Abstract
Carcinogenesis has a multifactorial etiology, and the underlying molecular pathogenesis is still not entirely understood, especially for eye cancers. Primary malignant intraocular neoplasms are relatively rare, but delayed detection and inappropriate management contribute to poor outcomes. Conventional treatment, such as orbital exenteration, chemotherapy, or radiotherapy, alone results in high mortality for many of these malignancies. Recent sequential multimodal therapy with a combination of high-dose chemotherapy, followed by appropriate surgery, radiotherapy, and additional adjuvant chemotherapy has helped dramatically improve management. Transcription factors are proteins that regulate gene expression by modulating the synthesis of mRNA. Since transcription is a dominant control point in the production of many proteins, transcription factors represent key regulators for numerous cellular functions, including proliferation, differentiation, and apoptosis, making them compelling targets for drug development. Natural compounds have been studied for their potential to be potent yet safe chemotherapeutic drugs. Since the ancient times, plant-derived bioactive molecules have been used to treat dreadful diseases like cancer, and several refined pharmaceutics have been developed from these compounds. Understanding targeting mechanisms of oncogenic transcription factors by natural products can add to our oncologic management toolbox. This review summarizes the current findings of natural products in targeting specific oncogenic transcription factors in various types of eye cancer.
Keywords: Eye cancer, Natural products, Oncogenic transcription factors
1. Introduction
Natural products have been used on an empirical basis for healing throughout history. According to the World Health Organization in 2008, more than 80% of the world’s population relies on traditional, ethnobotanical medicines for their primary healthcare needs [1, 2]. Studies of biologically active components from natural products derived from plants, animals, and microbes have led to several advancements in medical therapies. Before the chemist Felix Hoffman at Bayer developed aspirin, the ancient Egyptians and Greeks treated pain with willow leaves and bark, which contain the active component used to derive aspirin [3]. Lately, natural products have received considerable attention for their anticancer activity. As a matter fact, 74.9% of anticancer drugs (excluding vaccines and biologicals) developed between the 1940s and 2010 are naturally derived or inspired [2]. Active components from natural compounds, such as alkaloids, taxanes, and flavonoids have been utilized to develop chemotherapeutic drugs to treat various cancers such as leukemia, breast, prostate, and ovarian cancer [4–9].
While natural compounds are used to treat various cancers or are currently in clinical trials, research regarding the application of natural products to the treatment of ocular cancer is lacking. This is most likely due to the rarity of ocular cancers. Previous epidemiological studies of Western populations suggest that eye cancers account for about 0.2% of cancer diagnoses and less than 0.1% of cancer deaths [10, 11]. However, while rare, they greatly diminish quality of life and are deadly if left untreated. The most common adult intraocular cancer is uveal melanoma, and it is the most lethal melanoma with survival rates at 50% over a 10-year period [12]. The most common pediatric intraocular cancer is retinoblastoma, and the first-line treatment for Grade I-IV retinoblastoma according to the International Retinoblastoma Staging System (IRSS) is enucleation, leading to irreversible blindness [13].
There is much overlap in the mechanisms underlying the various types of cancers. Overexpression of oncogenes and inactivation of tumor suppressor genes are typically the initiating events in tumor development [14–17]. Some of these genetic and epigenetic events involve genes encoding transcription factors, which bind to specific DNA elements and regulate several gene expression patterns. Three groups of transcription factors are known to play important roles in cancer [18]. The group first recognized is the steroid receptors (e.g. estrogen receptors in breast cancer and androgen receptors in prostate cancer) [19]. The second group identified is resident nuclear proteins, normally activated by serine kinase cascades [20]. And the most recently recognized group is the latent cytoplasmic factors, normally activated by receptor–ligand interaction at the cell surface [21]. Multiple dysregulated genes converge to specific sets of transcription factors, and interference at these transcription factors is highly desirable in drug development [21–24].
Natural products of plants and microbes offer an important and largely unexplored pool for the identification and development of novel drugs. They are exceptionally diverse and can produce a variety of secondary metabolites that have therapeutic functions. It is estimated that only 6% of identified plant species have been systematically investigated pharmacologically [25]. Additionally, microbes, such as endophytic bacteria, are able to biosynthesize some anti-cancer metabolites, hosting a reservoir of potentially therapeutic compounds. The vast microbial diversity has great potential for the discovery of new drug leads. Thus, natural products are an untapped potential that can lead to the development of better, safer and more effective anticancer drugs.
Research regarding natural products for treating ocular cancers is limited. By discussing key ocular oncogenic transcription factors and pathways in eye cancer and the natural agents known to affect these same pathways in other cancers, we hope the consolidated information will be used for future translational research in ocular oncology drug discovery. While some pathways are important in multiple ocular cancers, in this review, we summarize current findings of natural products that target the pathways most pertinent to each different type of eye cancer. Elucidating natural products’ effects on directly or indirectly modulating oncogenic factors may enhance our development of better pharmaceutical scaffolds and new targeted therapies for the management of ocular tumors.
2. Retinoblastoma
Retinoblastoma (RB) is the most common pediatric intraocular malignancy with approximately 8,000 children newly diagnosed worldwide each year [26]. It is most commonly noticed when the tumor blocks the retina’s red light reflex, causing patients to present with leukocoria. The second most common clinical presentation is strabismus [26].
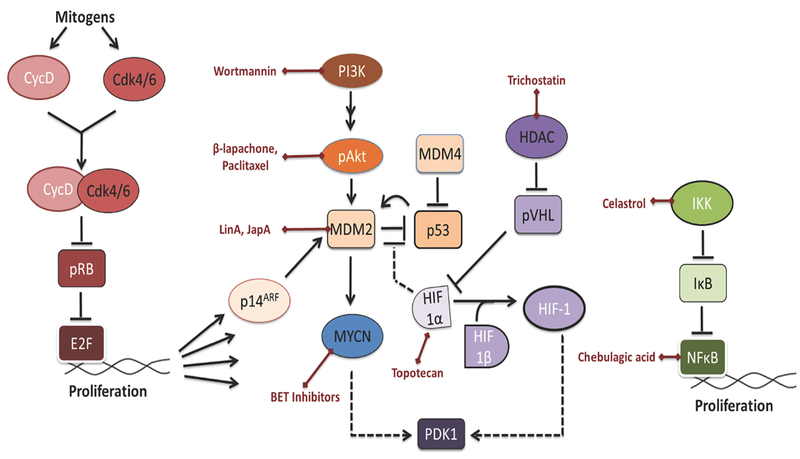
Primary treatment of RB seeks to reduce the risk of metastasis and cancer-related death, eliminate the tumor, salvage the eye, and preserve vision [27]. Enucleation remains the most favored approached globally, but intravenous chemotherapy (carboplatin, etoposide, and vincristine), intra-arterial chemotherapy (melphalan, topotecan, and/or carboplatin), and focal therapy (laser therapy or cryotherapy) are emerging as promising options in RB patients [27, 28]. Different natural products that target signaling pathways involved in retinoblastoma progression have been identified and are discussed below (Figure 2).
Figure 2: Natural products targeting important pathways in retinoblastoma.
Retinoblastoma is characterized by the lack of pRB or the overexpression of MYCN. (A) BET inhibitors target MYCN (blue). (B) Wortmannin, β-lapachone, and paclitaxel target ARF/MDM2-MDMX/p53 (pink). (C) Trichostatin and topotecan target HIF-1 (purple). (D) Celastrol and chebulagic acid target NFκB pathway (green).
2.1. CyclinD-cdk/RB/E2F & MYCN
RB is characterized by the biallelic loss of the retinoblastoma gene RB1, which encodes the cell-cycle regulator protein pRB. In the absence of mitogenic signals, the hypophosphorylated form of pRB binds to the transcription factor E2F and suppresses the expression of cell proliferation genes. As a result, cell cycle halts in the G1 to S phase transition. When the cell is ready to progress, mitogenic signals induce expression of cyclin D. Cyclin D binds with cdk4 or cdk6 and hyperphosphorylates pRB. This inactivation of pRB releases E2F, which then promotes cell proliferation. Anti-proliferative signals are integrated into this pathway at the cyclin-cdk4/6 complex. When DNA is damaged, for instance, Cip/Kip proteins (p21, p27, p57) are expressed and inhibit cyclin-cdk complexes. In RB1−/− cells, E2F is always unbound. Even in the absence of mitogenic signals, cells can express proliferative genes [29, 30]. However, this is not the entire RB story. Retinocytoma is a benign RB1−/− tumor [31], and further studies are needed to understand how it develops malignancy.
RB1+/+ patients can develop RB characterized by high MYCN expression [32]. These cases are associated with early age onset. In neuroblastoma, MYCN is known to target SKP2, and SKP2 degraded p27 [33]. This would result in an inactive pRB and expression of proliferative genes. In RB1+/+ cells, pRB was found to be phosphorylated at S608 and S795, which are key residues for its interaction with E2F, but the MYCN/SKP2/p27 pathway was shown to be inactive [34]. Another mechanism yet to be determined is likely involved.
2.1.a. BET inhibitors
Bromodomain and extraterminal (BET) protein BRD4 acts as a transcriptional coactivator of cell cycle genes by recognizing acetylated lysine residues on histone tails and activating oncogenic chromatin regions, such as MYCN and CDK. BET inhibitors can displace BET bromodomains from chromatin by competitively binding to the acetyl lysine recognition pocket, preventing oncogene activation. Natural agents such as 2,5-diketopiperazines (2,5-DKPs), particularly fumitremorgin B, isolated from the co-cultured Penicillium sp. DT-F29 and Bacillus sp. B31 were found to inhibit BRD4 [35].
Puissant et al. also demonstrated a robust correlation of the sensitivity of bromodomain inhibition to MYCN amplification, where BRD4-mediated inhibition of MYCN impaired growth and induced apoptosis in neuroblastoma [36]. Furthermore, the study showed that MYCN amplification was the top predictive marker of neuroblastoma responsiveness to BRD4 inhibition. Therefore, BRD4 inhibition is a vital target for neuroblastoma therapeutics, and future clinical studies on natural BRD4 inhibitors, such as 2,5-DKPs, could make way to the development of additional impactful neuroblastoma therapies.
Recently, Wu et al. created a mouse model of MYCN amplified RB and studied its response to MYCN inhibition [37]. Downregulation of MYCN expression initially led to cell cycle arrest and partial regression of RB tumors. Over time, the tumors reemerged, suggesting they evolved to overcome their MYCN dependence. In some of the reemerged tumors, there was an amplification of the MYCN target gene Mir-17–92 [37], but further studies are needed to understand its role in RB development. Because MYCN suppression only inhibit RB progression for a short period, co-administration of MYCN inhibitors with another agent is necessary for long-term impact.
2.2. ARF/MDM2-MDMX/p53
It has been proposed that changes in the p53 mechanism, which induces apoptosis, are needed for RB cells to proliferate. While p53 inactivation has been used in murine RB models, no p53 mutations have been characterized in human RB [26]. Instead, there are high expression levels of MDMX (or MDM4) and MDM2, two p53 inhibitors [38]. For cells with an intact p14ARF/MDM2-MDMX/p53 pathway, when RB1 is loss, E2F induces the expression of p14ARF; p14ARF inactivates MDM2, and the cell enters p53-mediated apoptosis [39]. In RB primary cells, p14ARF mRNA level increases while its proteins are undetectable; MDM2, MDMX, and p53 are present [38]. Inhibition of this pathway can slow RB cell proliferation, which shows its potential as a therapeutic target.
2.2.a. Beta-lapachone, Paclitaxel, and Wortmannin
Beta-lapachone, paclitaxel, and wortmannin are natural agents found to interact with ARF/MDM2-MDMX/p53 pathway in human RB Y79 cell cultures [40–42]. Beta-lapachone is a naphthoquinone found in the bark of the Tabebuia impetiginosa; its bioprocessing leads to high concentrations of reactive oxygen species [40]. Paclitaxel is a diterpene taxane compound isolated from the bark extract of Taxus brevifolia; it binds to tubulin and disrupts the dynamic assembly of microtubules, a crucial component in cell division [43]. Wortmannin is a PI3K inhibitor derived from the fungi Penicillium funiculosum. All three compounds decreased levels of phosphorylated Akt (pAkt), which inhibited the phosphorylation and nuclear transport of MDM2 [41].
2.2.b. Lineariifolianoid A and Japonicones A
Qi et al. showed that MDM2 promotes RB cell proliferation through MYCN induction, and depletion of MDM2 severely inhibits growth [44]. Lineariifolianoid A (LinA) and Japonicones A (JapA) are sesuiterpenoid dimers found to reduce MDM2 levels in breast cancer cells by targeting the NFAT-MDM2 pathway [45, 46]. Both compounds were originally isolated from traditional Chinese medicine plants: LinA from Inula lineariifolia and JapA from Inula japonica [46, 47]. Because they inhibit MDM2 activity and RB cells depend on MDM2, LinA and JapA may be potential RB cell inhibitors.
2.3. HIF-1
Another transcription factor active in RB is HIF-1 (hypoxic inducible factor 1), which plays a key role in cellular response to the hypoxic microenvironments found in solid tumors and the vitreous cavity of the eye. It upregulates genes involved in angiogenesis (e.g. VEGF) and metabolism (e.g. PDK1) [48]. For RB, this process is particularly important, as cells originate in the highly-perfused retina but seeds into the hypoxic vitreous humor of the eye. HIF-1 is composed of two subunits: the hypoxia induced HIF-1-alpha and the constitutively expressed HIF-1-beta. Previous studies have reported intricate interactions between p53 and HIF-1-alpha [49]. Knockdown of HIF-1- alpha was shown to reduce proliferation, induce cell cycle arrest, and promote apoptosis in Weri-RB1 cells under hypoxic conditions [50].
2.3.a. Trichostatin and Topotecan
Under normoxic conditions, Von Hippel-Lindau tumor suppressor (pVHL) recognizes the prolyl hydroxylated HIF-1-alpha and signals for the latter’s polyubiquitination and proteosomal degradation. When cells are hypoxic, an increased expression of histone deacetylases (HDAC) downregulates pVHL, increases HIF-1 activity, and promotes a metabolism shift favorable for hypoxia [51, 52]. Trichostatin is a fungistatic antibiotic originally isolated from Streptomyces hygroscopicus [51] and it selectively inhibits the class I and II mammalian HDACs. As a result, cells retain their inefficient metabolism in hypoxia, thereby slowing down RB progression into the vitreous humor.
Camptothecin (CPT) is a cytotoxic quinoline alkaloid isolated from Camptotheca acuminata that inhibits DNA enzyme topoisomerase I (Topo1). Topo1 normally introduces transient breaks in the DNA helix to remove local supercoils during the S-phase of cell cycle [53]. CPT binds to the Topo1-DNA complex and prevents the religation of the nicked DNA ends [53]. The complex blocks the advancing replication fork and results in lethal DNA damage. The camptothecin-analog topotecan (TPT) is currently accepted in the standard intra-arterial chemotherapy treatment for RB. Even for advanced bilateral RB, systemic topotecan when combined with vincristine and carboplatin achieved 78% ocular salvage and 80% preserved vision [54]. TPT is a topoisomerase I inhibitor, but it can also decrease the rate of HIF-1-alpha translation in U251 human glioma cells [52]. This pathway was shown to be independent of DNA damage during replication [52]. In a histological trial analyzing paired biopsies pre- and post- oral TPT treatments, TPT was shown to decrease HIF-1 alpha expression in advanced solid tumors [55].
2.4. Nuclear Factor κB
Nuclear Factor κB (NFκB) is a transcriptional factor normally found to be silent in the cytoplasm until external signal induces the phosphorylation and degradation of the Inhibitor of NFκB (IκB). After translocating into the nucleus, NFκB activates on proliferative genes (e.g. cyclin D1 and myc) and antiapoptotic genes (e.g. Bcl-1 and IAP-1). While NFκB and IκB are unlikely to be found mutated in cancer cells, the pathway is often constitutively active [30, 56] and contributes to tumor progression and drug resistance. In addition to cancer, NFκB has been studied in other contexts including chronic inflammation, autoimmune diseases, and viral infections [57].
2.4.a. Celastrol
Celastrol (tripterine) is isolated from the root extracts of Tripterygium wilfordii (Thunder god vine) and belongs to the family of quinone methides. Celastrol is derived from Celastrus orbiculatus and has broad spectrum antitumor activity [58]. It was found to inhibit IκB kinase (IKK) activity and demonstrates anti-inflammatory and anti-tumor activities in animal models, suggesting its therapeutic potential. However, one of its limitation is its poor solubility in water. To test celastrol’s effectiveness against RB, Li et al. loaded celastrol in PEG-b-PCL nanoparticles and administered them in a xenograft model of SO-RB50 cells in mice [59]. It was demonstrated that nanoparticles containing celastrol in PEG-b-PCL decreased the levels of bcl-2, NFκB p65, and phospho-NFκB p65 indicating its anti-tumor efficacy.
2.4.b. Chebulagic acid
The natural compound Chebulagic acid (CA) is a benzopyran tannin derived from Terminalia chebula. It was originally discovered as an inhibitor of cyclooxygenase, a key thrombosis promoter. In RB cells Y79, chebulagic acid was found to act on three pathways [60]. First, it induces apoptosis by depolarizing the mitochondrial membrane potential, releasing cytochrome c, activating caspase 3, and shifting the bax:bcl2 ratio towards apoptosis. Second, it halts cell cycle by increasing the activity of p27, a CDK inhibitor responsible for repression at the G1 to S phase transition. And third, it inhibits proliferation of retinoblastoma cells by suppressing NFκB [60]. Overall, CA inhibited proliferation of Y79 cells in a dose-dependent manner.
3. Uveal Melanoma
Uveal melanoma (UM) is the most common intraocular malignancy with an incidence rate of 4.3 per million. It originates from neuroectodermal melanocytes in the choroid, ciliary body, and iris, and often metastasizes to the liver [61]. Clinical symptoms are usually nonspecific; painless visual distortions like flashes and floaters. As a result, UM is usually noticed during eye examinations [61].
Genetic expression profiling categorizes UM into two distinct molecular classes – low grade class 1 versus high grade class 2 – based on patterns of downregulated genes on chromosome 3 and upregulated genes on chromosome 8q [62]. Class 2 tumors have higher proliferation rates and epithelial to mesenchymal transition factors. Further analysis associates high PRAME expression with higher metastasis risks in class 1 tumors [63].
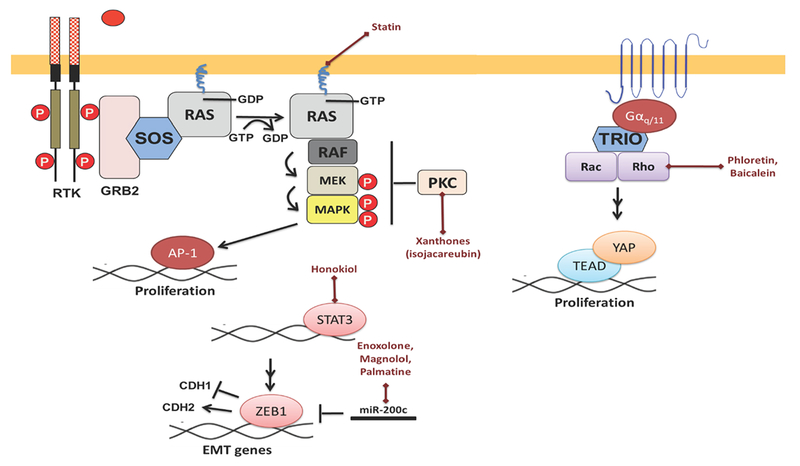
GNAQ and GNA11 are oncogenes found to be mutually exclusively mutated in 83% of all uveal melanomas [64–66]. They encode for alpha subunits in G proteins (Gαq/11) normally activated by serotonin receptors. However, the mutations at residue Q209 and R183 disrupt GTPase activities and render the proteins constitutively active. As a result, the proteins turn on downstream pathways such as MAPK, Rho/Rac/YAP, and PI3K/AKT [67–69]. Various oncogenic transcription factors are targeted by different types of natural products as shown in Figure 3.
Figure 3: Natural products targeting important pathways in uveal melanoma.
UM is characterized by GNAQ/11 mutation in approximately 85% of cases. (A) Xanthones and statin inhibit PKC and MAPK pathways. (B) Phloretin and baicalein target Rho/Rac and YAP/TEAD. (C) Enoxolone, magnolol, and palmatine inhibit ZEB1.
3.1. MAPK and PKC
Mitogen-activated protein kinase (MAPK) pathway involves a sequential phosphorylation cascade of RAF, MEK, and ERK proteins and regulates multiple transcription factors important in cell cycle (e.g. c-myc, CREB, and c-Fos) [30]. When the MEK1/2 inhibitor selumetinib was used against GNAQ-mutated UM cells, cell viability was less in tumor cells compared to wild-type cells [67]. Downregulation of RNA helicase DDX21 and cyclin-dependent kinase regulator CDK5R1 and upregulation of Jun were observed. MAPK pathway activation can be partially or temporarily inhibited by protein kinase C (PKC) inhibitors in UM cells, suggesting PKC induces MAPK activation. Combined treatment of MAPK and PKC inhibitors demonstrated synergistic antitumor effects [70]. Dual inhibitions of PKC/p53-MDM2 and PKC/mTORC1 were also found to regress preclinical models of metastatic UM tumors in a synergistic manner [71].
3.1.a. Xanthones
Since PKC pathways are crucial in cell cycle progression, tumorigenesis and metastasis, natural inhibitors of these pathways have been studied in many cancer cells (e.g. curcumin in RB, as mentioned above) [72–75]. Amongst these inhibitors are xanthones, which are plant-derived natural products with anti-tumor properties [76]. Semisynthetic and completely synthetic analogs of xanthones have also been studied as antitumor agents [77]. They have different levels of affinity and potency towards PKC isoforms. Isojacareubin (ISJ) is an antibacterial isolate from Hypericum japonicum with a xanthone scaffold, and it specifically interacts with aPKC, cPKC, and nPKC in hepatocellular carcinoma (HCC) [76]. ISJ downregulated the MAPK cascade, and overall, it inhibited HCC proliferation and metastasis.
3.2. Rho/Rac and YAP-TEAD
Yes-associated proteins (YAP) and TEAD transcription factors are known to be part of the Hippo signaling pathway that regulate organ size. During proliferation, YAP translocate into the nucleus and bind with TEAD to promote cell growth. When cells reach a certain density level, cell-cell signaling induces the degradation of YAP through phosphorylation and sequestration in the cytosol. However, in UM, activation of YAP is independent of Hippo and dependent on the GTPases Rho and Rac found downstream of Gαq/11 signaling [68]. YAP-inhibition by verteporfin was shown to block tumor growth of GNAQ/11 mutated UM cells [78].
3.2.a. Statins
Sorrentino et al. screened 640 clinically-used compounds for their potential to sequester YAP/TAZ in the cytoplasm and found the most potent agents belonging to the class of cholesterol-lowering drugs, statin [79]. Statins inhibit HMG-CoA reductase in the mevalonate cholesterol synthesis pathway and reduce geranylgeranyl pyrophosphate levels. As a result, statins inhibit Rho activation and the downstream nuclear translocation of YAP [79].
3.2.b. Rho kinase inhibitors
Amen et al. studied the ethanol extract of Ganodermal lingzhi for the ability to inhibit Rho-kinases ROCK-I and ROCK-II and identified 35 compounds [80]. Lanostane triterpenoids were found to have the most potent activities. In another study, Su et al. virtually screened a natural product library and found phloretin and baicalein to inhibit Rho-kinase [81]. Phloretin is a dihydrochalcone found in apple and pears [82]. Baicalein is a flavonoid from Scutellaria baicalensis [83].
3.3. ZEB1
ZEB1 is a transcription factor that promotes epithelial-mesenchymal transition (EMT). It represses the epithelial marker CDH1 and induces the mesenchymal marker CDH2 to weaken cell-cell adhesion and to promote migration. Its role in malignant tumors and tumorigenesis is thought to be associated with this transition. Recent studies by Chen et. al. showed that ZEB1 played a crucial role in UM’s malignant progression [69].
3.3.a. Resveratrol, Enoxolone, Magnolol, Palmatine, and Honokiol
Resveratrol is a polyphenol found in grapes and peanuts. Their mechanism of action as an anticancer agent in UM has not yet been elucidated. However, it was shown to suppress the growth of xenograft tumors of UM cell lines C918 and Mum2b in mice upon oral administration and to regress tumors upon peritumor injections. Induced cell death was at least partially caused by the mitochondrial apoptotic pathway [84]. In another study, resveratrol treatment was associated with increased levels of tumor-suppressor miRNAs in breast cancer cells by promoting the activity of Argonaute2 (Ago2) [85]. One of the amplified miRNAs was miR-200c, which inhibits ZEB1 expression. Hagiwara et al. showed that enoxolone, magnolol, and palmatine chloride also upregulate miR-200c and induce anti-cancer effect in breast cancer cells [86]. Enoxolone is found in the herb liquorice; magnolol is found in the bark of magnolia; and palmatine is found in several plants. Avtanski et al. showed that honokiol, another phenolic compound extracted from magnolia seeds, inhibited ZEB1 expression as well. It targets Stat3 recruitment on the promoter, thereby reducing transcription [87].
4. Sebaceous Cell Carcinoma
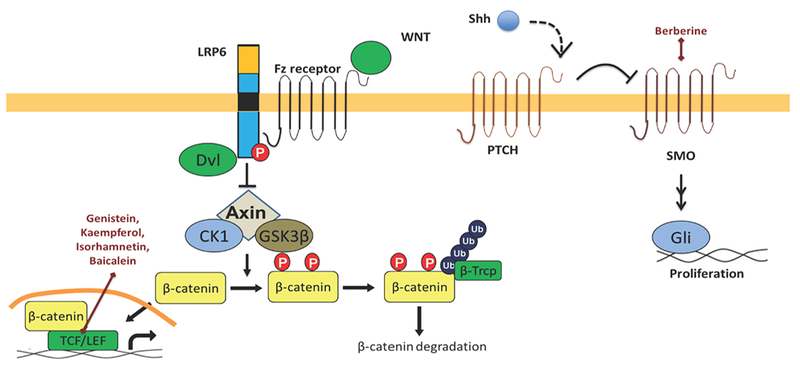
Around the eye, sebaceous cell carcinoma (SCC) originates from the meibomian and pilosebaceous glands. It accounts for 1–5% of eyelid malignancies and is often mistaken for more common lesions like chalazion. Misdiagnosis results in a 50% mortality rate, as SCC is aggressive and can metastasize throughout the body. Most develop in the head and neck, where glands are more concentrated. Current treatment includes excision and cryotherapy or chemotherapy. Different types of natural products, which target various oncogenic transcription factors, are represented schematically in Figure.4.
Figure 4: Natural products targeting important pathways in sebaceous cell carcinoma.
SCCs can have LEF1 mutations and hedgehog activity. (A) Genistein, kaempferol, isorhamnetin, baicalein target TCF/β-catenin interactions in the Wnt pathway. (B) Berberine targets Smo in the Hedgehog pathway.
4.1. Wnt/β-catenin
Wnt is involved in a highly conserved signaling pathway known for its role in embryonic development. It was first discovered as a proto-oncogene in a breast cancer cells. The pathway starts with Wnt factors binding to Frizzled receptors, which trigger a cascade to inactivate glycogen synthase kinase 3-β (GSK3β). This prevents GSK3β from phosphorylating and degrading proteins like β-catenin. β-catenin accumulates and eventually moves into the nucleus, where it binds to transcription factors TCF/LEF (T-cell factor/leukocyte enhancer factor). TCF/LEF are responsible for growth and proliferative genes [30].
In SCC, 30% of the cases involve a mutation in a downstream target of Wnt signaling, LEF-1 [88]. LEF-1 normally binds with TCF-3 and β-catenin, forming transcriptional complexes that regulate the expression of cell fate genes [89]. High levels of β-catenin stimulate hair follicles while low levels stimulate sebaceous cells [90]. A mutation in LEF-1 preventing its interactions with β-catenin resulted in the disruption of skin differentiation, and the overexpression of this mutant resulted in the formation of tumors with sebaceous characteristics [91]. And to further contribute to this tumorigenesis story, LEF-1 was found to induce p53- and p-21 dependent checkpoint regulation in cell cycle [92].
4.1.a. Genistein, kaempferol, isorhamnetin, and baicalein
Genistein is found in soybeans and soy products [93]. Kaempferol and isorhamnetin are found in fruits and vegetables [94, 95]. As mentioned above, baicalein is isolated from the roots of Scutellaria baicalensis. These compounds are polyphenolic flavonoids found to target the interaction between β-catenin and TCF interaction in HEK293 cells [96]. Genistein also inhibits Akt phosphorylation and prevents pAkt from normally inhibiting GSK3β. As a result, GSK3β continues to degrade β-catenin and prevents its interaction with LEF-1 and TCF. However, the latter mechanism of action is less relevant when LEF-1 is mutated and not interacting with β-catenin.
4.2. Hedgehog
Hedgehog (Hh) signaling pathway is also crucial in embryonic development. In skin, sonic hedgehog (Shh) is crucial for regulating the development of hair follicles and sebaceous glands [97]. Shh binds to the receptor Ptch, relieving its inhibition of Smoothened (Smo). This enables the downstream activation of Gli, PTCH, and TGF-beta. Hedgehog factor administered to the skin surface induced sebaceous gland development, even where sebaceous glands are not normally found [98].
4.2.a. Berberine
Berberine (BBR) is a natural alkaloid compound found to inhibit growth of medulloblastoma in vivo by targeting Smo. It inhibits Smo from releasing Gli transcription factors that turn on proliferative genes [99]. A novel molecular mechanism responsible for the anticancer action of BBR was uncovered, thus opening the way for the use of BBR for cancer therapeutics in addiction to aberrant Hh pathway activity.
4.2.b. Other Natural Inhibitors
Cyclopamine is a naturally occurring alkaloid found in Veratrum californicum. Cyclopamine inhibits the Shh signaling pathway by influencing the balance between the active and inactive forms of Smo [100]. Other natural products such as curcumin, genistein, EGCG, resveratrol, quercetin, baicalen, and apigenin along with novel compounds isolated from Southeast Asian plants, such as the potent sub-micromolar gitoxigenin derivatives, act as inhibitors of Hh pathway [101]. Currently, several Hh pathway inhibitory drugs are in clinical development, and the FDA recently approved Erivedge (vismodegib) from Curis/Genentech for treatment of advanced basal cell carcinoma [102].
5. Lacrimal Gland Adenocystic Carcinoma
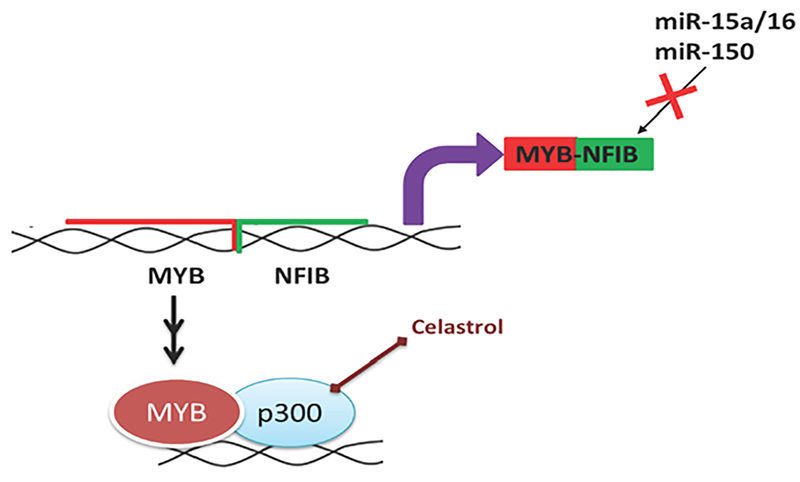
Lacrimal gland adenocystic carcinoma (LGACC) is a rare epithelial neoplasm in the eye (<1 in 2 million) with high metastatic rates and poor prognosis [103]. There is approximately 50% mortality rate within 5 years of diagnosis. Treatments include aggressive surgery and intra-arterial chemotherapy [103–107]. The overview of natural products targeting MYB in LGACC is shown in Figure 5. Though not discussed here, Notch, Wnt, TGFβ, and Hh signaling are important pathways for EMT for LGACC as well [108].
Figure 5: Natural products targeting important pathways in lacrimal gland adenocystic carcinoma (LGACC).
LGACCs can have high MYB expression as a result of t(6;9)(q22–23;p23–24) chromosomal translocation. MYB is inserted next to NFIB and loses its regulation by miR-15a/16 and miR-150. Celastrol inhibits MYB/p300 interactions.
5.1. MYB
LGACC is molecularly characterized by the overexpression of the oncogene MYB that is typically a result of a t(6;9)(q22–23;p23–24) chromosomal translocation, during which MYB fuses with NFIB. This translocation is found in 85% of adenoid cystic carcinomas (ACC) and 50% of LGACCs [109, 110]. The 3’ untranslated region (UTR) is disrupted during this process and can no longer be targeted by MYB-inhibitors miR-15a/16 and miR-150 [111].
5.1.a. Mexicanin-I
Sesquiterpene lactones were screened for MYB-inhibition in myelomonocytic chicken cell lines, and mexicanin-I, isolated from Arnica species, was identified to be a low-molecular weight inhibitor of MYB-inducible gene expression [112]. The molecular mechanism for mexicanin-I has not yet been elucidated.
5.1.b. Celastrol
As mentioned earlier, celastrol is a triterpenoid isolated from Tripterygium wilfordii. In RB cells, it was found to inhibit the NF-κB pathway. In acute myeloid leukemia cells, it was found to inhibit MYB activity by blocking its interaction with p300, a transcriptional cofactor [113].
6. Challenges and Prospects
The development of chemotherapeutic drugs from natural compounds is not a simple task, especially since little is known regarding the application of naturally-derived compounds on ocular cancers. However, the chemotherapeutic effects of natural compounds have been studied in greater detail in malignancies of other organs (Table 1). Utilizing these studies with overlapping cancer mechanisms in the eye can serve as a prudent starting point for the development of ocular chemotherapeutics.
Table 1: Natural products under various phases of clinical development in different malignancies.
Below table showing the different natural products and their targets which are under various phases of drug development/ clinical trials.
| Natural Agent | Target | Indication/status |
|---|---|---|
| BET inhibitors: l-BET-762 | BET (bromodomain and extra terminal domain) proteins | Acute myeloid leukemia/Phase 1 Breast adenocarcinoma/Phase 2 Breast cancer/Phase 1 Colorectal cancer/Phase 1 Multiple myeloma/Phase 1 Neuroblastoma/Phase 1 Non-small cell lung cancer/Phase 1 |
| Trichostatin, | Histone deacetylase | Metastatic melanoma/phase 1 |
| Topotecan | Topoisomerase I | Cervical carcinoma/Approved |
| Statins: atorvastatin | HMG-CoA reductase | Acute myeloid leukemia/Phase 2 Acute lymphocytic leukemia/Phase 2 |
| Rho kinase inhibitors | Rho-associated kinases ROCK1 and ROCK2 | Exfoliative glaucoma/Phase 2 Glaucoma/Phase 2 Ocular hypertension/Phase 2 Open-angle glaucoma/Phase 2 |
| Genistein, | Protein tyrosine kinase (PTK) | Neuroblastoma/Phase 2 Germ cell tumor/Phase 2 Prostate cancer/Phase 2 |
It is not uncommon that natural agents are able to target the same pathway across multiple cancer cell types. The most successful example is paclitaxel (Taxol). Its antiproliferative activity was first recognized in ovarian cancer and twenty years later, its potential was unveiled in refractory breast cancer [114]. Such a smooth transition for the alternative use of a drug is not always the case. Further molecular and pharmacological characterization is still necessary for validating natural products’ activities in eye cancer. However, understanding their effects in other solid tumors provides a start for choosing promising compounds to study in eye cancer.
Upon identification of a natural compound as a potential chemotherapeutic agent, the active molecular component must be identified and isolated. Natural extracts are usually comprised of multiple compounds, and active components need to be distinguished from confounding molecules when identifying exact targets and pathways responsible for antiproliferative activities. Adding to this challenge, natural products can also have multiple targets. For instance, celastrol is known to modulate the expression of MHCII, HO-1, iNOS, NFkB, Notch-1, AKT/mTOR, VEGF, CHOP, JNK, and so forth [115]. Advances in high-throughput screening technologies allow for the careful testing of natural agents against purified enzymes. By excluding confounding molecules, they increase the likelihood of identifying key oncotargets for “cleaner” pharmacological responses in the future.
Even after purification of a natural compound, additional chemical modifications may need to be performed to optimize the functionality of the compound. While natural products are shown to exhibit antiproliferative activities in various cancers, they have not undergone evolutionary selection in nature to be cancer therapeutics per se. In other words, they have not been fine-tuned in solubility, stability, bioavailability, and specificity for cancer therapy. Chemical modifications of compounds based on natural product scaffolds can facilitate this process. For example, two bacterial natural products FR901464 and paldienolide were used to develop the synthetic analogs sudemycin that exhibited improved cytotoxicity, solubility, and stability [116–118]. Therefore, natural products can be expanded into diverse chemical libraries with potentially better chemotherapeutic properties tailored to specific cancers.
7. Conclusions
Natural products comprise of approximately half of the drugs in chemotherapy, but little is known regarding their effects on eye cancer. The mechanisms of these drugs have been studied in greater detail for malignancies in other organs, shedding insight on how they can potentially modulate key pathways that overlap in eye cancer. Identifying a natural compound as a potential chemotherapeutic agent is the initial step in a long process that will require the isolation of its active component, further studies on active component selectivity, and chemical modification to fine tune its effects. With this review, we hope to further encourage translational research in ocular oncology drug discovery by discussing important pathways in eye cancer and natural agents known to affect these pathways in other well-studied cancers.
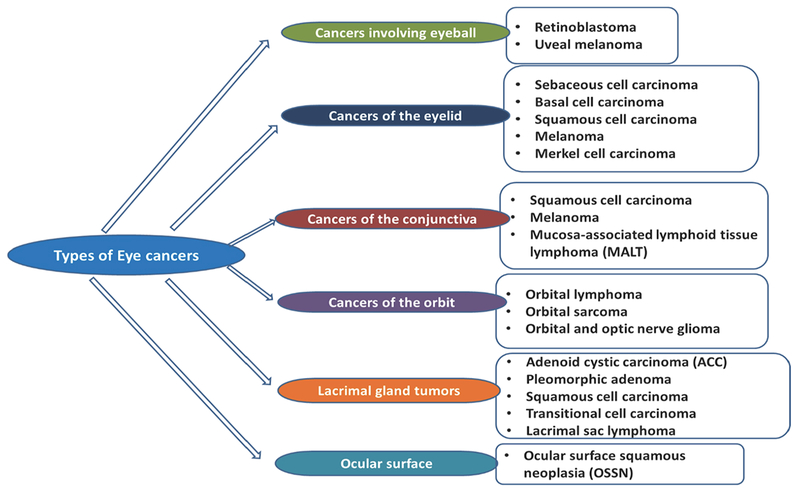
Figure 1: Schematic presentation of different types of eye cancer.
Eye cancer is a general term used to describe tumors in various parts of the eye. It occurs when healthy cells in or around the eye change and grow uncontrollably, forming benign or malignant masses.
Acknowledgement:
This work was supported by the Dr. Nasser Ibrahim Al-Rashid Orbital Vision Research Fund.
Abbreviations
- 2,5-DXP
2,5-diketopiperazine
- ACC
Adenoid cystic carcinomas
- BBR
Berberine
- BET
Bromodomain and extraterminal
- CA
Chebulagic acid
- CPT
Camptothecin
- EMT
Epithelial-mesenchymal transition
- GSK3
Glycogen synthase kinase 3-
- HCC
Hepatocellular carcinoma
- HDAC
Histone deacetylases
- Hh
Hedgehog
- HIF-1
Hypoxic inducible factor 1
- IkB
Inhibitor of NFkB
- IKK
IkB kinase
- ISJ
Isojacareubin
- JapA
Japonicones A
- LGACC
Lacrimal gland adenocystic carcinoma
- LinA
Lineariifolianoid A
- MAPK
Mitogen-activated protein kinase
- NFkB
Nuclear Factor kB
- PKC
Protein kinase C
- PRAME
Preferentially Expressed Antigen In Melanoma
- pVHL
Von Hippel-Lindau tumor suppressor
- RB
Retinoblastoma
- SCC
Sebaceous cell carcinoma
- Shh
Sonic hedgehog
- Smo
Smoothened
- TCF/LEF
T-cell factor/leukocyte enhancer factor
- TEAD
TEA domain
- Topo1
Topoisomerase I
- TPT
Topotecan
- UM
Uveal melanoma
- UTR
Untranslated region
- YAP
Yes-associated proteins
Footnotes
Conflict of interest:
None
References
- 1.Ekor M, The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Frontiers in Pharmacology, 2014. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newman DJ and Cragg GM, Natural Products As Sources of New Drugs over the 30 Years from 1981 to 2010. Journal of Natural Products, 2012. 75(3): p. 311–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desborough MJR and Keeling DM, The aspirin story - from willow to wonder drug. Br J Haematol, 2017. 177(5): p. 674–683. [DOI] [PubMed] [Google Scholar]
- 4.Newman DJ and Cragg GM, Natural products as sources of new drugs over the last 25 years. Journal of Natural Products, 2007. 70(3): p. 461–477. [DOI] [PubMed] [Google Scholar]
- 5.Altmann KH and Gertsch J, Anticancer drugs from nature-natural products as a unique source of new microtubule-stabilizing agents (vol 24, pg 327, 2007). Natural Product Reports, 2012. 29(12): p. 1481–1481. [DOI] [PubMed] [Google Scholar]
- 6.Kronenberg F, Mindes J, and Jacobson JS, The future of complementary and alternative medicine for cancer. Cancer Investigation, 2005. 23(5): p. 420–426. [PubMed] [Google Scholar]
- 7.Ali R, et al. , New Anticancer Agents: Recent Developments in Tumor Therapy. Anticancer Research, 2012. 32(7): p. 2999–3005. [PubMed] [Google Scholar]
- 8.Gordaliza M, Natural products as leads to anticancer drugs. Clinical & Translational Oncology, 2007. 9(12): p. 767–776. [DOI] [PubMed] [Google Scholar]
- 9.Cragg GM and Newman DJ, Natural products: A continuing source of novel drug leads. Biochimica Et Biophysica Acta-General Subjects, 2013. 1830(6): p. 3670–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devesa SS, et al. , Cancer Incidence and Mortality Trends among Whites in the United-States, 1947–84. Journal of the National Cancer Institute, 1987. 79(4): p. 701–770. [PubMed] [Google Scholar]
- 11.Foss AJE and Dolin PJ, Trends in eye cancer mortality among adults in the USA and England and Wales. British Journal of Cancer, 1996. 74(10): p. 1687–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh AD and Topham A, Incidence of uveal melanoma in the United States: 1973–1997. Ophthalmology, 2003. 110(5): p. 956–61. [DOI] [PubMed] [Google Scholar]
- 13.Treatment Retinoblastoma (PDQ(R)): Health Professional Version, in PDQ Cancer Information Summaries. 2002: Bethesda (MD). [Google Scholar]
- 14.Brivanlou AH and Darnell JE Jr., Signal transduction and the control of gene expression. Science, 2002. 295(5556): p. 813–8. [DOI] [PubMed] [Google Scholar]
- 15.Denhardt DT, Oncogene-initiated aberrant signaling engenders the metastatic phenotype: synergistic transcription factor interactions are targets for cancer therapy. Crit Rev Oncog, 1996. 7(3–4): p. 261–91. [DOI] [PubMed] [Google Scholar]
- 16.Futreal PA, et al. , Cancer and genomics. Nature, 2001. 409(6822): p. 850–2. [DOI] [PubMed] [Google Scholar]
- 17.Varmus HE, Oncogenes and transcriptional control. Science, 1987. 238(4832): p. 1337–9. [DOI] [PubMed] [Google Scholar]
- 18.Darnell JE, Transcription factors as targets for cancer therapy. Nature Reviews Cancer, 2002. 2(10): p. 740–749. [DOI] [PubMed] [Google Scholar]
- 19.Gibbs JB, Mechanism-based target identification and drug discovery in cancer research. Science, 2000. 287(5460): p. 1969–1973. [DOI] [PubMed] [Google Scholar]
- 20.Levy DE and Darnell JE Jr., Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol, 2002. 3(9): p. 651–62. [DOI] [PubMed] [Google Scholar]
- 21.Taipale J and Beachy PA, The Hedgehog and Wnt signaling pathways in cancer. Nature, 2001. 411(6835): p. 349–354. [DOI] [PubMed] [Google Scholar]
- 22.Singh BN, et al. , Dietary phytochemicals alter epigenetic events and signaling pathways for inhibition of metastasis cascade: phytoblockers of metastasis cascade. Cancer Metastasis Rev, 2014. 33(1): p. 41–85. [DOI] [PubMed] [Google Scholar]
- 23.McCormick F, Cancer gene therapy: fringe or cutting edge? Nat Rev Cancer, 2001. 1(2): p. 130–41. [DOI] [PubMed] [Google Scholar]
- 24.Shaywitz AJ and Greenberg ME, CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem, 1999. 68: p. 821–61. [DOI] [PubMed] [Google Scholar]
- 25.Balandrin MF, Kinghorn AD, and Farnsworth NR, Plant-Derived Natural-Products in Drug Discovery and Development - an Overview. Human Medicinal Agents from Plants, 1993. 534: p. 2–12. [Google Scholar]
- 26.Dimaras H, et al. , Retinoblastoma. Nat Rev Dis Primers, 2015. 1: p. 15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abramson DH, et al. , Intra-Arterial Chemotherapy (Ophthalmic Artery Chemosurgery) for Group D Retinoblastoma. PLoS One, 2016. 11(1): p. e0146582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abramson DH, et al. , A phase I/II study of direct intraarterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma initial results. Ophthalmology, 2008. 115(8): p. 1398–404, 1404 e1. [DOI] [PubMed] [Google Scholar]
- 29.Campomanes AOBJ, Genetics of hereditary retinoblastoma, in Ocular Disease: Mechanisms and Management. 2010. p. 369–376. [Google Scholar]
- 30.Weinberg R, Cytoplasmic Signaling Circuitry Programs Many of the Traits of Cancer, in The Biology of Cancer. 2014, Garland Science, Taylor & Francis Group, LLC; p. 175–229. [Google Scholar]
- 31.Margo C, et al. , Retinocytoma. A benign variant of retinoblastoma. Arch Ophthalmol, 1983. 101(10): p. 1519–31. [DOI] [PubMed] [Google Scholar]
- 32.Evans L, et al. , SKP2 is a direct transcriptional target of MYCN and a potential therapeutic target in neuroblastoma. Cancer Lett, 2015. 363(1): p. 37–45. [DOI] [PubMed] [Google Scholar]
- 33.Muth D, et al. , Transcriptional repression of SKP2 is impaired in MYCN-amplified neuroblastoma. Cancer Res, 2010. 70(9): p. 3791–802. [DOI] [PubMed] [Google Scholar]
- 34.Ewens KG, et al. , Phosphorylation of pRb: mechanism for RB pathway inactivation in MYCN-amplified retinoblastoma. Cancer Medicine, 2017. 6(3): p. 619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma VK, et al. , Induction of Cryptic and Bioactive Metabolites through Natural Dietary Components in an Endophytic Fungus Colletotrichum gloeosporioides (Penz.) Sacc. Front Microbiol, 2017. 8: p. 1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puissant A, et al. , Targeting MYCN in Neuroblastoma by BET Bromodomain Inhibition. Cancer Research, 2013. 73(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu N, et al. , A mouse model of MYCN-driven retinoblastoma reveals MYCN-independent tumor reemergence. J Clin Invest, 2017. 127(3): p. 888–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo Y, Pajovic S, and Gallie BL, Expression of p14ARF, MDM2, and MDM4 in human retinoblastoma. Biochem Biophys Res Commun, 2008. 375(1): p. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wade M, Li YC, and Wahl GM, MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat Rev Cancer, 2013. 13(2): p. 83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah HR, et al. , Beta-lapachone inhibits proliferation and induces apoptosis in retinoblastoma cell lines. Eye (Lond), 2008. 22(3): p. 454–60. [DOI] [PubMed] [Google Scholar]
- 41.D’Anneo A, et al. , Paclitaxel and beta-lapachone synergistically induce apoptosis in human retinoblastoma Y79 cells by downregulating the levels of phospho-Akt. J Cell Physiol, 2010. 222(2): p. 433–43. [DOI] [PubMed] [Google Scholar]
- 42.Drago-Ferrante R, et al. , Low doses of paclitaxel potently induce apoptosis in human retinoblastoma Y79 cells by up-regulating E2F1. Int J Oncol, 2008. 33(4): p. 677–87. [PubMed] [Google Scholar]
- 43.Barbuti AM and Chen ZS, Paclitaxel Through the Ages of Anticancer Therapy: Exploring Its Role in Chemoresistance and Radiation Therapy. Cancers, 2015. 7(4): p. 2360–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi DL and Cobrinik D, MDM2 but not MDM4 promotes retinoblastoma cell proliferation through p53-independent regulation of MYCN translation. Oncogene, 2017. 36(13): p. 1760–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin JJ, et al. , Inhibiting NFAT1 for breast cancer therapy: New insights into the mechanism of action of MDM2 inhibitor JapA. Oncotarget, 2015. 6(32): p. 33106–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin JJ, et al. , Identification of lineariifolianoid A as a novel dual NFAT1 and MDM2 inhibitor for human cancer therapy. J Biomed Res, 2016. 30(4): p. 322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qin JJ, et al. , Japonicones A-D, bioactive dimeric sesquiterpenes from Inula japonica Thunb. Bioorg Med Chem Lett, 2009. 19(3): p. 710–3. [DOI] [PubMed] [Google Scholar]
- 48.Sradhanjali S, et al. , Overexpression of pyruvate dehydrogenase kinase 1 in retinoblastoma: A potential therapeutic opportunity for targeting vitreous seeds and hypoxic regions. PLoS One, 2017. 12(5): p. e0177744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamakuchi M, et al. , P53-induced microRNA-107 inhibits HIF-1 and tumor angiogenesis. Proc Natl Acad Sci U S A, 2010. 107(14): p. 6334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia T, Cheng H, and Zhu Y, Knockdown of hypoxia-inducible factor-1 alpha reduces proliferation, induces apoptosis and attenuates the aggressive phenotype of retinoblastoma WERI-Rb-1 cells under hypoxic conditions. Ann Clin Lab Sci, 2014. 44(2): p. 134–44. [PubMed] [Google Scholar]
- 51.Nagle DG and Zhou YD, Natural product-based inhibitors of hypoxia-inducible factor-1 (HIF-1). Current Drug Targets, 2006. 7(3): p. 355–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rapisarda A, et al. , Topoisomerase I-mediated inhibition of hypoxia-inducible factor 1: mechanism and therapeutic implications. Cancer Res, 2004. 64(4): p. 1475–82. [DOI] [PubMed] [Google Scholar]
- 53.Staker BL, et al. , The mechanism of topoisomerase I poisoning by a camptothecin analog. Proceedings of the National Academy of Sciences of the United States of America, 2002. 99(24): p. 15387–15392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brennan RC, et al. , Ocular Salvage and Vision Preservation Using a Topotecan-Based Regimen for Advanced Intraocular Retinoblastoma. Journal of Clinical Oncology, 2017. 35(1): p. 72–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kummar S, et al. , Multihistology, Target-Driven Pilot Trial of Oral Topotecan as an Inhibitor of Hypoxia-Inducible Factor-1 alpha in Advanced Solid Tumors. Clinical Cancer Research, 2011. 17(15): p. 5123–5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qu Y, et al. , Clinicopathologic significances of nuclear expression of nuclear factor-kappaB transcription factors in retinoblastoma. J Clin Pathol, 2011. 64(8): p. 695–700. [DOI] [PubMed] [Google Scholar]
- 57.Luqman S and Pezzuto JM, NF kappa B: A Promising Target for Natural Products in Cancer Chemoprevention. Phytotherapy Research, 2010. 24(7): p. 949–963. [DOI] [PubMed] [Google Scholar]
- 58.Lee JH, et al. , Inhibition of NF-kappa B activation through targeting I kappa B kinase by celastrol, a quinone methide triterpenoid. Biochem Pharmacol, 2006. 72(10): p. 1311–21. [DOI] [PubMed] [Google Scholar]
- 59.Li ZR, et al. , Antitumor activity of celastrol nanoparticles in a xenograft retinoblastoma tumor model. International Journal of Nanomedicine, 2012. 7: p. 2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar N, et al. , Chebulagic acid from Terminalia chebula causes G1 arrest, inhibits NFkappaB and induces apoptosis in retinoblastoma cells. BMC Complement Altern Med, 2014. 14: p. 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Correa Z.H., J. W., Uveal melanoma, in Ocular Disease: Mechanisms and Management. 2010, Elsevier Inc. p. 362–368. [Google Scholar]
- 62.Onken MD, et al. , Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res, 2004. 64(20): p. 7205–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim HR, et al. , Aberrant Expression of TIMP-2 and PBEF Genes in the Placentae of Cloned Mice Due to Epigenetic Reprogramming Error. PLoS One, 2016. 11(11): p. e0166241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baxter SL, et al. , Mutations in GNAQ and GNA11 in Chinese patients with uveal melanoma. Investigative Ophthalmology & Visual Science, 2016. 57(12). [Google Scholar]
- 65.Van Raamsdonk CD, et al. , Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature, 2009. 457(7229): p. 599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Raamsdonk CD, et al. , Mutations in GNA11 in uveal melanoma. N Engl J Med, 2010. 363(23): p. 2191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ambrosini G, et al. , Identification of unique MEK-dependent genes in GNAQ mutant uveal melanoma involved in cell growth, tumor cell invasion, and MEK resistance. Clin Cancer Res, 2012. 18(13): p. 3552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feng X, et al. , Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell, 2014. 25(6): p. 831–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen Y, et al. , ZEB1 Regulates Multiple Oncogenic Components Involved in Uveal Melanoma Progression. Sci Rep, 2017. 7(1): p. 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen X, et al. , Combined PKC and MEK inhibition in uveal melanoma with GNAQ and GNA11 mutations. Oncogene, 2014. 33(39): p. 4724–4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carita G, et al. , Dual inhibition of protein kinase C and p53-MDM2 or PKC and mTORC1 are novel efficient therapeutic approaches for uveal melanoma. Oncotarget, 2016. 7(23): p. 33542–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shadid KA, et al. , Cytotoxic caged-polyprenylated xanthonoids and a xanthone from Garcinia cantleyana. Phytochemistry, 2007. 68(20): p. 2537–44. [DOI] [PubMed] [Google Scholar]
- 73.Bauvois B, et al. , Synthesis and biological evaluation of novel flavone-8-acetic acid derivatives as reversible inhibitors of aminopeptidase N/CD13. J Med Chem, 2003. 46(18): p. 3900–13. [DOI] [PubMed] [Google Scholar]
- 74.Pedro M, et al. , Xanthones as inhibitors of growth of human cancer cell lines and their effects on the proliferation of human lymphocytes in vitro. Bioorganic & Medicinal Chemistry, 2002. 10(12): p. 3725–3730. [DOI] [PubMed] [Google Scholar]
- 75.Garg R, et al. , Protein kinase C and cancer: what we know and what we do not. Oncogene, 2014. 33(45): p. 5225–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuan X, et al. , Inhibition of protein kinase C by isojacareubin suppresses hepatocellular carcinoma metastasis and induces apoptosis in vitro and in vivo. Scientific Reports, 2015. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang HF, et al. , Cytotoxic Activity and DNA-Binding Properties of Isoeuxanthone Derivatives. Chemical & Pharmaceutical Bulletin, 2014. 62(3): p. 260–266. [DOI] [PubMed] [Google Scholar]
- 78.Lyubasyuk V, et al. , YAP inhibition blocks uveal melanogenesis driven by GNAQ or GNA11 mutations. Mol Cell Oncol, 2015. 2(1): p. e970957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sorrentino G, et al. , Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol, 2014. 16(4): p. 357–66. [DOI] [PubMed] [Google Scholar]
- 80.Amen Y, et al. , Partial contribution of Rho-kinase inhibition to the bioactivity of Ganoderma lingzhi and its isolated compounds: insights on discovery of natural Rhokinase inhibitors. J Nat Med, 2017. 71(2): p. 380–388. [DOI] [PubMed] [Google Scholar]
- 81.Su H, et al. , Stepwise high-throughput virtual screening of Rho kinase inhibitors from natural product library and potential therapeutics for pulmonary hypertension. Pharm Biol, 2015. 53(8): p. 1201–6. [DOI] [PubMed] [Google Scholar]
- 82.Kobori M, et al. , Phloretin-induced apoptosis in B16 melanoma 4A5 cells and HL60 human leukemia cells. Biosci Biotechnol Biochem, 1999. 63(4): p. 719–25. [DOI] [PubMed] [Google Scholar]
- 83.Bui TT, et al. , Baicalein, wogonin, and Scutellaria baicalensis ethanol extract alleviate ovalbumin-induced allergic airway inflammation and mast cell-mediated anaphylactic shock by regulation of Th1/Th2 imbalance and histamine release. Anat Cell Biol, 2017. 50(2): p. 124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Ginkel PR, et al. , Resveratrol inhibits uveal melanoma tumor growth via early mitochondrial dysfunction. Investigative Ophthalmology & Visual Science, 2008. 49(4): p. 1299–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hagiwara K, et al. , Stilbene derivatives promote Ago2-dependent tumour-suppressive microRNA activity. Sci Rep, 2012. 2: p. 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hagiwara K, et al. , A robust screening method for dietary agents that activate tumour-suppressor microRNAs. Sci Rep, 2015. 5: p. 14697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Avtanski DB, et al. , Honokiol inhibits epithelial-mesenchymal transition in breast cancer cells by targeting signal transducer and activator of transcription 3/Zeb1/E-cadherin axis. Mol Oncol, 2014. 8(3): p. 565–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Takeda H, et al. , Human sebaceous tumors harbor inactivating mutations in LEF1. Nat Med, 2006. 12(4): p. 395–7. [DOI] [PubMed] [Google Scholar]
- 89.DasGupta R and Fuchs E, Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development, 1999. 126(20): p. 4557–68. [DOI] [PubMed] [Google Scholar]
- 90.Niemann C, et al. , Indian hedgehog and beta-catenin signaling: role in the sebaceous lineage of normal and neoplastic mammalian epidermis. Proc Natl Acad Sci U S A, 2003. 100 Suppl 1: p. 11873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Niemann C, et al. , Expression of DeltaNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development, 2002. 129(1): p. 95–109. [DOI] [PubMed] [Google Scholar]
- 92.Niemann C, et al. , Dual role of inactivating Lef1 mutations in epidermis: tumor promotion and specification of tumor type. Cancer Res, 2007. 67(7): p. 2916–21. [DOI] [PubMed] [Google Scholar]
- 93.Banerjee S, et al. , Multi-targeted therapy of cancer by genistein. Cancer Lett, 2008. 269(2): p. 226–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen AY and Chen YC, A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem, 2013. 138(4): p. 2099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Flamini R, et al. , Advanced knowledge of three important classes of grape phenolics: anthocyanins, stilbenes and flavonols. Int J Mol Sci, 2013. 14(10): p. 19651–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park S and Choi J, Inhibition of beta-Catenin/Tcf Signaling by Flavonoids. Journal of Cellular Biochemistry, 2010. 110(6): p. 1376–1385. [DOI] [PubMed] [Google Scholar]
- 97.Botchkarev VA and Fessing MY, Edar signaling in the control of hair follicle development. Journal of Investigative Dermatology Symposium Proceedings, 2005. 10(3): p. 247–251. [DOI] [PubMed] [Google Scholar]
- 98.Allen M, et al. , Hedgehog signaling regulates sebaceous gland development. American Journal of Pathology, 2003. 163(6): p. 2173–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang J, et al. , Berberine, a natural compound, suppresses Hedgehog signaling pathway activity and cancer growth. BMC Cancer, 2015. 15: p. 595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen JK, et al. , Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev, 2002. 16(21): p. 2743–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Drenkhahn SK, et al. , Inhibition of hedgehog/Gli signaling by botanicals: a review of compounds with potential hedgehog pathway inhibitory activities. Curr Cancer Drug Targets, 2013. 13(5): p. 580–95. [DOI] [PubMed] [Google Scholar]
- 102.Genentech ERIVEDGE (vismodegib), Prescribing Information. 2017. [Google Scholar]
- 103.Mendoza-Santiesteban E, et al. , Diagnosis and surgical treatment of orbital tumors. Semin Ophthalmol, 2010. 25(4): p. 123–9. [DOI] [PubMed] [Google Scholar]
- 104.Meldrum ML, Tse DT, and Benedetto P, Neoadjuvant intracarotid chemotherapy for treatment of advanced adenocystic carcinoma of the lacrimal gland. Arch Ophthalmol, 1998. 116(3): p. 315–21. [DOI] [PubMed] [Google Scholar]
- 105.Tse DT, et al. , Clinical analysis of the effect of intraarterial cytoreductive chemotherapy in the treatment of lacrimal gland adenoid cystic carcinoma. Am J Ophthalmol, 2006. 141(1): p. 44–53. [DOI] [PubMed] [Google Scholar]
- 106.Tse DT, et al. , Microdissection genotyping analysis of the effect of intraarterial cytoreductive chemotherapy in the treatment of lacrimal gland adenoid cystic carcinoma. Am J Ophthalmol, 2006. 141(1): p. 54–61. [DOI] [PubMed] [Google Scholar]
- 107.Tse DT, Clinical and microdissection genotyping analyses of the effect of intra-arterial cytoreductive chemotherapy in the treatment of lacrimal gland adenoid cystic carcinoma. Trans Am Ophthalmol Soc, 2005. 103: p. 337–67. [PMC free article] [PubMed] [Google Scholar]
- 108.Dvoriantchikova G, et al. , Molecular Profiling of the Developing Lacrimal Gland Reveals Putative Role of Notch Signaling in Branching Morphogenesis. Invest Ophthalmol Vis Sci, 2017. 58(2): p. 1098–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ho AS, et al. , The mutational landscape of adenoid cystic carcinoma. Nat Genet, 2013. 45(7): p. 791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.von Holstein SL, et al. , Adenoid cystic carcinoma of the lacrimal gland: MYB gene activation, genomic imbalances, and clinical characteristics. Ophthalmology, 2013. 120(10): p. 2130–8. [DOI] [PubMed] [Google Scholar]
- 111.Persson M, et al. , Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci U S A, 2009. 106(44): p. 18740–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bujnicki T, et al. , Inhibition of Myb-dependent gene expression by the sesquiterpene lactone mexicanin-I. Leukemia, 2012. 26(4): p. 615–22. [DOI] [PubMed] [Google Scholar]
- 113.Uttarkar S, et al. , Targeting acute myeloid leukemia with a small molecule inhibitor of the Myb/p300 interaction. Blood, 2016. 127(9): p. 1173–82. [DOI] [PubMed] [Google Scholar]
- 114.Mann J, Natural products in cancer chemotherapy: past, present and future. Nature Reviews Cancer, 2002. 2(2): p. 143–148. [DOI] [PubMed] [Google Scholar]
- 115.Salminen A, et al. , Celastrol: Molecular targets of Thunder God Vine. Biochemical and Biophysical Research Communications, 2010. 394(3): p. 439–442. [DOI] [PubMed] [Google Scholar]
- 116.Convertini P, et al. , Sudemycin E influences alternative splicing and changes chromatin modifications. Nucleic Acids Research, 2014. 42(8): p. 4947–4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lagisetti C, et al. , Optimization of Antitumor Modulators of Pre-mRNA Splicing. Journal of Medicinal Chemistry, 2013. 56(24): p. 10033–10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Makowski K, et al. , Sudemycin K: A Synthetic Antitumor Splicing Inhibitor Variant with Improved Activity and Versatile Chemistry. Acs Chemical Biology, 2017. 12(1): p. 163–173. [DOI] [PubMed] [Google Scholar]