Understanding the mechanisms underlying learning and memory continues to be of major interest in the neuroscientific discourse. Worldwide, over 50 million people live with some form of memory disorder, and this number will increase with the aging of our society. Synaptic plasticity is considered the main cellular correlate of learning in the brain, yet extrasynaptic changes in membrane excitability may also contribute. The possible roles of changes in membrane excitability during learning and memory have only started to be explored in the last few decades. In the hippocampus, possible functions of membrane excitability in memory allocation and the promotion of cell assembly formation during memory consolidation have been highlighted (1). In the cerebellum, and more specifically the cerebellar cortex, synaptic plasticity may establish connectivity patterns via action potential firing, whereas intrinsic plasticity may facilitate a neuron to get integrated into an active engram (2, 3). The cerebellum offers an ideal system to study basic mechanisms underlying learning and memory because of its evolutionarily well-preserved neuroarchitecture and the well-characterized forms of motor learning that it controls. In PNAS, Wang et al. (4) demonstrate learning-induced changes in membrane excitability in cerebellar nuclei projection neurons, which together with the vestibular nuclei neurons, form the main output of the cerebellum.
Early in the 20th century, delay eyeblink conditioning (EBC) was recognized as an elegant and simple form of associative learning (5), but it was not until the end of the century that changes in membrane excitability during EBC learning were studied as one of the potential mechanisms (6, 7). Pioneering work by Schreurs et al. (6) showed that cerebellar Purkinje cells, which inhibit the nuclei neurons (Fig. 1A), display learning-related changes in membrane excitability 24 h and 1 mo after EBC learning, pointing toward both a short-term and long-term role of intrinsic plasticity in cerebellum-dependent learning (6). This possibility is supported by various Purkinje cell-specific mouse models that suffer from deficits in intrinsic excitability and/or plasticity as well as from abnormal cerebellar motor learning, including not only EBC but also adaptation of the vestibuloocular reflex or locomotion learning (8–10). Cerebellar nuclei projection neurons, transferring information from the Purkinje cells to downstream structures, either inhibit neurons in the inferior olive or excite premotor neurons in the brainstem (Fig. 1A). The major question, namely how cerebellar nuclei neurons encode learning and memory, is still relatively uncharted territory due to the considerable technical difficulties in recording intracellularly from the identified neurons in adult cerebellar nuclei during behavior.
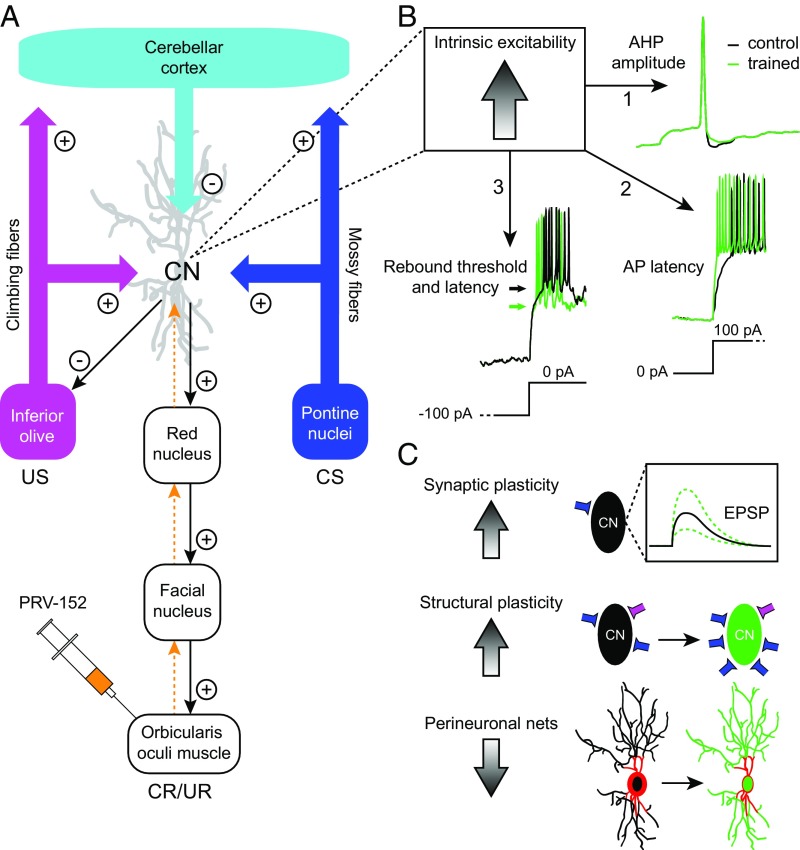
Fig. 1.
Changes in intrinsic excitability and other plasticity types in the cerebellar nuclei neurons during EBC. (A) Schematic representation of the main components of the olivocerebellar circuit involved in EBC, centered around the cerebellar nuclei (CN). Excitatory and inhibitory projections are indicated with (+) and (−) symbols, respectively. Transsynaptic neuronal tracer PRV-152 (orange) injected in the orbicularis oculi muscle led to retrograde labeling of projection neurons in the CN. (B) Learning-associated changes in intrinsic excitability in CN neurons manifested as 1, a reduced after-hyperpolarization (AHP) amplitude; 2, a reduced action potential (AP) latency after a depolarizing current step; and 3, a reduced threshold (indicated with arrows) and latency for rebound APs after release of a hyperpolarizing current step. Traces from our own recordings have been modified for illustrative purposes. (C) Earlier research suggests that EBC induces changes in different types of plasticity and dynamic mechanisms controlling plasticity. Synaptic plasticity may result in changes in postsynaptic potentials. Changes in structural plasticity may take place, including formation of new synaptic contacts. Dynamic changes in perineuronal nets may occur during learning, facilitating formation of new synaptic contacts, possibly through down-regulation of chemorepulsive molecules. EPSP, excitatory postsynaptic potential; UR, unconditioned response.
Wang et al. (4) partly bypass those technical difficulties. First, they trained mice with the EBC paradigm, associating a conditioned stimulus (CS; neutral tone) with an unconditioned stimulus (US; shock) to the eye for 4 d. After this training period, mice showed well-timed eyeblinks [conditioned responses (CRs)] in response to the CS in an average of 40% of the trials. On the fifth day, the mice were killed, and Wang et al. recorded from individual cerebellar nuclei neurons at the whole-cell level in vitro (6). To make sure that they recorded in the slice from nuclei neurons that encode eyeblink behavior, before dissection, the authors labeled the nuclei neurons by injecting a retrograde transneuronal tracer into the orbicularis oculi muscle (Fig. 1A). The ability to use transsynaptic tracers in conjunction with morphological identification and/or optogenetic manipulation has led to a revolution in neuroscience, allowing convergence of anatomical and physiological characterization of long-distance projection neurons in vitro. With these techniques in hand, Wang et al. show learning-related changes in anatomically identified cerebellar nuclei projection neurons after EBC. The data allow three conclusions (Fig. 1B): (i) The cerebellar nuclei projection neurons of conditioned mice that learn better show a smaller hyperpolarization after spiking (i.e., after-hyperpolarization); (ii) After conditioning, the mice show shorter latencies for the first evoked action potentials; and (iii) Conditioning appears to lead to a reduced threshold for rebound potentiation. All three conclusions suggest that intrinsic excitability of nuclei neurons may play a role during EBC acquisition and/or the expression of CRs. Determining whether intrinsic plasticity in cerebellar nuclei neurons is induced by an intensified release of Purkinje cell inhibition (3, 11, 12), by increased rebound activity subsequent to intensified Purkinje cell inhibition (11, 13), by enhanced excitatory inputs from collaterals (14, 15), and/or by untightening of the perineuronal net (16) will need further study (Fig. 1C).
The authors observed that not only anatomically identified but also unidentified cerebellar nuclei neurons showed changes in excitability after EBC. To date, none of the three parameters that differed between nuclei cells obtained from conditioned and control animals was found to show a significant difference among anatomically identified and nonidentified cells. Therefore, it appears possible that also nonspecific premotor output neurons or inferior olive projecting neurons show increases in excitability. If this holds true in vivo, intrinsic excitability would have a generalizing effect in the cerebellar nuclei. The possibility that intrinsic excitability is a more generalized effect after EBC learning catalyzes the question of what teleological function it might serve. Possibly, such generalization facilitates associations in both time and space. By altering the excitability in cerebellar nuclei neurons (4) and Purkinje cells (6) simultaneously, the sensitivity to many sets of afferent inputs may be increased, thereby resulting in more changes of spiking events within the olivocerebellar system during conditioning. This overall increased level of excitability may, for example, explain why (i) after completed delay conditioning, the duration of the CS can be considerably shortened to evoke the same CR (17); (ii) the noise level of the CS can be increased while in effect eliciting a similarly well-timed CR (18); and (iii) extinction learning for a specific CS condition allows quicker learning for a new condition (19). Likewise, at the end of conditioning, the complex spikes are not only occurring after presentation of the US but also of the CS (11, 20). Furthermore, such generalization induced by changes in intrinsic excitability may also explain why motor memory is not only specific but also very robust. In their original studies, Schreurs et al. (6) showed long-term changes of intrinsic excitability of Purkinje cells after 1 mo. Are the changes that Wang et al. (4) now report for the nuclei still there after 1 mo of learning? This would make intrinsic excitability a very durable way of encoding memory.
If the changes in excitability will hold for longer periods, one wonders how specificity for the paradigms involved is maintained within the system over time. Possibly, other plasticity mechanisms have to complement intrinsic excitability during different stages of learning to warrant sufficient specificity. So far, there is only indirect evidence that synaptic plasticity exists in the cerebellar nuclei after conditioning (11)—let alone that it has been studied whether synaptic plasticity and intrinsic excitability mechanisms complement each other during learning. What has been shown is that training over multiple days leads to structural plasticity such as mossy fiber sprouting (15), and that a reduction in perineuronal nets improves learning (16). Thus, particularly during later stages of learning, different mechanisms might complement one another to support the long-term storage and specific retrieval of eyeblink learning (Fig. 1C). The elegant study by Wang et al. (4) comes a long way in showing a proof of principle that EBC can evoke changes in intrinsic excitability in vitro after several days of training. Providing a critical test for an essential or necessary role of intrinsic plasticity in motor memory formation in vivo still needs to be achieved. Furthermore, data are needed to reveal how different forms of plasticity interact within individual learning trials as well as over the course of longer periods of learning.
Footnotes
The authors declare no conflict of interest.
See companion article on page E9419.
References
- 1.Lisman J, Cooper K, Sehgal M, Silva AJ. Memory formation depends on both synapse-specific modifications of synaptic strength and cell-specific increases in excitability. Nat Neurosci. 2018;21:309–314. doi: 10.1038/s41593-018-0076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohtsuki G, Hansel C. Synaptic potential and plasticity of an SK2 channel gate regulate spike burst activity in cerebellar Purkinje cells. iScience. 2018;1:49–54. doi: 10.1016/j.isci.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belmeguenai A, et al. Intrinsic plasticity complements long-term potentiation in parallel fiber input gain control in cerebellar Purkinje cells. J Neurosci. 2010;30:13630–13643. doi: 10.1523/JNEUROSCI.3226-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, et al. Changes in membrane properties of rat deep cerebellar nuclear projection neurons during acquisition of eyeblink conditioning. Proc Natl Acad Sci USA. 2018;115:E9419–E9428. doi: 10.1073/pnas.1808539115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cason H. The conditioned eyelid reaction. J Exp Psychol. 1922;5:153–196. [Google Scholar]
- 6.Schreurs BG, Gusev PA, Tomsic D, Alkon DL, Shi T. Intracellular correlates of acquisition and long-term memory of classical conditioning in Purkinje cell dendrites in slices of rabbit cerebellar lobule HVI. J Neurosci. 1998;18:5498–5507. doi: 10.1523/JNEUROSCI.18-14-05498.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aou S, Woody CD, Birt D. Increases in excitability of neurons of the motor cortex of cats after rapid acquisition of eye blink conditioning. J Neurosci. 1992;12:560–569. doi: 10.1523/JNEUROSCI.12-02-00560.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schonewille M, et al. Purkinje cell-specific knockout of the protein phosphatase PP2B impairs potentiation and cerebellar motor learning. Neuron. 2010;67:618–628. doi: 10.1016/j.neuron.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peter S, et al. Dysfunctional cerebellar Purkinje cells contribute to autism-like behaviour in Shank2-deficient mice. Nat Commun. 2016;7:12627. doi: 10.1038/ncomms12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.French CA, et al. Differential effects of Foxp2 disruption in distinct motor circuits. Mol Psychiatry. August 14, 2018 doi: 10.1038/s41380-018-0199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ten Brinke MM, et al. Dynamic modulation of activity in cerebellar nuclei neurons during Pavlovian eyeblink conditioning in mice. eLife. 2017;6:e28132. doi: 10.7554/eLife.28132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Person AL, Raman IM. Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei. Nature. 2011;481:502–505. doi: 10.1038/nature10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoebeek FE, Witter L, Ruigrok TJH, De Zeeuw CI. Differential olivo-cerebellar cortical control of rebound activity in the cerebellar nuclei. Proc Natl Acad Sci USA. 2010;107:8410–8415. doi: 10.1073/pnas.0907118107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aizenman CD, Linden DJ. Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nat Neurosci. 2000;3:109–111. doi: 10.1038/72049. [DOI] [PubMed] [Google Scholar]
- 15.Boele H-J, Koekkoek SKE, De Zeeuw CI, Ruigrok TJH. Axonal sprouting and formation of terminals in the adult cerebellum during associative motor learning. J Neurosci. 2013;33:17897–17907. doi: 10.1523/JNEUROSCI.0511-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirono M, et al. Perineuronal nets in the deep cerebellar nuclei regulate GABAergic transmission and delay eyeblink conditioning. J Neurosci. 2018;38:6130–6144. doi: 10.1523/JNEUROSCI.3238-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jirenhed D-A, Hesslow G. Time course of classically conditioned Purkinje cell response is determined by initial part of conditioned stimulus. J Neurosci. 2011;31:9070–9074. doi: 10.1523/JNEUROSCI.1653-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khilkevich A, Canton-Josh J, DeLord E, Mauk MD. A cerebellar adaptation to uncertain inputs. Sci Adv. 2018;4:eaap9660. doi: 10.1126/sciadv.aap9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medina JF, Garcia KS, Mauk MD. A mechanism for savings in the cerebellum. J Neurosci. 2001;21:4081–4089. doi: 10.1523/JNEUROSCI.21-11-04081.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohmae S, Medina JF. Climbing fibers encode a temporal-difference prediction error during cerebellar learning in mice. Nat Neurosci. 2015;18:1798–1803. doi: 10.1038/nn.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]