Highlights
-
•
The study of the relationship between function and structure of the brain is interesting in multiple sclerosis patients.
-
•
Different relationship between amplitude of low frequency fluctuations (ALFF) and gray matter volume has been detected.
-
•
This difference may be associated with the presence of white matter lesions involving specific tracts.
Keywords: Multiple sclerosis, Mirror neuron system, Amplitude of low frequency fluctuations, Voxel based morphometry
Abstract
The study of the relationship between function and structure of the brain could be particularly interesting in neurodegenerative diseases like multiple sclerosis (MS).
The aim of the present work is to identify differences of the amplitude of low frequency fluctuations (ALFF) in the mirror neuron system (MNS) between MS patients and healthy controls and to study the relationship between ALFF and the gray matter volume (GMV) of the regions that belong to the MNS.
Relapsing-remitting MS patients with minor disability were compared to healthy controls (HC) using resting-state functional magnetic resonance imaging (fMRI), anatomic T1 weighted images and diffusion tensor imaging (DTI). Region of interest (ROI) analyses was performed in the MNS regions.
A decrease of ALFF in MS patients was observed in the left inferior frontal gyrus (IFG). Furthermore, a correlation between ALFF in the IFG and the GMV of the left inferior parietal lobule (IPL) was identified. This relationship was different for MS patients than for HC, which may be associated with changes in diffusivity measures which were impaired in MS patients.
MS patients with low disability may show ALFF differences in the MNS without clinical correspondence. This functional difference may be associated with cortical and subcortical changes related to the disease.
Introduction
Multiple sclerosis (MS) is a chronic neurodegenerative disease associated with the development of localized inflammatory immuno-mediated processes in the central nervous system (Lutton et al., 2004). There are different forms of the disease, but the most frequent one is the relapsing-remitting form (RR), which consists of periods of disease-related activity combined with periods of no evident activity. The relapses may lead to a neurological impairment of MS patients, with motor and sensory disturbances as the main symptoms (Lutton et al., 2004). Cognitive dysfunction may also appear in the course of the disease. In fact, more than 65% of MS patients may present an impairment in at least one cognitive domain (Amato et al., 2006). Memory, attention and executive functions are the most frequently affected (Achiron and Barak, 2003; Olivares et al., 2005; Panou et al., 2012), but visuoperceptive and visuospatial dysfunction may also be present. The presence of visuospatial/visuoperceptive dysfunction has been associated with progressive forms of MS (Gaudino et al., 2001) and other research considered this dysfunction as a diffuse brain damage marker (Haase et al., 2003) and may be the consequence of the loss of cortico-subcortical connections integrating visual and spatial information. However, recent findings suggest that a neural network involved in visuospatial/visuoperceptive functions (i.e., the mirror neuron system) may be altered prior to the development of clinical symptoms (Plata-Bello et al., 2017).
The mirror neuron system (MNS) is a brain network distributed in the frontal and parietal lobes and mainly implicated in action understanding and social cognition (Cattaneo and Rizzolatti, 2009). Visuospatial/visuoperceptive functions are a consequence of a dual activation of this network: when an action is performed as well as when the same action is observed, which is the so-called mirror mechanism (Cattaneo and Rizzolatti, 2009; Rizzolatti and Craighero, 2004). The core regions of the MNS are located in the inferior frontal gyrus (IFG) and the inferior parietal lobule (IPL) bilateral (Molenberghs et al., 2012). The dysfunction of the MNS has been associated with an impairment in the imitation of a gesture, the recognition of a correctly performed gesture and the comprehension of a gesture’s meaning (Binder et al., 2017). These functions are closely linked with visuospatial and visuoperceptive abilities. In fact, an impairment of visuospatial/visuoperceptive functions are associated with an increase of the activity in the MNS (Plata-Bello et al., 2016a).
This brain network has been studied using different neurophysiological and neuroimaging techniques, but the most common method is functional magnetic resonance imaging (fMRI). In any case, the study of the MNS may be difficult in the clinical setting; on the one hand, execution and observation paradigms are needed to show the mirror mechanism and this is associated with longer scan periods; on the other hand, each paradigm limits the information about the functioning of the MNS to specific conditions (i.e., those included in the paradigm). Keeping these barriers in mind, the use of resting-state approaches has facilitated the study of the MNS with clinical purposes (Plata-Bello et al., 2016b), because we can obtain functional information of this network without any task, thus no additional cooperation of the patient.
Resting state fMRI (rs-fMRI) is based on the presence of spontaneous low-frequency (0.01 – 0.08 Hz) fluctuations in blood oxygen level dependent (BOLD) signal which are synchronized within different brain networks (Biswal et al., 1995; Lowe et al., 1998; Cordes et al., 2000; Greicius et al., 2003). Zang et al. (2007) developed an index, the amplitude of low-frequency fluctuations (ALFFs), in which the square root of the power spectrum was integrated in a low frequency range for detecting the regional intensity of spontaneous fluctuations in BOLD signal (Zang et al., 2007). In other words, the ALFF measurement allows the detection of the intensity of spontaneous fluctuations, defined as the total power in the low frequency range (Zhang et al., 2010). The ALFF can be considered a direct measure of functional activity from a specific brain region (Nugent et al., 2015; Aiello et al., 2015) and this signal is closely related to spontaneous neural activities (Lu et al., 2007; Mantini et al., 2007).
ALFF has been used to study MS patients using whole-brain approaches (i.e., no specific brain network has been explored using this approach). On the one hand, some works have reported an increase of ALFF in anterior cingulate (Liu et al., 2016) and basal ganglia (Liu et al., 2016, 2011; Zhou et al., 2014) and fronto-temporal regions (Liu et al., 2011) in relapsing-remitting (RR) MS patients. On the other hand, a decrease in ALFF was described in parietal regions of clinically isolated syndrome patients (the earliest stage of MS) (Liu et al., 2012) and in the right middle temporal gyrus, left middle frontal gyrus, lingual gyrus and cuneus of MS patients with spinal cord involvement (Liu et al., 2015). None of these studies described an increase or decrease of ALFF in MNS regions, but they were not specifically studied (for example, using a region of interest analysis). In fact, there is no work to date that has focused on the study of the MNS using ALFF. This may be useful for understanding the functioning of this brain network and, in the context of a disease-based study, it offers the possibility to identify new biomarkers that might allow us to predict the evolution of the neurological impairment.
Another interesting aspect is the study of the factors that may modulate ALFF. Bearing in mind that ALFF represents the spontaneous neural activity of the cortex, one can consider that the volume of gray matter might influence the ALFF measure. A limited number of studies have dealt with this question. Zhao et al. (2015) identified a correlation between gray matter volume (GMV) and ALFF in the right superior parietal lobule of amnestic-type mild cognitive impairment patients (Zhao et al., 2015); and Han et al. (2012) did not find any relationship between ALFF and GMV in this kind of patient (Han et al., 2012). Thus, there is no clear evidence of the existence of any relationship between ALFF and GMV. This may be particularly interesting in MS patients, because one of the pathophysiological events in MS is the decrease of GMV in specific regions and this is associated with the development of cognitive impairment (Grothe et al., 2016). An abnormal relationship between function (i.e., ALFF) and structure (GMV) might precede clinical manifestations and this could be studied as an early biomarker of disease. The identification of early biomarkers is essential in neurodegenerative disease, because it may allow the application of appropriate treatments to prevent the development of clinical impairment (e.g., early rehabilitation programs).
Regarding all the data described above, the aim of the present work is to identify differences in ALFF in the MNS between a cohort of MS patients with low disability (where early biomarkers would be most useful) and healthy controls. Moreover, the aim is to determine differences in the GMV of the regions that belong to the MNS and to test whether these volumes are correlated with ALFF within this brain network. We hypothesized that MS patients will show differences in ALFF maps compared to healthy controls. Furthermore, we will expect to find differences in GMV in MNS regions and altered relationship between ALFF and GMV in MNS regions, between MS patients and healthy controls.
Methods
Subjects
Twenty-four MS patients (mean age: 38.9 [SD = 11.39] years old; 16 women) and 15 healthy controls (mean age: 44.2 [SD = 10.04] years old; 7 women) were included in the study. All participants were right handed (Edinburgh Handedness Inventory (Oldfield, 1971) < 25). Part of the data from these patients was used in a previous publication where the functional connectivity of the MNS was studied in low-disability MS patients (Plata-Bello et al., 2017). In brief, the criteria for selecting the low-disability MS patients were: 1) A diagnosis of relapsing-remitting clinically-definite MS following the McDonald criteria (Polman et al., 2011); 2) Age between 18 and 50 years old; 3) The absence of steroid treatment in the last 6 months; 4) No relapse in the previous six months; 5) No history of optic neuritis; 6) No other neurological or psychiatric disease (including substance abuse); 7) The absence of severe impairment in memory or other cognitive functions assessed with the “Brief Repeatable Neuropsychological Battery”(Rao et al., 1991)
Patients were screened for eligibility in the MS monographic consultation and healthy controls were selected among non-first degree relatives or from the University and Hospital staff. Written informed consent was explained and signed by the patients and the control subjects. The study was approved by the University of La Laguna Ethics Committee, according to the Declaration of Helsinki. Demographic and clinical data is summed in Table 1. Lesion load was calculated by counting the number of lesions in each patient in coronal T2 weighted FLAIR images.
Table 1.
Clinical features and neuropsychological assessment results of the MS patients and healthy controls.
| MS patients (n = 24) |
Healthy controls (n = 15) |
p-value | |
|---|---|---|---|
| Age (years)a | 38.9 (SD = 11.39) | 44.2 (SD = 10.04) | 0.125 |
| Gender (male:female)b | 8:16 | 8:7 | 0.309 |
| EDSS (expanded disability status scale) | Median = 2.0 (range = 1.0–7.0) | – | – |
| Time from diagnosis (years) | 8.37 (SD = 6.37) | – | – |
| Mean lesion load (n) | 36.2 (range = 5–101) | – | – |
| Current Treatment | |||
|
Interferon beta 1 Natalizumab Teriflunomide Alemtuzumab Fingolimod None |
11 (46%) 1 (4%) 3 (13%) 4 (17%) 3 (13%) 2 (8%) |
– | – |
SD = Standard deviation.
Continuous variables were compared with the Mann Whitney U test.
Proportions were compared with the chi-square test.
A neuropsychological evaluation was performed by a specialist in neuropsychology (YPM). Visuoperceptive functions were evaluated with the Hooper organization visual test (HOVT) (Hooper, 1983) and the facial recognition test (FRT) (Benton and Hamsher KdeS VN and SO, 1983; Dricker et al., 1978); while visuospatial functions were evaluated with the judgement line orientation test (JLOT) (Benton and Hamsher KdeS VN and SO, 1983). No participant presented a pathological score in either test, although seven MS patients presented a borderline score in at least one of the three tests.
Data acquisition and processing
Data for the experiment were collected at the Magnetic Resonance for Biomedical Research Service of the University of La Laguna.
Resting-state fMRI images were obtained on a 3 T General Electric (Milwaukee, WI, USA) scanner using an echo-planar imaging gradient-echo sequence and an 8 channel head coil (TR = 2000 ms, TE = 22.1 ms, flip angle = 90◦, matrix size = 64 × 64 pixels, 36 slices/volume, interslice gap = 1 mm, slice thickness = 4 mm). The slices were aligned to the anterior commissure – posterior commissure line and covered the whole cranium.
A whole-brain three-dimensional high-resolution T1-weighted MRI was acquired for anatomical reference and voxel based morphometry analysis. A 3D fast spoiled gradient – recalled pulse sequence was obtained with the following acquisition parameters: TR = 8.8 ms, TE = 1.7 ms, flip angle = 10°, matrix size = 256 × 256 pixels, slice thickness = 1 mm.
Finally, a diffusion-weighted Spin-Echo EPI sequence was used. A DTI diffusion scheme was used and a total of 55 diffusion sampling directions were acquired. The b-value was 1500s/mm2. The in-plane resolution was 2.3984 mm. The slice thickness was 2.4 mm.
After checking the images for artefacts, resting-state data were preprocessed using Statistical Parametric Mapping software SPM8 (Wellcome Trust Centre for Neuroimaging; http://www.fil.ion.ucl.ac.uk/spm/). The images were spatially realigned, unwarped, and normalized to the Montreal Neurological Institute (MNI) space using standard SPM8 procedures. The normalized images of 2 × 2 × 2 mm were smoothed by a full width at half maximum (FWHM) 6 × 6 × 6 Gaussian kernel. Furthermore, the first 10 images were discarded to remove signal equilibration effects. After that, the source of spurious variance was removed by linear regression by including the signal from the ventricular system, the white matter and the global signal, in addition to the six parameters obtained by rigid body head motion correction.
The CAT12 toolbox (Structural Brain Mapping group, Jena University Hospital, Germany) implemented in SPM12 was used for voxel-based morphometry (VBM) analysis. All T1- weighted images were corrected for bias – field inhomogeneities, then spatially normalized using the DARTEL algorithm (Ashburner, 2007) and segmented into gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) (Ashburner and Friston, 2005). The sum of the volumes of these segmented structures was considered as the total intracranial volume (TIV). All images were manually inspected and corrected when it was necessary.
ALFF analysis
ALFF analysis was performed using the Resting State fMRI Data Analysis Toolkit (REST) version 1.8 (Song et al., 2011). The signal was linearly detrended and a temporal band-pass filter was applied (0.01 Hz < f < 0.08 Hz). The filtered time series was transformed to a frequency domain with a fast Fourier transform (FFT) and the power spectrum was then obtained. The square root was calculated at each frequency of the power spectrum and the averaged square root was obtained across 0.01-0.08 Hz at each voxel.
Afterwards, a two-sample t-test was performed on the ALFF maps for comparing MS patients and healthy controls. Age and gender were also included as regressors of no interest to reduce the variance unrelated to the variable of interest.
The statistical significance threshold was set at P < 0.001 with a cluster size of 18 voxels, using the REST AlphaSim (Song et al., 2011), which corresponded to a corrected P < 0.01.
Regions of interest (ROIs) analysis in ALFF maps
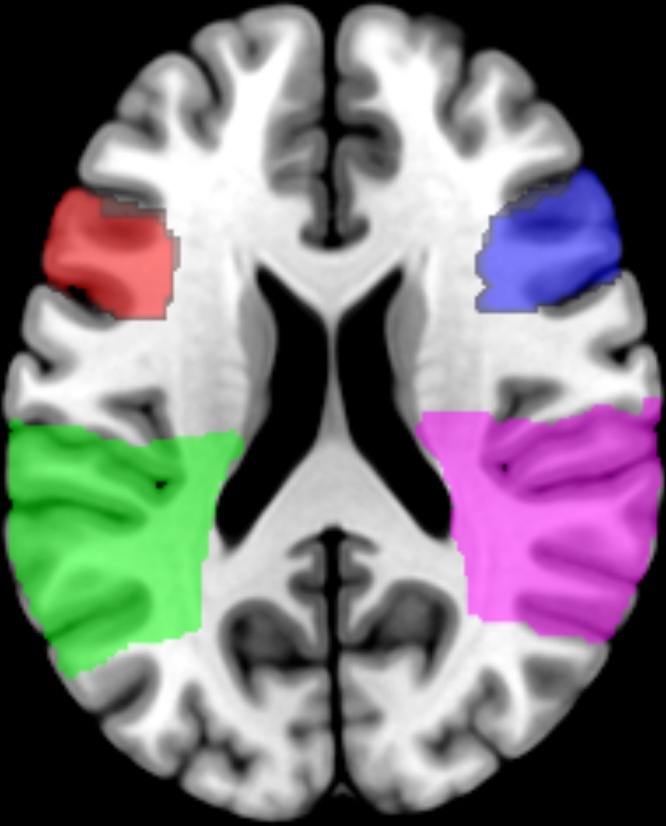
A ROI analysis was performed in regions that specifically belong to the MNS (i.e., bilateral IFG and bilateral IPL), using the Hammers adult atlas (Hammers et al., 2003) (Fig. 1). Each region was used for a ROI analysis using MarsBaR 0.44 toolbox (http://marsbar.sourceforge.net/) using a two-sample t-test to identify differences in ALFF maps between MS patients and healthy controls within MNS regions. The significance within this analysis was a corrected P-value = 0.05.
Fig. 1.
Delimitation of the mirror neuron system core regions using the Hammers adult atlas (Hammers et al., 2003). Red: left inferior frontal gyrus (IFG); Green: left inferior parietal lobule (IPL); blue (right IFG); purple (right IPL).
VBM analysis
GM segmented images were used in a two-sample t-test to identify differences in GM volume between MS patients and healthy controls. Age, gender and TIV were also included as regressors of no interest to reduce the variance unrelated to the variable of interest. The whole brain was considered comparing GM volume between MS patients and healthy controls using the random-effects approach (False Discovery Rate [FDR] = 0.05) with a minimum cluster size of ten voxels. Furthermore, a region of interest (ROI) analysis, implemented in CAT12, was performed to extract the volume of GM in the core regions of the MNS (i.e., bilateral IFG and bilateral IPL), using the Hammers adult atlas (Hammers et al., 2003) (Fig. 1). Total intracranial volume (TIV) was also calculated for each participant. The GM volume in each MNS core region was expressed relative to TIV, i.e., the absolute GM of each region was divided by the TIV of each participant. Finally, these data were added in the next analysis. Furthermore, Pearson correlation coefficient was calculated between the GM volume of the selected MNS regions. Statistical significance was considered when p – value < 0.002 (Bonferroni correction).
Relationship between ALFF and GMV in MNS
A full factorial design was performed to investigate the interaction between the factor of being an MS patient or HC and the bilateral IPL’s and bilateral IFG’s GM volume covariates. As variance was expected to differ between samples, a nonsphericity correction was applied. Age, gender and TIV were also included as regressors of no interest to reduce the variance unrelated to the variable of interest. This type of analysis is equivalent to an interaction model, testing for different regression slopes in MS patients and healthy controls between ALFF and GM volume in MNS regions. Moreover, positive and negative relationships between the whole studied group and each covariate were explored.
Firstly, whole brain was considered with a FDR = 0.05. Afterwards, a ROI analysis was performed in the region/s of the MNS that showed differences in the ALFF maps comparison contrasts (see above). ROIs were delimited using the Hammers adult atlas (Fig. 1) and the analysis was performed using MarsBaR 0.44 toolbox (http://marsbar.sourceforge.net/). The significance within this analysis was a corrected P-value = 0.05.
DTI analysis
The diffusion tensor was calculated and a deterministic fiber tracking algorithm (Yeh et al., 2013) between two ROIs (left IFG and left IPL) was used. Firstly, DTI images were corrected for head motion and Eddy current distortion. Afterwards, they were normalized to standard MNI space. The angular threshold was randomly selected from 15 degrees to 90 degrees. The step size was randomly selected from 0.1 voxel to 3 voxels. The anisotropy threshold was randomly selected. The fiber trajectories were smoothed by averaging the propagation direction with a percentage of the previous direction. The percentage was randomly selected from 0% to 95%. Tracks with a length shorter than 30 mm or longer than 300 mm were discarded. The analysis was conducted using DSI Studio (http://dsi-studio.labsolver.org). The value of the fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD) were extracted from the tracts between left IPL and left IFG for each participant. A comparison between MS patients and healthy controls was performed using Mann-Whitney U test. Statistical significance was considered when p-value<0.008 (Bonferroni correction). Finally, correlation coefficients between the GM volume and some diffusivity parameters (those showing differences in the previous comparison) were calculated. For this last analysis, statistical significance was considered when p-value<0.0125 (Bonferroni correction).
Results
Comparison of ALFF maps
A two-sample t-test was performed to compare the ALFF maps between MS patients and controls. No significant differences were identified when the whole brain was considered. However, the ROI analysis in MNS regions showed a decrease of ALFF in MS patients in the left IFG compared to healthy controls (t statistic = 4.85; corrected p = 0.006). This region was the only one considered for the rest of the resting-state images analysis.
GMV comparison
No differences in a two-sample t-test were identified when the whole brain was considered using a corrected p-value (FDR = 0.05). The ROI analysis in MNS regions did not identify any differences of the GMV in those regions between patients and healthy controls. In any case, a positive correlation of the GMV of the different MNS regions was identified for MS patients, but not for the healthy control group (Table 2). Healthy subjects showed a positive relationship between the GMV of the left and right IPL (Pearson correlation = 0.812; p > 0.001); and between the GMV of the left and right IFG (Pearson correlation = 0.7; p = 0.004) (Table 2). MS patients showed a positive relationship between the GMV of all MNS regions (Table 2).
Table 2.
Correlation coefficients among the different GMV of the MNS regions in healthy controls and MS patients.
| L-IPL | L-IFG | R-IPL | R-IFG | |
|---|---|---|---|---|
| Multiple sclerosis patients | ||||
| L-IPL | PC = .655 p = 0.001* |
PC = .703 p < 0.001* |
PC = .655 p-value = 0.001* |
|
| L-IFG | PC = .747 p < 0.001* |
PC = .822 p < 0.001* |
||
| R-IPL | PC = .595 p = 0.002* |
|||
| Healthy controls | ||||
| L-IPL | PC = .234 p = 0.401 |
PC = .812 p < 0.001* |
PC = .484 p = 0.067 |
|
| L-IFG | PC = .279 p = 0.314 |
PC = .700 p = 0.004 |
||
| R-IPL | PC = .354 p = 0.196 |
|||
PC: Pearson Correlation.
Significant with Bonferroni correction.
Regression between ALFF maps and GM volume in MNS regions
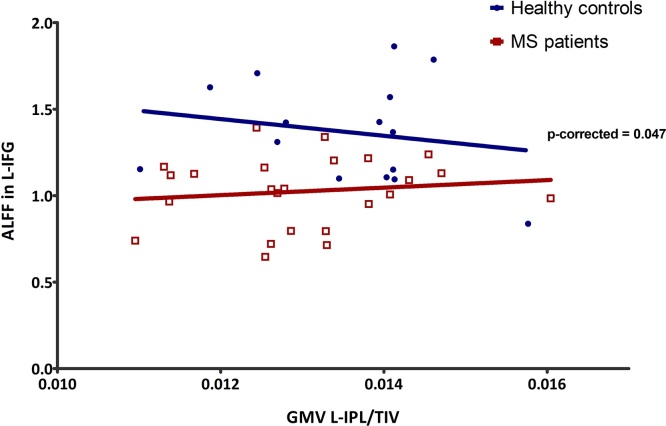
As previously stated, only the left IFG was considered in this analysis. A negative relationship between the ALFF in the left IFG and the GM volume in the left IPL when considering the whole group of participants (i.e., patients and controls). However, a significant interaction effect in this ROI was identified, with a significantly steeper gradient of regression in the MS patients group as compared with the healthy controls (FDR = 0.05) (Fig. 2). No other interaction reached statistical significance (supplementary Table 1).
Fig. 2.
Regression between mplitude of low frequency fluctuations (ALFF) in the left IFG and the grey matter volume (GMV) of the left IPL (relative to total intracranial volume [TIV]). Significant interaction between MS patients and HC is shown, with a significantly steeper gradient of regression in the MS patients group.
Anatomical connectivity of the MNS regions
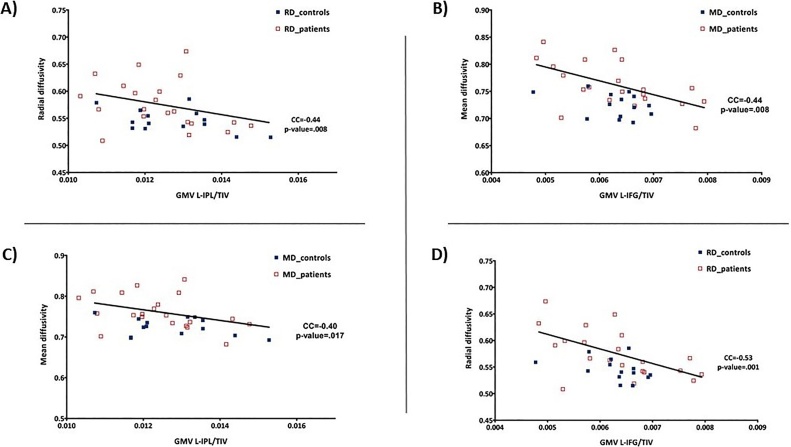
Bearing in mind the different relationship between the GMV of the left IPL and the ALFF in the left IFG, the study of structural connectivity between these regions was performed. Diffusivity parameters (i.e., MD and RD) were significantly higher in MS patients than healthy controls in the tracts that connect the MNS regions in the left-brain hemisphere, showing a disturbance in the connectivity of frontal and parietal regions (Table 3). No statistically significant difference was identified for the FA and AD. On the other hand, a negative correlation between the GMV in the left IPL and MD (CC = −0.40; p-value = 0.017) and RD (CC = −0.44; p-value = 0.008) was identified (Fig. 3A). In addition, a negative correlation between the GMV in the left IFG and MD (CC = −0.44; p-value = 0.008) and RD (CC = −0.53; p-value = 0.001) was also found (Fig. 3B). No differences were identified between MS patients and healthy controls in these contrasts.
Table 3.
Comparison of diffusion tensor imaging (DTI) parameters between healthy controls and MS patients.
| Healthy controls (n = 15) mean (SD) |
MS patients (n = 24) mean (SD) |
p-valuea | |
|---|---|---|---|
| Number of tracts | 15.9 (11.36) | 19.4 (13.20) | 0.538 |
| Track length (mm) | 236.5 (297.74) | 251.3 (298.98) | 0.127 |
| Fractional Anisotropy (FA) | 0.425 (0.02) | 0.420 (0.02) | 0.561 |
| Mean diffusivity (MD) | 0.725 (0.02) | 0.761 (0.04 | 0.005* |
| Axial diffusivity (AD) | 1.083 (0.04) | 1.133 (0.05) | 0.001* |
| Radial diffusivity (RD) | 0.545 (0.02) | 0.575 (0.04) | 0.034 |
SD: Standard deviation.
Mann-Whitney U. Statistical significance was considered when p-value < 0.008 (Bonferroni correction).
Fig. 3.
Correlation between radial diffusivity (RD) and median diffusivity (MD) with the grey matter volume (GMV) of the left inferior parietal lobule (IPL) (A and C) and left IFG (B and D).
Discussion
In the present study, a combination of functional and structural data of the MNS in MS patients and healthy controls has been examined. A decrease of ALFF in MS has been identified in the left IFG. Furthermore, a positive relationship between the ALFF in the IFG and the GM volume in IPL was identified. This relationship showed a significant interaction between the studied groups. This different relationship may be due to an impairment of white matter pathways connecting the MNS, as can be deduced by the presence of higher MD, AD and RD in MS patients than healthy controls (only differences in MD and AD resisted the correction for multiple comparison). These findings will be discussed below.
A decrease of ALFF in MS patients
The present work describes the existence of a decrease in ALFF in the IFG of a cohort of MS patients with low disability. Liu et al. (2012) described a decrease of ALFF in clinically isolated syndrome (the earliest stage of MS) in different regions of the default mode network (a brain network involving parietal and prefrontal regions which is preferentially active when individuals are not focused on the external environment (Buckner et al., 2008)) when compared to healthy controls (Liu et al., 2012). Liu et al. (2015), in their study involving MS patients with spinal cord involvement and healthy controls, reported a decrease in the right middle temporal gyrus, left middle frontal gyrus, lingual gyrus, and cuneus in patients compared to controls (Liu et al., 2015). Finally, Liu et al. (2016), observed a decrease of ALFF in the bilateral anterior cingulate gyrus and bilateral caudate head in RR MS patients (Liu et al., 2016).
MNS regions were not included among those with a decrease in ALFF described in previous works, although this may be associated with methodological differences. In previous studies, ALFF analyses were performed considering the whole brain while in the present work a ROI analysis in regions belonging to the MNS was done.
On the contrary, some authors have reported an increase ALFF in MS patients. For instance, Liu et al. (2016) studied the differences in ALFF maps between RR MS patients and healthy controls. They reported higher ALFF responses in the right fusiform gyrus for MS patients (Liu et al., 2016). Liu et al. (2011) showed an increase of ALFF in thalami, right insula, and right superior temporal gyrus. Furthermore, the authors reported a significant correlation with Expanded Disability Status Scale (EDSS) score (Liu et al., 2011). Similar results were reported by Zhou et al. (2014), with no decrease of ALFF in MS patients and an increase in thalami (Zhou et al., 2014). Finally, Liu et al. (2015), comparing MS patients with spinal cord involvement and healthy controls, described an increase of ALFF in patients compared to healthy controls in bilateral inferior parietal lobe and right insula (Liu et al., 2015). The authors explained the results considering that the abnormal baseline activity may be due to spinal cord damage or clinically undetected brain damage (Liu et al., 2015).
In view of that the increase in ALFF seems to be associated with greater disability in MS patients, the presence of a decrease of ALFF compared to controls in low disability patients may not have a clear explanation. If the presence of an increase in ALFF is associated with greater brain damage, MS patients should present higher ALFF in MNS regions than healthy controls, because MS patients are expected to present more brain damage than healthy controls. Furthermore, the clinical significance of this low ALFF is also unclear and should be explored in longitudinal studies. In any case, the difference of ALFF in MS patients described here may be explained by the existence of structural changes because of the brain damage (i.e. demyelinated lesions and secondary atrophy) produced during relapses.
Relationship between ALFF and GMV
Although demyelinization and axonal damage in the CNS are the key pathological findings in MS (Dutta and Trapp, 2011; Compston and Coles, 2008), GM atrophy during the disease course is another frequent pathological and imaging finding (Tedeschi et al., 2005; Vercellino et al., 2005). GM is associated with the development of cognitive decline and, furthermore, it correlates with the EDSS in the later phase of the disease, mainly in the secondary progressive forms of MS (Grothe et al., 2016). In early stages of the disease, disability is driven more by subcortical white matter lesions, whereas in the later stages disability is predominantly determined by the extent of cortical pathology (Grothe et al., 2016). In any case, GM damage begins early and seems to determine the severity and progression of the disease (Geisseler et al., 2016).
In the present work, neither the whole brain analysis nor the ROI analysis in the MNS regions showed differences between MS patients and healthy controls. This finding agrees with the description made above, because the present cohort of MS patients showed low disability. In this respect, it has been proposed that the anatomical differences determined by VBM analysis may reflect more stable and long-lasting abnormalities (Ren et al., 2013).
However, differences in the correlation of the GMV among the MNS regions was identified between MS patients and healthy controls. While only a positive correlation between right and left IPL and between right and left IFG was identified in healthy controls, MS patients showed a positive correlation of the GMV among all MNS regions. Furthermore, not only has a comparison of GMV and ALFF between MS patients and healthy controls in the MNS ROIs been studied, but also the relationship between the GMV and ALFF in the different MNS regions. This analysis showed a correlation between the ALFF in the left IFG and the GMV of the left IPL, with a significant interaction between MS patients and healthy controls.
As described in the introduction section, some authors have explored the existence of an association between the ALFF and GM volume. Zhao et al. (2015) identified a positive correlation between GMV and ALFF in the right superior parietal lobule of amnestic-type mild cognitive impairment patients (Zhao et al., 2015). Nevertheless, Han et al. (2012) did not find any relationship between ALFF and GM volume in a group of this kind of patient (Han et al., 2012). There are two main differences between the present work and the previous one. Firstly, while the regression analysis was performed considering the whole brain in the latter studies, only the ALFF in the left IFG was considered in the present one for the analysis (because this was the MNS region that showed differences in the previous analysis) and the GMV of the MNS (i.e., bilateral IPL and bilateral IFG). Secondly, reported studies only considered the relationship between ALFF and GMV in the same regions, but in the present one the relationship between the GM volume and ALFF in the different MNS regions has also been studied. Functional relationship between MNS has been previously reported (Plata-Bello et al., 2016b), but this is the first report to date about the relationship between the functional and structural data in the MNS regions.
The difference between MS patients and healthy controls in the relationship between ALFF and GMV in the MNS reported here may be explained by the existence of damage in the white matter tracts putatively connecting MNS regions. The main tracts involved in the connectivity between IFG and IPL are the superior longitudinal fascicle, arcuate fascicle and external capsule (Wang et al., 2012). In the present work, DTI analysis was performed to identify differences in the connectivity between the left IFG and the left IPL. Part of the tracts named before were involved in such connectivity. Higher MD, AD, and RD were present in MS patients. These parameters showed white matter and axonal damage in the connectivity of left IFG and left IPL and this could explain the abnormal relationship between ALFF and GM volume that was identified in MS patients.
Limitations
The main limitation of the present work is the number of subjects included in both groups. Bearing in mind, that we wanted to study MS patients with low disability, if one wants to identify differences studying the whole brain, it may be necessary larger cohorts of participants. In any case, using ROI analysis, a standard and widespread methodology in neuroimaging studies, we have been able to show differences between patients and healthy controls.
Apart from larger cohorts of participants, future studies should be more focused than the present work in the study of the diffusion tensor imaging parameters, using modern methods for analysing and improving the acquisition parameters. The present study did not aim to study specifically white matter tracts that connect the MNS hubs, but we used the information provided by DTI studies to give a more accurate explanation to the results from ALFF and GMV relationships.
Conclusion
Low disability MS patients may present an ALFF decrease in the MNS. This functional difference may be secondary to identifiable structural changes: an impairment in the white matter tracts which connect MNS regions to each other and, probably because of this, changes in the relationship of the GMV among the different MNS regions. These functional and structural changes do not seem to be associated with clinical disability, at least when standard scales are used. It would be useful to longitudinally study these changes because they might be early biomarkers of the possible evolution of the disease and they could help with the application of early appropriate measures to prevent the development of clinical impairment (e.g., rehabilitation programs).
Conflict of interests
The Authors declare no conflicts of interest.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ibror.2018.09.002.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Achiron A., Barak Y. Cognitive impairment in probable multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2003;74:443–446. doi: 10.1136/jnnp.74.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello M., Salvatore E., Cachia A. Relationship between simultaneously acquired resting-state regional cerebral glucose metabolism and functional MRI: a PET/MR hybrid scanner study. Neuroimage. 2015;113:111–121. doi: 10.1016/j.neuroimage.2015.03.017. [DOI] [PubMed] [Google Scholar]
- Amato M.P., Zipoli V., Portaccio E. Multiple sclerosis-related cognitive changes: a review of cross-sectional and longitudinal studies. J. Neurol. Sci. 2006;245:41–46. doi: 10.1016/j.jns.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Benton A.L., Hamsher KdeS VN and SO . Oxford University Press; Nueva York: 1983. Contributions to Neuropsychological Assessment: A Clinical Manual. [Google Scholar]
- Binder E., Dovern A., Hesse M.D. Lesion evidence for a human mirror neuron system. Cortex. 2017;90:125–137. doi: 10.1016/j.cortex.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cattaneo L., Rizzolatti G. The mirror neuron system. Arch. Neurol. 2009;66:557–560. doi: 10.1001/archneurol.2009.41. [DOI] [PubMed] [Google Scholar]
- Compston A., Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Cordes D., Haughton V.M., Arfanakis K. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am. J. Neuroradiol. 2000;21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- Dricker J., Butters N., Berman G. The recognition and encoding of faces by alcoholic Korsakoff and right hemisphere patients. Neuropsychologia. 1978;16:683–695. doi: 10.1016/0028-3932(78)90003-9. [DOI] [PubMed] [Google Scholar]
- Dutta R., Trapp B.D. Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog. Neurobiol. 2011;93:1–12. doi: 10.1016/j.pneurobio.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudino E.A., Chiaravalloti N.D., DeLuca J., Diamond B.J. A comparison of memory performance in relapsing-remitting, primary progressive and secondary progressive, multiple sclerosis. Neuropsychiatry Neuropsychol. Behav. Neurol. 2001;14:32–44. [PubMed] [Google Scholar]
- Geisseler O., Pflugshaupt T., Bezzola L. The relevance of cortical lesions in patients with multiple sclerosis. BMC Neurol. 2016;16:204. doi: 10.1186/s12883-016-0718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe M., Lotze M., Langner S., Dressel A. The role of global and regional gray matter volume decrease in multiple sclerosis. J. Neurol. 2016;263:1137–1145. doi: 10.1007/s00415-016-8114-3. [DOI] [PubMed] [Google Scholar]
- Haase C.G., Tinnefeld M., Lienemann M. Depression and cognitive impairment in disability-free early multiple sclerosis. Behav. Neurol. 2003;14:39–45. doi: 10.1155/2003/843760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammers A., Allom R., Koepp M.J. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum. Brain Mapp. 2003;19:224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Lui S., Kuang W. Anatomical and functional deficits in patients with amnestic mild cognitive impairment. PLoS One. 2012;7:e28664. doi: 10.1371/journal.pone.0028664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper H. Western Psychological Services; Los Angeles: 1983. Hooper Visual Organization Test (VOT) [Google Scholar]
- Liu Y., Liang P., Duan Y. Brain plasticity in relapsing-remitting multiple sclerosis: evidence from resting-state fMRI. J. Neurol. Sci. 2011;304:127–131. doi: 10.1016/j.jns.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Liu Y., Duan Y., Liang P. Baseline brain activity changes in patients with clinically isolated syndrome revealed by resting-state functional MRI. Acta Radiol. 2012;53:1073–1078. doi: 10.1258/ar.2012.120269. [DOI] [PubMed] [Google Scholar]
- Liu Y., Meng B., Zeng C. Abnormal baseline brain activity in patients with multiple sclerosis with simple spinal cord involvement detected by resting-state functional magnetic resonance imaging. J. Comput. Assist. Tomogr. 2015;39:866–875. doi: 10.1097/RCT.0000000000000299. [DOI] [PubMed] [Google Scholar]
- Liu H., Chen H., Wu B. Functional cortical changes in relapsing-remitting multiple sclerosis at amplitude configuration: a resting-state fMRI study. Neuropsychiatr. Dis. Treat. 2016;12:3031–3039. doi: 10.2147/NDT.S120909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe M.J., Mock B.J., Sorenson J.A. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Lu H., Zuo Y., Gu H. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proc. Natl. Acad. Sci. U. S. A. 2007;104:18265–18269. doi: 10.1073/pnas.0705791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutton J.D., Winston R., Rodman T.C. Multiple sclerosis: etiological mechanisms and future directions. Exp. Biol. Med. 2004;229:12–20. doi: 10.1177/153537020422900102. [DOI] [PubMed] [Google Scholar]
- Mantini D., Perrucci M.G., Del Gratta C. Electrophysiological signatures of resting state networks in the human brain. Proc. Natl. Acad. Sci. U. S. A. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P., Cunnington R., Mattingley J.B. Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neurosci. Biobehav. Rev. 2012;36:341–349. doi: 10.1016/j.neubiorev.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Nugent A.C., Martinez A., D’Alfonso A. The relationship between glucose metabolism, resting-state fMRI BOLD signal, and GABAA-binding potential: a preliminary study in healthy subjects and those with temporal lobe epilepsy. J. Cereb. Blood Flow Metab. 2015;35:583–591. doi: 10.1038/jcbfm.2014.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olivares T., Nieto A., Sánchez M.P. Pattern of neuropsychological impairment in the early phase of relapsing-remitting multiple sclerosis. Mult. Scler. 2005;11:191–197. doi: 10.1191/1352458505ms1139oa. [DOI] [PubMed] [Google Scholar]
- Panou T., Mastorodemos V., Papadaki E. Early signs of memory impairment among multiple sclerosis patients with clinically isolated syndrome. Behav. Neurol. 2012;25:311–326. doi: 10.3233/BEN-2012-110201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plata-Bello J., Modroño C., Acosta-López S. Subarachnoid hemorrhage and visuospatial and visuoperceptive impairment: disruption of the mirror neuron system. Brain Imaging Behav. 2016 doi: 10.1007/s11682-016-9609-3. [DOI] [PubMed] [Google Scholar]
- Plata-Bello J., Modroño C., Hernández-Martín E. The mirror neuron system also rests. Brain Struct. Funct. 2016 doi: 10.1007/s00429-016-1335-5. [DOI] [PubMed] [Google Scholar]
- Plata-Bello J., Pérez-Martín Y., Castañón-Pérez A. The mirror neuron system in relapsing remitting multiple sclerosis patients with low disability. Brain Topogr. 2017 doi: 10.1007/s10548-017-0558-y. [DOI] [PubMed] [Google Scholar]
- Polman C.H., Reingold S.C., Banwell B. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S.M., Leo G.J., Bernardin L., Unverzagt F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology. 1991;41:685–691. doi: 10.1212/wnl.41.5.685. [DOI] [PubMed] [Google Scholar]
- Ren W., Lui S., Deng W. Anatomical and functional brain abnormalities in drug-naive first-episode schizophrenia. Am. J. Psychiatry. 2013;170:1308–1316. doi: 10.1176/appi.ajp.2013.12091148. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Craighero L. The mirror-neuron system. Annu. Rev. Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Song X.-W., Dong Z.-Y., Long X.-Y. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi G., Lavorgna L., Russo P. Brain atrophy and lesion load in a large population of patients with multiple sclerosis. Neurology. 2005;65:280–285. doi: 10.1212/01.wnl.0000168837.87351.1f. [DOI] [PubMed] [Google Scholar]
- Vercellino M., Plano F., Votta B. Grey matter pathology in multiple sclerosis. J. Neuropathol. Exp. Neurol. 2005;64:1101–1107. doi: 10.1097/01.jnen.0000190067.20935.42. [DOI] [PubMed] [Google Scholar]
- Wang J., Fan L., Zhang Y. Tractography-based parcellation of the human left inferior parietal lobule. Neuroimage. 2012;63:641–652. doi: 10.1016/j.neuroimage.2012.07.045. [DOI] [PubMed] [Google Scholar]
- Yeh F.-C., Verstynen T.D., Wang Y. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One. 2013;8:e80713. doi: 10.1371/journal.pone.0080713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y.-F., He Y., Zhu C.-Z. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Lu G., Zhong Y. fMRI study of mesial temporal lobe epilepsy using amplitude of low-frequency fluctuation analysis. Hum. Brain Mapp. 2010;31:1851–1861. doi: 10.1002/hbm.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z.-L., Fan F.-M., Lu J. Changes of gray matter volume and amplitude of low-frequency oscillations in amnestic MCI: an integrative multi-modal MRI study. Acta Radiol. 2015;56:614–621. doi: 10.1177/0284185114533329. [DOI] [PubMed] [Google Scholar]
- Zhou F., Zhuang Y., Wu L. Increased thalamic intrinsic oscillation amplitude in relapsing-remitting multiple sclerosis associated with the slowed cognitive processing. Clin. Imaging. 2014;38:605–610. doi: 10.1016/j.clinimag.2014.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.