Abstract
The aim of the study was to assess the acute effect of whole-body vibration (WBV) exercise, with low frequency (5 Hz), on the pain level (PL), trunk flexibility, and cardiovascular responses (blood pressure [BP] and heart rate [HR]) in individuals with metabolic syndrome (MetS). Forty-four individuals were included in the study (control: 15) or in (WBV exercise: 29) groups. They were submitted to 3 bouts (1 minute each) of WBV exercise (5 Hz and peak-to-peak displacements of 2.5, 5.0, and 7.5 mm, corresponding to peak accelerations of 0.12, 0.25, and 0.35 g, respectively, sitting in a chair with the feet on the platform with knees flexed, followed by 1 minute of interset rest. The Control Group performed the same protocol, but the platform was turned off. The PL was measured through the visual analog pain scale, and the flexibility was measured through the anterior trunk flexion test. Significant improvements on PL (P = .031) and flexibility (P = .004) were found only in the WBV exercise group. The BP and HR remained at physiological levels. In conclusion, the WBV exercise would lead to physiological response decreasing PL and increasing flexibility as well as maintaining the cardiovascular responses in individuals with MetS.
Keywords: Mechanical vibration, metabolic syndrome, flexibility, pain, cardiovascular responses
Introduction
Metabolic syndrome (MetS) is a clinical disorder defined by interconnected physiological, biochemical, clinical, and metabolic factors, including alterations in the level of the lipids in the plasma, arterial hypertension, central adiposity, and insulin resistance and hyperglycemia that directly increase the cardiovascular risks.1 Moreover, these conditions are linked by a pathophysiological basis in low-grade chronic inflammation and also increase the risk of type 2 diabetes mellitus (T2DM) and all-cause mortality.2
Musculoskeletal pain can be found in individuals with MetS,3 and they can also show peripheral small-fiber neuropathy, characterized by damage to the primary afferent nociceptors due to chronic hyperglycemia, when the T2DM is present.2 This damage causes peripheral sensitization, leading to central neuron hyperexcitability and spontaneous nerve impulse generation, presenting as chronic pain.2 In addition, obese people have more pain than normal weight individuals.4 Duruöz et al5 have reported that in individuals with MetS, low back pain can also be found. In general, the mechanisms underlying the association between these metabolic disturbances and pain are not still well known, although the inflammation6 and the mechanical overload7 seem to have important roles in these relations.
According to World Health Organization (WHO), in 2016,8 more than 50% of deaths and disability related to cardiovascular diseases can be eliminated by a combination of national efforts and individual actions to reduce major risk factors such as high blood pressure (BP), high cholesterol, obesity, and smoking. Then, the morbidity and the mortality may be reduced by simple lifestyle interventions and easily modifiable behavior changes. The WHO8 estimates that 25% of healthier life-years will be lost to cardiovascular disease globally by 2020 if no action is taken to improve cardiovascular health and current trends. Putting together all the considerations, MetS would be related to a cluster of risk factors for cardiovascular disease and T2DM, which occur together more often than by chance alone.9 Heart disease and stroke costs in the United States were about $320 billion on health care and lost productivity in 2011 according the Centers for Disease Control and Prevention.10 In this context, the WHO8 has stated that countries might adopt policies and programs to promote population-wide interventions such as encouraging exercise and other actions. The physical disuse has been presented as one of the perpetuating factors for chronicity of pain, and some authors11 have suggested that the presence of MetS is importantly associated with a low physical performance and decreased cardiorespiratory endurance, flexibility, and muscular strength.12
Chang et al13 have reported that the presence of MetS was also associated with a decrease in flexibility in a community-based geriatric population. Therefore, physical reconditioning has been proposed in clinical practice as a goal in the treatment of patients with MetS.14 Despite this, not all forms of exercise are equally effective and safe; although aerobic exercise1,15 or resistance training has been associated with decreased cardiovascular disease risk factors, obesity, or MetS severity14,16 due to the pain or even the low physical fitness, most individuals are unable or unwilling to perform these exercises.11,17
Given the limitations for some types of exercises that many individuals report, different forms of exercise are being suggested in the literature for people with MetS.18 In this sense, whole-body vibration (WBV), which is a modality of exercise involving mechanical vibration generated in oscillating/vibratory platform, has been used as an alternative to manage individuals with different clinical disorders, including fibromyalgia,19,20 cerebral palsy,21 and chronic obstructive pulmonary disease.22 The WBV exercise can be considered a feasible, safe, and low-cost technique to the improvement of health in various populations.19–22 Biomechanical parameters, such as the frequency and the peak-to-peak displacement of the mechanical vibration, must be considered in the WBV exercise protocols.23 Wei et al24 consider low frequency (20 Hz), medium frequency (40 Hz), and high frequency (60 Hz).
The WBV exercise is associated with lower neuropathic pain symptoms and acute and chronic reductions in pain levels (PLs) measured in a visual analog pain scale (VAS).25 There is also strong evidence of the effects of WBV on fitness, including improvements in the flexibility of the lower limbs,26,27 that could be justified by stretching of the muscles.28,29 It was suggested that WBV would reduce the stiffness and hysteresis of the tendon, alter the properties of the intramuscular connective tissue,29 and possibly modify those of other passive skeletal structures related to the range of motion for a determined joint30 such as the knee.28 Acute and short- and long-term effects of WBV exercise have been studied21,28,29,31 as well as cardiovascular responses by WBV in young health adults,32,33 older adults,34 and obese women.35
An increase in oxygen consumption (VO2) with minimal cardiovascular stress (heart rate [HR], blood flow, or mean arterial pressure) with the addition of WBV to a static semi-squat exercise has been demonstrated. The findings could be justified based on type or intensity of vibration.35
Di Giminiani et al36 showed that WBV acutely increased lower back and hamstring flexibility and seems to be effective on flexibility also in clinical populations,37,38 and this kind of exercise could improve the quality of life that is poor in individuals with MetS.39,40 In fact, as reviewed by Sá-Caputo et al,26 WBV exercise could be suggested as a strategy to improve flexibility in individuals with MetS. Beavers et al11 reported a poor physical performance in older adults with MetS.
Considering the need and importance of physical activities, the aim of the study was to assess the acute effect of WBV exercise, with low frequency (5 Hz), on the (1) PL, (2) flexibility, and (3) cardiovascular responses (BP and HR) in individuals with MetS. We hypothesized that 5 Hz WBV exposure would be feasible, suitable, and safe and could significantly favor better conditions to pain, flexibility, and cardiovascular responses in individuals with MetS.
Materials and Methods
As the entrance of the individuals to participate in this project was not considering a specific randomization, the study is an interventionist, cross-sectional, pseudo-randomized controlled trial. In this sequence, they were allocated in the control or in the WBV exercise groups.
In the current study, the problem was the unknown effect of low-frequency (5 Hz) WBV on pain, flexibility, and cardiovascular responses of individuals with MetS immediately after a session of WBV and in a control group (individuals without vibration exposition). This frequency (5 Hz) was previously used in a work using WBV exercise in a case report of individuals with MetS26 and in a protocol involving individuals with knee osteoarthritis.41
This study was approved by the Research Ethics Committee of the Hospital Universitário Pedro Ernesto, Universidade do Estado do Rio de Janeiro (HUPE/UERJ) with the number CAAE 54981315.6.0000.5259.
Participants
Forty-nine participants with MetS were selected. Recruitment and all the procedures were done from October 2013 to December 2015. The selection of the participants was made through a screening performed by the medical staff of HUPE/UERJ. The principles embodied in the Declaration of Helsinki were followed. The authors confirm that all ongoing and related trials for this intervention are registered. The registration was performed following the guidelines of the Registro Brasileiro de Ensaios Clínicos numbered RBR2bghmh.
The eligibility criteria were outpatient older than 40 years with previous clinical diagnosis of MetS based on the criteria described by the International Diabetes Federation.9 The exclusion criteria were individuals with high BP (≥180 × 110 mm Hg), cardiovascular disease (coronary artery disease or stroke), and neurological, musculoskeletal, or rheumatologic disease that do not permit perform WBV exercise. Those participants who refused to sign the consent form for participation in the study were also excluded.
The Transparent Reporting of Evaluations with Non-randomized Designs statements42 were used to report all the different steps of the interventions utilized in this study. The dependent variables (pain, flexibility, BP, and HR) were measured before and after the WBV session (exercise group) or the control session (control group).
Sample Size
For a statistical power of 95% and significance level of 5%, a sample size (www.lee.dante.br/) of 13 participants was calculated, and the finding published by Vieira et al43 was considered. A sufficiently large sample size (n = 49) was recruited to account for potential dropout.
Allocation of the Participants
A convenience sample of 44 individuals was allocated in the control group (n = 15) or in the WBV exercise group (n = 29) in the sequence of the entrance of the participants in the study.
Outcomes
The PL, flexibility, and cardiovascular responses (BP and HR) were evaluated in the control and WBV exercise groups before and after the interventions. Anthropometric parameters (height [m], waist circumference [cm], body mass [kg], body mass index [kg/m2], and age [years]) were also verified before the interventions.
Anthropometric Characteristics
The height and body mass were measured on a digital balance (MIC 200 PPA, Micheletti, São Paulo, Brazil). Then, the body mass index was calculated by dividing the mass (kg) by the height squared (m2). The baseline anthropometric data of the participants of control and WBV groups were evaluated.
Pain Level
The PL was assessed using a VAS, with 10 indicating the highest level of pain and 0 the lowest.25 The analyses were performed in both groups before and after the intervention. In all, 25 individuals of the WBV exercise group and 15 individuals of the control group had pain before the interventions. The individuals have reported that the pain was felt in parts of back (lower back, cervical, and dorsal), lower (knee, heel, and hip), and upper limbs (arm, shoulder, and fist).
Flexibility
The measure of the flexibility was performed through the anterior trunk flexion (ATF) test also called fingertip-to-floor distance test.26 This test consisted of measuring the distance between the tip of middle finger and the floor after an ATF, with feet together and without bending the knees26,44 (Figure 1). The distance between the third finger of the hand to the floor and the measurements were performed once before and after the procedures in both the groups.
Figure 1.
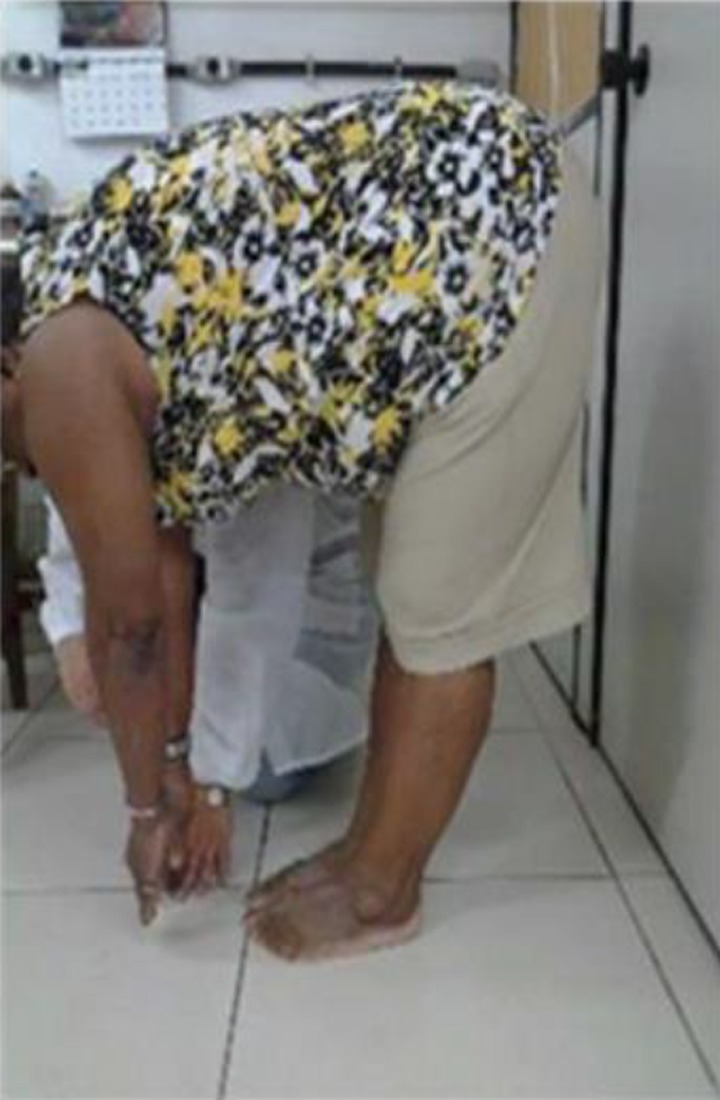
Individual performing the anterior trunk flexion test.
Cardiovascular Responses (BP and HR)
An automated device (OMRON, model HEM-7113, China) was utilized to record the systolic blood pressure (SBP) and diastolic blood pressure (DBP; mm Hg), and the HR (beats/min) was measured on the left arm of seated patient after a 10-minute rest.45 Three measurements were performed with 1 minute of rest after each measurement. Means of these 3 records of SBP and DBP and HR were used in the analyses of the WBV exercise group and the control group. The measurements were performed before and after the interventions in both the groups.
WBV exercise group Intervention
Patients were seated in a chair, in front of the platform, with their bare feet on the base of the side alternating oscillating/vibratory platform (Novaplate, Fitness Evolution, São Paulo, Brazil) with knees 50º flexed (from full extension of 180º; Figure 2A). The height of the chair was selected depending on the height of the individual to have the same knee flexion. The hands of the patient was positioned on the knees to facilitate the transmission of the mechanical vibration to the whole body and to generate WBV exercise in the individual.
Figure 2.
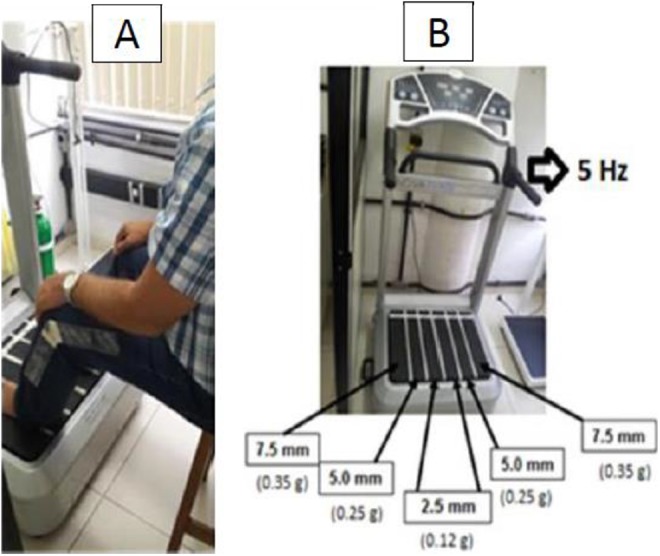
(A) Position of the individual during the interventions and (B) side alternating oscillating/vibratory platform. It is indicated the frequency (Hz), the peak-to-displacement (mm), and the acceleration peak (×g) used in the WBV exercise group. WBV indicates whole-body vibration.
Participants performed the WBV exercise with low frequency (5 Hz). Different peak-to-peak displacements were sequentially used in 3 ways: (1) 2.5, (2) 5.0, and (3) 7 5 mm (Figure 2B). The gravitational force was 0.12, 0.25, and 0.35 g, respectively, for WBV. The session consisted of 9-minute bout of work interspersed with 1-minute passive rest period between each bout. The total time of intervention was 17 minutes.
Control Group Intervention
The patient performed the same position and time of the WBV exercise group, but the side alternating oscillating/vibratory platform was off and without mechanical vibration stimulus transmission.
Statistical Analysis
The Shapiro-Wilk normality test was done to determine whether the data set are well modeled by a normal distribution. The Wilcoxon rank test was used to compare the results of HR, BP, PL, and flexibility before and after each intervention (WBV or control). These results are presented in mean and standard error. The Mann-Whitney U test was used to compare the anthropometric baseline data between WBV exercise group and control group, and these results are presented as mean (standard deviation). The level of significance was set at P < .05. These analyses were executed using the GraphPad Prism 6 statistic program.
Results
A total of 49 participants were recruited, and 44 performed the proposed intervention (15 in the Control Group and 29 in the WBV exercise group). Side effects were not found in these individuals during this study. The flow of participants through each stage of the study is shown in Figure 3.
Figure 3.
Flow of participants through each stage of the study.
The anthropometric characteristics (baseline) of the individuals in control and WBV exercise groups are presented in Table 1.
Table 1.
The Baseline Anthropometric Characteristics of the Individuals of Control Group and WBV Group.a
| Control Group, n = 15, Mean (SD) | WBV exercise Group, n = 29, Mean (SD) | P Value | |
|---|---|---|---|
| Height,(m | 1.61 (0.08) | 1.63 (0.07) | .74 |
| WC, cm | 108.3 (15.55) | 103.0 (11.09) | .32 |
| Body mass, kg | 87.43 (18.02) | 83.65 (16.27) | .70 |
| BMI, kg/m2 | 32.79 (6.94) | 31.16 (5.35) | .55 |
| Age, years | 58.20 (9.11) | 61.10 (8.39) | .38 |
Abbreviations: BMI, body mass index; SD, standard deviation; WBV, whole-body vibration; WC, waist circumference.
a P ≤ .05.
Considering the data analysis regarding the dependent variables, no significant difference was found between the 2 groups as shown in Table 2.
Table 2.
Data Analyses of Dependent Variables in the Baseline.
| Control Group, n = 15, Mean (SD) | WBV exercise Group, n = 29, Mean (SD) | P Value | |
|---|---|---|---|
| Pain level (scale) | 2.61 ± 0.67 | 2.28 (0.65) | .63 |
| Flexibility, cm | 17.81 (2.21) | 17.25 (2.52) | .43 |
| SBP, mm Hg | 121.3 (3.94) | 129.0 (3.45) | .97 |
| DBP, mm Hg | 65.65 (2.86) | 68.57 (2.55) | .27 |
| Heart rate, beats/min | 67.29 (2.70) | 70.29 (3.20) | .63 |
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure; SD, standard deviation; WBV, whole-body vibration.
All individuals completed the study, and the results before and after the intervention are presented for all the outcomes (PL, flexibility, and cardiovascular responses).
Considering the PL, the individuals of the WBV exercise group presented a decrease in this parameter, with 2.28 ± 0.65 before and 1.76 ± 0.57 after the WBV exercise intervention (P = .03). In the Control Group, no significant change was observed, with 2.61 ± 0.67 before and 2.72 ± 0.72 after the intervention (P = .75).
A significant difference (P < .05) in ATF was found in WBV exercise group, indicating an improvement on the flexibility of these individuals. The value was 17.25 ± 2.52 cm before and 15.15 ± 2.36 cm after the intervention (P = .004). In the Control Group, no improvement in the flexibility was presented, and the data were 17.81 ± 2.21 cm before and 16.84 ± 2.61 cm after the intervention (P = .28). None of the participants was able to reach the floor with the third finger.
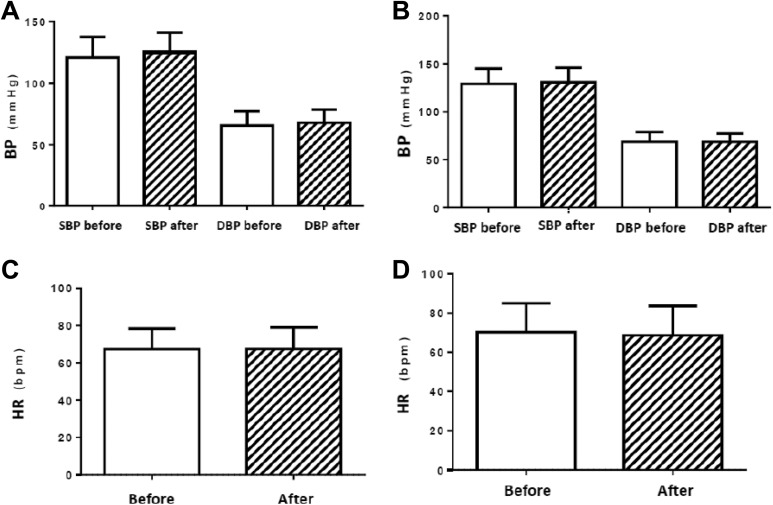
Considering the cardiovascular responses, in the Control Group, the findings of SBP were 121.3 ± 3.94 mm Hg before and 125.3 ± 3.82 mm Hg after the intervention (P = .06). The data of DBP were 65.65 ± 2.86 mm Hg before and 67.94 ± 2.63 mm Hg after the intervention (P = .08). In the WBV exercise group, the SBP was 129.0 ± 3.45 mm Hg before and 130.8 ± 3.25 mm Hg after the WBV exercise (P = .048). The data of DBP was 68.57 ± 2.55 mm Hg before and 68.48 ± 2.42 mm Hg after the WBV exercise (P = .92). The HR was measured and found to be 67.29 ± 2.70 beats/min before and 67.47 ± 2.80 beats/min after the intervention (P = .71) in the control group. In the WBV exercise group, it was 70.29 ± 3.20 beats/min before and 68.67 ± 3.25 beats/min after the WBV exercise (P = .16). These parameters were not altered and are presented in Figure 4A–D.
Figure 4.
(A) Blood pressure of control group, (B) blood pressure of whole-body vibration exercise group, (C) heart rate of control group, and (D) heart rate of whole-body vibration exercise group. BP indicates blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate.
Discussion
The current study aimed to evaluate the effects of WBV on the flexibility in individuals with MetS and to determine whether these improvements are related to symptom severity (bodily pain). The major findings of the study are that the WBV protocol applied with a low frequency (5 Hz) and that different peak-to-peak displacement resulted in improvements, reducing the PL and increasing flexibility. The results may be of clinical importance, as WBV exercise could be considered as a safe and reliable strategy in the management of individuals with MetS. Moreover, this is a study that uses a simple and comfortable position related to the WBV exercise to the management of individuals with MetS.
Considering the relevance of exercises that could be suitable and safe, and based on the analyses of the aforementioned results about the cardiovascular responses, there were no statistically significant differences in SBP, DBP, and HR, before and after the WBV exercise with 5 Hz in a side alternating oscillating/vibratory platform. This is in agreement with the findings reported by Robbins et al46 after WBV, although they used 40 Hz in a synchronous platform in healthy participants (12 males and 8 females). Figueroa et al47 suggested that WBV training with 25 to 30 Hz in a synchronous platform may benefit arterial function in deconditioned individuals who cannot perform conventional exercise. The authors reported that 6 weeks of WBV training decreased systemic arterial stiffness and SBP via improvements in wave reflection and sympathovagal balance in young overweight/obese normotensive women. Thus, it is proposed that the WBV exercise may be used safely by individuals with MetS without changes of the physiological levels of arterial BP and HR.
As improvements in the flexibility are important for the maintenance of the mobility and a better functional independence, the flexibility was investigated in the current study. Chang et al13 have pointed out that flexibility should be included in the complete evaluation for individuals with MetS. Jacobs and Burns48 have suggested the application of WBV as a preparatory activity before more intense exercise in untrained individuals. Sá-Caputo et al26 also found improvement on flexibility in individuals with MetS after a similar protocol that was used in the current study, although this is the unique study with the investigated patient. Similarly, Rittweger35 suggested that stretching could reduce the stiffness and hypothesize that these changes could be due to alterations in (1) tendon, (2) properties of the intramuscular connective tissue, and (3) passive skeletal structures that together define the range of motion for a specific joint.34 There is evidence in the literature suggesting that WBV can improve flexibility in both trained and untrained participants,47 and previous studies have found that WBV can acutely increase lower back and hamstring flexibility.36 These changes were attributed to the activation of the Ia inhibitory interneurons of the antagonist muscle after vibration. Another possible explanation to these changes was reported by Cardinale and Bosco49 who suggested that vibration may have caused changes in intramuscular coordination, leading to a decrease in the braking force around the hip and lower back joints, which may also explain, at least in part, the improvements shown in this study. In addition, an increase in the skin temperature and blood flow could also partly justify the findings of this study37 because these physiological conditions could improve the range of the motion of the joints.50
The current results may be related to a decrease in pain sensation. Although the mechanisms underlying the association between metabolic disturbances and pain would be complex, the inflammation6 and the mechanical overload7 would be associated with a reduction in chronic pain, especially in neuropathic pain symptoms.25 As proposed by Kessler and Hong,25 it may be suggested that the mechanisms to justify the acute pain effects would be related to vibration-induced sensory inhibition at both the peripheral and the central levels. At the peripheral level, a sensory inhibition would be linked to the gate control theory of pain.51 According this theory, pain signals need to encounter “neurological gates” at the spinal cord level and are in charge of determining whether the pain signals should reach the brain or not. In other words, pain is perceived when the gate gives way to the pain signals, and it is less intense or not at all perceived when the gate closes for the signals to pass through. Thus, this may be the reason why WBV is able to reduce the pain signal. Moreover, as postulated by Longe et al,52 pain relief due the WBV would be linked to the phenomenon related to the inhibition caused when the sensory fibers type Aβ conducting stimulus from specific mechanoreceptors, such as Pacinian and Meissner corpuscles. This phenomenon would reduce the painful input of the type C fibers by activating inhibitory circuits of the dorsal horn. At the central level, the inhibition caused by vibration would be justified by the proximity of areas responsible for processing pain and vibrotactile sensations in the somatosensory cortices of the brain.53 This may be of importance, since individuals with MetS would have possibility in developing pain conditions, and WBV could be suggested as a safe strategy to decrease pain.
Despite promising results, the current study has some limitations. It should be noted that the population of this work consisted of outpatients with MetS. A relatively small number of patients participated in the study, but it is higher than the number needed that was calculated for the sample size. Moreover, the participants maintained the medication prescribed by the physician during the interventions. In addition, only acute effects are shown; however, these effects might relevant in the rehabilitation of patients with MetS.
Conclusion
It is concluded that WBV exercise exposure with low frequency (5 Hz) is responsible in inducing physiologic parameters that contribute to decrease the PL and to increase the flexibility as well as to maintain cardiovascular responses (HR and BP) in individuals with MetS. As a consequence, WBV exercise may be considered as useful, feasible, and safe strategy to be used on the management of individuals with MetS.
Acknowledgments
The authors are thankful to Dr Tarik Isbelle, Dr Eric Heleno F. F. Frederico, and Dr Mario Pereira for all the support in this study.
Authors’ Note: Sá-Caputo DC and Paineiras-Domingos LL participated in the elaboration and execution of the study. Sá-Caputo DC and Paineiras-Domingos LL did the statistical analysis. Bernardo-Filho M coordinated the study and helped on the draft of manuscript. Sá-Caputo DC, Oliveira R, Neves MTF, Brandão A, Marin PJ, Sañudo B, Furness T, Taiar R, and Bernardo-Filho M reviewed and approved the final version of this investigation. Research Ethics Committee with the number CAAE 54981315.6.0000.5259, register in the Registro Brasileiro de Ensaios Clínicos (ReBEC) with the number RBR 2bghmh and UTN: U1111-1181-1177.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors are thankful to Conselho Nacional de Pesquisa e Desenvolvimento (CNPq), Fundação de Amparo à pesquisa do Estado do Rio de Janeiro (FAPERJ), and Universidade do Estado do Rio de Janeiro (UERJ) for their financial support. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) - Finance Code 001.
ORCID iD: L.L. Paineiras-Domingos  http://orcid.org/0000-0003-3451-5056
http://orcid.org/0000-0003-3451-5056
Borja Sañudo  http://orcid.org/0000-0002-9969-9573
http://orcid.org/0000-0002-9969-9573
References
- 1. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2. Seaman DR, Palombo AD. An overview of the identification and management of the metabolic syndrome in chiropractic practice. J Chiropr Med. 2014;13(3):210–219. doi:10.1016/j.jcm.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Briggs MS, Spees C, Bout-Tabaku S, Taylor CA, Eneli I, Schmitt LC. Cardiovascular risk and metabolic syndrome in obese youth enrolled in a multidisciplinary medical weight management program: implications of musculoskeletal pain, cardiorespiratory fitness, and health-related quality of life. Metab Syndr Relat Disord. 2015;13(3):102–109. [DOI] [PubMed] [Google Scholar]
- 4. Hitt HC, McMillen RC, Thornton-Neaves T, Koch K, Cosby AG. Comorbidity of obesity and pain in a general population: results from the southern pain prevalence study. J Pain. 2007;8(5):430–436. [DOI] [PubMed] [Google Scholar]
- 5. Duruöz MT, Turan Y, Gürgan A, Deveci H. Evaluation of metabolic syndrome in patients with chronic low back pain. Rheumatol Int. 2012;32(3):663–667. [DOI] [PubMed] [Google Scholar]
- 6. Barbe MF, Barr AE. Inflammation and the pathophysiology of work-related musculoskeletal disorders. Brain Behav Immun. 2006;20(5):423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Courties A, Gualillo O, Berenbaum F, Sellam J. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthr Cartilage. 2015;23(11):1955–1965. [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization. Cardiovascular death and disability can be reduced more than 50 percent. 2016. http://www.who.int/mediacentre/news/releases/pr83/en/. Updated 2016, Accessed February 16, 2018.
- 9. Alberti KG, Eckel RH, Grundy SM, et al. ; International Diabetes Federation Task Force on Epidemiology and Prevention. National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. [DOI] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention. Metabolic Syndrome Prevalence by Race/Ethnicity and Sex in the United States, National Health and Nutrition Examination Survey, 1988–2012 http://millionhearts.hhs.gov/; Updated 2016, Accessed February 16, 2018.
- 11. Beavers KM, Hsu FC, Houston DK, et al. Health ABC Study. The role of metabolic syndrome, adiposity, and inflammation in physical performance in the health ABC study. J Gerontol A Biol Sci Med Sci. 2013;68(5):617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hwang HJ, Kim SH. The association among three aspects of physical fitness and metabolic syndrome in a Korean elderly population. Diabetol Metab Syndr. 2015;7:112 doi:10.1186/s13098-015-0106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang KV, Hung CY, Li CM, et al. Reduced flexibility associated with metabolic syndrome in community-dwelling elders. PLoS One. 2015;10(1):e0117167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farias DL, Tibana RA, Teixeira TG, et al. Elderly women with metabolic syndrome present higher cardiovascular risk and lower relative muscle strength. Einstein. 2013;11(2):174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dixit S, Maiya A, Shastry B. Effect of aerobic exercise on quality of life in population with diabetic peripheral neuropathy in type 2 diabetes: a single blind, randomized controlled trial. Qual Life Res. 2014;23(5):1629–1640. [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization. Global status report on noncommunicable diseases. Geneva, Switzerland: World Health Organization, http://apps.who.int/iris/bitstream/10665/148114/1/9789241564854_eng.pdf?ua=1/; Updated 2014, Accessed February 16, 2018. [Google Scholar]
- 17. Callaghan BC, Xia R, Reynolds E, et al. Association between metabolic syndrome components and polyneuropathy in an obese population. JAMA Neurol. 2016;73(12):1468–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sossa C, Delisle H, Agueh V, Sodjinou R, Ntandou G, Makoutodé M. Lifestyle and dietary factors associated with the evolution of cardiometabolic risk over four years in West-African adults: the Benin study. J Obes. 2013; 2013: 298024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sañudo B, Carrasco L, de Hoyo M, Oliva-Pascual-Vaca Á, Rodríguez-Blanco C. Changes in body balance and functional performance following whole-body vibration training in patients with fibromyalgia syndrome: a randomized controlled trial. J Rehabil Med. 2013;45(7):678–684. [DOI] [PubMed] [Google Scholar]
- 20. Hidalgo-Santamaria M, Fernandez-Montero A, Martinez-Gonzalez MA, et al. Exercise intensity and incidence of metabolic syndrome: The SUN project. Am J Prev Med. 2017;52(4):e95–e101. doi:10.1016/j.amepre.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 21. Gusso S, Munns CF, Colle P, et al. Effects of whole-body vibration training on physical function, bone and muscle mass in adolescents and young adults with cerebral palsy. Sci Rep. 2016; 6:22518 doi:10.1038/srep22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Braz Júnior DS, Dornelas de Andrade A, Teixeira AS, Cavalcanti CA, Morais AB, Marinho PE. Whole-body vibration improves functional capacity and quality of life in patients with severe chronic obstructive pulmonary disease (COPD): a pilot study. Int J Chron Obstruct Pulmon Dis. 2015;10:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rauch F, Sievanen H, Boonen S, et al. ; International Society of Musculoskeletal and Neuronal Interactions. Reporting whole-body vibration intervention studies: recommendations of the International Society of Musculoskeletal and Neuronal Interactions. J Musculoskelet Neuronal Interact. 2010;10(3):193–198. [PubMed] [Google Scholar]
- 24. Wei N, Pang MY, Ng SS, Ng GY. Optimal frequency/time combination of whole-body vibration training for improving muscle size and strength of people with age-related muscle loss (sarcopenia): a randomized controlled trial. Geriatr Gerontol Int. 2016. doi:10.1111/ggi.12878. [DOI] [PubMed] [Google Scholar]
- 25. Kessler NJ, Hong J. Whole body vibration therapy for painful diabetic peripheral neuropathy: a pilot study. J Bodyw Mov Ther. 2013;17(4):518–522. [DOI] [PubMed] [Google Scholar]
- 26. Sá-Caputo Dda C, Ronikeili-Costa P, Carvalho-Lima RP, et al. Whole body vibration exercises and the improvement of the flexibility in patient with metabolic syndrome. Rehabil Res Pract. 2014;2014:628518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dallas G, Paradisis G, Kirialanis P, Mellos V, Argitaki P, Smirniotou A. The acute effects of different training loads of whole body vibration on flexibility and explosive strength of lower limbs in divers. Biol Sport. 2015;32(3):235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pistone EM, Laudani L, Camillieri G, et al. Effects of early whole-body vibration treatment on knee neuromuscular function and postural control after anterior cruciate ligament reconstruction: a randomized controlled trial. J Rehabil Med. 2016;48(10):880–886. [DOI] [PubMed] [Google Scholar]
- 29. Donahue RB, Vingren JL, Duplanty AA, Levitt DE, Luk HY, Kraemer WJ. Acute effect of whole-body vibration warm-up on footspeed quickness. J Strength Cond Res. 2016;30(8):2286–2291. [DOI] [PubMed] [Google Scholar]
- 30. Rieder F, Wiesinger HP, Kösters A, Müller E, Seynnes OR. Immediate effects of whole body vibration on patellar tendon properties and knee extension torque. Eur J Appl Physiol. 2016;116(3): 553–561. [DOI] [PubMed] [Google Scholar]
- 31. Kubo K, Kanehisa H, Kawakami Y, Fukunaga T. Influence of static stretching on viscoelastic properties of human tendon structures in vivo. J Appl Physiol. 2001;90(2):520–527. [DOI] [PubMed] [Google Scholar]
- 32. Hazell TJ, Lemon PW. Synchronous whole-body vibration increases VO2 during and following acute exercise. Eur J Appl Physiol. 2012;112(2):413–420. [DOI] [PubMed] [Google Scholar]
- 33. Owtell JL, Jackman SR, Scott S, et al. Short duration small sided football and to a lesser extent whole body vibration exercise induce acute changes in markers of bone turnover. Biomed Res Int. 2016;2016:3574258 doi:10.1155/2016/3574258.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rittweger J, Beller G, Felsenberg D. Acute physiological effects of exhaustive whole-body vibration exercise in man. Clin Physiol. 2000;20(2):134–142. [DOI] [PubMed] [Google Scholar]
- 35. Rittweger J. Vibration as an exercise modality: how it may work, and what its potential might be. Eur J Appl Physiol. 2000;108(5):877–904. [DOI] [PubMed] [Google Scholar]
- 36. Di Giminiani R, Manno R, Scrimaglio R, Sementilli G, Tihanyi J. Effects of individualized whole-body vibration on muscle flexibility and mechanical power. J Sports Med Phys Fitness. 2010;50(2):139–151. [PubMed] [Google Scholar]
- 37. Cochrane DJ, Sartor F, Winwood K, Stannard SR, Narici MV, Rittweger J. A comparison of the physiologic effects of acute whole-body vibration exercise in young and older people. Arch Phys Med Rehabil. 2008;89(5):815–821. [DOI] [PubMed] [Google Scholar]
- 38. Sañudo B, Alfonso-Rosa R, Del Pozo-Cruz B, Del Pozo-Cruz J, Galiano D, Figueroa A. Whole body vibration training improves leg blood flow and adiposity in patients with type 2 diabetes mellitus. Eur J Appl Physiol. 2013;113(9):2245–2252. [DOI] [PubMed] [Google Scholar]
- 39. Carvalho-Lima RP, Sá-Caputo DC, Moreira-Marconi E, et al. Quality of life of patients with metabolic syndrome is improved after whole body vibration exercises. Afr J Tradit Complement Altern Med. 2017;14(4 suppl):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang Q, Chair SY, Wong EM. The effects of a lifestyle intervention program on physical outcomes, depression, and quality of life in adults with metabolic syndrome: a randomized clinical trial. Int J Cardiol. 2017;230:461–467. [DOI] [PubMed] [Google Scholar]
- 41. Neto SBS, Marconi EM, Kutter CR, et al. Beneficial effects of whole body mechanical vibration alone or combined with auriculotherapy in the pain and in flexion of knee of individuals with knee osteoarthritis. Acupunct Electrother Res. 2017;17:185–201. [Google Scholar]
- 42. Des Jarlais DC, Lyles C, Crepaz N, TREND Group. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health. 2004;94(3):361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vieira DC, Tibana RA, Tajra V, et al. Decreased functional capacity and muscle strength in elderly women with metabolic syndrome. Clin Interv Aging. 2013;8:1377–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Robinson HS, Mengshoel AM. Assessments of lumbar flexion range of motion: intertester reliability and concurrent validity of 2 commonly used clinical tests. Spine. 2014;39(14):E270–E275. [DOI] [PubMed] [Google Scholar]
- 45. Sociedade Brasileira de Cardiologia. III Diretrizes brasileiras sobre dislipidemias e diretriz sobre prevenção da aterosclerose do Departamento de Aterosclerose da Sociedade Brasileira de Cardiologia. Arq Bras Cardiol. 2001;77(3):1–48. [PubMed] [Google Scholar]
- 46. Robbins D, Yoganathan P, Goss-Sampson M. The influence of whole body vibration on the central and peripheral cardiovascular system. Clin Physiol Funct Imaging. 2013;17 doi:10.1111/cpf.12103. [DOI] [PubMed] [Google Scholar]
- 47. Figueroa A, Gil R, Wong A, et al. Whole-body vibration training reduces arterial stiffness, blood pressure and sympathovagal balance in young overweight/obese women. Hypertens Res. 2012;35(6):667–672. [DOI] [PubMed] [Google Scholar]
- 48. Jacobs PL, Burns P. Acute enhancement of lower-extremity dynamic strength and flexibility with whole-body vibration. J Strength Cond Res. 2009;23(1):51–57. [DOI] [PubMed] [Google Scholar]
- 49. Cardinale M, Bosco C. The use of vibration as an exercise intervention. Exerc Sport Sci Rev. 2003;31(1):3–7. [DOI] [PubMed] [Google Scholar]
- 50. Marshall LC, Wyon MA. The effect of whole-body vibration on jump height and active range of movement in female dancers. J Strength Cond Res. 2012;26(3):789–793. [DOI] [PubMed] [Google Scholar]
- 51. Melzack R, Wall PD. Pain mechanisms: a new theory. Sci Rev. 1965;150(3699):971–999. [DOI] [PubMed] [Google Scholar]
- 52. Longe SE, Wise R, Bantick S, et al. Counter-stimulatory effects on pain perception and processing are significantly altered by attention: an fMRI study. Neuroreport. 2001;12(9):2021–2025. [DOI] [PubMed] [Google Scholar]
- 53. Coghill RC, Talbot JD, Evans AC, et al. Distributed processing of pain and vibration by the human brain. J Neurosci. 1994;14(7):4095–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]