Abstract
White Matter Hyperintensities (WMHs) are associated with cognitive decline in aging and Alzheimer's disease. However, the pathogenesis of cognitive decline in Parkinson's disease (PD) is not as clearly related to vascular causes, and therefore the role of WMHs as a marker of small-vessel disease (SVD) in PD is less clear. Currently, SVD in PD is assessed and treated independently of the disease. However, if WMH as the major MRI sign of SVD has a higher impact on cognitive decline in PD patients than in healthy controls, vascular pathology needs to be assessed and treated with a higher priority in this population. Here we investigate whether the presence of WMHs leads to increased cognitive decline in de novo PD, and if these effects relate to cortical atrophy. WMHs and cortical thickness were measured in de novo PD patients and age-matched controls (NPD = 365, NControl = 174) from Parkinson's Progression Markers Initiative (PPMI) to study the relationship between baseline WMHs, future cognitive decline (follow-up: 4.09 ± 1.14 years) and cortical atrophy (follow-up: 1.05 ± 0.10 years). PD subjects with high baseline WMH loads had significantly greater cognitive decline than i) PD subjects with low WMH load, and ii) control subjects with high WMH load. Furthermore, in PD subjects, high WMH load resulted in more cortical thinning in the right frontal lobe. Theses results show that the presence of WMHs in de novo PD patients predicts greater future cognitive decline and cortical atrophy than in normal aging.
Keywords: Parkinson's disease, White matter hyperintensities, Magnetic resonance imaging, Cognitive decline, De novo patients
Highlights
-
•
High WMH loads cause greater cognitive decline in PD patients compared to controls.
-
•
Higher WMH loads cause greater future cortical atrophy in PD patients.
-
•
WMHs interact with PD to increase future cognitive decline.
-
•
Baseline WMHs are linked with future decline in different cognitive domains in PD.
1. Introduction
While Parkinson's disease (PD) is typically characterized by motor symptoms, cognitive deficits occur in approximately 15% of patients in early drug-naïve stages (Poletti et al., 2012). Two decades after disease onset, this prevalence increases to over 80% (Hely et al., 2008). Early mild cognitive impairment (MCI) is a strong predictor of later development of dementia (Anang et al., 2014; Pedersen et al., 2017), which is a key determinant of mortality and poorer quality of life in PD (de Lau et al., 2005). Taken together, these epidemiological findings highlight the need to better understand cognitive impairment in PD and what biological underpinnings may shape the course of this decline. Progress has been made in recent years in understanding how subcortical dysfunction in early stages, followed by cortical α-synuclein pathology and loss of neurotransmitters, may relate to cognitive impairment in PD patients. Additionally, Compta et al. and Irwin et al. have underlined the relevance of neuropathological markers of Alzheimer's disease (AD) (i.e. amyloid beta and tau protein aggregates) present in ex-vivo samples of PD patients with dementia, indicating another potential co-morbid source of cognitive decline in PD (Compta et al., 2011; Irwin et al., 2012). More specifically, they report that high levels of cortical amyloid beta correlate with faster progression to dementia (Compta et al., 2011), and that in a cohort of samples obtained from 92 patients with a diagnosis of PD, almost a third had histopathologic findings associated with AD as a comorbidity (Irwin et al., 2012). Nevertheless, how vascular pathology (i.e., small-vessel disease (SVD) (Halliday et al., 2014; Merino & Hachinski, 2000)) - assessed by white matter changes or leukoaraiosis (Hachinski et al., 1987)- may contribute to cognitive dysfunction in PD remains unclear.
The term SVD is mainly related to two etiologies: age-related vascular disease, also referred as arteriolosclerosis, or vascular-risk-factor related SVD (de Leeuw et al., 2002; Debette & Markus, 2010), and cerebral amyloid angiopathy (Pantoni, 2010), which is also present in the small cortical vessels in AD pathology. Both etiologies play a crucial role in stroke, dementia and aging, and could also be relevant in PD. Therefore, early detection of WMHs and treatment of cardiovascular risk factors could have a positive impact on cognitive decline in PD (Dufouil et al., 2005; Hawkins et al., 2017; Biesbroek et al., 2017; Veselỳ & Rektor, 2016). One measure of SVD is white matter hyperintensities (WMHs) which are areas of increased signal in T2-weighted and FLAIR structural MRI (Pantoni & Garcia, 1997). Neuropathologic correlates of WMHs include loss of axons and glial cells, myelin rarefaction, spongiosis, perivascular demyelination, gliosis, subependymal glial accumulation and loss of the ependymal lining (Merino & Hachinski, 2000). Despite the various findings, consensus exists regarding the association of WMHs and SVD (Pantoni & Garcia, 1997).
In Alzheimer's disease (AD), WMHs have been extensively studied and strongly predict rapid cognitive decline in individuals with mild cognitive impairment (MCI) (Dubois et al., 2014; Tosto et al., 2014). In PD, the pathogenic role of vascular risk factors is less clear (de Lau et al., 2005) and results have been contradictory (Veselỳ & Rektor, 2016). WMHs might cause cognitive decline independent of PD, or the synergy between the two mechanisms may accelerate cognitive impairment (Veselỳ & Rektor, 2016). Alternatively, WMHs might aggravate the pathologic spread of misfolded α-synuclein or amyloid-β proteins. WMH burden can also precede neurological damage as indexed by cortical atrophy. Higher WMH load (WMHL) has been shown to correlate with lower cortical thickness in regions that are related to cognitive decline (Tuladhar et al., 2015). Cortical thinning caused by direct or indirect effects of WMHs might lead to cognitive decline in PD.
Of the few studies that have investigated WMHs and cognitive decline in PD, most are cross-sectional, include patients that are on dopaminergic medication, and are typically from cohorts that are at later stages of disease (Auning et al., 2014; Mak et al., 2015; Jones et al., 2017). Additionally, different groups implement different tests to assess cognition and many do not perform a comprehensive neuropsychological battery.
Capitalizing on the longitudinal assessment of cognitive abilities and neuroimaging biomarkers in the large-scale, multi-centre cohort of de novo PD patients from the Parkinson's Progression Markers Initiative (Marek et al., 2011), we investigated the relationship between WMH burden and: 1) cognitive decline over time, and 2) cortical grey matter changes over time (as indexed by cortical thinning) in early stages of PD.
2. Methods
2.1. Patients
The Parkinson's Progression Markers Initiative (PPMI) is a longitudinal multi-site clinical study of de novo PD patients and age-matched healthy controls (HC)(Marek et al., 2011) (http://www.ppmi-info.org). The study was approved by the institutional review board of all participating sites and written informed consent was obtained from all participants before inclusion in the study. In the present study, we included all subjects that had either FLAIR or T2-weighted MR images at their baseline visit and had follow-up visits for at least one year after the baseline scan (NPD = 365, NHC = 174). All subjects were regularly assessed (yearly follow-ups, mean total follow-up period of 4.09 ± 1.14 years) for clinical characteristics (motor, non-motor and neuropsychological performance) by site investigators, including Montreal Cognitive Assessment (MoCA), Hopkins Verbal Learning Test–Revised (HVLT), Benton judgement of line orientation test for visuospatial skills, Letter-Number Sequencing test for verbal working memory, and semantic fluency test to detect cognitive decline (Table 2). The executive function score is calculated as the sum of letter number sequencing and semantic fluency scores (Chan et al., 2008). To validate the correlation between these two components, we verified their relationship in the PD population (r = 0.56, p < 0.0001). For more information on clinical measurements, see supplementary material, section Cognitive Testing.
Table 2.
Summary of the mixed effects models of association between baseline WMH Load and cognition in HC and PD cohorts. Entries show the regression coefficients for the listed fixed effect followed by the associated p values. Baseline WMH load was log transformed and z-scored along with age, MoCA, HVLTRT, and Benton scores prior to analysis. WMHL = White Matter Hyperintensity Load. HC = Healthy Control. “x” indicates the interaction between two variables. Global Cognition = Montreal Cognitive Assessment Score (MoCA). Memory = Hopkins Verbal Learning Test Revised Total Score (HVLT). Visuospatial = Benton Judgement of Line Orientation Score. Executive = Executive Function Score (Letter Number Sequencing + Semantic Fluency). HC = Healthy Control. PD = Parkinson's Disease. Bold font indicates statistical significance.
| Cognitive Score |
Global Cognition |
Memory |
Visuospatial |
Executive |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | ß | p-value | ß | p-value | ß | p-value | ß | p-value | |
| PD | Intercept | −0.063 | 0.180 | −0.098 | 0.029 | 0.013 | 0.737 | −0.086 | 0.059 |
| AGE | −0.413 | <0.001 | −0.341 | <0.001 | −0.164 | <0.001 | −0.374 | <0.001 | |
| WMHL | 0.035 | 0.428 | −0.029 | 0.485 | −0.093 | 0.021 | −0.049 | 0.236 | |
| AGExWMHL Interaction | −0.122 | <0.001 | −0.091 | 0.006 | −0.062 | 0.059 | −0.048 | 0.139 | |
| HC | Intercept | 0.251 | <0.001 | 0.263 | <0.001 | 0.116 | 0.067 | 0.186 | 0.005 |
| AGE | −0.215 | <0.001 | −0.113 | 0.030 | −0.131 | 0.019 | −0.167 | 0.002 | |
| WMHL | −0.031 | 0.495 | −0.093 | 0.083 | −0.017 | 0.777 | −0.088 | 0.113 | |
| AGExWMHL Interaction | −0.047 | 0.180 | −0.043 | 0.330 | −0.087 | 0.072 | 0.011 | 0.816 | |
2.2. Procedures
All MR images were preprocessed using our standard pipeline (Aubert-Broche et al., 2013) in three steps: noise reduction, intensity non-uniformity correction, and intensity normalization. T2-weighted and FLAIR images were linearly co-registered to the T1-weighted images using a 6-parameter rigid registration. The T1-weighted images were first linearly and then nonlinearly registered to the standard template (MNI-ICBM-152). The WMHs were segmented using a previously validated automatic multi-modality segmentation technique in the native space of FLAIR or T2-weighted scans to avoid further blurring caused by resampling of the images (Dadar et al., 2017a; Dadar et al., 2017b). This technique uses a set of location and intensity features obtained from a library of manually segmented scans in combination with a random forest classifier to detect the WMHs in new images. The libraries used in this study were obtained from Alzheimer's Disease Neuroimaging Initiative (ADNI) dataset since the T2-weighted and FLAIR sequences for the PPMI images follow the same acquisition protocol as ADNI. The quality of the registrations and segmentations was visually assessed and cases that did not pass this quality control were discarded (n = 43). WMHL was defined as the volume (in cm3) of all segmented WMH voxels in the standard space, i.e. the WMH volumes were corrected for total intracranial volume (ICV). All MRI processing and segmentation steps were blinded to clinical outcomes.
For voxel-wise analysis of WMHs, the WMH probability maps generated by the segmentation tool were nonlinearly transformed to the template space at 2 × 2 × 2 mm3 resolution and blurred with a 3D Gaussian kernel with full width at half maximum of 5 mm to compensate for the variability caused by differences in voxel sizes in the native FLAIR and T2-weighted images. Rates of cognitive decline were calculated for subjects that had at least one-year follow-up information as the change of the score per year (NPD = 365, NHC = 174), using a linear regression between time and the score values at different time points along with an intercept term.
Only subjects with T1-weighted 3 T MRI data at both initial/baseline visit and at a one-year follow-up MRI were included for cortical thickness analysis (NTotal = 155, see Table 1). Cortical models were generated using the CIVET 2.1 preprocessing pipeline (Ad-Dab'bagh et al., 2006), registered to MNI-ICBM-152 template, and analyzed using the SurfStat software package (http://www.math.mcgill.ca/keith/surfstat/)(Yau et al., 2018). Distances between inner and outer cortical surfaces were evaluated to provide a measure of cortical thickness at each vertex. Changes in cortical thickness were calculated by subtracting the values (Δt = t1 − t2) at the one-year follow-up (t2) from the baseline (t1). The average time between the baseline and follow-up visits was 1.05 ± 0.11 and 1.05 ± 0.09 years for the PD and control subjects, respectively.
Table 1.
Descriptive statistics for the PPMI subjects enrolled in this study. Data are number of participants in each category (N), percentage of the total population (%), and mean (SD) of key variables. PPMI=Parkinson's Progression Marker Initiative. FLAIR = Fluid Attenuated Inversion Recovery. MoCA = Montreal Cognitive Assessment Score. HVLT = Hopkins Verbal Learning Test Revised Total Score. Benton = Benton Judgement of Line Orientation Score. WMH = White Matter Hyperintensity.
| Control | De novo PD | |
|---|---|---|
| Participants (NTotal) | 174 | 365 |
| Female (N) | 57 (33%) | 114 (32%) |
| T1-weighted and FLAIR Scans (NBaseline) | 79 (45%) | 167 (46%) |
| T1-weighted and T2-weighted Scans (NBaseline) | 95 (55%) | 198 (54%) |
| Follow-up T1-weighted scans (NFollow-up) | 55 (32%) | 100 (27%) |
| Age at Baseline (years) | 60.07 (±11.34) | 60.51 (±9.86) |
| MoCA at Baseline | 28.25 (±1.12) | 27.24 (±2.22) |
| HVLT at Baseline | 35.05 (±6.78) | 32.01 (±7.95) |
| Benton at Baseline | 26.13 (±4.12) | 25.60 (±4.07) |
| Executive Function at Baseline | 20.94 (±4.73) | 22.29 (±4.58) |
| WMH Load at Baseline (cm3) | 7.66 (±10.38) | 6.93 (±8.03) |
2.3. Statistical analysis
We tested two major hypotheses: 1. greater WMHL will lead to more extensive and faster decline in cognition of the PD patients, and 2. patients with a higher WMHL will show more cortical thinning in their follow-up visit after one year.
Survival analysis was used to investigate the relationship between WMH burden and decline in cognition. It has been previously shown that a threshold of WMHs should be present before cognitive deficits are observed (Price et al., 2012; Boone et al., 1992). The question of interest was whether there is a significant difference between the cognitive survival curves of subjects (normal controls and PD patients) with low versus high WMHL. The threshold for differentiating between high and low WMHL was set at 5 cm3 (median value, 0.7% of WM volume, 0.27% of brain volume). Similar to previous studies (Suministrado et al., 2017; Baracchini et al., 2012; Suzuki et al., 2015; Joana et al., 2013), a stable 2-point drop in MoCA (a drop that persists over the follow-up visits) was considered as the terminal event in the survival analysis and the time from baseline MoCA measurement to the visit where the 2-points drop was detected was considered as survival time. This was consistent with recommendations from our in-house clinical consultation. Drop in MoCA was selected as the main terminal event since MoCA has been previously validated as a sensitive measure for detecting and monitoring cognitive change over time (Costa et al., 2014) in general and MCI or dementia in PD specifically (Hoops et al., 2009). Robustness of the results was verified for a WMHL threshold of 10 cm3 and 1–4 points drop in MoCA. For survival analysis, the survdiff function from R package survival was used (ftp://centos.ustc.edu.cn/CRAN/web/packages/survival/survival.pdf). The function implements the two-sample Gρ statistics family of Harrington and Fleming, with weights on each event (2-point drop in MoCA) of S(t)ρ, where S(t) is the Kaplan-Meier estimate of survival, i.e. the probability that a subject survives longer than time t(Harrington & Fleming, 1982).
Furthermore, longitudinal mixed-effects models were used to assess the association of WMHs with changes in cognition. MoCA, Benton, HVLT, and executive function scores were used as measures of cognition (dependent variables). The log-transformed WMHLs and age at each timepoint were used as continuous predictors for either PD or control cohorts. All continuous variables were z-scored prior to the analysis. All models contained first order interactions with age. Subject and contrast used for segmentation (T2-weighted versus FLAIR) were considered as categorical random effects in all the models. A first order interaction between WMHL and cognitive status was also assessed, using the longitudinal cognitive status (based on Litvan's criteria) as the dependent variable, the log-transformed WMHLs and age at each timepoint as continuous predictors, and cohort (either PD or control) as a categorical variable. The mixed-effects model estimates are denoted by β in the results below, and are reported along with their corresponding p-values. Models were fitted using fitlme in MATLAB version R2015b.
Differences in cortical thickness between high and low WMHL classes [(highWMHLt1-highWMHLt2)-(lowWMHLt1-lowWMHLt2)] were analyzed statistically based on Gaussian random field theory with a threshold of p < 0.05 (Worsley et al., 1996). Similar to the survival analysis, the threshold for differentiating between high and low WMHL was 5 cm3. Observed differences in cortical thickness were then correlated to cognitive measures using Pearson partial correlations correcting for age.
3. Results
3.1. Baseline WMHL as a predictor of longitudinal cognition
Based on Litvan's criteria for cognitive status, all subjects were cognitively normal at baseline (Litvan et al., 2011). Baseline WMHL was not significantly different in control and PD populations (p > 0.05). Controlling for age, the rate of decline in MoCA score was significantly correlated with baseline WMHL (r = −0.145, p = 0.007) in the PD cohort, but not in controls (r = 0.045, p = 0.577). Similarly, controlling for age, longitudinal cognitive status (based on Litvan's criteria) was significantly associated with baseline WMHL (β = 0.216, p < 0.0001) in the PD cohort, but not in controls (β = 0.022, p-value = 0.344). In addition, we found a significant interaction between baseline WMHL and Cohort, suggesting greater cognitive decline in PDs with high WMHL (β = 0.210, p = 0.005). See supplementary materials for further information on baseline measures.
3.2. Survival analysis
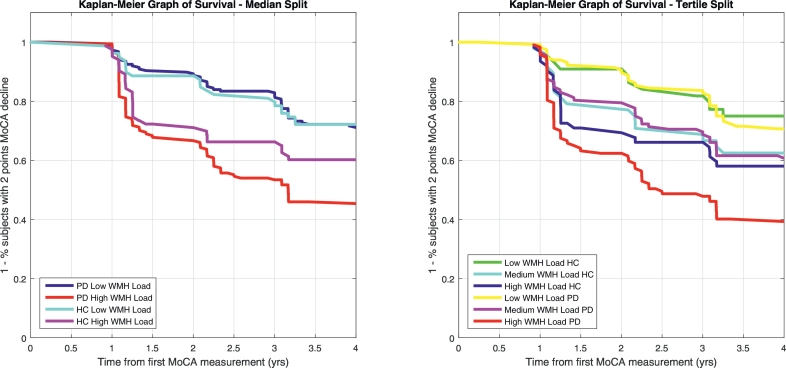
Survival analysis was used to investigate whether there is a significant difference between the cognitive survival curves of subjects (controls and PD patients) with low versus high WMHL – in other words, we used survival curves to measure the effect of baseline WMHL on cognitive decline (Fig. 1). A 2-point drop in MoCA was considered as the terminal event; the time from baseline MoCA measurement to the visit where a 2-points drop was detected was considered as survival time. The 4-year survival rate (i.e. rate of patients maintaining MoCA stability) for the low and high WMHL groups was 0.63 (95 CI = 0.55–0.70) and 0.37 (95 CI = 0.29–0.45) in PD subjects and 0.65 (95 CI = 0.52–0.75) and 0.56 (95 CI = 0.45–0.67) in controls, respectively (NPD-Low = 186, NPD-High = 174, NHC-Low = 79, NHC-High = 83). In PD, the high WMHL cohort had a significantly lower survival rate than the low WMHL cohort (χ2 = 30.9, p < 0.00001, hazard ratio = 2.42, 95 CI = 1.75–3.33). There was no high vs low difference in controls (χ2 = 2.5, p = 0.11, hazard ratio = 1.52, 95 CI = 0.91–2.55). Furthermore, PD patients showed significantly lower survival compared to controls in the high WMHL group (χ2 = 6.7, p = 0.009, hazard ratio = 1.58, 95 CI = 1.09–2.29) while the survival rate was not significantly different between two groups in low WMHL group (χ2 = 0.1, p = 0.8, hazard ratio = 1.0, 95 CI = 0.66–1.70). Similar results were obtained with a threshold of 10 cm3 to determine high/low WMHL groups, and with 1–4 points drop in MoCA, suggesting that WMHL-based dichotomization is sensitive to a range in the cognitive decline as measured by MoCA. Similarly, splitting the data into tertiles (low, medium, and high WMHL groups), we found consistent results, i.e. the high WMHL PD cohort had significantly greater cognitive decline than medium and low WMHL groups, and all normal control groups (p < 0.05).
Fig. 1.
Kaplan-Meier graph of survival showing the survival curves of control and PD patients with low versus high WMH loads (left, median split) and low, medium, and high WMH loads (right, tertile split) demonstrating the compounded effect of PD and WMH load. A 2-point drop in MoCA was considered as the survival event and the time from baseline MoCA measurement to the visit where the 2-point drop occurred was considered as survival time. HC=Healthy Control. PD = Parkinson's Disease. MoCA = Montreal Cognitive Assessment Score.
3.3. Mixed-effects modelling
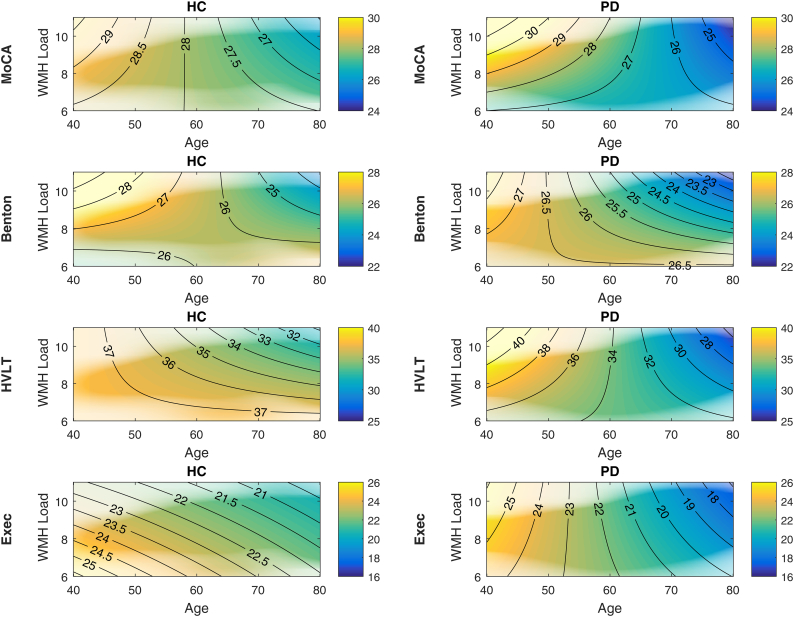
The results showed a significant negative relationship between MoCA, Benton, HVLT, and executive function scores and age in both PD and HC cohorts (Table 2, Fig. 2). More importantly, in the PD cohort, there was a significant interaction between Age and baseline WMHL for MoCA, Benton, and HVLT that was not observed in the HC cohort. The results did not change in terms of significance after including baseline cognitive scores as independent variables in the models (see Supplementary material, Section Mixed-Effects Models, Table S.3).
Fig. 2.
Density plots of longitudinal cognitive changes versus age and log transformed baseline WMH load. The colors indicate predicted cognitive scores by the mixed effects models, with warmer colors representing higher scores, and cooler colors representing lower scores. The transparency in the figures indicates the density of the data, i.e. areas of low transparency indicate regions where there are no subjects and the model is extrapolating (e.g. young subjects with high WMH loads, or old subjects with low WMH loads). The contour lines imply the direction of changes (i.e. horizontal orientation indicates predominance of age effects and vertical orientation indicates predominance of WHM load effects). WMH=White Matter Hyperintensities. HC = Healthy Control. PD = Parkinson's Disease. MoCA = Montreal Cognitive Assessment Score. HVLT = Hopkins Verbal Learning Test Revised Total Score. Benton = Benton Judgement of Line Orientation Score. Exec = Executive Function Score.
4. Cortical thickness
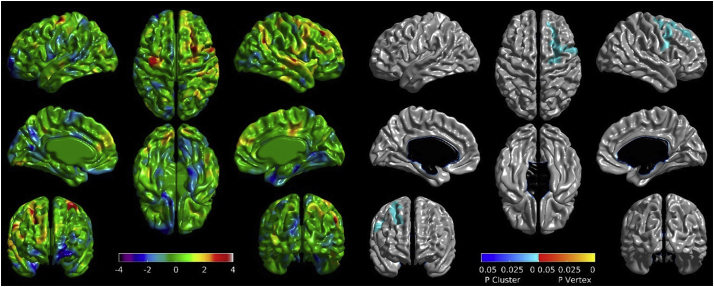
Mean whole-brain cortical thickness decreased significantly among PD patients with both low (t1 = 3.3177 mm ± 0.0993; t2 = 3.3087 mm ± 0.1082) and high (t1 = 3.2932 mm ± 0.0996; t2 = 3.2786 mm ± 0.0966) WMHL at baseline. Among PD patients, baseline WMHL did not correlate with whole-brain cortical thickness at baseline (r = −0.09, p > 0.05) or at one-year follow-up (r = −0.19, p > 0.05), but did correlate with cortical thickness change across the one-year period (r = 0.26, p = 0.01). When comparing high and low WMH groups in PD, cortical thinning was greater in the high WMH group with a significant cluster observed in the right frontal lobe (NVertices = 1523, resels = 7.99, p < 0.001) which covers the lateral precentral, superior frontal, and middle frontal gyri (Fig. 3). Cortical thinning of this cluster was not significantly correlated with poorer performance on the HVLT at baseline (r = −0.169, p > 0.05), but was correlated at one-year follow-up (r = −0.335, p < 0.001) and with declining performance over the one-year period (r = 0.196, p < 0.05). No significant correlations or vertex/cluster-wise differences were observed in the HC cohort. No significant correlation was observed between MoCA, Benton, and executive function with cortical thickness in PD cohort.
Fig. 3.
Differences in cortical thickness changes between high and low WMHL cohorts in PD subjects. T-maps (left) and areas of significant cortical thickness decreases (right) covering the precentral, superior frontal, and middle frontal gyri. WMHL = White Matter Hyperintensity Load. PD = Parkinson's Disease.
4.1. Voxel-wise analysis
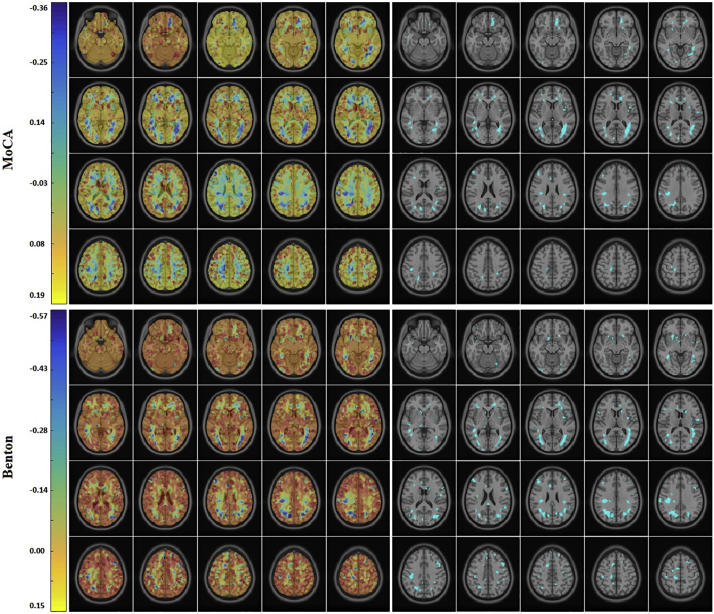
Within the PD cohort, significant voxel-wise correlations were observed between WMH localization maps and the slope of MoCA and Benton scores, corrected for multiple comparisons using false discovery rate (FDR) adjusted and controlled for age and modality (Fig. 4). The significant regions include voxels in all lobes: frontal, temporal, parietal, occipital, and insular subcortical WM bilaterally. No significant associations were found for the HC cohort. No significant associations were found for HVLT and Executive Function scores in the PD cohort. No significant differences were observed between the baseline voxel-wise WMH maps of PD and HC cohorts after FDR correction.
Fig. 4.
Correlation between WMH location and slope of MoCA (top) and Benton (bottom) score in the PD cohort, controlled for age and modality. Correlation coefficients (left) and thresholded areas of significant correlations after FDR correction. WMH=White Matter Hyperintensity. MoCA = Montreal Cognitive Assessment. PD = Parkinson's Disease. FDR = False Discovery Rate.
5. Discussion
High WMHL PD patients experienced significantly higher decline than i) low WMHL PD patients and ii) high WMHL control subjects. Additionally, WMHL at baseline was significantly associated with whole-brain cortical thinning after only one-year follow-up in PD patients, but not in controls. Moreover, PD patients with a high WMHL at baseline showed significant cortical thinning in the right frontal lobe compared to those with low WMHL. Taken together, these findings suggest that measures of WMHL in de novo PD patients can predict later cognitive decline, even in patients exhibiting no cognitive symptoms at baseline. This information could potentially be used to enrich PD cohorts in clinical trials of neuroprotective agents.
As with previous studies (Dalaker et al., 2009), cross-sectional WMHL at baseline in early PD was not significantly associated with baseline cognitive performance. Rather, WMHL at baseline was associated with future cognitive deterioration across multiple cognitive domains including visuospatial, memory, and global cognition, and this was independent of age. This suggests that we can extend previous work on later stages of PD, where WMH burden was significantly associated with conversion to dementia in patients with MCI (Sunwoo et al., 2014; Kandiah et al., 2014), to the earliest stages of the disease. In line with these findings, post-mortem studies have shown that vascular lesions are common in idiopathic PD (i.e., Lewy body disease of the brainstem type)(Jellinger, 2003).
MoCA has been validated as a sensitive measure for detecting and monitoring cognitive change over time (Costa et al., 2014). Controlling for age, MoCA decline was significantly correlated with baseline WMHL in the PD cohort, but not in controls. Additionally, PD subjects with high WMHLs were more likely to experience a 2-point drop in MoCA than i) the low WMHL PD and ii) the high WMHL HC subjects, as evaluated by the survival analysis. Repeating the analysis with a tertile split yielded consistent results (Fig. 1), supporting the robustness of the findings. The driver for cognitive decline in controls and PD appear to differ in that the former is largely driven by age, while the latter is affected by both advancing age and greater baseline WMHL.
While the literature on PD and WMH is scarce, there has been substantial progress in understanding the relationship between WMHs and cognitive impairment and dementia in AD, especially in the context of amyloid pathology. WMHs associated with vascular risk factors (e.g., hypoperfusion and inflammation) are thought to precede Aβ aggregation. Previous work found significant associations between baseline WMHs and later progression of amyloid load (Grimmer et al., 2012). This further supports the hypothesis of a chain of events; namely vascular pathology associated with WMHs impairs clearance of amyloid, which builds up and contributes to cognitive impairment and AD symptoms. While amyloid deposition strongly predicts progression to AD, WMH burden can provide additional independent information to this prediction (Provenzano et al., 2013), suggesting that WMH is not solely related to amyloid pathology, but can directly impact cognitive impairment. Interestingly, there has also been evidence linking low levels of amyloid beta in CSF, which is considered as a marker of parenchymal and small vessel deposition of this protein, and parieto-occipital WMH load in PD patients that experience cognitive decline, showing both an independent cross-sectional association between moderate-to-severe parieto-occipital WMHs, decrease in CSF amyloid beta levels and PD-dementia, as well as a longitudinal association between moderate-to-severe parieto-occipital WMHs and low CSF amyloid beta with progression to dementia (Compta et al., 2016). Whether a similar interaction between vascular lesions and α-synuclein formation or deposition occurs in PD remains unclear and will require further study.
WMH burden can also precede irreversible neurological damage as indexed by cortical atrophy. Previous studies have found higher WMHL to be correlated with lower cortical thickness in frontotemporal regions which in turn are related to cognitive decline (Tuladhar et al., 2015). Cortical thinning caused by direct or indirect effects of small vessel disease as indicated by WMHs (tract-specific damage) might lead to cognitive decline and eventually dementia. Cortical thickness might be a sensitive measurement to detect regional grey matter micro-changes that are missed by conventional voxel-based techniques at the earlier stages of the neurodegeneration due to partial volume effects (Hutton et al., 2009; Seo et al., 2012). While we observed whole-brain cortical thinning among all PD patients, those with high WMHL showed greater cortical thinning of a frontal cluster, mostly encompassing the right dorsolateral prefrontal cortex (rDLPFC) which was further associated with decline in memory performance in HVLT over the one-year period. This is consistent with previous studies that have found significant associations between rDLPFC and HVLT scores (Qiao et al., 2016; Ries et al., 2012). Our results suggest that cortical changes in early PD are potentially moderated by WMHL, and might in turn presage cognitive decline.
Prevention and treatment of vascular risk factors associated with WMHs is a promising avenue to slow down cognitive deterioration, especially in de novo PD patients who are largely cognitively asymptomatic. The classical and most explored strategy regarding reduction of vascular disease risk and WMHs has been to control hypertension, which subsequently reduces the risk of cognitive deterioration (de Leeuw et al., 2002; Debette & Markus, 2010; Dufouil et al., 2001). In a randomized trial, active lowering of blood pressure was shown to stop or lower the progression of WMHs in patients with cerebrovascular disease over 3 years of follow-up (Dufouil et al., 2005). In the present cohort, we observed an association between WMHL and (systolic-diastolic) blood pressure for both PDs and controls (p < 0.001). However, there is also evidence linking WMHs and dementia in PD to orthostatic hypotension, a common occurrence in PD which can be aggravated with anti-hypertensive medication, especially as the disease progresses (Oh et al., 2013). This further indicates the need for a tailored blood pressure management in PD patients, while extreme care should be taken to avoid overtreating hypertension. Finally, other small-vessel disease risk factors (some of which have been explored in the context of other pathologies, mainly AD, showing significant correlations with WMHs (Biesbroek et al., 2017; Veselỳ & Rektor, 2016)) should be further explored to assess their relevance in WMHs severity and cognitive decline in PD. More importantly, most of these factors are potentially modifiable: percentage of small dense LDL cholesterol, triglycerides level, body mass index, tobacco consumption, type II diabetes, and insulin levels. More studies should focus on assessment of these risk factors in the context of PD and its related WMHs.
From a practical standpoint, WMHs can be quantified reliably and non-invasively on large samples and can be measured as a continuous trait, thus providing increased statistical power to detect potential associations (Debette & Markus, 2010). The image processing and WMH segmentation pipelines used in this study have been designed to process data from multi-center studies, are able to control biases due to multi-site MRI scanning (i.e. differences in acquisition parameters), and have been previously applied successfully to a number of multi-site projects (Zeighami et al., 2015; Boucetta et al., 2016; Dadar et al., 2018; Dadar et al., 2018; Zeighami et al., 2017). The WMH segmentation pipeline has been trained and extensively validated on data from multiple scanners and different acquisition parameters to ensure inter-site and inter-scanner generalizability (Dadar et al., 2017b).
The ranges of the WMH loads found in this study for both control and de novo PD populations (mean value of 7 CCs = 0.37% of the total intracranial volume) were similar to values reported in other normal aging studies, suggesting that similar trends of decline can be expected in such populations as well (De Marco et al., 2017; Huang et al., 2018; Wiseman et al., n.d.; Zhang et al., 2018).
We acknowledge that there are limitations to the present study. First, while their differences were accounted for in our analysis, segmentations were based on either T2-weighted (N = 293) or FLAIR (N = 246) images, of which the latter has the better contrast for detecting WMHs. Second, subjects had these scans only at their baseline visit; therefore, we were not able to study the longitudinal changes of WMHL. Third, only one third of the subjects had follow-up T1w images (NHC = 55, NPD = 100) and were used in the cortical thickness analysis, reducing the power of the study. Future studies investigating WMHs in PD during prodromal and pre-clinical stages are warranted, though there are inherent constraints in recruiting such a cohort. Also, the population under study included relatively cognitively intact individuals (none of the subjects met criteria for dementia), limiting the ability to detect important contributors. Longer follow-ups might further increase the observed differences. One potential confounding factor could be PD medication. However, previous studies have found no significant difference between PD patients on PD medications and PD patients off medications in MoCA and several other cognitive tasks (Cools et al., 2006). Similarly, we found no relationship between MoCA and medication dosage in PD patients (see supplementary material, Medication Information). Another limitation is that we cannot identify the underlying mechanism. The WMHs might cause cognitive decline independent of PD, however the synergy between the two mechanisms may accelerate the cognitive decline. Alternatively, the WMHs might aggravate the pathologic spread of misfolded α-synuclein proteins in PD. Another possibility is that WMHs in PD may promote amyloid propagation, similar to AD.
Correlations with clinical cognitive measures are generally small, particularly in populations where individuals don't have significant cognitive deficits. In PPMI, all subjects were cognitively normal at baseline (individuals with cognitive deficit were excluded from the study) and exhibited relatively modest decline in comparison with populations at late stage dementia. Other studies have similarly found modest associations between WMHs and cognitive performance in normal aging (for a review, see (Gunning-Dixon & Raz, 2000)). Specifically in PPMI where PD subjects are de novo, other studies have also found only weak associations between different variables and cognition in different domains, after adjusting for age (Yau et al., 2018; Siepel et al., 2018). While some of the correlation coefficients in the present study may be considered modest, they nonetheless hold important clinical implications.
In conclusion, our findings suggest that WMH burden is an important predictor of subsequent acceleration in cortical thinning and cognitive decline in early-stage de novo PD. Recognizing WMHs as early indicators of cognitive deficit, prior to onset of MCI or dementia, provides an opportunity for timely interventions (Marek et al., 2011; Zeighami et al., 2015).
Acknowledgments
Acknowledgement
We would like to acknowledge funding from the Famille Louise & André Charron. Ms. Yau is a Vanier Scholar and receives funding from the Canadian Institute of Health Research. This work was also supported by grants from the Canadian Institutes of Health Research (MOP-111169), les Fonds de Research Santé Québec Pfizer Innovation fund, an NSERC CREATE grant (4140438 - 2012), the Levesque Foundation, the Douglas Hospital Research Centre and Foundation, the Government of Canada, the Canada Fund for Innovation, the Michael J. Fox Foundation and Weston Brain Institute.
Data used in this article were obtained from the Parkinson's Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org. PPMI is sponsored and partially funded by the Michael J Fox Foundation for Parkinsons Research and funding partners, including AbbVie, Avid Radiopharmaceuticals, Biogen, Bristol-Myers Squibb, Covance, GE Healthcare, Genentech, GlaxoSmithKline (GSK), Eli Lilly and Company, Lundbeck, Merck, Meso Scale Discovery (MSD), Pfizer, Piramal Imaging, Roche, Servier, and UCB (www.ppmi-info.org/fundingpartners). MD and DLC had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author contributions
Mahsa Dadar (MSc): Study concept and design, analysis and interpretation of the data and drafting and revising the manuscript.
Yashar Zeighami (MSc): Study concept and design, analysis and interpretation of the data and revising the manuscript.
Yvonne Yau (MSc): Analysis and interpretation of the data, drafting and revising the manuscript.
Seyed-Mohammad Fereshtehnejad (PhD): Study concept and design, interpretation of the data, drafting and revising the manuscript.
Josefina Maranzano (MD): Interpretation of the data, drafting and revising the manuscript.
Ronald Postuma (MD): Interpretation of the data and revising the manuscript.
Alain Dagher (MD): Study concept and design, interpretation of the data and revising the manuscript.
D. Louis Collins (PhD): Study concept and design, interpretation of the data, drafting and revising the manuscript.
Conflicts of interests
MD, YZ, YY, JM, and AD have no conflicts of interest to report. SMF reports grants from Richard and Edith Strauss Postdoctoral Fellowship, grants from Preston Robb Fellowship, outside the submitted work. RP reports grants and personal fees from Fonds de la Recherche en Sante, grants from Canadian Institute of Health Research, grants from The Parkinson Society of Canada, grants from Weston-Garfield Foundation, grants from Michael J. Fox Foundation, grants from Webster Foundation, personal fees from Biotie, personal fees from Roche/Prothena, personal fees from Teva Neurosciences, personal fees from Novartis Canada, personal fees from Biogen, personal fees from Boehringer Ingelheim, outside the submitted work. DLC reports grants from Canadian Institutes of Health Research, grants from National Science and Engineering Research Council, grants from Canadian foundation for Innovation, other from NeuroRx Inc., other from Truepositive Medical Devices, during the conduct of the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.09.025.
Contributor Information
Mahsa Dadar, Email: mahsa.dadar@mail.mcgill.ca.
Yashar Zeighami, Email: yashar.zeighami@mail.mcgill.ca.
Yvonne Yau, Email: yvonne.yau@mail.mcgill.ca.
Seyed-Mohammad Fereshtehnejad, Email: sm.fereshtehnejad@mail.mcgill.ca.
Josefina Maranzano, Email: jmaranzano@mrs.mni.mcgill.ca.
Ronald B. Postuma, Email: ron.postuma@muhc.mcgill.ca.
Alain Dagher, Email: alain.dagher@mcgill.ca.
D. Louis Collins, Email: louis.collins@mcgill.ca.
Appendix A. Supplementary data
Supplementary material
References
- Ad-Dab'bagh Y. Proceedings of the 12th Annual Meeting of the Organization for Human Brain Mapping. Florence; Italy: 2006. The CIVET image-processing environment: a fully automated comprehensive pipeline for anatomical neuroimaging research; p. 2266. [Google Scholar]
- Anang J.B. Predictors of dementia in Parkinson disease a prospective cohort study. Neurology. 2014;83:1253–1260. doi: 10.1212/WNL.0000000000000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert-Broche B. A new method for structural volume analysis of longitudinal brain MRI data and its application in studying the growth trajectories of anatomical brain structures in childhood. NeuroImage. 2013;82:393–402. doi: 10.1016/j.neuroimage.2013.05.065. [DOI] [PubMed] [Google Scholar]
- Auning E. White matter integrity and cognition in Parkinson's disease: a cross-sectional study. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2013-003976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baracchini C. Carotid endarterectomy protects elderly patients from cognitive decline: a prospective study. Surgery. 2012;151:99–106. doi: 10.1016/j.surg.2011.06.031. [DOI] [PubMed] [Google Scholar]
- Biesbroek J.M., Weaver N.A., Biessels G.J. Lesion location and cognitive impact of cerebral small vessel disease. Clin. Sci. 2017;131:715–728. doi: 10.1042/CS20160452. [DOI] [PubMed] [Google Scholar]
- Boone K.B. Neuropsychological correlates of white-matter lesions in healthy elderly subjects: a threshold effect. Arch. Neurol. 1992;49:549–554. doi: 10.1001/archneur.1992.00530290141024. [DOI] [PubMed] [Google Scholar]
- Boucetta S. Structural brain alterations associated with rapid eye movement sleep behavior disorder in Parkinson's disease. Sci. Rep. 2016;6 doi: 10.1038/srep26782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R.C., Shum D., Toulopoulou T., Chen E.Y. Assessment of executive functions: review of instruments and identification of critical issues. Arch. Clin. Neuropsychol. 2008;23:201–216. doi: 10.1016/j.acn.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Compta Y. Lewy- and Alzheimer-type pathologies in Parkinson's disease dementia: which is more important? Brain. 2011;134:1493–1505. doi: 10.1093/brain/awr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compta Y. White matter hyperintensities, cerebrospinal amyloid-β and dementia in Parkinson's disease. J. Neurol. Sci. 2016;367:284–290. doi: 10.1016/j.jns.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Cools R., Altamirano L., D'Esposito M. Reversal learning in Parkinson's disease depends on medication status and outcome valence. Neuropsychologia. 2006;44:1663–1673. doi: 10.1016/j.neuropsychologia.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Costa A.S. Evidence of the sensitivity of the MoCA alternate forms in monitoring cognitive change in early Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 2014;37:95–103. doi: 10.1159/000351864. [DOI] [PubMed] [Google Scholar]
- Dadar M. Validation of a Regression Technique for Segmentation of White Matter Hyperintensities in Alzheimer's Disease. IEEE Trans. Med. Imaging. 2017 doi: 10.1109/TMI.2017.2693978. [DOI] [PubMed] [Google Scholar]
- Dadar M. Performance comparison of 10 different classification techniques in segmenting white matter hyperintensities in aging. NeuroImage. 2017;157:233–249. doi: 10.1016/j.neuroimage.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadar M., Maranzano J., Ducharme S., Carmichael O.T., Decarli C., Collins D.L., Initiative Alzheimers.Disease Neuroimaging. Validation of T 1w-based segmentations of white matter hyperintensity volumes in large-scale datasets of aging. Human brain mapping. 2018;39(3):1093–1107. doi: 10.1002/hbm.23894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadar M., Fonov V.S., Collins D.L., Initiative A.D.N. A comparison of publicly available linear MRI stereotaxic registration techniques. NeuroImage. 2018;174:191–200. doi: 10.1016/j.neuroimage.2018.03.025. [DOI] [PubMed] [Google Scholar]
- Dalaker T.O. White matter hyperintensities do not impact cognitive function in patients with newly diagnosed Parkinson's disease. NeuroImage. 2009;47:2083–2089. doi: 10.1016/j.neuroimage.2009.06.020. [DOI] [PubMed] [Google Scholar]
- de Lau L.M., Schipper C.M.A., Hofman A., Koudstaal P.J., Breteler M.M. Prognosis of Parkinson disease: risk of dementia and mortality: the Rotterdam Study. Arch. Neurol. 2005;62:1265–1269. doi: 10.1001/archneur.62.8.1265. [DOI] [PubMed] [Google Scholar]
- de Leeuw F.-E. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125:765–772. doi: 10.1093/brain/awf077. [DOI] [PubMed] [Google Scholar]
- De Marco M., Manca R., Mitolo M., Venneri A. White matter hyperintensity load modulates brain morphometry and brain connectivity in healthy adults: a neuroplastic mechanism? Neural Plast. 2017;2017 doi: 10.1155/2017/4050536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S., Markus H.S. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- Dufouil C. Longitudinal study of blood pressure and white matter hyperintensities the EVA MRI Cohort. Neurology. 2001;56:921–926. doi: 10.1212/wnl.56.7.921. [DOI] [PubMed] [Google Scholar]
- Dufouil C. Effects of Blood pressure Lowering on Cerebral White Matter Hyperintensities in patients with Stroke. Circulation. 2005;112:1644–1650. doi: 10.1161/CIRCULATIONAHA.104.501163. [DOI] [PubMed] [Google Scholar]
- Grimmer T. White matter hyperintensities predict amyloid increase in Alzheimer's disease. Neurobiol. Aging. 2012;33:2766–2773. doi: 10.1016/j.neurobiolaging.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon F.M., Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14:224. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Hachinski V.C., Potter P., Merskey H. Leuko-Araiosis. Arch. Neurol. 1987;44:21–23. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- Halliday G.M., Leverenz J.B., Schneider J.S., Adler C.H. The neurobiological basis of cognitive impairment in Parkinson's disease. Mov. Disord. 2014;29:634–650. doi: 10.1002/mds.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington D.P., Fleming T.R. A class of rank test procedures for censored survival data. Biometrika. 1982:553–566. [Google Scholar]
- Hawkins K.A. Hyperinsulinemia and elevated systolic blood pressure independently predict white matter hyperintensities with associated cognitive decrement in the middle-aged offspring of dementia patients. Metab. Brain Dis. 2017:1–9. doi: 10.1007/s11011-017-9980-9. [DOI] [PubMed] [Google Scholar]
- Hely M.A., Reid W.G., Adena M.A., Halliday G.M., Morris J.G. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov. Disord. 2008;23:837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- Hoops S. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73:1738–1745. doi: 10.1212/WNL.0b013e3181c34b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.-C. Nonlinear pattern of the emergence of white matter hyperintensity in healthy Han Chinese: an adult lifespan study. Neurobiol. Aging. 2018;67:99–107. doi: 10.1016/j.neurobiolaging.2018.03.012. [DOI] [PubMed] [Google Scholar]
- Hutton C., Draganski B., Ashburner J., Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. NeuroImage. 2009;48:371–380. doi: 10.1016/j.neuroimage.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin D.J. Neuropathologic substrates of Parkinson disease dementia. Ann. Neurol. 2012;72:587–598. doi: 10.1002/ana.23659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger K.A. Prevalence of cerebrovascular lesions in Parkinson's disease. A postmortem study. Acta Neuropathol. (Berl.) 2003;105:415–419. doi: 10.1007/s00401-003-0676-3. [DOI] [PubMed] [Google Scholar]
- Joana B., Fonseca M.J., Moreira A., Abelha F. Quality of life in patients with cognitive decline after major surgery: 17AP2-9. Eur. J. Anaesthesiol. EJA. 2013;30:239. [Google Scholar]
- Jones J.D., Tanner J.J., Okun M., Price C.C., Bowers D. Are Parkinson's patients more vulnerable to the effects of cardiovascular risk: a neuroimaging and neuropsychological study. J. Int. Neuropsychol. Soc. 2017;23:322–331. doi: 10.1017/S1355617717000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandiah N. Hippocampal volume and white matter disease in the prediction of dementia in Parkinson's disease. Parkinsonism Relat. Disord. 2014;20:1203–1208. doi: 10.1016/j.parkreldis.2014.08.024. [DOI] [PubMed] [Google Scholar]
- Litvan I. MDS task force on mild cognitive impairment in Parkinson's disease: critical review of PD-MCI. Mov. Disord. 2011;26:1814–1824. doi: 10.1002/mds.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak E. White matter hyperintensities and mild cognitive impairment in Parkinson's disease. J. Neuroimaging. 2015;25:754–760. doi: 10.1111/jon.12230. [DOI] [PubMed] [Google Scholar]
- Marek K. The Parkinson Progression Marker Initiative (PPMI) Prog. Neurobiol. 2011;95:629–635. doi: 10.1016/j.pneurobio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino J.G., Hachinski V. Leukoaraiosis: reifying rarefaction. Arch. Neurol. 2000;57:925–926. doi: 10.1001/archneur.57.7.925. [DOI] [PubMed] [Google Scholar]
- Oh Y.-S., Kim J.-S., Lee K.-S. Orthostatic and supine blood pressures are associated with white matter hyperintensities in Parkinson disease. J. Mov. Disord. 2013;6:23–27. doi: 10.14802/jmd.13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- Pantoni L., Garcia J.H. Pathogenesis of leukoaraiosis. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- Pedersen K.F., Larsen J.P., Tysnes O.-B., Alves G. Natural course of mild cognitive impairment in Parkinson disease a 5-year population-based study. Neurology. 2017;10:1212. doi: 10.1212/WNL.0000000000003634. [DOI] [PubMed] [Google Scholar]
- Poletti M. Mild cognitive impairment and cognitive-motor relationships in newly diagnosed drug-naive patients with Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 2012:2011. doi: 10.1136/jnnp-2011-301874. [DOI] [PubMed] [Google Scholar]
- Price C.C. MRI-leukoaraiosis thresholds and the phenotypic expression of dementia. Neurology. 2012;79:734–740. doi: 10.1212/WNL.0b013e3182661ef6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano F.A. White matter hyperintensities and cerebral amyloidosis: necessary and sufficient for clinical expression of Alzheimer disease? JAMA Neurol. 2013;70:455–461. doi: 10.1001/jamaneurol.2013.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J. The positive effects of high-frequency right dorsolateral prefrontal cortex repetitive transcranial magnetic stimulation on memory, correlated with increases in brain metabolites detected by proton magnetic resonance spectroscopy in recently detoxified alcohol-dependent patients. Neuropsychiatr. Dis. Treat. 2016;12:2273–2278. doi: 10.2147/NDT.S106266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries M.L. Medial prefrontal functional connectivity—Relation to memory self-appraisal accuracy in older adults with and without memory disorders. Neuropsychologia. 2012;50:603–611. doi: 10.1016/j.neuropsychologia.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S.W. Cortical thinning related to periventricular and deep white matter hyperintensities. Neurobiol. Aging. 2012;33 doi: 10.1016/j.neurobiolaging.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Siepel F.J. Cognitive executive impairment and dopaminergic deficits in de novo Parkinson's disease. Mov. Disord. 2018;29:1802–1808. doi: 10.1002/mds.26051. [DOI] [PubMed] [Google Scholar]
- Suministrado M.S.P. Poststroke cognitive decline is independent of longitudinal changes in cerebral hemodynamics parameters. J. Neuroimaging. 2017;27:326–332. doi: 10.1111/jon.12395. [DOI] [PubMed] [Google Scholar]
- Sunwoo M.K. The burden of white matter hyperintensities is a predictor of progressive mild cognitive impairment in patients with Parkinson's disease. Eur. J. Neurol. 2014;21 doi: 10.1111/ene.12412. [DOI] [PubMed] [Google Scholar]
- Suzuki H. One-Year change in the Japanese version of the Montreal cognitive assessment performance and related predictors in community-dwelling older adults. J. Am. Geriatr. Soc. 2015;63:1874–1879. doi: 10.1111/jgs.13595. [DOI] [PubMed] [Google Scholar]
- Tosto G., Zimmerman M.E., Carmichael O.T., Brickman A.M. Predicting aggressive decline in mild cognitive impairment: the importance of white matter hyperintensities. JAMA Neurol. 2014;71:872–877. doi: 10.1001/jamaneurol.2014.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuladhar A.M. Relationship between white matter hyperintensities, cortical thickness, and cognition. Stroke. 2015;46:425–432. doi: 10.1161/STROKEAHA.114.007146. [DOI] [PubMed] [Google Scholar]
- Veselỳ B., Rektor I. The contribution of white matter lesions (WML) to Parkinson's disease cognitive impairment symptoms: a critical review of the literature. Parkinsonism Relat. Disord. 2016;22:S166–S170. doi: 10.1016/j.parkreldis.2015.09.019. [DOI] [PubMed] [Google Scholar]
- Wiseman, S. J. et al. Cognitive abilities, brain white matter hyperintensity volume, and structural network connectivity in older age. Hum. Brain Mapp. 39, 622–632. [DOI] [PMC free article] [PubMed]
- Worsley K.J. A unified statistical approach for determining significant signals in images of cerebral activation. Hum. Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Yau Y. Network connectivity determines cortical thinning in early Parkinson's disease progression. Nat. Commun. 2018;9:12. doi: 10.1038/s41467-017-02416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeighami Y. Network structure of brain atrophy in de novo Parkinson's disease. elife. 2015;4 doi: 10.7554/eLife.08440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeighami Y. A clinical-anatomical signature of Parkinson's Disease identified with partial least squares and magnetic resonance imaging. NeuroImage. 2017 doi: 10.1016/j.neuroimage.2017.12.050. https://www.sciencedirect.com/science/article/pii/S1053811917310741 [DOI] [PubMed] [Google Scholar]
- Zhang C.E. Blood–brain barrier leakage in relation to white matter hyperintensity volume and cognition in small vessel disease and normal aging. Brain Imaging Behav. 2018:1–7. doi: 10.1007/s11682-018-9855-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material