Abstract
Background
Vancomycin-resistant enterococcus (VRE) causes substantial health care–associated infection with increasing reports of resistance to daptomycin or linezolid. We conducted a case–control study reporting 81 cases of daptomycin and linezolid–nonsusceptible VRE (DLVRE), a resistance pattern not previously reported.
Methods
We reviewed VRE isolates from June 2010 through June 2015 for nonsusceptibility to both daptomycin (minimum inhibitory concentration [MIC] > 4) and linezolid (MIC ≥ 4). We matched cases by year to control patients with VRE susceptible to both daptomycin and linezolid and performed retrospective chart review to gather risk factor and outcome data.
Results
We identified 81 DLVRE cases. Resistance to both daptomycin and linezolid was more common than resistance to either agent individually. Compared with susceptible VRE, DLVRE was more likely to present as bacteremia without focus (P < 0.01), with DLVRE patients more likely to be immune suppressed (P = .04), to be neutropenic (P = .03), or to have had an invasive procedure in the prior 30 days (P = .04). Any antibiotic exposure over the prior 30 days conferred a 4-fold increased risk for DLVRE (odds ratio [OR], 4.25; 95% confidence interval [CI], 1.43−12.63; P = .01); multivariate analysis implicated daptomycin days of therapy (DOT) over the past year as a specific risk factor (OR, 1.10; 95% CI, 1.01−1.19; P = .03). DLVRE cases had longer hospitalizations (P = .04) but no increased risk for in-hospital death.
Conclusions
DLVRE is an emerging multidrug-resistant pathogen associated with immune suppression, neutropenia, and recent invasive procedure. Prior antibiotic exposure, specifically daptomycin exposure, confers risk for acquisition of DLVRE.
Keywords: daptomycin, linezolid, resistance, risk factors, vancomycin-resistant enterococcus
Vancomycin-resistant enterococci (VRE) were first reported in 1988 and quickly emerged as a major health care–associated pathogen [1]. VRE colonization alone increases risk for infection, and vancomycin resistance is an independent risk factor for increased mortality in patients with enterococcal bacteremia [1, 2]. The Food and Drug Administration approved linezolid, an oxazolidinone, in 2000, and daptomycin, a cyclic lipopeptide, in 2003, both of which have become mainstays of therapy against VRE infection.
Enterococcal resistance to linezolid and daptomycin emerged not long after these antibiotics were introduced. Linezolid resistance was observed in the laboratory before drug approval, then noted again during compassionate use [3]. Daptomycin-resistant enterococci were also seen in vitro before clinical use and again during daptomycin clinical trials [4]. Surveillance programs for both linezolid and daptomycin continue to report <1% overall enterococcal resistance [5–8]. However, case reports of linezolid-nonsusceptible enterococcus (LNSE) and daptomycin-nonsusceptible enterococcus (DNSE) continue to accumulate, with 1 institution reporting daptomycin resistance rates among VRE isolates as high as 15% [9].
Small studies have proposed risk factors for acquiring LNSE, including immune suppression, prior positive methicillin-resistant Staphylococcus aureus (MRSA) culture, peripheral vascular disease, solid organ transplant, allogeneic stem cell transplant, receipt of total parental nutrition, invasive procedure, and length of hospital stay [3, 10–15]. Beta-lactam and sulfonamide exposure have been implicated, but data are conflicting as to whether linezolid exposure is a specific risk factor for LNSE [12, 13, 15].
Small studies have also identified risk factors for acquiring DNSE, including immune suppression, comorbid conditions, recent surgery, retained nidus of infection, and length of hospital stay [9, 16–19]. Cephalosporins, nitrofurantoin, and antibiotics active against anaerobes have been reported as risk factors for DNSE, but it is not clear if daptomycin exposure specifically is an independent risk factor for DNSE [9, 16–19].
We noted an increasing incidence of VRE isolates lacking susceptibility to both daptomycin and linezolid at our institution, a pattern not previously described in the literature [20]. We sought to characterize risk factors for acquisition of daptomycin and linezolid–nonsusceptible vancomycin-resistant enterococcus (DLVRE) through a retrospective, case–control study comparing patients with DLVRE with patients with VRE susceptible to both daptomycin and linezolid.
METHODS
We reviewed all VRE isolate data from June 1, 2010, through June 30, 2015, including both inpatients and outpatients. Cases were defined as patients with a nonduplicate VRE isolate with daptomycin minimum inhibitory concentration (MIC) >4 and linezolid MIC ≥4 by Phoenix automated broth microdilution (BD Diagnostic Systems, Sparks, MD). Controls were selected by random number generator from remaining patients with a nonduplicate VRE isolate susceptible to both daptomycin and linezolid. Patients <18 years of age were excluded. Controls were then matched 1:1 to cases by year of isolate.
If the primary provider or infectious diseases consultant elected to treat the VRE isolate with antibiotic therapy, the patient was deemed to have an active infection rather than colonization. Type of infection was recorded as the diagnosis provided by the primary provider in the discharge summary, infectious diseases consult final note, or report of death note.
Patient demographic and medical history data were extracted retrospectively by chart review and managed using REDCap electronic data capture tools [21]. Immune suppression was defined as chemotherapy, steroids (equivalent to ≥10 mg oral prednisone for >5 days), tacrolimus, mycophenalate mofetil, cyclosporine, or tumor necrosis factor (TNF) inhibitor in the past 30 days. Neutropenia was defined as an absolute neutrophil count (ANC) <500 cells/µL ±14 days from time of culture.
Health care exposure was counted as a minimum of 1 exposure to dialysis, home health services, invasive procedure (defined as surgery requiring general anesthesia, percutaneous interventional radiology procedure, biopsy, or endoscopy), long-term care facility and/or hospital admission as reported in prior outpatient clinic notes, provider history, or subsequent inpatient daily notes. Antibiotic exposure data were recorded as a minimum of 1 dose obtained from review of provider documentation (including outpatient clinic notes, prior discharge summaries, and inpatient daily notes). Both health care and antibiotic exposure were documented as occurring in the past year, 90 days, and/or 30 days. Prior vancomycin, linezolid, and daptomycin exposure was recorded as total days of therapy (DOT) over the past year, counting any dose on a given day as 1 DOT. Inpatient DOT data at our institution were collected by review of the electronic medication administration record (eMAR). We verified outpatient DOT data at our institution by reviewing weekly parenteral antimicrobial home infusion records, which are monitored weekly by infectious diseases clinicians. DOT data at other institutions were culled from review of provider documentation including outpatient clinic notes, prior discharge summaries, and inpatient daily notes.
Statistical analysis was performed on Stata software (Release 14, StataCorp LP, College Station, TX). Univariate and multivariate analysis were performed using conditional logistic regression, except where noted. Variables with a P value <.05 were chosen for multivariate analysis, with daptomycin DOT in the prior year selected as the representative variable for antibiotic exposure. Institutional review board approval was obtained before requesting isolate data from our microbiologic database.
RESULTS
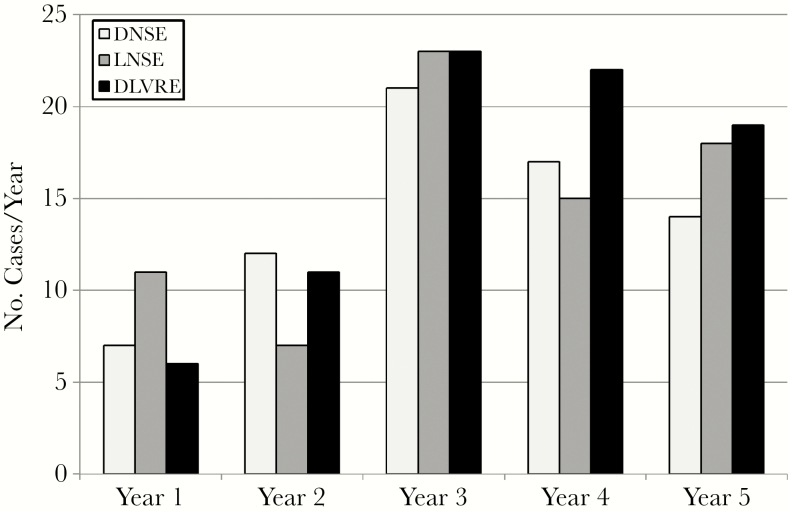
A total of 81 DLVRE cases were identified; 95% were speciated as Enterococcus faecium. The total number of DLVRE cases (n = 81) was greater than either DNSE (n = 71) or LNSE (n = 74) patients over the study period (Figure 1).
Figure 1.
Case counts for daptomycin-nonsusceptible enterococcus (DNSE), linezolid-nonsusceptible enterococcus (LNSE), and daptomycin and linezolid–nonsusceptible enterococcus (DLVRE).
DLVRE patients and daptomycin and linezolid–susceptible VRE patients differed with respect to age, which was normally distributed, with cases more likely to be younger than controls (mean age, 53 years vs 59 years; P = .04) (Table 1). Roughly half of DLVRE patients were considered to be colonized, which was not significantly different relative to controls (46% vs 57%; P = .15). There were no instances in which the primary provider and infectious diseases consultant disagreed on colonization vs active infection. Of the active infections, DLVRE was more likely to present as bacteremia without focus (25% vs 0%; P < 0.01), whereas controls were more likely to present as cystitis (14% vs 40%; P = .01) or CAUTI (0% vs 11%; P = .02) (Table 3).
Table 1.
Characteristics and Risk Factors for Case and Control Patients in a Study of Daptomycin and Linezolid–Nonsusceptible Vancomycin-Resistant Enterococcus
| Case Patients | Control Patients | P Value | |||
|---|---|---|---|---|---|
| (n = 81) | (n = 81) | ||||
| Age, mean ± SD, y | 53 ± 17.3 | 59 ± 16.9 | .04 | ||
| Sex | |||||
| Male | 34 | (42) | 29 | (36) | .43 |
| Female | 47 | (58) | 52 | (64) | |
| Race | |||||
| White | 66 | (81) | 66 | (81) | 1.00 |
| Black | 12 | (15) | 8 | (10) | .35 |
| Other | 3 | (4) | 7 | (9) | |
| Colonization | 37 | (46) | 46 | (57) | .15 |
| Comorbidity | |||||
| Immune suppression | 39 | (48) | 27 | (33) | .04 |
| Hematologic malignancy | 23 | (28) | 13 | (16) | .06 |
| Solid organ malignancy | 11 | (14) | 13 | (16) | .67 |
| Neutropenia | 17 | (21) | 7 | (9) | .03 |
| Bone marrow transplant | 8 | (10) | 2 | (2) | .07 |
| Diabetes | 26 | (32) | 31 | (38) | .37 |
| Chronic kidney disease | 18 | (22) | 21 | (26) | .59 |
| Congestive heart failure | 14 | (17) | 16 | (20) | .70 |
| COPD | 10 | (12) | 12 | (15) | .67 |
| Total parenteral nutrition | 8 | (10) | 5 | (6) | .37 |
| Cirrhosis | 2 | (2) | 8 | (10) | .08 |
| HIV infection | 2 | (2) | 3 | (4) | .66 |
| Health care exposure in the prior 30 days | |||||
| Hospital admission | 67 | (83) | 57 | (70) | .07 |
| Invasive procedure | 60 | (74) | 47 | (58) | .04 |
| Long-term care | 11 | (14) | 7 | (9) | .29 |
| Dialysis | 9 | (11) | 6 | (7) | .41 |
| Home health services | 5 | (6) | 4 | (5) | .71 |
| Prior MRSA isolate | 12 | (15) | 15 | (19) | .53 |
| Indwelling device | 62 | (77) | 54 | (67) | .14 |
| Antibiotic exposure in the prior 30 days | |||||
| Any antibiotic | 74 | (91) | 61 | (75) | .01 |
| Vancomycin | 54 | (67) | 40 | (49) | .05 |
| Daptomycin | 14 | (17) | 1 | (1) | <.001 |
| Linezolid | 4 | (5) | 2 | (2) | .42 |
| Other | 73 | (90) | 61 | (75) | .1 |
| Antibiotic days of therapy in the prior year | |||||
| Vancomycin | 12.33 | 11.80 | .86 | ||
| Daptomycin | 4.31 | 0.43 | .03 | ||
| Linezolid | 1.09 | 0.38 | .21 | ||
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: COPD, chronic obstructive pulmonary disease; MRSA, methicillin-resistant Staphylococcus aureus.
Table 3.
Hospital Course for Admitted Case and Control Patients in a Study of Daptomycin and Linezolid–Nonsusceptible Vancomycin-Resistant Enterococcus
| Hospital Course | Case Patients | Control Patients | P Value | ||
|---|---|---|---|---|---|
| (n = 69) | (n = 64) | ||||
| Infectious disease consult | 49 | (71) | 33 | (52) | .02 |
| ICU during hospitalization | 42 | (61) | 32 | (50) | .58 |
| Length of stay, days | |||||
| Hospitalization | 27.4 | 17.6 | .04 | ||
| ICU | 14.8 | 14.4 | .27 | ||
| In-hospital death | 12 | (17) | 10 | (16) | .59 |
Data are presented as No. (%) unless otherwise indicated. P values for length of stay were calculated using a signed-rank test.
Abbreviation: ICU, intensive care unit.
Most DLVRE patients had at least 1 comorbidity (91%), with malignancy, diabetes, and chronic kidney disease as common conditions (Table 2). Cases were more likely than controls to have received immune suppression in the prior 30 days (48% vs 33%; P = .04) or be neutropenic at the time of DLVRE culture (21% vs 9%; P = .03). The majority of cases had some health care exposure in the prior 30 days (83%), with cases being more likely than controls to have had some invasive procedure during that time frame (74% vs 58%; P = .04).
Table 2.
Type of Infection and Antibiotic Therapy for Case and Control Patients With Active Infection in a Study of Daptomycin and Linezolid–Nonsusceptible Vancomycin-Resistant Enterococcus
| Case Patients | Control Patients | P Value | |||
|---|---|---|---|---|---|
| (n = 44) | (n = 35) | ||||
| Type of infection | |||||
| Bacteremia without focus | 11 | (25) | 0 | (0) | <.01 |
| Cystitis | 6 | (14) | 14 | (40) | .01 |
| Abscess (not skin) | 8 | (18) | 3 | (9) | .33 |
| Osteomyelitis | 3 | (7) | 5 | (14) | .46 |
| Pneumonia | 3 | (7) | 1 | (3) | .63 |
| CLABSI | 5 | (11) | 4 | (11) | .99 |
| CAUTI | 0 | (0) | 4 | (11) | .04 |
| Pyelonephritis | 2 | (5) | 0 | (0) | .50 |
| Empyema | 1 | (2) | 0 | (0) | .99 |
| Cellulitis | 1 | (2) | 2 | (6) | .19 |
| Peritonitis | 0 | (0) | 1 | (3) | .44 |
| Meningitis | 0 | (0) | 0 | (0) | |
| Septic arthritis | 0 | (0) | 0 | (0) | |
| Other | 4 | (9) | 1 | (3) | .28 |
| Antibiotic choice | |||||
| Linezolid | 19 | (43) | 17 | (49) | .63 |
| Daptomycin | 17 | (39) | 13 | (37) | .89 |
| Tigecycline | 7 | (16) | 1 | (3) | .07 |
| Quinupristin-dalfopristin | 4 | (9) | 0 | (0) | .12 |
| Vancomycin | 3 | (7) | 0 | (0) | .25 |
| Beta-lactam | 2 | (5) | 1 | (3) | .99 |
| Aminogylcoside | 1 | (2) | 1 | (3) | .99 |
| Fosfomycin | 0 | (0) | 2 | (6) | .19 |
| Nitrofurantoin | 0 | (0) | 0 | (0) | |
| Active therapy | 10 | (23) | 35 | (100) | <.001 |
| Time to active therapy, days | 3.10 | 3.33 | .35 | ||
Data are presented as No. (%) unless otherwise indicated. Fisher exact tests, as appropriate, were used to test significant differences. P value for time to active therapy was calculated using a signed-rank test.
Abbreviations: CLABSI, central line–associated bloodstream infection; CAUTI, catheter-associated urinary tract infection.
DLVRE patients had more antibiotic exposure than controls in the prior 30 days (91% vs 75%; P = .01) (Table 2). This was particularly true of daptomycin exposure in the prior 30 days (17% vs 1%; P < 0.01) and cumulative daptomycin DOT in the prior year (4.31 vs 0.43; P = .03). More cases received linezolid than controls, but overall linezolid exposure in both groups was small and not statistically significant with respect to linezolid exposure in the prior 30 days (5% vs 2%; P = .42) and cumulative linezolid DOT in the prior year (1.09 vs 0.38; P = .21).
Time to active therapy was not different between cases and controls (3.10 vs 3.33 days; P = 0.35), but DLVRE patients were less likely to be treated with active therapy at all (23% vs 100%; P < .01) (Table 2). Though sample size limited statistical significance, DLVRE patients received more tigecycline (16% vs 3%; P = .07) and quinupristin-dalfopristin (9% vs 0%; P = .12). Cases and controls had a similar likelihood of being inpatient at the time of culture (85% vs 79%; P = .36), but cases had longer hospital stays than controls if admitted (27.4 vs 17.6 days; P = .04) (Table 4). DLVRE patients were not more likely to be admitted to an intensive care unit (61% vs 50%; P = .58) or to die during hospitalization (17% vs 16%; P = .59).
Table 4.
Multivariate Analysis of Risk Factors Associated With Daptomycin and Linezolid–Nonsusceptible Vancomycin-Resistant Enterococcus
| Risk Factor | OR | 95% CI | P Value |
|---|---|---|---|
| Age, years | 0.98 | 0.96–1.00 | .05 |
| Neutropenia | 3.13 | 0.89–11.02 | .08 |
| Immune suppression | 1.30 | 0.53–3.23 | .57 |
| Invasive procedure in the prior 30 days | 2.26 | 1.02–5.02 | .045 |
| Daptomycin days of therapy in the prior year | 1.10 | 1.01–1.19 | .03 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Multivariate analysis controlling for age, immune suppression in the past 30 days, neutropenia at time of culture, invasive procedure in the past 30 days, and daptomycin DOT in the past year confirmed recent invasive procedure to be an independent risk factor for DLVRE (odds ratio [OR], 2.26; 95% confidence interval [CI], 1.02−5.02; P = .045). Furthermore, this analysis showed that each day of prior daptomycin exposure increases risk for acquisition of DLVRE (OR, 1.10; 95% CI, 1.01−1.19; P = .03) (Table 1).
DISCUSSION
DLVRE represents a unique threat as therapeutic options beyond daptomycin and linezolid are few and may have increased toxicity. Multivariate analysis from this report found that recent invasive procedure and each day of prior daptomycin exposure increase a patient’s risk for DLVRE.
Daptomycin exposure correlates with risk for DLVRE acquisition despite the small amount of daptomycin receipt in the study group. It remains possible that linezolid too is a DLVRE risk factor, but the number of patients who received prior linezolid was insufficient to achieve statistical significance. On the other hand, the overall rarity of preceding daptomycin or linezolid exposure for DLVRE patients confirms the concern from prior DNSE and LNSE studies for a “community reservoir” of multidrug-resistant VRE not directly related to prior receipt of either antibiotic [18].
The number of DLVRE cases over the study period is relatively large compared with prior reports of either DNSE or LNSE. Triple resistance was at least as common as VRE resistance to either daptomycin or linezolid alone in our population, suggesting that DLVRE is a more prevalent pattern of resistance than anticipated. It is worth noting the disparate mechanisms of enterococcal resistance to daptomycin and linezolid. Daptomycin resistance has been attributed to genetic mutations affecting cell envelope homeostasis or cell membrane metabolism, whereas mutations in the 23S rRNA binding site account for enterococcal linezolid resistance [3, 20, 22–34]. Our study supports the need for ongoing research into the mechanism of VRE resistance, including any novel genetic lesions implicated in DLVRE.
DLVRE did not increase risk of in-hospital death relative to VRE, suggesting that virulence may not correlate with increased resistance. It is important to note that this study included linezolid intermediate isolates (MIC = 4) in the group labeled DLVRE. Partial linezolid activity against these intermediate isolates might account for the statistical insignificance of our in-hospital mortality data. Only 18 of the 81 DLVRE cases had linezolid MIC >4, which might serve as a smaller cohort for future study. Furthermore, morbidity and possibly mortality associated with DLVRE might occur after discharge but were not measured as part of this study.
This study is limited by reliance on 1-time reporting of susceptibility data by automated broth microdilution. We would have ideally repeated susceptibility testing or confirmed results by another method, but this was not possible as the majority of samples had been discarded. The discrepancy between cases and controls receiving active antibiotic therapy is likely a consequence of daptomycin and linezolid MIC data not being routinely reported in the medical record of our institution. This reporting strategy was intended to limit overuse of daptomycin and linezolid, though this has since been updated; daptomycin and linezolid MIC data are now automatically reported when the enterococcal isolate proves vancomycin resistant.
Our study exists amid growing concern for increasing enterococcal antibiotic resistance with unforetold clinical implications [35]. Treatment for VRE other than daptomycin and linezolid invites added toxicity supported by less robust outcome data, especially for invasive infection [36, 37].
The emergence of DLVRE as a highly drug-resistant pathogen places high priority on ongoing work to characterize the efficacy of synergistic therapy for multidrug-resistant VRE, such as the addition of beta-lactam therapy to restore susceptibility to daptomycin [38–40].
Acknowledgments
We thank Vanderbilt University Medical Center Microbiology, which cultured the isolates and performed susceptibility testing.
Financial support. This work was supported by Vanderbilt University Medical Center.
Potential conflicts of interest. None of the authors has a conflict of interest with the subject matter covered in this paper. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Zirakzadeh A, Patel R. Vancomycin-resistant enterococci: colonization, infection, detection, and treatment. Mayo Clinic Proc 2006; 81:529–36. [DOI] [PubMed] [Google Scholar]
- 2. DiazGranados CA, Zimmer SM, Klein M, Jernigan JA. Comparison of mortality associated with vancomycin-resistant and vancomycin-susceptible enterococcal bloodstream infections: a meta-analysis. Clin Infect Dis 2005; 41:327–33. [DOI] [PubMed] [Google Scholar]
- 3. Meka VG, Gold HS. Antimicrobial resistance to linezolid. Clin Infect Dis 2004; 39:1010–5. [DOI] [PubMed] [Google Scholar]
- 4. Humphries RM, Pollett S, Sakoulas G. A current perspective on daptomycin for the clinical microbiologist. Clin Microbiol Rev 2013; 26:759–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sader HS, Jones RN. Antimicrobial susceptibility of Gram-positive bacteria isolated from US medical centers: results of the Daptomycin Surveillance Program (2007–2008). Diagn Microbiol Infect Dis 2009; 65:158–62. [DOI] [PubMed] [Google Scholar]
- 6. Flamm RK, Mendes RE, Hogan PA, et al. . Linezolid surveillance results for the United States (LEADER surveillance program 2014). Antimicrob Agents Chemother 2016; 60:2273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mendes RE, Hogan PA, Jones RN, et al. . Surveillance for linezolid resistance via the Zyvox® Annual Appraisal of Potency and Spectrum (ZAAPS) programme (2014): evolving resistance mechanisms with stable susceptibility rates. J Antimicrob Chemother 2016; 71:1860–5. [DOI] [PubMed] [Google Scholar]
- 8. Sader HS, Farrell DJ, Flamm RK, Jones RN. Daptomycin activity tested against 164457 bacterial isolates from hospitalised patients: summary of 8 years of a Worldwide Surveillance Programme (2005-2012). Int J Antimicrob Agents 2014; 43:465–9. [DOI] [PubMed] [Google Scholar]
- 9. Kamboj M, Cohen N, Gilhuley K, et al. . Emergence of daptomycin-resistant VRE: experience of a single institution. Infect Control Hosp Epidemiol 2011; 32:391–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pai MP, Rodvold KA, Schreckenberger PC, et al. . Risk factors associated with the development of infection with linezolid- and vancomycin-resistant Enterococcus faecium. Clin Infect Dis 2002; 35:1269–72. [DOI] [PubMed] [Google Scholar]
- 11. Kainer MA, Devasia RA, Jones TF, et al. . Response to emerging infection leading to outbreak of linezolid-resistant enterococci. Emerg Infect Dis 2007; 13:1024–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pogue JM, Paterson DL, Pasculle AW, Potoski BA. Determination of risk factors associated with isolation of linezolid-resistant strains of vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol 2007; 28:1382–8. [DOI] [PubMed] [Google Scholar]
- 13. McGregor JC, Hartung DM, Allen GP, et al. . Risk factors associated with linezolid-nonsusceptible enterococcal infections. Am J Infect Control 2012; 40:886–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Santayana EM, Grim SA, Janda WM, et al. . Risk factors and outcomes associated with vancomycin-resistant Enterococcus infections with reduced susceptibilities to linezolid. Diagn Microbiol Infect Dis 2012; 74:39–42. [DOI] [PubMed] [Google Scholar]
- 15. Hayakawa K, Marchaim D, Pogue JM, et al. . Predictors and outcomes of linezolid-resistant vancomycin-resistant Enterococcus: a case-case-control study. Am J Infect Control 2012; 40:e261–3. [DOI] [PubMed] [Google Scholar]
- 16. Judge T, Pogue JM, Marchaim D, et al. . Epidemiology of vancomycin-resistant enterococci with reduced susceptibility to daptomycin. Infect Control Hosp Epidemiol 2012; 33:1250–4. [DOI] [PubMed] [Google Scholar]
- 17. Kelesidis T, Tewhey R, Humphries RM. Evolution of high-level daptomycin resistance in Enterococcus faecium during daptomycin therapy is associated with limited mutations in the bacterial genome. J Antimicrob Chemother 2013; 68:1926–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kelesidis T, Chow AL, Humphries R, et al. . Case-control study comparing de novo and daptomycin-exposed daptomycin-nonsusceptible Enterococcus infections. Antimicrob Agents Chemother 2012; 56:2150–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang G, Kamalakaran S, Dhand A, et al. . Identification of a novel clone, ST736, among Enterococcus faecium clinical isolates and its association with daptomycin nonsusceptibility. Antimicrob Agents Chemother 2014; 58:4848–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mendes RE, Jones RN, Deshpande LM, et al. . Daptomycin activity tested against linezolid-nonsusceptible gram-positive clinical isolates. Microb Drug Resist 2009; 15:245–9. [DOI] [PubMed] [Google Scholar]
- 21. Harris PA, Taylor R, Thielke R, et al. . Research Electronic Data Capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Munita JM, Panesso D, Diaz L, et al. . Correlation between mutations in liaFSR of Enterococcus faecium and MIC of daptomycin: revisiting daptomycin breakpoints. Antimicrob Agents Chemother 2012; 56:4354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diaz L, Tran TT, Munita JM, et al. . Whole-genome analyses of Enterococcus faecium isolates with diverse daptomycin MICs. Antimicrob Agents Chemother 2014; 58:4527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reyes J, Panesso D, Tran TT, et al. . A liaR deletion restores susceptibility to daptomycin and antimicrobial peptides in multidrug-resistant Enterococcus faecalis. J Infect Dis 2015; 211:1317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arias CA, Panesso D, McGrath DM, et al. . Genetic basis for in vivo daptomycin resistance in enterococci. N Engl J Med 2011; 365:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Panesso D, Reyes J, Gaston EP, et al. . Deletion of liaR reverses daptomycin resistance in Enterococcus faecium independent of the genetic background. Antimicrob Agents Chemother 2015; 59:7327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mendes RE, Deshpande LM, Jones RN. Linezolid update: stable in vitro activity following more than a decade of clinical use and summary of associated resistance mechanisms. Drug Resist Updat 2014; 17:1–12. [DOI] [PubMed] [Google Scholar]
- 28. Ruggero KA, Schroeder LK, Schreckenberger PC, et al. . Nosocomial superinfections due to linezolid-resistant Enterococcus faecalis: evidence for a gene dosage effect on linezolid MICs. Diagn Microbiol Infect Dis 2003; 47:511–3. [DOI] [PubMed] [Google Scholar]
- 29. Herrero IA, Issa NC, Patel R. Nosocomial spread of linezolid-resistant, vancomycin-resistant Enterococcus faecium. N Engl J Med 2002; 346:867–9. [DOI] [PubMed] [Google Scholar]
- 30. Bourgeois-Nicolaos N, Massias L, Couson B, et al. . Dose dependence of emergence of resistance to linezolid in Enterococcus faecalis in vivo. J Infect Dis 2007; 195:1480–8. [DOI] [PubMed] [Google Scholar]
- 31. Gonzales RD, Schreckenberger PC, Graham MB, et al. . Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 2001; 357:1179. [DOI] [PubMed] [Google Scholar]
- 32. Jones RN, Della-Latta P, Lee LV, Biedenbach DJ. Linezolid-resistant Enterococcus faecium isolated from a patient without prior exposure to an oxazolidinone: report from the SENTRY Antimicrobial Surveillance Program. Diagn Microbiol Infect Dis 2002; 42:137–9. [DOI] [PubMed] [Google Scholar]
- 33. Marshall SH, Donskey CJ, Hutton-Thomas R, et al. . Gene dosage and linezolid resistance in Enterococcus faecium and Enterococcus faecalis. Antimicrob Agents Chemother 2002; 46:3334–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prystowsky J, Siddiqui F, Chosay J, et al. . Resistance to linezolid: characterization of mutations in rRNA and comparison of their occurrences in vancomycin-resistant enterococci. Antimicrob Agents Chemother 2001; 45:2154–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shukla BS, Shelburne S, Reyes K, et al. . Influence of minimum inhibitory concentration in clinical outcomes of Enterococcus faecium bacteremia treated with daptomycin: is it time to change the breakpoint?Clin Infect Dis 2016; 62:1514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Olsen KM, Rebuck JA, Rupp ME. Arthralgias and myalgias related to quinupristin-dalfopristin administration. Clin Infect Dis 2001; 32:e83–6. [DOI] [PubMed] [Google Scholar]
- 37. Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis 2012; 54:1699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hindler JA, Wong-Beringer A, Charlton CL, et al. . In vitro activity of daptomycin in combination with β-lactams, gentamicin, rifampin, and tigecycline against daptomycin-nonsusceptible enterococci. Antimicrob Agents Chemother 2015; 59:4279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smith JR, Barber KE, Raut A, et al. . β-lactam combinations with daptomycin provide synergy against vancomycin-resistant Enterococcus faecalis and Enterococcus faecium. J Antimicrob Chemother 2015; 70:1738–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smith JR, Barber KE, Raut A, Rybak MJ. β-lactams enhance daptomycin activity against vancomycin-resistant Enterococcus faecalis and Enterococcus faecium in in vitro pharmacokinetic/pharmacodynamic models. Antimicrob Agents Chemother 2015; 59:2842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]