Abstract
The present study established systems to predict the chemo-sensitivity of muscle invasive bladder cancer (MIBC) for neoadjuvant chemotherapy (NAC) with methotrexate, vinblastine, doxorubicin plus cisplatin (M-VAC) and carboplatin plus gemcitabine (CaG) by analyzing microarray data. The primary aim of the study was to investigate whether the clinical response would increase by combining these prediction systems. Treatment of each MIBC case was allocated into M-VAC NAC, CaG NAC, surgery, or radiation therapy groups by their prediction score (PS), which was calculated using the designed chemo-sensitivity prediction system. The therapeutic effect of the present study was compared with the results of historical controls (n=76 patients) whose treatments were not allocated using the chemo-sensitivity prediction system. In addition, the overall survival between the predicted to be responder (positive PS) group and predicted to be non-responder (negative PS) group was investigated in the present study. Of the 33 patients with MIBC, 25 cases were positive PS and 8 were negative PS. Among the 25 positive PS cases, 7 were allocated to receive M-VAC NAC and 18 were allocated to receive CaG NAC according to the results of the prediction systems. Of the 8 negative PS cases, 3 received CaG NAC, 1 received surgery without NAC and 4 received radiation therapy. The total clinical response to NAC was 88.0% (22/25), which was significantly increased compared with the historical controls [56.6% (43/76) P=0.0041]. Overall survival of the positive PS group in the study was significantly increased compared with the negative PS group (P=0.027). In conclusion, the combination of the two prediction systems may increase the treatment efficacy for patients with MIBC by proposing the optimal NAC regimen. In addition, the positive PS group would have a better prognosis compared with the negative PS group. These results suggest that the two prediction systems may lead to the achievement of ‘precision medicine’.
Keywords: methotrexate, vinblastine, doxorubicin plus cisplatin, carboplatin plus gemcitabine, reverse transcription-polymerase chain reaction, neoadjuvant chemotherapy, muscle invasive bladder cancer
Introduction
Prognosis of muscle invasive bladder cancer (MIBC) has not significantly improved in the past several decades (1). Neoadjuvant chemotherapy (NAC) is well understood as an applicable strategy for MIBC. However, some patients who undergo NAC do not receive any benefit, and others are not candidates for surgery because of disease progression.
We have been predicting the effectiveness of NAC for MIBC by using microarray analyses. The prediction systems were established a combination of methotrexate, vinblastine, doxorubicin plus cisplatin (M-VAC) in 2005 and carboplatin plus gemcitabine (CaG) in 2011, respectively (2,3). In our prior retrospective study, we reported that M-VAC and CaG prediction systems showed the result of nearly 90% accuracy predicting the chemo-sensitivity for M-VAC and CaG, respectively (2–4). Moreover, to investigate the clinical implications of these two systems that predict the response to M-VAC and CaG for NAC, we simulated the clinical response of the CaG-treated 37 patients to M-VAC therapy using our M-VAC prediction system; conversely, we also applied the CaG prediction system indicated above to the M-VAC treated 39 patients who had been previously reported (2–4). As a result of considering the positive and negative predictive accuracies of the prediction systems for responsiveness to M-VAC and CaG, 80.1% of the 76 patients who received M-VAC or CaG prediction system were predicted as responders for at least M-VAC or CaG regimen using the combination of the two systems (4).
Based on these results, the primary aim of this research is to investigate whether by combining M-VAC and CaG prediction systems NAC performance to MIBC will be improved more than historical control groups who have not been allocated NAC regimen according to the results of prediction systems. The secondary aim was to compare with overall survival between predicted to be responder group and predicted to be non-responder group in this prospective study.
Patients and methods
Patients
The Ethics Committee of Iwate Medical University (Iwate, Japan) approved this clinical trial prior to patient recruited and is registered with the UMIN CTR as UMIN000019902. Bladder cancer tissue samples, which were confirmed as urothelial carcinoma, from punch biopsies and the corresponding clinical information were obtained from Iwate Medical University after each patient provided written informed consent. Clinical stage was judged according to the Tumor-Node-Metastasis classification. We enrolled only patients who had no lymph node metastasis as determined by computed tomography (CT) from the chest to the pelvis as well as magnetic resonance imaging (MRI) of the abdomen and pelvis at clinical stages T2aN0M0 to T4aN0M0 (Stage II–III); patients were expected to undergo radical cystectomy without prior radiation therapy. None of the participants had any serious abnormality in renal, hepatic, or hematologic function or had an Eastern Cooperative Oncology Group performance status judged to be ≤2. As historical controls, previous patients who received either the M-VAC or CaG regimen were matched for both clinical data and the prediction system used. Hence, the patient characteristics and results of our previous studies were used. Using the predicting systems for the response to M-VAC and CaG, we obtained 39 and 37 cases, respectively. Eighteen of 39 M-VAC cases and 18 of 37 CaG cases served as learning cases to establish the prediction system; the remaining 21 and 19 cases, respectively, were used as test cases to verify the prediction scoring system based on expression data.
From preservation of tissue samples, RNA extraction and reverse transcription-quantitative polymerase chain reaction for the calculation of the prediction score (PS)
Several cancer tissue samples were obtained from each patient at the time of biopsy before NAC. These samples were immediately embedded in TissueTek OCT compound (Sakura, Tokyo, Japan), frozen and stored at −80°C. The frozen tissues were sliced into 8-µm sections using a cryostat (Sakura) and were then stained with H&E for histologic examination. Bladder cancer cells were selectively enriched for our experiments using the EZ-cut system with a pulsed UV narrow beam focus laser (SL microtest GmbH, Germany) according to the manufacturer's protocols. Total RNA extraction were performed by using RNeasy Micro Kits (Qiagen Inc., Valencia, CA, USA) as previously described (5,6). We extracted approximately 9 µl of total RNA from each sample. Seven of the 9 µl were used for M-VAC analysis, and the remainder was used for CaG analysis. The M-VAC group was directly analyzed by RT-qPCR by using oligo-dT primer and SuperScript II reverse transcriptase (Invitrogen; Thermo Fisher Scientific, Waltham, MA, USA). In the CaG group; we followed the protocol previously described in the Affymetrix GeneChip 3′IVT Express Kit User Manual protocol (Affymetrix, Santa Clara, CA, USA) the after that performed RT-qPCR (3). In the RT-qPCR of CaG group, we used the random hexamer and SuperScript II reverse transcriptase (Invitrogen; Thermo Fisher Scientific). For M-VAC analysis, expression of 14 predictive genes and one endogenous control gene was measured by quantitative RT-qPCR using the Format 16 (cat. no. 4346798) of Custom TaqMan® Array Cards (Invitrogen; Thermo Fisher Scientific) on an ABI Prism 7900 Sequence Detection system (Applied Biosystems Life Technologies, Foster City, CA, USA) according to the supplier's protocol. The PCR cycling parameters of M-VAC (40 cycles) were as follows: Predenaturation (95°C, 10 min), denaturation (95°C, 15 sec), annealing and extension (60°C, 60 sec). Moreover, relative expression ratios of each sample were calculated as described previously (2,3,7). The expression of the 12 predictive genes for CaG and 1 endogenous control gene was measured by RT-qPCR using TaqMan Gene Expression Assay products on a Light Cycler 480 system (Roche Applied Science, Basel, Switzerland) as described previously (3). The PCR cycling parameters of CaG (45 cycles) were as follows: Predenaturation (95°C, 10 min), denaturation (95°C, 10 sec), annealing and extension (55°C, 50 sec). The M-VAC and CaG sequences of the primers and fluorogenic TaqMan MGB probes are shown in Table I (2,3). The normalized gene expression values were log-transformed (on a base 2 scale). Moreover, to normalize the expression of each gene, we selected as internal controls chaperonin-containing TCP1, subunit 6A (CCT6A). To keep reproducibility, the expression levels of M-VAC were calculated by means of 2−ΔΔCq method (8) and were normalized to that of using our previous control from bladder samples and the expression levels of CaG were calculated by means of standard curve method and our previous control from bladder samples were used as standard samples respectively. Based on the results of each relative expression ratio, we calculated prediction score (PS) of M-VAC and CaG according to previously described procedures (2,3,9). PS values ranged from −100 to 100; positive PS which ranged from 0 to100 is defined as predicted to be responder. On the other hand, negative PS which ranged from −100 to 0 is defined as predicted to be non-responder.
Table I.
List of primer sets and TaqMan probes of M-VAC and CaG Predictive genes.
| Public ID | Symbol | Forward primer (5′-3′) | Reverse primer (5′-3′) | TaqMan probe (5′-3′) |
|---|---|---|---|---|
| A, Internal control | ||||
| AF385084 | CCT6A | CTCCTGCACTGTGATTGCCA | GACATTCCAGCTCGCATGATC | FAM-CAACATTCTCTTGGTTGATG-MGB |
| B, M-VAC Predictive genes | ||||
| L19067 | RELA | TGGCTGAAGGAAACAGTGCA | AAACCCCTTCTGGATCCTGG | FAM-CAGCACTGGCTCTC-MGB |
| BU625507 | SLC16A3 | TGGATCTGCGGTGAAGCC | CCCCTGGTGAGGATGCCT | FAM-AGCCGCAAGGTTAC-MGB |
| J04088 | TOP2A | AAAAGCCTGATCCTGCCAAA | CATCAGAAGTGGATGGCTTCC | FAM-CCAAGAATCGCCGCAAA-MGB |
| AK025288 | CCTCCGTCACACACACGAGT | ACTGGGAACAAGAGCCACATG | FAM-ACATAGGATAGATATGTGTATGTGA-MGB | |
| BC006992 | PIR51 | CGCCTTGGCTTGTCCAGAT | GGTGCTAGTGGCATTTGGATG | FAM-AGCACGAGTTAAACCT-MGB |
| X80497 | PHKA2 | ATCCTCCTGGCGGCAGTAG | GCACAGACAGACTGCATCCTG | FAM-TGACAAGGGCCACCTC-MGB |
| BX094005 | GGGACACAGGAGATTGGCAG | GGTGGAGGGAGGGCTAGAGA | FAM-CCAACACAGCTAGCC-MGB | |
| NM_005461 | MAFB | GTCCTGCATCAGAAACGAGCT | TGCGGCAGGTTTGATTTCA | FAM-TGGTTTTTACAGATTCAAC-MGB |
| BC062996 | DBI | ATGGTGGGAATTCGGGAAA | GAGCCGTATGGTGAGCAGC | FAM-CCAGTTAAACCAGCTACT-MGB |
| L41143 | TCTA | CCATCTGGCTGCCTTTGCT | GCTGCAATTCCAGGGCC | FAM-AAGCCATCTTTGTGGTAGAG-MGB |
| AK025736 | HMGCS1 | CAATGAAAATAAGGTATGACCCAAGTT | TCCTACTTCAGACCTTGAAGTGGA | FAM-TTACCTAGTCTGACTAGAAGTA-MGB |
| AL136794 | RACGAP1 | GGAAGATTGTCAATATTTTGTGGTAAGA | TTTCAGCATCCAAAGTGCAAAG | FAM-AAGCTACAGTCATTTTT-MGB |
| BM677885 | RASL11B | TGGCAATGACGTTGGGTTG | ATTTCAGCCACCCTTAGGCA | FAM-CTAGGCCTGGCTGAGTT-MGB |
| BU622526 | C14orf142 | TGTTATAAAGAGTACATGTCACGGTTCA | AATTTTCACTACTTGTTCATGTCAGTTCT | FAM-AGGCAGTAACATTTCA-MGB |
| C, CaG Predictive genes | ||||
| AL137335 | IPO7 | TTGTGGTGCACTCACCTCTGA | CAATGAAATACCACTAACCCCTTTTT | FAM-AGTGACTTGAATTCGG-MGB |
| BC043571 | LOC613266 | CCTCCAAGAGTGTTCGATTTCAA | CCTGCGTTCAGACTACTTGAGTAAGA | FAM-CATTGTGCAATTTC-MGB |
| BF508662 | SPRY1 | CTTTTGGCCCCTTGGATAGTT | AGGCAAGGAAAACACAGAAGAGA | FAM-ACAGCTGAGTAATTCT-MGB |
| AI884890 | OSBPL11 | AGGTTCTTCTCTGTTTACCCTAAATCC | CAATCAGGAAGCAGGTCACTCA | FAM-CCCAGAATGGAGTCATT-MGB |
| NM_016220 | ZNF107 | TGCTCTTCATTCCTATTGTATTCACAT | CATAAATAATACCGACCTAACAGAAATGAT | FAM-CATGCATCAAAGATATGAGA-MGB |
| AI025829 | TGTTTTTCAGTTGCTGCACTTTTT | GCATATTCCAGCAATTACCTTTGA | FAM-TTTAATCTTGCTCAGTCCC-MGB | |
| AF090916 | TGGCAATATCCTTTTCTCTGATTTT | GGCCTTGGTTGCCCAAA | FAM-AAAGTTAGGCTGAGTGCAGT-MGB | |
| N63709 | LIN7C | CCTCTGCCAACAATCTGGTTTT | CCATACCTGGAATAACCTTTGAAGA | FAM-ATTGTTGTCTAAAGTTTGCTAGTAG-MGB |
| AL043021 | WDR90 | GCCTGGAGCAAGCTGTTGTAA | CAAAAGGGCAACAGGTATGAAAG | FAM-TTTGGCGCCCTGTGAA-MGB |
| NM_002555 | SLC22A18 | TTTGGCGTCCCCGTCTT | GGACCAGGAGGACAAGGGTATT | FAM-CACGTGCAGGTTGCTA-MGB |
| NM_018129 | PNPO | ATCACACCTGCCTGAGAAGGA | CCTGACGGACTGGGAATAAAAA | FAM-TGGGCTGTCACTAGGA-MGB |
| NM_005207 | CRKL | TTGAGGCCATGGCGAGAT | GCAGCTAAGCCACTGCTTTGT | FAM-CTGCATGTTTGCTGTTC-MGB |
The probes contain a 6-carboxy-FAM label at the 5′end of the gene and a MGB and nonfluorescent quencher at the 3′end. FAM, fluorescein phosphoramidite; MGB, minor groove binder.
Allocated treatments
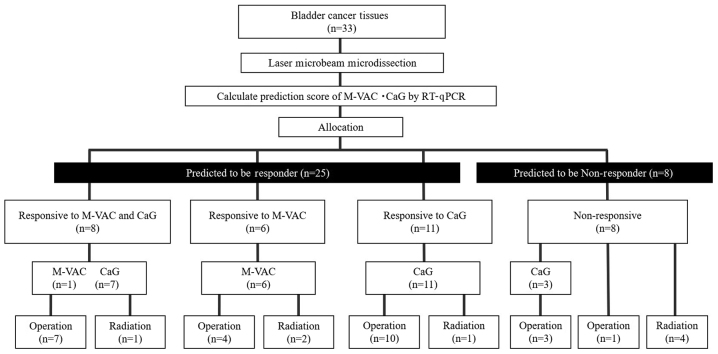
Based on the results of the PS and patient wishes, patients were allocated to receive one of the four treatments: M-VAC, CaG, surgery, or radiation therapy (Fig. 1). Patients who were positive PS for M-VAC or CaG were given two or three 28-day cycles of M-VAC or 21-day cycles of CaG as previously described, respectively (2–4). They underwent surgery consisting of cystectomy or trans-urethral resection of the bladder tumor (TUR-BT), radiation therapy, or supportive care according to the NAC outcome and performance status of each patient. Patients allocated to surgery underwent radical cystectomy and ileal conduit formation, cutaneous ureterostomy, or complete TUR-BT performed within 40 days post-biopsy or post-chemotherapy. Radical cystectomy included internal, external iliac, and obturator pelvic lymph node dissection. Radiotherapy was administered as intensity-modulated radiation therapy, aiming at delivering approximately 66 Gy to the bladder and pelvic nodes.
Figure 1.
Flow chart of methods. M-VAC, methotrexate, vinblastine, doxorubicin plus cisplatin; CaG, carboplatin plus gemcitabine; PCR, polymerase chain reaction; PS, prediction score.
Follow-up
Post-primary and/or secondary treatment follow-up included evaluation of blood count, blood chemistry (particularly for kidney function), urinary cytology, and CT scans of the chest, abdomen, and pelvis every three months for the first two years and at six-month intervals for the next three years until recurrence or according to clinician discretion. Patients who relapsed received evidence-based treatments or best supportive care according to clinician assessment.
Statistical analysis
We calculated positive predictive accuracies and negative predictive accuracies for cases with selective NAC in our study. The results of positive predictive accuracies of M-VAC (PPAM-VAC), negative predictive accuracies of M-VAC (NPAM-VAC) and positive predictive accuracies of CaG (PPACaG), negative predictive accuracies of CaG (NPACaG) therapies were calculated as a function of the patients who received selective NAC according to the results of the respective prediction systems. In terms of clinical efficacy, to decrease selection bias as much as possible, we compared the intention-to-treat proportion of patients achieving significant tumor shrinkage who received each of the two regimens with that of historical controls (2–4). In the NAC group, we categorized patients into two groups according to NAC response: ‘Responders’ who achieved significant tumor shrinkage (>60%), and ‘non-responders’ (≤60%) by the MRI or CT images. Based on their response and prediction results, patients were each evaluated as either ‘accurate’ or ‘inaccurate’ to the prediction system. Moreover, we did not only compare the overall survivals between the positive PS group and negative PS group but this prospective and historical control, respectively. Data were analyzed using JMP® 10 (SAS Institute Inc., Cary, NC, USA) statistical software. The association between this study and the historical control study were analyzed using t-tests and χ2 tests. Kaplan-Meier survival curves were plotted, and the significance of differences between survival curves was determined using the log-rank test. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
From March 2011 to July 2013, 33 MIBC patients (10 women and 23 men; median age, 70 years; age range, 46–78 years) were enrolled in our study. Patient characteristics are listed in Table II including 76 historical control cases (2–4). Among 76 cases, 39 patients in the M-VAC group (nine women and 30 men; median age, 66; range, 53–77 years) were enrolled into study between July 2002 and August 2004 and 37 patients in the CaG group (six women and 31 men; median age, 67; range, 52–78 years) were enrolled between May 2008 and April 2010. No significant differences were detected in age, sex and clinical T(cT) stage between the present cohort of patients and those of the historical control group (Table II).
Table II.
Patient characteristics.
| Historical control study | |||||
|---|---|---|---|---|---|
| Characteristic | The present study (n=33) | Total (n=76) | CaG (n=37) | M-VAC (n=39) | P-value |
| Age, years | 0.31 | ||||
| Median | 70 | 67 | 67 | 66 | |
| Range | 46–78 | 52–78 | 52–78 | 53–77 | |
| Sex, n (%) | 0.23 | ||||
| Male | 23 (70) | 61 (90) | 30 (88) | 31 (91) | |
| Female | 10 (30) | 7 (10) | 4 (12) | 3 (9) | |
| Clinical T stage, n (%) | 0.14 | ||||
| cT2 | 17 (52) | 24 (32) | 16 (43) | 8 (21) | |
| cT3 | 14 (42) | 45 (59) | 15 (41) | 30 (77) | |
| cT4 | 2 (6) | 7 (9) | 6 (16) | 1 (3) | |
| The first therapy, n (%) | 0.0003 | ||||
| M-VAC | 7 (21) | 39 (51) | – | 39 (100) | |
| CaG | 21 (64) | 37 (49) | 37 (100) | – | |
| Surgery | 1 (3) | 0 | 0 | 0 | |
| Radiation | 4 (12) | 0 | 0 | 0 | |
| The second therapy, n (%) | 28 (85) | 0.25 | |||
| M-VAC surgery | 5 (15) | 28 (37) | – | 28 (72) | – |
| M-VAC radiation | 2 (6) | 11 (14) | – | 11 (28) | |
| CaG surgery | 19 (58) | 30 (39) | 30 (81) | – | |
| CaG radiation | 2 (6) | 7 (10) | 7 (19) | – | |
M-VAC, methotrexate, vinblastine, doxorubicin plus cisplatin; M-VAC, methotrexate, vinblastine, doxorubicin plus cisplatin; CaG, carboplatin plus gemcitabine.
Allocated treatment
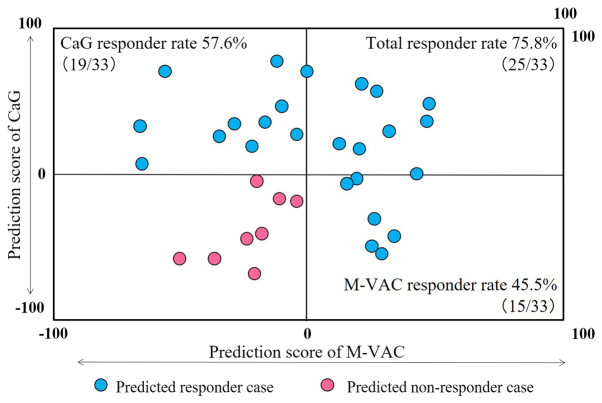
PS analysis of all 33 cases was completed successfully. The distribution of cases according to predicted responses to M-VAC or CaG therapy is summarized in a scatter plot of the PSs in Fig. 2. No correlation was detected between M-VAC and CaG PSs (Pearson's correlation coefficient; r=0.063). Among the 33 patients analyzed by each prediction system, nine were positive PS to both M-VAC and CaG therapies (upper right in Fig. 2), six were positive PS to only M-VAC therapy (lower right in Fig. 2), 10 were positive PS to only CaG therapy (upper left in Fig. 2), and eight were negative PS to both therapies (lower left in Fig. 2). Therefore, in all 33 MIBC patients, 25 cases were positive PS and eight were negative PS (Fig. 1). Among 25 responder cases, seven were allocated to M-VAC, 21 to CaG, one to surgery and four to radiation according to PSs and patient wishes (Fig. 1). Five patients who received surgery or radiation therapy declined NAC (Fig. 1).
Figure 2.
Scatter plot of two PSs for each 33 patients. The horizontal rows represent M-VAC PS and the vertical columns represent CaG PS. Each case is plotted corresponded to M-VAC and CaG PSs. At least either M-VAC or CaG PS would be greater than zero; the cases plotted with blue represent positive PS cases, the cases plotted with red represent negative PS cases. M-VAC, methotrexate, vinblastine, doxorubicin plus cisplatin; CaG, carboplatin plus gemcitabine; PS, prediction score.
The accuracy of prediction systems for clinical response to NAC
Twenty-eight of the 33 patients received NAC in our prospective study (Fig. 1). Among of 15 patients who were positive PS in M-VAC scoring system, 7 received M-VAC NAC, 6 out of 7 patients clinically responded. No patient with a negative PS received M-VAC. Therefore, PPAM-VAC and NPAM-VAC were 85.7% (6/7) and no data (no cases). In contrast, among of the 19 cases showing positive PS in CaG scoring system, 18 cases received CaG NAC, 16 out of 18 patients clinically responded. Three patients showing negative PS in CaG scoring system received CaG NAC (Fig. 1). Two had incomplete responses to CaG chemotherapy. The remaining negative PS patient was a non-responder. PPACaG and NPACaG were 88.9% (16/18) and 33.3% (1/3). Therefore, in this prospective study, the total predictive accuracy of a combination of PPAM-VAC and PPACaG with NPACaG was 82.1% (23/28). On the other hand, among of the test cases in the historical control study, the PPAM-VAC and NPAM-VAC, and PPACaG and NPACaG of test cases were 87.5% (14/16) and 100% (5/5), and 100% (10/10) and 88.9% (8/9), respectively (2–4). Therefore, the predictive accuracy of a combination of PPAM-VAC, PPACaG, NPAM-VAC, and NPACaG was 92.5% (37/40) (2–4). Based on these results, the predictive accuracies of a combination of PPAM-VAC, PPACaG, NPAM-VAC, and NPACaG in the prospective and historical control study were 82.1% (23/28) and 92.5% (37/40), respectively, which were not statistically significant (P=0.25; Table III).
Table III.
Accuracy of predicted clinical response in NAC cases.
| Clinical response | The present study % (n=33) | Historical control study % (n=76: M-VAC, 39; CaG, 37) | Odds ratio | 95% CI | P-value |
|---|---|---|---|---|---|
| Accuracy of the prediction system | 82.1 (23/28a) | 92.5 (37/40b) | 0.37 | 0.08 to 1.71 | 0.259 |
| The rate of predicted to be responder | 75.8 (25/33) | 57.9 (44/76) | 2.27 | 0.91 to 5.69 | 0.087 |
| Clinical response rate of NAC cases | 88.0 (22/25c) | 56.6 (43/76) | 5.63 | 1.55 to 20.42 | 0.0041d |
| The rate of surgery after NAC | 85.7 (24/28) | 75.0 (57/76) | 2.00 | 0.62 to 6.50 | 0.296 |
| The rate of downstaged (pT1≤) | 54.2 (13/24) | 63.2 (36/57) | 0.69 | 0.26 to 1.81 | 0.466 |
| The rate of pT0 | 4.2 (1/24) | 14.0 (8/57) | 0.27 | 0.03 to 2.26 | 0.268 |
Excluding
5 patients who declined NAC
36 (M-VAC 18, CaG 18) learning cases
3 NAC cases with negative PSs.
P<0.01. NAC, neoadjuvant chemotherapy; CI, confidence intervals; M-VAC, methotrexate, vinblastine, doxorubicin plus cisplatin.
The proportion of patients with positive PS for each M-VAC and CaG was 45.5% (15/33) and 57.6% (19/33), respectively. Moreover, 75.8% (25/33) of patients could be expected to respond to at least one of these two regimens by applying our two prediction systems (Fig. 2). In contrast, among of our historical control study, 64.1% (25/39) cases using the M-VAC scoring system and 51.4% (19/37) using the CaG scoring system were positive PS cases (2–4). Therefore, combining the 76 cases in historical control study, the average proportion of patients predicted to be responders was 57.9% (44/76) (75.8 vs. 57.9%; P=0.087; Table II).
The clinical responses to M-VAC and CaG in this prospective study were 85.7% (6/7) and 88.9% (16/18). Therefore, the clinical response to M-VAC and CaG was 88.0% (22/25). On the other hand, in the historical controls, M-VAC and CaG clinical responders were 59.0% (23/39) and 54.1% (20/37), respectively. Therefore, the average of clinical responses of M-VAC and CaG was 56.6% (43/76). Consequently, it was found that the clinical response of the cases who received NAC in the prospective study was significantly higher than that of the historical controls (88.0 vs. 56.6%; P=0.0041; Table II).
Histological response to NAC
The number of the surgical cases in the prospective and in the historical control study in the neoadjuvant setting were 85.7% (24/28) and 75.0% (57/76), respectively (P=0.296; Table II). In the former, 13 of 24 patients who received surgery were downstaged, eight had stable disease, and three were upgraded. Especially pT0 case was only one case (1/24) in this study. None of three negative PS cases accomplished downstaged. Among the four positive PS cases in which surgery was unable to be performed, two had disease progression (M-VAC, 1; CaG, 1), one was due to interstitial pneumonia (CaG, 1) and one refused surgery (M-VAC, 1) (Fig. 1). In the historical control group, 36 of 57 cases were downstaged, 18 had stable disease, and 3 were upgraded. Moreover, pT0 cases were eight cases (8/57) in historical controls. No significant difference was detected in the proportion both of downstaged and pT0 cases between our prospective study (54.2% (13/24); 4.2% (1/24)) and historical control study [63.2% (36/57); 14.0% (8/57)] (54.2 vs. 63.2%; P=0.466; 4.2 vs. 14.0%; P=0.268; Table II).
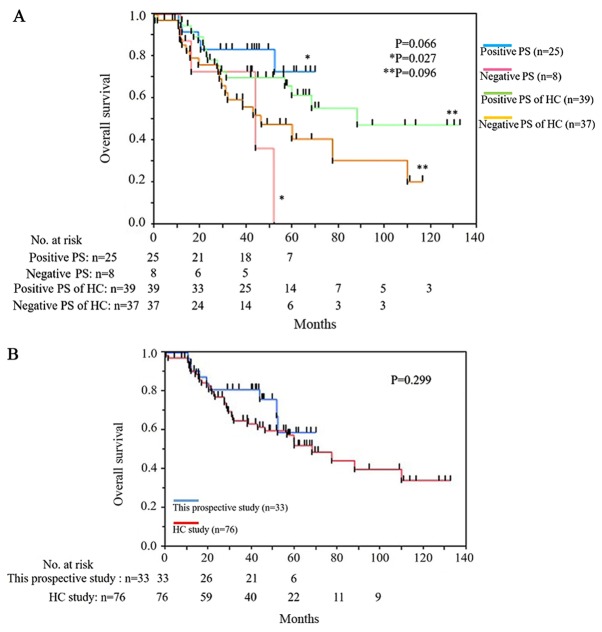
Overall survival
The median follow-up period of the 33 patients in this prospective study was 40.0 months in the present cohort. During the follow-up period, nine patients died: Eight died of bladder cancer and one died of small cell lung cancer. Among eight death cases, four with positive PS and four with negative PS. The small cell lung cancer death case was positive PS. The overall survival between 25 cases in the positive PS group and eight cases in the negative PS group was longer in the positive PS group with significant difference (P=0.027; Fig. 3A). However, in the historical control study, there were no statistically significant between the positive and the negative PS group in terms of overall survival (P=0.096; Fig. 3A). The median overall survival of positive PS was not reached and that of negative PS group was 43.5 months in this prospective study (Fig. 3A). The overall survival of this prospective study was superior to that of historical control from 30 to 50 months but not statistically significant (P=0.299; Fig. 3B).
Figure 3.
Overall survival rate. (A) Comparison of positive and negative PS patients, and positive and negative PS of HC patients. (B) Comparison of this prospective study and the HC study. PS, prediction score; HC, historical control.
Discussion
The prediction systems for sensitivity to neoadjuvant M-VAC and CaG were established from genome-wide expression studies using microarray analyses (2–4). We hypothesized that more patients would be predicted to be responders to the NACs and actual respond to them unless the two prediction systems receive little interference each other. Therefore, this prospective study was designed to investigate whether the combination of two prediction scoring systems lead to increasing the number of responders to MIBC treatments. The proportions of predicted to be responder between the present cohort [75.8% (25/33)] and the historical control cohort [57.9% (44/76)] did not indicate the statistically significant differences (P=0.087). However, in this prospective study, 22 of 25 (88.0%) patients achieved significant tumor shrinkage when treated with appropriate NAC based on the results of the prediction systems; this rate was significantly higher than that of single regimen treatment 56.6% (43/76); (88.0 vs. 56.6%; P=0.0041). These results suggest that each patient may have a different profile for sensitivity to M-VAC and CaG, even in our prospective study as well as previous reports (10–13). The accuracies of predicting system between this prospective study and the historical controls were not statistically significant. (82.1% (23/28) vs. 92.5% (37/40); P=0.259).
In terms of invasion to the patients, predicting system requires the patients only to receiving cold biopsy with histopathologic examination. Therefore, it is good point that there would be minimal invasiveness to patients. Moreover, it does not influence several other prediction systems concerning prognostic factors (14,15).
We did not adopt a combination with gemcitabine plus cisplatin (GC) but a CaG regimen because carboplatin causes less damage than cisplatin in renal function, and because the non-coincidence of drugs between CaG and M-VAC groups would provide a greater chance for each patient to receive the most promising chemotherapy. Indeed, GC chemotherapy is the gold standard for advanced bladder cancer (16) and has been reported that GC NAC was comparable to M-VAC in terms of the pT0 rate in NAC setting (17–20). However, Dogliotti et al (21) reported in a randomized study comparing GC vs. CaG for advanced urothelial cancer that the GC group had a better prognosis, but there were no statistically significant differences in overall survival. Other report suggested that CaG regimen can be considered a reasonable in the NAC setting in especially for cisplatin unfit case (22,23).
Several clinical reports showed that patients who achieve pT0 have a good prognosis; (24–26) however, in our prospective study, only a very small number of cases achieved free of residual disease (pT0) (only 1 case of the 33 cases received CaG NAC). Our previous results were reported that the incidence of pT0 cases was 6% in the M-VAC group and 15% in the CaG group (23). The reason could be that our study showed such a low pT0, because no patient had undergone TUR-BT before NAC so as to investigate the chemo-sensitivity of each case. Interestingly, similar to our results, Scattoni et al (27) reported that the incidence of pT0 was 9% in patients who were treated with platinum-based chemotherapy without TUR-BT. In the SWOG-S8710 and JCOG0209 trials in which TUR-BT was performed for every case, the proportions of patients who did not receive chemotherapy and achieved pT0 were 11.7 and 9.4%, respectively (24,28). Based on these results, we estimate that the effects of chemotherapy combined with TUR-BT would have not only an additive effect, but also a synergistic effect.
We analyzed the overall survivals of the patients between positive PS and negative PS, patients with positive PS showed significant longer overall survival than patients with negative PS (P=0.027). Among of the eight negative PS patients, the number of deaths in surgery and radiotherapy was two out of four cases during followed up period equally, the proportion of negative PS patients less received NAC and cystectomy than that of positive PS patients. Because each patient received several different treatments, the comparison with cohorts would not be accurate. But the patients with positive PS in prospective study were better prognosis than that of patients in retrospective study. There is possibility that the patients in this prospective study had the more opportunity to select regimens that could be expected to be effective than the patients in the retrospective study. The slightly better OS in the prospective study than retrospective study would suggest the allocating effect of our prediction systems. In the future, we would have to choose the alternative treatment instead of surgery or radiation therapy to negative PS patients.
As for other limitations of our prospective study, when one regimen was performed for a predicted responder, the other regimen, regardless of the prediction result, was not used. Therefore, selection bias is present for NPAM-VAC and NPACaG. Moreover, possible explanations include that this small study be unable to adequately stratify patients. Especially, the numbers of negative PS patients were too small to calculate the accuracies and efficacies correctly. The tumor shrinkage cut-off rate was set at 60% because, in the M-VAC retrospective study, this shrinkage rate most clearly discriminated good from poor prognoses in terms of overall survival (data not published). Therefore, a shrinkage rate cut-off line of 60% was adopted in the CaG retrospective study (4). However, RECIST classification is widely used clinically as a method of evaluating therapeutic effect. Though our prediction system is not able to apply to the RECIST criteria, we tried to check the coefficient between shrinking rates of two dimension of this study and RECIST criteria. The result of Pearson's correlation coefficient this prospective test and the historical control test was relatively high (r=0.691).
This is the first report to suggest that the combination of predicting systems for the response to M-VAC and CaG increases clinical efficacy by allowing clinicians to prospectively select the optimal regimen based on the result of prediction system for each patient. In the future, it should be necessary to investigate of this study in a larger group to adequately stratify patients and to make statistical accuracy. Moreover, future prospective studies of TUR-BT should be performed incorporating the combination of predicting systems for the response to M-VAC and CaG and to examine the effect on survival as a function of chemotherapy regimen.
In conclusion, to the best of our knowledge this study represents the first prospective study predicting chemo-sensitivity for MIBC. These results indicate that the described prediction system can increase treatment efficacy for MIBC patients with minimum invasiveness by proposing the optimal regimen. This ability is clinically applicable as ‘Precision Medicine’; however, larger prospective trials are required.
Acknowledgements
The authors would like to thank Mrs. Kumi Matsuda (Laboratory of Molecular Medicine, Human Genome Center, Institute of Medical Science, The University of Tokyo, Tokyo, Japan) for their assistance with the reverse transcription-polymerase chain reaction experiments and Mrs. Reiko Shinagawa (Iwate Medical University) for the management of clinical tissue.
Glossary
Abbreviations
- MIBC
muscle invasive bladder cancer
- NAC
neoadjuvant chemotherapy
- AC
adjuvant chemotherapy
- M-VAC
methotrexate, vinblastine, doxorubicin plus cisplatin
- CaG
carboplatin plus gemcitabine
- CT
computed tomography
- MRI
magnetic resonance imaging
- RT-qPCR
reverse transcription-quantitative polymerase chain reaction
- PS
prediction score
- TUR-BT
trans-urethral resection of the bladder tumor
- PPAM-VAC
positive predictive accuracies of M-VAC
- NPAM-VAC
negative predictive accuracies of M-VAC
- PPACaG
positive predictive accuracies of CaG
- NPACaG
negative predictive accuracies of CaG
Funding
The present study was supported by Grant-in-Aid for 7th Young Research of The Japanese Urological Association (grant no. 31).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YK performed the experiments, analyzed the experimental data and wrote the manuscript. RT was responsible for calculating the PS of M-VAC. TM, RK and MK performed the patient biopsies, embedded the OCT compound to preserve the RNA, and investigated the chemo efficacies. KI was responsible for summarizing and evaluating the clinical course of the past 76 case series. NY and TS produced the tissue slides. HZ, TK, TF and YN designed the experiments. HZ assisted with the experimental technique, performed data analysis and revised the manuscript. WO designed the experiment, interpreted the data and revised the manuscript.
Ethics approval and consent to participate
The present study was approved by The Ethics Committee of Iwate Medical University (Iwate, Japan; register no. HG H22-14) prior patient recruitment and is registered with the UMIN CTR as UMIN000019902. Written informed consent was obtained from all patients.
Patient consent for publication
Each patient provided written informed consent for the publication of this study.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Abdollah F, Gandaglia G, Thuret R, Schmitges J, Tian Z, Jeldres C, Passoni NM, Briganti A, Shariat SF, Perrotte P, et al. Incidence, survival and mortality rates of stage-specific bladder cancer in United States: A trend analysis. Cancer Epidemiol. 2013;37:219–225. doi: 10.1016/j.canep.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Takata R, Katagiri T, Kanehira M, Tsunoda T, Shuin T, Miki T, Namiki M, Kohri K, Matsushita Y, Fujioka T, Nakamura Y. Predicting response to methotrexate, vinblastine, doxorubicin, and cisplatin neoadjuvant chemotherapy for bladder cancers through genome-wide gene expression profiling. Clin Cancer Res. 2005;11:2625–2636. doi: 10.1158/1078-0432.CCR-04-1988. [DOI] [PubMed] [Google Scholar]
- 3.Kato Y, Zembutsu H, Takata R, Miya F, Tsunoda T, Obara W, Fujioka T, Nakamura Y. Predicting response of bladder cancers to gemcitabine and carboplatin neoadjuvant chemotherapy through genome-wide gene expression profiling. Exp Ther Med. 2011;2:47–56. doi: 10.3892/etm.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takata R, Katagiri T, Kanehira M, Shuin T, Miki T, Namiki M, Kohri K, Tsunoda T, Fujioka T, Nakamura Y. Validation study of the prediction system for clinical response of M-VAC neoadjuvant chemotherapy. Cancer Sci. 2007;98:113–117. doi: 10.1111/j.1349-7006.2006.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitahara O, Furukawa Y, Tanaka T, Kihara C, Ono K, Yanagawa R, Nita ME, Takagi T, Nakamura Y, Tsunoda T. Alterations of gene expression during colorectal carcinogenesis revealed by cDNA microarrays after laser-capture microdissection of tumor tissues and normal epithelia. Cancer Res. 2011;61:3544–3549. [PubMed] [Google Scholar]
- 6.Okabe H, Satoh S, Kato T, Kitahara O, Yanagawa R, Yamaoka Y, Tsunoda T, Furukawa Y, Nakamura Y. Genome-wide analysis of gene expression in human hepatocellular carcinomas using cDNA microarray: Identification of genes involved in viral carcinogenesis and tumor progression. Cancer Res. 2001;61:2129–2137. [PubMed] [Google Scholar]
- 7.Yamanaka Y, Tamari M, Nakahata T, Nakamura Y. Gene expression profiles of human small airway epithelial cells treated with low doses of 14- and 16-membered macrolides. Biochem Biophys Res Commun. 2001;287:198–203. doi: 10.1006/bbrc.2001.5550. [DOI] [PubMed] [Google Scholar]
- 8.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 9.Golub TR, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, Mesirov JP, Coller H, Loh ML, Downing JR, Caligiuri MA, et al. Molecular classification of cancer: Class discovery and class prediction by gene expression monitoring. Science. 1999;286:531–537. doi: 10.1126/science.286.5439.531. [DOI] [PubMed] [Google Scholar]
- 10.Karadimou A, Lianos E, Pectasides D, Dimopoulos MA, Bamias A. Efficacy of methotrexate/vinblastine/doxorubicin cisplatin combination in gemcitabine-pretreated patients with advanced urothelial cancer: A retrospective analysis. Open Access J Urol. 2010;2:193–119. doi: 10.2147/OAJU.S13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han KS, Joung JY, Kim TS, Jeong IG, Seo HK, Chung J, Lee KH. Methotrexate, vinblastine, doxorubicin and cisplatin combination regimen as salvage chemotherapy for patients with advanced or metastatic transitional cell carcinoma after failure of gemcitabine and cisplatin chemotherapy. Br J Cancer. 2008;98:86–90. doi: 10.1038/sj.bjc.6604113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edeline J, Loriot Y, Culine S, Massard C, Albiges L, Blesius A, Escudier B, Fizazi K. Accelerated MVAC chemotherapy in patients with advanced bladder cancer previously treated with a platinum-gemcitabine regimen. Eur J Cancer. 2012;48:1141–1146. doi: 10.1016/j.ejca.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Hoshi S, Ohyama C, Ono K, Takeda A, Yamashita S, Yamato T, Itoh A, Satoh M, Saito S, Okada Y, et al. Gemcitabine plus carboplatin; and gemcitabine, docetaxel, and carboplatin combined chemotherapy regimens in patients with metastatic urothelial carcinoma previously treated with a platinum-based regimen: preliminary report. Int J Clin Oncol. 2004;9:125–129. doi: 10.1007/s10147-003-0379-8. [DOI] [PubMed] [Google Scholar]
- 14.Sarkis AS, Bajorin DF, Reuter VE, Herr HW, Netto G, Zhang ZF, Schultz PK, Cordon-Cardo C, Scher HI. Prognostic value of p53 nuclear overexpression in patients with invasive bladder cancer treated with neoadjuvant MVAC. J Clin Oncol. 1995;13:1384–1390. doi: 10.1200/JCO.1995.13.6.1384. [DOI] [PubMed] [Google Scholar]
- 15.Riester M, Werner L, Bellmunt J, Selvarajah S, Guancial EA, Weir BA, Stack EC, Park RS, O'Brien R, Schutz FA, et al. Integrative analysis of 1q23.3 copy-number gain in metastatic urothelial carcinoma. Clin Cancer Res. 2014;20:1873–1883. doi: 10.1158/1078-0432.CCR-13-0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A, Arning M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 17.Galsky MD, Pal SK, Chowdhury S, Harshman LC, Crabb SJ, Wong YN, Yu EY, Powles T, Moshier EL, Ladoire S, et al. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer. 2015;121:2586–2593. doi: 10.1002/cncr.29387. [DOI] [PubMed] [Google Scholar]
- 18.Yeshchina O, Badalato GM, Wosnitzer MS, Hruby G, Roy Choudhury A, Benson MC, Petrylak DP, McKiernan JM. Relative efficacy of perioperative gemcitabine and cisplatin versus methotrexate, vinblastine, adriamycin, and cisplatin in the management of locally advanced urothelial carcinoma of the bladder. Urology. 2012;79:384–390. doi: 10.1016/j.urology.2011.10.050. [DOI] [PubMed] [Google Scholar]
- 19.Zargar H, Espiritu PN, Fairey AS, Mertens LS, Dinney CP, Mir MC, Krabbe LM, Cookson MS, Jacobsen NE, Gandhi NM, et al. Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2015;67:241–249. doi: 10.1016/j.eururo.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dash A, Pettus JA, IV, Herr HW, Bochner BH, Dalbagni G, Donat SM, Russo P, Boyle MG, Milowsky MI, Bajorin DF. A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: A retrospective experience. Cancer. 2008;113:2471–2477. doi: 10.1002/cncr.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dogliotti L, Cartenì G, Siena S, Bertetto O, Martoni A, Bono A, Amadori D, Onat H, Marini L. Gemcitabine plus cisplatin versus gemcitabine plus carboplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium: Results of a randomized phase 2 trial. Eur Urol. 2007;52:134–141. doi: 10.1016/j.eururo.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 22.Mertens LS, Meijer RP, Kerst JM, Bergman AM, van Tinteren H, van Rhijn BW, Horenblas S. Carboplatin based induction chemotherapy for nonorgan confined bladder cancer-a reasonable alternative for cisplatin unfit patients? J Urol. 2012;188:1108–1113. doi: 10.1016/j.juro.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Iwasaki K, Obara W, Kato Y, Takata R, Tanji S, Fujioka T. Neoadjuvant gemcitabine plus carboplatin for locally advanced bladder cancer. Jpn J Clin Oncol. 2013;43:193–199. doi: 10.1093/jjco/hys213. [DOI] [PubMed] [Google Scholar]
- 24.Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, deVere White RW, Sarosdy MF, Wood DP Jr, Raghavan D, Crawford ED. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 25.Meyer A, Ghandour R, Bergman A, Castaneda C, Wosnitzer M, Hruby G, Benson M, McKiernan J. The natural history of clinically complete responders to neoadjuvant chemotherapy for urothelial carcinoma of the bladder. J Urol. 2014;192:696–701. doi: 10.1016/j.juro.2014.03.078. [DOI] [PubMed] [Google Scholar]
- 26.Petrelli F, Coinu A, Cabiddu M, Ghilardi M, Vavassori I, Barni S. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: A meta-analysis. Eur Urol. 2014;65:350–357. doi: 10.1016/j.eururo.2013.06.049. [DOI] [PubMed] [Google Scholar]
- 27.Scattoni V, Bolognesi A, Cozzarini C, Francesca F, Grasso M, Galli L, Torelli T, Campo B, Villa E, Rigatti P. Neoadjuvant CMV chemotherapy plus radical cystectomy in locally advanced bladder cancer: The impact of pathologic response on long-term results. Tumori. 1996;82:463–469. doi: 10.1177/030089169608200511. [DOI] [PubMed] [Google Scholar]
- 28.Kitamura H, Tsukamoto T, Shibata T, Masumori N, Fujimoto H, Hirao Y, Fujimoto K, Kitamura Y, Tomita Y, Tobisu K, et al. Randomised phase III study of neoadjuvant chemotherapy with methotrexate, doxorubicin, vinblastine and cisplatin followed by radical cystectomy compared with radical cystectomy alone for muscle-invasive bladder cancer: Japan clinical oncology group study JCOG0209. Ann Oncol. 2014;25:1192–1198. doi: 10.1093/annonc/mdu126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.