Abstract
Resistance to 5-fluorouracil (5-FU) is a serious problem in cancer therapy and overcoming it is required in order to improve the efficacy of cancer chemotherapy. Histone deacetylase (HDAC) inhibitors are used in cancer treatments and, recently, it has been reported that HDAC inhibitors can overcome resistance to various anti-cancer drugs in vitro. In the present study, a 5-FU-resistant breast cancer cell line was established, and the effects of HDAC inhibitors in these cells were examined. The 5-FU-resistant cell line MDA-MB-468 (MDA468/FU) was established by continuous exposure of the parental cells to 5-FU. This subline was characterized by high resistance to 5-FU, higher mRNA expression levels of thymidylate synthetase and dihydropyrimidine dehydrogenase (DPD), and lower mRNA expression levels of uridine monophosphate synthetase (UMPS) than the parental cells. Gimeracil, a DPD inhibitor, did not affect the sensitivity of MDA468/FU cells to 5-FU. Oteracil, a UMPS inhibitor, decreased the cytotoxicity of 5-FU in MDA468 cells, but not in MDA468/FU cells. The HDAC inhibitors, valproic acid and suberanilohydroxamic acid sensitized the two cell lines to 5-FU in a concentration-dependent manner. In conclusion, the results of the present study revealed that HDAC inhibitors increase the sensitivity to 5-FU in 5-FU-sensitive and -resistant cells.
Keywords: histone deacetylase inhibitors, 5-fluorouracil, breast cancer, drug resistance, thymidylate synthetase, chemotherapy
Introduction
5-Fluorouracil (5-FU) is one of the most widely used drugs against colon, stomach, and lung cancers. In breast cancers, especially triple-negative breast cancers (negative for estrogen receptor, progesterone receptor, and amplification or overexpression of HER2), chemotherapy including 5-FU therapy is important because these cancers lack the targets required for endocrine therapy or HER2-targeted therapy. However, acquired resistance to 5-FU is often associated with its use. Although the mechanisms of 5-FU resistance have not been fully understood, some reports have highlighted a few, such as overexpression of target enzyme thymidylate synthetase (TS), high metabolism of 5-FU through the overexpression of dihydropyrimidine dehydrogenase (DPD), and increase of the efflux of 5-FU from cancer cells through ATP-binding cassette (ABC) transporters (1–3). However, much is still unknown about this process, and to overcome 5-FU-resistance is necessary to improve the efficacy of chemotherapy.
Histone deacetylase (HDAC) inhibitors, such as suberanilohydroxamic acid (SAHA, also known as vorinostat), have been used in the clinical setting as anti-cancer drugs that inhibit epigenetic regulation by HDACs (4). HDACs catalyze the removal of acetyl groups from lysine residues on histones, leading to a condensed chromatin structure, and suppressing the interaction between DNA and transcriptional activators or repressors (5). In several cancer cells, HDAC inhibitors, alone or in combination with various anti-cancer drugs, arrest cell growth, cell cycle progression, and induce apoptosis (4). In addition, it has been suggested that HDAC inhibitors have the ability to overcome the resistance to some drugs. A study from Rauzan et al (6) has shown that SB939, a HDAC inhibitor, overcomes the resistance to BCR-ABL kinase inhibitors in chronic myeloid leukemia cell lines. Furthermore, a different group has found that the HDAC inhibitor belinostat reverses platinum drug resistance through attenuation of ABCC2 activity (7). Therefore, HDAC inhibitors may be useful drugs to circumvent anti-cancer drug resistance.
Recently, it has been reported that HDAC inhibitors potentiate the cytotoxic effects of 5-FU in colon, gastric, and hepatic cancer cell lines (8–10). However, it is unknown whether HDAC inhibitors sensitize 5-FU-resistant cells to 5-FU. In the present study, we established a 5-FU-resistant breast cancer cells using a triple-negative breast cancer model, MDA-MB-468 (MDA468), and examined the effects of HDAC inhibitors on the resistant cells.
Materials and methods
Chemicals
5-FU was obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Valproic acid (VPA) was purchased from Wako Pure Chemical Co. (Osaka, Japan). Gimeracil was purchased from Cayman Chemical Company (Ann Arbor, MI, USA). Oteracil and SAHA were obtained from Tokyo Chemical Co. (Tokyo, Japan).
Cell culture
The human breast cancer cell line MDA468 was kindly gifted by Dr Tamotsu Sudo, Hyogo Cancer center (Akashi, Japan). MDA468 cells were maintained in Dulbecco's modified Eagle's medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Inc.), 100 U/ml penicillin, and 0.1 mg/ml streptomycin (Nacalai Tesque, Kyoto, Japan) at 37°C under 5% CO2 and 95% air conditions.
Establishment of 5-FU-resistant MDA-MB-468 sublines
MDA468 cells were cultured in the presence of increasing amounts of 5-FU for about one year. The final concentration of 5-FU was 10 µM. The cells were then cloned using the limiting dilution method. We selected a subclone with the same growth ability of the parental cells, and named it MDA468/FU. MDA468/FU cells were maintained in culture medium containing 10 µM of 5-FU.
Growth inhibitory activity assay
Cell survival was measured using the CellQuanti-Blue™ Cell Viability Assay kit (BioAssay Systems, Hayward, CA, USA) and previously described methods (11). Briefly, the cells (1×103 cells/well) were seeded on 96-well plates and cultured for 24 h. The cells were then treated with various concentrations of 5-FU with or without gimeracil or oteracil. Alternatively, in case of co-treatment with 5-FU and VPA or SAHA, VPA or SAHA were added to the medium 24 h before the 5-FU treatment because VPA and SAHA are known to alter gene expression. After a week, the medium in each well was replaced with culture medium containing the CellQuanti-Blue™ reagent (Medium/CellQuanti-Blue™=10:1), and the plates were incubated at 37°C for 5 h in normoxia. The fluorescence intensity of each well was measured at an excitation wavelength of 535 nm and an emission wavelength of 590 nm using a microplate reader (GENios; Tecan, Männedorf, Switzerland). The 50% growth inhibitory concentration (IC50) was calculated according to the sigmoid inhibitory effect model (equation 1 below) using the nonlinear least-squares fitting method (Solver, Microsoft® Excel; Microsoft, Corporation, Redmond, WA, USA).
Where E and Emax represent the surviving fraction (% of control) and the maximum survival, respectively. C and γ are the drug concentration in the culture medium and the sigmoidicity factor, respectively (12).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Cells (4×105 cells/well) were seeded on 24-well plates, cultured for 24 h and treated with VPA (1 or 5 mM) or SAHA (0.5 or 1 µM) for 24 h. Then, total RNA was extracted using RNAzol® RT (cat. no. RN190; Molecular Research Center, Inc., Cincinnati, OH, USA) according to the manufacturer's instructions. The RNA was reverse-transcribed using a ReverTra® Ace qPCR kit (cat. no. FSQ-101; Toyobo) and an i-Cycler instrument (Bio-Rad Laboratories, Inc., Hercules, CA, USA). RT was initiated by 37°C for 15 min followed by 98°C for 5 min, then cooling at 4°C. RT-qPCR was performed using the THUNDERBIRDTM SYBR® qPCR Mix (cat. no. QPS-201; Toyobo) and a LightCycler® Nano System (Roche Diagnostics, Basel, Switzerland). The primer sequences were as follows: Forward 5′-CCCACTCCTCCACCTTTGAC-3′, reverse 5′-TGTTGCTGTAGCCAAATTCGTT-3′ (glyceraldehyde-3-phosphate dehydrogenase, GAPDH, house-keeping gene) (13); forward 5′-AATGATTCGAAGAGCTTTTGAAGC-3′, reverse 5′-GTTCCCCGGATGATTCTGG-3′ (DPD) (14); forward 5′-CAGATTATTCAGGACAGGGAGTT-3′, reverse 5′-CATCAGAGGAAGATCTCTTGGATT-3′ (TS) (15); forward 5′-TAGTGTTTTGGAAACTGTTGAGGTT-3′, reverse 5′-CTTGCCTCCCTGCTCTCTGT-3′ (uridine monophosphate synthetase, UMPS) (16). The thermocycling conditions for PCR amplification were as follows: 95°C for 1 min, 45 cycles of 95°C for 10 sec, and 60°C for 30 sec. GAPDH was used as an internal standard gene and relative gene expression was calculated using the 2−ΔΔCq method (17).
Statistical analyses
Data are presented as the mean ± standard deviation. Comparisons between 2, and 3 or more groups were conducted via Student's unpaired t-tests and repeated one-way analysis of variance followed by Dunnett's post hoc test, respectively. Statistical analysis was performed using SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Growth inhibitory effect of 5-FU in MDA468 and MDA468/FU cells
We first analyzed the survival of parental and 5-FU-resistant MDA468 cells to 5-FU. The cell survival curve of MDA468/FU cells to 5-FU was shifted to the right (higher concentration side) compared with that of MDA468 cells (Fig. 1). The 5-FU IC50 values in MDA468 and MDA468/FU cells were 3.88±0.30 and 92.5±5.2 µM, respectively.
Figure 1.
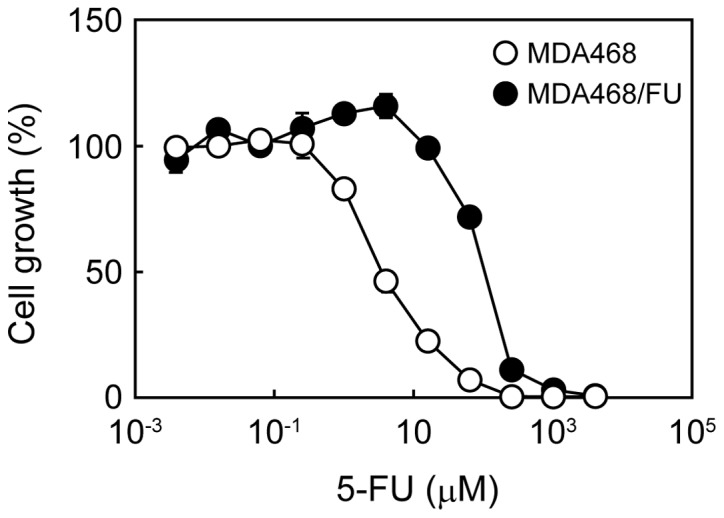
Growth inhibitory effect of 5-FU in MDA468 and MDA468/FU cells. Cells were seeded into 96-well plates and treated with various concentrations of 5-FU for one week. The cell viability was then measured. Each point represents the mean ± standard deviation (n=4). 5-FU, 5-fluorouracil.
DPD, TS, and UMPS mRNA expression levels in MDA468 and MDA468/FU cells
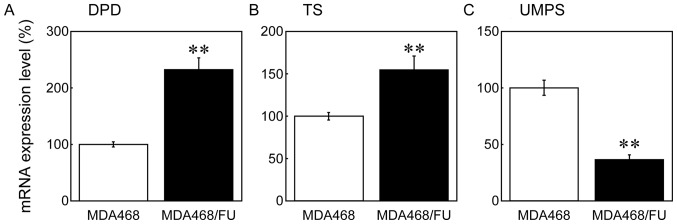
Next, we measured the levels of expression of several enzymes that are involved in 5-FU resistance. In MDA468/FU cells, DPD and TS mRNA expression levels were, respectively, 2- and 1.5-fold higher than those in MDA468 cells (Fig. 2A and B). Contrarily, UMPS mRNA expression levels in MDA468/FU cells were half of those in MDA468 cells (Fig. 2C). The differences found between parental and 5-FU-resistant cells were statistically significant.
Figure 2.
mRNA expression levels of DPD, TS and UMPS in MDA468 and MDA468/FU cells. Cells were seeded into 6-well plates. Following incubation for 48 h, total RNA was extracted and reverse-transcribed. Reverse transcription-quantitative polymerase chain reaction analysis was then performed to detect (A) DPD, (B) TS and (C) UMPS mRNA levels. GAPDH was used as the internal standard. Each bar represents the mean ± standard deviation (n=3). **P<0.01 vs. MDA468. DPD, dihydropyrimidine dehydrogenase; TS, thymidylate synthetase; UMPS, uridine monophosphate synthetase; 5-FU, 5-fluorouracil.
Effect of gimeracil and oteracil on the growth inhibitory effect of 5-FU
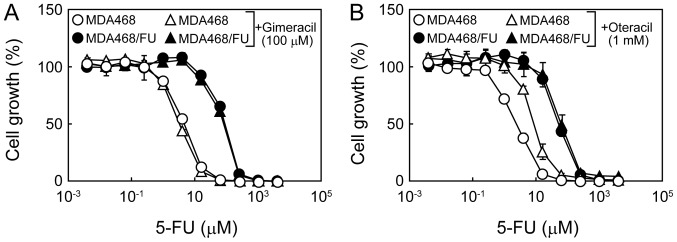
We then investigated whether the inhibition of either DPD or UMPS was related to the sensitivity to 5-FU. Gimeracil did not affect the resistance of MDA468 or MDA468/FU cells to 5-FU (Fig. 3A). Similarly, oteracil did not affect the growth inhibitory effect of 5-FU in MDA468/FU cells (Fig. 3B). Notably, however, the cell survival curve of MDA468 cells to 5-FU was shifted to the right upon co-treatment with 5-FU and oteracil, indicating that oteracil increases the resistance of MDA468 cells to 5-FU (Fig. 3B).
Figure 3.
Effect of gimeracil and oteracil on the growth inhibitory effect of 5-FU in MDA468 and MDA468/FU cells. (A and B) Cells were seeded into 96-well plates and treated with various concentrations of 5-FU with or without (A) gimeracil (100 µM) or (B) oteracil (1 mM) for one week. The cell viability was then determined. Each point represents the mean ± standard deviation (n=4). 5-FU, 5-fluorouracil.
Effect of HDAC inhibitors on the growth inhibitory effect of 5-FU
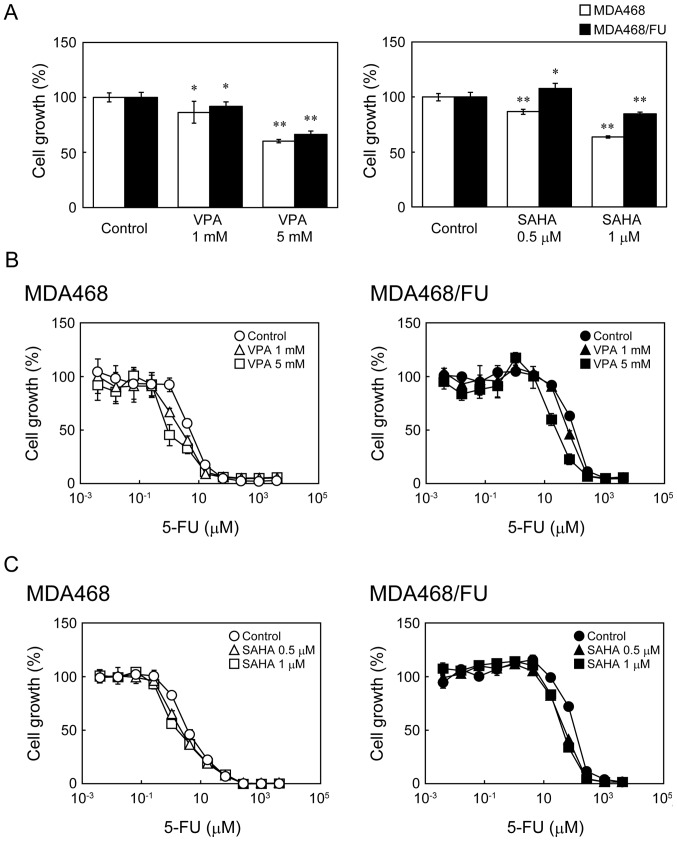
Next, we analyzed the effect of the two HDAC inhibitors VPA and SAHA on the sensitivity of the cells to 5-FU. In MDA468 and MDA468/FU cells, VPA and SAHA inhibited the cell growth (Fig. 4A) and potentiated the growth inhibitory effects of 5-FU in a concentration-dependent manner (Fig. 4B and C). The 5-FU IC50 values in both cell lines were indeed significantly decreased upon co-treatment of VPA (Table I) or SAHA (Table II).
Figure 4.
Effect of histone deacetylase inhibitors on the growth inhibitory effect of 5-FU in MDA468 and MDA468/FU cells. (A) Cells were seeded into 96-well plates and treated with VPA (1 or 5 mM) or SAHA (0.5 or 1 µM) for 8 days. The cell viability was then determined. White and black bars indicate MDA468 and MDA468/FU, respectively. Each bar represents the mean ± standard deviation (n=4). *P<0.05 and **P<0.01 vs. control. (B and C) Cells were seeded into 96-well plates and treated with (B) VPA (1 or 5 mM) or (C) SAHA (0.5 or 1 µM) for 24 h. Then the cells were treated with various concentrations of 5-FU with or without VPA (1 or 5 mM) or SAHA (0.5 or 1 µM) for one week. The cell viability was then determined. Each point represents the mean ± standard deviation (n=4). VPA, valproic acid; SAHA, suberanilohydroxamic acid; 5-FU, 5-fluorouracil.
Table I.
IC50 values for 5-FU in MDA468 and MDA468/FU cells in the presence of VPA.
| MDA468 cells | MDA468/FU cells | |||
|---|---|---|---|---|
| Treatment | IC50 (µM) | R. S. | IC50 (µM) | R. S. |
| Control | 5.19±1.1 | – | 84.2±0.83 | – |
| VPA, mM | ||||
| 1 | 2.89±1.6a | 1.80 | 63.4±0.72b | 1.33 |
| 5 | 1.51±0.5b | 3.44 | 17.6±1.3b | 4.78 |
The results are presented as the mean ± standard deviation (n=4).
P<0.05
P<0.01 vs. control. R.S., Relative sensitivity (the ratio of the IC50 values for 5-FU in the control divided by that of the groups treated with VPA); IC50, 50% growth inhibitory concentration; 5-FU, 5-fluorouracil; VPA, valproic acid.
Table II.
IC50 values for 5-FU in MDA468 and MDA468/FU cells in the presence of SAHA.
| MDA468 cells | MDA468/FU cells | |||
|---|---|---|---|---|
| Treatment | IC50 (µM) | R.S. | IC50 (µM) | R.S. |
| Control | 3.88±0.30 | – | 92.5±5.2 | – |
| SAHA, µM | ||||
| 0.5 | 2.22±0.31a | 1.75 | 42.8±1.6a | 2.16 |
| 1 | 1.76±0.30a | 2.21 | 35.9±2.4a | 2.58 |
The results are presented as the mean ± standard deviation (n=4).
P<0.01 vs. control. R.S., Relative sensitivity (the ratio of the IC50 values for 5-FU in the control divided by that of the groups treated with SAHA); IC50, 50% growth inhibitory concentration; 5-FU, 5-fluorouracil; SAHA, suberanilohydroxamic acid.
Effect of HDAC inhibitors on the mRNA levels of TS and UMPS
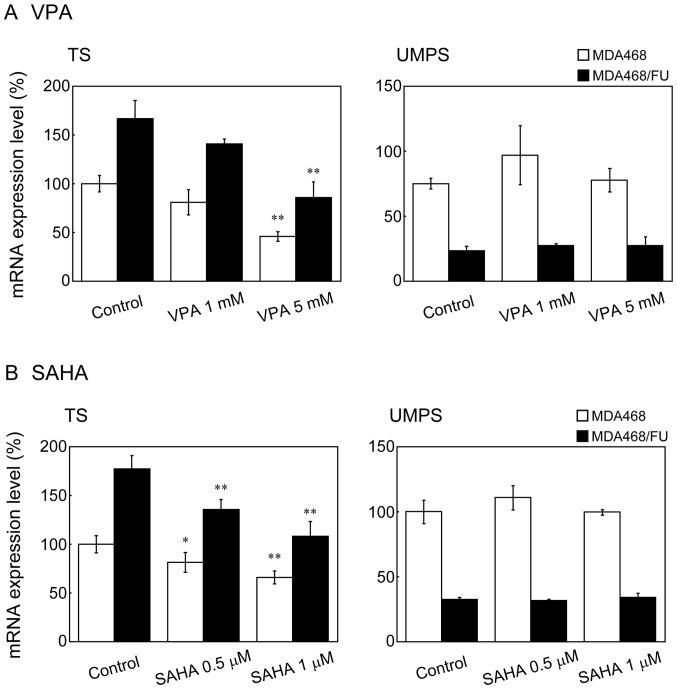
VPA and SAHA decreased TS mRNA expression levels in a concentration dependent manner in both cell lines; however, they did not affect UMPS mRNA levels in either (Fig. 5A and B).
Figure 5.
Effect of histone deacetylase inhibitors on mRNA expression in MDA468 and MDA468/FU cells. (A and B) Cells were seeded into 24-well plates. Following incubation for 24 h, the cells were treated with (A) VPA (1 or 5 mM) or (B) SAHA (0.5 or 1 µM) for 24 h. Total RNA was then extracted and reverse transcription-quantitative polymerase chain reaction analysis was performed. GAPDH was used as the internal standard. White and black bars indicate MDA468 and MDA468/FU, respectively. Each bar represents the mean ± standard deviation (n=3-4). *P<0.05 and **P<0.01 vs. control. VPA, valproic acid; SAHA, suberanilohydroxamic acid; TS, thymidylate synthetase; UMPS, uridine monophosphate synthetase; 5-FU, 5-fluorouracil.
Discussion
In this study, we established a 5-FU-resistant breast cancer cell line. This cell line showed high resistance to 5-FU compared with the parental cells. DPD mRNA expression was higher in MDA468/FU cells than in the parental cells. DPD is important in 5-FU detoxification, and DPD mRNA and protein levels are correlated to 5-FU-sensitivity in esophageal cancer cell lines and patients with colorectal cancer (18,19). However, gimeracil, a DPD inhibitor, did not affect the growth inhibitory effect of 5-FU in MDA468/FU cells. These data suggest that the increase of DPD mRNA is not involved in the mechanisms of resistance to 5-FU in MDA468/FU cells.
UMPS mRNA expression in MDA468/FU cells was significantly lower than that in MDA468 cells. The UMPS inhibitor oteracil attenuated the growth inhibitory effect of 5-FU in MDA468 cells. 5-FU exerts its cytotoxic effect through the inhibition of DNA synthesis and RNA function. 5-FU is phosphorylated by UMPS to form fluorouridine monophosphate, followed by fluorouridine diphosphate conversion to fluorouridine triphosphate which is uptaken in different forms of RNA (20). Tsutani et al (21) reported that UMPS protein levels decreased in a 5-FU-resistant gastric cancer cell line. It has therefore been suggested that the down-regulation of UMPS mRNA is related to the resistance mechanisms in 5-FU resistant cells. We found that oteracil did not affect the growth inhibitory effect of 5-FU in MDA468/FU cells, confirming that one of the mechanisms of 5-FU resistance is the decrease in UMPS levels: In the absence (or low levels of) UMPS, 5-FU does not inhibit the RNA function.
The mRNA levels of TS, an enzyme that targets 5-FU, were higher in MDA468/FU cells than in the parental cell line. Several reports have shown high TS expression in many types of 5-FU-resistant cancer cells (22–24). Therefore, it has been suggested that high TS expression, is another mechanism of 5-FU-resistance. HDAC inhibitors decreased TS mRNA expression in both MDA468 and MDA468/FU cell lines. Similarly, Fazzone et al (8) reported that SAHA decreases TS mRNA and protein expression levels in colon cancer cell lines. Although it remains unclear whether TS mRNA decreases as a consequence of the histone modification by HDAC inhibitors in MDA468 and MDA468/FU, our data confirm that the downregulation of TS expression by HDAC inhibitors is useful for increasing the cytotoxic effect of 5-FU in both 5-FU-sensitive and -resistant cancers.
Pratt et al (3) reported that efflux of 5-FU by ABCC5 was related to 5-FU-resistance using ABCC5-overexpressing human embryonic kidney cells. Takara et al (25) reported that HeLa cells resistant to SN-38, an active metabolite of irinotecan, showed variation in the expression of some mRNAs, including those of glutathione-related enzymes and organic anion transporter, compared with that in parental cells. These studies suggest resistant-related alternation to the gene expression profile of drug-resistant cancer cells. For a better understanding of 5-FU-resistance, further studies are needed to evaluate the expression of various genes in the resistant cells.
In conclusion, the low UMPS expression and high TS expression are related to the resistance mechanisms in 5-FU-resistant cells, and we suggest that HDAC inhibitors increase the sensitivity to 5-FU in both 5-FU-sensitive and -resistant cells. Therefore, future studies might be able to adequately utilized HDAC inhibitors, such as SAHA or VPA, with 5-FU chemotherapy.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by a grant from Kyoto Pharmaceutical University Fund for the Promotion of Scientific Research (grant no. 16-7) and a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT)-Supported Program for the Strategic Research Foundation at Private Universities, 2013–2017 (grant no. S1311035).
Availability of data and materials
All data analyzed during this study are included in this published article.
Authors' contributions
TM, MT and KN conceived and designed the research. TM, AS, MM, ST, SY, AW, ToT, TaT, and AY conducted the experiments and analyzed the data. MT and KN gave experimental advice and assisted with the interpretation of the results. TM wrote the manuscript. All authors approved the final paper.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Habara K, Ajiki T, Kamigaki T, Nakamura T, Kuroda Y. High expression of thymidylate synthase leads to resistance to 5-fluorouracil in biliary tract carcinoma in vitro. Jpn J Cancer Res. 2001;92:1127–1132. doi: 10.1111/j.1349-7006.2001.tb01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yasumatsu R, Nakashima T, Uryu H, Masuda M, Hirakawa N, Shiratsuchi H, Tomita K, Fukushima M, Komune S. The role of dihydropyrimidine dehydrogenase expression in resistance to 5-fluorouracil in head and neck squamous cell carcinoma cells. Oral Oncol. 2009;45:141–147. doi: 10.1016/j.oraloncology.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Pratt S, Shepard RL, Kandasamy RA, Johnston PA, Perry W, III, Dantzig AH. The multidrug resistance protein 5 (ABCC5) confers resistance to 5-fluorouracil and transports its monophosphorylated metabolites. Mol Cancer Ther. 2005;4:855–863. doi: 10.1158/1535-7163.MCT-04-0291. [DOI] [PubMed] [Google Scholar]
- 4.Eckschlager T, Plch J, Stiborova M, Hrabeta J. Histone deacetylase inhibitors as anticancer drugs. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18071414. pii: E1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khangura RK, Bali A, Jaggi AS, Singh N. Histone acetylation and histone deacetylation in neuropathic pain: An unresolved puzzle? Eur J Pharmacol. 2017;795:36–42. doi: 10.1016/j.ejphar.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Rauzan M, Chuah CT, Ko TK, Ong ST. The HDAC inhibitor SB939 overcomes resistance to BCR-ABL kinase inhibitors conferred by the BIM deletion polymorphism in chronic myeloid leukemia. PLoS One. 2017;12:e0174107. doi: 10.1371/journal.pone.0174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.To KK, Tong WS, Fu LW. Reversal of platinum drug resistance by the histone deacetylase inhibitor belinostat. Lung Cancer. 2017;103:58–65. doi: 10.1016/j.lungcan.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Fazzone W, Wilson PM, Labonte MJ, Lenz HJ, Ladner RD. Histone deacetylase inhibitors suppress thymidylate synthase gene expression and synergize with the fluoropyrimidines in colon cancer cells. Int J Cancer. 2009;125:463–473. doi: 10.1002/ijc.24403. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Yashiro M, Ren J, Hirakawa K. Histone deacetylase inhibitor, trichostatin A, increases the chemosensitivity of anticancer drugs in gastric cancer cell lines. Oncol Rep. 2006;16:563–568. [PubMed] [Google Scholar]
- 10.Ocker M, Alajati A, Ganslmayer M, Zopf S, Lüders M, Neureiter D, Hahn EG, Schuppan D, Herold C. The histone-deacetylase inhibitor SAHA potentiates proapoptotic effects of 5-fluorouracil and irinotecan in hepatoma cells. J Cancer Res Clin Oncol. 2005;131:385–394. doi: 10.1007/s00432-004-0664-6. [DOI] [PubMed] [Google Scholar]
- 11.Minegaki T, Fukushima S, Morioka C, Takanashi H, Uno J, Tsuji S, Yamamoto S, Watanabe A, Tsujimoto M, Nishiguchi K. Effects of bisphosphonates on human esophageal squamous cell carcinoma cell survival. Dis Esophagus. 2016;29:656–662. doi: 10.1111/dote.12370. [DOI] [PubMed] [Google Scholar]
- 12.Takara K, Sakaeda T, Yagami T, Kobayashi H, Ohmoto N, Horinouchi M, Nishiguchi K, Okumura K. Cytotoxic effects of 27 anticancer drugs in HeLa and MDR1-overexpressing derivative cell lines. Biol Pharm Bull. 2002;25:771–778. doi: 10.1248/bpb.25.771. [DOI] [PubMed] [Google Scholar]
- 13.Catalano S, Giordano C, Panza S, Chemi F, Bonofiglio D, Lanzino M, Rizza P, Romeo F, Fuqua SA, Maggiolini M, et al. Tamoxifen through GPER upregulates aromatase expression: A novel mechanism sustaining tamoxifen-resistant breast cancer cell growth. Breast Cancer Res Treat. 2014;146:273–285. doi: 10.1007/s10549-014-3017-4. [DOI] [PubMed] [Google Scholar]
- 14.Yoshinare K, Kubota T, Watanabe M, Wada N, Nishibori H, Hasegawa H, Kitajima M, Takechi T, Fukushima M. Gene expression in colorectal cancer and in vitro chemosensitivity to 5-fluorouracil: A study of 88 surgical specimens. Cancer Sci. 2003;94:633–638. doi: 10.1111/j.1349-7006.2003.tb01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsubara J, Nishina T, Yamada Y, Moriwaki T, Shimoda T, Kajiwara T, Nakajima TE, Kato K, Hamaguchi T, Shimada Y, et al. Impacts of excision repair cross-complementing gene 1 (ERCC1), dihydropyrimidine dehydrogenase, and epidermal growth factor receptor on the outcomes of patients with advanced gastric cancer. Br J Cancer. 2008;98:832–839. doi: 10.1038/sj.bjc.6604211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gustavsson B, Kaiser C, Carlsson G, Wettergren Y, Odin E, Lindskog EB, Niyikiza C, Ma D. Molecular determinants of efficacy for 5-FU-based treatments in advanced colorectal cancer: mRNA expression for 18 chemotherapy-related genes. Int J Cancer. 2009;124:1220–1226. doi: 10.1002/ijc.23852. [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Minegaki T, Takara K, Hamaguchi R, Tsujimoto M, Nishiguchi K. Factors affecting the sensitivity of human-derived esophageal carcinoma cell lines to 5-fluorouracil and cisplatin. Oncol Lett. 2013;5:427–434. doi: 10.3892/ol.2012.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciaparrone M, Quirino M, Schinzari G, Zannoni G, Corsi DC, Vecchio FM, Cassano A, La Torre G, Barone C. Predictive role of thymidylate synthase, dihydropyrimidine dehydrogenase and thymidine phosphorylase expression in colorectal cancer patients receiving adjuvant 5-fluorouracil. Oncology. 2006;70:366–377. doi: 10.1159/000098110. [DOI] [PubMed] [Google Scholar]
- 20.Miura K, Kinouchi M, Ishida K, Fujibuchi W, Naitoh T, Ogawa H, Ando T, Yazaki N, Watanabe K, Haneda S, et al. 5-fu metabolism in cancer and orally-administrable 5-fu drugs. Cancers (Basel) 2010;2:1717–1730. doi: 10.3390/cancers2031717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsutani Y, Yoshida K, Sanada Y, Wada Y, Konishi K, Fukushima M, Okada M. Decreased orotate phosphoribosyltransferase activity produces 5-fluorouracil resistance in a human gastric cancer cell line. Oncol Rep. 2008;20:1545–1551. [PubMed] [Google Scholar]
- 22.Oguri T, Achiwa H, Bessho Y, Muramatsu H, Maeda H, Niimi T, Sato S, Ueda R. The role of thymidylate synthase and dihydropyrimidine dehydrogenase in resistance to 5-fluorouracil in human lung cancer cells. Lung Cancer. 2005;49:345–351. doi: 10.1016/j.lungcan.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Saga Y, Suzuki M, Mizukami H, Kohno T, Takei Y, Fukushima M, Ozawa K. Overexpression of thymidylate synthase mediates desensitization for 5-fluorouracil of tumor cells. Int J Cancer. 2003;106:324–326. doi: 10.1002/ijc.11221. [DOI] [PubMed] [Google Scholar]
- 24.Wang W, McLeod HL, Cassidy J, Collie-Duguid ES. Mechanisms of acquired chemoresistance to 5-fluorouracil and tomudex: Thymidylate synthase dependent and independent networks. Cancer Chemother Pharmacol. 2007;59:839–845. doi: 10.1007/s00280-006-0384-5. [DOI] [PubMed] [Google Scholar]
- 25.Takara K, Kitada N, Yoshikawa E, Yamamoto K, Horibe S, Sakaeda T, Nishiguchi K, Ohnishi N, Yokoyama T. Molecular changes to HeLa cells on continuous exposure to SN-38, an active metabolite of irinotecan hydrochloride. Cancer Lett. 2009;278:88–96. doi: 10.1016/j.canlet.2008.12.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed during this study are included in this published article.