Abstract
The aim of this study was to investigate the acute effects of whole-body vibration exercises (WBVE) in different positions on muscular activity of flexor digitorum superficialis (FD), wrist extensor (ED), and handgrip strength (HG) of healthy men. Fifteen participants have performed 5 test sets each one consisting of HG strength measurement and 1-minute WBVE intervention (frequency: 50 Hz, amplitude: 1.53 mm, synchronous tri-planar oscillating/vibratory platform), that could be control (no exposition to vibration), squat (30 seconds of rest and 30 seconds of WBVE in squat position), or push-up (30 seconds of rest, and 30 seconds of WBVE in push-up position). After testing, participants had 2 minutes of rest and then were encouraged to keep themselves on a pull-up bar for 30 seconds. During all procedures, muscular activity of FD and ED was measured by surface electromyography (EMG). Statistical analysis has revealed that the EMG measured in the FD during the static pull-up bar exercise after SQUAT condition was significantly higher (P = .004) than the CONTROL and PUSH-UP conditions. Whole-body vibration exercises in squat position increase acutely muscle activation of the FD during isometric exercises of longer duration, while muscle activation of ED and HG strength are not affected by WBVE.
Keywords: muscle strength dynamometer, electromyography, upper extremity, military personnel, hand strength, Brazilian Army
Introduction
Whole-body vibration exercise (WBVE) standing in different static positions or exercising on an oscillating/vibratory platform (OVP) has been promoted as an attractive, alternative, and efficient complement method to resistance training programs for fitness improvements in healthy participants.1 Whole-body vibration exercise is a safe modality to increase muscular activity and muscle performance in athletes and healthy participants due to the characteristics of the vibration stimulation generated during the exercise.1–3 Whole-body vibration exercise could represent an effective exercise intervention for enhancing neuromuscular performance in well-trained and healthy people, probably due to the need for quickly modulating muscle stiffness to accommodate the vibratory waves.4
Although literature suggests that acute effects of WBVE can enhance upper and lower body muscle power,2 most studies regarding this type of exercise have focused on the lower extremities, and little has been studied regarding the optimal posture for training the upper extremities.5–7 Acute effects of WBVE have been investigated by focusing on the increase in muscle activity as measured by surface electromyography (EMG).8–10
Active militaries must have a high fitness level and improve some physical abilities to accomplish some specific tasks in the Army, as climb ropes, walls, and pull up.3,11 The assessment of the muscular activity of flexor digitorum superficialis (FD), wrist extensor (ED), and the measurement of handgrip strength (HG) can be used to evaluate the performance of upper limbs.12
Therefore, the purpose of this study has been to investigate the acute effects of WBVE from different body positions on muscular activity of FD, ED, and also HG strength of Brazilian Army soldiers. The hypothesis was WBVE increases muscular activity and strength of upper limbs muscles with different responses depending on the position used by the participants.
Methods
To investigate the difference in muscle activation and strength in different conditions, a randomized, crossover experimental design has been used for this study. It consisted of 6 sessions separated by 72 hours, performed at Universidade do Estado do Rio de Janeiro - UERJ, with controlled temperature (25 ± 2°C), at the same time of the day (8:00-11:00 am) to avoid any effect of circadian rhythm. All procedures have been supervised by the same investigator.
At the first session, the participants were submitted to clinical exam (interview, measurement of body mass, height, body mass index, and body fat).13 The randomization also occurred at the first session, where each participant took 3 numbered balls from a bag. The WBVE sequential order was according to the taken numbers (1 = CONTROL, 2 = SQUAT, 3 = PUSH-UP).
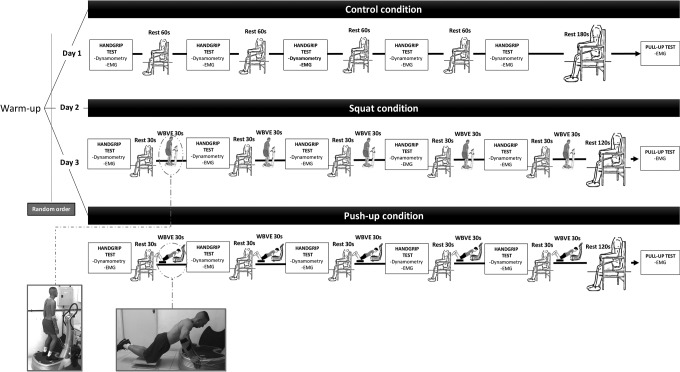
The following sessions were called “familiarization sessions” (2 sessions) and “experimental sessions” (3 sessions). At the familiarization sessions, participants performed sessions as experimental, but the data collected were not used to analysis. Experimental sessions were divided in CONTROL (participants were not exposed to WBVE), SQUAT (participants were exposed to WBVE on squat condition—over OVP with 50°-flexion of the knees and arms along the body) and PUSH-UP (participants were exposed to WBVE on push-up condition—hands on the base of OVP, elbows lightly flexed, feet with no contact with floor, and knees over a force platform [EMG832WF; EMG System, São José dos Campos/SP, Brazil], to control the force applied to the base of OVP; Figure 1).
Figure 1.
Schematic sequence of test procedure.
Fifteen healthy young males (age 20.4 [1.8] years, height 1.74 [0.06] m, body mass 75.7 [14.1] kg, body mass index 24.8 [3.9] kg/m2, body fat 28.2 [4.1%]; mean [SD]) volunteered for the study and completed all the interventions. The participants were soldiers from Hospital Central do Exército (HCE), Rio de Janeiro, Brazil, physically active but with no experience in WBVE. They were required to have their usual breakfast and liquid intake on the days of the experiment. Exclusion criteria included joint pain and/or implants, musculoskeletal diseases, vertigo, and other clinical diseases that could involve some risk or discomfort during WBVE. All participants were informed of the requirements associated with participation. Informed consent was obtained from all individual participants included in the study.
All procedures performed in this study involving human participants were in accordance with the ethical standards of the Research Ethics Committee of the Hospital Universitário Pedro Ernesto, UERJ (Certificado de Apresentação para Apreciação Ética - CAAE: 47933015.1.0000.5259), and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Participants were fitted with EMG electrodes and completed a warm-up (2-minute skipping, 30 jumping jack, 10 squats, and 5 submaximal contractions of both hands with a dynamometer). After a 2-min period of rest, all participants performed 5 test sets, each one consisting of HG strength measurement and 1-minute WBVE intervention. On CONTROL condition, participants were not exposed to vibration and stayed on a chair for 60 seconds. The SQUAT condition, the participants performed 30 seconds of rest and 30 seconds of WBVE in squat position. The PUSH-UP condition, participants performed 30 seconds of rest and 30 seconds of WBVE in push-up position. After the test, the participants had 2 minutes of rest, then were encouraged to keep themselves on a pull-up bar for 30 seconds, with palms facing forward using prescribe grip from Brazilian Army, with no thumb opposition, and arms extended (Figure 1).
Muscular activity of FD and ED was measured during HG strength evaluation, WBVE intervention, and static pull-up bar exercise, using EMG of the right upper limb, according to surface electromyography for the non-invasive assessment of muscles (SENIAM) project recommendations.14 The area of electrodes placement was shaved and cleansed with ethyl alcohol 70% to reduce skin impedance. The double electrodes (Hal Indústria e Comércio Ltda, São Paulo/SP, Brazil) were placed over the middle of the muscle belly of both muscles in a distance of 50 mm between them. A ground electrode was placed over the C7 tuberosity vertebrae. The cables were fastened with Kinesio tape (Kinesiology Tape Nitreat NKH-50BU, Nitto Denko Corporation, Japan) to avoid swinging and others movement that could cause artifacts. The surface electrodes were connected to a 16-bit AD converter (EMG System, São José dos Campos/SP, Brazil). Raw EMG signals were preamplified close to the electrodes (signal bandwidth of 10-500 Hz), sampled at 2000 Hz and stored on a laptop. Surface electromyography data analysis was performed using specific computer software (EMG832WF, EMG System, São José dos Campos/SP, Brazil). Surface electromyography raw data were averaged by root mean square (EMGrms) in order to obtain the average amplitude of the EMG signal. Moreover, in order to obtain average frequency, the signal was averaged by median frequency (MDF).
The measurement of HG strength was analyzed according to American Society of Hand Therapists – ASHT.15 The participants were in seated position, arm in adduction, with 90° forward at elbow joint, forearm in neutral position, wrist with extension between 0° and 30° and ulnar flexion between 0° and 15°. They performed 3 maximum attempts with a manual dynamometer (EMG832WF, EMG System, São José dos Campos/SP, Brazil) with the right hand,15 for 5 seconds each and 15 seconds of rest. The participants were instructed to contract their muscles as hard as possible and verbal encouragement was provided during each measurement. The maximum value from each trial was recorded and used to analysis.
Soldiers were exposed to mechanical vibrations through synchronous triplanar OVP Power Plate pro5 (Power Plate International LTD, The Netherlands). The WBVE were applied at a frequency of 50 Hz with 1.53 mm (high) of amplitude, 7.7 g, and apeak 75.54 m/s2 (measured using a 3-axial accelerometer—Vibration Datalogger DT-178A, Ruby Electronics, Saratoga, USA). During all conditions, the participants used their own athletic shoes to standardize the damping of the vibration because of footwear.16
Values are presented as means (standard deviation [SD]). All the measures were normally distributed, as determined by the Kolmogorov-Smirnov test. Sphericity was tested by the Greenhouse-Geisser method. Dependent variables were evaluated with a 1-way repeated measures analysis of variance (ANOVA), where significant F-values were achieved; pairwise comparisons were performed using the Boferroni post hoc procedure. Data were analyzed using PASW/SPSS Statistics version 20 (SPSS Inc, Chicago, Illinois), and significance level was set at P ≤ .05.
Results
No significant main effect of the HG strength measurement was detected between CONTROL, PUSH-UP, or SQUAT conditions (P = .712; η2 = 0.046, Table 1).
Table 1.
Handgrip Strength Results (N) From Right Hand, During the 3 Conditions. Five Repetitions Each Condition.
| CONTROL | PUSH-UP | SQUAT | |
|---|---|---|---|
| Set 1 | 409.1 (10.3) | 400.2 (9.4) | 404.2 (9.0) |
| Set 2 | 369.8 (11.3) | 365.9 (9.2) | 375.7 (7.3) |
| Set 3 | 355.1 (10.4) | 346.3 (7.7) | 364.9 (8.4) |
| Set 4 | 337.5 (9.6) | 347.3 (9.1) | 338.4 (7.7) |
| Set 5 | 341.4 (9.8) | 348.2 (8.4) | 345.3 (8.7) |
Abbreviation: N, Newton.
The EMGrms measured in the FD muscle during the WBVE sets had no significant difference between CONTROL and PUSH-UP or SQUAT conditions (P = .640; η2 = 0.045, Table 2). A similar effect was observed with the MDF (P = .378; η2 = 0.065, Table 2).
Table 2.
Root-Mean Square (EMGrms - %MVIC) and Median Frequency (MDF - Hz) of Flexor Digitorum Superficialis Muscle From Right Upper Limb, During 3 Conditions. Five Repetitions each Condition.
| Flexor digitorum superficialis muscle | ||||||
|---|---|---|---|---|---|---|
| EMGrms (%MVIC) | MDF (Hz) | |||||
| CONTROL | PUSH-UP | SQUAT | CONTROL | PUSH-UP | SQUAT | |
| Set 1 | 100.0 (0.0) | 100.0 (0.0) | 100.0 (0.0) | 108.1 (31.4 | 106.1 (21.5) | 103.4 (30.1) |
| Set 2 | 91.0 (11.8) | 97.3 (16.7) | 101.1 (14.6) | 109.9 (28.8 | 107.3 (23.0) | 108.0 (29.6) |
| Set 3 | 95.9 (17.4) | 98.7 (21.1) | 98.4 (21.9) | 107.7 (25.6 | 107.3 (22.4) | 108.0 (29.5) |
| Set 4 | 93.5 (16.3) | 91.3 (15.3) | 96.0 (15.8) | 106.7 (23.6 | 110.0 (24.1) | 110.9 (31.3) |
| Set 5 | 97.6 (20.3) | 98.1 (18.3) | 100.9 (14.5) | 110.4 (27.2 | 110.7 (24.8) | 107.8 (27.4) |
The EMGrms measured in the FD muscle during the static pull-up bar exercise after SQUAT condition was significantly higher (P = .004) than the CONTROL and PUSH-UP conditions (Table 3). In the MDF no effects were observed between the CONTROL and the PUSH-UP or SQUAT conditions (P = .654; Table 3).
Table 3.
Root-Mean Square (EMGrms - %MVIC) and Median Frequency (MDF – Hz) of Flexor Digitorum Superficialis and Wrist Extensor Muscles from Right Upper Limb, during Static Pull Bar Exercise in 3 Conditions.
| Flexor digitorum superficialis Muscle | Wrist Extensor Muscle | |||
|---|---|---|---|---|
| EMGrms (%MVIC) | MDF (Hz) | EMGrms (%MVIC) | MDF (Hz) | |
| CONTROL | 69.5 (22.2) | 91.2 (20.0) | 39.0 (28.2) | 107.0 (22.5) |
| PUSH-UP | 66.7 (14.9) | 94.5 (23.0) | 42.3 (17.1) | 112.7 (30.6) |
| SQUAT*# | 92.8 (27.4) | 98.9 (25.1) | 37.4 (20.1) | 109.7 (23.8) |
* Difference from CONTROL condition (P = .006). #Difference from PUSH-UP condition (P = .002).
The EMGrms measured in the ED muscle during the WBVE sets (P = .946; η2 = 0.009) and the static pull-up bar exercise (P = .826) had no significant difference between CONTROL and PUSH-UP or SQUAT conditions, as shown on Table 4. A similar effect was observed with the MDF (P = .644; η2 = .034), as shown on Table 4.
Table 4.
Root-Mean Square (EMGrms - %MVIC) ad Median Frequency (MDF – Hz) of Wrist Extensor Muscle from Right Upper Limb, During 3 Conditions. Five Repetitions Each Condition.
| Wrist Extensor Muscle | ||||||
|---|---|---|---|---|---|---|
| EMGrms (%MVIC) | MDF (Hz) | |||||
| CONTROL | PUSH-UP | SQUAT | CONTROL | PUSH-UP | SQUAT | |
| Set 1 | 100.0 (0.0) | 100.0 (0.0) | 100.0 (0.0) | 134.1 (20.2) | 132.8 (20.7) | 128.1 (25.5) |
| Set 2 | 96.1 (43.5) | 96.3 (17.7) | 94.7 (21.5) | 140.7 (19.7) | 140.6 (23.9) | 135.1 (21.1) |
| Set 3 | 92.5 (20.7) | 97.2 (26.4) | 92.8 (21.7) | 140.8 (17.7) | 143.3 (24.3) | 138.6 (22.5) |
| Set 4 | 88.2 (20.8) | 94.1 (37.6) | 88.6 (27.1) | 141.0 (21.8) | 144.5 (26.5) | 138.9 (22.9) |
| Set 5 | 94.4 (38.8) | 96.5 (35.8) | 98.3 (35.9) | 144.8 (20.6) | 146.8 (26.1) | 140.2 (25.1) |
Discussion
To the best of our knowledge, this study is the first to assess the effects of WBVE in different positions on HG strength and muscular activity of FD and ED in military soldiers. The main finding of this study has been that WBVE applied on lower limbs, through SQUAT position, can increase acutely muscle activation of the FD during isometric exercises of longer duration, while muscle activation of ED and HG strength are not affected by WBVE.
This study shows no effect on HG mean strength with any vibration condition. Based on the purpose of our study, participants kept forearm muscles very close to the vibration source during PUSH-UP condition and far from vibration source during SQUAT position. It is accepted that acute or chronic WBVE applied in squat position have no effect on HG strength performance.7,17,18 On the other hand, the effects on HG strength of WBVE applied in push-up position are still controversial. Giminiani et al8 found no significant differences in maximum voluntary isometric contraction during HG after an exposure to WBVE in push-up position. No effects in HG strength were observed by Morel et al3 and Torvinen et al7 either after a 4-minute WBVE session or after 5 sessions of WBVE in push-up position, respectively. In contrast, Kurt and Pekünlü19 found a significant performance-enhancing effect on HG strength in well-trained combat athletes exposed to WBVE in push-up position.
Considering the aim of this study, the hypothesis of improving muscular performance after WBVE can be justified by inhibitory effect of vibration in antagonist muscles.20,21 The main finding of this study is an increase in muscle activity of FD during the static pull-up bar exercise after WBVE protocol in squat position. In accordance with Colson et al22 our results show that the post effect of vibration stimulus does not produce changes on the antagonist muscles but on the agonist muscle only, increasing its activity. García-Gutiérrez et al23 also found an acute increase in muscle coordination and a decrease in the coactivation of digital extensor muscle during HG measurement in young and healthy men exposed to WBVE.
Surface EMG is often used to assess muscle response to WBVE and can provide information about occurrence of muscle fatigue.10 It has been suggested that vibration may result in greater muscle fatigue, represented by a reduction in MDF, because it may increase neuromuscular performance earlier in an exercise,24 especially with frequencies higher than 45 Hz.7 In this study, with 5 repetitions of WBVE during 30 seconds in each condition, with a total work time of 150 seconds, no significant difference was found in MDF, suggesting no evidence of fatigue. Similarly, Maffiuletti et al25 found no evidence of fatigue after WBVE.
There are some suggestions in the literature that the acute residual effect of WBVE lasts only some minutes after vibration exercise.26,27 In this study, an increase in FD muscle activation was observed 150 seconds after WBVE exposition. This result may allow us to conclude that the acute residual effect remains unchanged within a time period of 2 minutes and 30 seconds. Torvinen et al7 observed an enhancement in physical performance 2 minutes after finishing the vibration intervention, but the acute effect disappeared when subjects were rested 1 hour later. McBride et al27 observed that the acute residual enhancement lasted 8 minutes but it disappeared at 16 minutes testing time point, while Gyulai et al28 found no loss of acute effects after 10 minutes of WBVE exposure.
With the increase in FD muscle activity after WBVE in squat position, it suggests that WBVE via a ground-based platform can alter upper arm muscle activity. Our results are in accordance with Lee et al29 that a vibration stimulus applied to the feet can result in positive improvements in the performance of upper body resistance exercise. In contrast, Cochrane and Stannard17 suggested that muscles are not directly exposed to vibration and does not show a concomitant performance enhancement as the vibrated muscles.
In summary, it is evident that the immediate effects of WBVE in squat position are beneficial for muscle performance of upper limbs. In conclusion, WBVE in squat position improve upper body resistance exercise performance and cause no changes in handgrip strength. The main limitation to this study was the relatively small sample size.
Acknowledgments
We would like to thank all soldiers and LAVIMPI team for their important cooperation; we also thank to Exército Brasileiro and Hospital Central do Exército for the assistance. The authors are grateful to John Jaquish for his kind donation of OVP used in this study and to Fernando Porteiro for his writing assistance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research received grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
ORCID iD: Danielle S Morel  http://orcid.org/0000-0003-0507-9304
http://orcid.org/0000-0003-0507-9304
References
- 1. Morel DS, Dionello CDF, Moreira-Marconi E, et al. Relevance of whole body vibration exercise in sport: a short review with soccer, diver and combat sport. Afr J Tradit Complement Altern Med. 2017;14(4 suppl):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cochrane DJ. Vibration exercise: the potential benefits. Int J Sports Med. 2011;32(2):75–99. [DOI] [PubMed] [Google Scholar]
- 3. Morel DS, Moreira-Marconi E, Sobrinho-Neto SBS, et al. Effects of whole body vibration intervention on handgrip strength of brazilian healthy soldiers. Afr J Tradit Complement Altern Med. 2017;14(4 suppl):28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cardinale M, Bosco C. The use of vibration as an exercise intervention. Exerc Sport Sci Rev. 2003;31(1):3–7. [DOI] [PubMed] [Google Scholar]
- 5. Lienhard K, Vienneau J, Friesenbichler B, et al. The effect of whole-body vibration on muscle activity in active and inactive subjects. Int J Sports Med. 2015;36(7):585–591. [DOI] [PubMed] [Google Scholar]
- 6. Padulo J, Di Giminiani R, Ibba G, et al. The acute effect of whole body vibration on repeated shuttle-running in young soccer players. Int J Sports Med. 2014;35(1):49–54. [DOI] [PubMed] [Google Scholar]
- 7. Torvinen S, Kannu P, Sievänen H, et al. Effect of a vibration exposure on muscular performance and body balance. Randomized cross-over study. Clin Physiol Funct Imaging. 2002;22(2):145–152. [DOI] [PubMed] [Google Scholar]
- 8. Di Giminiani R, Fabiani L, Baldini G, et al. Hormonal and neuromuscular responses to mechanical vibration applied to upper extremity muscles. PloS One. 2014;9(11):e111521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. García-Gutiérrez MT, Hazell TJ, Marín PJ. Effects of whole-body vibration applied to lower extremity muscles during decline bench press exercise. J Musculoskelet Neuronal Interact. 2016;16(3):204–210. [PMC free article] [PubMed] [Google Scholar]
- 10. Marín PJ, García-Gutiérrez MT, Da Silva-Grigoletto ME, et al. The addition of synchronous whole-body vibration to battling rope exercise increases skeletal muscle activity. J Musculoskelet Neuronal Interact. 2015;15(3):240–248. [PMC free article] [PubMed] [Google Scholar]
- 11. Sammito S, Gundlach N, Böckelmann I. Correlation between the results of three physical fitness test (endurance, strength, speed) and the output measured during a bicycle ergometer test in a cohort of military servicemen. Mil Med. 2016;3:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dias JA, Ovando AC, Külkamp W, et al. Força de preensão palmar: métodos de avaliação e fatores que influenciam a medida. Rev Bras Cineantropom Desempenho Hum. 2010;12:209–216. [Google Scholar]
- 13. Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40(3):497–504. [DOI] [PubMed] [Google Scholar]
- 14. Hermens HJ, Freriks B, Disselhorst-Klug C, et al. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10(5):361–374. [DOI] [PubMed] [Google Scholar]
- 15. Incel NA, Ceceli E, Durukan PB, et al. Grip strength: effect of hand dominance. Singapore Med J. 2002;43(5):234–237. [PubMed] [Google Scholar]
- 16. Marín PJ, Bunker D, Rhea MR, et al. Neuromuscular activity during whole-body vibration of different amplitudes and footwear conditions: implications for prescription of vibratory stimulation. J Strength Cond Res. 2009;23(8):2311–2316. [DOI] [PubMed] [Google Scholar]
- 17. Cochrane DJ, Stannard SR. Acute whole body vibration training increases vertical jump and flexibility performance in elite female field hockey players. Br J Sports Med. 2005;39(11):860–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Torvinen S, Sievanen H, Jarvinen TA, et al. Effect of 4-min vertical whole body vibration on muscle performance and body balance: a randomized cross-over study. Int J Sports Med. 2000;23(5):374–379. [DOI] [PubMed] [Google Scholar]
- 19. Kurt C, Pekünlü E. Acute effect of whole body vibration on isometric strength, squat jump, and flexibility in well-trained combat athletes. Biol Sport. 2015;32(2):115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ribot-Ciscar E, Roll JP. Ago-antagonist muscle spindle inputs contribute together to joint movement coding in man. Brain Res. 1998;791(1-2):167–176. [DOI] [PubMed] [Google Scholar]
- 21. Ribot-Ciscar E, Rossi-Durand C, Roll JP. Muscle spindle activity following muscle tendon vibration in man. Neurosci Lett. 1998;258(3):147–150. [DOI] [PubMed] [Google Scholar]
- 22. Colson SS, Petit PD, Hebreard L, et al. Whole body vibration does not enhance muscle activation. Int J Sports Med. 2009;30(12):841–844. [DOI] [PubMed] [Google Scholar]
- 23. García-Gutiérrez MT, Rhea MR, Marín PJ. A comparison of different vibration exercise techniques on neuromuscular performance. J Musculoskelet Neuronal Interact. 2014;14(3):303–310. [PubMed] [Google Scholar]
- 24. Luo J, McNamara B, Moran K. The use of vibration training to enhance muscle strength and power. Sports Med. 2005;35(1):23–41. [DOI] [PubMed] [Google Scholar]
- 25. Maffiuletti NA, Saugy J, Cardinale M, et al. Neuromuscular fatigue induced by whole-body vibration exercise. Eur J Appl Physiol. 2013;113(6):1625–1634. [DOI] [PubMed] [Google Scholar]
- 26. Bedient AM, Adams JB, Edwards DA, et al. Displacement and frequency for maximizing power output resulting from a bout of whole-body vibration. J Strength Cond Res. 2009;23(6):1683–1687. [DOI] [PubMed] [Google Scholar]
- 27. McBride JM, Nuzzo JL, Dayne AM, et al. Effect of an acute bout of whole body vibration exercise on muscle force output and motor neuron excitability. J Strength Cond Res. 2010;24(1):184–189. [DOI] [PubMed] [Google Scholar]
- 28. Gyulai G, Racz L, Di Giminiani R, et al. Effect of whole body vibration applied on upper extremity muscles. Acta Physiol Hung. 2013;100(1):37–47. [DOI] [PubMed] [Google Scholar]
- 29. Lee DY. Analysis of muscle activation in each body segment in response to the stimulation intensity of whole-body vibration. J Phys Ther Sci. 2017;29(2):270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]