Abstract
Background
Vitamin C is one of the key antioxidant vitamins which is abundant in the extracellular fluid lining the lung and low vitamin C intake has been associated with pulmonary dysfunction.
Objectives
To evaluate the evidence for the efficacy of vitamin C in the treatment of asthma.
Search methods
The Cochrane Airways Review Group asthma register was searched and bibliographies of studies identified were also checked for further trials. This review has been updated by searches to August 2008.
Selection criteria
Only randomised controlled trials were eligible for inclusion. Studies were considered for inclusion if they dealt with the treatment of asthma using vitamin C supplementation. Two independent reviewers identified potentially relevant studies using pre‐defined criteria and selected studies for inclusion.
Data collection and analysis
Data were abstracted independently by two reviewers. Information on patients, methods, interventions, outcomes and results was extracted using standard forms.
Main results
Nine studies met the review entry criteria, randomising a total of 330 participants. Study design varied and the reporting was generally poor. Five trials contributed numerical data to the review. They provided outcome data on lung function, symptom scores, IgE levels and inhaled steroid use. One small study showed a significant difference in % drop in FEV1 post‐exercise.
Authors' conclusions
At present, evidence from randomised‐controlled trials is insufficient to recommend a specific role for vitamin C in the treatment of asthma. Further methodologically strong and large‐scale randomised controlled trials are needed in order to address the question of the effectiveness of vitamin C in children with asthma.
Plain language summary
Vitamin C supplementation for asthma
Asthma is a chronic inflammatory disease of the airways characterised by wheeze and breathlessness. One theory for the observed increase in the number of people with asthma is the 'western' diet with it's lack of nutrients from fresh food. We reviewed evidence from nine trials of the antioxidant vitamin C as a treatment for asthma. In general the trials were small, varied greatly in their design and the reporting was poor. From the available evidence it is not possible to recommend either the use or avoidance of vitamin C supplements in asthma.
Background
Asthma is now recognised as a chronic inflammatory disease resulting in reversible airways bronchoconstriction (Holgate 1990). The incidence and prevalence of asthma has increased in many countries over the past few decades. The most marked increase has been observed in children (Strachan 1999; Lewis 1996). This may be due to changing environmental exposures or the increased susceptibility of populations with reduced host resistance (Seaton 1994).
One hypothesised cause for this increase is that changes in "western" diet have produced a reduction in host resistance over time. In particular recent interest has focussed on the association between anti‐oxidants in diets and health outcomes. Cross‐sectional studies show that infrequent fruit consumption is associated with reduced lung function, both in children (Cook 1997) and adults (Butland 1999). In addition, the National Food Survey has documented a drop in the consumption of antioxidant food sources such as fresh fruit and vegetables in countries such as Great Britain since the 1950's (Seaton 1994).
Vitamin C is one of the key antioxidant vitamins which is abundant in the extracellular fluid lining the lung. Low vitamin C intake is associated with pulmonary dysfunction (Schwartz 1994). Both adults (Olusi 1979) and children (Aderele 1985) with asthma have been found to have lower concentrations of vitamin C when compared to normal subjects. Patients with asthma may have low supplies of vitamin C or an increased demand for vitamin C in the face of an oxidant load resulting in depletion. There is a need to clarify whether supplementation with vitamin C may bring benefits in reducing morbidity, improving pulmonary function or quality of life in patients with asthma.
There have been three recent reviews of the literature on the role of vitamin C in asthma (Bielory 1994; Hatch 1995; Monteleone 1997). However, the review by Bielory et al (Bielory 1994) only searched the English language literature using MEDLINE and gave no further details as to how the studies had been located. The other two reviews did not specify their methodology. These reviews reached different conclusions. Bielory et al (Bielory 1994) concluded that the role of vitamin C in asthma was unclear and that current literature did not support its use. The review by Hatch et al (Hatch 1995) found 7 out of 11 studies indicated that vitamin C supplementation might reverse or improve asthma symptoms. The review by Monteleone et al (Monteleone 1997) offers the opinion that vitamin C provides a short term protective effect on airway responsiveness, but less clear impact on other objective lung function measurements. All three reviews recommend further studies into the role of vitamin C in asthma. As a first step, a review using the Cochrane methodology is needed to systematically weigh the quality of the existing evidence before recommending any future studies.
Objectives
To determine the overall efficacy of vitamin C supplementation in patients with stable chronic asthma.
Methods
Criteria for considering studies for this review
Types of studies
To be eligible, all studies needed to be randomised‐controlled trials (RCTs). Double‐blinded trials were preferred, but single blind and open studies were also reviewed for possible inclusion.
Types of participants
Studies were considered for inclusion if they recruited adults and/or children with chronic stable asthma, seasonal asthma or those with exercise‐induced bronchospasm. Studies of other allergic conditions such as hay fever, allergic rhinitis and eczema were only considered if the results for subjects with asthma were presented separately. Vitamin C studies, which reported outcomes on patients with asthma separately as a sub‐group, were also considered for inclusion.
Types of interventions
Vitamin C supplementation compared to placebo or "standard care". We considered studies that administered vitamin C via any route, dosage or dose interval. Both single dose and longer ‐term studies were considered for inclusion.
Types of outcome measures
Primary outcome measures
1) Lung function (e.g. FEV1, PEFR) 2) Symptoms (e.g. symptom scores)
Secondary outcome measures
3) Functional outcomes (e.g. quality of life, sickness absence, exercise capacity) 4) Non‐specific bronchial hyper‐reactivity (BHR) to histamine or methacholine 5) Immunological markers (IgE levels) 6) Asthma medication requirements (e.g. additional steroid or bronchodilator usage) 7) Health service utilisation (e.g. GP attendance, hospital admissions) 8) Asthma exacerbations
Search methods for identification of studies
Electronic Searches
Trials were identified using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see the http://www.mrw.interscience.wiley.com/cochrane/clabout/articles/AIRWAYS/frame.html for further details). All records in the Specialised Register coded as 'asthma' were searched using the following terms:
ascorbic* or "vitamin c" or antioxid*
The most recent search was conducted in August 2008.
Other sources
Reference lists of all primary studies and review articles were reviewed for additional references. Authors of identified trials were contacted.
Data collection and analysis
Retrieval of studies
All trials that appeared potentially relevant were assessed by two independent reviewers for relevance using abstract and title from the electronic search. Using texts from all potentially relevant articles, final inclusion was also determined independently by two reviewers. Disagreements about study inclusion were resolved with discussion.
Assessment of methodological quality
The quality of included studies was assessed using the Cochrane approach. A risk of bias table was completed for each study assessing the reporting of method of randomisation, allocation concealment and blinding. Each item was judged as being adequate, unclear or inadequate. Any disagreements between reviewers were resolved by discussion.
Data abstraction
Data were extracted independently by two reviewers and entered in the Cochrane Collaboration Software, Review Manager (RevMan). For studies where the original data were not presented, when possible, they were extracted from graphically representations. Study outcomes that were reported post‐bronchial challenge (e.g. exercise or histamine) were analysed separately from outcomes that did not involve bronchial challenge or when results were reported before such challenges (but post‐dose vitamin C administration).
Statistical considerations
Outcomes from included trials were combined using RevMan. For continuous outcomes the weighted mean difference (WMD) with fixed effect was used to estimate the individual effect sizes and 95% confidence intervals (95% CI). If there were any dichotomous outcomes the Peto fixed or random effect model was to be used to estimate the pooled odds ratio (OR) and 95% CI.
The main planned comparison for statistical consideration was any form or dose of vitamin C supplementation versus placebo or "standard care". If there were adequate included studies, the following pre‐defined sub‐group analysis were planned:
1. Single dose versus chronic administration of vitamin C 2. Dietary advice to increase vitamin C consumption versus no intervention 3. Oral vitamin C versus intravenous vitamin C supplementation 4. Adults versus children 5. Males versus females
Results
Description of studies
Details of the search history and results can be found in Table 1. For the 2008 update, 39 new references were identified and the full text of 15 of these were retrieved. One additional included study (Tecklenburg 2007), an extension to a previously included study (Fogarty 2003), and 5 further excluded studies were identified. The review now contains a total of nine studies which meet the inclusion criteria. Further details can be found in the table, "Characteristics of included studies". A total of twelve studies were excluded after examining the full‐text paper. Please see "Characteristics of excluded studies" for further details.
Table 1.
Search history
| Search dates | Results |
| January 2001 | Thirty‐five abstracts were identified from the search of the Cochrane Airways Group register, of which 6 met the inclusion criteria (Anah 1980, Anderson 1983, Cohen 1997, Kordanksy 1979, Malo 1986, Schachter 1987) and 5 were added as excluded studies. |
| January 2001 ‐ April 2004 | Twenty‐four abstracts were identified by the updated search. Two additional included studies (Fogarty 2003, O'Sullivan 2000) and 4 excluded studies were added to the review. |
The included studies were conducted in the USA (Kordansky 1979; Schachter 1982,Tecklenburg 2007), Nigeria (Anah 1980), South Africa (Anderson 1983), Canada (Malo 1986), Israel (Cohen 1997) and the UK (Fogarty 2003; O'Sullivan 2000).
Three studies examined the impact of vitamin C supplementation on exercise challenge tests in subjects with a confirmed diagnosis of exercise‐induced asthma (Cohen 1997; Schachter 1982, Tecklenburg 2007). Two studies examined the impact of vitamin C administration on bronchial hyper responsiveness to histamine challenge tests in participants with asthma (Malo 1986; O'Sullivan 2000). Four studies examined the impact of vitamin C on bronchial hyper responsiveness to allergen challenge in subjects sensitive to ragweed allergen (Kordansky 1979), frequency of asthma exacerbations due to infection (Anah 1980), lung function and immunological markers in patients with asthma (Anderson 1983) and clinical control of asthma in primary care (Fogarty 2003).
Three of the studies (Anah 1980; Anderson 1983; Fogarty 2003) followed a parallel study design and the remaining 6 used crossover designs. Data from the two types of study designs were presented separately in RevMan. No usable data could be extracted from the reports of four studies (Anah 1980;Kordansky 1979; Malo 1986; O'Sullivan 2000), despite attempts at author contact.
Seven studies (Anah 1980; Fogarty 2003; Kordansky 1979; Malo 1986; O'Sullivan 2000; Schachter 1982, Tecklenburg 2007) involved adult patients, one study (Anderson 1983) involved only children and one (Cohen 1997) had both adults and children. The smallest study had six participants (Kordansky 1979). Others ranged from 8 to 41. The largest was Fogarty 2003 with 210 particpants. This review contains a total of 330 randomised participants.
All treatments were administered orally, either as tablets or as an oral solution. Three studies (Anah 1980; Anderson 1983; Fogarty 2003) featured long‐term supplementation with 1 g vitamin C daily for 14 weeks, 6 months and 16 weeks, respectively. Another long‐term study (Kordansky 1979) used 500 mg vitamin C supplementation daily for seven days. One study looked at supplementation with 1500mg over a short ‐term period of two weeks (Tecklenburg 2007) and the remaining four studies (Cohen 1997; Malo 1986; O'Sullivan 2000; Schachter 1982) used single doses of vitamin C 2g, 2g, 2g and 500 mg, respectively.
Two of the crossover studies (Cohen 1997, Tecklenburg 2007) mentioned a washout period. None of the other crossover studies (Kordansky 1979; Malo 1986; O'Sullivan 2000; Schachter 1982) reported a washout period. It has been suggested that after a single oral dose of vitamin C, at least 1‐2 days is required for excretion depending on pre‐existing body levels (Bates 2001).
Included studies reported disparate outcome measures, which made aggregation for the purpose of a meta‐analysis difficult. Most of the studies did not report the actual data in the published papers or did not provide sufficient data for a meta‐analysis, although attempts were made to contact the authors for data. Outcome measures included a variety of lung function tests, symptoms and symptom scores, immune markers and reduction in the use of inhaled steroids.
Risk of bias in included studies
In general, the reporting quality of the studies was poor. All the included studies were reported as being randomised, however, only one study (Fogarty 2003) reported the method of randomisation and just three of the nine studies reported the method of allocation concealment (Anah 1980, Fogarty 2003, Malo 1986). Four studies reported the method of blinding (Anah 1980, Malo 1986, Schachter 1982, Tecklenburg 2007), while one study was inadequately blinded (Anderson 1983) and the remaining four were unclear.
None of the studies adequately reported all three methods of randomisation, allocation concealment or blinding. Anah 1980, Fogarty 2003 and Malo 1986 gave the most detailed account. An overview of our judgments of are presented in Figure 1 and Figure 2.
Figure 1.
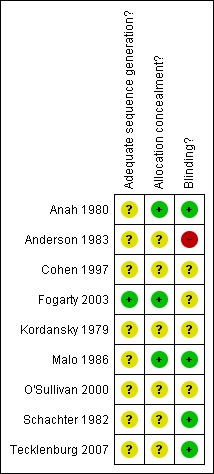
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Figure 2.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Effects of interventions
Of the nine included studies, five have contributed numerical data to the review: Anderson 1983, Cohen 1997, Fogarty 2003, Schachter 1982 and Tecklenburg 2007. Four studies (Anah 1980; Kordansky 1979; Malo 1986; O'Sullivan 2000) did not report data in an manner that permitted further analysis and author contact has been unsuccessful. However, none of these studies found a significant difference for the effect of vitamin C on lung function or symptoms.
It was only possible to combine data for one outcome (FEV1). For all other outcomes, studies could not be combined statistically, because those which addressed similar comparisons used different interventions or outcome variables. For example, there were three studies where the protective effects of vitamin C were investigated using exercise challenge. All three reported pulmonary function outcomes, but Cohen 1997 reported absolute mean value post‐exercise, Schachter 1982 reported absolute change post‐exercise and Tecklenburg 2007 was a two week intervention study rather a single dose study, and reported maximum precentage fall from baseline.
Primary outcomes
Lung function
Single dose studies
FEV1 (L) ‐ pre‐exercise challenge: no significant difference (Cohen 1997, Schachter 1982)
FEV1 (L) ‐ post‐exercise challenge: no significant difference (Cohen 1997, Schachter 1982)
FVC (L) ‐ pre‐exercise challenge: no significant difference (Schachter 1982)
FVC (L) ‐ post‐exercise challenge: no significant difference (Schachter 1982)
PEFR (L/min) ‐ pre‐exercise challenge: no significant difference (Schachter 1982)
PEFR (L/min) ‐ post‐exercise challenge: no significant difference (Schachter 1982)
Short term studies
FEV1 (%) drop post‐exercise: a significant difference was shown in favour of vitamin C: 6.5 (95% confidence interval 0.05 to 12.95), P=0.05 (Tecklenburg 2007)
Long term studies
FEV1 mL at four months:no significant difference (Fogarty 2003)
Peak Flow L/min (morning and evening) at 4 months: no significant difference (Fogarty 2003)
Symptom Scores
One study (Tecklenburg 2007) reported data on symptom scores (Asthma Quality of Life Questionnaire). There was no significant difference.
Secondary outcomes
IgE (IU/ml serum) ‐ absolute values at one month: no significant difference (Anderson 1983)
IgE (IU/ml serum) ‐ absolute values at three months:no significant difference (Anderson 1983)
IgE (IU/ml serum) ‐ absolute values at six months: no significant difference (Anderson 1983)
Decrease in inhaled corticosteroid use (µg): no significant difference (Fogarty 2003)
There were no data from any of the included studies for health service utilisation.
Data on acute exacerbations was provided by one study (Anah 1980). There were nine exacerbations in the intervention group which had 22 patients and 35 exacerbations in the placebo group which had 19 patients. Thus some patients had more than one exacerbation. Data concerning the number of patients who had one or more exacerbations would be more meaningful since it would not be subjected to bias from a few patients with recurrent exacerbations.
Adverse effects
Only one study (Anah 1980) reported on adverse effects. None of the participants in either group experienced any side effects.
Discussion
We found nine randomised controlled trials assessing vitamin C supplementation as a treatment for asthma. These covered single‐dose, short‐term and long‐term administration. Because the studies were very diverse and reported outcomes in different ways, it was not possible to pool the results. Four of the studies did not contribute any numerical data to the review, although none of these reported a significant difference for either of our primary outcomes of lung function and symptom scores. The only outcome reported by more than one study was pre‐exercise FEV1, and this did not show any significant difference.
One study (Tecklenburg 2007) found a significant difference in favour of vitamin C on the percentage drop in FEV1 post‐exercise in mild‐to‐moderate persistent asthmatics with exercise‐induced asthma. This was a small study of eight participants who were assigned to vitamin C or placebo for two weeks, then crossed over after a one week washout period. The size and duration of the trial means that the positive outcome should be interpreted with caution.
The reporting of the trial methods was generally poor. The method of randomisation, allocation concealment and blinding was not always clear, leaving the results open to bias. Only one study reported on adverse effects. No side effects were experienced by any participant in the vitamin C or placebo group, and Vitamin C is generally considered to be safe in recommended amounts, however, mega‐doses can cause adverse effects (MedlinePlus 2008) so it is still important for adverse effects to be documented in trial reports.
Previous reviews of vitamin C in asthma (Bielory 1994, Hatch 1995, Monteleone 1997) all recommended more research before any solid conclusions can be drawn. Although all but one of the studies included in this review appear to show no effect of vitamin C on asthma, the diversity of the study designs meant that it was not possible to combine them to gain an overall picture. The role of vitamin C in asthma is still unclear.
Authors' conclusions
Implications for practice.
At present, evidence from the limited number of randomised‐controlled trials is insufficient to recommend a specific role for vitamin C in the treatment or management of asthma.
Implications for research.
Further randomised, double blind, placebo controlled trials of vitamin C supplementation in asthma are needed, particularly in children. When designing such a trial, attention must be paid to well defined interventions excluding known confounders (e.g., smoking, caffeine, drugs), statistical power (to show clinically relevant differences) and the relevance of outcomes (e.g., lung function, symptoms, medication usage, quality of life, exacerbations). It is also important to consider the pharmacokinetics / pharmacodynamics of vitamin C for adequate dosing and washout period especially when using a crossover design.
Data and analyses
Comparison 1.
Oral vitamin C vs placebo (single‐dose studies)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (L) ‐ pre‐exercise challenge | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Absolute values | 2 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.36 [‐0.35, 1.08] |
| 2 FEV1 (L) ‐ post‐exercise challenge | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Absolute values | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐0.39, 0.93] |
| 2.2 Absolute change | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.05, 0.31] |
| 3 FVC (L) ‐ pre‐exercise challenge | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Absolute values | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐2.62, 2.42] |
| 4 FVC (L) ‐ post‐exercise challenge | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Absolute change | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.03, 0.29] |
| 5 PEFR (L/min) ‐ pre‐exercise challenge | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Absolute values | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐4.00, 3.20] |
| 6 PEFR (L/min) ‐ post‐exercise challenge | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Absolute change | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.49 [‐0.07, 1.05] |
Analysis 1.1.
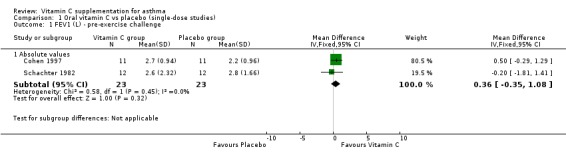
Comparison 1 Oral vitamin C vs placebo (single‐dose studies), Outcome 1 FEV1 (L) ‐ pre‐exercise challenge.
Analysis 1.2.
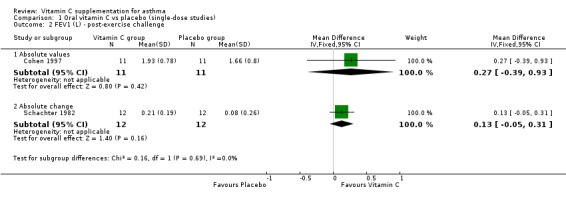
Comparison 1 Oral vitamin C vs placebo (single‐dose studies), Outcome 2 FEV1 (L) ‐ post‐exercise challenge.
Analysis 1.3.
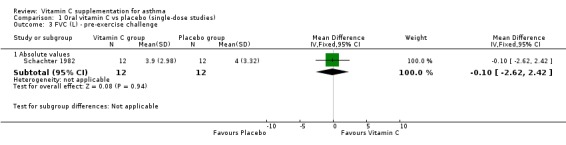
Comparison 1 Oral vitamin C vs placebo (single‐dose studies), Outcome 3 FVC (L) ‐ pre‐exercise challenge.
Analysis 1.4.
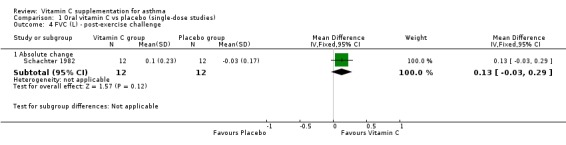
Comparison 1 Oral vitamin C vs placebo (single‐dose studies), Outcome 4 FVC (L) ‐ post‐exercise challenge.
Analysis 1.5.
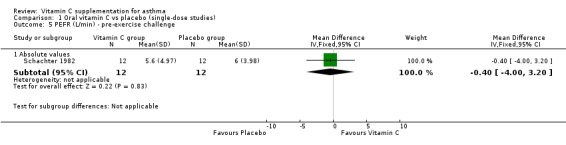
Comparison 1 Oral vitamin C vs placebo (single‐dose studies), Outcome 5 PEFR (L/min) ‐ pre‐exercise challenge.
Analysis 1.6.
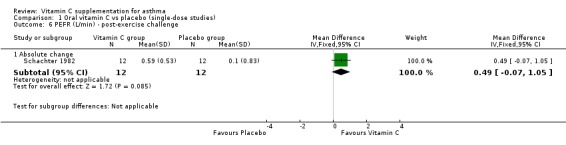
Comparison 1 Oral vitamin C vs placebo (single‐dose studies), Outcome 6 PEFR (L/min) ‐ post‐exercise challenge.
Comparison 2.
Oral vitamin C vs placebo (short term studies)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% drop) post‐exercise | 1 | 0 | Mean Difference (Fixed, 95% CI) | 6.5 [0.05, 12.95] |
| 2 Symptom scores (Asthma Quality of Life Questionnnaire) | 1 | 0 | Mean Difference (Fixed, 95% CI) | 0.5 [‐0.24, 1.24] |
Analysis 2.1.
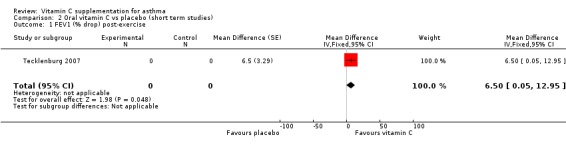
Comparison 2 Oral vitamin C vs placebo (short term studies), Outcome 1 FEV1 (% drop) post‐exercise.
Analysis 2.2.
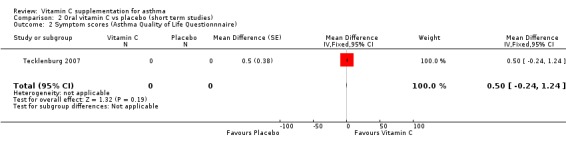
Comparison 2 Oral vitamin C vs placebo (short term studies), Outcome 2 Symptom scores (Asthma Quality of Life Questionnnaire).
Comparison 3.
Oral vitamin C vs placebo (long‐term studies)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IgE (IU/ml serum) ‐ absolute values | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 1 month | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐140.42, 148.42] |
| 1.2 3 months | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐312.0 [‐628.21, 4.21] |
| 1.3 6 months | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐143.0 [‐425.38, 139.38] |
| 2 FEV1 mL 4 months | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 3 Peak Flow (L/min) 4 months | 1 | Mean difference (Fixed, 95% CI) | Totals not selected | |
| 3.1 Morning | 1 | Mean difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Evening | 1 | Mean difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Geometric mean decrease in inhaled corticosteroid use (μg) | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 38.0 [‐20.46, 96.46] |
Analysis 3.1.
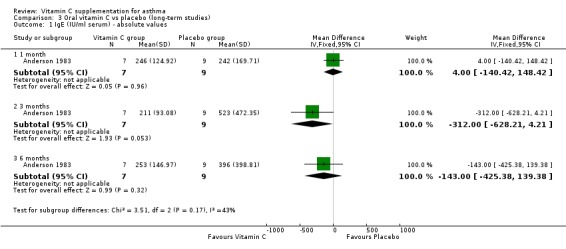
Comparison 3 Oral vitamin C vs placebo (long‐term studies), Outcome 1 IgE (IU/ml serum) ‐ absolute values.
Analysis 3.2.
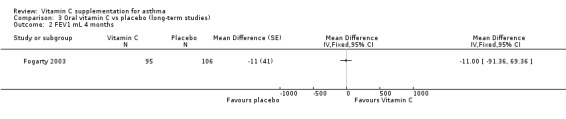
Comparison 3 Oral vitamin C vs placebo (long‐term studies), Outcome 2 FEV1 mL 4 months.
Analysis 3.3.

Comparison 3 Oral vitamin C vs placebo (long‐term studies), Outcome 3 Peak Flow (L/min) 4 months.
Analysis 3.4.
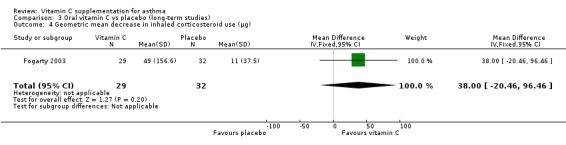
Comparison 3 Oral vitamin C vs placebo (long‐term studies), Outcome 4 Geometric mean decrease in inhaled corticosteroid use (μg).
What's new
Last assessed as up‐to‐date: 29 October 2008.
| Date | Event | Description |
|---|---|---|
| 29 October 2008 | New citation required but conclusions have not changed | One new included study, one extension to a previously included study (Fogarty 2003) and five excluded studies were identified. Conclusions remain unchanged. Change in authorship. |
| 29 August 2008 | New search has been performed | New search. |
| 14 August 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 3, 1996 Review first published: Issue 1, 1999
| Date | Event | Description |
|---|---|---|
| 1 April 2004 | New citation required and conclusions have changed | Substantive amendment |
Contributions of authors
Felix Ram and BK conducted the original version of this review in 2001. BR was the assigned editor contributing to the protocol and review editing. FR updated the review in April 2004. EA updated the review in August 2008.
Declarations of interest
There are no known conflicts of interest.
Sources of support
Internal sources
St George's, University of London, UK.
External sources
No sources of support supplied
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised double‐blind, placebo controlled, parallel trial. | |
| Participants | 41 Nigerian adults (22 males, 19 females), aged 15‐46, attending a hospital clinic. Non‐smokers, asthmatic for at least 4 years, with exacerbations in the rainy season due to respiratory infections. All continued with regular medication (mostly bronchodilators but N not specified, 1 on oral steroids). | |
| Interventions | For 14 weeks over the rainy season 1g vitamin C orally, once daily (n=22) compared to placebo dummy pill (n=19). | |
| Outcomes | Asthma attacks during the rainy season ‐ mild (self‐reported increase in wheeze/breathlessness), moderate (required increase in inhaler use, addition of medication on regular basis) severe (emergency admission) | |
| Notes | Data not presented in an abstractable format, no reply from author to date. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of randomisation not described |
| Allocation concealment? | Low risk | Allocation of treatment was coded and the code revealed after the completion of the study. |
| Blinding? All outcomes | Low risk | Tablets described as identical |
| Methods | Randomised, parallel trial. | |
| Participants | 16 white South African children (12 males, 4 females) aged 6‐13 on beta agonists, cromoglycates & aminophylline for asthma but no steroids. No intestinal parasites. | |
| Interventions | 1g vitamin C plus standard therapy daily for 6 months (n=7) vs standard therapy (n=9). Study also included i.v. arm but has no placebo arm for this i.v. group and the i.v. patients were not randomised. | |
| Outcomes | IgE titres | |
| Notes | Reported insignificant decreases in serum IgG, IgA, secretory IgA and IgM in both groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of randomisation not described |
| Allocation concealment? | Unclear risk | Method of allocation not described |
| Blinding? All outcomes | High risk | There was no placebo, control patients received standard therapy only. Trial is not described as blinded. |
| Methods | Randomised double blind placebo controlled crossover study. | |
| Participants | 20 patients (adults & children) with diagnosed exercise‐induced asthma with a fall in FEV1 >15% after exercise test on a motorised treadmill (13M;7F). Age ranging from 7‐28 yrs with mean 13.8 yrs. | |
| Interventions | 2g of oral ascorbic acid or placebo 1 hour before a 7‐minute exercise session on a treadmill. Pulmonary function tests were performed after an 8‐minute rest. Procedure repeated 1 week later, with each subject receiving the alternative medication. 5 patients continued on a 2 week treatment but not able to use data as there was no control or placebo arm for this group of patients were selected as they showed protective effect with vitamin C in the 1 hour study (not randomised). | |
| Outcomes | FEV1 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of randomisation not described |
| Allocation concealment? | Unclear risk | Method of allocation not described |
| Blinding? All outcomes | Unclear risk | Study described as double blind but method of blinding (e.g. identical placebo pill) not described |
| Methods | Randomised placebo‐controlled double‐blind parallel group trial. Study was powered to detect a 310ml change in FEV1, a 20l/min increase in PEFR or a 1.5 fold in airway reactivity relative to placebo. | |
| Participants | Patients were identified from computer records of 24 general practices in Nottingham, UK. At the start of the study there were 95 participants in the vitamin C group and 106 in the placebo group (and 99 in a third group randomised to magnesium). Vitamin C group: 37 males, mean age 42, mean daily inhaled steroids 715ug, number of long acting beta 2 agonists 20, current smokers 5, mean pack years 1.2, mean dietary vitamin C 90mg. Placebo group: 42 males, mean age 40, mean daily inhaled steroids 618ug, number of long acting beta 2 agonists 14, current smokers 4, mean pack years 1.1, mean dietary vitamin C 82mg. |
|
| Interventions | Parallel comparison of daily supplementation with vitamin C 1g/day and placebo. | |
| Outcomes | FEV1, FVC, PD20, morning and evening PEFR, beta 2 use, symptoms. | |
| Notes | Randomisation was stratified according to dose of regular corticosteroids usage. An extension to the trial provided data on the reduction in use of inhaled steroids. Ninety two of the original participants agreed to continue taking their allocated supplement for a further 10 weeks and enter a corticosteroid reduction protocol. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Participants were randomly assigned using a random number generator. |
| Allocation concealment? | Low risk | Particpants were allocated by an individual code number. The code was broken only after the last particpant left the trial. All randomisation table preparation and dispensing were carried out independently from the recruitment and assessment of participants. |
| Blinding? All outcomes | Unclear risk | Study is described as double‐blind but the appearance of the tablets is not specifically mentioned |
| Methods | Randomised double‐blind placebo controlled trial with crossover design. | |
| Participants | 6 adults (2 female, 4 male) in Baltimore, USA, with ragweed sensitive asthma, defined by skin‐prick positivity. Tested out of the ragweed season. Asymptomatic and not on treatment. | |
| Interventions | 500mg once daily vitamin C for 7 days compared to lactose placebo. | |
| Outcomes | PD20 FEV1, PD35 SGaw, tested on day 7, 3hrs after dose of placebo/vitamin C | |
| Notes | Data not presented in an abstractable format, no reply from author to date. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of randomisation not described |
| Allocation concealment? | Unclear risk | Method of allocation not described |
| Blinding? All outcomes | Unclear risk | Study described as double blind but method of blinding (e.g. identical placebo pill) not described |
| Methods | Randomised double blind placebo controlled crossover study. | |
| Participants | 16 adults (3M; 13F) with asthma that met the ATS criteria. Age range 19‐59, mean 43.1 (SD 7.7) yrs, mean duration of asthma 10.5 yrs (SD 14.6). | |
| Interventions | The subjects were studied on 4 different days. Subjects received treatment or placebo which consisted of 250ml of a transparent and odourless sweet liquid in which was dissolved either 2g ascorbic acid or placebo. One hour later spirometry measured and histamine challenge done until PC20 reached. | |
| Outcomes | FEV1, FVC, PC20 | |
| Notes | Data not presented in an abstractable format. Values reported for different days rather than different groups. No reply from author to date. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of randomisation not described |
| Allocation concealment? | Low risk | Concealment of treatments were done using codes. The oral solutions were prepared by hospital pharmacy. |
| Blinding? All outcomes | Low risk | Oral solutions were described as being of similar taste |
| Methods | Randomised double‐blind, cross‐over placebo controlled study. | |
| Participants | Ten mild (ATS criteria) asthmatic participants. | |
| Interventions | Each participant completed two treatment periods with ingestion of either 2g of ascorbic acid or placebo 45 minutes prior histamine bronchoprovocation. | |
| Outcomes | Spirometry was measured before, during and after the histamine challenges. | |
| Notes | Abstract only published, data not presented in an abstractable format, no reply from author to date. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of randomisation not described |
| Allocation concealment? | Unclear risk | Method of allocation not described |
| Blinding? All outcomes | Unclear risk | Study described as double blind but method of blinding (e.g. identical placebo pill) not described |
| Methods | Randomised double‐blind controlled trial, crossover design. | |
| Participants | 12 adults (5 male, 7 female) with exercise‐induced asthma, never on corticosteroids or admitted to hospital. | |
| Interventions | Single dose of 500 mg vitamin C orally or sucrose placebo. Study done on 2 subsequent days. 90 minutes post does subjects underwent exercise challenge. No washout indicated. Exercise challenge in incremental workload and until subjects heart rate reach 170bpm or the subject fatigued. Pulmonary function was measured before & after oral dose and after exercise. | |
| Outcomes | FVC, FEV1, PEFR before and after exercise challenge on cyclegometer to 170bpm or exhaustion. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of randomisation not described |
| Allocation concealment? | Unclear risk | Method of allocation not described |
| Blinding? All outcomes | Low risk | Identical placebo capsule |
| Methods | Randomised, double‐blind, crossover trial over 5 consecutive weeks | |
| Participants | Eight participants (2 male, 6 female) with physician diagnosed mild‐to‐moderate asthma and documented exercise‐induced bronchoconstriction. Particpants were recruited from University population and the local community and were active. | |
| Interventions | Ascorbic acid supplement 1500mg/day (3x500mg capsules) or placebo (sucrose) (3 capsules). Manufactured by NOW Foods. Particpants were randomised to active treatment or placebo for two weeks. There was a wash‐out period of one week and then the particpants crossed over. Particpants were advised to avoid foods that were high in vitamin C during the study. |
|
| Outcomes | Pulmonary function (FEV1) pre‐and post‐exercise; Exhaled nitric oxide (FENO) pre‐ and post‐exercse; sypmtom questionnaire | |
| Notes | Study conducted in the USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of randomisation not described |
| Allocation concealment? | Unclear risk | Method of allocation concealment not described. |
| Blinding? All outcomes | Low risk | Matching placebo manufactured by the same company as the active treatment. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cuomo 2004 | Study used a combined supplement of vitamin C, vitamin E and other antioxidants |
| Forastiere 2000 | Not a randomised controlled trial (before and after questionnaire survey). |
| Gvozdjakova 2005 | Study used a combined supplement of vitamin C, Coenzyme Q10 and α‐tocopherol. |
| Kongerud 2003 | Not a randomised controlled trial and intervention not testing efficacy of vitamin C. Study examined the levels of ascorbic acid in induced sputum of asthmatic patients compared to healthy volunteers. |
| Miric 1991 | Not a randomised controlled trial. All subjects given placebo first than followed later with all patients receiving vitamin C. |
| Mohsenin 1983 | Study did not have a placebo arm (only had before and after effects of vitamin C administration). |
| Mohsenin 1987 | Study used healthy subjects and excluded subjects who had asthma. |
| Murphy 2002 | Study used a combination of vitamin C and α‐tocopherol |
| Omenaas 2003 | Postal questionnaire not a randomised controlled trial. |
| Panina 2002 | Study used a complex of oral antioxidants. It is not clear that the study was randomised. |
| Romieu 2002 | Study used both vitamin C and E in the intervention group. |
| Ting 1983 | Study did not have a placebo arm and was not randomised (only had before and after effects of vitamin C). |
Acknowledgements
The authors would like to acknowledge the work of Dr Felix Ram in producing the original version, and the 2004 update of this review.
Kaur B, Rowe BH, Arnold E. Vitamin C supplementation for asthma. Cochrane Database of Systematic Reviews 2009, Issue 1 Art. No.: CD000993. DOI: 10.1002/14651858.CD000993.pub3.
References
References to studies included in this review
Anah 1980 {published data only}
- Anah CO, Jarike LN, Baig HA. High dose ascorbic acid in Nigerian asthmatics. Tropical and Geographical Medicine 1980;32(2):132‐7. [PMID: 7423602] [PubMed] [Google Scholar]
Anderson 1983 {published data only}
- Anderson R, Hay I, van Wyk HA, Theron A. Ascorbic acid in bronchial asthma. South African Medical Journal 1983;63(17):649‐52. [PubMed] [Google Scholar]
Cohen 1997 {published data only}
- Cohen HA, Neuman I, Nahum H. Blocking effect of vitamin C in exercise‐induced asthma. Archives of Pediatrics and Adolescent Medicine 1997;151(4):367‐70. [DOI] [PubMed] [Google Scholar]
Fogarty 2003 {published data only}
- Fogarty A, Lewis SA, Scrivener SL, Antoniak M, Pacey S, Pringle M. Corticosteroid sparing effects of vitamin C and magnesium in asthma: a randomised trial. Respiratory Medicine 2006;100(1):174‐9. [PUBMED: 16338599] [DOI] [PubMed] [Google Scholar]
- Fogarty A, Lewis SA, Scrivener SL, Antoniak M, Pacey S, Pringle M, et al. Oral magnesium and vitamin C supplements in asthma: a parallel group randomized placebo‐controlled trial. Clinical and Experimental Allergy 2003;33(10):1355‐9. [PUBMED: 14519140] [DOI] [PubMed] [Google Scholar]
Kordansky 1979 {published data only}
- Kordansky DW, Rosenthal RR, Norman PS. The effect of vitamin C on antigen‐induced bronchospasm. Journal of Allergy and Clinical Immunology 1979;63(1):61‐64. [DOI] [PubMed] [Google Scholar]
Malo 1986 {published data only}
- Malo JL, Cartier A, Pineau L, L'Archeveque J, Ghezzo H, Martin RR. Lack of acute effects of ascorbic acid on spirometry and airway responsiveness to histamine in subjects with asthma. Journal of Allergy and Clinical Immunology 1986;78(6):1153‐8. [DOI] [PubMed] [Google Scholar]
O'Sullivan 2000 {published data only}
- O'Sullivan S, Doyle S, Cormican L, Gunaratnam C, Poulter LW, Burke CM. Attenuation of bronchial hyperresponsiveness to histamine by vitamin C in asthmatic subjects. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A106. [Google Scholar]
Schachter 1982 {published data only}
- Schachter EN, Schlesinger A. The attenuation of exercise‐induced bronchospasm by ascorbic acid. Annals of Allergy 1982;49(3):146‐151. [PubMed] [Google Scholar]
Tecklenburg 2007 {published data only}
- Tecklenburg SL, Mickleborough TD, Fly AD, Bai Y, Stager JM. Ascorbic acid supplementation attenuates exercise‐induced bronchoconstriction in patients with asthma. Respiratory Medicine 2007;101(8):1770‐8. [PUBMED: 17412579 ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cuomo 2004 {published data only}
- Cuomo B, Fasoli L, Guerrea T, Cosettini M, Don M, Saretta F, et al. Efficacy of antioxidant supplementation diet in asthmatic children [Abstract]. European Respiratory Journal 2004;24(Suppl 48):167s. [Google Scholar]
Forastiere 2000 {published data only}
- Forastiere F, Pistelli R, Sestini P, Fortes C, Renzoni E, Rusconi F, et al. Consumption of fresh fruit rich in vitamin C and wheezing symptoms in children. SIDRIA Collaborative Group, Italy (Italian Studies on Respiratory Disorders in Children and the Environment). Thorax 2000;55(4):283‐8. [PMID: 10722767] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gvozdjakova 2005 {published data only}
- Gazdik F, Gazdikova K, Jahnova E, Pijak MR. Sparing effect of coenzyme Q10, a‐tocopherol and ascorbic acid on the consumption of corticosteroids in allergic asthmatics [Abstract]. Allergy & Clinical Immunology International 2003;1 Suppl:Abstract No: P‐2‐38. [Google Scholar]
- Gazdik F, Gvozdjakova A, Kucharska J, Kucharikova Z, Jahnova E, Gazdikova. Coenzyme Q10 supplementation therapy decreased corticosteroid consumption in patients with asthma. Advances in Immunopathology & Respiratory Allergy. 2005:29‐33.
- Gvozdjakova A, Kucharska J, Bartkovjakova M, Gazdikova K, Gazdik FE. Coenzyme Q10 supplementation reduces corticosteroids dosage in patients with bronchial asthma. Biofactors 2005;25:235‐40. [PUBMED: 16873952] [DOI] [PubMed] [Google Scholar]
Kongerud 2003 {published data only}
- Kongerud J, Crissman K, Hatch G, Alexis N. Ascorbic acid is decreased in induced sputum of mild asthmatics. Inhalation Toxicology 2003;15:101‐109. [DOI] [PubMed] [Google Scholar]
Miric 1991 {published data only}
- Miric M, Haxhiu MA. Effect of vitamin C on exercise‐induced bronchoconstriction [Article in Serbo‐Croatian (Roman)]. Plucne Bolesti 1991;43(1‐2):94‐97. [MEDLINE: PMID: 1766998; UI: 92115812] [PubMed] [Google Scholar]
Mohsenin 1983 {published data only}
- Mohsenin V, Dubois AB, Douglas JS. Effect of ascorbic acid on response to methacholine challenge in asthmatic subjects. American Review of Respiratory Diseases 1983;127(3):143‐147. [MEDLINE: PMID: 6830027; UI: 83150876] [DOI] [PubMed] [Google Scholar]
Mohsenin 1987 {published data only}
- Mohsenin V. Effect of vitamin C on NO2‐induced airway hyperresponsiveness in normal subjects. American Review of Respiratory Diseases 1987;136:1408‐1411. [DOI] [PubMed] [Google Scholar]
Murphy 2002 {published data only}
- Murphy JD, Ferguson CS, Brown KR, Harms CA. The effect of dietry antioxidants on lung function in exercise induced asthmatics. Medicine & Science in Sports & Exercise 2002;34(5 Suppl 1):S155. [Google Scholar]
Omenaas 2003 {published data only}
- Omenaas E, Fluge O, Buist AS, Vollmer WM, Gulsvik. Dietary vitamin C intake is inversely related to cough and wheeze in young smokers. Respiratory Medicine 2003;97(2):134‐42. [DOI] [PubMed] [Google Scholar]
Panina 2002 {published data only}
- Panina NT, Yakovleva NG, Kotenko TV, Danilov LN. Role of antioxidant and trace element complex in outpatient care of bronchial asthma [Abstract]. European Respiratory Journal 2002;20(Suppl 38):52s. [Google Scholar]
Romieu 2002 {published data only}
- Ramirez M, Morena H, Sienra JJ, Reyes N, DelRio BE, Hatch G, et al. Modulation of air pollution impact on peak expiratory flow by serum levels of a‐tocopherol and ascorbic acid among asthmatic children in Mexico City. American Thoracic Society 99th International Conference. 2003:B091 Poster 917.
- Romieu I, Sienra J J, Ramirez M, Moreno H, Reyes N I, Del Rio BE, et al. Antioxidant supplementation and generic susceptibility to ozone: a randomized controlled trial of children with asthma [abstract]. American Thoracic Society 99th International Conference. 2003:C014.
- Romieu I, Sienra JJ, Ramirez M, Moreno H, Del Rio BE, Hatch G, et al. Antioxidant supplementation and inflammatory responses among young asthmatics exposed to high levels of air pollutants [Abstract]. European Respiratory Journal 2004;24(Suppl 48):623s. [Google Scholar]
- Romieu I, Sienra‐Monge J J, Ramirez‐Aguilar M, Moreno‐Macias H, Reyes‐Ruiz N I, Estela del Rio‐Navarro B, et al. Genetic polymorphism of GSTM1 and antioxidant supplementation influence lung function in relation to ozone exposure in asthmatic children in Mexico City. Thorax 2004;59(1):8‐10. [PMC free article] [PubMed] [Google Scholar]
- Romieu I, Sienra‐Monge JJ, Ramirez‐Aguilar M, Moreno‐Macias H, Reyes‐Ruiz NI, Del Rio‐Navarro BE. Incidence of wheezing and genetic polymorphism of GSTM1 among asthmatic children supplemented with antioxidants in Mexico City [Abstract]. American Thoracic Society 100th International Conference, May 21‐26, 2004, Orlando. 2004:C24 Poster 701.
- Romieu I, Sienra‐Monge JJ, Ramirez‐Aguilar M, Tellez‐Rojo MM, Moreno‐Macias H, Reyes‐Ruiz NI, et al. Antioxidant supplementation and lung functions among children with asthma exposed to high levels of air pollutants. American Journal of Respiratory & Critical Care Medicine 2002;166(5):703‐9. [PUBMED: 12204869] [DOI] [PubMed] [Google Scholar]
- Sienra‐Monge JJ, Ramirez‐Aguilar M, Moreno‐Macias H, Reyes‐Ruiz NI, Del Rio‐Navarro BE, Ruiz‐Navarro MX, et al. Antioxidant supplementation and nasal inflammatory responses among young asthmatics exposed to high levels of ozone. Clinical & Experimental Immunology 2004;138(2):317‐22. [PUBMED: 15498043] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ting 1983 {published data only}
- Ting S, Mansfield LE, Yarbrough J. Effects of ascorbic acid on pulmonary functions in mild asthma. Journal of Asthma 1983;20(1):39‐42. [MEDLINE: PMID: 6853425; UI: 83213036] [DOI] [PubMed] [Google Scholar]
Additional references
Aderele 1985
- Aderele WI, Ette SI, Oduwole O, Ikpeme SJ. Plasma vitamin C (ascorbic acid) levels in asthmatic children. African Journal of Medical Science 1985;14(3‐4):115‐120. [MEDLINE: PMID: 3004170; UI: 86126797] [PubMed] [Google Scholar]
Bates 2001
- Bates C (Human Nutrition Unit, University of Cambridge) . Personal communication 2001.
Bielory 1994
- Bielory L, Gandhi R. Asthma and vitamin C. Annals of Allergy 1994;73:89‐99. [PubMed] [Google Scholar]
Butland 1999
- Butland BK, Strachan DP, Anderson HR. Fresh fruit intake and asthma symptoms in young British adults: confounding or effect modification by smoking?. European Respiratory Journal 1999;13(4):744‐50. [MEDLINE: PMID:10362034; UI:99288880] [DOI] [PubMed] [Google Scholar]
Cook 1997
- Cook DG, Carey IM, Whincup PH, Papagosta O, Chirico S, Bruckdorfer KR, Walker M. Effect of fresh fruit consumption on lung function and wheeze in children. Thorax 1997;52:628‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hatch 1995
- Hatch GE. Asthma, inhaled oxidants and dietary antioxidants. American Journal of Clinical Nutrition 1995;61(Suppl 3):625S‐630S. [DOI] [PubMed] [Google Scholar]
Holgate 1990
- Holgate ST. Interfaces in medicine: asthma. Report of a conference. Journal of the Royal College of Physicians of London 1990;24(4):313‐7. [MEDLINE: PMID: 2258849; UI: 91080048] [PMC free article] [PubMed] [Google Scholar]
Lewis 1996
- Lewis S, Butland B, Strachan D, Bynner J, Richards D, Butler N, et al. Study of the aetiology of wheezing illness at age 16 in two national British birth cohorts. Thorax 1996;51(7):670‐6. [MEDLINE: PMID: 8882071; UI: 97036423] [DOI] [PMC free article] [PubMed] [Google Scholar]
MedlinePlus 2008
- MedlinePlus. Vitamin C (ascorbic acid). http://www.nlm.nih.gov/medlineplus/druginfo/natural/patient‐vitaminc.html (accessed 29 August 2008).
Monteleone 1997
- Monteleone CA, Sherman AR. Nutrition and asthma. Archives of Internal Medicine 1997;157:23‐34. [PubMed] [Google Scholar]
Olusi 1979
- Olusi SO, Ojutiku OO, Jessop WJ, Iboko MI. Plasma and white blood cell ascorbic acid concentrations in patients with bronchial asthma. Clinica Chimica Acta 1979;92(2):161‐6. [MEDLINE: PMID: 487570; UI: 80023020] [DOI] [PubMed] [Google Scholar]
Schwartz 1994
- Schwartz J, Weiss ST. Relationship between dietary vitamin C intake and pulmonary function in the First National Health and Nutrition Examination Survey (NHANES I). American Journal of Clinical Nutrition 1994;59(1):110‐4. [MEDLINE: PMID: 8279390; UI: 94106429] [DOI] [PubMed] [Google Scholar]
Seaton 1994
- Seaton A, Godden DJ, Brown K. Increase in asthma: a more toxic environment or a more susceptible population?. Thorax 1994;49(2):171‐4. [MEDLINE: PMID: 8128408; UI: 94174476] [DOI] [PMC free article] [PubMed] [Google Scholar]
Strachan 1999
- Strachan D. The epidemiology of childhood asthma. Allergy 1999;54(Suppl 49):7‐11. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ram 2001
- Ram FSF, Rowe BH, Kaur B. Vitamin C supplementation for asthma. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD000993] [DOI] [PubMed] [Google Scholar]
Ram 2004
- Ram FSF, Rowe BH, Kaur B. Vitamin C supplementation for asthma. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD000993.pub2] [DOI] [PubMed] [Google Scholar]


