Significance
The human pathogen Pseudomonas aeruginosa is the leading cause of hospital-acquired infections and, moreover, is resistant to commonly used antibiotics. P. aeruginosa uses the cell-to-cell communication process called quorum sensing (QS) to control virulence. QS relies on production and response to extracellular signaling molecules called autoinducers. Here, we identify the PqsE enzyme as the synthase of an autoinducer that activates the QS receptor RhlR. We show that the PqsE-derived autoinducer is the key molecule driving P. aeruginosa biofilm formation and virulence in animal models of infection. We propose that PqsE and RhlR constitute a QS synthase–receptor pair, and that this system can be targeted for antimicrobial development.
Keywords: quorum sensing, biofilms, Pseudomonas aeruginosa, antimicrobial, virulence
Abstract
Pseudomonas aeruginosa is a leading cause of life-threatening nosocomial infections. Many virulence factors produced by P. aeruginosa are controlled by the cell-to-cell communication process called quorum sensing (QS). QS depends on the synthesis, release, and groupwide response to extracellular signaling molecules called autoinducers. P. aeruginosa possesses two canonical LuxI/R-type QS systems, LasI/R and RhlI/R, that produce and detect 3OC12-homoserine lactone and C4-homoserine lactone, respectively. Previously, we discovered that RhlR regulates both RhlI-dependent and RhlI-independent regulons, and we proposed that an alternative ligand functions together with RhlR to control the target genes in the absence of RhlI. Here, we report the identification of an enzyme, PqsE, which is the alternative-ligand synthase. Using biofilm analyses, reporter assays, site-directed mutagenesis, protein biochemistry, and animal infection studies, we show that the PqsE-produced alternative ligand is the key autoinducer that promotes virulence gene expression. Thus, PqsE can be targeted for therapeutic intervention. Furthermore, this work shows that PqsE and RhlR function as a QS-autoinducer synthase–receptor pair that drives group behaviors in P. aeruginosa.
Multidrug-resistant Pseudomonas aeruginosa is the leading cause of hospital-acquired infections in the United States (1). P. aeruginosa infections are difficult to eradicate because of antibiotic resistance and because P. aeruginosa forms communities called biofilms during infection (2). Essential for virulence and biofilm development in P. aeruginosa is a cell–cell communication process called quorum sensing (QS) (3, 4). QS allows bacteria to assess and collectively respond to changes in population density (reviewed in ref. 5). QS relies on the production, detection, and groupwide response to extracellular signaling molecules called autoinducers. In a typical Gram-negative bacterial QS system, an acyl-homoserine lactone autoinducer synthase, usually a LuxI homolog, produces an autoinducer that is bound by a partner transcription factor, usually a LuxR homolog. Most LuxR-type receptors function only when bound to their cognate autoinducers. Furthermore, autoinducer binding is required for some LuxR-type proteins to fold, and thus resist proteolysis (6).
The P. aeruginosa QS circuit consists of two LuxI/R type pairs: LasI/R and RhlI/R (7, 8). LasI produces and LasR responds to the autoinducer 3OC12-homoserine lactone (3OC12-HSL). The LasR:3OC12-HSL complex activates transcription of many genes including rhlR, encoding a second QS receptor. RhlR binds to the autoinducer C4-homoserine lactone (C4-HSL), the product of RhlI. RhlR:C4-HSL also directs a large regulon of genes, including those encoding secondary metabolites such as rhamnolipids (9–11) and pyocyanin (12, 13), that play crucial roles in virulence and biofilm formation. Typically, mutations in QS luxI-type and luxR-type genes (i.e., lasI-lasR and rhlI-rhlR) confer identical phenotypes because each component of the pair needs the other to function (5). However, we previously discovered that RhlR directs both RhlI-dependent and RhlI-independent regulons (14). Importantly, we showed that ΔrhlI mutant cell-free culture fluids contain an activity that stimulates RhlR-dependent gene expression, indicating that another autoinducer (an alternative ligand) must exist (14).
Here, we discover that PqsE is required for production of the alternative ligand that activates the RhlR QS receptor in the absence of the canonical autoinducer C4-HSL. PqsE is a thioesterase and residues in the active site are required for alternative-ligand synthesis. The PqsE-derived alternative ligand is sufficient to activate RhlR, and we identify residues in the RhlR ligand binding domain that are required for the response to the alternative ligand. Thus, PqsE and RhlR function as an autoinducer synthase–receptor pair that activates expression of genes specifying P. aeruginosa group behaviors. We demonstrate that PqsE is required and RhlI is dispensable for RhlR-directed virulence in animals.
Results
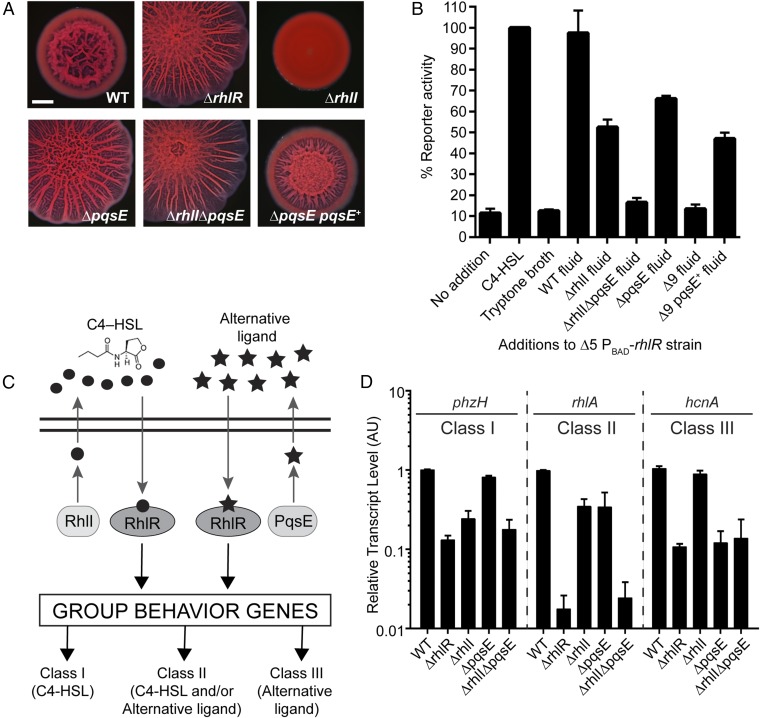
A Screen to Identify the Alternative-Ligand Synthase.
We recently discovered that the P. aeruginosa QS receptor RhlR regulates hundreds of genes in the absence of its partner synthase RhlI, and thus in the absence of its canonical autoinducer C4-HSL (14). Specifically, on Congo red agar biofilm medium, although WT P. aeruginosa UCBPP-PA14 (hereafter called PA14) exhibits a rugose-center/smooth-periphery colony biofilm phenotype, the ΔrhlR mutant is hyper-rugose because it fails to produce phenazines (e.g., pyocyanin) (12, 13). In contrast, the ΔrhlI mutant is smooth because of the overproduction of phenazines (Fig. 1A). Thus, unlike most QS receptor–synthase pairs, the ΔrhlR and ΔrhlI mutants do not have identical phenotypes. We discovered that ΔrhlI mutant cell-free culture fluids contain an activity (hereafter called the alternative ligand) that stimulates RhlR-dependent target gene expression (14).
Fig. 1.
Identification of pqsE as the alternative-ligand synthase. (A) Colony biofilm phenotypes of WT PA14 and the designated mutants on Congo red agar medium after 5 d of growth. pqsE+ refers to complementation with pqsE under the Plac promoter on the pUCP18 plasmid. (Scale bar, 2 mm.) (B) rhlA expression was measured using a chromosomally encoded PrhlA-mNeonGreen transcriptional reporter. The PA14 strain used in this analysis is ΔlasR ΔlasI ΔrhlR ΔrhlI ΔpqsE carrying PBAD-rhlR on the chromosome; designated Δ5 PBAD-rhlR. rhlR was induced with 0.1% l-arabinose. The rhlA reporter activity was set to 100% when 10 µM C4-HSL was added (second bar). For all other bars, either 30% (vol/vol) of broth or the indicated cell-free culture fluid was added. The bar designated Δ9 refers to culture fluid from the ΔlasR ΔlasI ΔrhlR ΔrhlI ΔpqsABCDE strain. The bar designated Δ9 pqsE+ refers to culture fluid from the ΔlasR ΔlasI ΔrhlR ΔrhlI ΔpqsABCDE strain carrying pqsE on the pUCP18 plasmid under the Plac promoter. (C) Schematic of the RhlR-dependent QS system: RhlI makes C4-HSL (circles), and here, PqsE is discovered to be required for alternative-ligand (stars) synthesis. The two black horizontal lines represent the cytoplasmic membrane. Class I, Class II, and Class III denote RhlI-dependent, partially dependent, and independent genes, respectively. Class II and III genes require the alternative ligand for full expression. (D) Relative expression of the representative RhlR-regulated genes phzH, rhlA, and hcnA measured by qRT-PCR in the WT and designated mutants grown planktonically to HCD (OD600 = 2.0). Data are normalized to 5S RNA levels. AU denotes arbitrary units. (B and D) Error bars represent SEM for three biological replicates.
In this study, our goal was to identify the gene or genes required for synthesis of the alternative ligand. We took a mutagenesis approach with the following rationale: in the ΔrhlI mutant that makes no C4-HSL autoinducer, disruption of the gene encoding the alternative-ligand synthase would eliminate production of the alternative ligand. As a consequence, RhlR would be rendered inactive because of the absence of both of its ligands. Thus, phenazine production would be abolished, which would confer the hyper-rugose colony biofilm phenotype to the strain. Such a mutant would also fail to make the alternative ligand that remains present in cell-free culture fluids of the ΔrhlI strain (14). Before screening, we first eliminated two obvious candidates: HdtS, a non-LuxI autoinducer synthase (15), and AmbBCDE, the enzymes that produce the Integrated Quorum Sensing Signal [2-(2-hydroxyphenyl)-thiazole-4-carbaldehyde] (16). We made single ΔhdtS and ΔambB mutants and double ΔrhlI ΔhdtS and ΔrhlI ΔambB mutants. None had the hyper-rugose colony biofilm phenotype (SI Appendix, Fig. S1A), and all possessed alternative-ligand activity in their cell-free culture fluids (SI Appendix, Fig. S1B). Thus, neither HdtS nor AmbB is involved in alternative-ligand synthesis, and the Integrated Quorum Sensing Signal cannot be the alternative ligand.
To discover the alternative-ligand synthase, we randomly mutagenized the ΔrhlI strain using the Tn5 IS50L derivative ISlacZ/hah (17). We screened ∼10,000 colonies for those exhibiting the hyper-rugose colony biofilm phenotype. Transposon insertions were located in genes encoding hypothetical proteins, as well as proteins involved in motility and c-di-GMP production, which are known to affect the colony biofilm phenotype (SI Appendix, Table S1) (18, 19). We were particularly intrigued to identify multiple transposon insertions in pqsA and pqsD of the pqsABCDE operon, and we focused on these mutants here (SI Appendix, Fig. S2A). PqsABCD, but not PqsE, is required for the synthesis of PQS (2-heptyl-3-hydroxy-4-quinolone) (20) and other quinolones (SI Appendix, Fig. S2B) (21). This result was surprising, as we have previously shown that PQS is not the alternative ligand (14). To test whether another quinolone produced by PqsABCDE is the alternative ligand, pqsA and pqsD were deleted in the WT and ΔrhlI strains and the colony biofilm phenotypes assayed. The ΔpqsA and ΔpqsD mutants have approximately WT colony biofilm phenotypes, and the ΔrhlI ΔpqsA and ΔrhlI ΔpqsD double mutants form smooth colony biofilms (SI Appendix, Fig. S2C). We therefore infer that neither of these genes is responsible for alternative-ligand production. Because transposon insertions can be polar, and pqsA and pqsD lie upstream of pqsE, PqsE remained a candidate for the alternative-ligand synthase. We investigated this possibility by generating a deletion of pqsE in the WT and ΔrhlI strains. Indeed, the ΔpqsE mutant displays a hyper-rugose colony biofilm phenotype, and the ΔrhlI ΔpqsE double mutant has a colony biofilm phenotype indistinguishable from the ΔrhlR mutant (Fig. 1A). Introduction of a plasmid carrying pqsE restored the WT colony biofilm phenotype (Fig. 1A).
To examine whether PqsE is required for production of the alternative ligand, we constructed a PA14 RhlR-dependent reporter strain harboring deletions in the lasR, lasI, rhlR, rhlI, and pqsE genes that contains a chromosomal PrhlA-mNeonGreen transcriptional reporter fusion and chromosomal, arabinose-inducible rhlR (this strain is called Δ5 PBAD-rhlR). Our results are shown in Fig. 1B. The addition of tryptone broth results in background levels of reporter activity similar to a no-addition control. Supplementation with synthetic C4-HSL or with WT cell-free culture fluids elicit maximal (∼ninefold) reporter activity. Addition of cell-free culture fluids from the ΔrhlI mutant fosters ∼fivefold induction of the reporter. Most important, in contrast to the ΔrhlI strain, cell-free culture fluids from the ΔrhlI ΔpqsE double mutant fail to stimulate the reporter. Last, cell-free culture fluids from the single ΔpqsE mutant show significant (∼sixfold) activity because they contain C4-HSL made by RhlI. To explore this finding further, we generated a nonuple mutant lacking the lasR, lasI, rhlR, rhlI, and pqsABCDE genes (we call this strain Δ9). Cell-free culture fluids from the Δ9 mutant elicit only background reporter activity. However, introduction of pqsE into the Δ9 strain restored activity, as shown by the ability of the fluids to stimulate RhlR-dependent reporter gene expression comparable to that elicited by the ΔrhlI strain (Fig. 1B). We conclude that PqsE is required for alternative-ligand synthesis.
The PqsE-Derived Ligand Activates Class II and III but Not Class I RhlR Target Genes.
We previously showed that there exist three classes of RhlR-regulated genes based on whether C4-HSL, the alternative ligand, or both autoinducers are present (14). Class I genes, exemplified by phzH, require C4-HSL; class II genes, such as rhlA, depend on both C4-HSL and the alternative ligand; and class III genes, represented by hcnA, are activated by the alternative ligand independent of C4-HSL (Fig. 1C). These response patterns give us another means to assay for the presence/absence of the alternative ligand, and in turn, to pinpoint the role of PqsE in its production. We performed quantitative RT-PCR analyses on high-cell density (HCD; OD600 ∼ 2.0) planktonic cultures of WT, ΔrhlR, ΔrhlI, ΔpqsE, and ΔrhlI ΔpqsE mutants probing for phzH, rhlA, and hcnA (Fig. 1D). Expression of the class I gene, phzH, does not change when pqsE is deleted in either the WT or the ΔrhlI parent, whereas transcription of the class II gene, rhlA, declines in both the ΔrhlI and ΔpqsE strains, and even more so in the double mutant. Finally, consistent with PqsE being the alternative-ligand synthase, class III hcnA transcript levels decrease 10-fold in the ΔpqsE single and ΔrhlI ΔpqsE double mutants compared with the WT and ΔrhlI strains. Collectively, the data in Fig. 1 provide evidence that PqsE is the alternative-ligand synthase.
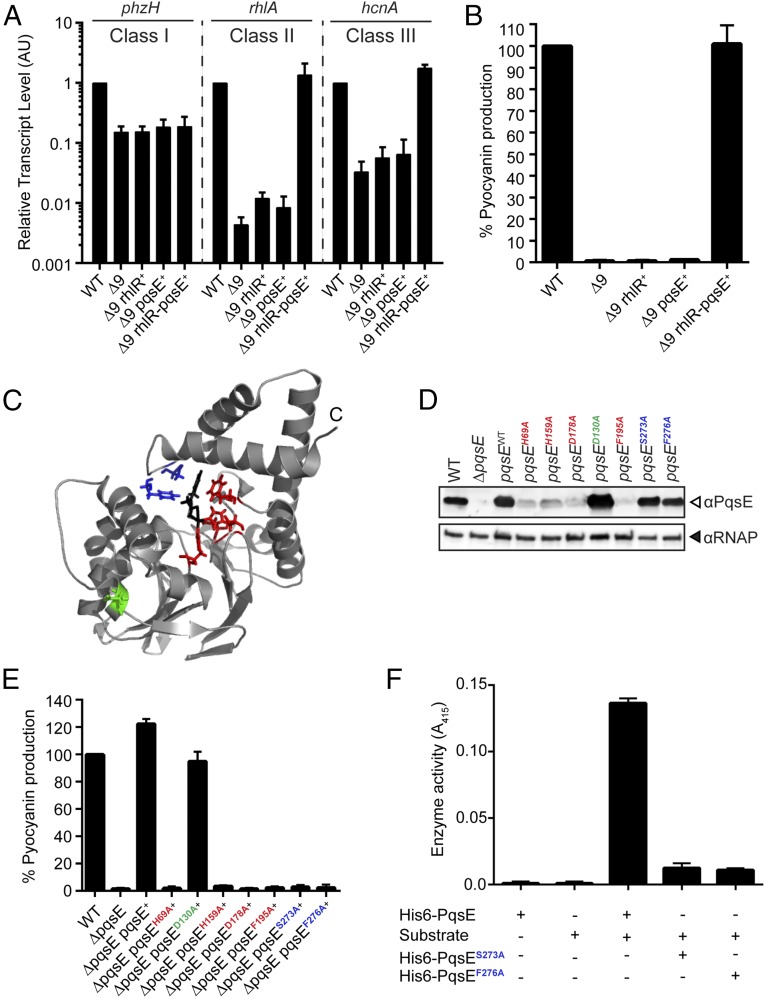
PqsE and RhlR Form an Autoinducer Synthase–Receptor Pair.
A key feature of all QS circuits is that the autoinducer synthase produces a small molecule that is detected by the cognate receptor. Our present findings show that PqsE is required for synthesis of the alternative ligand that activates RhlR. To address whether the PqsE-derived alternative ligand is necessary and sufficient to stimulate RhlR-controlled group behaviors, we introduced pqsE and/or rhlR into the Δ9 strain and assessed whether or not RhlR-dependent target genes are expressed. Fig. 2A shows quantitative RT-PCR results for phzH, rhlA, and hcnA. Compared with WT, the Δ9 strain fails to express all three genes, and introduction of either pqsE or rhlR alone does not activate their expression. Consistent with our analyses showing that phzH is a RhlI-dependent class I gene, its expression is not activated when pqsE and rhlR are introduced into the Δ9 mutant. In contrast, introduction of pqsE together with rhlR restores the WT level of expression of rhlA and hcnA, which do depend on the alternative ligand.
Fig. 2.
PqsE and RhlR are an autoinducer synthase–receptor pair. (A) Relative expression of the RhlR-regulated genes phzH, rhlA, and hcnA measured by qRT-PCR in the WT and designated mutants grown planktonically to HCD. Class I, Class II, and Class III are as in Fig. 1. Data are normalized to 5S RNA levels. AU denotes arbitrary units. (B) Pyocyanin production (OD695) was measured in WT PA14 and the designated mutants. (C) PqsE mutations mapped on the available structure of PqsE (gray) bound to 2-aminobenzoylacetate (black) (PDB ID: 5HIO) (24). Residues highlighted in red (H69, H159, D178, F195) form the metal ion center, S273 and F276 are required for alternative-ligand synthesis (blue), and the control residue for mutation is D130 (green). (D) Western blot analysis of lysates from WT PA14, the ΔpqsE mutant, and the ΔpqsE strain complemented with either WT pqsE or the indicated pqsE mutants. The blot was separately probed with anti-PqsE and anti-RNAP antibodies. (E) Pyocyanin production (OD695) was measured in WT PA14 and the designated mutants. (F) In vitro thioesterase activity assay for WT PqsE and the designated mutant proteins using the artificial substrate S-(4-nitrobenzoyl) mercaptoethane. In A, B, E, and F, error bars represent SEM for three independent replicates.
Beyond regulating transcription, if RhlR and PqsE act as a receptor-synthase pair, together they should control group behaviors in vivo. To investigate this, we quantified pyocyanin production as a proxy for QS-controlled group behaviors in the Δ9 strain and in the Δ9 strain harboring rhlR, pqsE, or both genes, and we compared the output with that made by the WT. Indeed, introduction of both rhlR and pqsE, but neither gene alone, restored pyocyanin production to the maximum WT level (Fig. 2B). Thus, we conclude that PqsE and RhlR are an autoinducer synthase–receptor pair that drives group behaviors in P. aeruginosa.
The PqsE Enzyme Active Site Is Required for Alternative-Ligand Synthesis.
PqsE is reported to be a thioesterase that plays a redundant role in converting 2-aminobenzoylacetyl-coenzymeA to 2-aminobenzoylacetate, a step in PQS synthesis (22). The PqsE crystal structure predicts that residues H69, H159, D178, F195, S273, and F276 are in the active site (Fig. 2C) (23, 24). We substituted the above residues with alanine and introduced the PqsE variants into the ΔpqsE PA14 strain to test their roles in alternative-ligand synthesis. We also substituted D130, a residue distal to the active site pocket, to serve as a WT control mutant. PqsE D130A produced a stable protein, and it restored pyocyanin production to the ΔpqsE strain (Fig. 2 D and E). Alanine substitution of H69, H159, D178, or F195, residues involved in coordination of two active-site metal ions required for enzyme function (23, 24), resulted in unstable protein, and therefore, these PqsE mutants failed to restore pyocyanin production to the ΔpqsE strain (Fig. 2 D and E). PqsE residues S273 and F276 are reported to be required for catalysis and for substrate binding, respectively (23, 25). Substitution of these residues to alanine resulted in stable but inactive PqsE protein, as they failed to complement the pyocyanin production defect of the ΔpqsE strain (Fig. 2 D and E). We tested the two stable PqsE proteins, PqsES273A and PqsEF276A, for defects in in vitro thioesterase activity, using a commercial substrate [S-(4-nitrobenzoyl) mercaptoethane] (23). Although the PqsEWT protein displays thioesterase activity, the PqsES273A and PqsEF276A proteins do not (Fig. 2F). Thus, PqsE active site residues required for thioesterase activity are also required for alternative-ligand synthesis. We conclude that key PqsE active site residues S273 and F276 are crucial for alternative-ligand synthesis and group behavior.
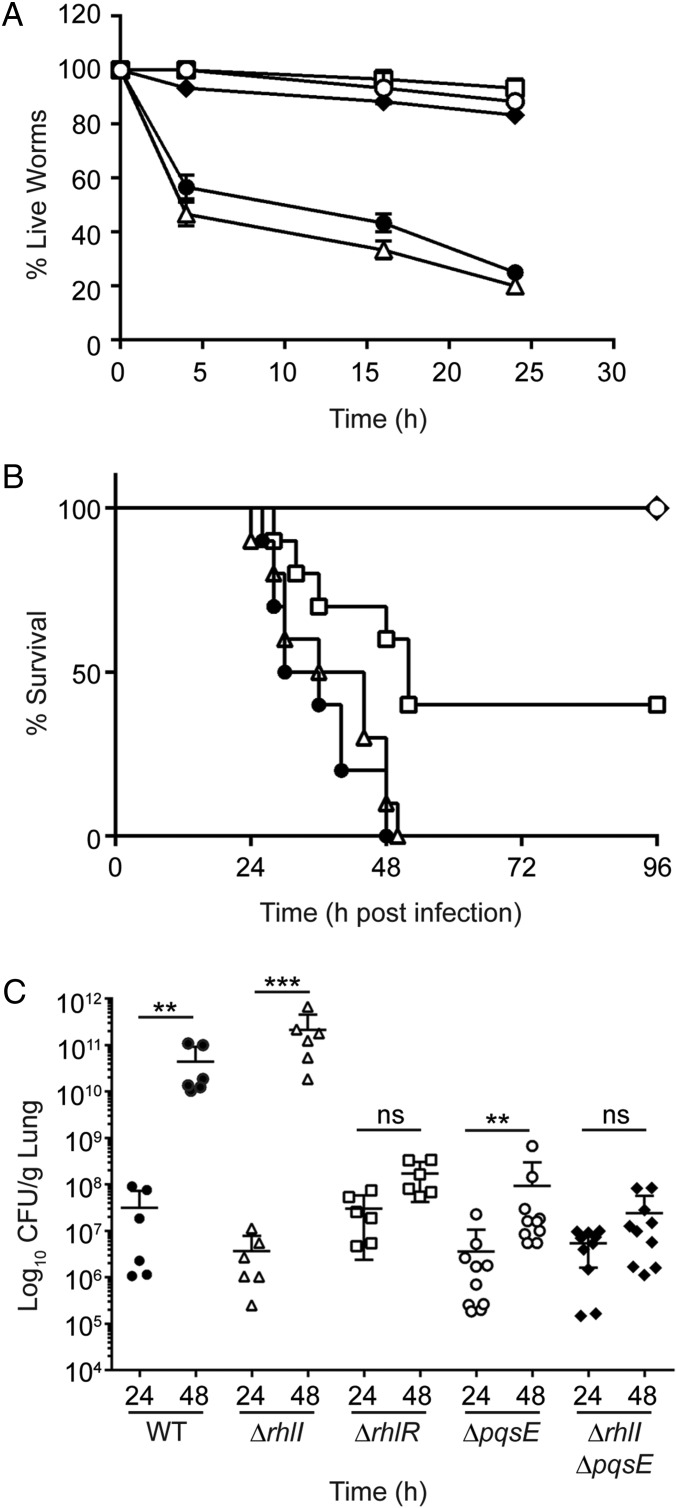
PqsE Is Required for RhlR-Dependent Virulence in Animal Infection Models.
We previously demonstrated that RhlR is required for P. aeruginosa virulence in nematode and murine infection models, whereas RhlI, and therefore C4-HSL, are dispensable (14). We reasoned that it is the alternative ligand that promotes RhlR-dependent virulence in animals in the absence of RhlI. Discovering that PqsE is the alternative-ligand synthase gave us the means to test our hypothesis about the crucial role of the alternative ligand in RhlR-dependent virulence in animals. In a Caenorhabditis elegans fast-kill infection assay, WT P. aeruginosa is virulent, the ΔrhlR mutant is avirulent, and the ΔrhlI mutant that lacks C4-HSL but produces the alternative ligand is as virulent as the WT (Fig. 3A). Here we show that both the ΔpqsE and ΔrhlI ΔpqsE mutant strains are avirulent, displaying the same phenotype as the ΔrhlR mutant (Fig. 3A). We conclude that the PqsE-derived alternative ligand is the key autoinducer driving RhlR-dependent virulence in nematodes.
Fig. 3.
PqsE is required for RhlR-dependent virulence in animal infection models. (A) C. elegans were applied to lawns of WT PA14 (closed circles), the ΔrhlR mutant (open squares), the ΔrhlI mutant (open triangles), the ΔpqsE mutant (open circles), and the ΔrhlI ΔpqsE double mutant (closed diamonds). Error bars represent SEM of three independent replicates. (B) For survival experiments, BALB/c mice were infected intratracheally with ∼3 × 106 cfu of WT PA14 or the indicated mutants and were monitored for up to 4 d postinfection. Symbols as in A. Results are represented on Kaplan Meier curves and were compiled from two independent experiments; n = 10. Significant differences were calculated by log rank test by comparing each strain to the WT (ΔrhlI; P = 0.374, ΔrhlR; P < 0.01, ΔpqsE; P < 0.0001, ΔrhlI ΔpqsE; P < 0.0001). (C) Bacterial burden recovered from mice at 24 h and 48 h postinfection with WT PA14 or the indicated mutants. Results were analyzed by one-way ANOVA. Multiple comparisons were performed between the indicated points for each strain. ***P < 0.001; **P < 0.05; ns, not significant.
To determine whether the alternative ligand is the primary autoinducer that stimulates RhlR-dependent virulence in mammals, we examined pathogenicity of the above strains in a murine model of acute lung infection. We previously determined LD50 values for the WT, ∆rhlR, and ∆rhlI strains to be 1.9 × 106 cfu, 2.6 × 106 cfu, and 1.1 × 106 cfu, respectively (14). Here we show that the LD50 is >10–20 times higher for both the ΔpqsE (3.0 × 107 cfu) and ΔrhlI ΔpqsE (4.8 × 107 cfu) mutants than the WT. We compared the potential of each of these mutants to influence virulence in the lung infection model, using a single input dose, 3 × 106 cfu, corresponding to ∼1.5 × LD50 of the WT strain. Fig. 3B shows that mice given the WT or the ΔrhlI mutant succumbed to infection by 48 h, whereas mice given the ΔpqsE or ΔrhlI ΔpqsE mutant all survived. Mice infected with the ΔrhlR mutant showed ∼40% survival.
To determine the level of lung colonization achieved by infection, we administered sublethal doses (∼0.5 LD50) of these strains. At 24 h postinfection, the bacterial load was similar among all infected mice (Fig. 3C). However, at 48 h postinfection, the bacterial burden in mice infected with the WT and ΔrhlI strains increased by 10,000-fold (Fig. 3C). In contrast, the bacterial load in mice infected with the ΔrhlR, ΔpqsE, and ΔrhlI ΔpqsE strains did not change significantly (Fig. 3C). Thus, the ΔrhlR, ΔpqsE, and ΔrhlI ΔpqsE strains are highly attenuated, producing a four-order of magnitude lower burden of bacteria in the murine host than the WT and the ΔrhlI mutant. In sum, our results show that PqsE-derived alternative ligand is essential to activate RhlR to promote virulence gene expression in both the C. elegans and the murine animal models.
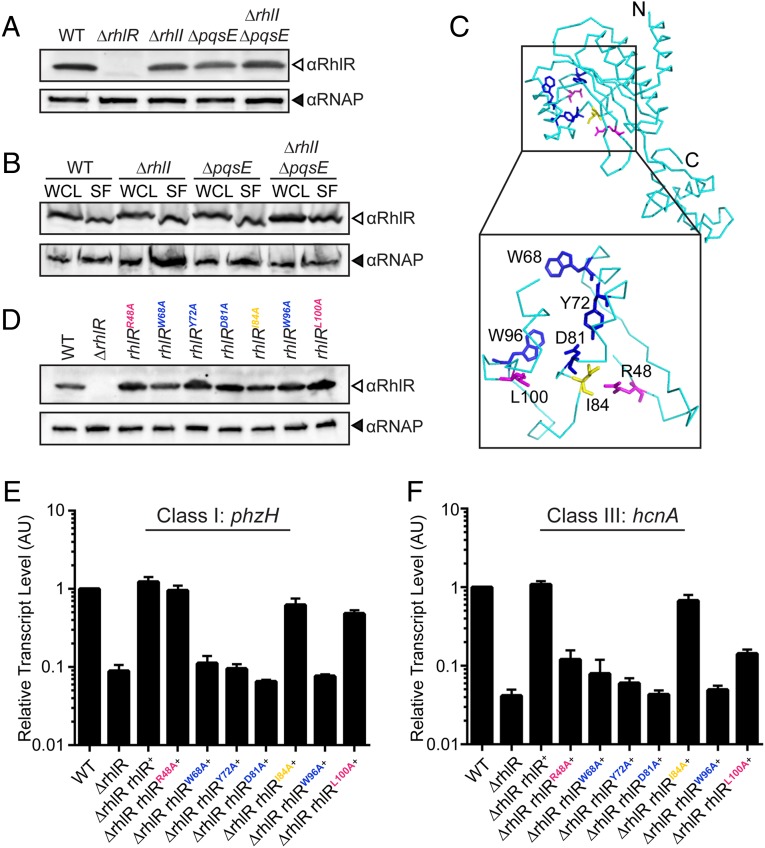
RhlR Does Not Require an Autoinducer for Solubility in P. aeruginosa.
RhlR appears to be an atypical LuxR-type receptor, as it responds to two autoinducers: C4-HSL and the alternative ligand. We wondered how each autoinducer regulates RhlR function. One possibility could be autoinducer control of RhlR protein stability, a common mechanism for LuxI-LuxR type partners. Western blot analyses were used to assess in vivo RhlR levels in cell lysates prepared from the WT, ΔrhlI, ΔpqsE, and ΔrhlI ΔpqsE PA14 strains. Compared with WT, only a modest decrease in RhlR levels could be detected in the mutant strains (Fig. 4A). We speculate that this decrease occurs because, in the absence of one or both autoinducers, RhlR cannot properly feedback to activate its own transcription.
Fig. 4.
The RhlR ligand-binding domain is required for sensing the alternative ligand. (A) Western blot analysis of lysates from WT, ΔrhlR, ΔrhlI, ΔpqsE, ΔrhlI, ΔpqsE PA14 strains. RhlR levels were detected using anti-RhlR antibody, and RNAP was probed as the loading control using anti-RNAP antibody. (B) Western blot analysis of whole-cell lysates (WCL) and soluble fractions (SF) from the indicated strains; all were additionally deleted for rhlR and carry rhlR on the pUCP18 plasmid under the Plac promoter. (C) The predicted ribbon structure of the RhlR monomer (cyan) based on Phyre2 threading and comparison with the crystal structure of the closest homolog, SdiA, from E. coli (PDB ID: 4Y15) (30, 38). (Inset) Amino acids lining the putative RhlR ligand-binding pocket that were mutated in the present work. Residues in blue (W68, Y72, D81, W96) are required for response to both C4-HSL and the alternative ligand; residues in magenta (R48, L100) are required for sensing the alternative ligand but not C4-HSL, whereas reside I84 (yellow) is dispensable for detection of both ligands. (D) Western blot analysis of lysates from WT PA14, the ΔrhlR mutant, and the ΔrhlR mutant complemented with either WT rhlR or the indicated rhlR point mutants. (E) Relative expression of the RhlI-dependent phzH gene measured by qRT-PCR in the WT and mutant strains grown planktonically to HCD. Data are normalized to 5S RNA levels. Error bars represent SEM of three biological replicates. AU denotes arbitrary units. (F) As in E showing expression of the PqsE-dependent hcnA gene.
Our finding that RhlR is stable in the PA14 mutants was surprising, given that this is not the case for other studied LuxR-type proteins (6, 26, 27). Indeed, RhlR, when produced in Escherichia coli, is not soluble even in the presence of saturating C4-HSL (28). To further explore this result, we deleted rhlR in the WT, ΔrhlI, ΔpqsE, and ΔrhlI ΔpqsE backgrounds and reintroduced rhlR on a plasmid under a constitutive promoter to eliminate possible changes in RhlR transcription because of the absence of RhlI and/or PqsE. Soluble RhlR protein is present in all four strains (Fig. 4B). We conclude that in PA14, neither C4-HSL nor the alternative ligand is required for RhlR to fold and become soluble. One formal possibility is that there exists a third autoinducer that is capable of solubilizing RhlR in the absence of C4-HSL and the alternative ligand. However, our evidence suggests this is not the case, as cell-free culture fluids prepared from the ΔrhlI ΔpqsE double mutant do not contain any activity that elicits RhlR-dependent gene expression (Fig. 1 B and D). We therefore infer that autoinducers are required only to activate RhlR as a transcription factor.
The RhlR Ligand-Binding Domain Is Crucial for Sensing the Alternative Ligand.
RhlR contains an N-terminal ligand-binding domain (LBD) and a C-terminal DNA-binding domain (5). There is currently no structure of RhlR. Moreover, RhlR shows significant sequence divergence from TraR, the prototype for which a structure is solved (SI Appendix, Fig. S3A) (23). Thus, how RhlR binds C4-HSL and/or the alternative ligand is unknown. To explore how RhlR selects its autoinducers, we generated a homology model of RhlR based on the E. coli SdiA structure, the closest homolog of RhlR (47% sequence identity; Fig. 4C and SI Appendix, Fig. S3 A and B). In SdiA and other LuxR-type proteins, the highly conserved amino acids W68 and D81 (positions refer to RhlR) interact with the amide group-oxygen and the amide group-nitrogen, respectively, of the cognate HSL autoinducers (29, 30). Other conserved residues, such as Y72 and W96, are required for hydrophobic and van der Waals interactions with the ligands (Fig. 4C and SI Appendix, Fig. S3C). We substituted W68, Y72, D81, and W96 with alanine and introduced these RhlR variants into the ΔrhlR strain carrying WT rhlI and pqsE, which is therefore capable of producing both C4-HSL and the alternative ligand. Western blot shows that these RhlR mutant proteins are stable (Fig. 4D). To determine how the RhlR mutations affect autoinducer response, we measured transcription of the C4-HSL-dependent class I gene phzH and the alternative-ligand–dependent-class III gene hcnA. RhlR-driven phzH and hcnA expression was abolished in every case (Fig. 4 E and F). We conclude that all four residues are required for sensing both C4-HSL and the alternative ligand.
Guided by the putative RhlR LBD tertiary structure, we identified amino acids R48, I84, and L100, as predicted to face the interior of the LBD (Fig. 4C). Again, we generated alanine substitutions and introduced the RhlR variants into the ΔrhlR strain. All the variants produce stable protein (Fig. 4D). The RhlR R48A and L100A substitutions eliminated hcnA expression without affecting phzH expression. The RhlR I84A mutant behaved similar to WT (Fig. 4 E and F). These data indicate that W68, Y72, D81, and W96 are required for the response to C4-HSL and the alternative ligand. However, the R48 and L100 residues are dispensable for C4-HSL detection, but are necessary for the RhlR response to the alternative ligand. We did not discover any residue that was required exclusively for C4-HSL detection.
Discussion
The PqsE enzyme is widely distributed in P. aeruginosa strains and is essential for P. aeruginosa QS-dependent group behaviors (21, 31–33). Initially thought to be required for synthesis of the PQS autoinducer, based on its location in the pqsABCDE PQS biosynthetic operon, it is now known that PqsE, unlike the other genes in the operon, is dispensable for PQS biosynthesis (SI Appendix, Fig. S2B) (21, 31). Here, we define the role of PqsE: PqsE catalyzes the synthesis of the alternative ligand, a ligand that is necessary and sufficient to activate RhlR-dependent group behaviors in in vivo and in vitro assays and during animal infection. We are currently working to identify this alternative ligand. Our identification of PqsE as the alternative-ligand synthase explains several previously reported puzzling observations. First, PqsE was reported to require RhlR to enhance Rhl-directed QS (21). Our results show that the PqsE-derived alternative ligand functions together with RhlR to activate RhlR transcriptional activity (Fig. 1). Second, PqsE overexpression/induction was reported to lower PQS levels by an unknown mechanism (31, 34). Our current study, combined with earlier results, provides the mechanism: the PqsE-derived alternative ligand drives RhlR-dependent repression of pqsA transcription, thereby reducing PQS production (14). PqsE was also proposed to exert its effect on QS via protein–protein interaction (24). Here, we show that mutating PqsE S273 and F276, putative nonsurface exposed active site residues, eliminate RhlR-directed QS group behaviors, suggesting that the PqsE effect on Rhl QS is not mediated by direct protein–protein interaction with RhlR. Rather, we interpret these earlier results to be a consequence of interaction of the PqsE product, the alternative ligand, with RhlR, but not PqsE itself.
RhlR belongs to the LuxR family of proteins and, similar to its homologs, possesses conserved amino acids in the LBD that, in other receptors, are required for recognition of HSL autoinducers (29, 30, 35). Our finding that mutation of these conserved residues abrogates both C4-HSL and alternative-ligand detection/response suggests that the binding surface for the alternative ligand overlaps with that of the canonical autoinducer C4-HSL. Importantly, RhlR residues R48 and L100, required for response to the PqsE-derived alternative ligand, are distinct from those typically used for HSL recognition. Thus, RhlR LBD has overlapping but not identical binding sites for its autoinducers.
P. aeruginosa is a pathogen of high clinical relevance (36, 37). The key finding in the present work is that PqsE and the alternative ligand are essential, whereas RhlI and the canonical C4-HSL autoinducer are dispensable for RhlR-driven virulence in animal models of infection. Unlike in the C. elegans infection assay, in the murine model of acute lung infection, the ΔpqsE and ΔrhlIΔpqsE mutants were more attenuated than the ΔrhlR mutant. We speculate that, beyond virulence, PqsE has a role in PA14 survival in the complex murine lung environment because the alternative ligand controls factors that contribute to bacterial fitness in this niche. Together, these results provide a set of unanticipated targets for therapeutic intervention. PqsE enzyme function, PqsE stability, RhlR interaction with the alternative ligand, RhlR:alternative-ligand dimerization, RhlR:alternative-ligand DNA binding, and RhlR:alternative-ligand RNA-Polymerase engagement are all now revealed as candidates for inhibition in pursuit of new therapeutics. Moreover, these same biocomponents are revealed to function together as a QS pathway in one of the most actively studied QS pathogens, P. aeruginosa.
Materials and Methods
Detailed experimental procedures are described in the SI Appendix, Extended Experimental Procedures. Strains, and plasmids used in this study are listed in SI Appendix, Table S2.
Pyocyanin Assay.
PA14 strains were grown overnight in LB liquid medium at 37 °C with shaking. Cultures were back diluted 1:1,000 into fresh medium and grown for 18 h. The cells were pelleted by centrifugation, and the culture fluids were passed through 0.22 μm filters into clear plastic cuvettes. The OD695 of each sample was measured on a spectrophotometer (Beckman Coulter DV 730).
Colony Biofilm Assay.
The procedure for enabling colony biofilm formation has been described (12, 14). Briefly, 1 μL of overnight PA14 cultures was spotted onto 60 × 15 mm Petri plates containing 10 mL 1% TB medium fortified with 40 mg/L Congo red and 20 mg/L Coomassie brilliant blue dyes and solidified with 1% agar. Biofilms were grown at 25 °C, and images were acquired after 120 h, using a Leica stereomicroscope M125 mounted with a Leica MC170 HD camera at 7.78× zoom.
qRT-PCR.
PA14 strains were harvested from planktonic cultures (OD600 = 2.0), and RNA was purified using Qiagen kits, and subsequently, DNase treated (TURBO DNA-free; Thermo Fisher). cDNA was synthesized using SuperScript III Reverse Transcriptase (Invitrogen) and quantified using PerfeCTa SYBR Green FastMix Low ROX (Quanta Biociences).
Animal Infection Models.
C. elegans fast-killing assay and murine infection assays were performed as described previously (9). Detailed procedure can be found in SI Appendix. All animal procedures were conducted according to the guidelines of the Emory University Institutional Animal Care and Use Committee, under approved protocol number DAR-2003421–042219BN. The study was carried out in strict accordance with established guidelines and policies at Emory University School of Medicine, and recommendations in the Guide for Care and Use of Laboratory Animals of the National Institute of Health, as well as local, state, and federal laws.
Supplementary Material
Acknowledgments
We thank Tharan Srikumar and the Proteomics and Mass Spectrometry Core Facility at Princeton University for LC-MS analyses. We also thank all members of the Bassler group for thoughtful discussions. This work was supported by the Howard Hughes Medical Institute, NIH Grant 5R37GM065859, and National Science foundation Grant MCB-1713731 (to B.L.B.), and a Life Science Research Foundation Postdoctoral Fellowship through the Gordon and Betty Moore Foundation through Grant GBMF2550.06 (to S.M.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1814023115/-/DCSupplemental.
References
- 1.Klevens RM, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 3.Davies DG, et al. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 4.de Kievit TR, Kakai Y, Register JK, Pesci EC, Iglewski BH. Role of the Pseudomonas aeruginosa las and rhl quorum-sensing systems in rhlI regulation. FEMS Microbiol Lett. 2002;212:101–106. doi: 10.1016/s0378-1097(02)00735-8. [DOI] [PubMed] [Google Scholar]
- 5.Papenfort K, Bassler BL. Quorum sensing signal-response systems in Gram-negative bacteria. Nat Rev Microbiol. 2016;14:576–588. doi: 10.1038/nrmicro.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu J, Winans SC. The quorum-sensing transcriptional regulator TraR requires its cognate signaling ligand for protein folding, protease resistance, and dimerization. Proc Natl Acad Sci USA. 2001;98:1507–1512. doi: 10.1073/pnas.98.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seed PC, Passador L, Iglewski BH. Activation of the Pseudomonas aeruginosa lasI gene by LasR and the Pseudomonas autoinducer PAI: An autoinduction regulatory hierarchy. J Bacteriol. 1995;177:654–659. doi: 10.1128/jb.177.3.654-659.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brint JM, Ohman DE. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J Bacteriol. 1995;177:7155–7163. doi: 10.1128/jb.177.24.7155-7163.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu K, Rock CO. RhlA converts beta-hydroxyacyl-acyl carrier protein intermediates in fatty acid synthesis to the beta-hydroxydecanoyl-beta-hydroxydecanoate component of rhamnolipids in Pseudomonas aeruginosa. J Bacteriol. 2008;190:3147–3154. doi: 10.1128/JB.00080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xavier JB, Kim W, Foster KR. A molecular mechanism that stabilizes cooperative secretions in Pseudomonas aeruginosa. Mol Microbiol. 2011;79:166–179. doi: 10.1111/j.1365-2958.2010.07436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mellbye B, Schuster M. Physiological framework for the regulation of quorum sensing-dependent public goods in Pseudomonas aeruginosa. J Bacteriol. 2014;196:1155–1164. doi: 10.1128/JB.01223-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietrich LEP, Teal TK, Price-Whelan A, Newman DK. Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science. 2008;321:1203–1206. doi: 10.1126/science.1160619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Recinos DA, et al. Redundant phenazine operons in Pseudomonas aeruginosa exhibit environment-dependent expression and differential roles in pathogenicity. Proc Natl Acad Sci USA. 2012;109:19420–19425. doi: 10.1073/pnas.1213901109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukherjee S, Moustafa D, Smith CD, Goldberg JB, Bassler BL. The RhlR quorum-sensing receptor controls Pseudomonas aeruginosa pathogenesis and biofilm development independently of its canonical homoserine lactone autoinducer. PLoS Pathog. 2017;13:e1006504. doi: 10.1371/journal.ppat.1006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laue BE, et al. The biocontrol strain Pseudomonas fluorescens F113 produces the Rhizobium small bacteriocin, N-(3-hydroxy-7-cis-tetradecenoyl)homoserine lactone, via HdtS, a putative novel N-acylhomoserine lactone synthase. Microbiology. 2000;146:2469–2480. doi: 10.1099/00221287-146-10-2469. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, et al. A cell-cell communication signal integrates quorum sensing and stress response. Nat Chem Biol. 2013;9:339–343. doi: 10.1038/nchembio.1225. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs MA, et al. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2003;100:14339–14344. doi: 10.1073/pnas.2036282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caiazza NC, Merritt JH, Brothers KM, O’Toole GA. Inverse regulation of biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J Bacteriol. 2007;189:3603–3612. doi: 10.1128/JB.01685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuchma SL, et al. BifA, a cyclic-Di-GMP phosphodiesterase, inversely regulates biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J Bacteriol. 2007;189:8165–8178. doi: 10.1128/JB.00586-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pesci EC, et al. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1999;96:11229–11234. doi: 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrow JM, 3rd, et al. PqsE functions independently of PqsR-Pseudomonas quinolone signal and enhances the rhl quorum-sensing system. J Bacteriol. 2008;190:7043–7051. doi: 10.1128/JB.00753-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drees SL, Fetzner S. PqsE of Pseudomonas aeruginosa acts as pathway-specific thioesterase in the biosynthesis of alkylquinolone signaling molecules. Chem Biol. 2015;22:611–618. doi: 10.1016/j.chembiol.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Yu S, et al. Structure elucidation and preliminary assessment of hydrolase activity of PqsE, the Pseudomonas quinolone signal (PQS) response protein. Biochemistry. 2009;48:10298–10307. doi: 10.1021/bi900123j. [DOI] [PubMed] [Google Scholar]
- 24.Zender M, et al. Dissecting the multiple roles of PqsE in Pseudomonas aeruginosa virulence by discovery of small tool compounds. ACS Chem Biol. 2016;11:1755–1763. doi: 10.1021/acschembio.6b00156. [DOI] [PubMed] [Google Scholar]
- 25.Folch B, Déziel E, Doucet N. Systematic mutational analysis of the putative hydrolase PqsE: Toward a deeper molecular understanding of virulence acquisition in Pseudomonas aeruginosa. PLoS One. 2013;8:e73727. doi: 10.1371/journal.pone.0073727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duerkop BA, Ulrich RL, Greenberg EP. Octanoyl-homoserine lactone is the cognate signal for Burkholderia mallei BmaR1-BmaI1 quorum sensing. J Bacteriol. 2007;189:5034–5040. doi: 10.1128/JB.00317-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuster M, Urbanowski ML, Greenberg EP. Promoter specificity in Pseudomonas aeruginosa quorum sensing revealed by DNA binding of purified LasR. Proc Natl Acad Sci USA. 2004;101:15833–15839. doi: 10.1073/pnas.0407229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Loughlin CT, et al. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc Natl Acad Sci USA. 2013;110:17981–17986. doi: 10.1073/pnas.1316981110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang RG, et al. Structure of a bacterial quorum-sensing transcription factor complexed with pheromone and DNA. Nature. 2002;417:971–974. doi: 10.1038/nature00833. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen Y, et al. Structural and mechanistic roles of novel chemical ligands on the SdiA quorum-sensing transcription regulator. MBio. 2015;6:e02429-14. doi: 10.1128/mBio.02429-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hazan R, et al. Homeostatic interplay between bacterial cell-cell signaling and iron in virulence. PLoS Pathog. 2010;6:e1000810. doi: 10.1371/journal.ppat.1000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rampioni G, et al. Transcriptomic analysis reveals a global alkyl-quinolone-independent regulatory role for PqsE in facilitating the environmental adaptation of Pseudomonas aeruginosa to plant and animal hosts. Environ Microbiol. 2010;12:1659–1673. doi: 10.1111/j.1462-2920.2010.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rampioni G, et al. Unravelling the genome-wide contributions of specific 2-alkyl-4-quinolones and PqsE to quorum sensing in Pseudomonas aeruginosa. PLoS Pathog. 2016;12:e1006029. doi: 10.1371/journal.ppat.1006029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knoten CA, Wells G, Coleman JP, Pesci EC. A conserved suppressor mutation in a tryptophan auxotroph results in dysregulation of Pseudomonas quinolone signal synthesis. J Bacteriol. 2014;196:2413–2422. doi: 10.1128/JB.01635-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Churchill MEA, Chen L. Structural basis of acyl-homoserine lactone-dependent signaling. Chem Rev. 2011;111:68–85. doi: 10.1021/cr1000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J Infect Dis. 2008;197:1079–1081. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 37.Pendleton JN, Gorman SP, Gilmore BF. Clinical relevance of the ESKAPE pathogens. Expert Rev Anti Infect Ther. 2013;11:297–308. doi: 10.1586/eri.13.12. [DOI] [PubMed] [Google Scholar]
- 38.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.