Significance
The biological changes associated with cognitive decline in aging are complex. Age-related changes in the striatum have been hypothesized as potentially important for predicting declining memory function over the adult life span, but empirical support for this hypothesis is sparse. We provide evidence from human fMRI and PET data for the association of age-related differences in striatal function to memory decline. A reduction of specificity (i.e., dedifferentiation) in functional coupling between the caudate and regions of the default network predicted memory decline over subsequent years in older adults. An age-related reduction in density of presynaptic striatal dopamine transporters also predicted memory decline but was unrelated to functional dedifferentiation, suggesting that relations between age-related changes in striatal functions and memory are multifaceted.
Keywords: striatum, aging, memory, dopamine, functional connectivity
Abstract
Age-related changes in striatal function are potentially important for predicting declining memory performance over the adult life span. Here, we used fMRI to measure functional connectivity of caudate subfields with large-scale association networks and positron emission tomography to measure striatal dopamine transporter (DAT) density in 51 older adults (age 65–86 years) who received annual cognitive testing for up to 7 years (mean = 5.59, range 2–7 years). Analyses showed that cortical–caudate functional connectivity was less differentiated in older compared with younger adults (n = 63, age 18–32 years). Unlike in younger adults, the central lateral caudate was less strongly coupled with the frontal parietal control network in older adults. Older adults also showed less “decoupling” of the caudate from other networks, including areas of the default network (DN) and the hippocampal complex. Contrary to expectations, less decoupling between caudate and the DN was not associated with an age-related reduction of striatal DAT, suggesting that neurobiological changes in the cortex may drive dedifferentiation of cortical–caudate connectivity. Reduction of specificity in functional coupling between caudate and regions of the DN predicted memory decline over subsequent years at older ages. The age-related reduction in striatal DAT density also predicted memory decline, suggesting that a relation between striatal functions and memory decline in aging is multifaceted. Collectively, the study provides evidence highlighting the association of age-related differences in striatal function to memory decline in normal aging.
The biological changes associated with cognitive decline in aging are complex and multifaceted (e.g., ref. 1). Because of its importance for understanding Alzheimer’s disease, much of the extant research on memory in aging has focused on the medial temporal lobe system and cortical networks. Age-related changes in the striatum have long been recognized as another potentially important predictor of declining memory function over the adult life span (2), but only recently have multimodal imaging studies begun to deliver empirical evidence for this hypothesis (3, 4).
The striatum works in concert with the cortex to support different aspects of behavior. A cognitive system involving connections of the association cortices with the caudate and anterior putamen can be distinguished from a motor system and a reward system that involve the posterior putamen and ventral striatum, respectively (5, 6). In line with this heuristic, one previous study in older adults has treated the caudate as a functionally uniform region subserving cognitive functions (3). However, functional MRI (fMRI) has revealed that the human caudate can be functionally further differentiated based on associations with two of the most widely studied cortical association networks implicated in human cognition (7): A central lateral zone that is coupled preferentially to the frontal parietal control network (FPN; including the anterior, dorsolateral, and dorsomedial prefrontal cortex and lateral parietal areas) (8) and a medial wall zone coupled most strongly to the default network (DN; including the ventromedial prefrontal cortex, posterior cingulate, and inferior parietal areas) (9). Because differential coupling along a medial-to-lateral gradient with cortical networks has not been fully appreciated in human studies until quite recently, the functional implications of caudate subfield connectivity with large-scale association networks have not been studied extensively and, to our knowledge, not at all in the context of aging and cognition.
The present study explores the hypothesis that cortical-subcortical connectivity between caudate subfields and cortical association networks (FPN and DN) is less differentiated in older adults, which in turn contributes to age-related cognitive decline. Of particular relevance for memory may be age-related differences in coupling between caudate subfields and the DN, because the DN involves connections with the medial temporal lobes and is implicated in memory function (9). These results would be informative not only for understanding functional implications of age-related changes in the striatum but also for understanding age-related declines in the interaction between a medial temporal lobe memory system on the one hand and a corticalstriatal system on the other hand.
Dedifferentiation has previously been discussed in the context of aging and human fMRI to reflect a loss of selectivity in neural processing with aging (10–14). In the striatum specifically, reductions of dopamine receptors and transporters in aging are a reliable observation (15). Because dopamine is thought to play a critical role in optimizing signal-to-noise in neuronal circuits (16)—that is, increasing the distinctiveness of neural representations—it is possible that aging-related differences in striatal dopamine function are related to functional dedifferentiation of cortical–caudate connectivity. This study combines fMRI functional connectivity (fc) data with a positron emission tomography (PET) marker of the striatal dopamine system. Older adults were followed for up to 7 y with annual testing of memory, executive function, and processing speed to yield estimates of longitudinal cognitive change during aging. We tested whether dedifferentiation of cortical–caudate connectivity and striatal dopamine function predict cognitive change.
Results
Longitudinal Change in Cognitive Performance.
Of the 54 older adults that entered this study, cognitive performance for tests of memory, executive functions, and processing speed was tracked in 51 older adults (mean age = 74.87; age range = 65–86 y) annually for up to 7 y (mean number of years = 5.59, SD = 0.94, range 2–7 y).
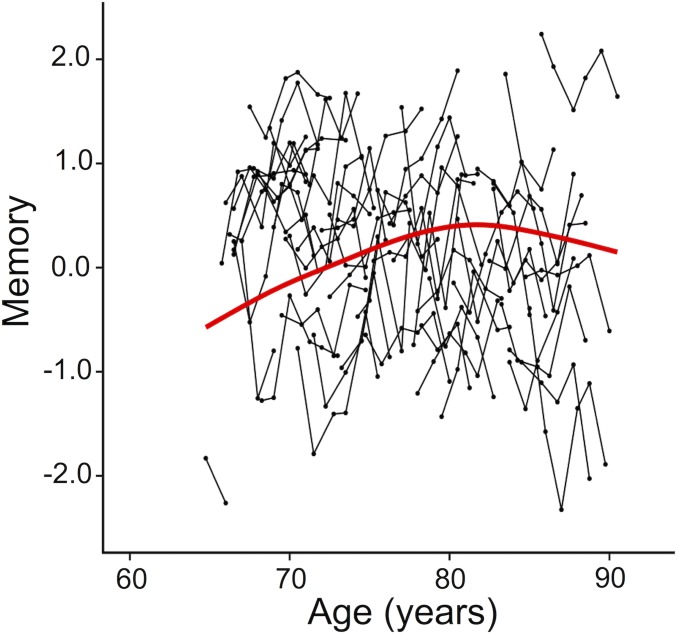
Cognitive functions differed depending on baseline age (significant age × time interactions reported in SI Appendix, Table S1: models 1–3a). To explore these interactions further, mean changes in cognitive performance were estimated using generalized additive mixed models (GAMM). GAMM plots revealed that cognitive decline accelerated after ∼80 y (Fig. 1 and SI Appendix, Fig. S1). In addition, the GAMM plot for memory also showed that older adults younger than ∼80 y showed increased performance over time, reflecting practice effects (Fig. 1 and SI Appendix, Results 1 and Fig. S2).
Fig. 1.
Factor scores for memory for 51 individuals from the main study sample of older adults. Black lines connect different measurements in the same individual. The red line indicates mean change, estimated using a GAMM.
Caudate Functional Dedifferentiation in Older Adults.
Voxelwise caudate parcellations.
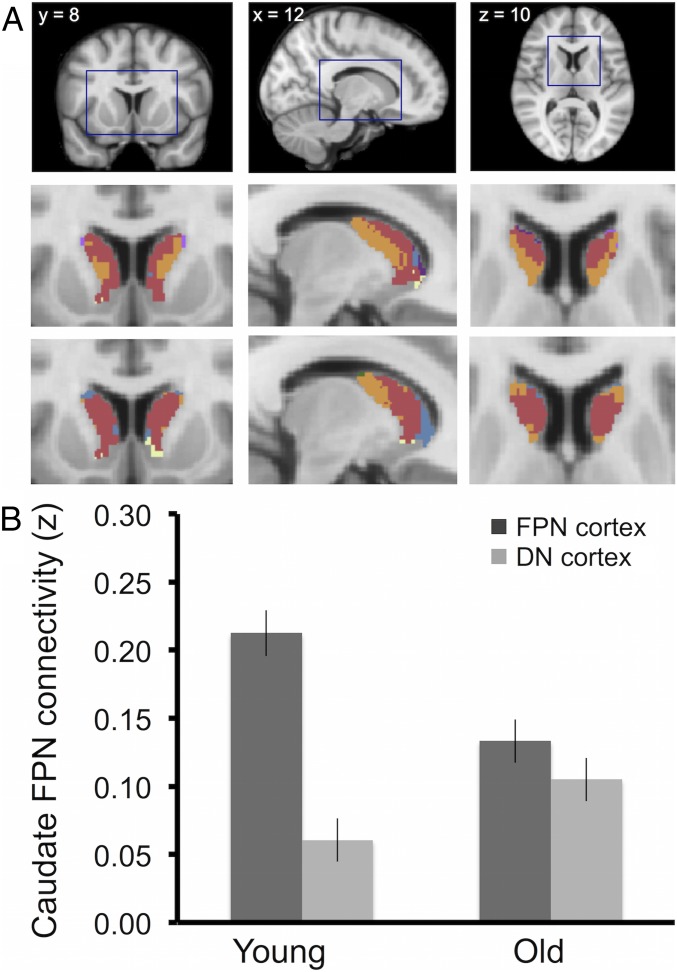
Following the same procedures as described for young adults in Choi et al. (7) (see Methods for details), voxelwise parcellations of the caudate showed that only 46% of voxels allocated to the FPN in young controls (n = 63) were also allocated to the FPN in older adults (n = 54; for comparison, the overlap in FPN-assigned voxels between young controls and the original parcellation reported in ref. 7 was 94%). As shown in Fig. 2A, the central lateral territories of the caudate assigned to the FPN (Fig. 2A, orange) in younger adults were assigned to the DN (Fig. 2A, red) instead in older adults. In contrast, the majority (72%) of voxels allocated to the DN (Fig. 2A, red) in young controls were also allocated to the DN (Fig. 2A, red) in older adults, suggesting age differences in caudate fc with cortical association networks predominantly affect the FPN subfield (i.e., central lateral caudate).
Fig. 2.
Dedifferentiation of caudate FPN connectivity in older adults. (A) Voxelwise parcellation of the caudate (highlighted, Top) in young (Middle) and the main study sample of older adults (Bottom). Orange voxels are allocated to the FPN and red voxels to the DN. Voxels of other colors are allocated to other cortical networks and are not discussed further because of their small extent and location at the borders with cerebrospinal fluid. (B) Mean connectivity strength (z) between caudate FPN ROI and cortical ROIs in the FPN and the DN. The pattern for older adults is less differentiated than that for younger adults (significant age × ROI interactions, P < 0.05).
Region of interest analyses.
Data from a priori-defined regions of interest (ROIs) in the caudate and cortical FPN and DN were used to confirm a statistically significant 2(cortical networks) × 2(age groups) interaction for the caudate FPN subfield, F(1, 115) = 14.09, P < 0.01. Compared with young adults, older adults displayed significantly reduced connectivity of the caudate FPN field with the cortical FPN, t(115) = −3.42, P < 0.01 but increased connectivity of the caudate FPN field with the cortical DN, t(115) = 1.98, P = 0.05 (Fig. 2B). For the DN caudate subfield, the interaction was not significant, F(1, 115) = 0.02, P = 0.88, confirming that the age group difference is predominant in the FPN subfield (i.e., central lateral caudate).
Seed-based whole-brain fc maps.
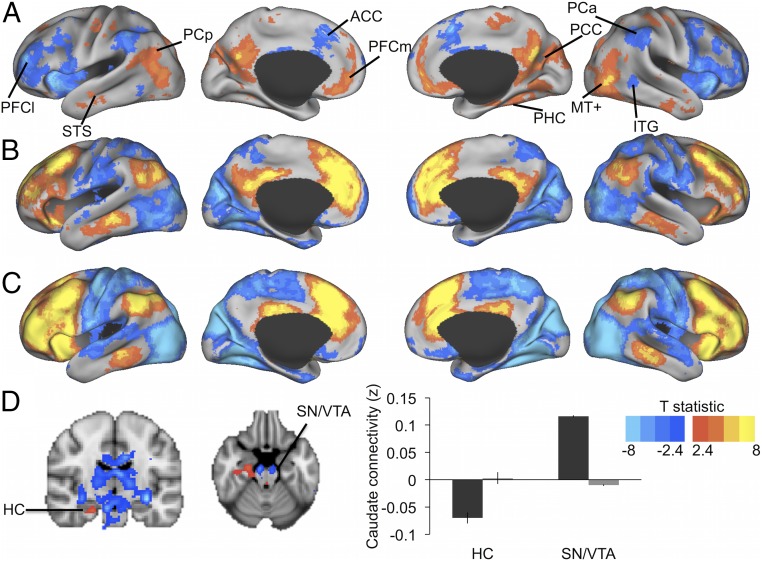
Seed-based whole-brain fc maps of the caudate FPN subfield were computed to visualize whether age group differences in fc of the caudate FPN subfield ROI were predominant for select regions within the cortical DN and FPN. Fig. 3A shows that this was not the case. Reduced connectivity of the caudate FPN subfield with the cortical FPN was apparent for the widespread lateral prefrontal cortex, anterior cingulate, anterior inferior parietal cortex, and inferior temporal gyrus. Increased connectivity of the caudate FPN subfield with the cortical DN was also widespread across key regions of the DN. These not only included the posterior cingulate cortex, medial prefrontal cortex, and posterior inferior parietal cortex, but also temporal regions of the DN that were not considered in the a priori ROIs (bilateral superior/middle temporal sulcus and right parahippocampal gyrus). The whole-brain analysis further showed that increased connectivity of the caudate FPN seed with cortical regions in older adults extended to sensory areas outside the association networks, including parts of the motor cortex, middle temporal area (MT+), and visual cortex. Fig. 3 B and C suggest that increased connectivity in older adults is largely due to lower decoupling of cortical areas outside the FPN from the caudate FPN (i.e., less negative fc).
Fig. 3.
Whole-brain fc of a bilateral caudate FPN seed [Montreal Neurological Institute (MNI): x = −12, y = 10, z = 8; x = 12, y = 10, z = 8]. (A) Older > young adults = red-yellow; older < young adults = blue. ACC, anterior cingulate cortex; ITG, inferior temporal gyrus; MT+, middle temporal area; PCa, anterior inferior parietal cortex; PCC, posterior cingulate cortex; PCp, posterior inferior parietal cortex; PFCl, lateral prefrontal cortex; PFCm, medial prefrontal cortex; PHC, parahippocampal sulcus; STS, superior temporal sulcus. (B and C) Mean connectivity map for older (B) and younger (C) adults. Red-yellow regions are positively connected with the seed, blue regions negatively. (D) Age group differences in subcortical connectivity with the caudate FPN seed. Older > young adults = red-yellow; older < young adults = blue. For illustration, mean functional connectivity estimates are shown thresholded at T > 2.4 (P < 0.01). Bar graph illustrates mean connectivity of the caudate FPN seed with the hippocampus (HC) and midbrain (SN/VTA) by age group (older adults = gray bars; younger adults = black bars).
Subcortically, the whole-brain analysis further revealed reduced connectivity of the caudate FPN seed with midbrain structures of the dopaminergic pathways (i.e., near the substantia nigra/ventral tegmental area) for older adults, and a failure to decouple the caudate FPN from the hippocampus. The hippocampus is functionally interrelated with the cortical DN in younger adults via the parahippocampal gyrus (17). Again, higher connectivity between the caudate FPN seed and hippocampus reflected lower decoupling in the older adults (i.e., less negative fc) (Fig. 3D).
Confounding variables.
Results from the voxelwise parcellation, ROI analyses, and seed-based whole-brain analysis were replicated in an independent sample of older adults (n = 45) (SI Appendix, Results 2). Moreover, the four ROI-based fc measures (compare with Region of interest analyses, above) did not correlate with measures of atrophy (i.e., gray matter volumes adjusted for intracranial volume), signal-to-noise ratio, or head motion (P > 0.05) (SI Appendix, Table S2).
Caudate Dedifferentiation and Striatal Dopamine Transporter Density Are Independent Predictors of Memory Change.
Caudate fc predicting cognitive change.
Analyses relating individual differences in caudate fc at baseline to change in cognitive performance in older adults were focused on the ROI-based connectivity estimates of the caudate FPN subfield with the cortical FPN (FPN–FPN) and with the cortical DN (FPN–DN) (see Methods for details). These correspond to the connections where group differences emerged in the ROI-based comparison of young and older subjects (compare with Fig. 2B).
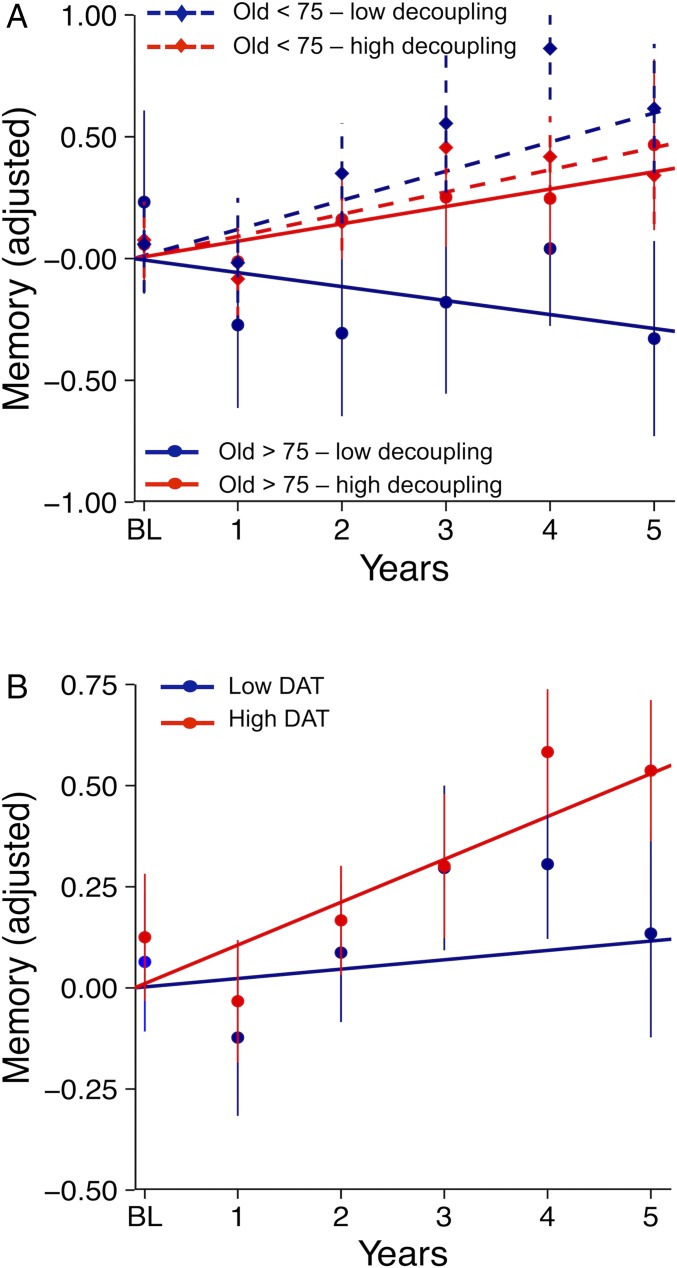
Linear mixed-effects models with cognitive performance as the outcome (memory, executive function, and processing speed tested separately) found a significant three-way interaction between baseline age, time, and FPN–DN for memory only (SI Appendix, Table S1, model 3b). Fig. 4A shows that reduced decoupling of the caudate FPN subfield with the cortical DN (i.e., increased connectivity) had no effect in older adults aged 65–75 y but predicted memory change over time in older adults 75 y and older.
Fig. 4.
Predictors of memory change. Memory decline is pronounced in individuals over age 75 y with low decoupling between the caudate FPN subfield and the cortical DN (A) and low striatal DAT (B). For all groups, lines are the estimated mean slopes for memory change from a linear mixed-effects model with random intercept and fixed slope. Intercepts are adjusted to zero for illustration. Points are the corresponding observed mean values for memory (with SE) at each time point. <75 = older adults under age 75, >75 = older adults 75+ y.
An analysis of individual nodes showed that, while differences between young and older adults in decoupling of the caudate FPN subfield and cortical areas generalize across the DN network (compare with Fig. 2), the association with memory in the older group was mainly driven by lower decoupling of the caudate FPN field from the posterior parietal cortex and hippocampus (SI Appendix, Results 3).
Striatal dopamine transporter density predicting cognitive change.
Within the group of older adults, age and striatal dopamine transporter (DAT) were modestly but not significantly correlated (r = −0.25, P = 0.07). Striatal DAT was not correlated with FPN–DN (r = 0.06, P = 0.66) or FPN–FPN (r = 0.09, P = 0.50).
When striatal DAT density was also entered in the model containing FPN–DN, it emerged as an independent predictor of memory decline (SI Appendix, Table S1, model 3c), not interacting further with baseline age (estimate: −0.003, SE = 0.003, P = 0.24), FPN–DN (estimate: −0.02, SE = 0.03, P = 0.43), or FPN–FPN (estimate: −0.02, SE = 0.02, P = 0.32). Fig. 4B shows that those older adults with low levels of striatal DAT (based on median split) had weaker practice effect than those with higher levels of DAT density. Collectively, these results suggest that an additive effect of functional and neurochemical changes in striatum on memory emerges with advancing age.
We detected no significant association between striatal DAT density and processing speed or executive function at baseline (Ps < 0.50, covarying for age) and no significant association between striatal DAT density and change in executive function or processing speed over time (Ps > 0.16, including models with only age as a covariate or full models including the same set of predictors as for memory).
Replication analysis.
Taking into consideration the small sample size, it is important to note that the complex associations between mean network fc, age, and change in memory replicated in the age-matched sample of older adults with high-quality MR data (SI Appendix, Results 4). PET data were unavailable for the replication sample.
Discussion
Utilizing multimodal imaging and repeated cognitive assessments in a sample of healthy older adults, we identified that individual differences in caudate connectivity with large-scale association networks predict change in memory over subsequent years. Multiple analyses converged to show that the central lateral portion of the caudate body is functionally connected with the FPN in young adults and decoupled from other cortical networks, including the DN. In older adults, this pattern is less differentiated, seen by reduced connectivity with the FPN and a lack of decoupling from the DN. Notably, functional dedifferentiation of the caudate (i.e., less decoupling of the central lateral caudate from cortical DN) was unrelated to age-related loss of DAT, one of the most prominent neurobiological changes in an aging striatum that is also associated with memory decline.
Age-Related Dedifferentiation of Caudate Functional Connectivity.
While age-related differences in fc are predominantly characterized by reduced connectivity within networks that support cognition (18), increases in fc for older adults are often found between networks (e.g., refs. 10–12 and 19–24). These studies found that: (i) increased connectivity in older adults typically reflects less negative connectivity between two regions belonging to different networks, and (ii) increased connectivity is often negatively associated with cognitive performance. Taking these data together, we find that decreases within networks and increases between networks are thus reflective of a less selective—or differentiated—cortical network organization in aging. Here, we show that age-related dedifferentiation of large-scale networks that support cognition includes the caudate. Moreover, as shown in the voxelwise whole-brain analysis, an age-related difference in decoupling of the central lateral caudate and the DN also included the hippocampus. A medial temporal lobe system involved in episodic memory and a corticostriatal system involved in learning and memory are often discussed separately in terms of their involvement in cognition and age-related cognitive decline (2, 25). Here, we show that a functional separation of a corticostriatal system from a medial temporal lobe system becomes blurred in older adults and is associated with memory decline.
Loss of DATs Is Independent of Caudate Connectivity.
The aging brain is characterized by age-related degeneration of the brain’s white matter pathways, gray matter atrophy, and reduced neurotransmitter activity (e.g., refs. 25–27). In the striatum, lower density of dopamine receptors and transporters in aging are particularly prominent (15). Because dopamine is thought to play a critical role in optimizing signal-to-noise ratio in neuronal circuits (16), we hypothesized that lower striatal dopamine function would be associated with functional dedifferentiation of cortical–caudate connectivity. For this reason, participants of the main study sample were tested with both MRI and PET to assess DAT density, a presynaptic marker of the dopamine system. Contrary to our hypothesis, we found no evidence that caudate fc estimates in older adults were associated with age-related declines in DAT density. One possibility is that our marker of the striatal dopamine system did not adequately characterize the complexity of neurochemical alterations. While the presynaptic DAT marker can reliably distinguish age-related loss from pathological loss (e.g., refs. 28 and 29), postsynaptic receptor markers or markers of dopamine synthesis capacity may assess unique aspects of the dopamine system that are sensitive to a modulatory role of dopamine in striatal connectivity (4, 30, 31). That said, our marker did emerge as an independent predictor of memory decline in aging, indicating that it was successful in capturing age-related declines in the dopamine system that are relevant to cognition. This raises the question whether caudate connectivity dedifferentiation is more reflective of age-related changes in the target cortical region. In the cortex, reduced glucose metabolism and increased amyloid deposition are, for example, particularly prominent in the cortical DN (32–35). In support of this hypothesis, a recent study in a superset (n = 237) of the current samples showed that lower fc in the DN predicts cognitive decline, particularly in those individuals harboring amyloid burden (36). Future work with multiple markers, including subcortical dopaminergic markers and cortical amyloid markers, in larger samples will be needed to explore this hypothesis further.
Associations Between Caudate Connectivity and Cognition Are Specific to Memory.
While higher DAT density was associated with better memory performance over time across the sample, functional caudate dedifferentiation was associated with memory decline only in older adults >75 y. Notably, these relations between striatal markers and cognition were specific to memory, with no significant effects for processing speed and executive function. This supports a recent cross-sectional finding that the association between striatal D2 receptor densities in aging and cognition was specific to memory (4), as well as reports that both genetically determined and pharmacologically induced increases in dopamine concentration benefit episodic memory in aging (37, 38).
Our seed-specific ROI analyses further showed that reduced decoupling of the central lateral caudate with the inferior parietal cortex and hippocampus are associated with subsequent change in memory performance over time. Future work in patients is needed to explore whether our results have additional implications for understanding pathological memory deficits: for example, whether functional dedifferentiation of the medial temporal lobe and the striatal system in aging may be an early indicator of Alzheimer’s disease or Parkinson’s pathology.
Study Strengths and Limitations.
We acknowledge several limitations of our study sample and design. First, while our main study sample is unique in its combination of DAT imaging, fMRI imaging and longitudinal assessments of cognitive decline over several years, the number of participants is small. While we are able to replicate our findings regarding fc and cognitive decline in an independent sample, no such data were available for the results that included DAT imaging. Replication of these effects in independent samples is warranted.
A further limitation of our study design is the assessment of age differences by an extreme age group comparison. Within the older age group, the interaction in the wide age span examined is suggestive of a “break point” in advanced age in terms of predictors of memory decline. However, no such conclusions can be generated for our estimate of age differences in caudate fc. Both life span samples and longitudinal imaging data are necessary to further identify at which point dedifferentiation of caudate fc occurs.
Conclusions
Compared with younger adults, older adults displayed lower coupling of the central lateral caudate to the FPN and lower decoupling from regions outside the FPN, predominantly those within the DN and including the hippocampal complex. Less decoupling between caudate and the DN was not related to age-sensitive markers of striatal DAT, suggesting that aging-related changes in the cortex or medial temporal lobe may contribute to dedifferentiation of cortical–caudate connectivity.
The study further showed that lower decoupling of the caudate and DN/hippocampus was specifically associated with memory decline in aging, leading us to conclude that an age-related difference in selectivity of cortical–caudate connections is related to a dedifferentiation of memory systems in the aging brain.
Whereas caudate connectivity predicted memory decline only in older adults >75 y, striatal DAT had an independent association with memory decline across all older adults, suggesting an additive influence on memory that emerges with advancing old age. Collectively, the study provides evidence highlighting the importance of age-related changes in striatal function for understanding memory decline in normal aging.
Methods
Participants.
Data from three samples are presented: (i) the main study sample of 54 clinically normal older adults (65–86 y), (ii) a young MRI control sample of 63 healthy adults (18–32 y), and (iii) an independent “replication sample” of 45 clinically normal older adults (69–89). All samples are participants of the larger Harvard Aging Brain study (39). All experimental procedures were performed with the understanding and written consent of the subject and the study was approved by the Partners Healthcare Institutional Review Board. The samples are described in detail in SI Appendix, Methods 1 and Table S3.
Cognitive Measures.
A neuropsychological battery was administered to older adults at baseline and at each annual follow-up visit. Individual tests included phonemic fluency, letter–number sequencing, the Trail Making test, the Digit-Symbol test, the Logical Memory test, the Free and Cued Selective Reminding test, and the Six-Trial Selective Reminding test. Factor scores for episodic memory, processing speed, and executive function were modeled after a previously reported cross-sectional factor analysis in the Harvard Aging Brain sample (40) and adapted to include only tests with repeated administration across all time points (41).
Included participants had complete data for at least two time points. When there were missing data at any time point, factor scores for one or more cognitive domains were excluded for that time point only (in total one observation for memory, and two observations each for processing speed and executive function).
MRI Processing.
MRI acquisitions are described in SI Appendix, Methods 2. fMRI and structural data were preprocessed and aligned using boundary-based within-subject alignment and nonlinear deformations to standard space (SI Appendix, Methods 2 and Fig. S6).
Voxelwise parcellation.
Age-related differences in fc between caudate subfields and large-scale association networks were explored with a voxelwise parcellation of the caudate in each age group. We chose this method as the starting point for fMRI fc analyses because it is purely data driven and does not require initiating seeds or ROIs that may bias the results to any one age group.
The methods follow those described in refs. 7 and 42. Briefly, a clustering algorithm was applied to partition the cortical surface into seven large-scale distributed networks, which correspond to the motor and visual networks, dorsal and ventral attention networks, a limbic network, the frontal-parietal control network, and the DN (42). Then, each voxel in the caudate head and body was assigned to one of the seven cortical networks based on its top 25 most-correlated cortical vertices.
In keeping with the nomenclature from refs. 7, 42, and 43, voxels in caudate that were assigned to the FPN were color-coded in orange and voxels assigned to the DN were color-coded in red (Fig. 2). A few voxels at the edges of the caudate were assigned to a third network (the motor network) and coded in blue. We refrain from interpreting group differences in these edge voxels further because of uncertainties in alignment ( SI Appendix, Fig. S6).
Post hoc follow-up analysis.
While group-based voxelwise parcellations have the advantage that they are not biased to the selection of a specific seed, the method does not readily allow for individual difference statistics. Moreover, as the voxelwise assignment to a network relies on a “winner takes all” strategy, a more subtle connectivity pattern, such as coupling of one voxel to two cortical networks, may be missed. Therefore, following the data-driven approach, ROI data were used to explore group differences in caudate connectivity with the DN and FPN further. Two 6-mm spherical ROIs were placed in the caudate to reflect the FPN and DN subfields, and three bilateral cortical ROIs each to reflect key nodes of the FPN and DN. Center coordinates for all ROIs are listed in SI Appendix, Table S4 and were chosen a priori, based on those reported previously in an independent sample (7).
Fc was computed by correlating the fMRI (residual) time series between each caudate ROI and each cortical network ROI (average of three nodes), resulting in four measures for each participant, (FPN–FPN, FPN–DN, DN–FPN, DN–DN, where the first abbreviation denotes the caudate subfield and the second abbreviation the cortical network). These were compared between age groups and used to study associations with cognitive decline.
Voxelwise seed-based analysis.
A final set of fMRI analyses used whole brain, seed-based fc maps of the caudate FPN subfield. This was used to confirm that age differences in caudate connectivity with cortical association networks generalize across the cortical network and also to identify potential subcortical correlates of the caudate (e.g., hippocampus, brainstem, thalamus). Voxelwise fc maps for the caudate FPN ROI (compare with the previous section) were computed for each subject and compared between age groups using an independent t test. Permutation testing in FSL was used to output thresholded t statistic maps (t > 2.4), that were reweighted and corrected for multiple comparisons using threshold-free cluster enhancement (44).
PET Processing.
A C-11 Altropane PET scan was acquired with an HR+ (CTI) PET camera (3D mode, 63 adjacent slices of 2.42-mm interval, 15.2-cm axial field of view, 5.60-mm transaxial resolution) (SI Appendix, Methods 5). A voxelwise correction for partial volume effects was computed using the T1-weighted image (45), as implemented in PVElab Software with SPM5 (46). Radioactivity concentration C was estimated as a function of time t for the striatum and for a reference region (cerebellum). The cumulative integral of the reference region curve was plotted against the cumulative integral of the ROI curve (normalized by Ct). The distribution volume ratio corresponds to the slope of this function for a time window at which the function is linear (47). Distribution volume ratio is an estimate of striatal DAT density.
Because prior work has shown DAT density losses in aging to be uniform across caudate and putamen (48), DAT densities were computed in a large ROI in central striatum, which minimizes noise and the contamination of residual partial volume effects in the PET images. The striatal ROI was defined as the 1,000 voxels with the highest signal intensity on the average emission image. Previous work has shown that this approach is unrelated to subcortical atrophy and microstructural abnormalities but sensitive to age-related decline in DAT density (49) and to pathological loss of DAT in Parkinson’s disease (29).
Statistical Models.
A series of linear mixed-effects models were used to estimate the relation of striatal fc estimates and DAT density with longitudinal changes in cognition. These are described in detail in SI Appendix, Methods 6.
Supplementary Material
Acknowledgments
We thank Koene Van Dijk, Emily Smith, Aaron Schultz, and Thomas Yeo for their contributions to data collection and analysis. This work was supported by National Institute on Aging Grants R01 AG054110, R01 AG053509, R01 AG034556, P01 AG036694, P50 AG005134, K24 AG035007, and K01 AG040197. This research was carried out in part at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Biotechnology Resource Grant supported by the National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health. This work also involved the use of instrumentation supported by the NIH Shared Instrumentation Grant Program and High-End Instrumentation Grant Program: specifically, Grants S10RR023401, S10RR019307, S10RR019254, and S10RR023043. A.R. was supported by the Swedish Research Council (Vetenskapsrådet).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1804641115/-/DCSupplemental.
References
- 1.Hedden T, et al. Multiple brain markers are linked to age-related variation in cognition. Cereb Cortex. 2016;26:1388–1400. doi: 10.1093/cercor/bhu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckner RL. Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Fjell AM, et al. Brain events underlying episodic memory changes in aging: A longitudinal investigation of structural and functional connectivity. Cereb Cortex. 2016;26:1272–1286. doi: 10.1093/cercor/bhv102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nyberg L, et al. Dopamine D2 receptor availability is linked to hippocampal-caudate functional connectivity and episodic memory. Proc Natl Acad Sci USA. 2016;113:7918–7923. doi: 10.1073/pnas.1606309113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: Parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- 6.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 7.Choi EY, Yeo BTT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108:2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 10.Geerligs L, Maurits NM, Renken RJ, Lorist MM. Reduced specificity of functional connectivity in the aging brain during task performance. Hum Brain Mapp. 2014;35:319–330. doi: 10.1002/hbm.22175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geerligs L, Renken RJ, Saliasi E, Maurits NM, Lorist MM. A brain-wide study of age-related changes in functional connectivity. Cereb Cortex. 2015;25:1987–1999. doi: 10.1093/cercor/bhu012. [DOI] [PubMed] [Google Scholar]
- 12.Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS. Decreased segregation of brain systems across the healthy adult lifespan. Proc Natl Acad Sci USA. 2014;111:E4997–E5006. doi: 10.1073/pnas.1415122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goh JOS. Functional dedifferentiation and altered connectivity in older adults: Neural accounts of cognitive aging. Aging Dis. 2011;2:30–48. [PMC free article] [PubMed] [Google Scholar]
- 14.Park J, et al. Neural broadening or neural attenuation? Investigating age-related dedifferentiation in the face network in a large lifespan sample. J Neurosci. 2012;32:2154–2158. doi: 10.1523/JNEUROSCI.4494-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rieckmann A, Buckner RL, Hedden T. Molecular imaging of aging and neurodegenerative disease. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive Neuroscience of Aging. 2nd Ed. Oxford Univ Press; New York: 2016. pp. 35–69. [Google Scholar]
- 16.Servan-Schreiber D, Printz H, Cohen JD. A network model of catecholamine effects: Gain, signal-to-noise ratio, and behavior. Science. 1990;249:892–895. doi: 10.1126/science.2392679. [DOI] [PubMed] [Google Scholar]
- 17.Ward AM, et al. The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Hum Brain Mapp. 2014;35:1061–1073. doi: 10.1002/hbm.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews-Hanna JR, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hampson M, Driesen N, Roth JK, Gore JC, Constable RT. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magn Reson Imaging. 2010;28:1051–1057. doi: 10.1016/j.mri.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu M, et al. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res. 2006;148:133–142. doi: 10.1016/j.pscychresns.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spreng RN, Schacter DL. Default network modulation and large-scale network interactivity in healthy young and old adults. Cereb Cortex. 2012;22:2610–2621. doi: 10.1093/cercor/bhr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keller JB, et al. Resting-state anticorrelations between medial and lateral prefrontal cortex: Association with working memory, aging, and individual differences. Cortex. 2015;64:271–280. doi: 10.1016/j.cortex.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avelar-Pereira B, Bäckman L, Wåhlin A, Nyberg L, Salami A. Age-related differences in dynamic interactions among default mode, frontoparietal control, and dorsal attention networks during resting-state and interference resolution. Front Aging Neurosci. 2017;9:152. doi: 10.3389/fnagi.2017.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell KL, Grigg O, Saverino C, Churchill N, Grady CL. Age differences in the intrinsic functional connectivity of default network subsystems. Front Aging Neurosci. 2013;5:73. doi: 10.3389/fnagi.2013.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jagust W. Vulnerable neural systems and the borderland of brain aging and neurodegeneration. Neuron. 2013;77:219–234. doi: 10.1016/j.neuron.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: Contributions from diffusion tensor imaging. Neuropsychol Rev. 2009;19:415–435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raz N, et al. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 28.Frost JJ, et al. Positron emission tomographic imaging of the dopamine transporter with 11C-WIN 35,428 reveals marked declines in mild Parkinson’s disease. Ann Neurol. 1993;34:423–431. doi: 10.1002/ana.410340331. [DOI] [PubMed] [Google Scholar]
- 29.Rieckmann A, Gomperts SN, Johnson KA, Growdon JH, Van Dijk KR. Putamen-midbrain functional connectivity is related to striatal dopamine transporter availability in patients with Lewy body diseases. Neuroimage Clin. 2015;8:554–559. doi: 10.1016/j.nicl.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tziortzi AC, et al. Connectivity-based functional analysis of dopamine release in the striatum using diffusion-weighted MRI and positron emission tomography. Cereb Cortex. 2014;24:1165–1177. doi: 10.1093/cercor/bhs397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klostermann EC, Braskie MN, Landau SM, O’Neil JP, Jagust WJ. Dopamine and frontostriatal networks in cognitive aging. Neurobiol Aging. 2012;33:623.e15–623.e24. doi: 10.1016/j.neurobiolaging.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minoshima S, et al. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 33.Benson DF, et al. The fluorodeoxyglucose 18F scan in Alzheimer’s disease and multi-infarct dementia. Arch Neurol. 1983;40:711–714. doi: 10.1001/archneur.1983.04050110029003. [DOI] [PubMed] [Google Scholar]
- 34.Buckner RL, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: Evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mintun MA, et al. [11C]PIB in a nondemented population: Potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 36.Buckley RF, et al. Functional network integrity presages cognitive decline in preclinical Alzheimer disease. Neurology. 2017;89:29–37. doi: 10.1212/WNL.0000000000004059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chowdhury R, Guitart-Masip M, Bunzeck N, Dolan RJ, Düzel E. Dopamine modulates episodic memory persistence in old age. J Neurosci. 2012;32:14193–14204. doi: 10.1523/JNEUROSCI.1278-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raz N, Rodrigue KM, Kennedy KM, Land S. Genetic and vascular modifiers of age-sensitive cognitive skills: Effects of COMT, BDNF, ApoE, and hypertension. Neuropsychology. 2009;23:105–116. doi: 10.1037/a0013487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dagley A, et al. Harvard aging brain study: Dataset and accessibility. Neuroimage. 2017;144:255–258. doi: 10.1016/j.neuroimage.2015.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hedden T, et al. Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. J Neurosci. 2012;32:16233–16242. doi: 10.1523/JNEUROSCI.2462-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rabin JS, et al. Global white matter diffusion characteristics predict longitudinal cognitive change independently of amyloid status in clinically normal older adults. Cereb Cortex. 2018;139:1877. doi: 10.1093/cercor/bhy031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeo BTT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 45.Meltzer CC, Leal JP, Mayberg HS, Wagner HN, Jr, Frost JJ. Correction of PET data for partial volume effects in human cerebral cortex by MR imaging. J Comput Assist Tomogr. 1990;14:561–570. doi: 10.1097/00004728-199007000-00011. [DOI] [PubMed] [Google Scholar]
- 46.Quarantelli M, et al. Integrated software for the analysis of brain PET/SPECT studies with partial-volume-effect correction. J Nucl Med. 2004;45:192–201. [PubMed] [Google Scholar]
- 47.Logan J, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- 48.van Dyck CH, et al. Age-related decline in dopamine transporters: Analysis of striatal subregions, nonlinear effects, and hemispheric asymmetries. Am J Geriatr Psychiatry. 2002;10:36–43. [PubMed] [Google Scholar]
- 49.Rieckmann A, et al. Dopamine transporter availability in clinically normal aging is associated with individual differences in white matter integrity. Hum Brain Mapp. 2016;37:621–631. doi: 10.1002/hbm.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.