Short abstract
Background
Cancer pain is a well-known serious complication in metastatic or terminal cancer patients. Current pain management remains unsatisfactory. The activation of spinal and supraspinal P2X7 receptors plays a crucial role in the induction and maintenance mechanisms of various kinds of acute or chronic pain. The midbrain periaqueductal gray is a vital supraspinal site of the endogenous descending pain-modulating system. Tramadol is a synthetic, centrally acting analgesic agent that exhibits considerable efficacy in clinically relieving pain. The purpose of this study was to determine whether the activation of P2X7 receptor in the ventrolateral region of the periaqueductal gray (vlPAG) participates in the analgesic mechanisms of tramadol on bone cancer pain in rats. The bone cancer pain rat model was established by intratibial cell inoculation of SHZ-88 mammary gland carcinoma cells. The analgesic effects of different doses of tramadol (10, 20, and 40 mg/kg) were assessed by measuring the mechanical withdrawal threshold and thermal withdrawal latency values in rats by using an electronic von Frey anesthesiometer and radiant heat stimulation, respectively. Alterations in the number of P2X7 receptor-positive cells and P2X7 protein levels in vlPAG were separately detected by using immunohistochemistry and Western blot assay. The effect of intra-vlPAG injection of A-740003 (100 nmol), a selective competitive P2X7 receptor antagonist, on the analgesic effect of tramadol was also observed.
Results
The expression of P2X7 receptor in the vlPAG on bone cancer pain rats was mildly elevated, and the tramadol (10, 20, and 40 mg/kg) dose dependently relieved pain-related behaviors in bone cancer pain rats and further upregulated the expression of P2X7 receptor in the vlPAG. The intra-vlPAG injection of A-740003 pretreatment partly but significantly antagonized the analgesic effect of tramadol on bone cancer pain rats.
Conclusions
The injection of tramadol can dose dependently elicit analgesic effect on bone cancer pain rats by promoting the expression of the P2X7 receptor in vlPAG.
Keywords: Bone cancer pain, tramadol, midbrain periaqueductal gray, P2X7 receptor, analgesic effect
Background
Bone cancer pain (BCP) is the most common cancer pain arises from bone metastasis of malignant tumors like breast, prostate, and lung cancers.1,2 BCP patients frequently experience daily pain that substantially impairs their quality of life.3,4 With the rapid increase in the incidence and survival rates of cancer and prolonged survival of patients, finding strategies to manage BCP has become increasingly important.3 Unfortunately, the neural mechanisms underpinning BCP are still remain unsolved.5 From the basic research point of view, pathological phenotypes, such as neuropathic and inflammatory pain, are the underlying components of BCP,6 thus making BCP a well-known complex pain state. Numerous studies have shown that neuronal and/or glial cell function, pain-related neurotransmitters, receptors, and proinflammatory cytokines are all major factors linked to the molecular mechanisms of BCP.7–12
At present, cancer pain is commonly managed using a “three-step” analgesic ladder, which was originally developed by the World Health Organization in 1986; this ladder involves opioids and non-opioid analgesics (nonsteroidal anti-inflammatory drugs).3 Other adjuvant therapies, including radiation therapy, surgery, and chemotherapy, are also used to control cancer pain.3 However, current therapies are often partially effective because of their relative ineffectiveness and severe side effects, such as tolerance, withdrawal, dependence, and addiction to opioids.4 Studies have shown that almost half of cancer pain is not adequately relieved and that BCP is one of the most debilitating conditions to treat.3,13 Thus, new analgesic therapies that are efficacious and/or capable of abating the side effects of current therapies should be developed.
Adenosine triphosphate (ATP) is a widely distributed signaling molecule.14 ATP activates a vast variety of pathways, allowing downstream effects that can lead to both physiological and pathological responses in the central/peripheral nervous system via activation of the P2X and P2Y receptors.15 At present, the pathophysiology and therapeutic potential of P2X receptors are the focus of basic research and clinical treatment. Among all P2X receptors, the P2X7 receptor is a well-defined therapeutic target for a series of diseases that has been found in the central and peripheral nervous systems.16–19 Numerous studies have shown that P2X7 receptor is a key factor responsible for the occurrence and development of neuropathic pain and inflammatory pain.20–22
The neuronal circuit and endogenous substances construct a presumptive endogenous nociceptive modulatory system that originates from the anterior cingulate cortex or hypothalamus to the midbrain periaqueductal gray (PAG) and the ventromedial medulla (VMM): delected; and terminates in the spinal cord (dorsal horn).23,24 PAG is a deciding site in this modulatory system, which involves the integration of somatic and autonomic responses to nociceptive and other stressful stimuli.25 When stimulated by electrical or excitatory amino acids, PAG integrates descending noradrenergic and serotonergic pathway activity, which can suppress nociceptive transmission from the dorsal horn of the spinal cord; this phenomenon suggests that changes in PAG may be a common feature in different pain models. The ventrolateral region of the periaqueductal gray (vlPAG) is a particularly important site in descending control of nociception.25,26 Early studies revealed that P2X1-6 receptors exist in PAG,27 and subsequent investigations confirmed that the P2X7 receptor can be added to this archive.28,29
Tramadol hydrochloride, (1RS, 2RS)-2-[(dimethylamino)methyl]-1–(3-methoxyphenyl)-cyclohexanol (HCl), is an atypical central opioid receptor analgesic prescribed to relieve moderate to severe pain caused by numerous different illnesses and diseases, such as inflammatory, cancer, neuropathic, and postoperative pain.30–32 Drug addiction, respiratory depression, drug abuse, and other side effects of this drug are trivial.33 Different from classic opioids, tramadol exhibits dual analgesic mechanisms, namely, central and peripheral mechanisms. In brief, the mechanism of central analgesic effect occurs through the activation of the mu opioid receptors or reduction of norepinephrine (NE) and 5-hydroxytryptamine (5-HT) reabsorption, whereas the peripheral mechanism is a local anesthetic effect.34,35 To the best of our knowledge, the role of the vlPAG P2X7 receptor in the mechanisms of tramadol analgesia has yet to be reported; therefore, the main purpose of this study is to observe the analgesic effect of tramadol on BCP rats and elucidate whether vlPAG P2X7 receptor is involved in the underlying mechanisms. This study is expected to enhance our understanding of the mechanisms of supraspinal pain modulation and provide theoretical support for tramadol clinical analgesia.
Methods
Animals and ethics
Female Sprague–Dawley rats weighing 210 ± 10 g at the beginning of the experiments were purchased from the Laboratory Animal Center of the Army Medical University (Chongqing, China) and used for all experiments. The animals were housed in groups of five in a polypropylene cage with a 12 h light–dark cycle (8:00 a.m.–8:00 p.m.). The laboratory conditions were 25°C ± 2°C and 55% ± 5% relative humidity. Food and water were provided ad libitum. All experimental procedures were conducted according to the guidelines of the Ethical Committee of the International Association for the Study of Pain,36 and the Animal Care Ethics Committee of Zunyi Medical University approved all the experimental protocols. Efforts were made to minimize the number of animals used and reduce their chances of suffering from experimental procedures.
Preparation of cancer cells
SHZ-88 mammary gland carcinoma cells were purchased commercially from the Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Sangon Biotech, Shanghai, China) in a 37°C constant-temperature incubator (Thermo, USA) containing 5% CO2. The cell culture medium was changed every two days, and cell passage was conducted every week. In order to detach, the cultured cells were digested by 0.25% trypsin for 5–8 min, and the enzyme reaction was terminated with a culture medium containing 10% fetal bovine serum. For cells harvesting, the cells and medium were centrifuged for 3 min at 1000 r/min. The supernatant thus obtained was discarded and the pellet resuspended in the RPMI-1640 medium and diluted to achieve a final concentration of 107 cells/0.5 mL. The cells were counted by using a hemocytometer. Cell viability was estimated by MTT ((3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide)) assay as described previously.37
Model of cancer pain
All animals were assessed for their baseline nociceptive sensitivity to mechanical and thermal stimuli one day prior to experiments. Only the animals with normal baseline responses were used in the study. BCP model was conducted as described previously.38 In brief, under 4% chloral hydrate anesthesia (10 mL/kg body weight, intraperitoneal, i.p.), this method provided a state of anesthesia with stable physiological parameters. The rats were placed in a prone position on a thermo-regulated heat mat. A 1.5-cm incision was made on the right leg of the rat to expose the upper medial aspect of the tibia with minimal damage to the muscles and nerves, and 20 μL of SHZ-88 carcinoma cells (107 cells/0.5 mL) was slowly injected into the intramedullary cavity of the tibia. The syringe was removed, and the injection site was immediately closed with sterile bone wax. Then, penicillin was applied to the wound. In the sham-BCP group, the same procedures were followed, except that cancer cells at an equal volume and concentration were heated to 90°C for 20 min and administered instead of normal carcinoma cells. The day of carcinoma cells inoculation is day 0.
Bone radiological detection
Bone radiological detection was assessed on day 21 prior to decalcification and histological staining. After anesthesia with injection of 4% chloral hydrate (10 mL/kg body weight, i.p.), the hind limb of the rat was exposed to an intraoral X-ray source for 0.32 s at 60 kV, 7 mA (Kodak, CS2200, Carestream Health, Inc.). The bone destruction of each proximal tibia was detected based on the blind analysis of radiographs.
Hematoxylin–eosin staining
Histological staining was conducted as described previously with minor alterations39; fresh rat tibial tissue was gently separated, rinsed in 0.9% sodium chloride, and fixed in 4% paraformaldehyde for 12 h. These steps were followed by decalcification in 6.5% nitric acid/10% formaldehyde solution for 12 h. The decalcification process was terminated when the bone was easily penetrated through by a needle without any force, followed by routine dehydration and paraffin embedding. Several 3 μm sections were cut using a microtome (REM-710, YAMATO, Japan) and placed on clean glass slides. The sections were stained with hematoxylin solution for 5 min and then rinsed with distilled water. The sections were stained with eosin solution for 2 min, dehydrated with graded alcohol, and cleared with xylene. The mounted slides were then examined and photographed using a light microscope (IX53+DP73, OLYMPUS, Japan). Analysis was performed in a blinded manner.
Cannula implant and microinjection procedures
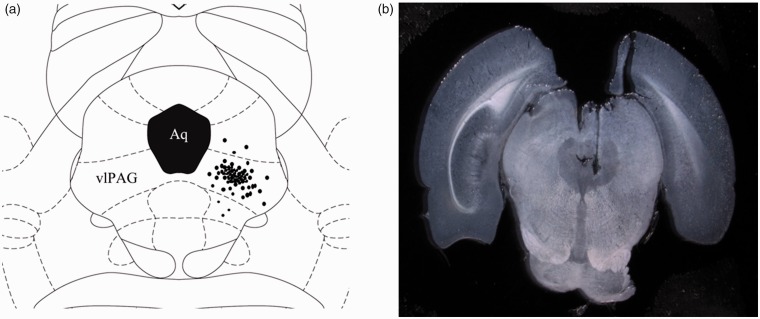
After anesthesia with injection of 4% chloral hydrate (10 mL/kg body weight, i.p.), the rats were mounted on a stereotaxic frame (68025, RWD Life Science Co., Ltd, Shenzhen, China). The skull was exposed and the bregma was located. A stainless steel guide cannula (0.48 mm outside diameter (O.D.) 0.34 mm inner diameter (I.D.)) was fixed to the skull by using dental zinc cement and jewelers’ screw. Stereotaxic coordinates for vlPAG were 7.90 mm posterior to the bregma, 0.80 mm lateral to the midline, and 6.00 mm ventral to the skull surface. A dummy cannula inserted into the guide cannula at the time of surgery served to reduce the incidence of occlusion. Upon removal from stereotaxic apparatus, rats were administered with 2 mL of 0.9% sterile saline s.c. to prevent dehydration and placed in a thermal-controlled cage to prevent hypothermia until complete anesthetic recovery. Prior to any experimentation, the animals were allowed to recover from implantation surgery for four days at minimum, and signs of motor deficiency were monitored daily. Rats with neurological deficits resulting from the surgical procedure were excluded from the experiments. On the day of microinjection, the rats were transferred from the main holding area to the laboratory and left undisturbed for 1 h prior to drug administration. Each rat was lightly restrained and inserted with an injection cannula (0.3 mm O.D.; 0.5 mm longer than the guide cannula) into the guide cannula and an injector connected to a micro-injection pump (Legato 130, KD Scientific, USA). A total volume of 0.3 μL was injected over a 3-min period, and the injector remained in place for an additional 1 min before slow removal to ensure complete diffusion of the drug. At the end of the experiments, the animals were deeply anesthetized by an injection of a 4% chloral hydrate (20 mL/kg body weight, i.p) and intracardially perfused with physiological saline (0.9% NaCl) followed by 4% paraformaldehyde solution. The position of the cannula was visually confirmed by 0.1 μL of 2% Evans blue infusion through the microinjection cannula at the end of the experiment. Administration sites were verified by histological examination and plotted on coronal maps adapted from the atlas of Paxinos and Watson as a reference.40 Only rats with microinjection site located within vlPAG were used for analysis (Figure 1).
Figure 1.
(a) Schematic diagram illustrates the sites of A-740003 microinjections, which were identified histologically by Evans blue dye microinjection (dots). (b) Photograph of an injection site in the vlPAG. The microinjection sites were mostly distributed within the vlPAG region. Data from rats with an injection site outside of the vlPAG region were discarded. Aq: aqueduct of midbrain; vlPAG: ventrolateral periaqueductal gray.
Inclined plane test
The functional deficit of locomotion, including muscular strength and proprioception, was evaluated using an inclined plane according to a previously reported method.41 The rats were placed crosswise to the long axis of an inclined plane. The initial angle of the inclined plane was 30°, and the angle was tilted slowly by 5° increments. The maximum angle of the plane on which the rats maintained a constant body position for 5 s without falling was recorded. This measurement was recorded for five times for each rat, and the average values from the measurements were defined as the inclined plane test values.
The electronic pressure meter test of mechanical withdrawal threshold
For determining mechanical withdrawal thresholds (MWTs) in a quiet room, each rat was placed in an individual Plexiglass house (18 cm × 12 cm × 12 cm) with a wire mesh floor and allowed to explore and groom until they settled down. Each hind paw was measured for five times, and the average values from the measurements were considered as the mechanical paw withdrawal thresholds. Mechanical hyperalgesia was tested in rats as reported previously.42 In brief, the test consists of evoking hind paw flexion reflex with the electronic von Frey anesthesiometer (IITC Life Science Instruments, USA) adapted with a 0.5 mm2 contact area polypropylene tip. The investigator was trained to apply the tip perpendicularly to the central area of the hind paw with a gradual increase in pressure, and the endpoint was characterized by the removal of the paw (the animal actively lifted the whole paw upon the tip of the anesthesiometer, bit or licked the paw, or shook the paw with high amplitude movements in response to the stimulus).
Radiant heat test
Radiant heat test described by Hargreaves et al.43 was carried out to evaluate thermal withdrawal latency (TWL). In brief, each rat was placed in a clean plastic chamber, allowed to adapt for 30 min, and exploratory behavior ceased. Then, 52°C ± 0.2°C radiant heat (50 W, 8 V bulb) was applied to the plantar surface of the hind paw. The latency period was recorded between the start of the beam and a functional response, namely, characteristic lifting, hind paw licking, flicking, or commenced jumping. For preventing tissue injury, the cut-off limit was set to 60 s. Each hind paw was measured for three times at 3-min intervals. The average value from three measurements was considered as the value of TWL.
Immunohistochemistry
The distribution of P2X7 receptor in the vlPAG was detected using immunohistochemistry. The animals were sacrificed using an overdose of 4% chloral hydrate anesthesia (20 mL/kg body weight, i.p.) and perfused with 150 mL of normal saline (NS) in the aorta through the left ventricle followed by 200-mL of 4% paraformaldehyde in 0.2 M phosphate-buffered saline (PBS). The rats were decapitated, and their brains were rapidly removed, post-fixed in 4% paraformaldehyde/0.1 M PBS for 4–6 h, transferred to 30% sucrose for 48–60 h, and cryoprotected. PAGs were serially cut along the axis at a thickness of 25 μm by using a cryostat (Leica CM 1950, Germany). One of every five to six sections through the PAG was collected for immunohistochemical analysis. After incubation with 3% H2O2 in 0.01 M PBS and then 3% bovine serum albumin (BSA) in 0.1 M PBS, the sections were incubated with primary antibody (rabbit anti-rat P2X7 polyclonal antiserum, 1:400; Abcam, USA) for P2X7 receptors. After 1 h of incubation at 37°C, sections were stained overnight at 4°C. The 5% goat anti-rabbit serum (Beijing Zhongshan Biotech Co., China) was used as secondary antibody. Finally, the sections were mounted on glass sides, dehydrated through ascending series of ethanol solutions and xylene, and cover slipped. For confirming immune labeling specificity, control slides were exposed to diluted normal fetal bovine serum instead of the primary antibody. For quantification, images of positive staining in the vlPAG sections were analyzed using a Leica Q500IW image analysis system. Whole-mount preparations were used for quantitative analysis as described previously.44 In brief, immunoreactive positive neuron bodies in the vlPAG were counted per visual field (0.3 mm2) in whole-mount preparations. Only cells with a distinct cell body and clear cellular boundary were included in the counts. Cell measurements were obtained from the mean value of five separate high-power fields per section, and 10 sections per brain were assayed. Cell density was expressed as cells per square millimeter with a 20× objective (total magnification 200×).
Western blot
For obtaining protein extracts, rats were sacrificed using an overdose of chloral hydrate (20 ml/kg body weight, i.p.) on day 21 after BCP operation, and whole PAGs were dissected and rapidly stored in a −70°C refrigerator. Before Western blot analysis, we attempted to harvest the vlPAG area, and the samples were sonicated in 400 μL of ice-cold lysis buffer (50 mM Tris–HCl [pH 7.5], 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), and 0.02% sodium azide) supplemented with proteinase inhibitors. Protein concentrations of the lysate were determined using Bradford reagent (Bio-Rad, Hercules, CA, USA). For Western blot analysis, samples (20 μg of protein/lane) were loaded on a 12% SDS–polyacrylamide electrophoresis gel for 90 min at 120 V. After separation, the proteins were transferred to a polyvinylidene fluoride membrane for 6 h at 12 V. The membrane was shaken for 1 h at room temperature in Tris-buffered saline (TBS) containing 0.1% Tween-20, 5% skim milk, and 0.2% BSA. The membrane was incubated for 2 h at room temperature with primary antibody (1. rabbit polyclonal anti-P2X7, Abcam Corporation, USA. 1:400; 2. mouse monoclonal anti-β-actin, Millipore Corporation, USA. 1:2000) in TBS containing 0.1% Tween-20. Immunoreactive proteins were detected by using enhanced chemiluminescence reagents (Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer’s instructions. The band intensity of the detected proteins was measured by using Quantity One software (Bio-Rad). Protein levels were normalized to β-actin as the loading control. Relative optical density (ROD) of the protein bands was measured after subtracting the film background. Data are expressed as mean ratio ± S.E.M of the P2X7/β-actin protein.
Experimental design and drugs
The experiment consisted of four series. In series 1, we observed whether cancer cell inoculation, drug injection, or vlPAG microinjection exerted certain adverse effects on sensorimotor function in rats.
In series 2, we investigated the time-course changes of MWT or TWL values after the establishment of rat BCP model or intraperitoneal injection of different doses of tramadol. The rats were randomly and equally divided into the following six groups according to a random number table: normal, sham-BCP, BCP, TL, TM, and TH groups (n = 12 per group). Pain threshold values were observed on postoperative days 0, 5, 7, 10, 14, 18, and 21. The TL, TM, and TH group rats were intraperitoneally administered with 10, 20, and 40 mg/kg tramadol injection, respectively, once per day for 7 days from postoperative days 14 to 21.
In series 3, alterations of the P2X7 receptor expression in the vlPAG were observed. A total of 12 rats from each group in series 2 were sacrificed on postoperative day 21, 6 rats were used for immunohistochemical analysis, and the other 6 rats were used for immunoblotting. Besides Western blot and immunohistochemical analysis, the tibia of each rat was harvested and observed by X-ray scanning and hematoxylin–eosin (HE) staining.
In series 4, the effects of pretreatment with A-740003 on the analgesic effect of 40 mg/kg tramadol (i.p. injection) was observed. In brief, 24 rats were randomly divided into three groups (n = 8 per group), namely, BCP group, BCP rats with an i.p. injection of 40 mg/kg tramadol only (BCP+TH group), and BCP rats with pretreatment of A-740003 30 min before i.p. injection of 40 mg/kg tramadol (BCP+TH+A-740003 group).
Tramadol hydrochloride was purchased from DuoDuo Pharmaceutical Co., Ltd (Jiamusi, China), and A-740003 was obtained from Sigma-Aldrich. A-740003 was dissolved in NS solution and administered via intra-vlPAG injection in a volume of 100 nmol/0.3 μL. Doses and treatment times of A-740003 were selected from our preliminary experiment or from previous studies. Antagonistic effect of A-740003 was assessed at 0, 15, 30, 45, 60, 75, and 90 min postmicroinjection.
Statistical analysis
Data were processed with the GraphPad Prism (version 6.01; GraphPad Software, Inc., CA). Results are expressed as mean ± S.D. Statistical differences were assessed by one-way analysis of variance with a post hoc analysis for multiple comparisons. Student's t-test was used when only two independent groups were compared. A probability value of less than 0.05 indicates significant difference.
Results
Changes in sensorimotor function after BCP operation or drugs injection
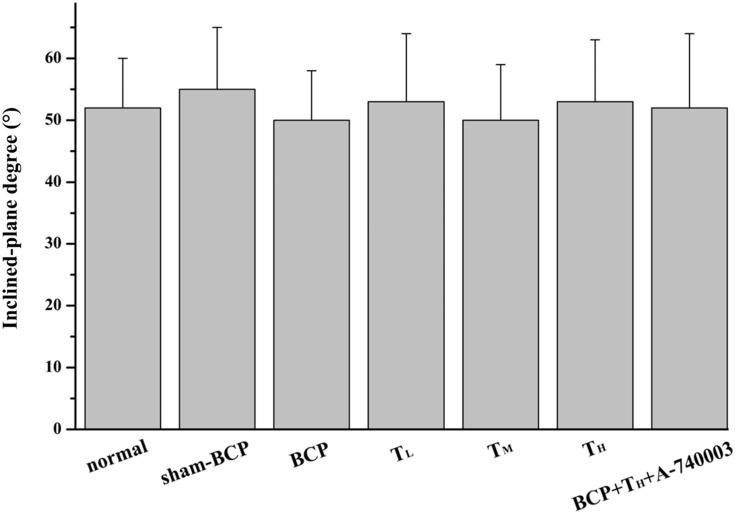
Inclined plane test was used in all experimental rats before algesimetry test to study whether experimental procedures, e.g., modeling operation, drug microinjection, and i.p. injection exerted certain adverse effects on the sensorimotor function of the experimental rats. The average maximum angles at which a rat can retain its position were not significantly different among these groups (all p > 0.05, Figure 2).
Figure 2.
Changes in sensorimotor capability of the experimental rats. This histogram demonstrates the effect of experimental procedures on hind limb muscular strength and proprioception. Rats showed no considerable deficits in hind limb movement in this test. (n = 12 or 8). p > 0.05 among all groups. BCP: bone cancer pain.
Evaluation of bone destruction in BCP model
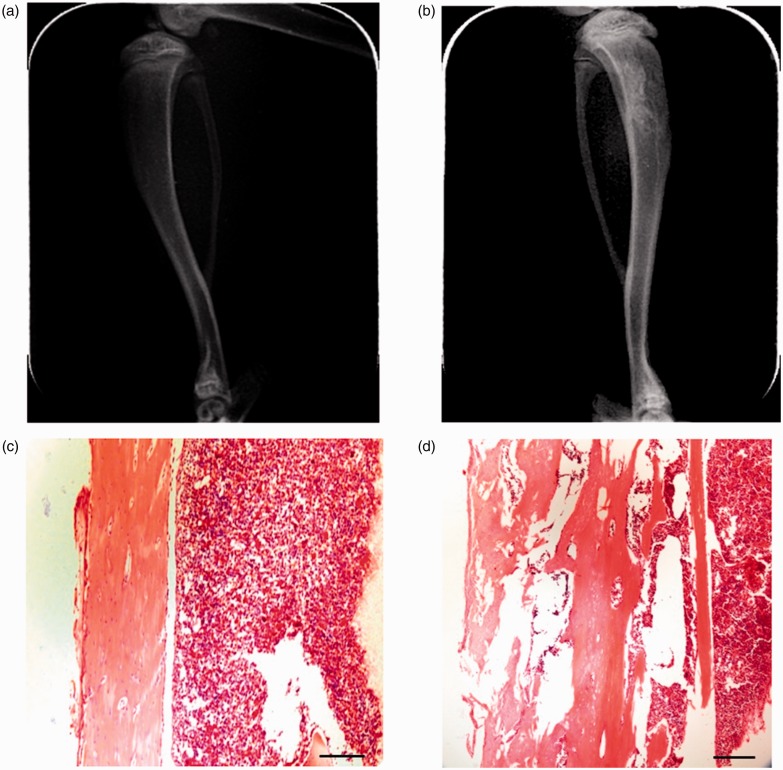
The BCP model was validated using radiological imaging and histological analysis. On day 21, postcancer cell inoculation on an X-ray picture of the rat tibia inoculated with SHZ-88 cells (ipsilateral) showed a loss of normal medullary cavity opacity and discontinuity of the bone structure, indicating bone destruction around the cancer cell injection site. No bone deficits were found on contralateral side tibia on day 21 postcancer cell inoculation (Figure 3(a) and (b)). Hematoxylin–eosin staining of tibia sections did not show cancer cells or bone destruction in the contralateral normal tibia of the BCP model rats (Figure 3(c) and (d)). However, the ipsilateral tibia showed marked bone destruction along the surfaces of trabecular and cortical bone, indicating the development of bone cancer in the tibia.
Figure 3.
Tibia destruction at 21 days after SHZ-88 cell inoculation. Radiograph of the contralateral tibia shows an intact tibia, while ipsilateral tibia shows a loss of normal medullary cavity opacity and discontinuity of the bone structure in the tibia inoculated with cancer cells (a and b). Histopathological images of tibia sections stained by hematoxylin–eosin indicate obvious bone destruction along the surfaces of trabecular and compact bone (c and d). Scale bars = 250 µm. (a) Uninjured rat tibia (contralateral), (b) tibia bone cancer (ipsilateral), (c) contralateral side, and (d) ipsilateral side.
Alterations of pain thresholds by cancer cell inoculation
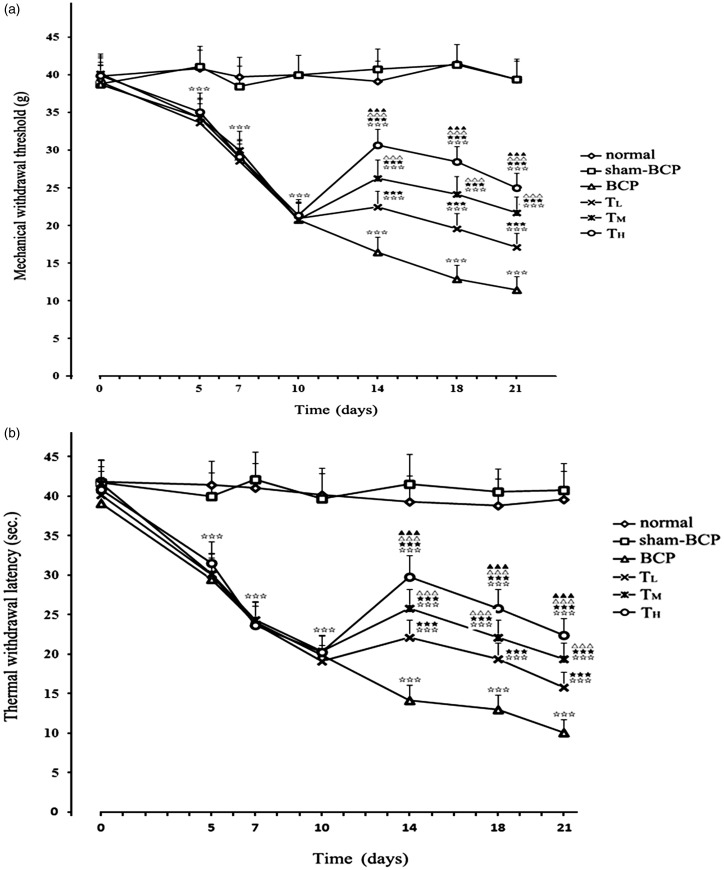
MWT was measured before BCP operation (day 0) and at days 5, 7, 10, 14, 18, and 21 post-operation. The mean MWT values of all groups of rats in the MWT test were approximately 39.41 g preoperatively. In the BCP group, hind paw MWT decreased from 38.69 ± 2.56 g before operation to 20.81 ± 2.17 g at day 10 (p < 0.001 compared with that before cancer cell inoculation) and 11.47 ± 1.76 g at day 21 (p < 0.001 compared with that before cancer cell inoculation) after cancer cell inoculation. In the sham-BCP group, from day 5 to day 21 postcancer cell inoculation, the values of MWT were stable with no significant difference between sham-BCP and normal groups (p > 0.05). Changes in the TWL values exhibited a similar trend to that of MWT values (Figure 4(a) and (b)).
Figure 4.
Cancer cell inoculation-induced mechanical and thermal hyperalgesia in rats. In the BCP group, cancer cell inoculation caused a significant decrease in MWT (a) or TWL (b) from day 5 to day 21 after BCP operation. The MWT and TWL values were significantly and dose dependently elevated after i.p. tramadol injection in TL, TM, and TH groups. (n=12). ⋆⋆⋆p < 0.001 versus sham-BCP group at the same time point; ★★★p < 0.001 versus BCP group at the same time point; △△△p < 0.001 versus TL group at the same time point; ▲▲▲p < 0.001 versus TM group at the same time point. BCP: bone cancer pain.
Changes of P2X7 receptors expression in the vlPAG after cancer cell inoculation
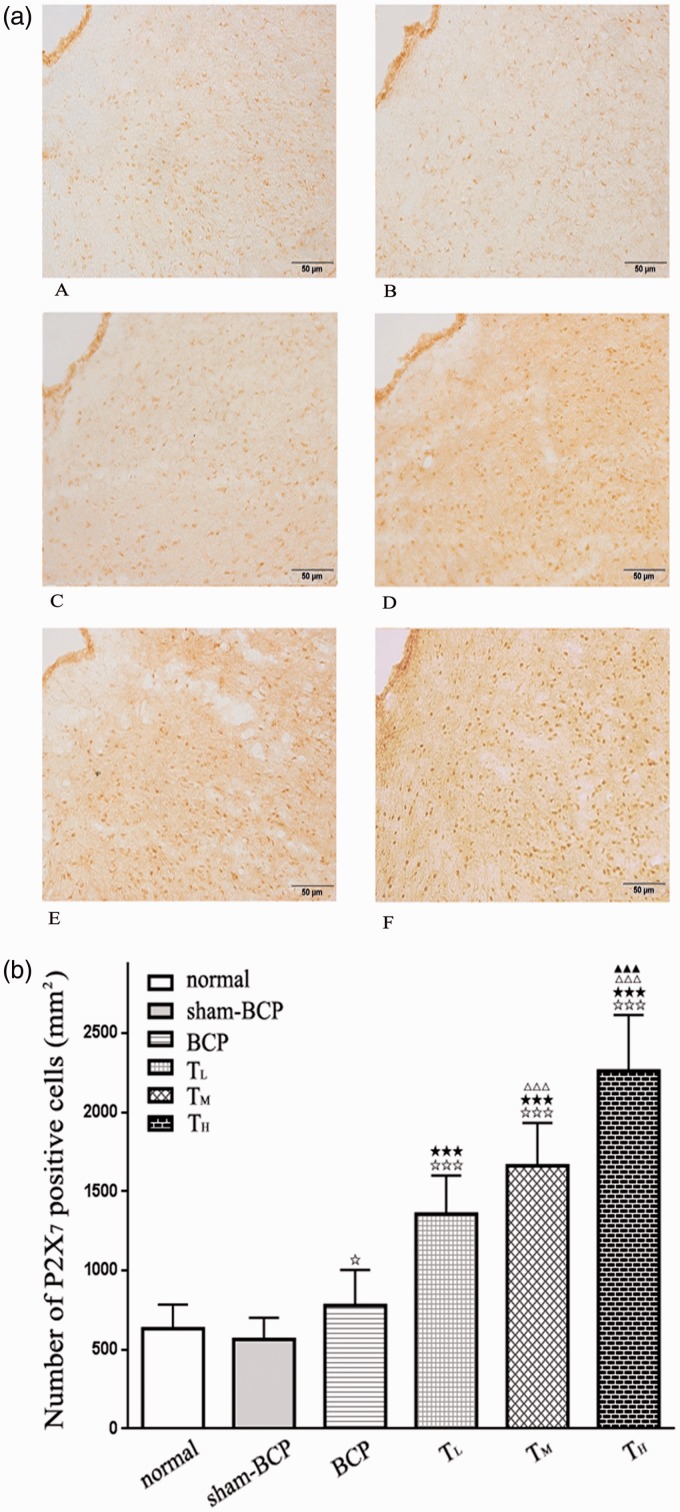
The immunohistochemistry for P2X7 receptors manifested weak and sparse P2X7 receptor-specific immunoreactivity in vlPAG; that is, the P2X7-positive cell number for (637.26 ± 147.40) per mm2 in the normal group and for (582.71 ± 134.15) per mm2 in the sham-BCP group (p > 0.05, n=6). In BCP groups (with P2X7-positive cell number of 730.53 ± 229.11 per mm2), P2X7 receptor-specific immunoreactivity was mildly increased compared with normal group or sham-BCP group on day 21 after BCP operation (p < 0.05, n=6). Negative controls, with no primary antibody, did not show staining (Figure 5(a) and (b)).
Figure 5.
Changes of P2X7 receptor expression in vlPAG following cancer cell inoculation or tramadol injections. (a) Photomicrographs are shown for P2X7 receptor immunostaining in the vlPAG of normal group (Figure 5(a) A), sham-BCP group (Figure 5(a) B), BCP group (Figure 5(a) C), TL group (Figure 5(a) D), TM group (Figure 5(a) E), and TH group (Figure 5(a) F). Scale bar = 50 μm. (b) The graph shows the number of P2X7-positive cells in vlPAG. No distinct change in P2X7 immunostaining was observed in vlPAG between normal and sham-BCP groups, but cancer cell inoculation upregulated the expression of P2X7 receptor in vlPAG at day 21 after cancer cell inoculation in BCP group compared with normal and sham-BCP groups. Intraperitoneal tramadol injection at doses of 10, 20, and 40 mg/kg showed significantly and dose dependently upregulated the number of P2X7-positive cells in vlPAG. (n = 6); ⋆p < 0.05 and ⋆⋆⋆p < 0.001 compared with normal or sham-BCP group.★★★p < 0.001 versus BCP group; △△△p < 0.001 versus TL group; ▲▲▲p < 0.001 versus TM group. BCP: bone cancer pain.
Observation of P2X7 receptor protein levels in vlPAG by Western blot analysis following cancer cell inoculation
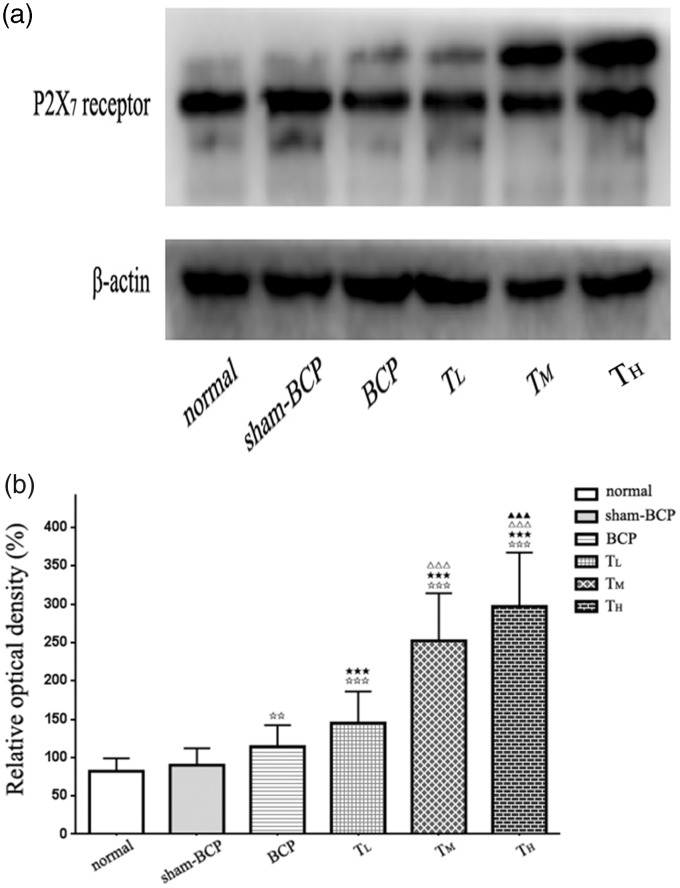
Immunoblots from vlPAG homogenates revealed the presence of an immunopositive band of P2X7 receptors with an apparent molecular weight of 68 kDa in normal, sham-BCP, and BCP groups. No significant difference was observed between normal (ROD: 82.45 ± 17.76) and sham-BCP groups (ROD: 90.23 ± 22.62) (p > 0.05). In the BCP group, cancer cell inoculation caused an increase in P2X7 receptor protein level (ROD: 114.51 ± 28.89) compared with those in normal and sham-BCP rats at day 21 after BCP operation (all p < 0.01; n = 6; Figure 6(a) and (b)).
Figure 6.
Alterations of P2X7 receptor protein level in the vlPAG induced by cancer cell inoculation or tramadol injections. (a) By using Western blot analysis, we detected a protein band of ≈68 kDa, coinciding with the known molecular weight of the P2X7 receptor. (b) β-actin was used as loading control. The P2X7 receptor protein levels in different groups were expressed as ROD. No distinct change in P2X7 receptor protein level was observed in vlPAG between normal and sham-BCP groups. The P2X7 receptor protein level in vlPAG in BCP group was increased compared with normal and sham-BCP groups. Intraperitoneal tramadol injection at doses of 10, 20, and 40 mg/kg showed significantly and dose dependently upregulated the P2X7 receptor protein level in vlPAG. n = 6; ⋆⋆p < 0.01 and⋆⋆⋆p < 0.001 compared with normal or sham-BCP group.★★★p < 0.001 versus BCP group; △△△p < 0.001 versus TL group; ▲▲▲p < 0.001 versus TM group. BCP: bone cancer pain.
Effect of tramadol on hyperalgesia of BCP rats
No significant difference was observed in pain thresholds between BCP, TL, TM, and TH groups on 10 days’ postcancer cell inoculation before i.p. tramadol injection (p > 0.05, n = 12). On 14 days’ postcancer cell inoculation, the intraperitoneal administration of tramadol at doses of 10, 20, and 40 mg/kg considerably and dose dependently increased the MWT and TWL values of BCP rats compared with the pain threshold values of BCP group from 14 to 21 days’ postcancer cell inoculation. Furthermore, the pain threshold values were significantly different among TL, TM, and TH groups at the same time point (all p < 0.001; n = 12; Figure 4(a) and (b)).
Effect of tramadol on P2X7 receptor expression on BCP rats
Compared with the BCP animals, rats administered with tramadol at doses of 10, 20, and 40 mg/kg once a day for 7 days significantly and dose dependently upregulated the number of P2X7-positive cells in vlPAG at 21 days’ postcancer cell inoculation (p < 0.001, n = 6; Figure 5(a) and (b)).
Effect of tramadol on P2X7 protein level on BCP rats
Compared with BCP rats, rats administered with tramadol at doses of 10, 20, and 40 mg/kg once a day for 7 days significantly and dose dependently upregulated P2X7 receptor protein levels in vlPAG at 21 days’ postcancer cell inoculation (p < 0.001, n = 6; Figure 6(a) and (b)).
Influence of A-740003 pretreatments on the antinociceptive effect of i.p. injection of 40 mg/kg tramadol
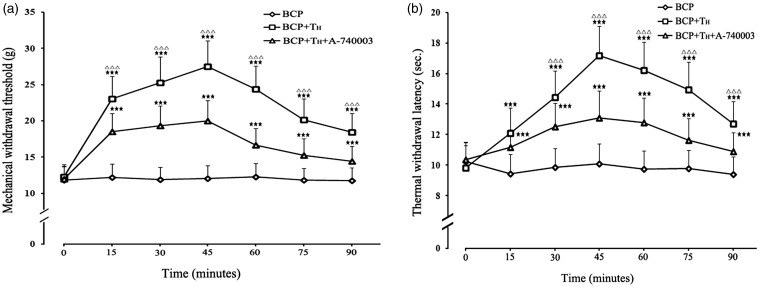
In BCP+TH+A-740003 group rats, MWT increased only from 11.98 ± 1.79 g to 20.02 ± 2.80 g, while TWL increased from 10.37 ± 1.09 s to 13.08 ± 1.77 s at 45 min after i.p. tramadol injection. The peak values of MWT and TWL were substantially lower than those of the TH group (p < 0.001, compared with TH group at the same time point), and the antinociceptive effect of tramadol was partially but significantly blocked by the vlPAG microinjection of P2X7 receptor antagonist A-740003 (Figure 7(a) and (b)).
Figure 7.
Pretreatment with A-740003 attenuates the antinociceptive effect of 40 mg/kg tramadol. The pain thresholds of each BCP rats before intra-vlPAG injection of A-740003 or i.p. tramadol injection served as control value (p>0.05 among all groups). MWT (a) and TWL (b) values were significantly elevated after i.p. injection of 40 mg/kg tramadol, but pretreatment with A-740003 (100 nmol) intra-vlPAG injection attenuated the antinociceptive effect of 40 mg/kg tramadol in BCP rats. (n = 8).★★★p < 0.001, compared with the BCP group; △△△p < 0.001, compared with the BCP+TH+A-740003 group. BCP: bone cancer pain.
Discussion
In this study, a rat model for BCP was induced by inoculating SHZ-88 mammary gland carcinoma cells into the medullary cavity of the proximal tibia. The analgesic effects of i.p. injection of different doses of tramadol on BCP rats and the expression changes of P2X7 receptor in vlPAG were observed. The main results of this study revealed that BCP rats exhibited a slight increased expression of P2X7 receptor in vlPAG compared with normal rats. Tramadol (10, 20, and 40 mg/kg, i.p.) markedly and dose dependently ameliorated the pain behaviors of BCP rats, upregulated pain thresholds, and further raised the expression level of P2X7 receptor in vlPAG. Intra-vlPAG microinjection of a selective P2X7 receptor antagonist A-740003 partly but significantly reversed the analgesic effect of tramadol.
Using a mouse femur bone cancer (FBC) pain model, the researchers found that tramadol (100 mg/kg, s.c.) significantly decreased the retention time of FBC mice by a Rota-rod test,45 meaning that tramadol at this dose can reduce the motor coordination capability of rats. In the current study, inclined-plane test was used in all experimental rats before algesimetry test to study whether BCP operation, drug administration, and intra-vlPAG microinjection exerted certain adverse effects on the motor function of experimental rats. The results showed all the experimental procedures without obvious effects on sensorimotor capability of the rats.
SHZ-88 carcinoma cell line was derived from rat breast carcinoma induced by dimethylbenzanthracene (DMBA). In the present study, when inoculated with 20 μL (107 cells/0.5 mL) SHZ-88 carcinoma cells, the rats survived for 30 and 40 days postinoculation, which allowed sufficient time to assess nociceptive activity. The measurement of nociceptive activity showed that activation threshold decreased considerably with respect to that of the sham-BCP group, thereby indicating mechanical allodynia. Moreover, thermal hyperalgesia was reported as determined as the decrease in retirement latency and compared with that of the sham-BCP group. The pain threshold values were usually reduced to a minimum at day 21; therefore, the X-ray images and HE staining were taken at day 21 postinoculation in this study. The radiographic results revealed a tumor growth with visible bone destruction, and histological images showed the destruction of smooth bone and trabecular bone of the tibia.
Cancer pain includes two components, namely, neuropathic pain and inflammatory pain. Notably, cancer pain also exhibits seemingly unique elements.6,46 ATP and its receptors play an important role in the formation and maintenance of pain.47 The purine P2 receptor comprises a ligand-gated ion channel P2X receptor subtype and a G protein-coupled P2Y receptor subtype. P2X receptors and P2Y receptors are distributed on the surface of the cell membrane and are activated by extracellular ATP or other nucleotides that sequentially induce a series of physiological or pathophysiological changes. P2X receptors include seven subtypes (P2X1–7) in which P2X7 receptors are expressed predominantly in neurons and glial cells (microglia, astrocytes, and oligodendrocytes).48–50 Previous research has suggested the presence of P2X7 receptor in the dorsal root ganglion and spinal cord dorsal horn and its involvement in neuropathic pain mechanism in rats.51,52 The studies suggested that P2X7 receptors are involved in the modulation of neuropathic pain in relation to the following factors. (1) Purine signaling can be conducted by non-synaptic routes. Preconditioning with ATP to the spinal cord slices of the neuropathic pain rats induce non-synaptic and non-neuronal P2X7 receptor activation in the spinal cord, and increased glutamate release led to the formation of pain.53 (2) P2X7 receptor activation and Ca2 + influx activate a variety of downstream cell signal transduction pathways that excite sensory neurons, especially nociceptive neurons, thereby increasing their excitability and resulting in pain.54 (3) Microglia P2X7 receptors are involved in microglia and neuronal interactions (crosstalk). The activation of microglia P2X7 receptors promotes interleukin-1β (IL-1β) release, phosphorylation of p38 mitogen-activated protein kinase, and the expression of glutamate receptor 1 (GluR1). Finally, the peripheral c fiber-induced field potential forms long-term potentiation, which leads to central sensitization and causes pain.55 Likewise, P2X7 receptor plays an important role in the modulation of inflammation, and inflammation reinforces the sensation of pain. The activation of P2X7 receptor incurred an inflammatory response and subsequent release of inflammatory cytokines. By using antagonists of P2X7 receptor, the inflammatory pain was obviously attenuated. Regrettably, the exact role of P2X7 receptor in cancer pain currently remains controversial. Hansen et al.56 discovered that P2X7 receptor knockout mice were more vulnerable to BCP compared with cancer-bearing, wild-type mice. On the contrary, Huang et al.57 demonstrated that the activation of microglial P2X7 receptor in the rostral VMM contributes to the development of BCP via the upregulation of spinal 5-HT levels by the descending pain facilitatory system. Additionally, relatively few studies have reported on the role of P2X7 receptors in pain regulation at the supraspinal anatomic sites at present. We demonstrated that the expression levels of P2X7 receptor-positive cells and P2X7 protein in vlPAG were considerably higher in BCP rats compared with those in sham BCP rats, indicating that tibia cancer cell inoculation may promote P2X7 receptor expression in vlPAG.
Tramadol is used alone or in combination with other narcotics for neuropathic and inflammatory pain treatment.32,58 Several investigations revealed that tramadol can alleviate BCP in rodent models and in humans59,60; however, these studies failed to correlate the mechanism underlying the analgesic effect of tramadol on BCP with the purinergic signaling pathway. Based on the previous findings,61,62 tramadol can rapidly penetrate the blood–brain barrier and blood–cerebrospinal fluid barrier. This study revealed that 10, 20, and 40 mg/kg tramadol i.p. administration caused a dose-dependent increase in the expression of P2X7 receptor in vlPAG compared with that in BCP rats. The classical view of tramadol analgesic mechanisms includes two main routes: (1) playing an analgesic role by activating the central opiate receptor (mainly μ receptor) and (2) inhibiting the re-uptake of NE and serotonin (5-HT), which increase NE and 5-HT levels in the synaptic cleft when released from the nerve endings. Tramadol is a racemic mixture with a molecular structure containing two optical enantiomers. The drug exerts analgesic effect through different mechanisms, namely, dextrose tramadol activation of the opioid μ receptor and left-enantiomer inhibition of NE and 5-HT re-uptake.35 On one hand, Gordon et al.63 found that in rat hypothalamic paraventricular nucleus, NE can promote glial cell release of ATP; combined with the current study, we deduced that i.p. injection of tramadol possibly reduces NE re-uptake in vlPAG and thus increases NE levels in the local microenvironment, consequently increasing the ATP level and upregulating P2X7 receptor expression in vlPAG; on the other hand, Papp et al.64 found that P2X1, 2, 3, 4, 6, 7 receptors expressed on the brainstem catecholaminergic neurons project to the hippocampus and that the activation of the above P2X receptor enhance NE release. From the above-mentioned reasons, there may be existed a crosstalk between NE and ATP. In our previous study, we observed up-regulation of P2X3 receptors in vlPAG in neuropathic pain rats.65 Thus, we hypothesized that a similar mechanism may exist in vlPAG in BCP rats, that is, P2X3 receptors in BCP rats are activated, and NE release increases and further promote ATP release, ultimately leading to P2X7 receptor activation. The hypotheses need to be further validated.
Yuan et al.59 found that i.p. injection of tramadol (10 mg/kg) can relieve BCP in rats. Our results showed that i.p. injections of tramadol exert a dose-dependent analgesic effect on BCP rats, and this analgesic effect was reversed by intra-vlPAG injection of a selective P2X7 receptor antagonist A-740003. Known tramadol analgesia mechanisms involve multiple neurotransmitter systems, such as opioid, NE, and 5-HT systems, which are closely related to pain.34,66 To the best of our knowledge, no research has determined the relationship between tramadol and purine receptors to date. This study further clarifies that the purine signaling pathway is also involved in the tramadol analgesic mechanism. Therefore, the current study can further our understanding of the interaction between pain-related neurotransmitters.
In summary, the present study demonstrates that the i.p. injection of tramadol enhances P2X7 receptor activation in the vlPAG and exerts a dose-dependent analgesic effect on BCP. These findings suggest that the activation of purine P2X7 receptor in vlPAG is involved in the analgesic mechanism of tramadol, thus providing theoretical basis for the clinical application of tramadol and potential for the development of new drugs targeting P2X7 receptors.
Acknowledgments
The authors thank Wei Chen and Xiang Lu for their support with the histological analysis work.
Author contributions
PT, QZ, ZX, SY, YQ, and YY performed the experiments. ZX analyzed the data and drafted the manuscript. All authors read and approved the final version of the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by the National Natural Science Foundation of China (grant number 81760214 to Zhi Xiao) and Guizhou Provincial Science and Technology Planning Project (grant number QianKeHe JiChu [2017]1214 to Zhi Xiao).
References
- 1.Mantyh PW. Bone cancer pain: from mechanism to therapy. Curr Opin Support Palliat Care 2014; 8: 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leppert W, Zajaczkowska R, Wordliczek J, Dobrogowski J, Woron J, Krzakowski M. Pathophysiology and clinical characteristics of pain in most common locations in cancer patients. J Physiol Pharmacol 2016; 67: 787–799. [PubMed] [Google Scholar]
- 3.Kane CM, Hoskin P, Bennett MI. Cancer induced bone pain. BMJ 2015; 350: h315. [DOI] [PubMed] [Google Scholar]
- 4.Mantyh P. Bone cancer pain: causes, consequences, and therapeutic opportunities. Pain 2013; 154 Suppl 1: S54–S62. [DOI] [PubMed] [Google Scholar]
- 5.Zhu YF, Ungard R, Seidlitz E, Zacal N, Huizinga J, Henry JL, Singh G. Differences in electrophysiological properties of functionally identified nociceptive sensory neurons in an animal model of cancer-induced bone pain. Mol Pain 2016; 12: 174480691662877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figura N, Smith J, Yu HM. Mechanisms of, and adjuvants for, bone pain. Hematol Oncol Clin North Am 2018; 32: 447–458. [DOI] [PubMed] [Google Scholar]
- 7.Hua B, Gao Y, Kong X, Yang L, Hou W, Bao Y. New insights of nociceptor sensitization in bone cancer pain. Expert Opin Ther Targets 2015; 19: 227–243. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Y-Q, Liu Z, Liu H-Q, Liu D-Q, Chen S-P, Ye D-W, Tian Y-K. Targeting glia for bone cancer pain. Expert Opin Ther Targets 2016; 20: 1365–1374. [DOI] [PubMed] [Google Scholar]
- 9.Inoue K. ATP receptors of microglia involved in pain. Novartis Found Symp 2006; 276: 263–272. discussion 273–281. [PubMed] [Google Scholar]
- 10.Chwistek M. Recent advances in understanding and managing cancer pain. F1000Res 2017; 6: 945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y-Q, Chen S-P, Liu D-Q, Manyande A, Zhang W, Yang S-B, Xiong B-R, Fu Q-C, Song Z-P, Rittner H, Ye D-W, Tian Y-K. The role of spinal GABAB receptors in cancer-induced bone pain in rats. J Pain 2017; 18: 933–946. [DOI] [PubMed] [Google Scholar]
- 12.Liu M, Yao M, Wang H, Xu L, Zheng Y, Huang B, Ni H, Xu S, Zhou X, Lian Q. P2Y12 receptor-mediated activation of spinal microglia and p38MAPK pathway contribute to cancer-induced bone pain. J Pain Res 2017; 10: 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Portenoy RK. Treatment of cancer pain. Lancet 2011; 377: 2236–2247. [DOI] [PubMed] [Google Scholar]
- 14.Burnstock G. Purinergic signalling: pathophysiology and therapeutic potential. Keio J Med 2013; 62: 63–73. [DOI] [PubMed] [Google Scholar]
- 15.Burnstock G, Kennedy C. P2X receptors in health and disease. Adv Pharmacol 2011; 61: 333–372. [DOI] [PubMed] [Google Scholar]
- 16.Nobile M, Monaldi I, Alloisio S, Cugnoli C, Ferroni S. ATP-induced, sustained calcium signalling in cultured rat cortical astrocytes: evidence for a non-capacitative, P2X7-like-mediated calcium entry. FEBS Lett 2003; 538: 71–76. [DOI] [PubMed] [Google Scholar]
- 17.Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz J M, Morelli A, Torboli M, Bolognesi G, Baricordi OR. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood 2001; 97: 587–600. [DOI] [PubMed] [Google Scholar]
- 18.Barros-Barbosa AR, Lobo MG, Ferreirinha F, Correia-de-Sá P, Cordeiro JM. P2X7 receptor activation downmodulates Na(+)-dependent high-affinity GABA and glutamate transport into rat brain cortex synaptosomes. Neuroscience 2015; 306: 74–90. [DOI] [PubMed] [Google Scholar]
- 19.Burnstock G, Knight GE. The potential of P2X7 receptors as a therapeutic target, including inflammation and tumour progression. Purinergic Signal 2018; 14: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes JP, Hatcher JP, Chessell IP. The role of P2X(7) in pain and inflammation. Purinergic Signal 2007; 3: 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burnstock G. Purinergic mechanisms and pain. Adv Pharmacol 2016; 75: 91–137. [DOI] [PubMed] [Google Scholar]
- 22.Bernier LP, Ase AR, Seguela P. P2X receptor channels in chronic pain pathways. Br J Pharmacol 2018; 175: 2219–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tavares I, Lima D. From neuroanatomy to gene therapy: searching for new ways to manipulate the supraspinal endogenous pain modulatory system. J Anat 2007; 211: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millan MJ. Descending control of pain. Prog Neurobiol 2002; 66: 355–474. [DOI] [PubMed] [Google Scholar]
- 25.Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol 1995; 46: 575–605. [DOI] [PubMed] [Google Scholar]
- 26.Mills EP, Di Pietro F, Alshelh Z, Peck CC, Murray GM, Vickers ER, Henderson LA. Brainstem pain-control circuitry connectivity in chronic neuropathic pain. J Neurosci 2018; 38: 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Worthington RA, Arumugam TV, Hansen MA, Balcar VJ, Barden JA. Identification and localisation of ATP P2X receptors in rat midbrain. Electrophoresis 1999; 20: 2077–2080. [DOI] [PubMed] [Google Scholar]
- 28.Xiao Z, Li YY, Sun MJ. Activation of P2X receptors in the midbrain periaqueductal gray of rats facilitates morphine tolerance. Pharmacol Biochem Behav 2015; 135: 145–153. [DOI] [PubMed] [Google Scholar]
- 29.Gao X-F, Wang W, Yu Q, Burnstock G, Xiang Z-H, He C. Astroglial P2X7 receptor current density increased following long-term exposure to rotenone. Purinergic Signal 2011; 7: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mintzer MZ, Lanier RK, Lofwall MR, Bigelow GE, Strain EC. Effects of repeated tramadol and morphine administration on psychomotor and cognitive performance in opioid-dependent volunteers. Drug Alcohol Depend 2010; 111: 265–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montero Matamala A, Bertolotti M, Contini MP, Guerrero Bayón C, Nizzardo A, Paredes Lario I, Pizà Vallespir B, Scartoni S, Tonini G, Capriati A, Pellacani A. Tramadol hydrochloride 75 mg/dexketoprofen 25 mg oral fixed-dose combination in moderate-to-severe acute pain: sustained analgesic effect over a 56-h period in the postoperative setting. Drugs Today 2017; 53: 339–347. [DOI] [PubMed] [Google Scholar]
- 32.Nishikawa N, Nomoto M. Management of neuropathic pain. J Gen Fam Med 2017; 18: 56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hassamal S, Miotto K, Dale W. Tramadol: understanding the risk of serotonin syndrome and seizures. Am J Med. Epub ahead of print 10 May 2018. DOI: 10.1016/j.amjmed.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 34.Miotto K, Cho AK, Khalil MA, Blanco K, Sasaki JD, Rawson R. Trends in tramadol: pharmacology, metabolism, and misuse. Anesth Analg 2017; 124: 44–51. [DOI] [PubMed] [Google Scholar]
- 35.Beakley BD, Kaye AM, Kaye AD. Tramadol, pharmacology, side effects, and serotonin syndrome: a review. Pain Physician 2015; 18: 395–400. [PubMed] [Google Scholar]
- 36.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–110. [DOI] [PubMed] [Google Scholar]
- 37.Pérez-Rojas JM, González-Macías R, González-Cortes J, Jurado R, Pedraza-Chaverri J, García-López P. Synergic effect of alpha-mangostin on the cytotoxicity of cisplatin in a cervical cancer model. Oxid Med Cell Longev 2016; 2016: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shenoy PA, Kuo A, Vetter I, Smith MT. The walker 256 breast cancer cell- induced bone pain model in rats. Front Pharmacol 2016; 7: 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu H, Zhu R, Liu C, Ma R, Wang L, Chen B, Li L, Niu J, Zhao D, Mo F, Fu M, Brömme D, Zhang D, Gao S, Evaluation of decalcification techniques for rat femurs using HE and immunohistochemical staining. Biomed Res Int 2017; 2017: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paxinos G and Watson C. The rat brain in stereotaxic coordinates. 6th ed Amsterdam, The Netherlands: Academic Press, 2007. [Google Scholar]
- 41.Rivlin AS, Tator CH. Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J Neurosurg 1977; 47: 577–581. [DOI] [PubMed] [Google Scholar]
- 42.Cunha TM, Verri WA, Jr, Vivancos GG, Moreira IF, Reis S, Parada CA, Cunha FQ, Ferreira SH. An electronic pressure-meter nociception paw test for mice. Braz J Med Biol Res 2004; 37: 401–407. [DOI] [PubMed] [Google Scholar]
- 43.Hargreaves K, Dubner R, Brown F, Flores C, Joris J, A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988; 32: 77–88. [DOI] [PubMed] [Google Scholar]
- 44.Van Nassauw L, Bogers J, Van Marck E, Timmermans JP. Role of reactive nitrogen species in neuronal cell damage during intestinal schistosomiasis. Cell Tissue Res 2001; 303: 329–336. [DOI] [PubMed] [Google Scholar]
- 45.Ono H, Nakamura A, Kanbara T, Minami K, Shinohara S, Sakaguchi G, Kanemasa T. Effect of the norepinephrine transporter (NET) inhibition on mu-opioid receptor (MOR)-induced anti-nociception in a bone cancer pain model. J Pharmacol Sci 2014; 125: 264–273. [DOI] [PubMed] [Google Scholar]
- 46.Falk S, Dickenson AH. Pain and nociception: mechanisms of cancer-induced bone pain. J Clin Oncol 2014; 32: 1647–1654. [DOI] [PubMed] [Google Scholar]
- 47.Burnstock G. Purinergic mechanisms and pain–an update. Eur J Pharmacol 2013; 716: 24–40. [DOI] [PubMed] [Google Scholar]
- 48.Miras-Portugal MT, Sebastián-Serrano Á, de Diego García L, Díaz-Hernández M. Neuronal P2X7 receptor: involvement in neuronal physiology and pathology. J Neurosci 2017; 37: 7063–7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhattacharya A, Biber K. The microglial ATP-gated ion channel P2X7 as a CNS drug target. Glia 2016; 64: 1772–1787. [DOI] [PubMed] [Google Scholar]
- 50.Rivera A, Vanzulli I, Butt AM, A central role for ATP signalling in glial interactions in the CNS. Curr Drug Targets 2016; 17: 1829–1833. [DOI] [PubMed] [Google Scholar]
- 51.Yang J, Park KS, Yoon JJ, Bae H-B, Yoon MH, Choi JI. Anti-allodynic effect of intrathecal processed Aconitum jaluense is associated with the inhibition of microglial activation and P2X7 receptor expression in spinal cord. BMC Complement Altern Med 2016; 16: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie J, Liu S, Wu B, Li G, Rao S, Zou L, Yi Z, Zhang C, Jia T, Zhao S, Schmalzing G, Hausmann R, Nie H, Li G, Liang S. The protective effect of resveratrol in the transmission of neuropathic pain mediated by the P2X7 receptor in the dorsal root ganglia. Neurochem Int 2017; 103: 24–35. [DOI] [PubMed] [Google Scholar]
- 53.Ando RD, Sperlagh B. The role of glutamate release mediated by extrasynaptic P2X7 receptors in animal models of neuropathic pain. Brain Res Bull 2013; 93: 80–85. [DOI] [PubMed] [Google Scholar]
- 54.Sperlágh B, Vizi ES, Wirkner K, Illes P. P2X7 receptors in the nervous system. Prog Neurobiol 2006; 78: 327–346. [DOI] [PubMed] [Google Scholar]
- 55.Chu Y-X, Zhang Y, Zhang Y-Q, Zhao Z-Q. Involvement of microglial P2X7 receptors and downstream signaling pathways in long-term potentiation of spinal nociceptive responses. Brain Behav Immun 2010; 24: 1176–1189. [DOI] [PubMed] [Google Scholar]
- 56.Hansen RR, Nielsen CK, Nasser A, Thomsen SIM, Eghorn LF, Pham Y, Schulenburg C, Syberg S, Ding M, Stojilkovic SS, Jorgensen NR, Heegaard A-M. P2X7 receptor-deficient mice are susceptible to bone cancer pain. Pain 2011; 152: 1766–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang ZX, Lu ZJ, Ma WQ, Wu FX, Zhang YQ, Yu W-F, Zhao ZQ. Involvement of RVM-expressed P2X7 receptor in bone cancer pain: mechanism of descending facilitation. Pain 2014; 155: 783–791. [DOI] [PubMed] [Google Scholar]
- 58.Mert T, Sahin E, Yaman S. Pain-relieving effectiveness of co-treatment with local tramadol and systemic minocycline in carrageenan-induced inflammatory pain model. Inflammation 2018; 41: 1238–1249. [DOI] [PubMed] [Google Scholar]
- 59.Yuan X, Wu J, Wang Q, Xu M. The antinociceptive effect of systemic administration of a combination of low-dose tramadol and dexmedetomidine in a rat model of bone cancer pain. Eur J Anaesthesiol 2014; 31: 30–34. [DOI] [PubMed] [Google Scholar]
- 60.Corona-Ramos JN, Déciga-Campos M, Romero-Piña M, Medina LA, Martínez-Racine I, Jaramillo-Morales OA, García-López P, López-Muñoz FJ. The effect of gabapentin and tramadol in cancer pain induced by glioma cell in rat femur. Drug Dev Res 2017; 78: 173–183. [DOI] [PubMed] [Google Scholar]
- 61.Sheikholeslami B, Gholami M, Lavasani H, Rouini M. Evaluation of the route dependency of the pharmacokinetics and neuro-pharmacokinetics of tramadol and its main metabolites in rats. Eur J Pharm Sci 2016; 92: 55–63. [DOI] [PubMed] [Google Scholar]
- 62.Kitamura A, Higuchi K, Okura T, Deguchi Y. Transport characteristics of tramadol in the blood-brain barrier. J Pharm Sci 2014; 103: 3335–3341. [DOI] [PubMed] [Google Scholar]
- 63.Gordon GRJ, Baimoukhametova DV, Hewitt SA, Rajapaksha WRAKJS, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci 2005; 8: 1078–1086. [DOI] [PubMed] [Google Scholar]
- 64.Papp L, Balázsa T, Köfalvi A, Erdélyi F, Szabó G, Vizi ES, Sperlágh B. P2X receptor activation elicits transporter-mediated noradrenaline release from rat hippocampal slices. J Pharmacol Exp Ther 2004; 310: 973–980. [DOI] [PubMed] [Google Scholar]
- 65.Xiao Z, Ou S, He W-J, Zhao Y-D, Liu X-H, Ruan H-Z. Role of midbrain periaqueductal gray P2X3 receptors in electroacupuncture-mediated endogenous pain modulatory systems. Brain Res 2010; 1330: 31–44. [DOI] [PubMed] [Google Scholar]
- 66.Bravo L, Mico JA, Berrocoso E, Discovery and development of tramadol for the treatment of pain. Expert Opin Drug Discov 2017; 12: 1281–1291. [DOI] [PubMed] [Google Scholar]