Abstract
Purpose:
To ascertain whether a new delivery system (the Halcyon system) equipped with dual-layer stacked multileaf collimator operating in a mode, which allows independent, fully interdigitating motion of both layers and 6 flattening filter free energy, could generate plans of high clinical quality compared to a well-established delivery system with single layer multileaf collimator.
Methods:
Twenty patients in each of the 3 groups (advanced head and neck, breast, and high-risk prostate) were selected for an in silico planning study. For each patient, reference plans were developed for volumetric modulated arc therapy technique with 6 MV photon beams from a TrueBeam linear accelerator and compared against the corresponding plans for the Halcyon system. Plan comparison was performed in terms of dose volume histogram quantitative analysis.
Results:
Concerning the planning target volumes, with identical dose calculation and optimization algorithms and with identical planning techniques, no clinically relevant difference in coverage (D98%), hot spot (D2%), or homogeneity was observed. Similarly, for all the organs at risk, the dosimetric findings showed that (1) all planning constraints were met by the 2 delivery systems and (2) although statistical significant differences were reported for most of the parameters but none of these were judged of potential clinical relevance.
Conclusion:
The data presented confirmed that the new delivery system can generate treatment plans for volumetric modulated arc therapy with the same dosimetric quality of what is achievable with other systems routinely used in the clinics without significantly changing the current practice. Additional studies which customize the optimization parameters for each delivery device would complement the spectrum of investigations.
Keywords: dual-layer MLC, halcyon, breast, prostate, head and neck, VMAT, RapidArc
Introduction
In the spring of 2017, a new radiotherapy delivery system, the Halcyon (Varian Medical Systems, Palo Alto) was introduced in clinical practice, and some institutes started clinical operations with it. Halcyon (H) is a jawless device equipped with a dual-layer multileaf collimator (MLC) and with a nominal maximum field size of 28 × 28 cm2. At the time of submission, only a few studies have been published about H. The first research focused on investigation of the level of doses delivered to the normal tissues when mega voltage (MV) image-guided radiation therapy is performed.1 From a dosimetric point of view, the majority of the published works explored cervix cancer planning. Anamalayil et al 2 showed at a planning level that, for 16 test patients with cervical cancer, a good agreement was achieved between H and the data from plans developed for a conventional TrueBeam (TB) linear accelerator. Brady et al 3 tested for 6 patients the possibility to overcome field size limitations by using multiple isocenters and assessing positively the robustness of the plans for advanced cervical cancer treatment (ie, including para-aortic nodes in the target volume). Mihailidis et al 4 compared plans for intensity modulated therapy (IMRT) and volumetric modulated arc therapy (VMAT) for H and the TB and confirmed the dosimetric equivalence results observed for the cervix experiments. In all cases, the expected delivery time with the new system was at least twice faster. A variety of clinical indications were included in the study by Netherton et al,5 which compared H with conventional linacs for both IMRT and VMAT. A limitation of all these studies was the small number of patients in each cohort and the use, also for planning, of a preclinical system operating only in the “per-leaf” tracking mode which limits the shaping of the fields to a resolution of 1 cm at isocenter. The per-leaf tracking, featured in the first release of H, allowed to shape only the field with the lower bank of leaves, while the upper bank “tracks” the position of the lower leaves only to reduce interleaf leakage transmission. This limit was abrogated in the second generation of H.
Michels et al 6 reported, for 30 head and neck (HN) patients, the preclinical and early clinical experience for the determination of robust planning class solutions and reported also the results from pretreatment quality assurance measurements. The authors included also data concerning delivery efficiency. Lloyd et al 7 reported about the use of TG-51 reference dosimetry for the new system and provided reference values for the various parameters (as, for example, the beam quality conversion factors).
The primary aim of the present investigation was to ascertain whether the second generation of H could generate plans of high quality and fulfill binding clinical acceptance criteria and to put this in comparison against benchmark data from well-established delivery systems. The secondary aim was to determine whether H plans might benefit from the use of a slightly increased number of arcs to potentially compensate for the leaf resolution or whether the optimization engines tailored to this new system could guarantee the sufficient performance with a minimum number of arcs. In order to investigate a broad spectrum of clinical problems of increasing complexity, the study was performed for 3 cohorts of patients: advanced HN, whole breast, and high-risk prostate and with 3 different prescription schemes: an accelerated single dose level regimen (whole breast) and 2 simultaneous integrated boost (SIB) protocols with 2 or 3 dose levels (HN and prostate, respectively).
Materials and Methods
The H System
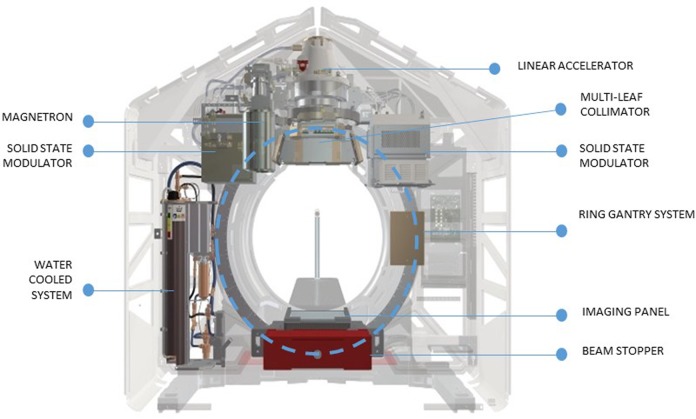
Halcyon is a ring-based delivery system and it is schematically represented in Figure 1. It consists of a linear accelerator capable of producing a 6 MV flattening filter free (FFF) photon beam with a maximum dose rate of 800 MU/min when calibrated to deliver 1.0 Gy per 100 monitor units (MU) under the reference conditions of 100 cm source to surface distance (SSD) at d max depth of 1.3 cm. The dose rate is reduced to 600 MU/min maximum when the reference condition is 90 cm SSD and 10 cm depth.
Figure 1.
A schematic representation of the Halcyon delivery system.
The linac rotates around the mounting ring (back and forth with 1 single rotation) with a velocity up to 24 per second (which corresponds to 4 full rotations per minute) in both imaging and delivery mode.
The clinically available H system utilizes an MV planar imager capable of acquiring either 2-dimensional or 3-dimensional data sets as MV-based cone beam computed tomography (MV-CBCT). Additionally, in development is the addition of a kV source to the H gantry capable with software to generate standard CBCT images. Imaging procedures are mandatory prior to each fraction of treatment of the patients.
From the treatment planning and delivery perspective, the main features of H are:
Absence of any jaws in both X and Y directions
Presence of a dual-layer stacked–staggered MLC (with 28 leaves of 1 cm width (projected at isocenter). Each layer is “shifted” 5 mm with respect to the other producing an effective shaping capability of 5 mm at isocenter. The design specifications of the MLC stated a 0.01% transmission and leakage.
Maximum field size for a single isocenter treatment of 28 × 28 cm2.
28.0 cm of over travel of the MLC leaves with full interdigitation.
Maximum 5.0 cm/s leaf velocity
Figure 2 illustrates the arrangement and the field shaping logic of the new MLC system. The central panel illustrates the “per-leaf” tracking method used by H in the first generation, while the right side panel shows the 5 mm field shaping produced by both the leaf banks as seen in beam’s eye view with the 2 layers colored with different tones of grey/black.
Figure 2.
The Dual-layer stacked multileaf collimator system and a conceptual realization of 5 mm field shaping. The central panel illustrates the “per-leaf” tracking method used by Halcyon in the first generation, whereas the right side panel shows the 5 mm field shaping produced by both the leaf banks as seen in beam’s eye view (BEV) with the 2 layers colored with different tones of blue.
The second generation of H is capable of performing kV-based CBCT in addition to the original MV-CBCT of the first generation. This allows for better quality of the images and the avoidance of unnecessary dose from the MV process. For the scope of the present planning study, all plans were generated in the Eclipse system assuming that daily imaging procedures would be performed only with kV imaging (kV-CBCT) and therefore no extra dose from MV-imaging was accounted for in the planning procedure. If MV imaging were used, the dose attributable to it would be automatically considered by the planning engine as described in the study by Li and colleagues. In that article, the authors also detailed the potential extra dose due to the MV process and eliminated from our present study by using kV-CBCT only.
Patient Selection and Dose Prescription
This investigation describes the results of a planning comparison study performed on 3 cohorts of patients (20 cases each) selected from the database of treated cases, appropriately de-identified. All patients signed an informed consent at hospital admission to allow the use of their data for retrospective analysis and the Ethical committee of the hospital approval is waived for these deidentified in silico feasibility studies.
The breast patients were planned according to the methods described in the study by Fogliata et al 8 and Koivumaki et al 9 with a single dose level of 40.05 Gy to be delivered in 15 fractions to the entire breast. All cases were left-sided patients. The organs at risk (OARs) considered were the heart, the ipsilateral and the contralateral lungs and the contralateral breast. The clinical target volume (CTV) was delineated following the Radiation Therapy Oncology Group recommendations10 and the planning target volume (PTV) was defined with an expansion of 5 mm of the CTV. The PTV was then cropped by 5 mm inside the patient outline to exclude the derma region. The HN patients presented with advanced cancer (stage III or IV according to the American Joint Committee on Cancer staging system). The gross tumor volume (GTV) was delineated on CT imaging for all patients with support of magnetic resonance coregistered images. The CTV for the boost encompassed the GTV with an additional 1 cm margin for both the tumor and nodal disease, correcting for anatomical boundaries; the elective CTV included also the elective nodes according to internationally accepted guidelines.9 An isotropic 5 mm margin was then added to CTVs to obtain the PTVs which were cropped 4 mm inside the body contour to avoid the derma. These cases were planned as described in the study by Gregoire et al 11, Franzese et al 12, and Fogliata et al 13 with a SIB fractionation scheme to deliver 2 dose levels of 69.96 Gy to the primary target volume and 54.45 Gy to the elective volume in 33 fractions. The OARs of first priority considered were the parotids, the spinal cord, the oral cavity, and the larynx. In addition (not reported but used in the optimization) the constrictor muscles, the submandibular glands, the brainstem, and the thyroid were considered.
The prostate patients selected were unfavorable high-risk patients who were planned as discussed in Franzese et al 14 for a 28 fraction SIB course with 51.8 Gy prescribed to the pelvic lymph nodes, 65.5 Gy to the seminal vesicles, and74.2 Gy to the prostate. The OARs were the rectum, the bladder, and the femoral heads. The CTVs were delineated on CT imaging for all patients with the support of 11C-choline positron emission tomography imaging. An isotropic 7 mm margin was added to the various CTVs to derive the PTVs except posteriorly, where 5 mm expansion was applied. The PTV for the prostate was reduced of the volume overlapping the rectum widened by 2 mm margin.
For all the OARs in each cohort, clinical dose–volume objectives were obtained from the relative published studies and are summarized together with the study findings in Table 1.
Table 1.
Summary of the Quantitative Analysis of the Dose–Volume Histograms for the Main Structures Over the Entire Cohort of Patients.a,b,c
| Parameter | Objective | TB | TB-FFF | H-2arcs | H-3arcs | P |
|---|---|---|---|---|---|---|
| PTV volume, range, cm3 | ||||||
| D2%% | Minimize | 105.5 ± 0.8 | 105.3 ± 0.7 | 105.6 ± 0.8 | 104.9 ± 0.6 | – |
| D98%% | Maximize | 93.9 ± 1.3 | 94.2 ± 1.3 | 94.1 ± 1.3 | 94.8 ± 1.4 | a,c,d,e,f |
| V95%% | >95% | 95.8 ± 2.1 | 96.5 ± 2.3 | 95.4 ± 1.8 | 96.8 ± 2.3 | – |
| SD% | ∼3% | 2.3 ± 0.5 | 2.3 ± 0.5 | 2.6 ± 0.5 | 2.1 ± 0.5 | a,c |
| HI% | <10% | 8.9 ± 2.7 | 7.0 ± 4.0 | 8.7 ± 2.7 | 5.8 ± 3.5 | a,b,c,d,e,f |
| Breast | ||||||
| Ipsilateral lung | Volume: 2053± 400 (991-2812) cm3 | |||||
| Dmean, Gy | <8 Gy | 5.9 ± 1.1 | – | 5.9 ± 0.9 | 6.0 ± 1.2 | c |
| V20 Gy% | <15% | 7.5 ± 2.1 | – | 7.6 ± 2.1 | 7.3 ± 2.2 | c |
| Heart | Volume: 695 ± 93 (510-828) cm3 | |||||
| Dmean, Gy | <4 Gy | 1.9 ± 1.1 | – | 1.7-0.9 | 1.9 ± 1.0 | a, b |
| D1%, Gy | Minimize | 7.4 ± 3.2 | – | 7.1 ± 3.7 | 7.6 ± 3.8 | – |
| Contralateral lung | Volume: 2381 ± 460 (1186-3163) cm3 | |||||
| Dmean, Gy | <2 Gy | 0.2 ± 0.1 | – | 0.30 ± 0.1 | 0.3 ± 0.1 | a, b, d |
| Contralateral breast | Volume: 853 ± 36 (285-1829) cm3 | |||||
| Dmean, Gy | <3 Gy | 0.4 ± 0.2 | – | 0.4 ± 0.2 | 0.4 ± 0.2 | – |
| Head and neck | ||||||
| Parotids | Volume: 24.1 ± 5.4 (14.0-32.8) cm3 | |||||
| Dmean, Gy | <26 Gy | 24.2 ± 2.9 | 24.2 ± 2.9 | 23.8 ± 2.5 | 24.0 ± 2.7 | a, b, c |
| Spinal cord | Volume: 28.8 ± 7.3 (17.5-44.2) cm3 | |||||
| D0.1 cm3, Gy | <45 Gy | 30.3 ± 3.6 | 29.7 ± 3.7 | 29.9 ± 3.7 | 28.8 ± 3.3 | a, c, d, e, f |
| Oral cavity | Volume: 124 ± 22 (86-161) cm3 | |||||
| Dmean, Gy | Minimize | 40.4 ± 9.8 | 41.4 ± 10.4 | 40.4 ± 10.0 | 39.4 ± 10.5 | a, d, e |
| Larynx | Volume: 44 ± 26 (8-92) cm3 | |||||
| Dmean, Gy | <43.5Gy | 29.6 ± 9.6 | 27.8 ± 8.5 | 29.0 ± 8.9 | 27.3 ± 8.6 | a, b, e |
| Prostate | ||||||
| Bladder | Volume: 223 ± 14 (51-591) cm3 | |||||
| Dmean, Gy. | Minimize | 39.5 ± 8.6 | 39.5 ± 8.4 | 39.5 ± 8.5 | 38.7 ± 8.4 | a, d, e |
| V60 Gy, %. | <25-30% | 19.3 ± 12.6 | 19.1 ± 12.6 | 19.8 ± 12.7 | 19.2 ± 12.4 | b,c |
| Rectum | Volume: 56 ± 26 (25-108) cm3 | |||||
| Dmean, Gy | 29.0 ± 6.5 | 29.2 ± 6.9 | 27.9 ± 6.0 | 27.2 ± 6.0 | a, b, c, d, e | |
| D1 cm3, Gy | <68-70 Gy | 68.7 ± 1.3 | 68.8 ± 1.4 | 68.8 ± 1.6 | 68.7 ± 1.5 | – |
| V65 Gy, %. | <15%-20% | 8.0 ± 4.3 | 8.0 ± 4.0 | 8.4 ± 4.2 | 8.5 ± 4.6 | b,d,e |
| Femoral heads | Volume: 63 ± 26 (29-123) cm3 | |||||
| D1%, Gy | <45 Gy | 18.4 ± 3.5 | 18.7 ± 3.9 | 17.9 ± 3.8 | 17.8 ± 3.6 | c, d, e |
Abbreviations: Dx, dose received by x% or x cm3 of the volume; Dmean, mean dose; FFF, flattening filter free, H, Halcyon; HI, homogeneity index; SD, standard deviation; TB, TrueBeam; V20Gy, volume receiving at least 20 Gy.
a Data for the target volumes are the average over all the PTVs from the 3 groups of patients and from the different dose levels for a total of 120 data points.
b Results are expressed as averages ± 1 SD.
c Statistical significance (P): a, H2-arcs versus H3-arcs; b, H2-arcs versus TB; c, H2-arcs versus TB-FFF; d, H3-arcs versus TB; e, H3-arcs versus TB-FFF; f, TB versus TB-FFF
The Treatment Planning Techniques and Features
All the plans were designed and optimized using the Eclipse treatment planning system (Varian Medical Systems, Palo Alto) version 15.5.07 (a preclinical release).
For each patient in the 3 cohorts, VMAT plans were generated according to the specifications summarized in Table 2 for 2 delivery systems: a TB (with the millennium 120 MLC) and the H. For TB, 2 groups of plans were generated using either FFF or flattened 6 MV photon beams. The plans optimized for the TB were set as the reference baseline in the comparison, the FFF plans for HN and prostate and the standard photons for breast. In this latter case, no FFF plans were generated since this would not be a standard practice for single-dose levels and large volumes. The beam arrangements chosen for the plans were derived from the published studies8–9,12–14 for each cohort which represent the clinically adopted solution for those patients. For TB plans, only the published technical approach of 2 arcs was adopted, whereas for H both 2-arc and 3-arc plans were generated to achieve the second aim of the study. For each patient, a small adjustment of some parameters was allowed (eg, the collimator angles or the start and stop gantry angles for the partial arcs) with respect to the baseline class solution values, to better account for the specific patient anatomy. In these cases, the same arrangement was used for all the plans of the patient.
Table 2.
Summary of Main Characteristics of the Treatment Plans Concerning Dose Prescription and Arc Definition Settings.a
| Breast | Head and Neck | Prostate | |
|---|---|---|---|
| Dose prescription | 2.67 Gy/fr; 15 fr | SIB: 2.12Gy/fr; 33 fr | SIB: 2.65 Gy/fr; 28 fr |
| PTV: 40.05 Gy | PTV1: 54.4 Gy | PTV1: 51.8 Gy | |
| PTV2: 69.96 Gy | PTV2: 65.5 Gy | ||
| PTV3: 74.2 Gy | |||
| 2 arcs plans | |||
| Number or arcs | 2 partial arcs | 2 full arcs | 2 full arcs |
| Coll. angle arc 1 | (10/20)° | (10/20)° | 20° |
| Coll. angle arc 2 | (350/340)° | (350/340)° | 340° |
| Gantry start angle | ∼295° range: (285/307) | Full rotation | Full rotation |
| Gantry stop angle | ∼173° range: (160/180) | – | – |
| Avoidance sector per arc | Start: (345/20)° | – | – |
| Stop (90/110)° | – | – | |
| 3 arcs plans (Halcyon only) | |||
| Number or arcs | 3 partial arcs | 3 full arcs | 3 full arcs |
| Coll. angle arc 1 | (10/20)° | (10/20)° | 20° |
| Coll. angle arc 2 | (350/340)° | (350/340)° | 340° |
| Coll. angle arc 3 | 90° | 90° | 90° |
| Gantry start angle | ∼295°range: (285/307) | Full rotation | Full rotation |
| Gantry stop angle | ∼173°range: (160/180) | – | – |
| Avoidance sector per arc | Start: (345/20)° | – | – |
| Stop (90/110)° | – | – | |
Abbreviations: Coll., collimator; Fr, fractions; PTV, planning target volume; SIB, simultaneous integrated boost.
a The ranges expressed for the collimator rotation or in the start/stop angles for the breast and the head and neck plans represent the limits for the adjustment for individual patients.
In the following, the plan groups were labeled as TB, TB-fff, H-2arcs, and H-3arcs according to the dominant feature.
Once the optimization dose–volume objectives were determined for the TB reference plans for each individual patient, all other plans for the same patient were generated with a completely automated and unattended procedure using the same objectives. This was to avoid any human bias in the generation of the test plans. All plans were generated by the same planner.
Specifically for the HN, the planning objectives were determined by means of the knowledge based engine RapidPlan using the predictive model discussed in the study by Fogliata et al.13 In the breast and prostate, the objectives were defined manually (from the clinical practice standards) with a mix of dose–volume and generalized equivalent uniform dose (gEUD) constraints. The use of gEUD objectives in Eclipse was investigated separately15 and found to be highly efficient and, therefore, is here confirmed and proposed as additional standard for the routine planning.
For all plans, the optimization engine was the Photon Optimizer using the convergence mode option set to “ON.” The activation of the convergence mode forced an increased number of iterations per multiresolution step (about 2-3 times more than the standard mode).
For all plans, the dose distributions were computed using the Anisotropic Analytical Algorithm (AAA)16 with a resolution of 2.5 mm. The choice of AAA was forced by the H system which, at present, is not supported in the Eclipse system for the Acuros-XB algorithm.17
Quantitative Assessment
The analysis was performed by means of quantitative metrics derived from the dose–volume histograms (DVHs) for the various target volumes and OARs. For each structure, the mean dose and selected Vx and Dx parameters were extracted. Vx represents the volume receiving at least or at most an x level of dose and Dx the dose received by an x fraction of volume. All parameters could be expressed either in absolute (Gy or cm3) or relative (%) terms according to the case. For the targets, also the standard deviation (SD) and a homogeneity index (HI) were scored to measure the variance of the dose inside the volumes supposed to be homogeneously irradiated. Homogeneity index was defined as HI = (D5% − D95%)/Dmean. To qualitatively appraise the results, the average DVHs were computed, for each structure and each cohort with a dose binning resolution of 0.02 Gy.
An analysis of the computed MU needed to deliver the prescribed doses was performed to indirectly evaluate the potential delivery efficiency and implicitly the degree of modulation of each plan. The MU calculation was done consistently for the same calibration conditions of the 2 delivery units.
The assessment of the potential statistical significance of the differences among the groups of plans was performed by means of nonparametric tests for each couple of experiments (Wilcoxon test for paired samples).
Results
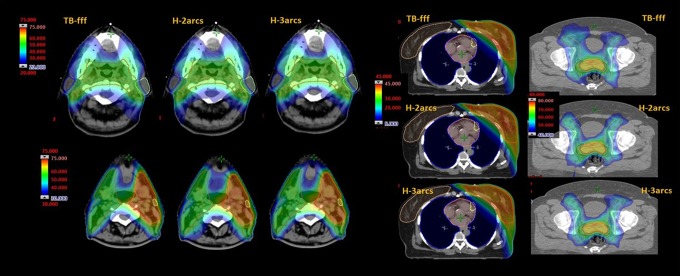
Figure 3 shows the dose distributions for 1 example patient in each cohort and for all the plan variants. Figures 4 to 6 illustrate the average DVH for the target volumes and the relevant OARs for the 3 cohorts of patients and for the various planning experiments. The data shown demonstrate qualitatively that all the approaches resulted in clinically equivalent treatment plans.
Figure 3.
Isodose distributions in color wash for example cases of the head and neck, breast and, prostate plans.
Figure 4.
Average dose–volume histogram (DVH) for the target volumes (clinical and planning) and the main organs at risk (OARs) for the breast cohort.
Figure 5.
Average dose–volume histogram (DVH) for the target volumes (the 2 target volumes corresponding to the 2 dose levels in the SIB treatment scheme) and the main organs at risk (OARs) for the head and neck (HN) cohort.
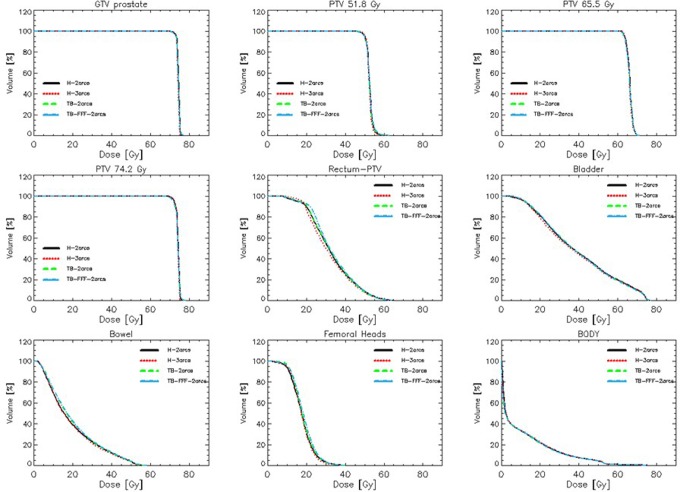
Figure 6.
Average dose–volume histogram (DVH) for the target volumes (GTB and PTVs corresponding to the 3 dose levels in the SIB treatment scheme) and the main organs at risk (OARs) for the prostate cohort.
Table 1 presents the summary of the quantitative analysis of the plans for the main structures, considered as first priority in the clinical assessment and for the most relevant metrics. The data from all the PTVs were analyzed in percentage and presented in a single data set. There are a total of 120 PTVs with 3 PTVs in the prostate, 2 PTVs in the HN, and 1 PTV in the breast cohorts. The global analysis demonstrates that all groups of plans achieved in average the minimum coverage requirement on V95% and that the homogeneity of the irradiation (either measured by the SD or by HI) was consistent among the plan groups and fulfilling the planning requirements. The observed differences, although statistically significant in many cases, are likely to be irrelevant from a clinical viewpoint. Similar results were obtained for the separate cohorts and are not reported since not adding further insights.
The results for the OARs are confirming the same trend. Note that the parotids and the femoral heads data are reported for the union of the 2 structures in each case. On average, all the clinical objectives were met, in particular the spinal cord, the larynx, all the structures in the breast plans, and the bladder and femoral heads in the prostate. The mean dose constraint for the parotids was exceeded in a minority of the cases where the gland was mostly included in the PTV. Similarly for the prostate cohort, a minority of the cases had the near-to-maximum dose exceeding the clinical criteria due to volume overlap with the target. For none of the other parameters there were violations of the planning objectives. Minor differences could be observed between TB and H data and between H-2arcs and H-3arcs but without any clear trend in favor of any of the possible solutions. The intergroup consistency of the results as well as the low interpatient variability demonstrates the possibility to optimize consistent and coherent dose distributions, irrespective of the delivery system. As for the PTVs, the observed statistical significance for many of the compared couples is of insufficient magnitude to have clinical relevance.
Table 3 summarizes the analysis of the MU investigation. The MU needed to deliver the H plans were lower than the reference TB-fff for the HN (in average ∼−2%) and prostate (in average ∼−14.5%). The different intensity of the increment from HN to prostate might be primarily due to the fractionation scheme with 2 or 3 dose levels. As expected, the use of unflattened beams for H in the breast case lead to an increased number of MU (∼+6.5% in average).
Table 3.
Monitor Units.a,b
| TB | TB-FFF | H-2arcs | H-3arcs | Significance | ||
|---|---|---|---|---|---|---|
| Breast | MU | 673 ± 89 | – | 706 ± 83 | 733 ± 62 | B, C, F |
| Delta% | Reference | – | +4.9 | +8.9 | ||
| Head and Neck | MU | 639 ± 50 | 716 ± 50 | 696 ± 29 | 711 ± 77 | A, D |
| Delta% | −10.8 | Reference | −2.8 | −0.7 | ||
| Prostate | MU | 1024 ± 232 | 1208 ± 317 | 1013 ± 154 | 1051 ± 150 | A, D, E, F |
| Delta% | −15.2 | Reference | −16.1 | −13.0 |
Abbreviations: FFF, flattening filter free, H, Halcyon; TB, TrueBeam.
a Delta is the percentage variation with the reference plan which is the TB for the breast cases and TB-FFF for the head and neck and the prostate plans.
b Statistical significance: A, TB versus TB-fff; B, TB versus H2; C, TB versus H3; D, TB-fff versus H2; E, TB-fff versus H3; F, H2 versus H3.
Discussion
This in silico treatment planning study aimed to investigate the dosimetric quality of VMAT plans optimized for a new delivery system in comparison with standard linear accelerators. The study was carried out on a cohort of 60 patients divided into 3 subgroups (HN, breast, and prostate) to cover (1) different anatomical sites, (2) different prescription regimens, and (3) different clinical objectives with the global aim to cover a wide range of clinical situations from simpler to more complex. The unique features of the new system, particularly the dual-layer MLC operating in fully independent leaf motions, legitimated the need for a systematic investigation. The goal of the study was not to push the optimization to the extreme potential but to ascertain the possibility to obtain plans (1) compatible or equivalent to what is achievable with standard practices and (2) fully acceptable from the clinical point of view. The data presented here allowed to confirm both hypothesis and to provide evidence that the new device can be considered as a versatile, general purpose delivery machine for high-quality radiation treatment of patients with cancer. It is also important to notice that this systematic study confirms the early investigations performed on a first prototype system2-5 on a smaller number of patients and under slightly different planning conditions.
It is important to note that the equivalence of the results was achieved by adopting exactly the same optimization strategy (dose–volume constraints, structures, and other planning tools) for both the H and TB plans. No additional manipulation of the structures (eg rings, cropped structures, etc) nor additional constraints were applied in any of the cases.
Although subjectively appraised and not part of the study aims, the time required, in this study, to perform the plan optimization with H resulted slightly longer then the corresponding as measured for TB. This was primarily due to a slower convergence of the cost function in the case of H plans and might be estimated in some 10% to 20% extra time. This might be confirmed or negated by other experiments and should not be considered as a definitive finding. The learning curve in the planning procedures might influence this factor as well.
This study has been carried out with the awareness of a number of limitations which shall be briefly summarized. Firstly, concerning the machine characteristics, there is a limit in the length of target volumes, which can be managed with a single isocenter (28 cm). The solution of this is the use of multiple isocenters. A second limitation of H is the possibility to treat only coplanar fields/arcs. If in most of the cases this is not an issue, for smaller lesions in particular regions (eg, brain or abdomen) this might cause some challenge and should be subject to some dedicated study. A third limitation of H is the absence, today, of an integrated motion management system (eg, to enable breath hold). This also has an impact in the treatment of breast due to the more favorable body habitus and subsequent OAR sparing that can arise from the inspiration breath hold.
From the treatment planning point of view, the current Eclipse releases support the AAA dose calculation algorithm only (a first approach to type b algorithms). The release of the deterministic Boltzmann solver Acuros-XB would be advisable and it might be included in one of the future releases of the system, according to manufacturer’s strategy. If Acuros-XB was available, certain anatomical situations (such as a prevalence of air cavities or low densities) could have improved accuracy and could enhance the comparison to TB. Nevertheless, the absence of Acuros-XB should have somehow minor impact in the case of treatment sites such as brain or pelvis/abdomen, where tissue heterogeneity is less of a concern. Also in the case of SBRT, with the exception of the thorax region, the use of AAA, although suboptimal, would not lead to major dose uncertainties.
From a validation perspective, the present study was carried out with in silico only methods and no direct measurements of the agreement between calculation and delivery were performed. This was beyond the scope of the study but the early investigations done on the prototype machines2-4 reported good to excellent agreement and nothing suggests this should be different for the present study data.
As a consequence of the limitations summarized earlier, and as part of a developing research line, further studies should aim to demonstrate the role of H in the moderate hypofractionated regimen, considering for example primary and metastatic hepatic lesions or oligometastatic abdominal lesions. In these respects, the minimum size of the lesions and the potential trade-offs of coplanar only treatment fields will be appraised. At the other end of the spectrum, there should be the effort to appraise the possibility to safely and efficiently treat longer target volumes as in the case of breast with supraclavicular nodes, or advanced stage pelvic tumors with, eg para-aortic nodes, or even the role of H in the treatment of mediastinal lymphomas.
From the delivery efficiency point of view, H is a fast-rotating O-ring linac system capable of delivering highly modulated treatment fields in a shorter time if compared to conventional linacs. This aspect, as anticipated above, was not directly investigated in our study but it was addressed by other studies. Michels reported, for 30 HN patients, an average beam on time of about 1 minute and 10 seconds or 1 minute and 40 seconds for the 2-arc or the 3-arc plans to be compared against 2 minutes and 12 seconds for the corresponding TB plans (with 2 arcs).
Conclusion
The data from this planning comparison confirmed the assumptions beneath the 2 study aims. The new delivery system can generate treatment plans for VMAT with the same dosimetric quality of what is achievable with other systems routinely used in the clinics without the need for significantly changing their clinical practice. Addition of a third arc in this study did not significantly impact the clinical quality of the plans. Further studies on different topics (eg, hypofractionated treatments and small target volumes) would complement the spectrum of investigations.
Abbreviations
- AAA
anisotropic analytical algorithm
- CBCT
cone beam computed tomography
- CTV
clinical target volume
- DVH
dose–volume histogram
- FFF
flattening filter free
- gEUD
generalized equivalent uniform dose
- GTV
gross tumor volume
- H
Halcyon
- HI
homogeneity index
- HN
head and neck
- IMRT
intensity modulated therapy
- kV
kilo voltage
- MLC
multileaf collimator
- MV
mega voltage
- MU
monitor unit
- OAR
organ at risk
- PTV
planning target volume
- SIB
simultaneous integrated boost
- SD
standard deviation
- SSD
source to surface distance
- TB
Truebeam
- VMAT
volumetric modulated arc therapy.
Footnotes
Author Contribution: Luca Cozzi and Antonella Fogliata contributed equally to this study.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: L. Cozzi acts as Scientific Advisor to Varian Medical Systems and is Clinical Research Scientist at Humanitas Cancer Center. Stephen Thompson is product manager for treatment planning at Varian Medical Systems.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Luca Cozzi, PhD  http://orcid.org/0000-0001-7862-898X
http://orcid.org/0000-0001-7862-898X
References
- 1. Li Y, Netherton T, Nitsch P, et al. Normal tissue doses from MV Image Guided Radiation Therapy (IGRT) using orthogonal MV and MV-CVCT. J Appl Clin Med Phys. 2018;19(3):52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anamalayil S, Brady L, Grover S, et al. Treatment of cervical cancer with a prototype flattening filter-free straight through linac with fast jawless MLC collimator. Int J Radiat Oncol Biol Phys.2017;99(2):E633. [Google Scholar]
- 3. Brady L, Scheuermann R, Anamalayil S, Kennedy C, Mihailidis D, Metz J. Robustness of extended field cervical target optimization techniques to isocenter offsets with a prototype fast jawless MLC system. Int J Radiat Oncol Biol Phys. 2017;99(2):E642. [Google Scholar]
- 4. Mihailidis D, Brady L, Anamalayil S, et al. Rapid IMRT delivery for head and neck (H&N) with a prototype jawless MLC system and a novel MV-CBCT panel. Int J Radiat Oncol Biol Phys. 2017;99(2):S230–S231. [Google Scholar]
- 5. Netherton T, Li Y, Nitsch P, et al. Efficiency and efficacy of intensity modulated treatments on a novel linear accelerator. Int J Radiat Oncol Biol Phys. 2017;99(2):E703. [Google Scholar]
- 6. Michels S, Poels K, Crijns W, et al. Volumetric modulated arc therapy of head and neck cancer on a fast rotating O-ring linac: plan quality and delivery time comparicon with a C-arm linac. Radiother Oncol. 2018;128(3):479–484. [DOI] [PubMed] [Google Scholar]
- 7. Lloyd S, Lim T, Fave X, Flores-Martinez E, Atwood T, Moiseenko V. TG-51 reference dosimetry for the Halcyon: a clinical experience. J Appl Clin Med Phys. 2018;19(4):98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fogliata A, Seppala J, Reggiori G, et al. Dosimetric trade-offs in breast treatment with VMAT technique. Br J Radiol. 2017;90(1070):20160701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koivumaki T, Fogliata A, Zeverino M, et al. Dosimetric evaluation of modern radiation therapy techniques for left breast in deep-inspiration breath hold. Phys Med. 2018;45:82–87. [DOI] [PubMed] [Google Scholar]
- 10. RTOG.org.Available at: http://www.rtog.org/. Accessed July 2016.
- 11. Gregoire V, Levendag P, Ang KK, et al. CTbased delineation of lymph node levels and related CTVs in the nodenegative neck: DAHANCA, EORTC, GORTEC, NCIC, RTOG consesus guidelines. Radiother Oncol.2003;69(3):227–236. [DOI] [PubMed] [Google Scholar]
- 12. Franzese C, Fogliata A, Clerici E, et al. Toxicity profile and early clinical outcome for advanced head and neck cancer patients treated with simultaneous integrated boost and volumetric modulated arc therapy. Radiat Oncol. 2015;10:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fogliata A, Reggiori G, Stravato A, et al. RapidPlan head and neck model: the objectives and possible clinical benefit.Radiat Oncol. 2017;12(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Franzese C, Fogliata A, D’Agostino G, et al. Moderate hypofractionated radiotherapy with volumetric modulated arc therapy and simultaneous integrated boost for pelvic irradiation in prostate cancer. J Cancer Res Clin Oncol. 2017;143(7):1301–1309. [DOI] [PubMed] [Google Scholar]
- 15. Fogliata A, Thompson S, Stravato A, Tomatis S, Scorsetti M, Cozzi L. On the gEUD biological optimization objective for organs at risk in photon optimizer of eclipse treatment planning system. J Appl Clin Med Phys. 2018;19(1):106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ulmer W, Harder D. A triple gaussian pencil beam model for photon beam treatment planning. Z Med Phys.1995;5(1):25–30. [Google Scholar]
- 17. Vassiliev O, Wareing T, McGhee J, et al. Validation of a new grid based Blotzmann equation solver for dose calculation in radiotherapy with photon beams. Phys Med Biol.2010;55(3):581–598. [DOI] [PubMed] [Google Scholar]