Abstract
Cyclin-dependent kinase inhibitor 2A (p16INK4a) protein is a surrogate immunohistochemical marker of human papillomavirus infection in oropharynx squamous cell carcinoma (OPSCC). However, the effects of p16INK4a in non-OPSCC require additional analysis. In addition, major gaps remain in the literature, including small volumes of data for China. Therefore, the present study evaluated the frequency of p16INK4a positivity in patients with non-OPSCC in Southern China, and assessed its prognostic value. p16INK4a expression status in patients with non-OPSCC was determined by immunohistochemistry. p16INK4a-positive expression was defined as a strong and diffuse staining in ≥70% of the tumor cells. Then, the diagnostic value of p16INK4a in predicting overall survival (OS) and disease-free survival (DFS) rate was determined. The positive rate of p16INK4a was 26.3% in larynx cancer and 24.8% in oral cavity cancer. Multivariate analysis revealed that the protein status independently predicted improved OS rate, but not DFS rate (P=0.096). Comparing different disease stages, patients at an early stage with p16INK4a-positive non-OPSCC exhibited improved DFS and OS rates compared with those exhibited by patients who were negative. The p16INK4a-positive rate in patients with non-OPSCC was 25.1% [26.3% in Laryngeal squamous cell carcinoma (LSCC) and 24.8% in Oropharyngeal squamous cell carcinomas (OSCC)] in the present cohort from South China. The present study suggested that p16INK4a expression in non-OPSCC predicts favorable clinical outcomes, particularly in early stage non-OPSCC and oral cancer.
Keywords: human papillomavirus infection, immunohistochemical marker, prognosis, cyclin-dependent kinase inhibitor 2A expression, non-oropharynx squamous cell carcinoma
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common type of cancer globally, with ~600,000 incident cases diagnosed per year (1–3). It is well known that this carcinoma is primarily a loco-regional disease. Improved local control of HNSCC has been achieved over previous decades due to recent advances in multidisciplinary treatments, including the surgical approach, reconstruction techniques and adjuvant treatment modalities (2,3). However, HNSCC is characterized by high rates of recurrence or metastasis following initial treatment, with rates of 40–60% (4). In consequence, the quality of life and life expectancy of patients with advanced HNSCC have not markedly improved over previous decades (2).
Human papillomavirus (HPV) infection is one of the major risk factors for HNSCC (5). Oropharynx squamous cell carcinoma (OPSCC) is most markedly associated with high-risk HPV infection (6–8). HNSCCs of other sites, including the oral cavity (9–11) and larynx (12–15), also have been demonstrated to harbor the virus. HPV status in tumors may be determined by numerous assays, including HPV E6/E7 RNA expression detected by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and HPV DNA detection by in situ hybridization or PCR. Cyclin-dependent kinase inhibitor 2A (p16INK4a), a tumor-suppressor protein that is overexpressed in cells, is a surrogate immunohistochemical marker of oncogenic subtypes of HPV infection in the oropharynx (6,16–18). However, the immunohistochemical studies of p16INK4a in non-oropharyngeal HNSCC have demonstrated variable results (13–15,19–23); thus, the validity of p16INK4a protein as a marker of HPV infection in non-OPSCC requires additional validation. Furthermore, patients who are positive for p16INK4a have improved outcomes compared with those of patients with OPSCC who are negative for p16INK4a (6,17). Compared with the significance of the data regarding HPV and OPSCC, the significance of HPV in HNSCC sites outside the oropharynx has not been established. Major gaps in the literature remain, including very little data for Asia, particularly China. Additionally, to the best of our knowledge, with the exception of a small number of studies investigating laryngeal cancer in Eastern China (24,25), there has been no analysis of p16INK4a expression in Chinese patients in oral cavity cancer. The aim of the present study was to evaluate the frequency of p16INK4a protein positive expression in patients with non-OPSCC in Southern China, and to assess its prognostic value.
Patients and methods
Patients and specimens
Patients with non-oropharyngeal HNSCC were treated at the Department of Head and Neck Surgery of Sun Yat-sen University Cancer Center (SYSUCC; Guangzhou, China) between January 2001 and December 2008, and were selected randomly and retrospectively by stratified sampling. The present study was approved by the Ethics Review Board of SYSUCC and written informed consent was provided by all patients. All activities were in accord with the 1964 Declaration of Helsinki. The oral cavity, glottis and supraglottic larynx cancer were defined as non-OPSCC. The inclusion criteria were as follows: i) Patients had undergone complete excision, with or without unilateral or bilateral neck dissection; ii) the diagnosis was confirmed by pathology; iii) pathologic samples and complete follow-up data were available. Patients with other concomitant organ disorders or malignant tumor types were excluded. A total of 183 eligible patients with non-OPSCC with a mean age of 54.3 years (range, 26–86 years) were included in the present study. Among these, females comprised 24.6% of the patients. No patients exhibited metastasis at the time of diagnosis (stage IVc), nor had they undergone chemotherapy and/or radiotherapy prior to surgery.
All patients had follow-up data through December 2015. Detailed information of the patients selected for analysis was reviewed. The tumors were restaged in accordance with the 2010 American Joint Committee on Cancer TNM Staging Manual (26). To verify the histological diagnosis of non-OPSCC and adequacy of the tissue for analysis, the haematoxylin and eosin-stained (H&E) slides were reviewed by a pathologist. Sections were stained by a standard H&E staining method as follows: Sections were incubated for 60 sec at room temperature in filtered Mayer's haematoxylin solution, which is diluted by distilled water (1:20). Subsequently, the sections were washed under running water for 10 min. Following incubation in 1% eosin solution for 15 sec at room temperature, the sections were washed quickly (5–10 sec) in water and dehydrated in ascending concentrations of ethanol (75, 85, 95 and 100%). Subsequently, xylene was used to clear and covered with slips. The representative formalin-fixed, paraffin-embedded block for each patient was obtained from the Pathology Department of SYSUCC.
Staining and evaluation
The 4-mm thick paraffin-embedded slices were dewaxed and rehydrated, and endogenous peroxidase activity was blocked with 0.3% H2O2. The slides were boiled in sodium citrate for 5 min at 95°C and 20 min at 60°C in a microwave for antigen retrieval. The primary mouse monoclonal antibody against human p16INK4a (CINtec® p16INK4a Histology kit; clone E6H4, prediluted, MTM-Laboratories; Roche Diagnostics, Basel, Switzerland) was used for the detection of the protein p16INK4a. Subsequently, the slides were incubated with a horseradish peroxidase-conjugated goat anti-mouse IgG secondary antibody (cat. no. 7076s; 1:2,000; Cell Signaling Technology, Inc., Danvers, MA, USA) at 37°C for 30 min. Finally, stained slides were counterstained with 5% Mayer's haematoxylin solution at room temperature for 60 sec. All slides were interpreted by two pathologists in a double-blinded manner. Positive p16INK4a expression was defined as a strong and diffuse nuclear and cytoplasmic staining in ≥70% of the tumor cells, as described previously (6,21,27).
Statistical analysis
All data were analyzed with the SPSS 19.0 statistical software package (IBM Corp., Armonk, NY, USA). The results of analysis were presented as the mean ± standard error of the mean. The χ2 test was used to assess the associations between p16INK4a expression and other characteristics of patients. The disease-free survival (DFS) time was defined as the time between diagnosis and loco-regional recurrence or progression, distant metastasis. The overall survival (OS) time was calculated from the time of diagnosis to the date of mortality or the last follow-up visit. Survival was analyzed with Kaplan-Meier survival curves and univariate analysis. Multivariable hazard ratios were estimated using the Cox proportional hazards model. A two-sided P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
The clinicopathological characteristics of the patients are summarized in Table I. The 183 eligible patients with stages I–IVb non-oropharyngeal cancer had a median age of 53 years (range, 26–86 years), and males comprised 75.4% of the patients, with 58.5% of all patients being current or former smokers. Of the 183 patients, 145 exhibited oral cancer types, 28 exhibited glottis and 10 were supraglottic larynx cancer types. Adjuvant treatment involving radiotherapy was administered to 45 (24.6%) patients. The median follow-up time was 95 months (range, 3–184 months). As of the last follow-up visit, 113 (61.7%) patients exhibited local or distant relapse events. Only 5 (2.7%) patients were lost to follow-up. The disease-specific mortality was 44.8% (82/183).
Table I.
Associations between p16INK4a expression and clinicopathological characteristics of patients with oral cavity and larynx squamous cell carcinoma (n=183).
| p16INK4a expressiona | |||
|---|---|---|---|
| Characteristic | Negative, n (%) | Positive, n (%) | P-value |
| Total | 137 (74.9) | 46 (25.1) | |
| Age at diagnosis, years | 0.560 | ||
| <50 | 50 (36.5) | 19 (41.3) | |
| ≥50 | 87 (63.5) | 27 (58.7) | |
| Sex | 0.360 | ||
| Male | 101 (73.7) | 37 (80.4) | |
| Female | 36 (26.3) | 9 (19.6) | |
| Smoking history | 0.156 | ||
| No | 61 (44.5) | 15 (32.6) | |
| Yes | 76 (55.5) | 31 (67.4) | |
| Alcohol consumption | 0.467 | ||
| No | 103 (75.2) | 37 (80.4) | |
| Yes | 34 (24.8) | 9 (19.6 | |
| Tumor position | 0.633 | ||
| Oral cavity | 109 (79.6) | 36 (78.3) | |
| Buccal mucosa | 8 | 2 | |
| Floor of mouth | 12 | 1 | |
| Anterior tongue | 71 | 28 | |
| Alveolar ridge | 18 | 5 | |
| Larynx | 28 (20.4) | 10 (21.7) | |
| Supraglottic | 8 | 2 | |
| Glottic | 20 | 8 | |
| Histological differentiation | 0.325 | ||
| Well | 74 (54.0) | 30 (65.2) | |
| Moderate | 49 (35.8) | 11 (23.9) | |
| Poor | 14 (10.2) | 5 (10.9) | |
| Clinical stage | 0.405 | ||
| I/II | 86 (62.8) | 32 (69.6) | |
| III/IV | 51 (37.2) | 14 (30.4) | |
| Disease recurrence | 0.025 | ||
| No | 46 (33.6) | 24 (52.5) | |
| Yes | 91 (66.4) | 22 (47.8) | |
| Recurrent tumor sites | 0.143 | ||
| Local | 36 (39.6) | 7 (30.4) | |
| Lymph node metastasis | 49 (53.8) | 16 (69.6) | |
| Distant metastasis | 6 (6.6) | 0 (0) | |
p16INK4a, Cyclin-dependent kinase inhibitor 2A.
p16INK4a positive expression was defined as a strong and diffuse nuclear and cytoplasmic staining in ≥70% of the tumor cells.
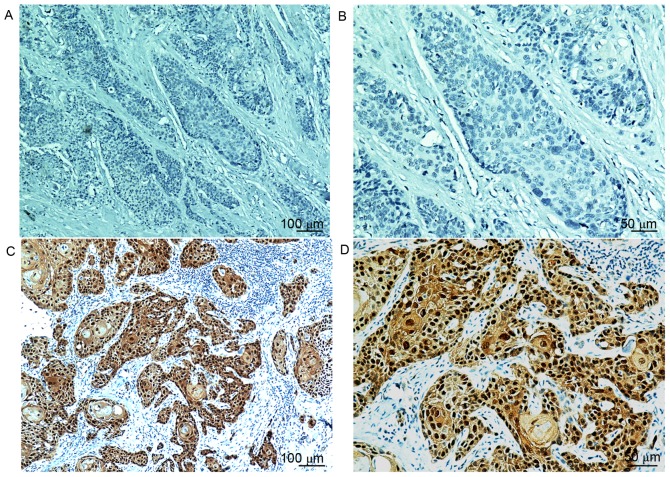
Overall, 46 patients (25.1%) were scored as p16INK4a-positive, as defined by moderate or strong intensity staining in ≥70% of tumor cells (Fig. 1). The group of patients with larynx cancer exhibited the highest rate of p16INK4a-positive staining (26.3%), followed by the oral cavity (24.8%). Negative p16INK4a expression was significantly associated with recurrence or metastasis (66.4% vs. 47.8%; P=0.025). No other significant clinicopathological differences were observed between the two groups (Table I).
Figure 1.
Representative p16INK4a immunohistochemistry results. Lack of brown staining in p16INK4a negative carcinoma at a magnification of (A) ×100 and (B) ×200. p16INK4a exhibiting diffuse nuclear and cytoplasmic brown staining in ≥70% of the tumor cells at (C) a magnification of ×100 and (D) ×200. p16INK4a, cyclin-dependent kinase inhibitor 2A.
Survival outcomes based on p16INK4a status
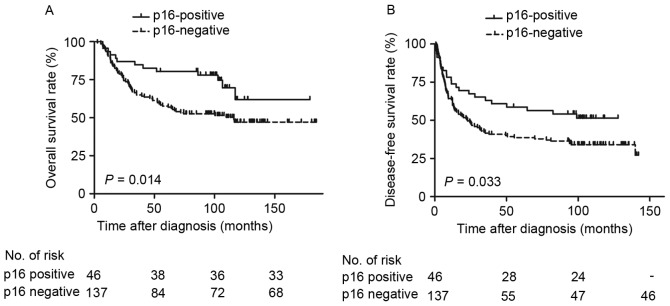
Survival outcomes based on p16INK4a status in patients with non-OPSCC were examined. In the p16INK4a-negative group, the 5-year OS and DFS rates were 57.7% and 38.6%, respectively. The p16INK4a-postitive expression group exhibited markedly improved survival rates: The 5-year OS and DFS rates were 78.1% and 56.4%, respectively. Patients with p16INK4a-positive expression exhibited improved DFS and OS rates compared with those with negative expression (Fig. 2). Furthermore, male patients (P=0.037), who smoked tobacco (P=0.016) and alcohol (P=0.020), with poorly differentiated tumor types (P=0.018) and stage III or IV disease (P<0.001) exhibited a reduced OS rate, and patients with poorly differentiated tumor types (P=0.004) and advanced disease (P=0.002) exhibited a reduced DFS rate, compared with their counterparts (Table II). Cox proportional hazards analysis revealed that p16INK4a-negative independently predicted low OS rate (Table III; P=0.020) but not DFS rate (P=0.096).
Figure 2.
Survival curves of 183 patients with non-oropharynx squamous cell carcinoma, stratified by p16INK4a expression status. (A) Overall survival rate. (B) Disease-free survival rate. p16INK4a, cyclin-dependent kinase inhibitor 2A.
Table II.
Univariate survival analysis of patients with oral cavity and larynx squamous cell carcinoma (n=183).
| Overall survival rate | Disease-free survival rate | |||
|---|---|---|---|---|
| Variable | RR 95% CI | P-value | RR 95% CI | P-value |
| Male | 1.88 (1.04–3.41) | 0.037 | 1.53 (0.95–2.46) | 0.081 |
| Tobacco use | 1.78 (1.11–2.86) | 0.016 | 1.36 (0.93–2.00) | 0.118 |
| Alcohol use | 1.74 (1.09–2.77) | 0.020 | 1.25 (0.83–1.89) | 0.291 |
| Age <50 years | 1.13 (0.72–1.77) | 0.602 | 1.06 (0.72–1.55) | 0.779 |
| Poorer differentiation | 1.69 (1.09–2.61) | 0.018 | 1.71 (1.18–2.48) | 0.004 |
| Cyclin-dependent kinase inhibitor 2A -positive expression | 0.48 (0.27–0.87) | 0.014 | 0.61 (0.38–0.97) | 0.033 |
| Stage III/IV disease | 2.26 (1.47–3.50) | <0.001 | 1.79 (1.23–2.60) | 0.002 |
RR, relative risk; CI, confidence interval.
Table III.
Multivariate survival analysis of patients with oral cavity and larynx squamous cell carcinoma (n=183).
| Overall survival rate | Disease-free survival rate | |||||
|---|---|---|---|---|---|---|
| Variable | RR | 95% CI | P-value | RR | 95% CI | P-value |
| Male | 0.95 | 0.42–2.15 | 0.909 | – | – | – |
| Tobacco use | 1.75 | 0.92–3.32 | 0.088 | – | – | – |
| Alcohol use | 1.53 | 0.92–2.52 | 0.099 | – | – | – |
| Poorer differentiation | 1.28 | 0.81–2.04 | 0.294 | 1.47 | 1.00–2.17 | 0.051 |
| Cyclin-dependent kinase inhibitor 2A -positive expression | 0.49 | 0.27–0.89 | 0.020 | 0.67 | 0.42–1.07 | 0.096 |
| Stage III/IV disease | 2.01 | 1.25–3.23 | 0.004 | 1.53 | 1.03–2.27 | 0.034 |
RR, relative risk; CI, confidence interval.
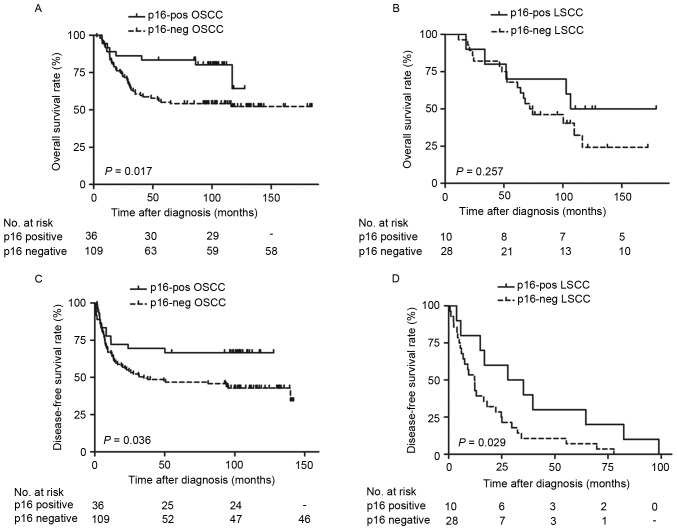
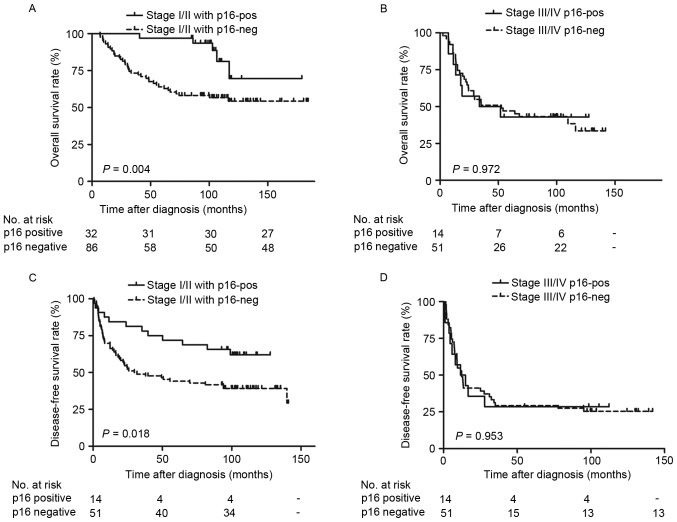
The survival outcomes of p16INK4a based on reclassifying by primary site (oral cavity or larynx) and stage (stage I/II or III/IV) were also examined. The patients with OSCC with p16INK4a-positive expression exhibited significantly improved OS (Fig. 3A; P=0.017) and DFS (Fig. 3B; P=0.036) rates, as determined by Kaplan-Meier survival curves. Additionally, patients with LSCC with positive p16INK4a expression exhibited no significant difference in OS rate (Fig. 3C; P=0.257), but significantly increased DFS rate (Fig. 3D; P=0.029). Furthermore, significantly improved OS (Fig. 4A; P=0.004) and DFS rates (Fig. 4B; P=0.018) were observed in patients with stage I/II tumor types with positive p16INK4a expression. However, there were no significant differences in stage III/IV tumor types (Fig. 4C and D; P>0.05). Additional univariate analysis confirmed that positive p16INK4a status predicted favorable OS rate in oral cancer [Table IV; relative risk (RR)=0.42; P=0.017] and in the stage I/II disease (RR=0.25; P=0.004) subgroups. Although patients who were p16INK4a-positive exhibited increased DFS rates, compared with those who were p16INK4a-negative (Fig. 2B; P=0.033), the statistical difference calculated by multivariate analysis between groups was not significant (Table III; RR=0.67; P=0.096).
Figure 3.
Survival curves of patients with OSCC and LSCC. Overall survival rates are presented according to the primary tumor site and p16INK4a status of (A) OSCC and (C) LSCC. Disease-free survival rates are presented according to the primary tumor site and p16INK4a status of (B) OSCC and (D) LSCC. OSCC, oral cavity squamous cell carcinoma; LSCC, larynx squamous cell carcinoma; p16INK4a, cyclin-dependent kinase inhibitor 2A.
Figure 4.
Survival curves of patients with different tumor stages. Overall survival rates are presented according to the disease stage and p16INK4a status in (A) stage I/II tumor types and (C) stage III/IV tumor types. Disease-free survival rates are presented according to the disease stage and p16INK4a status in (B) stage I/II tumor types and (D) stage III/IV tumor types. p16INK4a, cyclin-dependent kinase inhibitor 2A.
Table IV.
Univariate survival analysis of cyclin-dependent kinase inhibitor 2A in patients stratified by primary site and stage.
| Overall survival rate | Disease-free survival rate | ||||||
|---|---|---|---|---|---|---|---|
| Stratification factors | RR | 95% CI | P-value | RR | 95% CI | P-value | |
| Primary site | – | – | – | – | – | – | |
| Oral cancer | 0.42 | 0.20–0.88 | 0.017 | 0.52 | 0.28–0.97 | 0.036 | |
| Larynx cancer | 0.56 | 0.20–1.54 | 0.257 | 0.42 | 0.19–0.93 | 0.029 | |
| Primary stage | – | – | – | – | – | – | |
| I/II | 0.25 | 0.10–0.65 | 0.004 | 0.48 | 0.25–0.90 | 0.018 | |
| III/IV | 1.01 | 0.46–2.21 | 0.972 | 1.02 | 0.51–2.05 | 0.953 | |
RR, relative risk; CI, confidence interval.
Discussion
HPV E6/E7 RNA expression detected using RT-qPCR, the results of which indicate active viral gene transcription in a tumor, is considered to be the gold standard (16). Although p16INK4a protein is a reliable indicator of HPV infection in the oropharynx, the sensitivity and specificity of p16INK4a in OSCC and LSCC has not been conclusively determined (14,20,22,23,28–30). It was demonstrated that the incidence of p16INK4a positivity in southern Chinese patients with LSCC is 26.3%, and in patients with OSCC is 24.8% (14). Johns Hopkins University published a large-scale screening study that evaluated p16INK4a expression by immunohistochemistry, in which the patients with non-OPSCC were collected from Radiation Therapy Oncology Group (RTOG) 0129, 0234 and 0522 studies (21). A total of 19.3% (62/322) patients were p16INK4a positive, and the patients with OSCC exhibited the highest rate of p16INK4a positivity [21/80 (26.3%)], followed by the larynx [31/181 (17.1%)], which was similar to the present study. However, Xu et al (23) detected the status in Chinese patients with laryngeal cancer, and revealed a positive rate of 7.57%. In general, previously identified rates of p16INK4a protein expression in patients with non-OPSCC are not concordant (20,22,23,25,30), and multiple factors contribute to this discrepancy, for example: Heterogeneous patient selection (geographical differences, tobacco and alcohol status of the patient or the number of cases); or the scoring system used for defining p16INK4a as positive.
A debate exists with respect to the prognostic role of p16INK4a in non-OPSCC. In the present study, the OS and DFS rates among all 183 patients with non-OPSCC were improved in p16INK4a-positive patients compared with negative expression patients, although the multivariate survival analysis suggested that the difference between DFS rate was not statistically significant. The data from patients with non-OPSCC collected from RTOG studies concluded that, similar to the results in patients with OPSCC (6,17,31,32), patients with p16INK4a-positive exhibit improved outcomes compared with those with p16INK4a-negative non-OPSCC (21). Nevertheless, other studies did not observe a significant correlation between p16INK4a expression and improved outcomes (14,22,33). These differences may be due to the small number of samples, different anatomic sites enrolled or tumor heterogeneity in each retrospective cohort.
Additional stratified analysis identified that patients who were p16INK4a-positive with stage I/II tumor types or tumor types in oral cavity sites demonstrated significantly improved OS rate compared with patients who were negative expression. Patients who were p16INK4a-positive in stage I/II or at larynx sites demonstrated markedly improved DFS rates compared with patients who were p16INK4a-negative; and this observation may be of clinical importance. The treatment of an early stage tumor consists of surgery combined with radiotherapy (34,35). Nevertheless, the toxicities of these treatments may result in organ dysfunction and potentially treatment-associated mortalities (36,37). It is necessary to decrease the intensity of treatment for patients with favorable prognosis. However, no specific indicator for this subgroup was identified. In the present study, negative p16INK4a status was considered as an indicator for a requirement for aggressive therapy in patients with stage I/II tumor types, to achieve survival benefit.
However, due to the small sample sizes (n=183), the prognostic role of p16INK4a in LSCC demonstrated in the present study should be interpreted with caution. The present study aimed to detect the level of HPV DNA in the same set of samples, but the outcome was not conclusive as the DNA quality in the paraffin block was not adequate. Concomitantly, as a retrospective investigation, the present study is limited by the lack of large-scale screening.
The present study demonstrated an analysis of diagnostic tests, the diagnostic accuracy and prognostic relevance of p16INK4a in patients from South China. It was identified that the p16INK4a positive rate in patients with non-OPSCC was 25.1% (26.3% in LSCC and 24.8% in OSCC). It was suggested that p16INK4a expression in patients with non-OPSCC predicted favorable clinical outcomes, particularly in OSCC and early stage non-OPSCC. If so, these patients with p16INK4a-negative should receive more aggressive therapy and a closer follow-up. It is hoped that the design of forthcoming clinical trials in China, aimed at therapy in patients who are p16INK4a-positive with non-OPSCC and perhaps aggressive therapy for patients who are p16INK4a-negative, will be informed by these results.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the Natural Science Foundation of China (grant no. 81201716).
Availability of data and materials
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
WQJ and YXS participated in the design of the research. HY and YC conducted the IHC studies, participated in the collection of cases and drafted the manuscript. ZML and YJL helped the statistical analysis and participated in the IHC studies. WQJ and YXS helped to revise the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The current study was approved by the Ethics Review Board of Sun Yat-Sen University Cancer Center. All activities were in accord with the 1964 Declaration of Helsinki. Each patient signed informed consent for participate in the study and collect specimens.
Patient consent for publication
The patients involved in the present study signed informed consent for the publication of any associated data and accompanying images.
Competing interests
The authors declared that they have no competing interests.
References
- 1.Wong IC, Ng YK, Lui VW. Cancers of the lung, head and neck on the rise: Perspectives on the genotoxicity of air pollution. Chin J Cancer. 2014;33:476–480. doi: 10.5732/cjc.014.10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Atienza JA, Dasanu CA. Incidence of second primary malignancies in patients with treated head and neck cancer: A comprehensive review of literature. Curr Med Res Opin. 2012;28:1899–1909. doi: 10.1185/03007995.2012.746218. [DOI] [PubMed] [Google Scholar]
- 5.Mork J, Lie AK, Glattre E, Hallmans G, Jellum E, Koskela P, Møller B, Pukkala E, Schiller JT, Youngman L, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125–1131. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 6.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M, Cozen W, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the united states. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/jco.2011.29.15_suppl.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ndiaye C, Mena M, Alemany L, Arbyn M, Castellsagué X, Laporte L, Bosch FX, de Sanjosé S, Trottier H. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: A systematic review and meta-analysis. Lancet Oncol. 2014;15:1319–1331. doi: 10.1016/S1470-2045(14)70471-1. [DOI] [PubMed] [Google Scholar]
- 9.Bouda M, Gorgoulis VG, Kastrinakis NG, Giannoudis A, Tsoli E, Danassi-Afentaki D, Foukas P, Kyroudi A, Laskaris G, Herrington CS, Kittas C. ‘High risk’ HPV types are frequently detected in potentially malignant and malignant oral lesions, but not in normal oral mucosa. Mod Pathol. 2000;13:644–653. doi: 10.1038/modpathol.3880113. [DOI] [PubMed] [Google Scholar]
- 10.Paradiso A, Ranieri G, Stea B, Zito A, Zehbe I, Tommasino M, Grammatica L, De Lena M. Altered p16INK4a and Fhit expression in carcinogenesis and progression of human oral cancer. Int J Oncol. 2004;24:249–255. [PubMed] [Google Scholar]
- 11.Bradley KT, Budnick SD, Logani S. Immunohistochemical detection of p16INK4a in dysplastic lesions of the oral cavity. Mod Pathol. 2006;19:1310–1316. doi: 10.1038/modpathol.3800649. [DOI] [PubMed] [Google Scholar]
- 12.Baumann JL, Cohen S, Evjen AN, Law JH, Vadivelu S, Attia A, Schindler JS, Chung CH, Wirth PS, Meijer CJ, et al. Human papillomavirus in early laryngeal carcinoma. Laryngoscope. 2009;119:1531–1537. doi: 10.1002/lary.20509. [DOI] [PubMed] [Google Scholar]
- 13.Halec G, Holzinger D, Schmitt M, Flechtenmacher C, Dyckhoff G, Lloveras B, Höfler D, Bosch FX, Pawlita M. Biological evidence for a causal role of HPV16 in a small fraction of laryngeal squamous cell carcinoma. Br J Cancer. 2013;109:172–183. doi: 10.1038/bjc.2013.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y, Liu S, Yi H, Wang J, Dong P, Li X, Yin S. Human papillomavirus infection in 674 Chinese patients with laryngeal squamous cell carcinoma. PLoS One. 2014;9:e115914. doi: 10.1371/journal.pone.0115914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young RJ, Urban D, Angel C, Corry J, Lyons B, Vallance N, Kleid S, Iseli TA, Solomon B, Rischin D. Frequency and prognostic significance of p16(INK4A) protein overexpression and transcriptionally active human papillomavirus infection in laryngeal squamous cell carcinoma. Br J Cancer. 2015;112:1098–1104. doi: 10.1038/bjc.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi W, Kato H, Perez-Ordonez B, Pintilie M, Huang S, Hui A, O'Sullivan B, Waldron J, Cummings B, Kim J, et al. Comparative prognostic value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J Clin Oncol. 2009;27:6213–6221. doi: 10.1200/JCO.2009.23.1670. [DOI] [PubMed] [Google Scholar]
- 17.Rischin D, Young RJ, Fisher R, Fox SB, Le QT, Peters LJ, Solomon B, Choi J, O'Sullivan B, Kenny LM, McArthur GA. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28:4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan RC, Lingen MW, Perez-Ordonez B, He X, Pickard R, Koluder M, Jiang B, Wakely P, Xiao W, Gillison ML. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol. 2012;36:945–954. doi: 10.1097/PAS.0b013e318253a2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buajeeb W, Poomsawat S, Punyasingh J, Sanguansin S. Expression of p16 in oral cancer and premalignant lesions. J Oral Pathol Med. 2009;38:104–108. doi: 10.1111/j.1600-0714.2008.00710.x. [DOI] [PubMed] [Google Scholar]
- 20.Bussu F, Sali M, Gallus R, Vellone VG, Zannoni GF, Autorino R, Dinapoli N, Santangelo R, Martucci R, Graziani C, et al. HPV infection in squamous cell carcinomas arising from different mucosal sites of the head and neck region. Is p16 immunohistochemistry a reliable surrogate marker? Br J Cancer. 2013;108:1157–1162. doi: 10.1038/bjc.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung CH, Zhang Q, Kong CS, Harris J, Fertig EJ, Harari PM, Wang D, Redmond KP, Shenouda G, Trotti A, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014;32:3930–3938. doi: 10.1200/JCO.2013.54.5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim AM, Do H, Young RJ, Wong SQ, Angel C, Collins M, Takano EA, Corry J, Wiesenfeld D, Kleid S, et al. Differential mechanisms of CDKN2A (p16) alteration in oral tongue squamous cell carcinomas and correlation with patient outcome. Int J Cancer. 2014;135:887–895. doi: 10.1002/ijc.28727. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y, Liu S, Yi H, Wang J, Luo Y, Yin S. Low prevalence of human papillomavirus in head and neck squamous cell carcinoma in chinese patients. J Med Virol. 2015;87:281–286. doi: 10.1002/jmv.24052. [DOI] [PubMed] [Google Scholar]
- 24.Fu ZJ, Ma ZY, Wang QR, Lei DP, Wang R, Liu CX, Pan XL. Overexpression of cyclind1 and underexpression of p16 correlate with lymph node metastases in laryngeal squamous cell carcinoma in chinese patients. Clin Exp Metastasis. 2008;25:887–892. doi: 10.1007/s10585-008-9207-x. [DOI] [PubMed] [Google Scholar]
- 25.Li D, Zhang Q, Zhang X. Expression and clinical significance of MDM2 and P16 in laryngeal squamous cell carcinoma. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012;26:802–805. (In Chinese) [PubMed] [Google Scholar]
- 26.Edge SB, Compton CC. The american joint committee on cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 27.Schache AG, Liloglou T, Risk JM, Filia A, Jones TM, Sheard J, Woolgar JA, Helliwell TR, Triantafyllou A, Robinson M, et al. Evaluation of human papilloma virus diagnostic testing in oropharyngeal squamous cell carcinoma: Sensitivity, specificity, and prognostic discrimination. Clin Cancer Res. 2011;17:6262–6271. doi: 10.1158/1078-0432.CCR-11-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlecht NF, Brandwein-Gensler M, Nuovo GJ, Li M, Dunne A, Kawachi N, Smith RV, Burk RD, Prystowsky MB. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Mod Pathol. 2011;24:1295–1305. doi: 10.1038/modpathol.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doxtader EE, Katzenstein AL. The relationship between p16 expression and high-risk human papillomavirus infection in squamous cell carcinomas from sites other than uterine cervix: A study of 137 cases. Hum Pathol. 2012;43:327–332. doi: 10.1016/j.humpath.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 30.van Monsjou HS, van Velthuysen ML, van den Brekel MW, Jordanova ES, Melief CJ, Balm AJ. Human papillomavirus status in young patients with head and neck squamous cell carcinoma. Int J Cancer. 2012;130:1806–1812. doi: 10.1002/ijc.26195. [DOI] [PubMed] [Google Scholar]
- 31.Posner MR, Lorch JH, Goloubeva O, Tan M, Schumaker LM, Sarlis NJ, Haddad RI, Cullen KJ. Survival and human papillomavirus in oropharynx cancer in TAX 324: A subset analysis from an international phase III trial. Ann Oncol. 2011;22:1071–1077. doi: 10.1093/annonc/mdr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rietbergen MM, Brakenhoff RH, Bloemena E, Witte BI, Snijders PJ, Heideman DA, Boon D, Koljenovic S, Baatenburg-de Jong RJ, Leemans CR. Human papillomavirus detection and comorbidity: Critical issues in selection of patients with oropharyngeal cancer for treatment De-escalation trials. Ann Oncol. 2013;24:2740–2745. doi: 10.1093/annonc/mdt319. [DOI] [PubMed] [Google Scholar]
- 33.Kalfert D, Celakovsky P, Laco J, Ludvikova M. The role of protein p16(INK4a) in glottic laryngeal squamous cell carcinoma. Pathol Oncol Res. 2014;20:909–915. doi: 10.1007/s12253-014-9773-y. [DOI] [PubMed] [Google Scholar]
- 34.Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 35.Adelstein DJ, Saxton JP, Rybicki LA, Esclamado RM, Wood BG, Strome M, Lavertu P, Lorenz RR, Carroll MA. Multiagent concurrent chemoradiotherapy for locoregionally advanced squamous cell head and neck cancer: Mature results from a single institution. J Clin Oncol. 2006;24:1064–1071. doi: 10.1200/JCO.2005.01.5867. [DOI] [PubMed] [Google Scholar]
- 36.Machtay M, Moughan J, Trotti A, Garden AS, Weber RS, Cooper JS, Forastiere A, Ang KK. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corry J, Peters LJ, Rischin D. Optimising the therapeutic ratio in head and neck cancer. Lancet Oncol. 2010;11:287–291. doi: 10.1016/S1470-2045(09)70384-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.