Abstract
Objective:
To investigate the prognostic value of white blood cells detected for the first time after adjuvant chemotherapy in primary operable non-small cell lung cancer.
Methods:
From January 2010 to May 2016, data from 208 patients who underwent surgery for non-small cell lung cancer were retrospectively analyzed.
Results:
A white blood cell count detected for the first time after adjuvant chemotherapy greater than 7.00 was an independent predictor of poor disease-free survival (Hazard ratio: 1.736, 95% confidence interval: 1.267-2.378; P = .001) and overall survival (Hazard ratio: 1.802, 95% confidence interval: 1.305-2.471; P = .000). In a further study, after myelosuppression, survival analysis indicated that the patients with white blood cell counts <2.5 had poorer survival than patients with blood cell counts 2.5 to 4.0, P = .031. When the analysis was stratified by the type of histology, patients with a white blood cell count >7.00 and increased white blood cell after chemotherapy compared to pretreatment had a poorer prognosis than patients with white blood cell ≤7.00 and no increase in white blood cell, P = .000 and P = .002, respectively. We further evaluated the prognosis of the 2 groups in different levels of white blood cell. In the group of patients with white blood cell ≤4.0, patients with chemotherapy cycles ≤2, and >2 showed no differences (Hazard ratio: 2.346, 95% confidence interval: 0.288-19.073, P = .425). In the group of patients with white blood cell of 4.0 to 7.0, the prognosis of patients with chemotherapy cycles ≤2 and patients with chemotherapy cycles >2 showed no difference (Hazard ratio: 0.560, 95% confidence interval: 0.248-1.261, P = .161). In the group of patients with white blood cell >7.0, patients with >2 chemotherapy cycles had a better prognosis than patients with chemotherapy cycles ≤2 (Hazard ratio: 0.573, 95% confidence interval: 0.338-0.971, P = .037)
Conclusions:
The level of white blood cells detected for the first time after adjuvant chemotherapy is an independent risk factor for non-small cell lung cancer, especially for patients with nonadenocarcinoma. In addition, the level of white blood cells after postoperative adjuvant chemotherapy and its change compared with pretreatment might also provide useful information regarding the best choice of cycles of adjuvant chemotherapy.
Keywords: white blood cells, non-small cell lung cancer (NSCLC), prognosis, adjuvant chemotherapy, surgery
Introduction
Lung cancer is the leading cause of cancer-related death worldwide, with non-small cell lung cancer (NSCLC) representing approximately 85% of all cases.1,2 Unfortunately, only 20% to 30% of NSCLC patients are candidates for radical surgery when diagnosed. Most of the operable patients received comprehensive postoperative therapy, including chemotherapy. Despite advances in surgery, chemotherapy and radiotherapy, as well as the emergence of molecular therapy, have revolutionized the management of patients with lung cancer. Nevertheless, the prognosis of patients with lung cancer remains unsatisfactory due to local tumor recurrence and distant metastasis. Thus, the identification of an efficient and reliable marker to obtain additional prognostic information and to make the therapy more precise is essential.
A previous study showed that immune functions and the inflammatory response play important roles in tumor formation and tumor progression.3 Many immune methods to improve immune status, including checkpoint therapy, have been used to improve the survival of patients with cancer.4 However, many studies revealed that the tumor-related inflammatory response promotes the promotion of angiogenesis, DNA damage, and tumor invasion through the upregulation of cytokines.5,6 White blood cells (WBCs), including neutrophils, monocytes, and lymphocytes, are important markers of immune functions and the inflammatory response. Previous studies have shown that WBCs, neutrophils, monocytes, lymphocytes, and the neutrophil to lymphocyte ratio were all significantly related to the prognosis of patients with NSCLC,7–9 However, the prognostic value of the change in WBCs after postoperative chemotherapy compared to preoperative chemotherapy has not been debated. Therefore, in this study, we evaluated the clinical utility of the prognostic value of the change in WBCs after postoperative chemotherapy and potential values to help in choosing the plan for comprehensive therapy.
Patients and Methods
Patients
Records of all patients with complete surgical therapy from January 2010 to May 2016 at Xingtai People’s Hospital were reviewed in detail. Inclusion criteria were as follows: (1) without neo-adjuvant therapy; (2) underwent R0 resection and systematic lymph node dissection; (3) without clinical evidence of infection or other bone marrow, hematological, or autoimmune disease; (4) histology was confirmed by pathologists; (5) all patients received postoperative chemotherapy, regimens of chemotherapy including vinorelbine (25 mg/m2 days 1 + 8 every 21 days), paclitaxel (200 mg/m2 day 1 every 21 days), pemetrexed (pemetrexed 500 mg/m2 day 1 for nonsquamous every 21 days), or gemcitabine (1250 mg/m2 days 1 and 8 every 21 days) plus carboplatin (400 mg/m2 day 1), or cisplatin (75 mg/m2 day 1). (6) Patients with neutropenia (WBC ≤ 4.0) required the administration of Granulocyte colony stimulating factor (G-CSF) during the adjuvant chemotherapy. (7) Patients received routine and comprehensive evaluations according to the National Comprehensive Cancer Network (NCCN) guidelines, including the chemistry profile, peripheral blood cell count, contrast-enhanced chest computed tomography (CT) scans, cardiopulmonary functions tests, brain magnetic resonance imaging or CT, bone scanning, and abdominal CT or ultrasonography. The prognostic value of WBC detected after 1 month after the last chemotherapy session was analyzed. The Tumor Node Metastasis (TNM) stage was confirmed by pathologists according to the procedure outlined by the International Association for the Study of Lung Cancer. Patients with TNM stage I disease were excluded.
Follow-up ended in December 2016 with follow-up time ranging from 4 to 69 months. The postoperative failure pattern included local-regional recurrence and distant recurrence. Local-regional recurrence was defined as recurrence at the surgical site, at the anastomotic or bronchial stump, or in the local-regional lymph nodes (levels 1-14, including supraclavicular), which were the first failure sites. Cervical and abdominal lymph node disease and other organ disease as the first site of failure were considered distant recurrence. Time to recurrence was based on the date of imaging or biopsy-proven disease recurrence and the original date of surgery. The end point was cancer-related death.
The study was performed according to the World Medical Association Declaration of Helsinki. The aim of this study was to investigate prognostic factors. Therefore, no institutional review board and/or ethics committee approval from Xingtai People’s Hospital, Xingtai, China, was necessary. Patients provided oral informed consent.
Statistical Analysis
SPSS version 23.0 was used for statistical analysis. The receiver–operator characteristic curve, which ranged from 0.5 (no discrimination) to 1.0 (perfect discrimination), was used to find the best cut-off values of WBC detected 1 month after the last chemotherapy session.10 The best cut-off of WBC after adjuvant chemotherapy was 7.0. Hazard ratios (HRs) and 95% confidence intervals (CIs) and survival curves were calculated by univariate and multivariate analysis using the Cox regression analysis, α = 0.05.
Results
Characteristics of Patients
Characteristics of patients are shown in Table 1. A total of 208 patients from January 2010 to May 2016 at Xingtai People’s Hospital were enrolled in our study with 157 (75.5%) males and 51 (24.5%) females. The age of the patients ranged from 23 to 72 years with mean age of 59.27 years. Patients were classified into 2 categories according to histological type with 69 (36.6%) adenocarcinoma and 139 (66.8%) nonadenocarcinoma. A total of 139 (66.8%) patients had a smoking history. All patients received postoperative chemotherapy. The cycles of chemotherapy ranged from 1 to 14 with a mean of 3.76 cycles. Furthermore, 106 patients received advised 4 to 6 cycles of chemotherapy. Four patients who participated in a clinical trial on maintenance chemotherapy after surgery received more than 6 cycles. At the end point of follow-up, 87 patients died from cancer, and 2 died from heart disease. Twenty-eight (13.5%) had local-regional recurrence and 83 (39.9%) had distant recurrence.
Table 1.
Clinic-pathological Characters.
| Variables | P Value | White Blood Cell of Postchemotherapy | |
|---|---|---|---|
| ≤7 | >7 | ||
| Gender | .512 | ||
| Male | 81 | 76 | |
| Female | 29 | 22 | |
| Age(years) | .312 | ||
| ≤.3 | 79 | 64 | |
| >60 | 31 | 34 | |
| Smoking | .055 | ||
| No | 43 | 26 | |
| Yes | 67 | 72 | |
| Surgical procedures | .109 | ||
| Pneumonectomy | 9 | 18 | |
| Lobectomy | 100 | 78 | |
| Wedge resection | 1 | 2 | |
| T status | .001 | ||
| T1 | 29 | 25 | |
| T2 | 76 | 52 | |
| T3 | 5 | 21 | |
| N status | .143 | ||
| N0 | 39 | 27 | |
| N1 | 14 | 22 | |
| N2 | 57 | 49 | |
| Cycles of chemotherapy | .114 | ||
| ≤3 | 45 | 54 | |
| 4-6 | 63 | 42 | |
| >6 | 2 | 2 | |
| Regimes of chemotherapy | .610 | ||
| NP | 37 | 37 | |
| TP | 59 | 52 | |
| GP | 8 | 7 | |
| PP | 6 | 2 | |
| Histology | .183 | ||
| Non-AD | 69 | 70 | |
| AD | 41 | 28 | |
| Recurrence | .010 | ||
| No | 59 | 35 | |
| Yes | 51 | 63 | |
Abbreviations: AD, adenocarcinoma; non-AD: nonadenocarcinoma.
Correlation Between Clinical-pathological Parameters and WBC After Adjuvant Chemotherapy
The distribution of the clinical background characteristics of the studied patients in the 2 groups is shown in Table 1. As shown in Table 1, significant differences between WBC after adjuvant chemotherapy and T-stage (P = .001) and recurrence status (P = .010) were demonstrated. There were no differences between WBC after adjuvant chemotherapy, cycles of chemotherapy, and regimes of chemotherapy.
Survival Analysis
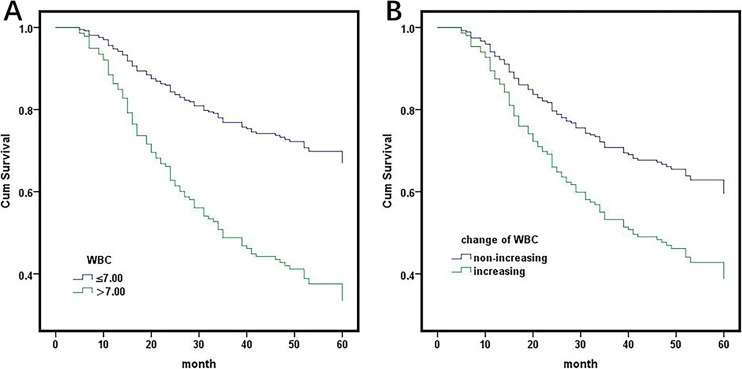
In univariate analysis, both preoperative and postchemotherapy WBC count showed significant relationships with overall survival (OS) and disease free survival (DFS; Table 2 and Figure 1A). A postchemotherapy WBC count >7.0 was an independent predictor of poor DFS (HR: 1.736, 95% CI: 1.267-2.378; P = .001) and OS (HR: 1.802, 95% CI: 1.305-2.471; P = .000), adjusting for a tumor pathology type, pathological stage, T-stage, and N-stage, preoperative lactate dehydrogenase (LDH), preoperative WBC count, preoperative hemoglobin (Hb), cycles of chemotherapy and platelets after postoperative adjuvant chemotherapy (Table 3). Other identified independent prognostic factors for DFS and OS included LDH, preoperative Hb, N-stage, and cycles of chemotherapy (Table 3).
Table 2.
Univariate Analysis of Survival.
| DFS | OS | |||||
|---|---|---|---|---|---|---|
| Variables | P Value | HR | 95%CI | P Value | HR | 95%CI |
| Gender (male, female) | .558 | 1.123 | 0.761-1.659 | .444 | 0.826 | 0.506-1.348 |
| Age (≤ge, >60) | .558 | 0.890 | 0.603-1.314 | .595 | 0.888 | 0.572-1.378 |
| Smoking (yes, no) | .446 | 1,157 | 0.782-1.713 | .179 | 1.369 | 0.866-2.162 |
| Sur (lobe, non-lobe) | .040 | 1.472 | 1.018-2.129 | .131 | 1.347 | 0.915-1.982 |
| T status (T1, T2/T3) | .314 | 1.141 | 0.883-1.473 | .895 | 1.103 | 0.833-1.233 |
| N status (N0,N1/N2) | .000 | 1.505 | 1.207-1.877 | .002 | 1.476 | 1.147-1.900 |
| Histology (AD,NAD) | .669 | 0.652 | 0.092-4.639 | .081 | 0.124 | 0.012-1.293 |
| Chemo (≤4, >4) | .002 | 0.865 | 0.789-0.949 | .012 | 0.875 | 0.788-0.971 |
| WBC1 (≤10, >10) | .294 | 1.222 | 0.840-1.787 | .050 | 1.518 | 1.000-2.307 |
| WBC2 (≤7, >7) | .003 | 1.755 | 1.212-2.542 | .000 | 2.728 | 1.766-4.212 |
| LDH (≤250, >250) | .005 | 1.690 | 1.170-2.443 | .001 | 2.083 | 1.368-3.173 |
| PLT (≤300, >300) | .097 | 1.377 | 1.944-2.008 | .112 | 1.414 | 0.922-2.170 |
| Hb (≤140, >140) | .048 | 1.829 | 1.005-3.328 | .073 | 1.826 | 0.945-3.527 |
Abbreviations: CI, confidence interval, DFS, disease free survival; HR, hazard ratio; Hb1, preoperative hemoglobin; OS, overall survival; PLT: platelets after adjuvant chemotherapy; WBC1: preoperative white blood cell count, WBC2, white blood cell count after adjuvant chemotherapy.
Figure 1.
(A) Survival analysis of white blood cells detected for the first time after adjuvant chemotherapy. HR: 2.728, 95% CI: 1.766-4.212, P = .000. (B) Survival analysis of the decreasing level of postchemotherapy white blood cell count compared to preoperative white blood cells. HR: 0.548, 95% CI: 0.361-0.830, P = .005. HR indicates hazard ratio; CI, confidence interval.
Table 3.
Multivariable Analysis of Survival.
| DFS | OS | |||||
|---|---|---|---|---|---|---|
| Variables | P Value | HR | 95%CI | P Value | HR | 95%CI |
| LDH (≤220, >220) | .001 | 1.907 | 1.314-2.767 | .001 | 2.115 | 1.385-3.228 |
| N status (N0, N1/N2) | .000 | 1.782 | 1.416-2.244 | .000 | 1.676 | 1.292-2.173 |
| Chemo (≤4, >4) | .000 | 0.816 | 0.742-0.898 | .002 | 0.840 | 0.754-0.936 |
| WBC (≤7, >7) | .003 | 1.757 | 1.209-2.553 | .000 | 2.578 | 1.663-3.996 |
| Hb (≤140, >140) | .002 | 2.591 | 1.409-4.764 | .027 | 2.122 | 1.087-4.141 |
Abbreviations: CI: confidence interval; DFS, disease free survival; HR: hazard ratio, OS, overall survival.
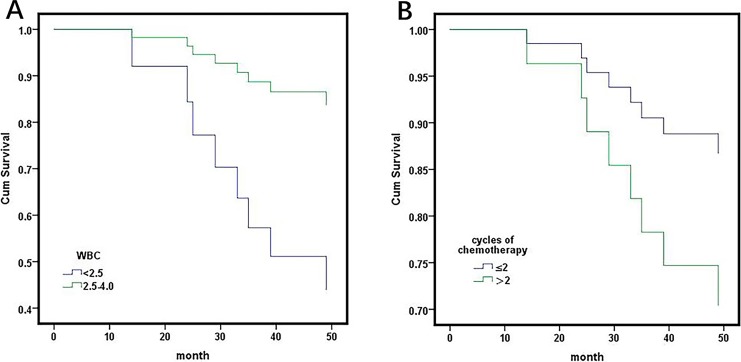
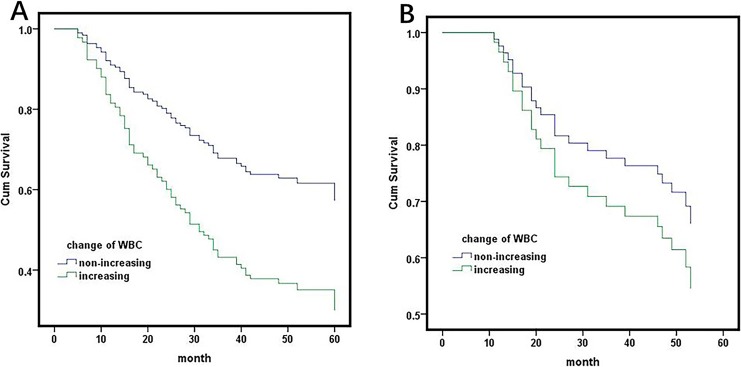
Further study revealed that the survival of patients with a WBC count ≤4.0 and 4.0 to 7.0 showed no significant difference, (HR: 0.690, 95% CI: 0.310-1.539, P = .365), but better than >7.0, (HR: 0.293, 95% CI: 0.140-0.615, P = .001). In a further study, the patients with a WBC count ≤4.0 after chemotherapy were separated into 2 arms (patients with blood cell count [2.5-4.0], <2.5) according to the myelosuppression following adjuvant chemotherapy. Survival analysis indicated that the patients with a WBC count <2.5 had a poor survival than patients with a blood cell count 2.5 to 4.0 (HR: 0.215, 95% CI: 0.054-0.886, P = .031; Figure 4A). When the analysis was stratified by the type of histology, patients with a WBC count >7.0 had a poorer prognosis than patients with WBC count ≤7.0 in patients with nonadenocarcinoma (HR: 4.206, 95% CI: 2.410-7.340, P = .000) but not in patients with adenocarcinoma (HR: 1.094, 95% CI: 0.478-2.504, P = 0.832; Figure 2A, B).
Figure 4.
(A) Patients with white blood cell count ≤4.0 after chemotherapy were separated into 2 arms (patients with blood cell count (2.5-4.0, <2.5) according to the myelosuppression following adjuvant chemotherapy. Survival analysis indicated that the patients with white blood cell count <2.5 had a poorer survival than patients with blood cell count 2.5-4.0 (HR: 0.215, 95% CI: 0.054-0.886, P = .031). B, Survival analysis of cycles of adjuvant chemotherapy in patients with white blood cells after chemotherapy <4.0. Mean (standard error) survival was 60.077 (2.808) and 52.465 (3.121) in patients with less than 2 and more than 2 cycles of chemotherapy, respectively, P = .065.
Figure 2.
Survival analysis of white blood cells after adjuvant chemotherapy stratified by histology. A, showed patients with nonadenocarcinoma. HR: 4.206, 95% CI: 2.410-7.340, P = .000. B, showed patients with adenocarcinoma. HR: 1.094, 95% CI: 0.478-2.504, P = .832. HR indicates hazard ratio; CI, confidence interval.
When the decreasing level of postchemotherapy WBCs compared to preoperative WBCs was taken into consideration, a decrease in WBC showed a significantly positive relationship with the prognosis of all patients (HR: 0.548, 95% CI: 0.361-0.830, P = .005; Figure 1B). However, the decreasing level ≥0 was significantly associated with better clinical outcomes in patients with nonadenocarcinoma (HR: 2.16, 95% CI: 1.327-3.521, P = .002), but not adenocarcinoma (HR: 1.416, 95% CI: 0.654-3.263, P = .355; Figure 3A and B).
Figure 3.
Survival analysis of the decreasing level of postchemotherapy white blood cells compared to the preoperative white blood cells stratified by histology. A, showed patients with nonadenocarcinoma. HR: 2.16, 95% CI: 1.327-3.521, P = .002. B, showed patients with adenocarcinoma. HR: 1.416, 95% CI: 0.654-3.263, P = .355.
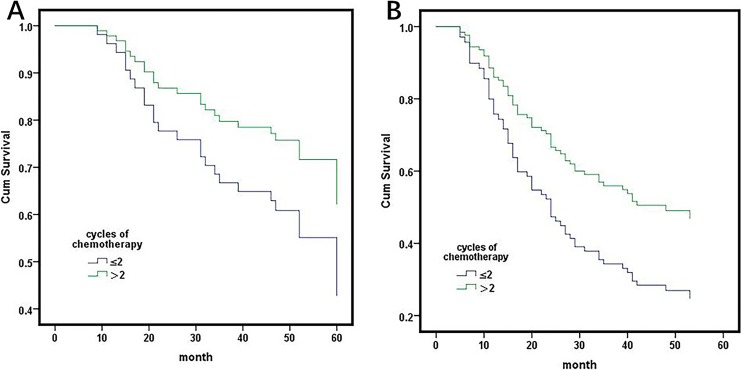
Furthermore, we evaluated the influence of decreased WBC on postoperative chemotherapy. Patients with more than 4 cycles of postoperative chemotherapy had a better prognosis than patients with less than 4 cycles (table). A previous study showed that the lymphocyte count was significantly decreased after 2 cycles of chemotherapy, and the patients with more than 2 cycles or less than 2 cycles were separated into 2 groups.11 We further evaluated the prognosis of the 2 groups in different levels of WBC. In the group of patients with WBC ≤4.0, patients with ≤2 and >2 chemotherapy cycles showed no differences (HR: 2.346 95% CI: 0.288-19.073, P = .425; Figure 4B). In groups of patients with WBC 4.0 to 7.0, the prognosis of patients with ≤2 chemotherapy cycles and patients with >2 chemotherapy cycles showed no differences (HR: 0.560, 95% CI: 0.248-1.261, P = .161; Figure 5A). In groups of patients with WBC >7.0, patients with >2 chemotherapy cycles had a better prognosis than patients with ≤2 chemotherapy cycles (HR: 0.573, 95% CI: 0.338-0.971, P = .037; Figure 5B).
Figure 5.
(A) In a group of patients with WBC 4.0 to 7.0, the prognosis of patients with ≤2 and ≤2 cycles of chemotherapy was no different (HR: 0.560, 95% CI: 0.248-1.261, P = .161). B, In a group of patients with WBC ≥7.0, patients with ≥2 chemotherapy cycles have a better prognosis than patients with ≤2 chemotherapy cycles have a better prognosis (HR: 0.573, 95% CI: 0.338-0.971, P = .037).
Discussion
Recent studies have shown that pretreatment leukocytosis was related to the poor prognosis of patients with advanced stage NSCLC and resectable disease.7,12,13 Tumor-related cytokines can increase the level of WBCs.14,15 Many factors, such as Vascular Endothelial Growth Factor Receptor (VEGFR), secreted by WBCs and neutrophils can promote cancer progression and metastasis.16–20 Therefore, the WBC count can reflect the tumor burden in patients. Previous studies showed that mild and severe chemotherapy-induced neutropenia were significantly predictive of longer survival in patients with advanced or metastasis NSCLC.21 This finding may explain why chemotherapy can decrease the tumor burden and decrease the tumor-related cytokines, which result in an increase in the level of WBCs. However, the effect of myelosuppression following chemotherapy decreases the level of WBCs. If the level of WBCs is lower than normal, the immune system of the patients may be influenced. For patients receiving radical surgery, the tumor was resected. Thus, the influence of the level of WBCs after postoperative adjuvant chemotherapy should be further investigated.
Previous studies revealed that postoperative adjuvant chemotherapy significantly improves the survival of patients with NSCLC.22–24 In this study, all patients received adjuvant chemotherapy. The results revealed that the level of WBCs after postoperative adjuvant chemotherapy was significantly related to the prognosis of patients with NSCLC. Multivariate analysis showed that it was an independent risk factor, but the pretreatment level of WBCs was not. This result was similar to a previous study on advanced or metastatic patients.13 However, the decreased level compared to pretreatment also showed a significant relationship with the prognosis of all patients, which is similar to the results of a previous study of advanced and metastatic NSCLC. Further study showed that both the level of WBC of patients receiving postoperative adjuvant chemotherapy and the decreased level after adjuvant chemotherapy compared with pretreatment were related to OS in patients with nonadenocarcinoma but not adenocarcinoma. These results suggested that tumor burden influences the prognosis of patients with micro-metastasis. Tumor-related changes in the WBC count may be different between nonadenocarcinoma and adenocarcinoma patients.
We further stratified the level of WBCs after adjuvant chemotherapy to investigate its prognostic value. Patients with a WBC count <2.5 and >7.0 had a poor survival than patients with a blood cell count 2.5 to 4.0 and 4.0 to 7.0. This revealed that patients may benefit from decreased level of WBC after adjuvant chemotherapy to a certain extent, but a severe decrease in WBCs may influence the immune system of the patients receiving radical surgery to prevent recurrence. Chemotherapy should be more individualized for patients with severe myelosuppression after postoperative adjuvant chemotherapy. This study showed that less than 4 cycles of chemotherapy was an independent risk factor for the prognosis of patients with NSCLC receiving postoperative chemotherapy. We further evaluated the influence of cycles of adjuvant chemotherapy stratified by levels of WBC after adjuvant chemotherapy. Interestingly, more than 2 cycles of adjuvant chemotherapy resulted in poorer survival in patients with a WBC less than 4.0, though it did not reach a statistical significance. Only 33 patients had a WBC less than 4.0; a larger cohort should be investigated. However, less than 2 cycles resulted in a poorer survival in patients with a WBC greater than 7.0. There was no significant difference between the 2 arms in patients with a WBC from 4.0 to 7.0. Thus, the change in WBC count after adjuvant chemotherapy can help to guide precise decision-making for adjuvant chemotherapy. If a patient has received 2 cycles adjuvant chemotherapy with a decrease in WBC 1 month after chemotherapy, he may not benefit from further chemotherapy. However, a patient with a level of WBC greater than 7.0 after 2 cycles of adjuvant chemotherapy can benefit from further chemotherapy. However, this conclusion needs to be further investigated because this study is retrospective and the number of patients with a WBC less than 4.0 was small.
In summary, our study investigated the prognostic value of the level of WBCs after adjuvant chemotherapy in NSCLC for the first time. This level is an independent risk factor for NSCLC, especially for patients with nonadenocarcinoma. The change in the WBC count after adjuvant chemotherapy compared to pretreatment was also an independent prognostic factor for patients with nonadenocarcinoma. Therefore, the change in WBC count may be influenced by different mechanisms in adenocarcinoma and nonadenocarcinoma, which needs to be further investigated. Finally, the level of WBCs after postoperative adjuvant chemotherapy and its changes compared with pretreatment might also provide useful information about the best choice of cycles of adjuvant chemotherapy. Therefore, the level of WBCs after postoperative adjuvant chemotherapy may be a potential prognostic marker that can be used to evaluate the prognosis in clinical practice. While our study was limited due to its retrospective design and insufficiently large sample size, a large prospectively studied cohort is essential before our study results can be used in clinical practice.
Abbreviations
- AD
adenocarcinoma
- non-AD
nonadenocarcinoma
- CI
confidence intervals
- DFS
disease free survival
- NSCLC
non-small cell lung cancer
- Hb
hemoglobin
- HR
Hazard ratio
- Hb1
preoperative hemoglobin
- LDH
lactate dehydrogenase
- OS
overall survival
- WBC1
preoperative white blood cell count
- WBC2
white blood cell count after adjuvant chemotherapy
- WBC
white blood cell.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 2. Groome PA, Bolejack V, Crowley JJ, et al. The IASLC lung cancer staging project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2(8):694–705. [DOI] [PubMed] [Google Scholar]
- 3. Pinato DJ, Shiner RJ, Seckl MJ, Stebbing J, Sharma R, Mauri FA. Prognostic performance of inflammation-based prognostic indices in primary operable non-small cell lung cancer. Br J Cancer. 2014;110(8):1930–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang GX, Guo LQ, Gainor JF, Fintelmann FJ. Immune checkpoint inhibitors in lung cancer: imaging considerations. AJR Am J Roentgenol. 2017;209(3):567–575. [DOI] [PubMed] [Google Scholar]
- 5. Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000;60(1):184–190. [PubMed] [Google Scholar]
- 6. Li R, Wang C, Liu L, et al. Autologous cytokine-induced killer cell immunotherapy in lung cancer: a phase II clinical study. Cancer Immunol Immunother. 2012;61(11):2125–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tomita M, Shimizu T, Hara M, Ayabe T, Onitsuka T. Preoperative leukocytosis, anemia and thrombocytosis are associated with poor survival in non-small cell lung cancer. Anticancer Res. 2009;29(7):2687–2690. [PubMed] [Google Scholar]
- 8. Kumagai S, Marumo S, Shoji T, et al. Prognostic impact of preoperative monocyte counts in patients with resected lung adenocarcinoma. Lung Cancer. 2014;85(3):457–464. [DOI] [PubMed] [Google Scholar]
- 9. Zhang H, Zhang L, Zhu K, et al. Prognostic significance of combination of preoperative platelet count and neutrophil-lymphocyte ratio (COP-NLR) in patients with non-small cell lung cancer: based on a large cohort study. PLoS One. 2015;10(5):e0126496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23(28):7114–7124. [DOI] [PubMed] [Google Scholar]
- 11. Wang Q, Yang L, Xu F, Wang J, An G, Ma Y. Changes of lymphocyte subgroups in non-small cell lung cancer patients before and during chemotherapy. Clin Lab. 2015;61(10):1343–1351. [DOI] [PubMed] [Google Scholar]
- 12. Mandrekar SJ, Schild SE, Hillman SL, et al. A prognostic model for advanced stage nonsmall cell lung cancer. Pooled analysis of north central cancer treatment group trials. Cancer. 2006;107(4):781–792. [DOI] [PubMed] [Google Scholar]
- 13. Tibaldi C, Vasile E, Bernardini I, Orlandini C, Andreuccetti M, Falcone A. Baseline elevated leukocyte count in peripheral blood is associated with poor survival in patients with advanced non-small cell lung cancer: a prognostic model. J Cancer Res Clin Oncol. 2008;134(10):1143–1149. [DOI] [PubMed] [Google Scholar]
- 14. Inoue M, Minami M, Fujii Y, Matsuda H, Shirakura R, Kido T. Granulocyte colony-stimulating factor and interleukin-6-producing lung cancer cell line, LCAM. J Surg Oncol. 1997;64(4):347–350. [DOI] [PubMed] [Google Scholar]
- 15. Katoh Y, Nakamura M, Ohnishi Y, Shimamura K, Ueyama Y, Tamaoki N. Autonomous production of granulocyte-colony stimulating factor in tumour xenografts associated with leukocytosis. Br J Cancer. 1993;68(4):715–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6(4):283–287. [DOI] [PubMed] [Google Scholar]
- 17. Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528(7582):413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mouchemore KA, Anderson RL, Hamilton JA. Neutrophils, G-CSF and their contribution to breast cancer metastasis. FEBS J. 2017;285(4):665–679. [DOI] [PubMed] [Google Scholar]
- 19. Garcia-Mendoza MG, Inman DR, Ponik SM, et al. Neutrophils drive accelerated tumor progression in the collagen-dense mammary tumor microenvironment. Breast Cancer Res. 2016;18(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coffelt SB, Kersten K, Doornebal CW, et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522(7556):345–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Di Maio M, Gridelli C, Gallo C, et al. Chemotherapy-induced neutropenia and treatment efficacy in advanced non-small-cell lung cancer: a pooled analysis of three randomised trials. Lancet Oncol. 2005;6(9):669–677. [DOI] [PubMed] [Google Scholar]
- 22. Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351–360. [DOI] [PubMed] [Google Scholar]
- 23. Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (adjuvant navelbine international trialist association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7(9):719–727. [DOI] [PubMed] [Google Scholar]
- 24. Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552–3559. [DOI] [PubMed] [Google Scholar]