini of messenger RNAs: Possible implications for processing. Cell. 1974;3:197–199. doi: 10.1016/0092-8674(74)90131-7. [DOI] [PubMed] [Google Scholar]
- 43.Mauer J, Luo X, Blanjoie A, Jiao X, Grozhik AV, Patil DP, Linder B, Pickering BF, Vasseur JJ, Chen Q, et al. Reversible methylation of m6Am in the 5′ cap controls mRNA stability. Nature. 2017;541:371–375. doi: 10.1038/nature21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Li Y, Toth JI, Petroski MD, Zhang Z, Zhao JC. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol. 2014;16:191–198. doi: 10.1038/ncb2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Y, Sun BF, Xiao W, Yang X, Sun HY, Zhao YL, Yang YG. Dynamic m6A modification and its emerging regulatory role in mRNA splicing. Sci Bull. 2015;60:21–32. doi: 10.1007/s11434-014-0695-6. [DOI] [Google Scholar]
- 47.Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, Pestova TV, Qian SB, Jaffrey SR. 5′ UTR m(6)A promotes cap-independent translation. Cell. 2015;163:999–1010. doi: 10.1016/j.cell.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ke S, Pandya-Jones A, Saito Y, Fak JJ, Vågbø CB, Geula S, Hanna JH, Black DL, Darnell JE, Jr, Darnell RB. m6A mRNA modifications are deposited in nascent pre-mRNA and are not required for splicing but do specify cytoplasmic turnover. Genes Dev. 2017;31:990–1006. doi: 10.1101/gad.301036.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ke S, Alemu EA, Mertens C, Gantman EC, Fak JJ, Mele A, Haripal B, Zucker-Scharff I, Moore MJ, Park CY, et al. A majority of m6A residues are in the last exons, allowing the potential for 3′ UTR regulation. Genes Dev. 2015;29:2037–2053. doi: 10.1101/gad.269415.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bokar JA, Shambaugh ME, Polayes D, Matera AG, Rottman FM. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA. 1997;3:1233–1247. [PMC free article] [PubMed] [Google Scholar]
- 51.Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin Z, Hsu PJ, Xing X, Fang J, Lu Z, Zou Q, Zhang KJ, Zhang X, Zhou Y, Zhang T, et al. Mettl3-/Mettl14-mediated mRNA N6-methyladenosine modulates murine spermatogenesis. Cell Res. 2017;27:1216–1230. doi: 10.1038/cr.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoon KJ, Ringeling FR, Vissers C, Jacob F, Pokrass M, Jimenez-Cyrus D, Su Y, Kim NS, Zhu Y, Zheng L, et al. Temporal control of mammalian cortical neurogenesis by m6A methylation. Cell. 2017;171(877–889):e17. doi: 10.1016/j.cell.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horiuchi K, Umetani M, Minami T, Okayama H, Takada S, Yamamoto M, Aburatani H, Reid PC, Housman DE, Hamakubo T, Kodama T. Wilms' tumor 1-associating protein regulates G2/M transition through stabilization of cyclin A2 mRNA. Proc Natl Acad Sci USA. 2006;103:17278–17283. doi: 10.1073/pnas.0608357103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, Hewitson KS, Yeo GS, McDonough MA, Cunliffe S, McNeill LA, et al. The obesity associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318:1469–1472. doi: 10.1126/science.1151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fischer J, Koch L, Emmerling C, Vierkotten J, Peters T, Brüning JC, Rüther U. Inactivation of the Fto gene protects from obesity. Nature. 2009;458:894–898. doi: 10.1038/nature07848. [DOI] [PubMed] [Google Scholar]
- 57.Li L, Zang L, Zhang F, Chen J, Shen H, Shu L, Liang F, Feng C, Chen D, Tao H, et al. Fat mass and obesity-associated (FTO) protein regulates adult neurogenesis. Hum Mol Genet. 2017;26:2398–2411. doi: 10.1093/hmg/ddx128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vågbø CB, Shi Y, Wang WL, Song SH, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feng C, Liu Y, Wang G, Deng Z, Zhang Q, Wu W, Tong Y, Cheng C, Chen Z. Crystal structures of the human RNA demethylase Alkbh5 reveal basis for substrate recognition. J Biol Chem. 2014;289:11571–11583. doi: 10.1074/jbc.M113.546168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Z, Theler D, Kaminska KH, Hiller M, de la Grange P, Pudimat R, Rafalska I, Heinrich B, Bujnicki JM, Allain FH, Stamm S. The YTH domain is novel RNA binding domain. J Biol Chem. 2010;285:14701–14710. doi: 10.1074/jbc.M110.104711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li A, Chen YS, Ping XL, Yang X, Xiao W, Yang Y, Sun HY, Zhu Q, Baidya P, Wang X, et al. Cytoplasmic m6A reader YTHDF3 promotes mRNA translation. Cell Res. 2017;27:444–447. doi: 10.1038/cr.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ivanova I, Much C, Di Giacomo M, Azzi C, Morgan M, Moreira PN, Monahan J, Carrieri C, Enright AJ, O'Carroll D. The RNA m6A reader YTHDF2 is essential for the post-transcriptional regulation of the maternal transcriptome and oocyte competence. Mol Cell. 2017;67(1059–1067):e4. doi: 10.1016/j.molcel.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao BS, Wang X, Beadell AV, Lu Z, Shi H, Kuuspalu A, Ho RK, He C. m6A-dependent maternal mRNA clearance facilitates zebrafish maternal-to-zygotic transition. Nature. 2017;542:475–478. doi: 10.1038/nature21355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: A comprehensive review and 2016 update. Blood Cancer J. 2016;6:e441. doi: 10.1038/bcj.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Touat M, Idbaih A, Sanson M, Ligon KL. Glioblastoma targeted therapy: Updated approaches from recent biological insights. Ann Oncol. 2017;28:1457–1472. doi: 10.1093/annonc/mdx106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lieberman F. Glioblastoma update: Molecular biology, diagnosis, treatment, response assessment, and translational clinical trials. F1000Res. 2017;6:1892. doi: 10.12688/f1000research.11493.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saletore Y, Meyer K, Korlach J, Vilfan ID, Jaffrey S, Mason CE. The birth of the Epitranscriptome: Deciphering the function of RNA modifications. Genome Biol. 2012;13:175. doi: 10.1186/gb-2012-13-10-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hussain S, Aleksic J, Blanco S, Dietmann S, Frye M. Characterizing 5-methylcytosine in the mammalian epitranscriptome. Genome Biol. 2013;14:215. doi: 10.1186/gb4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets and codes used for computational analyses are available from corresponding author upon request.