Abstract
Regorafenib and trifluridine/tipiracil (TAS-102) are novel antitumor agents for patients with refractory metastatic colorectal cancer. However, it is unclear which patients may derive a survival benefit from these drugs in real-life clinical practice. We evaluated retrospectively the efficacy and safety of regorafenib and TAS-102 at a single institution between June 2013 and November 2015. Cox regression analysis was carried out to obtain predictive scores (the nearest integers of hazard ratio) for survival benefit. Forty-four patients treated with regorafenib or TAS-102 were included in the analysis; among them, 17 received crossover treatment. The median overall survival (OS) was 9.1 months for regorafenib and 9.3 months for TAS-102, and the corresponding values after crossover were 7.1 and 5.3 months, respectively. OS was not correlated to relative dose intensity, but was proportional to the total administered dose of each drug. Adverse events were tolerable even after crossover. We identified three variables as significant for prediction of OS with good discrimination (C-statistic=0.70): Poor Eastern Cooperative Oncology Group performance status, time since diagnosis of metastatic disease ≤18 months, and previous chemotherapy continued ≥2 months beyond progression were all predictors of poor OS. Regorafenib and TAS-102 can be recommended for patients with better performance status and slow progression of metastatic disease. Optimal survival benefit was provided by prompt administration of either drug after failure of previous chemotherapy, with flexible titration to the optimal dose for each individual patient.
Keywords: salvage-line chemotherapy, prognostic score, regorafenib, trifluridine/tipiracil, colorectal cancer
Introduction
Colorectal cancer is the fourth most common cancer diagnosed in the United States, accounting for 8% of all new cancer cases (1). It is estimated that there were 132,700 new cases of colorectal cancer, and an estimated 49,700 people died of this disease in the US in 2015. Among 4,877 patients who received first-line chemotherapy for metastatic colorectal cancer (mCRC), identified in a nationwide and commercially available chemotherapy order entry system from 2004 to 2011, 53% (n=2,575) received second-line treatment, 28% (n=1,373) received third-line treatment, and only 13% (n=640) received fourth-line treatment (2). Patients with mCRC infrequently go on to receive third-line or later treatment, and this might negatively impact on their overall survival (OS).
Regorafenib (Stivarga®, Bayer AG, Leverkusen, Germany) is an oral multikinase inhibitor that blocks the activity of several protein kinases associated with angiogenesis [vascular endothelial growth factor (VEGF) receptors 1–3 and TIE2], oncogenesis (KIT, RET, RAF1 and BRAF), and the tumor microenvironment (PDGF receptor and FGF receptor) (3).
Trifluridine/tipiracil (TAS-102; Lonsurf®; Taiho Pharmaceutical Co. Ltd, Tokyo) is an orally administered combination of a thymidine-based nucleic acid analogue, trifluridine, and a thymidine phosphorylase inhibitor, tipiracil hydrochloride. Trifluridine is the active cytotoxic component of TAS-102; its triphosphate form is incorporated into DNA, and this appears to result in its antitumor effects (4). Tipiracil hydrochloride is a potent inhibitor of thymidine phosphorylase, and serves to prevent the rapid degradation of trifluridine in TAS-102, providing more prolonged maintenance of adequate plasma levels of the active drug.
Regorafenib and TAS-102 are new salvage-line treatment options (5,6), which provided statistically significant improvements of OS, progression-free survival (PFS), and disease control in placebo-controlled randomized phase III trials (CORRECT (7), CONCUR (8), RECOURSE (9) and TERRA) (10). Despite this evidence, these two drugs are often considered not clinically meaningful for patients based on the relatively small incremental benefits for OS and PFS.
A multicenter observational study (REGOTAS) (11) has recently demonstrated the clinical benefit and tolerability of these drugs in real-life clinical practice, and criteria to choose between regorafenib or TAS-102. However, the following issues remain to be established (12): The appropriate way of administration for patients with advanced disease, and which subpopulations of patients might derive the greatest benefit from salvage-line treatment with these drugs, compared to best supportive care only. To address these questions, we conducted a retrospective cohort study to evaluate the efficacy and safety of regorafenib and TAS-102 in patients with refractory mCRC with the aim of assessing their practical value as salvage-line therapy. A post-hoc exploratory subgroup analysis was carried out to obtain predictive scores for survival benefit in patients treated with these regimens.
Patients and methods
Patients
Patients with unresectable mCRC were eligible for the study if they had received at least two prior regimens of standard chemotherapies. All patients had been treated at Tokai University Hospital (Kanagawa, Japan) between June 2013 and November 2015, after the approval of each drug for medical reimbursement under the national insurance scheme in Japan (regorafenib and TAS-102 were approved in May 2013 and May 2014, respectively). The eligibility criteria were as follows: i) histologically confirmed adenocarcinoma of the colon or rectum, and presence of unresectable metastatic disease; ii) history of treatments with fluoropyrimidine, irinotecan, oxaliplatin, and anti-VEGF antibody (bevacizumab), or anti-epidermal growth factor receptor (EGFR) antibody (cetuximab or panitumumab) for patients who had KRAS exon 2 wild-type tumor; iii) Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 2; and iv) adequate bone-marrow, liver, and renal function at the start of the treatment. Patients were excluded if they had previously received regorafenib or TAS-102, or had uncontrolled medical disorders.
The Institutional Review Board for Clinical Research approved all procedures for this retrospective observational study (no. 16R-190), which was conducted in accordance with the Declaration of Helsinki.
Treatment
Regorafenib (160 mg as a standard dose) was administered once daily on days 1–21, with 7 days of rest. TAS-102 (35 mg/m2) was administered twice daily 5 days a week, with 2 days of rest, for 2 weeks, followed by a 14-day rest period. Both regimens were repeated every 4 weeks. The treatments were continued until disease progression, death, unacceptable toxicity, withdrawal of consent by the patient, or decision by the treating physician that discontinuation would be in the patient's best interest.
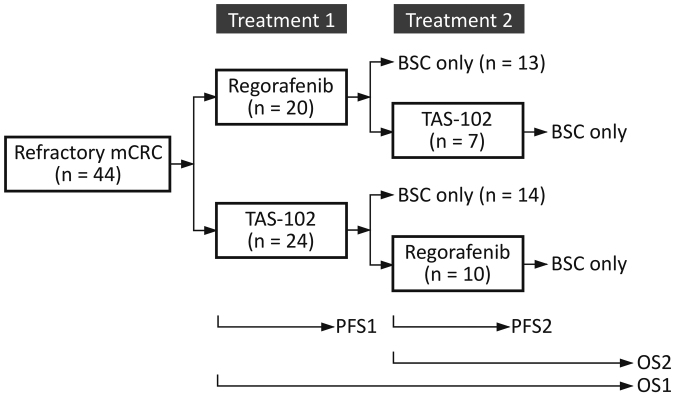
Patients whose initial dose had been reduced at the discretion of the treating physician were included in this study. Patients who required dose reductions could re-escalate the dose up to the recommended starting dose if the toxicity resolved to baseline level. All patients received the best supportive care available, but were not allowed to receive other antitumor agents, hormonal therapy, or immunotherapy. The decisions regarding which drug should be administered first, and whether to provide crossover between treatments, were made by the treating physicians (Fig. 1).
Figure 1.
Flow diagram of salvage-line therapy. Each agent was administered at the discretion of the attending physician. mCRC, metastatic colorectal cancer; TAS-102, trifluridine and tipiracil; BSC, best supportive care; OS, overall survival; PFS, progression-free survival.
Evaluation
All patients underwent computed tomography every 8 weeks to assess tumor responses to therapy in terms of change from baseline during treatment according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (13). We defined PFS1 as the interval from the first administration of the primary treatment to the first radiologic or clinical observation of disease progression or death from any cause, whichever came first (Fig. 1).
We defined PFS2 as the interval from the initiation of the secondary treatment to the second progression, for those who had undertaken crossover between treatments after a first progression. We defined OS1 as the time between the administration date of the primary treatment and the date of death from any cause, and OS2 as the time between the administration date of the secondary treatment, if applicable, and the date of death. The median PFS1, PFS2, OS1 and OS2 were estimated using the Kaplan-Meier method.
The planned dose intensity (DI) for each drug was defined as the total amount of drug in the entire treatment intended based on the recommended dose and schedule. Then, the relative dose intensity (RDI) for each drug was calculated as the ratio between the delivered DI and the planned DI (14). Adverse events were classified and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03 (15).
Statistical methods and prognostic score construction
Parametric data with P>0.05 for the Kolmogorov-Smirnov test were analyzed using Welch's two sample t-test, and non-parametric data using the Wilcoxon test. Categorical data were analyzed using Fisher's exact test. The PFS1, 2 and OS1, 2 were compared using a log-rank test with 95% confidence intervals (95% CIs).
The results of OS1 were plotted against the total delivered dose or the RDI for each drug and fitted to a simple linear regression model to calculate the regression coefficient (16). A Cox proportional hazards regression model was used to test each candidate variable predictor associated with OS1 using stepwise model selection according to Akaike's information criterion. To take account of the small number of patients with PS 2, the values of ECOG PS were incorporated into the model as a numerical variable. Hazard ratios were calculated by taking the exponentials of the ß coefficients of Cox models. Model discrimination was done by calculating the Harrell's C (for concordance) index, which is the area under the receiver operator curve (17,18). Hazard ratios of covariates were rounded to the nearest integer to construct score weights. The range of possible total score weights was divided into three groups to stratify patients into poor-, intermediate- and long-survival tertiles. P<0.05 was considered to indicate a statistically significant difference. All analyses were performed using R version 3.3.2 (The R Foundation for Statistical Computing Platform) (19).
Results
Patients
Patient demographics and characteristics are outlined in Table I. Between June 2013 and November 2015, 44 patients with mCRC who were treated with either regorafenib or TAS-102 for the first time were included in the analysis. Of these patients, 7 went on to receive TAS-102 and 10 went on to receive regorafenib as secondary treatment (Fig. 1). Baseline demographic and disease characteristics were well balanced between the two groups in terms of the primary treatment. All the patients had received prior chemotherapy regimens containing a fluoropyrimidine, oxaliplatin and irinotecan; all but one patient (in the group with primary use of TAS-102) had received bevacizumab.
Table I.
Demographics.
| Primary treatment | |||
|---|---|---|---|
| Characteristic | Regorafenib (n=20) | TAS-102 (n=24) | P-value |
| Age, median [range] | 68 [57–78] | 64 [44–86] | 0.087 |
| Sex | |||
| Male | 13 (65.0) | 15 (62.5) | 1.0 |
| Female | 7 (35.0) | 9 (37.5) | |
| ECOG PS | |||
| 0 | 6 (30.0) | 14 (58.3) | 0.077 |
| 1 | 12 (60.0) | 6 (25.0) | |
| 2 | 2 (10.0) | 4 (16.7) | |
| Primary site of disease | |||
| Right colon | 4 (20.0) | 10 (41.7) | 0.14 |
| Left colon | 7 (35.0) | 3 (12.5) | |
| Rectum | 9 (37.5) | 11 (45.8) | |
| KRAS exon 2 status | |||
| Wild | 9 (45.0) | 14 (58.3) | 0.55 |
| Mutation | 11 (55.0) | 10 (41.7) | |
| Number of prior regimens | |||
| 2 | 12 (60.0) | 12 (50.0) | 0.87 |
| 3 | 8 (40.0) | 11 (45.8) | |
| ≥4 | 0 (0) | 1 (4.2) | |
| Number of metastatic sites | |||
| 1 | 6 (30.0) | 6 (25.0) | 0.46 |
| 2 | 12 (60.0) | 11 (45.8) | |
| ≥3 | 2 (10.0) | 7 (29.2) | |
| Metastatic site | |||
| Liver | 16 (80.0) | 19 (79.2) | 0.26 |
| Lung | 10 (50.0) | 13 (54.2) | |
| Peritoneum | 6 (30.0) | 4 (16.7) | |
| Lymph node | 2 (10.0) | 8 (33.3) | |
| Others | 2 (10.0) | 8 (33.3) | |
| Time from initiation of first-line chemotherapy | |||
| ≤18 months | 5 (25.0) | 6 (25.0) | 1.0 |
| >18 months | 15 (75.0) | 18 (75.0) | |
| History of systemic anticancer agents | |||
| Fluoropyrimidine | 20 (100) | 24 (100) | 0.99 |
| Oxaliplatin | 20 (100) | 24 (100) | |
| Irinotecan | 20 (100) | 24 (100) | |
| Anti-VEGF antibody | 20 (100) | 23 (95.8) | |
| Anti-EGFR antibody (Wild KRAS or all-RASa) | 9 (45.0) | 11 (45.8) | |
| Post-treatment use of regorafenib or TAS-102 | 7 (35.0) | 10 (41.7) | 0.76 |
Non-parametric data with P<0.05 for Kolmogorov-Smirnov test are presented as the median [range] and were examined using the Wilcoxon rank sum test. Categorical data are accompanied by percentage in parentheses and were examined using Fisher's exact test.
An all-RAS test was approved in Japan in April, 2015. Anti-EGFR antibody was subsequently applied based on all-RAS test. ECOG PS, Eastern Cooperative Oncology Group performance status; TAS-102, trifluridine and tipiracil; KRAS, Kirsten rat sarcoma viral oncogene homolog VEGF, vascular endothelial growth factor; EGFR, epidermal growth factor receptor.
Treatment exposure
Crossover between treatments was conducted for patients with ECOG PS 0 or 1 at the time when the first treatment was finished (Table II). The durations of treatment were not significantly different for regorafenib and TAS-102: median 2.6 months (range: 0.1–10.8) for regorafenib and 3.8 months (0.9–20.3) for TAS-102 in Treatment 1, and then 4.2 months (0.4–12.9) and 3.7 months (0.9–15.1), respectively, in Treatment 2. The starting dose rate of regorafenib was reduced to 0.78±0.26 mean ± standard deviation (SD) in Treatment 1, and to 0.71±0.10 in Treatment 2. Although the incidences of any dose modification were equivalent, the RDI over the whole treatment period was greater for TAS-102: 0.83±0.14 for TAS-102 vs. 0.54±0.21 for regorafenib in Treatment 1 (P<0.001), and 0.90±0.11 vs. 0.63±0.16 in Treatment 2 (P<0.001).
Table II.
Administration of study drugs, response and survival.
| A, Treatment 1 (primary use). | |||
|---|---|---|---|
| Variable | Regorafenib n=20 | TAS-102 n=24 | P-value |
| ECOG PS, n (%) | |||
| 0 | 6 (30.0) | 14 (58.3) | 0.077 |
| 1 | 12 (60.0) | 6 (25.0) | |
| 2 | 2 (10.0) | 4 (16.7) | |
| Median period of medication, months | 2.6 [range: 0.1–10.8] | 3.8 [0.9–20.3] | 0.18 |
| Relative initial dose, mean ± SD | 0.78±0.26 | 0.97±0.09 | 0.0031 |
| Any treatment modification, n (%) | 19 (95.0) | 18 (75.0) | 0.11 |
| Mean RDI ± SD | 0.54 ± 0.21 | 0.83±0.14 | <0.001 |
| Median OS1, months | 9.1 (95% CI: 4.1–13.4) | 9.3 (5.5–12.3) | 0.68 |
| Patients alive at 12 months, n (%) | 4 (20.0) | 6 (25.0) | 0.73 |
| Median PFS1, months | 2.1 (95% CI: 1.3–3.6) | 3.1 (1.7–4.1) | 0.13 |
| Best overall responsea, n (%) | |||
| CR | 0 (0) | 0 (0) | 1.0 |
| PR | 0 (0) | 0 (0) | |
| SD | 15 (75.0) | 17 (70.8) | |
| PD | 5 (25.0) | 7 (29.2) | |
| B, Treatment 2 (secondary use). | |||
| Variable | Regorafenib n=10 | TAS-102 n=7 | P-value |
| ECOG PS, n (%) | |||
| 0 | 1 (10.0) | 3 (42.9) | 0.12 |
| 1 | 9 (90.0) | 4 (57.1) | |
| 2 | 0 (0) | 0 (0) | |
| Median period of medication, months | 4.2 [range: 0.4–12.9] | 3.7 [0.9–15.1] | 0.80 |
| Relative initial dose, mean ± SD | 0.71±0.10 | 0.94±0.15 | 0.0058 |
| Any treatment modification, n (%) | 10 (100) | 4 (57.1) | 0.051 |
| Mean RDI ± SD | 0.63±0.16 | 0.90±0.11 | <0.001 |
| Median OS2, months | 7.1 (95% CI: 5.0-NA) | 5.3 (3.0-NA) | 0.67 |
| Patients alive at 12 months, n (%) | 0 (0) | 0 (0) | 1.0 |
| Median PFS2, months | 3.7 (95% CI: 3.1-NA) | 3.7 (0.8-NA) | 0.23 |
| Best overall responsea, n (%) | |||
| CR | 0 (0) | 0 (0) | 1.0 |
| PR | 0 (0) | 0 (0) | |
| SD | 6 (60.0) | 4 (57.1) | |
| PD | 4 (40.0) | 3 (42.9) | |
Response evaluation criteria in solid tumors (RECIST) version 1.1; ECOG PS, Eastern Cooperative Oncology Group performance status; ± SD, standard deviation; RDI, relative dose intensity; OS, overall survival; PFS, progression-free survival; CI, confidence interval; NA, not available; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Efficacy
No patient had a complete response (CR) or partial response (PR), as shown in Table II. Disease control was achieved in 15 out of 20 patients (75.0%) for regorafenib and 17 out of 24 patients (70.8%) for TAS-102 in Treatment 1, and in 6 out of 10 patients (60.0%) and 4 out of 7 patients (57.1%) in Treatment 2, respectively. There was no difference in the best overall response for either treatment line.
Median OS1 was 9.1 months and 20% of patients were alive 12 months after starting regorafenib first; the corresponding values were 9.3 months and 25% for patients treated with TAS-102 first (Table II). As for secondary use, median OS2 values were 7.1 months and 5.3 months for regorafenib and TAS-102, respectively, and no patient was alive in either case at 12 months after crossover. There was no difference in outcomes between regorafenib and TAS-102, regardless of the order in which the two drugs were used.
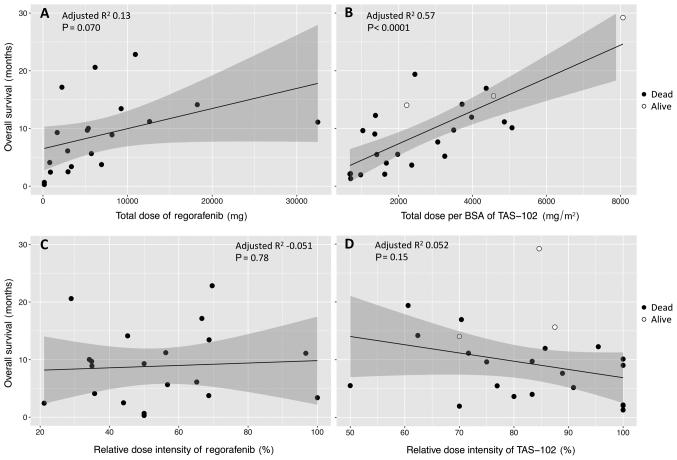
There was clearly a relationship between OS1 and a total of delivered dose for each drug (Fig. 2A and B), even though OS1 was not correlated to RDI (Fig. 2C and D). The correlation between OS1 and the total of delivered dose was higher for TAS-102, for which the data showed much less scatter, as shown in Fig. 2B.
Figure 2.
Effect of delivered dose on survival time, the time between the administration date of the primary treatment and the date of death from any cause (OS1). OS1 is plotted against total dose of (A) regorafenib or (B) TAS-102, and RDI of (C) regorafenib or (D) TAS-102. Among the patients with primary TAS-102, three (indicated by open circles) were alive at the time of data collection. The regression line is drawn with the 95% confidence intervals (gray shadows). BSA, body surface area; RDI, relative dose intensity; TAS-102, trifluridine/tipiracil.
Safety
Table III summarizes drug-related adverse events (AEs). Drug-related AEs occurred in 20 (100%) patients for regorafenib and in 22 (92%) patients for TAS-102 in Treatment 1, and then in 8 (80%) and 7 (100%), respectively, after crossover between the drugs in Treatment 2. The frequencies of grade 3 or 4 hand-foot skin reaction (HFSR), increased aspartate transaminase, increased alanine transaminase, and increased bilirubin for the secondary use of regorafenib after TAS-102 were not greater than the frequencies for the primary use of regorafenib (10% vs. 45%, 0% vs. 5%, 0% vs. 5%, 0% vs. 10%, respectively). Similarly, the frequencies of grade 3 or 4 leukopenia, neutropenia, anemia, and nausea for the secondary use of TAS-102 posterior to regorafenib were similar to those for the primary use of TAS-102 (57% vs. 29%, 29% vs. 34%, 57% vs. 38%, 0% vs. 8%, respectively). One patient during the primary administration of regorafenib suffered from severe treatment-related liver dysfunction, and discontinued the treatment after recovery.
Table III.
Adverse events.
| Regorafenib (n=30) | TAS-102 (n=31) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| REG-only or REG prior to TAS (n=20) | REG posterior to TAS (n=10) | TAS-only or TAS prior to REG (n=24) | TAS posterior to REG (n=7) | |||||||
| n (%) | Any grade | ≥Grade 3 | Any grade | ≥Grade 3 | P-valuea | Any grade | ≥Grade 3 | grade | ≥Grade 3 | P-valuea |
| Any event | 20 (100) | 13 (65) | 8 (80) | 5 (50) | 0.69 | 22 (92) | 15 (63) | 7 (100) | 6 (86) | 0.20 |
| Clinical AEs | ||||||||||
| HFSR | 14 (70) | 9 (45) | 3 (30) | 1 (10) | 0.077 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA |
| Nausea | 5 (25) | 0 (0) | 0 (0) | 0 (0) | 0.038 | 10 (42) | 2 (8) | 4 (57) | 0 (0) | 0.96 |
| Anorexia | 9 (45) | 1 (5) | 1 (10) | 0 (0) | 0.33 | 8 (36) | 2 (9) | 4 (57) | 0 (0) | 1.0 |
| Diarrhea | 2 (10) | 0 (0) | 1 (10) | 0 (0) | 0.090 | 3 (13) | 0 (0) | 1 (14) | 0 (0) | 0.021 |
| Fatigue | 11 (55) | 2 (10) | 4 (40) | 0 (0) | 0.31 | 12 (50) | 1 (4) | 3 (43) | 0 (0) | 0.87 |
| Mucositis oral | 4 (20) | 0 (0) | 0 (0) | 0 (0) | 0.30 | 6 (25) | 1 (4) | 1 (14) | 0 (0) | 0.37 |
| Hypertension | 9 (45) | 2 (10) | 2 (20) | 2 (20) | 0.33 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA |
| Voice alteration | 6 (30) | 0 (0) | 0 (0) | 0 (0) | 0.098 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA |
| Alopecia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA | 1 (4) | 0 (0) | 2 (29) | 0 (0) | 0.13 |
| Others | 0 (0) | 0 (0) | 3 (29) | 3b (30) | 0.038 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NA |
| Laboratory abnormalities | ||||||||||
| Leukopenia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.0 | 12 (50) | 7 (29) | 4 (57) | 4 (57) | 0.70 |
| Neutropenia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.0 | 13 (54) | 9 (34) | 3 (43) | 2 (29) | 0.92 |
| Anemia | 0 (0) | 0 (0) | 2 (20) | 0 (0) | 0.15 | 15 (63) | 9 (38) | 5 (71) | 4 (57) | 0.89 |
| Thrombocytopenia | 6 (30) | 2 (10) | 2 (20) | 1 (10) | 0.96 | 7 (29) | 2 (8) | 2 (29) | 0 (0) | 0.96 |
| AST increased | 15 (75) | 1 (5) | 4 (40) | 0 (0) | 0.26 | 6 (25) | 1 (4) | 4 (57) | 2 (29) | 0.44 |
| ALT increased | 6 (30) | 1 (5) | 2 (29) | 0 (0) | 0.40 | 4 (17) | 0 (0) | 5 (71) | 1 (14) | 0.020 |
| Hyperbilirubinemia | 7 (35) | 2 (10) | 1 (10) | 0 (0) | 0.61 | 1 (4) | 0 (0) | 3 (43) | 1 (14) | 0.030 |
| Discontinuation due to AEs | 5 (25) | 2 (20) | 0.75 | 3 (13) | 0 (0) | 0.78 | ||||
Fisher's exact test
Polymorphic exudative erythema and perforation. REG, regorafenib; TAS, TAS-102 (trifluridine/tipiracil); HFSR, hand-foot skin reaction; AE, adverse event; NA, not applicable; AST, aspartate aminotransferase; ALT, alanine aminotransferase. Categorical data are accompanied by percentages in parentheses.
Prognostic score
Table IV summarizes the findings of univariate and multivariate analyses of baseline characteristics as prognostic factors for OS and the score weights assigned to each retained predictor variable. The total possible score was 12 points; however, no patient had a score >10. Patients who had a worse ECOG PS, time since diagnosis of metastatic disease ≤18 months (rapid growth of tumor), and prior chemotherapy continued ≥2 months beyond progressive disease (PD) on the RECIST criteria (including so-called clinical PD) showed a P-value <0.05 in the univariate analysis examining the association between baseline characteristics and poor OS.
Table IV.
Predictors for OS1 in patients treated with regorafenib and/or TAS-102.
| Univariate model | Multivariate model | ||||
|---|---|---|---|---|---|
| Characteristic | HR (95% CI) | P-value | HR (95% CI) | P-value | Score weight |
| Male sex | 1.85 (0.912–3.75) | 0.089 | |||
| Age | 0.992 (0.960–1.03) | 0.62 | |||
| ECOG PS | 1.78 (1.02–3.10) | 0.044 | 2.00 (1.13–3.53) | 0.018 | 2 |
| Primary lesion | |||||
| Right colon | 1.53 (0.631–3.73) | 0.35 | |||
| Rectum | 1.49 (0.642–3.47) | 0.35 | |||
| KRAS exon 2 status | |||||
| Mutant | 0.985 (0.526–1.84) | 0.96 | |||
| Metastatic sites | |||||
| n≥3 | 1.21 (0.550–2.65) | 0.64 | |||
| Metastatic sites | |||||
| Liver | 1.18 (0.538–2.58) | 0.68 | |||
| Lung | 1.28 (0.688–2.40) | 0.43 | |||
| Peritoneum | 1.01 (0.477–2.12) | 0.99 | |||
| Lymph node | 0.596 (0.271–1.31) | 0.20 | |||
| Other | 1.17 (0.570–2.41) | 0.67 | |||
| Number of prior regimens | 0.876 (0.528–1.46) | 0.61 | |||
| History of biologicals | |||||
| Anti-VEGF antibody | 1.13 (0.153–8.30) | 0.91 | |||
| Anti-EGFR antibody | 0.827 (0.438–1.56) | 0.56 | |||
| Time since diagnosis of metastatic disease | |||||
| ≤18 months | 2.17 (1.04–4.55) | 0.039 | 2.51 (1.17–5.37) | 0.018 | 3 |
| Prior chemotherapy | |||||
| Continued ≥2 months beyond PD | 3.62 (1.72–7.63) | <0.001 | 4.95 (2.20–11.1) | <0.001 | 5 |
| Harrell's C-index | 0.70 | ||||
| Total possible score | 12 | ||||
Referent primary lesion is the left colon, referent number of metastatic sites is 1 to 2, and referent prior chemotherapy is discontinued at the time of diagnosis of PD, or for other reasons (unacceptable adverse events, or increase in serum level of carcinoembryonic antigen). OS1, the time between the administration date of the primary treatment and the date of death from any cause; HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; VEGF, vascular endothelial growth factor; EGFR, epidermal growth factor receptor; PD, progressive disease.
Prior chemotherapy was repeated every 2 to 3 weeks according to a regimen with an evaluation interval of <3 months, so regorafenib or TAS-102 could be started within 6 weeks after failure of the prior chemotherapy. Continuation of prior chemotherapy ≥2 months beyond PD would represent prolonged administration that clinicians intended to conduct for some reason.
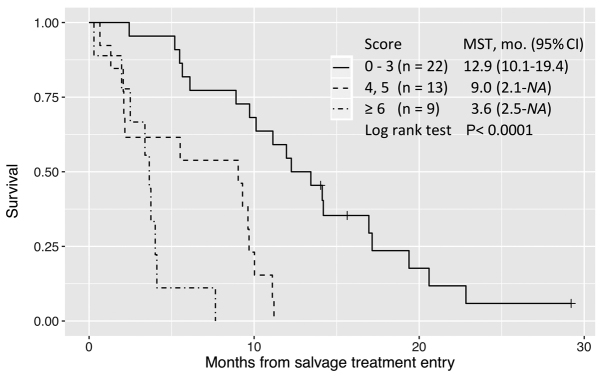
In multivariate analysis, these three factors remained significant for the parsimonious model to predict OS while retaining good discrimination (C-statistic=0.70). A score of 0–3 defined long survival; 4–5, intermediate survival; and ≥6, poor survival (Fig. 3).
Figure 3.
Overall survival since study entry (OS1) stratified by the prognostic score. Patients with a score of ≤3 exhibited relatively prolonged lifetimes. MST, mean survival time; CI, confidence interval.
Discussion
Regorafenib and TAS-102 have been reported to show similar efficacy but different toxicity profiles for regorafenib- and TAS-102-naive patients in retrospective cohort studies (11,20,21). Analyses of efficacy and safety in patients treated with regorafenib or TAS-102 in the real-life setting are important for clinicians, because patient characteristics in real-life, especially ECOG PS, may differ from those in phase III trials (7–9).
No patient had a CR or PR for either drug in our cohort. The two drugs were equivalent in terms of DCR: 75.0% for regorafenib and 70.8% for TAS-102 in primary salvage treatment, and 60.0 and 57.1%, respectively, in secondary use. Crossover administration was achieved in 7 out of 20 (35.0%) patients treated with regorafenib first, and in 10 out of 24 (41.7%) patients treated with TAS-102 first, but this does not imply inferiority: We found that TAS-102 had provided a prolonged period of medication for patients with poor performance status (ECOG PS=2), as shown in Table II. Median OS1 of 4 patients with ECOG PS=2 at the time of study entry was 3 months (range: 1.3 to 5.5 months) for TAS-102, providing better survival compared to two weeks for 2 patients treated with regorafenib (P=0.020).
A cohort study of regorafenib in real-life clinical practice for mCRC patients in France (REBECCA) (22) found that 50% of patients had a treatment modification (dose reduction or interruption), and 31% of patients discontinued regorafenib before progression mainly due to toxicity or deterioration of general health status. According to their data, survival was unfavorably affected by a low initial daily dose of regorafenib.
Median OS1 in our study was consistent with those in CORRECT (7) (median OS, 6.4 months; 12-month survival, 24%) and REBECCA (22) (5.6 months; 22%) for regorafenib, and RECOURSE (9) (7.1 months; 27%) for TAS-102, although 95 or 75% of our patients treated with regorafenib or TAS-102 first, respectively, had a dose modification (Table II). An initial dose was more likely to be reduced for regorafenib compared to TAS-102 to avoid early AE within the first 3 weeks of regorafenib treatment, but there was no correlation between deterioration of OS1 and reduction of initial daily dose (data not shown). This may be because we commonly escalated the dosage thereafter, if possible, up to 120 mg (4 out of 11 patients) or 160 mg (1 out of 10 patients), based on each patient's response. The most common dosage was 120 mg daily (19 out of 30 patients) in the first 2 cycles, as recently recommended in the ReDOS study (23). It seemed important for successful escalation of regorafenib to inform patients before administration about the likelihood of weekly dose escalation. Furthermore, OS1 for regorafenib or TAS-102, regardless of single use or crossover, was not correlated to RDI, but was proportional to a total dose of each drug (Fig. 2). In third-line or later treatments, clinicians may continue to prescribe the maximum recommended dose to obtain the best outcome, but may withdraw treatment from patients whose performance status deteriorates. In the former scenario, patients could experience adverse effects without any benefit, whereas possible responders could be missed in the latter scenario. Our results indicate that lower dose-intensity provides a longer duration of life under treatment compared to higher dose-intensity in some cases. This can be interpreted as indicating that there was a greater improvement in survival when these drugs were administered at the appropriate dose for each individual patient and continued for as long as possible until progression. Regarding regorafenib, Osawa (24) recommended an initial dose of 120 mg for salvage treatment of mCRC, as this provided a significant effect with good tolerability.
It has been considered that the toxic effects of TAS-102 are generally mild and manageable compared with those of regorafenib (21). The reported incidence of clinical AEs for regorafenib, including grade ≥3 HFSR (17% of patients in CORRECT) (7), fatigue (10%), and hepatotoxicity (6% of Asian population in CONCUR) (8), makes it difficult to administer regorafenib to patients who have previously been treated with TAS-102. In this study, the safety profiles of regorafenib and TAS-102 were broadly consistent with those in previous pivotal trials (7–9,25) (Table III). In addition, the incidences of HFSR, fatigue and hepatotoxicity in patients given regorafenib were not significantly increased even if the drug was used after TAS-102, while conversely, the frequencies of myelosuppression including leukopenia and neutropenia, nausea and anorexia in patients given TAS-102 were not greater in patients with previous regorafenib treatment. Although treatment discontinuation due to toxic effects was more frequently observed for regorafenib treatment, the incidence of toxic effects was not increased in patients with previous TAS-102 treatment, provided that the initial dose of regorafenib was reduced to 120 mg in most cases (Table II). These results indicate that regorafenib can be safely administered to patients with previous TAS-102 treatment.
Predictive biomarkers for OS have not yet been identified for mCRC patients treated with regorafenib or TAS-102 (11,26). No association was identified between KRAS, BRAF and PIK3CA mutation status and outcomes in CORRECT (7) and RECOURSE (9). The post hoc analysis of CORRECT indicated that patients treated with regorafenib who had long PFS (>4 months) tended to have a better ECOG PS (score, 0), fewer metastatic tumor sites (1 to 2 sites), and a longer time (≥18 months) since diagnosis of metastatic disease (25). In contrast, REBECCA (22) indicated that the following 6 baseline variables were associated with poorer survival: poor ECOG PS, a shorter time from diagnosis of metastases, a low initial dose of regorafenib, >3 metastatic sites, liver metastases, and KRAS mutations. A longer time since diagnosis of metastatic disease is considered to reflect a better response to chemotherapy, so PFS2 after success of crossover between regorafenib and TAS-102 tended to be longer than PFS1, which included patients with rapidly growing tumors refractory to treatments (Table II).
In our study, a model with a good discrimination (C-index 0.70), consisting of only 3 baseline predictors (poor ECOG PS, ≤18 months from diagnosis of metastases, and prior chemotherapy continued ≥2 months beyond PD) (Table IV), classified patients into similar prognostic groups (Fig. 3). We recommend that patients having a high probability of benefit should be identified before starting treatment with regorafenib or TAS-102 among patients refractory to standard chemotherapy.
It remains an important clinical issue to decide which drug should be administered first, but this has not been established because of the lack of a head-to-head randomized trial. A retrospective comparative analysis in 550 patients (REGOTAS) (11) suggested that regorafenib should be given first in patients aged <65 years, but TAS-102 in patients aged ≥65 years, based on a favorable trend of OS. We tried a propensity score method (inverse probability of treatment weighting) (27) for choosing between the two drugs in our study, but there was no clear result, except for a favorable trend with age in the TAS-102-first group (hazard ratio, 0.8; 95% confidence interval, 0.7–0.9). No difference in OS was found between the two drugs.
There are some potential limitations of this study. Our study is a retrospective single-center analysis. Although all patients with refractory mCRC treated with regorafenib or TAS-102 in the period were included, the number of patients was relatively small. All patients were treated by a team of six surgeons, all of whom are colorectal cancer specialists. An all-RAS (KRAS and NRAS) test was approved in Japan on April, 2015. Among our patients, 15 had wild-KRAS exon 2 tumor identified before that date, and all of them received anti-EGFR antibody regimens. Although 20% of them (3 patients) might have other RAS mutation, the number is too small to permit any conclusion; at worst, they would have had a short duration of anti-EGFR treatment without benefit until tumor progression. External validation is still needed to confirm the model used to predict OS.
This analysis suggests that the administration of regorafenib and TAS-102 can be recommended for patients with refractory mCRC who have a better performance status, and a longer time since diagnosis of metastatic disease. Prolongation of the previous chemotherapy after diagnosis of disease progression attenuated the survival benefit of regorafenib and TAS-102, regardless of the order of their administration. We suggest that the optimal survival benefit of regorafenib and TAS-102 is provided by flexible and careful titration to the optimal dose for each individual patient with initial dose reduction if necessary, followed by prolonged administration until disease progression.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- TAS-102
trifluridine and tipiracil
- mCRC
metastatic colorectal cancer
- VEGF
vascular endothelial growth factor
- EGFR
epidermal growth factor receptor
- KRAS
Kirsten rat sarcoma
- CR
complete response
- PR
partial response
- SD
stable disease
- PD
progressive disease
- RECIST
Response Evaluation Criteria in Solid Tumors
- CTCAE
Common Terminology Criteria for Adverse Events
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
Conception, design, collection of patient information, data interpretation, and drafting of the article was undertaken by AT. SS, TS, KO, GS and HM participated in patient treatment, and helped revise the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The Institutional Review Board for Clinical Research approved all procedures for this retrospective observational study (no. 16R-190), which was conducted in accordance with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Howlader N, Noone A, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, et al. SEER Cancer Statistics Review, 1975–2012. National Cancer Institute; Bethesda, MD, USA: 2015. [Google Scholar]
- 2.Abrams TA, Meyer G, Schrag D, Meyerhardt JA, Moloney J, Fuchs CS. Chemotherapy usage patterns in a US-wide cohort of patients with metastatic colorectal cancer. J Natl Cancer Inst. 2014;106:djt371. doi: 10.1093/jnci/djt371. [DOI] [PubMed] [Google Scholar]
- 3.Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, Thierauch KH, Zopf D. Regorafenib (BAY 73–4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–255. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 4.Lenz HJ, Stintzing S, Loupakis F. TAS-102, a novel antitumor agent: A review of the mechanism of action. Cancer Treat Rev. 2015;41:777–783. doi: 10.1016/j.ctrv.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crawford J, Becker PS, Armitage JO, Blayney DW, Chavez J, Curtin P, Dinner S, Fynan T, Gojo I, Griffiths EA, et al. Myeloid growth factors, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:1520–1541. doi: 10.6004/jnccn.2017.0175. [DOI] [PubMed] [Google Scholar]
- 6.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aguilar Aranda E, Bardelli A, Benson A, Bodoky G, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 7.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, Xu J, Bai Y, Chi Y, Wang L, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619–629. doi: 10.1016/S1470-2045(15)70156-7. [DOI] [PubMed] [Google Scholar]
- 9.Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero J, Komatsu Y, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909–1919. doi: 10.1056/NEJMoa1414325. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Kim TW, Shen L, Sriuranpong V, Pan H, Xu R, Guo W, Han SW, Liu T, Park YS, et al. Results of a randomized, double-blind, placebo-controlled, phase III trial of trifluridine/tipiracil (TAS-102) monotherapy in Asian patients with previously treated metastatic colorectal cancer: The TERRA study. J Clin Oncol. 2018;36:350–358. doi: 10.1200/JCO.2017.74.3245. [DOI] [PubMed] [Google Scholar]
- 11.Moriwaki T, Fukuoka S, Taniguchi H, Takashima A, Kumekawa Y, Kajiwara T, Yamazaki K, Esaki T, Makiyama C, Denda T, et al. Propensity score analysis of regorafenib versus trifluridine/tipiracil in patients with metastatic colorectal cancer refractory to standard chemotherapy (REGOTAS): A Japanese society for cancer of the colon and rectum multicenter observational study. Oncologist. 2018;23:7–15. doi: 10.1634/theoncologist.2017-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.García-Alfonso P, Feliú J, García-Carbonero R, Grávalos C, Guillén-Ponce C, Sastre J, García-Foncillas J. Is regorafenib providing clinically meaningful benefits to pretreated patients with metastatic colorectal cancer? Clin Transl Oncol. 2016;18:1072–1081. doi: 10.1007/s12094-016-1499-8. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Hryniuk W, Bush H. The importance of dose intensity in chemotherapy of metastatic breast cancer. J Clin Oncol. 1984;2:1281–1288. doi: 10.1200/JCO.1984.2.11.1281. [DOI] [PubMed] [Google Scholar]
- 15.Health and Human Services, corp-author. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf. [Aug 20;2013 ];National Institutes of Health and National Cancer Institute: Common terminology criteria for adverse events (CTCAE) 2013 v4.03. [Google Scholar]
- 16.Wilkinson GN, Rogers CE. Symbolic description of factorial models for analysis of variance. Appl Stat-J R Stat Soc. 1973;22:392–399. [Google Scholar]
- 17.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: Model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 19.R Development Core Team, corp-author. R Foundation for Statistical Computing. Vienna, Austria: 2016. R: A language and environment for statistical computing. [Google Scholar]
- 20.Masuishi T, Taniguchi H, Hamauchi S, Komori A, Kito Y, Narita Y, Tsushima T, Ishihara M, Todaka A, Tanaka T, et al. Regorafenib versus trifluridine/tipiracil for refractory metastatic colorectal cancer: A retrospective comparison. Clin Colorectal Cancer. 2017;16:e15–e22. doi: 10.1016/j.clcc.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 21.Sueda T, Sakai D, Kudo T, Sugiura T, Takahashi H, Haraguchi N, Nishimura J, Hata T, Hayashi T, Mizushima T, et al. Efficacy and safety of regorafenib or TAS-102 in patients with metastatic colorectal cancer refractory to standard therapies. Anticancer Res. 2016;36:4299–4306. [PubMed] [Google Scholar]
- 22.Adenis A, de la Fouchardiere C, Paule B, Burtin P, Tougeron D, Wallet J, Dourthe LM, Etienne PL, Mineur L, Clisant S, et al. Survival, safety, and prognostic factors for outcome with Regorafenib in patients with metastatic colorectal cancer refractory to standard therapies: Results from a multicenter study (REBECCA) nested within a compassionate use program. BMC Cancer. 2016;16:412. doi: 10.1186/s12885-016-2440-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bekaii-Saab TS, Ou FS, Anderson DM, Ahn DH, Boland PM, Ciombor KK, Jacobs NL, Desnoyers RJ, Cleary JM, Meyers JP, et al. Regorafenib dose optimization study (ReDOS): Randomized phase II trial to evaluate dosing strategies for regorafenib in refractory metastatic colorectal cancer (mCRC)- An ACCRU network study. J Clin Oncol. 2018;36(4 Suppl):S611. [Google Scholar]
- 24.Osawa H. Response to regorafenib at an initial dose of 120 mg as salvage therapy for metastatic colorectal cancer. Mol Clin Oncol. 2017;6:365–372. doi: 10.3892/mco.2017.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grothey A, Falcone A, Humblet Y, Bouche O, Mineur L, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM. Characteristics of patients (pts) with metastatic colorectal cancer (mCRC) treated with regorafenib (REG) who had progression-free survival (PFS) >4 months (m): Subgroup analysis of the phase 3 CORRECT trial. Ann Oncol. 2016;27(Suppl 6):S516P. doi: 10.1093/annonc/mdw370.64. [DOI] [Google Scholar]
- 26.Puthiamadathil JM, Weinberg BA. Emerging combination therapies for metastatic colorectal cancer - impact of trifluridine/tipiracil. Cancer Manag Res. 2017;9:461–469. doi: 10.2147/CMAR.S113320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013;32:2837–2849. doi: 10.1002/sim.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.