Abstract
Background:
Subcutaneous sacral nerve stimulation is recommended by the United Kingdom (UK) National Institute for Health and Care Excellence (NICE) as a second-line treatment for patients with faecal incontinence who failed conservative therapy. Sacral nerve stimulation is an invasive procedure associated with complications and reoperations. This study aimed to investigate whether delivering less invasive and less costly percutaneous tibial nerve stimulation prior to sacral nerve stimulation is cost-effective.
Methods:
A decision analytic model was developed to estimate the cost-effectiveness of percutaneous tibial nerve stimulation with subsequent subcutaneous sacral nerve stimulation versus subcutaneous sacral nerve stimulation alone. The model was populated with effectiveness data from systematic reviews and cost data from randomized studies comparing both procedures in a UK National Health Service (NHS) setting.
Results:
Offering percutaneous tibial nerve stimulation prior to sacral nerve stimulation (compared with delivering sacral nerve stimulation straight away) was both more effective and less costly in all modeled scenarios. The estimated savings from offering percutaneous tibial nerve stimulation first were £662–£5,697 per patient. The probability of this strategy being cost-effective was around 80% at £20,000–£30,000 per quality-adjusted life-year (QALY).
Conclusion:
Our analyses suggest that offering patients percutaneous tibial nerve stimulation prior to sacral nerve stimulation can be both cost-effective and cost-saving in the treatment of faecal incontinence.
Keywords: cost-effectiveness, faecal incontinence, health economics, percutaneous tibial nerve stimulation, sacral nerve stimulation
Introduction
It is estimated that in the United Kingdom (UK), the prevalence of faecal incontinence in adults living in the community is 1–10%, depending on the definition used.1,2 Faecal incontinence affects the ability to live a normal life, work and socialize, and has huge emotional impact on patients and their carers. Faecal incontinence is associated with high costs to the UK National Health Service (NHS) and society due to heavy use of healthcare services, loss of work productivity, unemployment and disability.
According to the NICE guidelines on managing faecal incontinence,2 the first-line treatment for faecal incontinence is conservative, such as antidiarrhoeal medication, pelvic floor muscle training, bowel retraining, specialist dietary assessment and management, biofeedback, electrical stimulation and rectal irrigation. For patients with a weak but structurally intact sphincter, in whom sphincter surgery is deemed inappropriate, subcutaneous sacral nerve stimulation (SNS) may be the next treatment option. It involves an implantation of a stimulator which applies an electric current to one of the sacral nerves via an electrode placed through the corresponding sacral foramen.3 According to a systematic literature review,4 41–75% of implanted patients achieved complete continence and 75–100% experienced 50% improvement in the number of faecal incontinence episodes. However, the implantation of a stimulator is associated with complications such as pain, lead migration, wound infection and loss of effectiveness. The reported pooled incidence of pain and infection after implantation was 13.0% and 3.9%, respectively.5 Complications may lead to removal of the stimulator and subsequent re-implantation.
Percutaneous tibial nerve stimulation (PTNS) is a less invasive treatment available for people with faecal incontinence who do not respond adequately to conservative therapy.6 It involves electrical stimulation of the posterior tibial nerve via a needle percutaneously inserted into the ankle and connected to an external pulse generator. Initial treatment usually consists of 12–15 outpatient sessions lasting 30 min each, typically a week apart. Treatment usually requires two top-up sessions every 6 months. Adverse events are rare and resolve spontaneously. PTNS was shown to be less effective in reducing faecal incontinence compared with SNS.4,7,8 The UK National Institute for Health and Care Excellence (NICE) guidance on PTNS states that there is limited evidence on the benefits of PTNS, particularly in the long term.6 It recommends that PTNS should only be used ‘with special arrangements for clinical governance, consent and audit or research’.6 In recent years there has been a growing body of evidence demonstrating the effectiveness of PTNS for faecal incontinence, including the long-term effects of this procedure.4,7–9 In light of these findings, the current NICE recommendations on the management of faecal incontinence2,6 should be reviewed.
Although PTNS appears to be less effective than SNS, it can be delivered prior to SNS due to good acceptance by patients. Patients eligible for SNS could be treated with PTNS before being considered for sacral nerve stimulation.10 The cost-effectiveness of this strategy is debatable, since patients receiving both treatments incur higher costs than people receiving one of the treatments. Comparing the costs of PTNS and SNS is not straightforward, given that SNS costs are incurred mainly upfront, while PTNS costs incur over time due to maintenance treatments. To enable a comparison between the two treatment strategies, a decision analytic model was developed which compared the cost-effectiveness of PTNS followed by SNS upon PTNS failure, versus SNS alone, over a 5-year time horizon. The model was populated with effectiveness data from published studies4,7–21 and the cost data from the randomized pilot study comparing PTNS with SNS in a UK NHS setting.22
Materials and methods
SNS and PTNS treatments
SNS is delivered in two stages. At the first stage a temporary peripheral nerve electrode is inserted under local anesthetic and attached to an external stimulator. Temporary SNS is delivered under local anesthetic as an outpatient procedure. The patients will be given questionnaires to record their faecal incontinence symptoms during the test period (2–3 weeks).3 They may develop complications such as pain, wound infection, electrode dislocation and loss of effectiveness. Treatment of these complications may include: adjusting the stimulator’s settings, electrode re-positioning, wound revision, antibiotic treatment, removal of electrode, and insertion of new electrode. If faecal incontinence symptoms improve during the testing period (2–3 weeks), the permanent lead and the permanent stimulator (implant) will be inserted. This procedure is delivered as a day case under general anesthetic.3 After the operation patients will be prescribed antibiotics and painkillers. They will be given a handset to control the stimulator and a diary to record symptoms of faecal incontinence. Following implantation patients may develop complications such as pain in the stimulation site, wound infection, lead migration and loss of effectiveness.5,12,15 They may also undergo removal of the device with subsequent re-implantation due to having a magnetic resonance imaging (MRI) scan, or to have the battery changed.13,23 In accordance with NICE guidance2 all patients with faecal incontinence will attend clinic every 6 months to review the symptoms.
PTNS treatment, delivered by a trained nurse, usually consists of 12 outpatient sessions lasting 30 min each, typically a week apart; treatment may be repeated as required. Adverse events are not included in the model since these are rare and resolve spontaneously.6
Model description
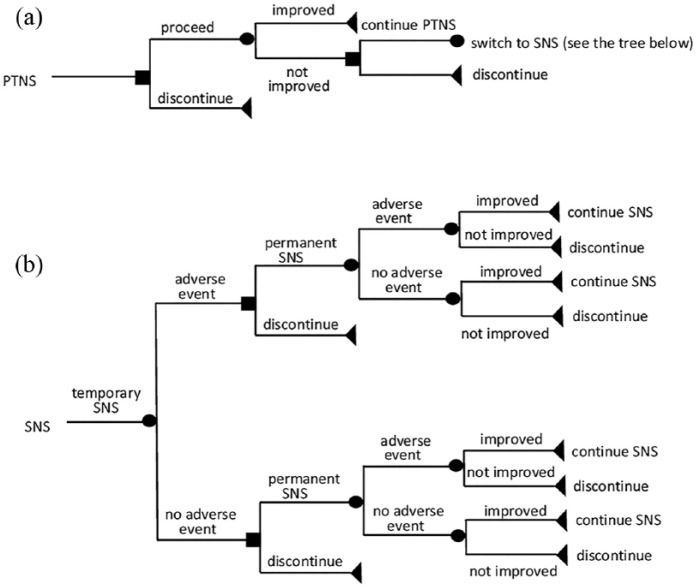
A decision analytic model was developed in TreeAge Pro Healthcare 2013 (TreeAge Software, Williamstown, MA, USA). The patient population included individuals eligible for SNS based on NICE criteria 1 for symptom severity and failure of prior conservative therapy.2 The decision trees for SNS and PTNS are shown in Figure 1.
Figure 1.
Decision trees for PTNS (a) and SNS (b).
PTNS, percutaneous tibial nerve stimulation; SNS, sacral nerve stimulation.
PTNS decision model [Figure 1(a)]. Patients who tolerate PTNS treatment will receive 17 sessions (15 sessions within 3 months plus two top-up sessions at 6 months). Patients who do not tolerate the treatment (e.g. due to vasovagal response) may discontinue after the first session. Those who achieved ⩾50% improvement in faecal incontinence will continue receiving two PTNS sessions every 6 months. Adverse events for PTNS are not included in the model given that these were minor and resolved spontaneously. Patients who did not achieve a 50% reduction of faecal incontinence after 17 PTNS sessions will proceed to SNS [Figure 1(b)].
SNS decision model [Figure 1(b)]. Patients first receive temporary SNS. They can discontinue the treatment due to intolerance or lack of effectiveness. They can also experience adverse events such as infection, electrode dislocation and pain. Patients who improved their continence in response to temporary SNS will receive permanent SNS. Permanent SNS can cause adverse events (e.g. pain, infection, lead dislocation, loss of effectiveness). The SNS stimulator may require its battery to be changed.
The costing perspective was that of the NHS and Personal Social Services in the UK, with a price year of 2012–2013. The list of model inputs and assumptions used in the model and correspondent references are shown in Table 1. The analysis considered a 5-year time horizon. The ‘success’ of treatment was defined as ⩾50% reduction in faecal incontinence episodes per week.4
Table 1.
List of parameters used in the model.
| Parameter | Value | Reference |
|---|---|---|
| Probabilities: PTNS | ||
| Probability of >50% improvement in FI for PTNS | ||
| base case | 0.59 | Thin and colleagues4 |
| lower limit | 0.59 | Thin and colleagues4 |
| upper limit | 0.71 | Thin and colleagues4 |
| Probability of discontinuation for PTNS | 0.04 | Peters and colleagues16 |
| Probability of proceeding to SNS after failing PTNS | ||
| base case | 0.96 | Assumption: probability of not proceeding to SNS equal to probability of discontinuation for PTNS |
| lower limit | 0.50 | Assumption |
| upper limit | 1.00 | Assumption |
| Probabilities: SNS | ||
| Probability of receiving permanent SNS | ||
| base case | 0.77 | Thin and colleagues4 |
| lower limit | 0.40 | Thin and colleagues4 |
| upper limit | 1.00 | Thin and colleagues4 |
| Probability of >50% improvement in FI for permanent SNS (short term) | ||
| base case | 0.79 | Thin and colleagues4 |
| lower limit | 0.69 | Thin and colleagues4 |
| upper limit | 0.83 | Thin and colleagues4 |
| Probability of >50% improvement in FI for permanent SNS (long term) | ||
| base case | 0.84 | Thin and colleagues4 |
| lower limit | 0.75 | Thin and colleagues4 |
| upper limit | 1.00 | Thin and colleagues4 |
| Probabilities: adverse events temporary SNS | ||
| base case | 0.22 | Hetzer and colleagues12 (joint probability of adverse events: pain in the stimulation site 0.06, wound infection 0.08, electrode defect/dislocation 0.08) |
| lower limit | 0.02 | Prapasrivorakul and colleagues17 |
| upper limit | 0.35 | Van Kerrebroeck and colleagues20 |
| Probabilities: adverse events permanent SNS | ||
| base case | 0.35 | Hetzer and colleagues12 (joint probability of adverse events: pain in the stimulation site 0.17, wound infection 0.09, electrode dislocation 0.09) |
| lower limit | 0.13 | Jarrett and colleagues14 |
| upper limit | 0.38 | Mellgren15 |
| Probabilities: maintenance SNS | ||
| Battery change | 0.08 | Hollingshead and colleagues13 |
| MRI scan: | 0.02 | Tjandra and colleagues19 |
| Health utilities | ||
| Persistent FI | ||
| base case | 0.69 | Harvie and colleagues11 |
| lower limit | 0.56 | Soria-Aledo and colleagues18 |
| upper limit | 0.68 | Van Wunnik and colleagues21 |
| >50% reduction in FI | ||
| base case | 0.77 | Harvie and colleagues11 |
| lower limit | 0.63 | Soria-Aledo and colleagues18 |
| upper limit | 0.86 | Van Wunnik and colleagues21 |
| Adverse events | ||
| base case | 0.46 | McDermott and colleagues26 (pain severity: moderate) |
| lower limit | 0.67 | McDermott and colleagues26 (pain severity: mild) |
| upper limit | 0.16 | McDermott and colleagues26 (pain severity: severe) |
| Adverse event duration | ||
| base case | 7 days | NHS England. Consultant-led referral to treatment waiting times. Annual report27 |
| lower limit | 3 days | Assumption |
| upper limit | 14 days | Assumption |
| Discounting rate | ||
| base case | 3.5% | NICE28 |
| lower limit | 1.5% | NICE28 |
| upper limit | 6.0% | Claxton and colleagues29 |
PTNS, percutaneous tibial nerve stimulation; SNS, sacral nerve stimulation.
Probabilities
The list of probabilities used in the model is shown in Table 1. Probabilities of >50% improvement in faecal incontinence for SNS and PTNS, and a probability of receiving permanent SNS were taken from a systematic review.4 The overall probabilities of adverse events for temporary and permanent SNS were estimated as a sum of probabilities for pain in the stimulation site, electrode defect/dislocation and wound infection. Probabilities of individual adverse events were taken from Hezler and colleagues.12 Sensitivity analyses were conducted including overall probabilities of adverse events for temporary and permanent SNS taken from published studies.14,15,17,20,24,25 The probability of discontinuation for PTNS was defined as probability of vasovagal response to needle placement during PTNS.16 Due to lack of published data on the discontinuation rate for temporary SNS, it was assumed to be the same as for PTNS (conservative assumption). Probabilities of removal of SNS stimulator due to MRI scan or battery change were taken from Tjandra and colleagues19 and Hollingshead and colleagues13
Costs
The costs of delivering SNS and PTNS in an NHS setting were estimated using a micro-costing approach. Data on the use of healthcare resources were collected in a randomized pilot study comparing SNS and PTNS, published elsewhere.22 Costs used in the model were divided into three categories:
(1) Upfront costs including devices, procedures, consultations and investigations;
(2) Maintenance costs including top-up sessions for PTNS, battery replacement for SNS, and removal of SNS device due to MRI scan;
(3) Costs associated with adverse events for temporary and permanent SNS. The major adverse events for both temporary and permanent SNS were: pain in the stimulation site (not related to stimulator settings), electrode or lead defect/dislocation, wound infection and the loss of effectiveness. It was assumed that all adverse events would require electrode removal with or without subsequent electrode replacement. Costs associated with management of wound infection also included antibiotic treatment. Costs associated with electrode defect (defined as excessive impedance) and electrode dislocation included cost of electrode replacement. Loss of effectiveness was assumed to involve electrode removal without subsequent replacement. The costs of adverse events were calculated by multiplying unit costs associated with management of each adverse event by the probability of each adverse event. Costs associated with adverse events for PTNS were not included in the model given that these adverse events resolve spontaneously.
Unit costs of SNS and PTNS devices were based on invoices. Unit costs of the SNS and PTNS procedures, consultations and investigations were taken from the National Schedule of Reference Costs (2011–2012).30 The model assumes that all patients incur investigation and examination costs. Patients who proceed to SNS upon failing PTNS will receive additional examinations. The model does not include costs associated with 6-monthly review meetings, since these apply to all patients with faecal incontinence.2 The lists of unit costs used in the model are shown in Tables 2–4.
Table 2.
Unit costs for PTNS.
| Expenditure type | Unit cost (£) |
Cost per patient (£) | References and assumptions |
|---|---|---|---|
| Device | |||
| UPC stimulator | 868.84 | 86.88 | Uroplasty, 2013 (personal communication). Multiple use, 10 patients per stimulator |
| UPC lead | 417.88 | 522.35 | Uroplasty, 2013 (personal communication). Single use, 15 leads per patient |
| Procedures | |||
| Consultation | 123 | 123 | NHS reference costs.30 Consultant-led: first attendance nonadmitted face to face, colorectal surgery |
| Physiology testing: | |||
| Outpatient appointment | 99 | 99 | NHS reference costs.30 Nonconsultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Ultrasound | 51 | 51 | NHS reference costs.30 Diagnostic imaging: outpatient. ultrasound scan, less than 20 min |
| Fluoroscopy | 119 | 119 | NHS reference costs.30Diagnostic imaging: outpatient. contrast fluoroscopy procedures, less than 20 min |
| Second consultation | 93 | 93 | NHS reference costs.30 Consultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| 15 PTNS procedures | 99 | 1,485.00 | NHS reference costs.30 Nonconsultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Upfront cost | 2,579.23 | ||
| Maintenance | |||
| UPC lead | 417.88 | 69.65 | Uroplasty, 2013 (personal communication). Single use, two leads per patient |
| 2 top-up PTNS procedures | 99 | 198.00 | NHS reference costs.30 Nonconsultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Maintenance cost over 5 years | 2,408.82 |
PTNS, percutaneous tibial nerve stimulation; UPC, Urgent® PC Stimulator.
Table 3.
Unit costs for SNS.
| Expenditure type | Unit cost (£) |
Cost per patient (£) | References and assumptions |
|---|---|---|---|
| Device | |||
| Temporary SNS | |||
| Temporary SNS kit (includes 2 × 9 cm needles) | 210 | 210 | Medtronic 2013 (personal communication). Single use |
| 12.5 cm needles pack of 6 | 135 | 0.45 | Medtronic 2013 (personal communication). Single use, assumes two needles per patient, required for 1% of patients |
| Power source | 335 | 18.61 | Medtronic 2013 (personal communication). Multiple use, assumes 18 patients as per trial |
| Permanent SNS | |||
| Patient programmer | 500 | 500 | Medtronic 2013 (personal communication). Single use |
| Pulse generator | 5,700.00 | 5,700.00 | Medtronic 2013 (personal communication). Single use |
| Tined lead | 1,350.00 | 1,350.00 | Medtronic 2013 (personal communication). Single use |
| Lead introducer kit | 200 | 200 | Medtronic 2013 (personal communication). Single use |
| Procedures | |||
| Temporary SNS | |||
| Initial consultation | 123 | 123 | NHS reference costs.30 Consultant-led: first attendance nonadmitted face to face, colorectal surgery |
| Physiology testing: | |||
| Outpatient appointment | 99 | 99 | NHS reference costs.30 Nonconsultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Ultrasound | 51 | 51 | NHS reference costs.30 Diagnostic imaging: outpatient. ultrasound scan, less than 20 min |
| Fluoroscopy | 119 | 119 | NHS reference costs.30 Diagnostic imaging: outpatient. contrast fluoroscopy procedures, less than 20 min |
| Second consultation | 93 | 93 | NHS reference costs.30 Consultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Counseling | 99 | 99 | NHS reference costs.30 Nonconsultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Pre-assessment for operation | 99 | 99 | NHS reference costs.30 Nonconsultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Procedure | 599 | 599 | NHS reference costs.30 Intermediate pain procedure, day case |
| Postoperative medication: paracetamol 500 mg for 7 days | 2.88 | 2.88 | BNF 2014. Nonproprietary, 0.5–1 g every 4–6 h |
| Specialist nurse review | 99 | 99 | NHS reference costs.30 Nonconsultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Permanent SNS | |||
| Pre-assessment for operation | 99 | 99 | NHS reference costs.30 Nonconsultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Procedure | 4,268.00 | 4,268.00 | NHS reference costs.30 Insertion of neurostimulator or intrathecal drug delivery device, day case |
| Postoperative medication: | |||
| Co-codamol 8/500 mg for 14 days | 3.4 | 3.4 | BNF 2014. Nonproprietary, 100-tab pack, 1–2 tablets every 4–6 h |
| Co-amoxiclav 500/125 mg for 5 days | 3.03 | 3.03 | BNF 2014. Sandoz, 21-tab pack, 1 tablet every 12 h |
| Post-operation check at 6 weeks | 93 | 93 | NHS reference costs.30 Consultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Total cost temporary SNS | 1,612.94 | ||
| Total cost permanent SNS | 12,216.43 | ||
| Maintenance | |||
| Battery change: | |||
| Removal of device | 599 | 35.94 | NHS reference costs.30 Intermediate pain procedure, day case |
| Re-implantation | 4,268.00 | 256.08 | NHS reference costs.30 Insertion of neurostimulator or intrathecal drug delivery device, day case |
| New device | 7,750.00 | 465.00 | Cost of device for permanent SNS (see top of this table) |
| MRI scan: | |||
| Removal of device | 599 | 11.09 | NHS reference costs.30 Intermediate pain procedure, day case |
| Re-implantation | 4,268.00 | 79.04 | NHS reference costs.30 Insertion of neurostimulator or intrathecal drug delivery device, day case |
| New device | 7,750.00 | 143.52 | Cost of device for permanent SNS (see top of this table) |
| Consultation | 93 | 7.44 | NHS reference costs.30Consultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Postoperative medication: | |||
| Co-codamol 8/500 mg for 14 days | 3.4 | 0.27 | BNF 2014. Nonproprietary, 1–2 tablets every 4–6 h |
| Co-amoxiclav 500/125 mg for 5 days | 3.03 | 0.24 | BNF 2014. Sandoz, 1 tablet every 12 h |
| Post-operation check | 93 | 7.44 | NHS reference costs.30 Consultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Maintenance cost over 5 years | 1,004 |
MRI, magnetic resonance imaging; NHS, National Health Service; SNS, sacral nerve stimulation.
Table 4.
Unit costs of adverse events for SNS.
| Event | Unit cost | Cost per person | Reference/assumption |
|---|---|---|---|
| Temporary SNS | |||
| Electrode defect/dislocation | |||
| Consultation | 93 | 46.50 | NHS reference costs.30 Consultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Electrode replacement | 599 | 299.50 | NHS reference costs.30 Intermediate pain procedure, day case |
| New electrode | 210 | 52.50 | Medtronic, 2013 (personal communication) |
| Paracetamol 500 mg for 7 days | 2.88 | 1.44 | BNF, 2014. Nonproprietary, 0.5–1.0 g every 4–6 h |
| Pain in the stimulation site | |||
| Consultation | 93 | 11.63 | NHS reference costs.30 Consultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Electrode re-positioning | 599 | 74.88 | NHS reference costs.30 Intermediate pain procedure, day case |
| New electrode | 210 | 13.13 | Medtronic 2013 (personal communication). |
| Paracetamol 500 mg for 7 days | 2.88 | 0.36 | BNF, 2014. Nonproprietary, 0.5–1.0 g every 4–6 h |
| Wound infection | |||
| Consultation | 93 | 11.63 | NHS reference costs.30 Consultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Wound revision/removal of electrode | 599 | 74.88 | NHS reference costs.30 Consultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Co-amoxiclav 500/125 mg for 5 days | 3.03 | 0.38 | BNF, 2014. Sandoz, 1 tablet every 12 h |
| Paracetamol 500 mg for 7 days | 2.88 | 0.36 | BNF, 2014. Nonproprietary, 0.5–1.0 g every 4–6 h |
| Post-operation check | 93 | 11.63 | NHS reference costs.30 Consultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Re-implantation of electrode | 599 | 74.88 | NHS reference costs.30 Intermediate pain procedure, day case |
| New electrode | 210 | 13.13 | Medtronic, 2013 (personal communication) |
| Loss of effectiveness | |||
| Removal of electrode | 93 | 23.25 | NHS reference costs.30 Consultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Cost of adverse events for temporary SNS adjusted for probability (Table 1) | 156.21 | ||
| Permanent SNS | |||
| Electrode dislocation | |||
| Consultation | 93 | 34.88 | NHS reference costs.30 Consultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Re-positioning of electrode | 4,268.00 | 1,600.50 | NHS reference costs.30 Insertion of neurostimulator or intrathecal drug delivery device, day case |
| Postoperative medication | |||
| Co-codamol 8/500 mg for 14 days | 3.4 | 1.28 | BNF, 2014. Nonproprietary, 100-tab pack, 1–2 tablets every 4–6 h |
| Co-amoxiclav 500/125 mg for 5 days | 3.03 | 1.14 | BNF, 2014. Sandoz, 21-tab pack, 1 tablet every 12 h |
| Post-operation check | 93 | 34.88 | NHS reference costs.30 Consultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Pain in the stimulation site | |||
| Consultation | 93 | 11.63 | NHS reference costs.30 Consultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Re-positioning of device | 599 | 74.88 | NHS reference costs.30 Intermediate pain procedure, day case |
| Paracetamol 500 mg for 7 days | 2.88 | 0.36 | BNF, 2014. Nonproprietary, 0.5–1.0 g every 4–6 h |
| Post-operation check | 93 | 11.63 | NHS reference costs.30 Consultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Wound infection | |||
| Consultation | 93 | 34.88 | NHS reference costs.30 Consultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Wound revision/device removal | 599 | 224.63 | NHS reference costs.30 Intermediate pain procedure, day case |
| Postoperative medication | |||
| Co-codamol 8/500 mg for 14 days | 3.4 | 0.43 | BNF, 2014. Nonproprietary, 100-tab pack, 1–2 tablets every 4–6 h |
| Co-amoxiclav 500/125 mg for 5 days | 3.03 | 0.38 | BNF, 2014. Sandoz, 21-tab pack, 1 tablet every 12 h |
| Post-operation check | 93 | 34.88 | NHS reference costs.30 Consultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Re-implantation | 4,268.00 | 533.50 | NHS reference costs.30 Insertion of neurostimulator or intrathecal drug delivery device, day case |
| New device | 7,750.00 | 968.75 | Cost of device for permanent SNS (see Table 3) |
| Postoperative medication | |||
| Co-codamol 8/500 mg for 14 days | 3.4 | 0.43 | BNF, 2014. Nonproprietary, 100-tab pack, 1–2 tablets every 4–6 h |
| Co-amoxiclav 500/125 mg for 5 days | 3.03 | 0.38 | BNF, 2014. Sandoz, 21-tab pack, 1 tablet every 12 h |
| Post-operation check | 93 | 11.63 | NHS reference costs.30 Consultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Loss of effectiveness | |||
| Consultation | 93 | 11.63 | NHS reference costs.30 Consultant-led: follow-up attendance nonadmitted face to face, colorectal surgery |
| Removal of device | 599 | 74.88 | NHS reference costs.30 Intermediate pain procedure, day case |
| Paracetamol 500 mg for 7 days | 2.88 | 0.36 | BNF, 2014. Nonproprietary, 0.5–1.0 g every 4–6 h |
| Cost of adverse events for permanent SNS adjusted for probability (Table 1) | 880.28 | ||
NHS, National Health Service; SNS, sacral nerve stimulation.
Health utilities
Health utilities for patients with faecal incontinence, derived using EQ-5D, were taken from published studies11,18,21 (Table 1). Due to a lack of published data on dis-utilities associated with adverse events for SNS, we used utilities for neuropathic pain derived using EQ-5D.26 In the base case analysis we used dis-utilities for moderate pain, while in sensitivity analyses we used dis-utilities for mild and severe pain. Dis-utilities associated with adverse events were assumed to be the same for temporary and permanent SNS. In the base case scenario, the duration of adverse events was 7 days (the average UK NHS consultant-led waiting time).30 In sensitivity analyses the duration of adverse events was 3 and 14 days. The model assumes that patients who discontinued PTNS or SNS with no adverse events have the same utility as those who did not achieve ⩾50% improvement in faecal incontinence.
Discounting
Both costs and quality-adjusted life-years (QALYs) were discounted after year 1 to reflect time preference. In the base case analysis both costs and QALYs were discounted at 3.5%.28 In sensitivity analyses discount rates were 1.5% and 6%.28,29 Given that the majority of costs (e.g. associated with devices, procedures, and adverse events) were incurred in year 1, the discounting was applied to maintenance costs only.
Cost-effectiveness analysis
The economic analysis complied with the NICE reference case,28 and is reported according to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS).31 The primary outcome of the model was the incremental cost-effectiveness ratio (cost per QALY gained). Univariate sensitivity analysis was conducted to assess uncertainty associated with probability of receiving permanent SNS; probabilities of >50% improvement in faecal incontinence for SNS and PTNS; probabilities of adverse events for temporary and permanent SNS; health utility for adverse events; duration of adverse events and costs of SNS and PTNS. A probabilistic sensitivity analysis was undertaken to assess the joint uncertainty in costs and QALYs.32 Probability distributions were assigned to probabilities, QALYs and costs and 10,000 Monte Carlo simulations were conducted to calculate the probability of cost-effectiveness at the NICE threshold of £30,000 per QALY gained.28
Results
Costs
The upfront and maintenance costs associated with PTNS are summarized in Table 2. The upfront cost, which included costs of device, consultations, investigations and 15 PTNS procedures, was £2,579 per patient. The cost of two maintenance PTNS procedures was £268 (£535 a year). Analysis assumed one stimulator per 10 patients (15 sessions for each patient) and 15 leads per patient, as per trial.22 The life-time of a Urgent® PC Stimulator was assumed to be 5 years. The total cost of delivering PTNS in year one was £2,847, and £4,988 over 5 years. Patients who discontinued PTNS due to intolerance incurred cost of £619, which included costs of consultations, investigations and one PTNS procedure. No costs related to adverse events were included, since adverse events for PTNS were minor and transient. The major PTNS costs were associated with clinic attendances to receive PTNS sessions.
Unit costs for SNS are summarized in Table 3. The upfront costs for SNS included costs of consultations, investigations and procedures for temporary and permanent SNS. The total upfront cost of SNS was £13,829 which included the cost of temporary SNS (£1,613) and the cost of permanent SNS (£12,216). All devices were considered to be single use, except for the power source for temporary SNS, which was assumed to be shared by 18 patients, as per trial.22 Maintenance costs included costs associated with battery change and MRI scan (Table 3), which would require removing the existing device and inserting a new one. Maintenance costs were calculated by multiplying unit costs by the probabilities of needing an MRI scan or battery change. The estimated maintenance cost over a 5-year period was £1,004 per person. The breakdown of costs associated with adverse events for SNS are shown in Table 4. The total cost of adverse events over 5 years multiplied by their probabilities (Table 1) was £156 per patient for temporary SNS, and £880 for permanent SNS. The main SNS costs were associated with the SNS device, followed by operation procedures.
The total PTNS and SNS costs over 5 years (including costs of adverse events and maintenance) are summarized in Appendix 1. These varied in the range £2,847–£4,849 for PTNS, £13,829–£19,153 for SNS, and £16,676–£22,000 for PTNS + SNS (Appendix 1).
QALYs
Total QALYs used in the model are summarized in Table 5. Patients who achieved ⩾50% improvement in faecal incontinence in the absence of adverse events generated 3.454 QALYs over 5 years, while patients who did improve their symptoms had a QALY value of 3.095 (3.5% discounting). This difference equals 131 days with improved continence. Adverse events had a small effect on total QALYs due to their transient character. The total QALYs were sensitive to the discounting rate, while changes in the duration of adverse events had only a very minor effect (Table 5).
Table 5.
QALYs over 5-year period calculated using different discounting rate and different duration of adverse events.
| Treatment scenario | Discounting rate |
||
|---|---|---|---|
| 1.5% | 3.5% | 6% | |
| SNS | QALY | ||
| ⩾50% improvement in faecal incontinence | |||
| No adverse events | 3.672 | 3.454 | 3.210 |
| One adverse event | 3.663 | 3.445 | 3.201 |
| Two adverse events | 3.654 | 3.436 | 3.192 |
| <50% improvement in faecal incontinence | |||
| No adverse events | 3.290 | 3.095 | 2.876 |
| One adverse event | 3.282 | 3.086 | 2.867 |
| Two adverse events | 3.273 | 3.078 | 2.859 |
| PTNS | |||
| ⩾50% improvement in faecal incontinence | 3.672 | 3.454 | 3.210 |
| <50% improvement in faecal incontinence | 3.290 | 3.095 | 2.876 |
| Treatment scenario | Duration of adverse events
(discounting rate 3.5%) |
||
| 3 days | 7 days | 14 days | |
| SNS | QALY | ||
| ⩾50% improvement in faecal incontinence | |||
| One adverse event | 3.450 | 3.445 | 3.436 |
| Two adverse events | 3.446 | 3.436 | 3.419 |
| <50% improvement in faecal incontinence | |||
| One adverse event | 3.370 | 3.365 | 3.356 |
| Two adverse events | 3.366 | 3.356 | 3.339 |
PTNS, percutaneous tibial nerve stimulation; QALY, quality-adjusted life-year; SNS, sacral nerve stimulation.
Cost–utility analysis
In the base case scenario (Table 6) the total cost of the PTNS + SNS treatment strategy was £8,619 over 5 years, and the cost of the SNS strategy was £12,386 (difference in cost −£3767). The total QALYs for patients receiving PTNS + SNS treatment were 3.379, and for SNS 3.310 (difference in QALY 0.070). The PTNS + SNS treatment strategy was less costly and marginally more effective compared with the SNS strategy.
Table 6.
Results of cost–utility analyses.
| Parameter |
Difference in cost, £ |
Difference in QALY |
ICER |
|---|---|---|---|
| Base case | −3,767 | 0.070 | PTNS + SNS dominates |
| Probability of >50% improvement in FI for PTNS | |||
| base case 0.59 | |||
| lower limit: 0.59 | −3,767 | 0.070 | PTNS + SNS dominates |
| upper limit:0.71 | −4,968 | 0.087 | PTNS + SNS dominates |
| Probability of receiving permanent SNS | |||
| base case 0.77 | |||
| lower limit: 0.4 | −662 | 0.135 | PTNS + SNS dominates |
| upper limit: 1.0 | −5,697 | 0.029 | PTNS + SNS dominates |
| Probability of >50% improvement in FI for permanent SNS | |||
| base case 0.79 | |||
| lower limit 0.69 | −3,725 | 0.087 | PTNS + SNS dominates |
| upper limit 0.83 | −3,784 | 0.063 | PTNS + SNS dominates |
| Probability of proceeding to SNS after failing PTNS | |||
| base case 0.96 | |||
| lower limit 0.50 | −6,078 | 0.031 | PTNS + SNS dominates |
| upper limit 1.0 | −3,566 | 0.073 | PTNS + SNS dominates |
| Overall probability of adverse events for temporary SNS | |||
| base case 0.22 | |||
| lower limit: 0.02 | −3,809 | 0.069 | PTNS + SNS dominates |
| upper limit: 0.35 | −3,739 | 0.070 | PTNS + SNS dominates |
| Overall probability of adverse events for permanent SNS | |||
| base case 0.24 | |||
| lower limit: 0.13 | −3,553 | 0.069 | PTNS + SNS dominates |
| upper limit: 0.38 | −4,039 | 0.070 | PTNS + SNS dominates |
| Utility for adverse events | |||
| base case 0.46 | |||
| lower limit: 0.16 | −3,767 | 0.068 | PTNS + SNS dominates |
| upper limit: 0.67 | −3,767 | 0.071 | PTNS + SNS dominates |
| Duration of adverse events | |||
| base case 7 days | |||
| lower limit: 3 days | −3,767 | 0.069 | PTNS + SNS dominates |
| upper limit: 14 days | −3,767 | 0.072 | PTNS + SNS dominates |
| Cost of temporary SNS procedure | |||
| base case £599 | |||
| lower limit: £338 | −3,554 | 0.070 | PTNS + SNS dominates |
| upper limit: £710 | −3,861 | 0.070 | PTNS + SNS dominates |
| Cost of permanent SNS procedure | |||
| base case £4,280 | |||
| lower limit: £1,373 | −2,209 | 0.070 | PTNS + SNS dominates |
| upper limit: £5,627 | −4,498 | 0.070 | PTNS + SNS dominates |
| Cost of PTNS procedure | |||
| base case £99 | |||
| lower limit: £53 | −4,876 | 0.070 | PTNS + SNS dominates |
| upper limit: £111 | −3,477 | 0.070 | PTNS + SNS dominates |
| Discounting rate | |||
| base case 3.5% | |||
| lower limit: 1.5% | −3,697 | 0.074 | PTNS + SNS dominates |
| upper limit: 6.0% | −3,845 | 0.065 | PTNS + SNS dominates |
ICER, incremental cost-effectiveness ratio; PTNS, percutaneous tibial nerve stimulation; QALY, quality-adjusted life-year; SNS, sacral nerve stimulation.
Univariate sensitivity analysis was conducted to address variation in model parameters (Table 6). Results of sensitivity analyses demonstrate that PTNS + SNS remained less costly and more effective in all scenarios. The difference in costs between the two strategies varied from £662 to £5,697 per participant over 5 years. The difference in QALYs varied from 0.029 to 0.135. The model was most sensitive to the probability of receiving permanent SNS following temporary SNS. Nevertheless, PTNS + SNS remained the dominant strategy even when the probability of receiving permanent SNS was set to 1.
Probabilistic sensitivity analysis was conducted to assess joint variation on model parameters using Monte Carlo simulations. Probability distributions assigned to model parameters are summarized in Appendix 2. The cost-effectiveness plane and the cost-effectiveness acceptability curve are shown in Figure 2. Figure 2(a) shows a plot of 1,000 simulations of incremental cost against incremental QALYs. Almost all simulations fell within the south quadrants, indicating that the PTNS + SNS treatment strategy was less costly compared with the SNS only. The ellipse on the graph shows 95% confidence intervals for the incremental cost-effectiveness ratio. The line shows the NICE willingness-to-pay threshold at £30,000 per QALY gained. Figure 2(b) shows the cost-effectiveness acceptability at different willingness-to-pay values. The probability of the PTNS + SNS treatment strategy being cost-effective at £20,000–£30,000 per QALY gained was around 80%.
Figure 2.
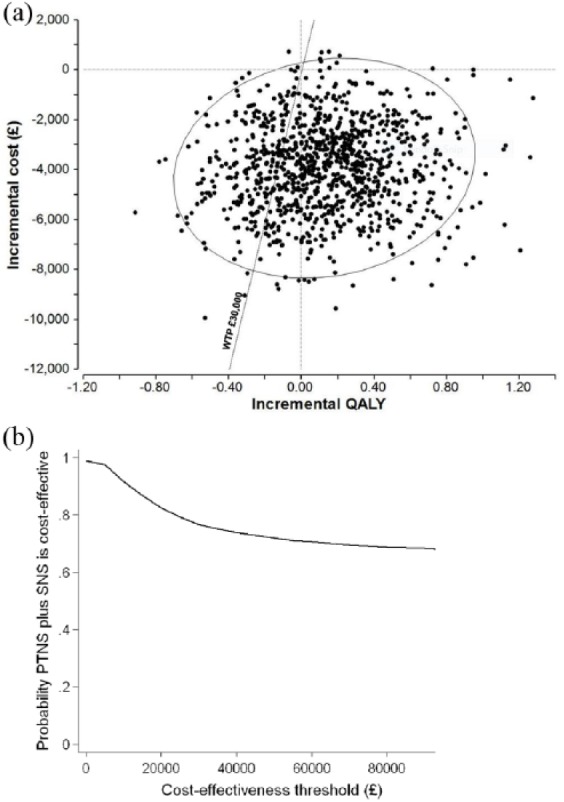
The cost-effectiveness plane (a) and the cost-effectiveness acceptability curve (b) for the base case scenario.
PTNS, percutaneous tibial nerve stimulation; QALY, quality-adjusted life-year; SNS, sacral nerve stimulation.
Discussion
The majority of economic evaluations in healthcare are focused on assessing the cost-effectiveness of alternative treatment options. Cost-effectiveness is the major driver of NICE decisions (82%), although other factors, such as clinical evidence, play an important role.33 The benefits of PTNS for people with faecal incontinence have been questioned34,35 due to the lack of pronounced clinical effects compared with a sham treatment.36,37 It has been concluded that PTNS is unlikely to be recommended over SNS.35 However, in real life, patients may prefer treatments which are less effective (although well tolerated) before considering more effective options.10
In this study we looked at two treatment options, SNS and PTNS, for patients with faecal incontinence who do not respond adequately to conservative therapy. SNS has been previously shown to be more effective and more costly compared with PTNS, at least in the short term. However, comparing two strategies is not straightforward. Firstly, the majority of SNS costs are incurred upfront, while PTNS requires repeated treatments over time. Secondly, SNS is delivered in two stages and proceeding to the second stage depends on the outcome of the first stage. Thirdly, in the long term, SNS may require reoperations due to the loss of effectiveness with time, battery change or the need of MRI scan. Fourthly, patients may first try PTNS before moving to SNS. Although PTNS appears to be less effective compared with SNS, it is less invasive, and therefore more appealing to patients. The long-term effectiveness of PTNS for faecal incontinence is still to be proved, although a sustained therapeutic effect of PTNS was demonstrated for up to 42 months.9 Due to a lack of long-term data for both SNS and PTNS, we limited our analysis to a 5-year time horizon. Within this time horizon the PTNS + SNS strategy was less costly and more effective compared with SNS alone. The probability of PTNS + SNS being cost-effective was around 80% within the NICE cutoff range of £30,000 per QALY (Figure 2).
Being model-based, this study has a number of limitations. We limited the number of adverse events for SNS to two, and the number of reoperations to one. We also assumed that there will be no hospitalisations associated with adverse events for SNS. As a matter of course, we based our model on conservative assumptions:
(1) In the base case analysis the probability of ⩾50% improvement for PTNS was set to the lowest value;
(2) Initial PTNS treatment included 15 sessions at 30 min each, as per trial,27 while NICE recommends 12 PTNS sessions at 30 min each.22
(3) The probability of discontinuation for SNS was assumed to be the same as for PTNS;
(4) It was assumed that the treatment of adverse events associated with SNS will require only one outpatient attendance, which may not be the case in real life.
Our estimations of SNS and PTNS costs are in general agreement with previous studies, except that the latter did not include costs associated with reoperations, adverse events and loss of effectiveness with time. A cost-effectiveness model commissioned by the UK Department of Health38 showed that total SNS cost over 10 years was £12,847 per person, compared with the cost of conservative management of £3,705 per person. A study by Hotouras and colleagues39 showed that the one year cost of SNS was £11,374 and for PTNS £1,740. According to our estimations, the cost of SNS ranges from £13,829 to £19,153 and the costs of PTNS from £2,847 to £4,849 per person over 5 years (Appendix 1). The conservative estimate is that 41% of patients will not benefit from PTNS4 and subsequently receive SNS. For this group of patients, the total cost of PTNS + SNS treatment will be in the range £16,676–£22,000 over 5 years (Appendix 1). A number of sensitivity analyses conducted by varying costs, probabilities and utilities (Table 5) showed that offering PTNS prior to SNS is both less costly and more effective compared with SNS on its own.
The attractiveness of PTNS is that it can be self-administered (by at least a fraction of patients), which could reduce costs associated with maintenance treatments. The home-based PTNS was piloted in nine patients; six of them reported an improvement in faecal incontinence, anxiety and depression.40
In summary, the results of our study demonstrate that delivering PTNS prior to SNS in the treatment of faecal incontinence can be both cost-effective and cost-saving compared with delivering SNS alone. Offering PTNS would reduce the need for SNS treatments, or at least delay these. It would decrease the costs associated with SNS complications. The savings can be up to £5,697 per patient over 5 years. Our results suggest that the probability of this strategy being cost-effective is around 80%.
Appendix
Appendix 1.
Costs used in the model (£), discounted after year one.
| 1.5% discounting | 3.5% discounting | 6% discounting | |
|---|---|---|---|
| PTNS | |||
| ⩾50% improvement | 4,848.79 | 4,678.38 | 4,487.25 |
| No improvement | 2,846.88 | 2,846.88 | 2,846.88 |
| Discontinued | 618.82 | 618.82 | 618.82 |
| SNS | |||
| Temporary SNS, no adverse events | 1,612.94 | 1,612.94 | 1,612.94 |
| Temporary SNS, adverse events | 2,322.98 | 2,322.98 | 2,322.98 |
| Permanent SNS, no adverse events, ⩾50% improvement | 14,775.38 | 14,704.35 | 14,624.68 |
| Permanent SNS, no adverse events, no improvement | 13,829.37 | 13,829.37 | 13,829.37 |
| Temporary SNS with adverse events + permanent SNS with adverse events, ⩾50% improvement | 19,153.28 | 19,082.25 | 19,002.58 |
| Temporary SNS with adverse events + permanent SNS with adverse events, no improvement | 18,207.27 | 18,207.27 | 18,207.27 |
| Temporary SNS with adverse events + permanent SNS no adverse events, ⩾50% improvement | 15,485.41 | 15,414.38 | 15,334.71 |
| Temporary SNS with adverse events + permanent SNS no adverse events, no improvement | 14,539.41 | 14,539.41 | 14,539.41 |
| Temporary SNS no adverse events + permanent SNS with adverse events, ⩾50% improvement | 18,443.24 | 18,372.21 | 18,292.54 |
| Temporary SNS no adverse events + permanent SNS with adverse events, no improvement | 17,497.23 | 17,497.23 | 17,497.23 |
| PTNS + SNS | |||
| PTNS + temporary SNS, no adverse events | 4,459.82 | 4,459.82 | 4,459.82 |
| PTNS + temporary SNS, adverse events | 5,169.86 | 5,169.86 | 5,169.86 |
| PTNS + permanent SNS, no adverse events, ⩾50% improvement | 17,622.26 | 17,551.23 | 17,471.56 |
| PTNS + permanent SNS, no adverse events, no improvement | 16,676.25 | 16,676.25 | 16,676.25 |
| PTNS + temporary SNS with adverse events + permanent SNS with adverse events, ⩾50% improvement | 22,000.16 | 21,929.13 | 21,849.46 |
| PTNS + temporary SNS with adverse events + permanent SNS with adverse events, no improvement | 21,054.15 | 21,054.15 | 21,054.15 |
| PTNS + temporary SNS with adverse events + permanent SNS no adverse events, ⩾50% improvement | 18,332.29 | 18,261.27 | 18,181.60 |
| PTNS + temporary SNS with adverse events + permanent SNS no adverse events, no improvement | 17,386.29 | 17,386.29 | 17,386.29 |
| PTNS + temporary SNS no adverse events + permanent SNS with adverse events, ⩾50% improvement | 21,290.12 | 21,219.09 | 21,139.42 |
| PTNS + temporary SNS no adverse events + permanent SNS with adverse events, no improvement | 20,344.11 | 20,344.11 | 20,344.11 |
Appendix 2.
Model parameters used fin probabilistic analysis.
| Parameters | Mean | SD | distribution | alpha | beta |
|---|---|---|---|---|---|
| Probabilities | |||||
| PTNS ⩾50% improvement | 0.6200 | 0.0300 | beta | 161.6822 | 99.0956 |
| Proceeding to permanent SNS | 0.7350 | 0.1500 | beta | 5.6277 | 2.0290 |
| SNS_⩾50% improvement | 0.7750 | 0.0350 | beta | 109.5439 | 31.8031 |
| Adverse events for temporary SNS | 0.2039 | 0.0828 | beta | 4.6281 | 18.0740 |
| Adverse events for permanent SNS | 0.2487 | 0.0625 | beta | 11.6484 | 35.1864 |
| QALY | |||||
| PTNS ⩾50% improvement | 3.3743 | 0.5087 | normal | 44.0037 | 13.0407 |
| PTNS discontinued | 2.8793 | 0.3233 | normal | 79.3048 | 27.5434 |
| Temporary SNS_adverse_no_implant | 2.8710 | 0.3191 | normal | 80.9752 | 28.2043 |
| QALY_TempSNS_no_adverse_no_implant | 2.8793 | 0.3233 | normal | 79.3048 | 27.5434 |
| QALY_PermSNS_adverse2_improved | 3.3579 | 0.4989 | normal | 45.3080 | 13.4931 |
| QALY_PermSNS_adverse2_not_improved | 2.8628 | 0.3148 | normal | 82.6991 | 28.8878 |
| QALY_PermSNS_adverse1_improved | 3.3743 | 0.5087 | normal | 44.0037 | 13.0407 |
| QALY_PermSNS_adverse1_not_improved | 2.8710 | 0.3191 | normal | 80.9752 | 28.2043 |
| QALY_PermSNS_no_adverse_not_improved | 2.8793 | 0.3233 | normal | 79.3048 | 27.5434 |
| QALY_PermSNS_no_adverse_improved | 3.3743 | 0.5087 | normal | 44.0037 | 13.0407 |
| Costs PTNS | |||||
| ⩾50% improvement | 4,040.61 | 715.54 | gamma | 31.8881 | 0.0079 |
| No improvement | 2,364.21 | 422.01 | gamma | 31.3860 | 0.0133 |
| Discontinued | 607.49 | 30.62 | gamma | 393.7170 | 0.6481 |
| Costs SNS | |||||
| Temporary SNS, no adverse events | 1,562.94 | 190.97 | gamma | 66.9787 | 0.0429 |
| Temporary SNS, adverse events | 2,229.23 | 358.08 | gamma | 38.7578 | 0.0174 |
| Permanent SNS, no adverse events, ⩾50% improvement | 14,103.89 | 2,525.38 | gamma | 31.1906 | 0.0022 |
| Permanent SNS, no adverse events, no improvement | 13,267.37 | 2,363.65 | gamma | 31.5067 | 0.0024 |
| Temporary SNS with adverse events + permanent SNS with adverse events, ⩾50% improvement | 18,150.79 | 3,898.14 | gamma | 21.6808 | 0.0012 |
| Temporary SNS with adverse events + permanent SNS with adverse events, no improvement | 17,314.27 | 3,736.41 | gamma | 21.4733 | 0.0012 |
| Temporary SNS with adverse events + permanent SNS no adverse events, ⩾50% improvement | 14,770.18 | 2,692.45 | gamma | 30.0937 | 0.0020 |
| Temporary SNS with adverse events + permanent SNS no adverse events, no improvement | 13,933.66 | 2,530.72 | gamma | 30.3140 | 0.0022 |
| Temporary SNS no adverse events + permanent SNS with adverse events, ⩾50% improvement | 17,484.51 | 3,731.08 | gamma | 21.9603 | 0.0013 |
| Temporary SNS no adverse events + permanent SNS with adverse events, no improvement | 16,647.98 | 3,569.34 | gamma | 21.7544 | 0.0013 |
| Costs PTNS+SNS | |||||
| PTNS + temporary SNS, no adverse events | 3,927.15 | 521.86 | gamma | 56.6305 | 0.0144 |
| PTNS + temporary SNS, adverse events | 4,593.44 | 643.92 | gamma | 50.8871 | 0.0111 |
| PTNS + permanent SNS, no adverse events, ⩾50% improvement | 16,468.11 | 2,697.60 | gamma | 37.2677 | 0.0023 |
| PTNS + permanent SNS, no adverse events, no improvement | 15,631.58 | 2,537.74 | gamma | 37.9412 | 0.0024 |
| PTNS + temporary SNS with adverse events + permanent SNS with adverse events, ⩾50% improvement | 20,515.01 | 4,060.83 | gamma | 25.5219 | 0.0012 |
| PTNS + temporary SNS with adverse events + permanent SNS with adverse events, no improvement | 19,678.49 | 3,899.93 | gamma | 25.4607 | 0.0013 |
| PTNS + temporary SNS with adverse events + permanent SNS no adverse events, ⩾50% improvement | 17,134.39 | 2,863.45 | gamma | 35.8063 | 0.0021 |
| PTNS + temporary SNS with adverse events + permanent SNS no adverse events, no improvement | 16,297.87 | 2,703.40 | gamma | 36.3447 | 0.0022 |
| PTNS + temporary SNS no adverse events + permanent SNS with adverse events, ⩾50% improvement | 19,848.72 | 3,894.26 | gamma | 25.9786 | 0.0013 |
| PTNS + temporary SNS no adverse events + permanent SNS with adverse events, no improvement | 19,012.20 | 3,733.41 | gamma | 25.9331 | 0.0014 |
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Natalia Hounsome  https://orcid.org/0000-0003-4460-2495
https://orcid.org/0000-0003-4460-2495
Contributor Information
Natalia Hounsome, Brighton and Sussex Medical School, University of Sussex, Falmer, Brighton, BN1 9PX, UK.
Chris Roukas, Pragmatic Clinical Trials Unit, Queen Mary University of London, London, UK.
References
- 1. Perry S, Shaw C, McGrother C, et al. Leicestershire MRC Incontinence Study Team (2002). Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut 2002; 50: 480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Institute for Health and Care Excellence. NICE guidelines CG49. Faecal incontinence in adults: management, https://www.nice.org.uk/guidance/cg49 (2007, accessed 6April 2018). [PubMed]
- 3. National Institute for Health and Care Excellence. NICE interventional procedure guidance IPG99. Sacral nerve stimulation for faecal incontinence, https://www.nice.org.uk/guidance/ipg99/resources/sacral-nerve-stimulation-for-faecal-incontinence-52774470220741cg49 (2004, accessed 6 April 2018).
- 4. Thin NN, Horrocks EJ, Hotouras A, et al. Systematic review of the clinical effectiveness of neuromodulation in the treatment of faecal incontinence. Br J Surg 2013; 100: 1430–1447. [DOI] [PubMed] [Google Scholar]
- 5. Maeda Y, Matzel K, Lundby L, et al. Postoperative issues of sacral nerve stimulation for fecal incontinence and constipation: a systematic literature review and treatment guideline. Dis Colon Rectum 2011; 54: 1443–1460. [DOI] [PubMed] [Google Scholar]
- 6. National Institute for Health and Care Excellence. NICE interventional procedure guidance IPG395 Percutaneous tibial nerve stimulation for faecal incontinence, https://www.nice.org.uk/guidance/ipg395/resources/percutaneous-tibial-nerve-stimulation-for-faecal-incontinence-pdf-1899867877238725 (2011, accessed 6 April 2018).
- 7. Findlay JM, Maxwell-Armstrong C. Posterior tibial nerve stimulation and faecal incontinence: a review. Int J Colorectal Dis 2011; 26: 265–273. [DOI] [PubMed] [Google Scholar]
- 8. Simillis C, Lal N, Qiu S, et al. Sacral nerve stimulation versus percutaneous tibial nerve stimulation for faecal incontinence: a systematic review and meta-analysis. Int J Colorectal Dis 2018; 33: 645–648. [DOI] [PubMed] [Google Scholar]
- 9. Hotouras A, Murphy J, Walsh U, et al. Outcome of percutaneous tibial nerve stimulation (PTNS) for fecal incontinence: a prospective cohort study. Ann Surg 2014; 259: 939–943. [DOI] [PubMed] [Google Scholar]
- 10. Hotouras A, Murphy J, Thin NN, et al. Outcome of sacral nerve stimulation for fecal incontinence in patients refractory to percutaneous tibial nerve stimulation. Dis Colon Rectum 2013; 56: 915–920. [DOI] [PubMed] [Google Scholar]
- 11. Harvie HS, Arya LA, Saks EK, et al. Utility preference score measurement in women with fecal incontinence. Am J Obstet Gynecol 2011; 204: 72.e1–e6. [DOI] [PubMed] [Google Scholar]
- 12. Hetzer FH, Bieler A, Hahnloser D, et al. Outcome and cost analysis of sacral nerve stimulation for faecal incontinence. Br J Surg 2006; 93: 1411–1417. [DOI] [PubMed] [Google Scholar]
- 13. Hollingshead JR, Dudding TC, Vaizey CJ. Sacral nerve stimulation for faecal incontinence: results from a single centre over a 10-year period. Colorectal Dis 2011; 13: 1030–1034. [DOI] [PubMed] [Google Scholar]
- 14. Jarrett ME, Varma JS, Duthie GS, et al. Sacral nerve stimulation for faecal incontinence in the UK. Br J Surg 2004; 91: 755–761. [DOI] [PubMed] [Google Scholar]
- 15. Mellgren A, Wexner SD, Coller JA, et al. SNS Study Group. Long-term efficacy and safety of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum 2011; 54: 1065–1075. [DOI] [PubMed] [Google Scholar]
- 16. Peters KM, Carrico DJ, Wooldridge LS, et al. Percutaneous tibial nerve stimulation for the long-term treatment of overactive bladder: 3-year results of the STEP study. J Urol 2013; 189: 2194–2201. [DOI] [PubMed] [Google Scholar]
- 17. Prapasrivorakul S, Gorissen KJ, Gosselink MP, et al. Temporary sacral neuromodulation under local anaesthesia using new anatomical reference points. Tech Coloproctol 2014; 18: 1093–1097. [DOI] [PubMed] [Google Scholar]
- 18. Soria-Aledo V, Mengual-Ballester M, Pellicer-Franco E, et al. Improvement in the quality of life of faecal incontinent patients after sacral root stimulation treatment. Cir Esp (English Edition) 2011; 89: 581–587. [DOI] [PubMed] [Google Scholar]
- 19. Tjandra JJ, Chan MK, Yeh CH, et al. Sacral nerve stimulation is more effective than optimal medical therapy for severe fecal incontinence: a randomized, controlled study. Dis Colon Rectum 2008; 51: 494–502. [DOI] [PubMed] [Google Scholar]
- 20. Van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, et al. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol 2007; 178: 2029–2034. [DOI] [PubMed] [Google Scholar]
- 21. Van Wunnik BP, Visschers RG, van Asselt AD, et al. Cost-effectiveness analysis of sacral neuromodulation for faecal incontinence in the Netherlands. Colorectal Dis 2012; 14: e807–e814. [DOI] [PubMed] [Google Scholar]
- 22. Thin NN, Taylor SJ, Bremner SA, et al. Randomized clinical trial of sacral versus percutaneous tibial nerve stimulation in patients with faecal incontinence. Br J Surg 2015; 102: 349–358. [DOI] [PubMed] [Google Scholar]
- 23. George AT, Kalmar K, Panarese A, et al. Long-term outcomes of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum 2012; 55: 302–306. [DOI] [PubMed] [Google Scholar]
- 24. Thaha MA, Abukar AA, Thin NN, et al. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev 2015; 8: CD004464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leroi AM, Lenne X, Dervaux B, et al. Outcome and cost analysis of sacral nerve modulation for treating urinary and/or fecal incontinence. Ann Surg 2011; 253: 720–732. [DOI] [PubMed] [Google Scholar]
- 26. McDermott AM, Toelle TR, Rowbotham DJ, et al. The burden of neuropathic pain: results from a cross-sectional survey. Eur J Pain 2006; 10: 127–135. [DOI] [PubMed] [Google Scholar]
- 27. NHS England. Consultant-led referral to treatment (RTT) waiting times statistics for England. 2013 annual report, https://www.england.nhs.uk/statistics/wp-content/uploads/sites/2/2014/02/RTT-Annual-Report-2013-final.pdf (2014, accessed 6 April 2018).
- 28. The National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013, http://publications.nice.org.uk/pmg9 (2013, accessed 6 April 2018). [PubMed]
- 29. Claxton K, Paulden M, Gravelle H, et al. Discounting and decision making in the economic evaluation of health-care technologies. Health Econ 2011; 20: 2–15. [DOI] [PubMed] [Google Scholar]
- 30. Department of Health and Social Care. National schedules of reference costs 2011–12: NHS own costs, https://www.gov.uk/government/publications/nhs-reference-costs-financial-year-2011-to-2012 (2012, accessed 6 April 2018).
- 31. Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Cost Eff Resour Alloc 2013; 11: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Claxton K, Sculpher M, McCabe C, et al. Probabilistic sensitivity analysis for NICE technology assessment: not an optional extra. Health Econ 2005; 14: 339–347. [DOI] [PubMed] [Google Scholar]
- 33. Dakin H, Devlin N, Feng Y, et al. The influence of cost-effectiveness and other factors on nice decisions. Health Econ 2015; 24: 1256–1271. [DOI] [PubMed] [Google Scholar]
- 34. Rex DK. Percutaneous tibial nerve stimulation is ineffective for fecal incontinence. NEJM J Watch Gastroenterology, https://www.jwatch.org/na38827/2015/08/27/percutaneous-tibial-nerve-stimulation-ineffective-fecal (2015, accessed 30 July 2018).
- 35. National Institute for Health Research. Percutaneous tibial nerve stimulation is of limited benefit for faecal incontinence, https://discover.dc.nihr.ac.uk/content/signal-000198/percutaneous-tibial-nerve-stimulation-is-of-limited-benefit-for-faecal-incontinence (2016, accessed 30 July 2018).
- 36. Knowles CH, Horrocks EJ, Bremner SA, et al. Percutaneous tibial nerve stimulation versus sham electrical stimulation for the treatment of faecal incontinence in adults (CONFIDeNT): a double-blind, multicentre, pragmatic parallel-group, randomised controlled trial. Lancet 2015; 386: 1640–1648. [DOI] [PubMed] [Google Scholar]
- 37. van der Wilt AA, Giuliani G, Kubis C, et al. Randomized clinical trial of percutaneous tibial nerve stimulation versus sham electrical stimulation in patients with faecal incontinence. Br J Surg 2017; 104:1167–1176. [DOI] [PubMed] [Google Scholar]
- 38. Department of Health. Centre for Evidence-based Purchasing. Sacral nerve stimulation for faecal incontinence. Evidence review, http://nhscep.useconnect.co.uk/CEPProducts/Catalogue.aspx?ReportType=Evidence+review (2010, accessed 6 April 2018).
- 39. Hotouras A, Murphy J, Allison M, et al. Prospective clinical audit of two neuromodulatory treatments for fecal incontinence: sacral nerve stimulation (SNS) and percutaneous tibial nerve stimulation (PTNS). Surg Today 2014; 44: 2124–2130. [DOI] [PubMed] [Google Scholar]
- 40. Grossi U, Hotouras A, Horrocks E, et al. Home-based percutaneous tibial nerve stimulation for fecal incontinence: is it feasible? Ann Surg 2015; 261: e1. [DOI] [PubMed] [Google Scholar]