Significance
The epilepsies are a class of chronic neurological disorders with diverse clinical presentations, which often negatively impacts quality of life. Currently available therapeutics are effective for some patients, but roughly one-third of patients remain drug-resistant; the identification of novel therapeutic targets to alleviate seizures is therefore required. We have determined that potentiating Cl− extrusion in vivo reduces susceptibility to chemoconvulsant-induced seizures. Our results provide insights into the importance of controlling the polarity of GABAergic synaptic signaling under conditions of hyperexcitation, and highlight KCC2 as a potential therapeutic target for epilepsy.
Keywords: KCC2, intracellular chloride, inhibition, hyperpolarization, epilepsy
Abstract
The type 2 K+/Cl− cotransporter (KCC2) allows neurons to maintain low intracellular levels of Cl−, a prerequisite for efficient synaptic inhibition. Reductions in KCC2 activity are evident in epilepsy; however, whether these deficits directly contribute to the underlying pathophysiology remains controversial. To address this issue, we created knock-in mice in which threonines 906 and 1007 within KCC2 have been mutated to alanines (KCC2-T906A/T1007A), which prevents its phospho-dependent inactivation. The respective mice appeared normal and did not show any overt phenotypes, and basal neuronal excitability was unaffected. KCC2-T906A/T1007A mice exhibited increased basal neuronal Cl− extrusion, without altering total or plasma membrane accumulation of KCC2. Critically, activity-induced deficits in synaptic inhibition were reduced in the mutant mice. Consistent with this, enhanced KCC2 was sufficient to limit chemoconvulsant-induced epileptiform activity. Furthermore, this increase in KCC2 function mitigated induction of aberrant high-frequency activity during seizures, highlighting depolarizing GABA as a key contributor to the pathological neuronal synchronization seen in epilepsy. Thus, our results demonstrate that potentiating KCC2 represents a therapeutic strategy to alleviate seizures.
Several loss-of-function mutations in the neuron-specific type 2 K+/Cl− cotransporter (KCC2, Slc12a5) were recently identified as a cause of epilepsy of infancy with migrating focal seizures (1, 2). KCC2 mutations are also epilepsy risk factors (3–6), and brain tissue resected from patients with acquired drug-resistant temporal lobe epilepsy exhibit impaired KCC2 functional expression (7, 8). These human studies highlight the critical importance of KCC2 for suppressing seizures and establishing normal brain function, which are consistent with the unique ability of KCC2 to extrude Cl− from neurons (9). Maintaining low intraneuronal Cl− levels is essential for determining the inhibitory efficacy of Cl−-permeable Glycine and GABAA receptors, which are the exclusive mediators of fast synaptic inhibition in the central nervous system (10–12) and a common target for antiseizure medication (13–15).
In rodents, [Cl−]i rises sharply during ictal discharges in vivo (16) and under epileptiform conditions in hippocampal slices (17, 18). Optogenetic induction of an excessive rise in [Cl−]i can contribute to the generation of ictal-like discharges in slices (19), which is consistent with the appearance of spontaneous tonic−clonic seizures in KCC2 (isoform b) knockout mice (20) and floxed loss-of-function mice (21). Therefore, increasing the Cl− extrusion capacity of KCC2 could have the opposite effect and reduce the likelihood of developing epileptiform discharges. However, it has been proposed that KCC2 actually facilitates Cl− loading during seizures due to a reversal in KCC2 transport direction caused by rises in [K+]o (10, 22–27). A role for KCC2 as either proconvulsant or anticonvulsant therefore remains controversial.
KCC2 activity is bidirectionally controlled by posttranslational modifications, with serine 940 phosphorylation enhancing its function and phosphorylation of threonines 906 (T906) and 1007 (T1007) inhibiting its function (28, 29). Mutation of serine 940 to alanine (S940A) in vivo reduces KCC2 function and exacerbates kainate-induced status epilepticus (30), while transient transfection of a T906/T1007 double-point alanine substitution mutant (T906A/T1007A) enhances KCC2 function in cell culture (29, 31–35). Unfortunately, no selective pharmacological activators of KCC2 exist (36), and so testing whether elevated KCC2 function can impact epileptiform activity in more complex systems remains hypothetical. We therefore generated a KCC2-T906A/T1007A knock-in mouse model to examine this theory.
Results
Generation and Characterization of KCC2-T906A/T1007A Knock-in Mice.
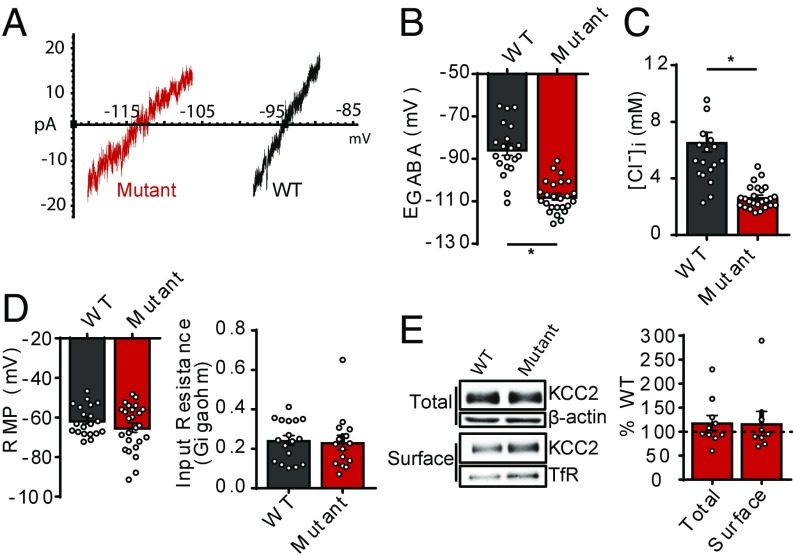
To determine if phosphorylation of KCC2 at threonines 906 and 1007 (T906/T1007) regulates KCC2 function in vivo, we generated mice in which T906/T1007 are mutated to alanine residues (T906A/T1007A). The presence of alanine mutations at sites T906 and T1007, within exons 22 and 24, respectively, was confirmed by DNA sequencing (SI Appendix, Fig. S1). Homozygous T906A/T1007A mice were viable, survived through adulthood, and exhibited no overt phenotypes. Motor function was normal, assessed as latency to fall off an accelerating rotarod (WT: 4.03 ± 0.35 min, n = 9; T906A/T1007A: 3.88 ± 0.23 min, n = 9; P = 0.7206) and distance traveled in an open field arena (WT: 41.6 ± 3.3 m, n = 9; T906A/T1007A: 40.6 ± 2.3 m, n = 9; P = 0.8145) (SI Appendix, Fig. S2). To determine the impact of the T906A/T1007A mutations on KCC2 function, we performed gramicidin perforated patch-clamp experiments on hippocampal neurons from WT and T906A/T1007A mice and recorded GABAA reversal potentials (EGABA) at 18 days to 25 days in vitro (DIV) (Fig. 1 A and B). EGABA was strongly hyperpolarized in T906A/T1007A neurons compared with WT (WT: −86 ± 3 mV, n = 20 neurons; T906A/T1007A: −108 ± 3 mV, n = 25 neurons; P < 0.0001), indicating increased KCC2 function in the T906A/T1007A neurons. We calculated the [Cl−]i values from the EGABA values using the Nernst equation, which revealed significantly lower [Cl−]i in the T906A/T1007A neurons compared with WT (WT: 6.6 ± 0.7 mM, n = 20 neurons; T906A/T1007A: 2.7 ± 0.2 mM, n = 25 neurons; P < 0.0001) (Fig. 1C). In contrast, both the resting membrane potential (RMP) (WT: −62 ± 2 mV, n = 20; T906A/T1007A: −65 ± 2 mV, n = 25 neurons; P = 0.2445) and input resistance (Ri) (WT: 243 ± 25 MΩ, n = 17 neurons; T906A/T1007A: 232 ± 36 MΩ, n = 15 neurons; P = 0.7946) did not significantly differ between WT and T906A/T1007A neurons (Fig. 1D).
Fig. 1.
KCC2 function is increased in KCC2-T906A/T1007A neurons. (A) Current–voltage (I–V) plots depicting the reversal potentials of an individual WT and KCC2-T906A/T1007A hippocampal neuron. (B) Summary of all EGABA values in mature (18 DIV to 21 DIV) hippocampal neurons. *P < 0.0001. (C) The [Cl−]i values corresponding to the measured EGABA values. *P < 0.0001. (D) Ri and RMP were unaffected by the KCC2-T906A/T1007A mutations. (E) Biotinylation of brain slices revealed that total and surface expression of KCC2 is unchanged in KCC2-T906A/T1007A mice. Total KCC2 was normalized to β-actin. Surface KCC2 was normalized to transferrin receptor (TfR) surface expression. Data are expressed as the percentage of WT expression.
To investigate the mechanism responsible for increased Cl− extrusion in T906A/T1007A neurons, we assessed whether the mutations influenced the total and/or surface expression of KCC2. We utilized whole-cell biotinylation followed by immunoblotting of brain slices, and we detected no difference in either total (118 ± 15% WT, n = 10 mice; P = 0.2523) or surface (117 ± 25% WT, n = 8 mice; P = 0.5236) KCC2 protein levels between WT and T906A/T1007A mice (Fig. 1E), indicating the mutations did not increase Cl− extrusion through enhanced surface levels but rather through modulation of an intrinsic property of the transporter.
Gross Brain Morphology and Neuronal Network Excitability Is Normal in KCC2-T906A/T1007A Mice.
We next sought to characterize effects of the KCC2-T906A/T1007A mutations on gross brain morphology and function. Nissl staining revealed no gross brain abnormalities (SI Appendix, Fig. S3). Immunoblotting for markers of excitatory (PSD-95 and GluA1) and inhibitory [Gephyrin, vesicular GABA transporter (VGAT), and GABAA receptor subunits α2 and β3] synapses revealed that the total expression of PSD-95 (103 ± 9% WT, n = 5; P = 0.7739), Gephyrin (85 ± 7% WT, n = 5; P = 0.0717), GABAA receptor subunit β3 (89 ± 5% WT, n = 5; P = 0.0795), and GABAA receptor subunit α2 (109 ± 9% WT, n = 5; P = 0.3592) was comparable between WT and T906A/T1007A mice, while small reductions in GluA1 (85 ± 6% WT, n = 5; P = 0.0380) and VGAT (83 ± 4% WT, n = 5; P = 0.0029) were detected (SI Appendix, Fig. S4). To determine whether these reductions alter synaptic excitability, we analyzed the synaptic input/output (I/O) relationship in the hippocampus. Field excitatory postsynaptic potentials were measured in the stratum radiatum in response to Schaffer collateral stimulation. I/O curves from WT and T906A/T1007A slices were comparable, indicating that the T906A/T1007A mutations do not impact excitability of this circuit (SI Appendix, Fig. S5).
Increased KCC2 Function Attenuates Seizure-Like Activity and Severity in Vitro.
After having confirmed that KCC2 function was increased in the mutant neurons, we sought to address the long-standing question of whether increasing KCC2 function has any impact on epileptiform activity and severity. The Kv channel blocker 4-aminopyridine (4-AP) is commonly used to induce seizure-like or epileptiform activity in acutely prepared hippocampal slices (37) (Fig. 2A). All WT slices (16/16 slices) exhibited seizure-like events (SLEs), while, intriguingly, only 46.7% (7/15 slices) of T906A/T1007A slices exhibited SLEs (Fig. 2B). Of the slices that did exhibit SLEs, onset of the first SLE was delayed in the T906A/T1007A slices (WT: 3.4 ± 0.4 min, n = 16 slices; T906A/T1007A: 27.8 ± 6.0 min, n = 7 slices; P < 0.0001) (Fig. 2D). Furthermore, the percentage of time spent in epileptiform activity was reduced in T906A/T1007A slices (WT: 47 ± 7%, n = 16; T906A/T1007A: 3.7 ± 1.7%, n = 15; P < 0.0001) (Fig. 2E). Application of 4-AP plus the small-molecule KCC2 inhibitor VU0463271 (10 μM) induced SLEs in all WT and T906A/T1007A slices tested (n = 4 slices for both genotypes) (Fig. 2B). VU0463271 accelerated the onset times of SLEs in mutant slices to values statistically similar to WT onset times (WT: 4.0 ± 1.3 min, n = 4; T906A/T1007A: 5.0 ± 0.7 min, n = 4; P = 0.5350) (Fig. 2D). Similarly, the percentage of time spent in epileptiform activity in mutant slices was increased to WT levels when KCC2 was inhibited (WT: 88 ± 1%, n = 4; T906A/T1007A: 90 ± 2%, n = 4; P = 0.3936) (Fig. 2E).
Fig. 2.
KCC2-T906A/T1007A mutations were protective against 4-AP−induced epileptiform activity. (A) Example traces of local field potential recordings of WT and KCC2-T906A/T1007A brain slices exposed to 4-AP in the absence or presence of VU0463271 (VU). (B) The percentage of KCC2-T906A/T1007A slices exhibiting SLEs was reduced compared with WT. This protection was abolished with the application of VU. (C) The number of individual SLEs was reduced in the KCC2-T906A/T1007A slices. *P < 0.0001. (D) The time to the first SLE was delayed in the KCC2-T906A/T1007A slices, but resembled WT onset times in the presence of VU. *P < 0.0001. (E) The percentage of time spent in seizure activity was reduced in the KCC2-T906A/T1007A slices, but these values increased in the presence of VU and were statistically similar to WT values (*P < 0.0001).
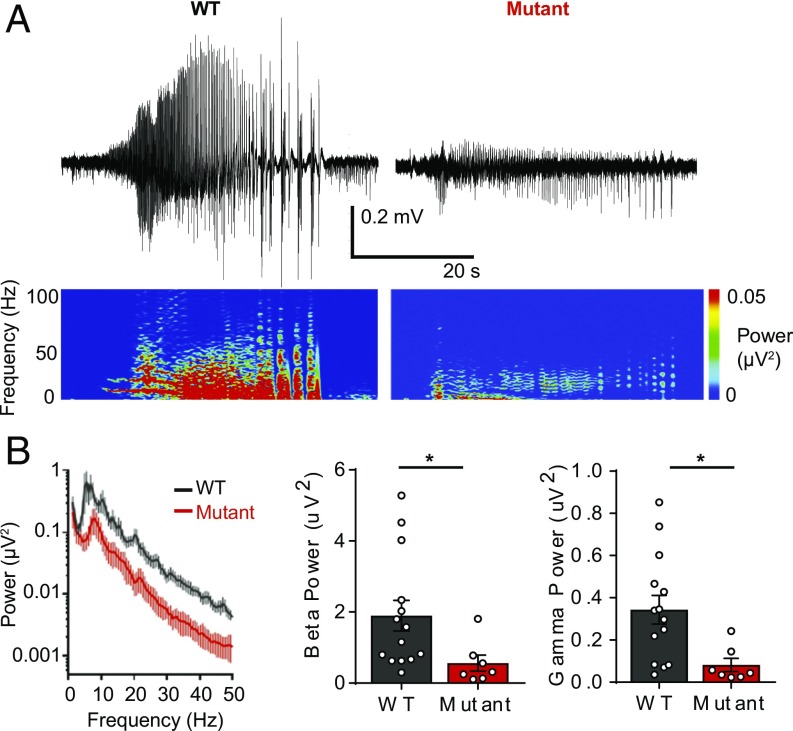
We then performed power spectral density analysis of the first SLE to gain insight into the relationship between seizure power and seizure severity. The power of 13- to 30-Hz beta frequency activity (WT: 1.90 ± 0.43 µV2, n = 14; T906A/T1007A: 0.56 ± 0.23 µV2, n = 7; P = 0.0485) and 30- to 50-Hz gamma frequency activity (WT: 0.34 ± 0.07 µV2, n = 14; T906A/T1007A: 0.08 ± 0.03 µV2, n = 7; P = 0.0163) was significantly reduced during T906A/T1007A SLEs compared with WT (Fig. 3), suggesting a correlation between high-frequency power and levels of seizure activity. Moreover, no T906A/T1007A slices (0/15 slices) degenerated into status epilepticus (SE)-like activity compared with 43.8% of WT (7/16 slices) (38) (SI Appendix, Fig. S6); 4-AP in the presence of the KCC2 inhibitor VU0463271 led to rapid SE entry in both WT and T906A/T1007A slices.
Fig. 3.
KCC2-T906A/T1007A mutations reduced high-frequency neuronal synchronization during 4-AP−induced SLEs. (A) Traces depict individual SLEs induced by 4-AP exposure as well as the corresponding spectrograms. (B) Power spectral density plot of the first SLE. The power of 13- to 30-Hz beta frequency activity and 30- to 50-Hz gamma frequency activity during the first SLE was lower in KCC2-T906A/T1007A slices compared with WT. *P < 0.05.
To ensure that the reduction in the seizure-like activity seen in the 4-AP model was not specific to seizure activity induced by blockade of voltage-gated potassium channels, we assessed seizure activity in a second in vitro model, in which all Mg2+ is removed from the artificial cerebrospinal fluid (ACSF) (0-Mg2+). All WT and KCC2-T906A/T1007A slices exhibited SLEs (SI Appendix, Fig. S7A); however, the onset to the first SLE was delayed in the KCC2-T906A/T1007A slices (WT: 8.007 ± 0.5735 min, n = 11; T906A/T1007A: 10.25 ± 0.7067 min, n = 9; P = 0.0228) (SI Appendix, Fig. S7B). The KCC2-T906A/T1007A slices also spent less time in epileptiform activity than WT slices (WT: 50.5 ± 5.4%, n = 11; T906A/T1007A: 29.0 ± 5.6%, n = 9; P = 0.0134) (SI Appendix, Fig. S7C). Interestingly, seizure activity progressed into late-recurrent discharges in 100% of WT slices, but in only 75% of KCC2-T906A/T1007A slices (SI Appendix, Fig. S7D). Interestingly, we detected lower levels of beta (WT: 1.16 ± 0.23 µV2, n = 11; T906A/T1007A: 0.44 ± 0.09 µV2, n = 9; P = 0.0154) and gamma (WT: 0.33 ± 0.07 µV2, n = 11; T906A/T1007A: 0.10 ± 0.03 µV2, n = 9; P = 0.0143) power during the KCC2-T906A/T1007A SLEs compared with WT SLEs (SI Appendix, Fig. S8 A and B).
Increased KCC2 Function Attenuates Seizure-Like Activity and Severity in Vivo.
We then examined seizure activity in vivo. We recorded EEG activity from mice injected with the chemoconvulsant kainate, which induces discrete convulsions that often degenerates into SE, where seizure activity is continuous and fails to self-terminate (Fig. 4A). Critically, KCC2-T1007 phosphorylation was increased in WT mice 1 h after kainate injection (150 ± 17% of WT littermates, n = 5; P = 0.0193) (SI Appendix, Fig. S9), which is consistent with a phosphorylation-dependent decrease of KCC2 function (33). Through analysis of the electrographic activity, we found that the onset of the first tonic seizure was delayed in the T906A/T1007A mice compared with the WT mice (137 ± 16% of WT littermates, n = 9; P = 0.0376) (Fig. 4B). As kainate is such a severe model, many mice died from SE; however, the percentage of T906A/T1007A mice that died was substantially reduced compared with WT (WT: 42.1%, n = 19; T906A/T1007A: 23.5%, n = 17) (pooled data from EEG- and non-EEG−implanted mice), and the time of death was comparatively delayed (Fig. 4C). Power spectral density analysis of the first 1 h after kainate injection revealed that the relative gamma power (50 Hz to 100 Hz) was reduced in the T906A/T1007A mice (WT: 11 ± 2% total power, n = 10; T906A/T1007A: 5 ± 1% total power, n = 7; P = 0.0140) (Fig. 4 D and E). Therefore, prevention of phosphorylation-dependent inhibition of KCC2 in T906A/T1007A mice mitigated seizure-induced increases in high-frequency activity.
Fig. 4.
The development and severity of kainate-induced seizures was reduced in KCC2-T906A/T1007A mice. (A) EEG recordings from WT and KCC2-T906A/T1007A mice injected with kainate. The beginning of the trace represents 30 s after the time of injection. The corresponding spectrograms are displayed. (B) The onset of the first tonic seizure is delayed in the KCC2-T906A/T1007A mice. Time of onset is expressed as a percentage of WT littermate control. *P < 0.05. (C) Kainate-induced lethality was reduced in KCC2-T906A/T1007A mice. The survival plot displays the percentage of death of WT and KCC2-T906A/T1007A mice at the corresponding time of death after kainate injection. (D). Power spectral density plot of the first hour of EEG activity after kainate. (E) The relative gamma power (50 Hz to 100 Hz) over this period was lower in the KCC2-T906A/T1007A mice. *P < 0.05.
KCC2-T906A/T1007A Mutations Resist EGABA Depolarization Under Hyperexcitable Conditions by Enhancing Rate of Cl− Extrusion.
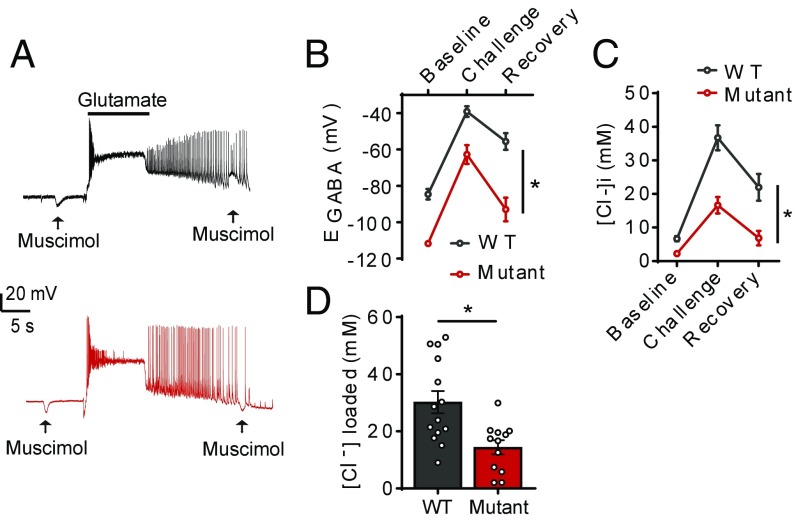
Seizure activity causes rapid accumulation of [Cl−]i; thus we wanted to simulate neuronal hyperexcitability and assess the impact of these conditions on GABAA currents. WT and T906A/T1007A hippocampal neurons were exposed to three consecutive pulses of glutamate (10 s per pulse, 20 μM), which induces membrane depolarization and increased neuronal firing (30, 39). Inhibitory postsynaptic potentials occurring in conjunction with the membrane depolarization drive Cl− into the cell, leading to a rapid loss of hyperpolarizing GABAA signaling (Fig. 5A).
Fig. 5.
KCC2-T906A/T1007A mutations limited EGABA depolarization under hyperexcitable conditions. (A) Example traces of the glutamate challenge performed on WT and KCC2-T906A/T1007A hippocampal neurons. Application of muscimol (arrows) to activate GABAA receptors was applied before and after glutamate exposure. A reversal in the polarity of GABAA currents in a WT neuron, and maintenance of hyperpolarizing current in a KCC2-T906A/T1007A neuron, is evident. (B) EGABA measurements were taken before glutamate exposure, immediately after glutamate exposure, and after a 3-min recovery period in which TTX was used to block neuronal activity. EGABA was more negative in KCC2-T906A/T1007A neurons at all three of these time points. *P < 0.001. (C) The corresponding [Cl−]i. *P < 0.01. (D) The concentration of Cl− loaded into the neurons during the glutamate challenge is reduced in KCC2-T906A/T1007A neurons compared with WT. *P < 0.01.
Baseline EGABA measurements again revealed increased KCC2 activity in the T906A/T1007A neurons (WT: −84.6 ± 2.9 mV, n = 15; T906A/T1007A: −111.7 ± 1.2 mV, n = 14; P < 0.0001), giving values that were equivalent to [Cl−]i values of 6.7 ± 0.8 mM for WT and 2.2 ± 0.1 mM for the T906A/T1007A neurons (P < 0.0001, compared with WT controls). Immediately after the glutamate exposure, EGABA values shifted to −39.1 ± 2.9 mV (n = 14) for WT neurons and −62.7 ± 5.2 mV for T906A/T1007A neurons (n = 12; P = 0.0004, compared with WT controls). The corresponding [Cl−]i values were 36.7 ± 3.7 mM for WT neurons and 16.7 ± 2.5 mM for T906A/T1007A neurons, indicating that glutamate induced a significantly smaller Cl− load in the mutants (P = 0.0002). We then assessed the ability of these neurons to recover from these activity-induced Cl− shifts (40). Three minutes after glutamate exposure, WT EGABA values were −55.6 ± 4.6 mV (n = 13), equivalent to Cl− values of 22.0 ± 4.0 mM, and T906A/T1007A EGABA values were −93.0 ± 6.5 mV (n = 13), equivalent to Cl− values of 6.8 ± 2.1 mM, both of which were significantly lower than the WT values (EGABA P < 0.0001; Cl− P = 0.0032) (Fig. 5 B and C). Importantly, the glutamate-induced Cl− shift was reduced in T906A/T1007A neurons compared with WT (WT: 30.2 ± 3.9 mM, n = 14; T906A/T1007A: 14.4 ± 2.5 mM, n = 12; P = 0.003), demonstrating that the T906A/T1007A neurons were more resistant to Cl− loading under hyperexcitable conditions than WT neurons (Fig. 5D).
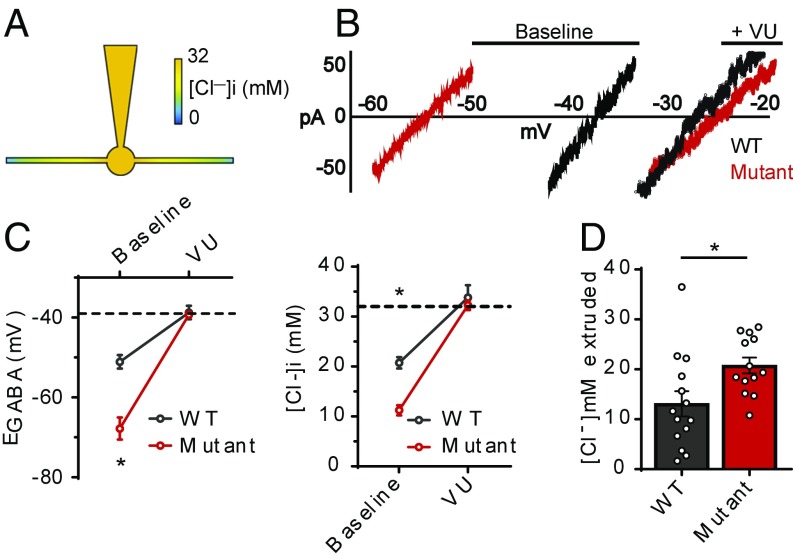
To directly compare the rate of Cl− extrusion between WT and T906A/T1007A neurons, we employed a whole-cell patch-clamp Cl− loading assay (22, 41). By imposing a 32-mM Cl− load through the patch pipette, the subsequent measurement of EGABA reports the degree to which these neurons were able to extrude a fixed amount of Cl− (Fig. 6A). Without KCC2 activity, the Nernst equation dictates that neurons should have an EGABA value of −39 mV when loaded with 32 mM [Cl−]i. Both WT and T906A/T1007A neurons displayed resting EGABA values that were more negative than the predicted value, indicating the presence of a functional Cl− extrusion mechanism. The EGABA values were more negative in T906A/T1007A neurons compared with those of WT (WT: −51.1 ± 1.7 mV, n = 14; T906A/T1007A: −67.8 ± 2.8 mV, n = 14; P < 0.0001), which were equivalent to 20.8 ± 1.1 mM [Cl−]i for WT neurons and 11.3 ± 1.0 mM [Cl−]i for T906A/T1007A neurons (P < 0.0001) (Fig. 6 B and C). The EGABA values shifted to statistically equivalent values between WT and T906A/T1007A neurons in the presence of the specific KCC2 inhibitor VU0463271 (WT: −38.8 ± 1.7 mV, n = 14; T906A/T1007A: −39.3 ± 0.7 mV, n = 13; P = 0.7669) (Fig. 6 B and C). Conversion of these values to [Cl−]i revealed the validity of this assay, as the Cl− values of WT (33.8 ± 2.5 mM) and T906A/T1007A (32.3 ± 1.0 mM) neurons were statistically similar to each other (P = 0.5741) and neither deviated from the pipette-imposed Cl− load of 32 mM (WT P = 0.47; T906A/T1007A P = 0.78) (Fig. 6C). This demonstrated that these deviations from the predicted Nernst potentials and imposed Cl− loads from the pipette were due to KCC2 activity. Moreover, the amplitude of the VU0463271-induced Cl− shift indicated that the concentration of Cl− that was extruded under baseline conditions was greater in the T906A/T1007A neurons (WT: 13.1 ± 2.5 mM, n = 14; T906A/T1007A: 20.8 ± 1.6 mM, n = 13; P = 0.018) (Fig. 6D). This demonstrated that the T906A/T1007A mutations enable more rapid KCC2-mediated Cl− extrusion, facilitating the efficacy of synaptic inhibition under conditions of high intracellular Cl−.
Fig. 6.
KCC2-T906A/T1007A mutations enhance the rate of KCC2-mediated Cl− extrusion. (A) Cartoon of whole-cell patch-clamp Cl− loading assay that creates a somatic−dendritic Cl− gradient. (B) I–V plot of leak-subtracted muscimol currents depicting the EGABA values, both before and after exposure to the KCC2 inhibitor VU0463271 (VU) (I–V plots depict results before correction for liquid junction potentials; see Materials and Methods). (C) EGABA values were more negative in KCC2-T906A/T1007A neurons compared with WT, indicating that the rate of Cl− extrusion is increased by the KCC2-T906A/T1007A mutations. The EGABA values were shifted to the expected value of approximately −39 mV (dashed line) when VU was applied. The corresponding [Cl−]i values are also shown, with the 32-mM [Cl−]i indicated by the dashed line. *P < 0.0001. (D) The concentration of Cl− that was extruded under baseline conditions was greater in the KCC2-T906A/T1007A neurons, calculated as the difference between [Cl−]i under baseline and VU conditions. *P < 0.05.
Discussion
We have determined that preventing phosphorylation of KCC2 at threonines 906 and 1007 is sufficient to enhance KCC2 activity in vivo. These mutations limited loss of synaptic inhibition during hyperexcitable conditions by enhancing the rate of KCC2-mediated Cl− extrusion. This was sufficient to delay the onset and severity of chemoconvulsant-induced seizure activity in vitro and in vivo, suggesting that enhancing KCC2 function may be an effective therapeutic strategy for epilepsy.
Interestingly, the KCC2-T906A/T1007A mutations did not impact KCC2 surface expression, indicating that the enhanced Cl− extrusion cannot simply be explained by more KCC2 protein on the cell surface. These mutations must be altering the functional capacity of KCC2 through direct alteration of its kinetic properties. This may be through modulation of the net velocity of Cl− transport in a manner similar to phosphorylation-initiated cross-linking of NKCC1 transmembrane domains that ultimately lock it in active/inactive states (42). On the other hand, these KCC2 mutations could increase the affinity of KCC2 for Cl−. Given that KCC2-T906A/T1007A neurons equilibrate at more negative EGABA values under basal conditions when Cl− entry is limited, an increased affinity is a more likely mechanism, as this would enable Cl− detection and extrusion beyond the threshold for WT. This “dual-affinity” model of KCC2 may be similar to nitrate transporters, which exhibit phosphorylation-dependent switching between high- and low-affinity states (43, 44). How (de)phosphorylation of the C terminus of KCC2 can translate to the transmembrane domain to modulate KCC2 activity remains to be determined. Structure−function studies could clarify this process, which may lead to a rational medicinal chemical strategy that exploits this mechanism to directly activate KCC2.
The mechanisms underlying ictogenesis are not well understood. Interneuron firing increases at the onset of ictal events in vitro, indicating a paradoxical role of excess GABAergic activity underlying ictogenesis (17, 45). Prolonged GABAA conductance, particularly when concurrent with membrane depolarization, degrades the efficacy of synaptic inhibition due to Cl− loading, which abolishes hyperpolarizing GABAA currents (39, 40). Artificially loading pyramidal neurons with Cl− raises network excitability and triggers full ictal events in the presence of subictal concentrations of 4-AP, clearly indicating a role for elevated [Cl−]i during ictogenesis (19). Our demonstration that enhanced KCC2 activity limited Cl− loading and delayed chemoconvulsant-induced seizure onset further supports Cl− dysregulation as a major factor for seizure initiation.
Optogenetic removal of intracellular Cl− during seizure-like activity reduces epileptiform activity and promotes termination of seizure-like activity in vitro (18, 46) suggesting Cl− loading is a key factor in maintaining seizure activity. Indeed, chloride extrusion and subsequent restoration of fast synaptic inhibition is necessary for seizure termination, as KCC2 inhibition leads to rapid SE entry in in vitro seizure models (demonstrated here and by refs. 12 and 37). In line with this, we have demonstrated that specifically increasing the rate of KCC2-mediated Cl− extrusion is sufficient to prevent SE in the 4-AP in vitro seizure model. Interestingly, disrupted Cl− gradients during SE may underlie the lack of efficacy of benzodiazepines in these patients (13, 14, 39, 47), suggesting restoration of Cl− homeostasis may have potential for treating this life-threatening medical emergency.
EGABA depolarization elicits high-frequency oscillations in epileptic circuits (19), suggesting Cl− loading may facilitate the induction of high-frequency activity during seizures. Our demonstration that increased KCC2-mediated Cl− extrusion mitigates this high-frequency activity during seizures in vitro and in vivo highlights depolarizing GABA as a key contributing factor in the induction of pathological neuronal synchronization. This may reflect limited spreading of pathological neuronal synchronization across multiple brain regions, which may be responsible for the reduced kainate-induced lethality seen in these mice. As only male mice were used in these experiments, future studies on female mice would prove interesting.
Epileptic patients have reduced KCC2 protein levels; however, some KCC2 protein is retained, indicating an untapped potential for targeting KCC2 T906 and T1007 phosphorylation to restore KCC2 function. The powerful chemoconvulsants used in this study rapidly remove and degrade KCC2 from the cell membrane (30, 48), far more than what is observed in human epileptic tissue; thus it is possible that increasing KCC2 function in patients will be efficacious in reducing seizures.
In conclusion, we report that increasing KCC2 function in vivo is sufficient to reduce chemoconvulsant-induced seizure activity and severity. This highlights KCC2 as a seizure-limiting protein and suggests it could be a therapeutic target for seizure disorders. No direct pharmacological activators of KCC2 currently exist (33), but our work suggests the discovery of such agents may have promising outcomes for alleviating seizures in patients with epilepsy.
Materials and Methods
Electrophysiology.
The perforated patch-clamp technique was used to obtain basal EGABA measurements and EGABA values in the glutamate challenge experiments. The whole-cell patch-clamp technique was used for the neuronal Cl− loading assay. Extracellular field recordings were used to detect SLEs in horizontal brain slices induced using 4-AP or 0-Mg2+. Power analysis was performed using Labchart software.
Biochemistry.
Surface KCC2 expression was visualized using slice biotinylation followed by SDS/PAGE and Western blotting. KCC2-T1007 phosphorylation was detected by isolating KCC2 using immunoprecipitation followed by SDS/PAGE and Western blotting, and measured using an antibody specific for KCC2-T1007 phosphorylation (32).
EEG.
All animal studies were performed with protocols approved by the Institutional Animal Care and Use Committee of Tufts New England Medical Center. Prefabricated head mounts (two-channel, catalog #8201; Pinnacle Technology) to record EEGs were glued to the skull of anesthetized mice, and the mice were left to recover for 5 days. Kainate was injected into the mice to induce seizures, and EEG activity was recorded using Sirenia Acquisition software. Power analysis was performed using Labchart software.
Supplementary Material
Acknowledgments
This work was supported by funding from National Institutes of Health (NIH)-National Institute of Neurological Disorders and Stroke (NINDS) Grants NS101888 (to T.Z.D. and S.J.M.), NS081735 (to S.J.M.), and NS087662 (to S.J.M.); National Institute of Mental Health (NIMH) Grants MH097446 and MH106954 (to S.J.M.); a predoctoral fellowship from American Epilepsy Society (to Y.E.M.); and NIH-National Institute on Drug Abuse Grant DA037170 (to S.J.M.).
Footnotes
Conflict of interest: N.J.B. is a full-time employee and shareholder of AstraZeneca. S.J.M. serves as a consultant for SAGE Therapeutics and AstraZeneca, relationships that are regulated by Tufts University.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1810134115/-/DCSupplemental.
References
- 1.Stödberg T, et al. Mutations in SLC12A5 in epilepsy of infancy with migrating focal seizures. Nat Commun. 2015;6:8038. doi: 10.1038/ncomms9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saitsu H, et al. Impaired neuronal KCC2 function by biallelic SLC12A5 mutations in migrating focal seizures and severe developmental delay. Sci Rep. 2016;6:30072. doi: 10.1038/srep30072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- 4.Huberfeld G, et al. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci. 2007;27:9866–9873. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muñoz A, Méndez P, DeFelipe J, Alvarez-Leefmans FJ. Cation-chloride cotransporters and GABA-ergic innervation in the human epileptic hippocampus. Epilepsia. 2007;48:663–673. doi: 10.1111/j.1528-1167.2007.00986.x. [DOI] [PubMed] [Google Scholar]
- 6.Palma E, et al. Anomalous levels of Cl- transporters in the hippocampal subiculum from temporal lobe epilepsy patients make GABA excitatory. Proc Natl Acad Sci USA. 2006;103:8465–8468. doi: 10.1073/pnas.0602979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karlócai MR, et al. Enhanced expression of potassium-chloride cotransporter KCC2 in human temporal lobe epilepsy. Brain Struct Funct. 2016;221:3601–3615. doi: 10.1007/s00429-015-1122-8. [DOI] [PubMed] [Google Scholar]
- 8.Campbell SL, et al. GABAergic disinhibition and impaired KCC2 cotransporter activity underlie tumor-associated epilepsy. Glia. 2015;63:23–36. doi: 10.1002/glia.22730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore YE, Kelley MR, Brandon NJ, Deeb TZ, Moss SJ. Seizing control of KCC2: A new therapeutic target for epilepsy. Trends Neurosci. 2017;40:555–571. doi: 10.1016/j.tins.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Payne JA. Functional characterization of the neuronal-specific K-Cl cotransporter: Implications for [K+]o regulation. Am J Physiol. 1997;273:C1516–C1525. doi: 10.1152/ajpcell.1997.273.5.C1516. [DOI] [PubMed] [Google Scholar]
- 11.Rivera C, et al. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 12.Sivakumaran S, et al. Selective inhibition of KCC2 leads to hyperexcitability and epileptiform discharges in hippocampal slices and in vivo. J Neurosci. 2015;35:8291–8296. doi: 10.1523/JNEUROSCI.5205-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Treiman DM, et al. Veterans Affairs Status Epilepticus Cooperative Study Group A comparison of four treatments for generalized convulsive status epilepticus. N Engl J Med. 1998;339:792–798. doi: 10.1056/NEJM199809173391202. [DOI] [PubMed] [Google Scholar]
- 14.Mayer SA, et al. Refractory status epilepticus: Frequency, risk factors, and impact on outcome. Arch Neurol. 2002;59:205–210. doi: 10.1001/archneur.59.2.205. [DOI] [PubMed] [Google Scholar]
- 15.Perucca P, Gilliam FG. Adverse effects of antiepileptic drugs. Lancet Neurol. 2012;11:792–802. doi: 10.1016/S1474-4422(12)70153-9. [DOI] [PubMed] [Google Scholar]
- 16.Sulis Sato S, et al. Simultaneous two-photon imaging of intracellular chloride concentration and pH in mouse pyramidal neurons in vivo. Proc Natl Acad Sci USA. 2017;114:E8770–E8779. doi: 10.1073/pnas.1702861114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lillis KP, Kramer MA, Mertz J, Staley KJ, White JA. Pyramidal cells accumulate chloride at seizure onset. Neurobiol Dis. 2012;47:358–366. doi: 10.1016/j.nbd.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellender TJ, Raimondo JV, Irkle A, Lamsa KP, Akerman CJ. Excitatory effects of parvalbumin-expressing interneurons maintain hippocampal epileptiform activity via synchronous afterdischarges. J Neurosci. 2014;34:15208–15222. doi: 10.1523/JNEUROSCI.1747-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alfonsa H, et al. The contribution of raised intraneuronal chloride to epileptic network activity. J Neurosci. 2015;35:7715–7726. doi: 10.1523/JNEUROSCI.4105-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woo NS, et al. Hyperexcitability and epilepsy associated with disruption of the mouse neuronal-specific K-Cl cotransporter gene. Hippocampus. 2002;12:258–268. doi: 10.1002/hipo.10014. [DOI] [PubMed] [Google Scholar]
- 21.Kelley MR, et al. Locally reducing KCC2 activity in the Hippocampus is sufficient to induce temporal lobe epilepsy. EBioMedicine. 2018;32:62–71. doi: 10.1016/j.ebiom.2018.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarolimek W, Lewen A, Misgeld U. A furosemide-sensitive K+-Cl- cotransporter counteracts intracellular Cl- accumulation and depletion in cultured rat midbrain neurons. J Neurosci. 1999;19:4695–4704. doi: 10.1523/JNEUROSCI.19-12-04695.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staley KJ, Proctor WR. Modulation of mammalian dendritic GABA(A) receptor function by the kinetics of Cl- and HCO3- transport. J Physiol. 1999;519:693–712. doi: 10.1111/j.1469-7793.1999.0693n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeFazio RA, Keros S, Quick MW, Hablitz JJ. Potassium-coupled chloride cotransport controls intracellular chloride in rat neocortical pyramidal neurons. J Neurosci. 2000;20:8069–8076. doi: 10.1523/JNEUROSCI.20-21-08069.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viitanen T, Ruusuvuori E, Kaila K, Voipio J. The K+-Cl cotransporter KCC2 promotes GABAergic excitation in the mature rat hippocampus. J Physiol. 2010;588:1527–1540. doi: 10.1113/jphysiol.2009.181826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamidi S, Avoli M. KCC2 function modulates in vitro ictogenesis. Neurobiol Dis. 2015;79:51–58. doi: 10.1016/j.nbd.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez OC, et al. Role of KCC2-dependent potassium efflux in 4-Aminopyridine-induced Epileptiform synchronization. Neurobiol Dis. 2018;109:137–147. doi: 10.1016/j.nbd.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HH, et al. Direct protein kinase C-dependent phosphorylation regulates the cell surface stability and activity of the potassium chloride cotransporter KCC2. J Biol Chem. 2007;282:29777–29784. doi: 10.1074/jbc.M705053200. [DOI] [PubMed] [Google Scholar]
- 29.Rinehart J, et al. Sites of regulated phosphorylation that control K-Cl cotransporter activity. Cell. 2009;138:525–536. doi: 10.1016/j.cell.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silayeva L, et al. KCC2 activity is critical in limiting the onset and severity of status epilepticus. Proc Natl Acad Sci USA. 2015;112:3523–3528. doi: 10.1073/pnas.1415126112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Titz S, Sammler EM, Hormuzdi SG. Could tuning of the inhibitory tone involve graded changes in neuronal chloride transport? Neuropharmacology. 2015;95:321–331. doi: 10.1016/j.neuropharm.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 32.de Los Heros P, et al. The WNK-regulated SPAK/OSR1 kinases directly phosphorylate and inhibit the K+-Cl- co-transporters. Biochem J. 2014;458:559–573. doi: 10.1042/BJ20131478. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Conway LC, et al. N-Ethylmaleimide increases KCC2 cotransporter activity by modulating transporter phosphorylation. J Biol Chem. 2017;292:21253–21263. doi: 10.1074/jbc.M117.817841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoue K, et al. Taurine inhibits K+-Cl- cotransporter KCC2 to regulate embryonic Cl- homeostasis via with-no-lysine (WNK) protein kinase signaling pathway. J Biol Chem. 2012;287:20839–20850. doi: 10.1074/jbc.M111.319418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedel P, et al. WNK1-regulated inhibitory phosphorylation of the KCC2 cotransporter maintains the depolarizing action of GABA in immature neurons. Sci Signal. 2015;8:ra65. doi: 10.1126/scisignal.aaa0354. [DOI] [PubMed] [Google Scholar]
- 36.Cardarelli RA, et al. The small molecule CLP257 does not modify activity of the K+-Cl- co-transporter KCC2 but does potentiate GABAA receptor activity. Nat Med. 2017;23:1394–1396. doi: 10.1038/nm.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelley MR, et al. Compromising KCC2 transporter activity enhances the development of continuous seizure activity. Neuropharmacology. 2016;108:103–110. doi: 10.1016/j.neuropharm.2016.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dreier JP, Heinemann U. Regional and time dependent variations of low Mg2+ induced epileptiform activity in rat temporal cortex slices. Exp Brain Res. 1991;87:581–596. doi: 10.1007/BF00227083. [DOI] [PubMed] [Google Scholar]
- 39.Deeb TZ, Nakamura Y, Frost GD, Davies PA, Moss SJ. Disrupted Cl(-) homeostasis contributes to reductions in the inhibitory efficacy of diazepam during hyperexcited states. Eur J Neurosci. 2013;38:2453–2467. doi: 10.1111/ejn.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson SM, Gähwiler BH. Activity-dependent disinhibition. I. Repetitive stimulation reduces IPSP driving force and conductance in the hippocampus in vitro. J Neurophysiol. 1989;61:501–511. doi: 10.1152/jn.1989.61.3.501. [DOI] [PubMed] [Google Scholar]
- 41.Khirug S, et al. Distinct properties of functional KCC2 expression in immature mouse hippocampal neurons in culture and in acute slices. Eur J Neurosci. 2005;21:899–904. doi: 10.1111/j.1460-9568.2005.03886.x. [DOI] [PubMed] [Google Scholar]
- 42.Monette MY, Somasekharan S, Forbush B. Molecular motions involved in Na-K-Cl cotransporter-mediated ion transport and transporter activation revealed by internal cross-linking between transmembrane domains 10 and 11/12. J Biol Chem. 2014;289:7569–7579. doi: 10.1074/jbc.M113.542258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu KH, Tsay YF. Switching between the two action modes of the dual-affinity nitrate transporter CHL1 by phosphorylation. EMBO J. 2003;22:1005–1013. doi: 10.1093/emboj/cdg118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun J, Zheng N. Molecular mechanism underlying the plant NRT1.1 dual-affinity nitrate transporter. Front Physiol. 2015;6:386. doi: 10.3389/fphys.2015.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gnatkovsky V, Librizzi L, Trombin F, de Curtis M. Fast activity at seizure onset is mediated by inhibitory circuits in the entorhinal cortex in vitro. Ann Neurol. 2008;64:674–686. doi: 10.1002/ana.21519. [DOI] [PubMed] [Google Scholar]
- 46.Alfonsa H, Lakey JH, Lightowlers RN, Trevelyan AJ. Cl-out is a novel cooperative optogenetic tool for extruding chloride from neurons. Nat Commun. 2016;7:13495. doi: 10.1038/ncomms13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staley K. Enhancement of the excitatory actions of GABA by barbiturates and benzodiazepines. Neurosci Lett. 1992;146:105–107. doi: 10.1016/0304-3940(92)90183-8. [DOI] [PubMed] [Google Scholar]
- 48.Puskarjov M, Ahmad F, Kaila K, Blaesse P. Activity-dependent cleavage of the K-Cl cotransporter KCC2 mediated by calcium-activated protease calpain. J Neurosci. 2012;32:11356–11364. doi: 10.1523/JNEUROSCI.6265-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.