Significance
Mast cells are mandatory for allergic reactions and participate in inflammatory responses in which the peptide substance P (SP) and the cytokine IL-33 are involved. This report shows that SP administered together with IL-33 to cultured human mast cells causes a marked increase in the secretion and gene expression of IL-1β. These responses are mediated via the activation of the SP receptor NK-1 and the IL-33 receptor ST2 and can be inhibited by the natural flavonoid methoxyluteolin. These findings highlight the important role of SP and IL-33 in mast cell secretion of IL-1β and point to targets for the development of therapies for inflammatory diseases.
Keywords: IL-1β, IL-33, inflammation, mast cells, substance P
Abstract
Mast cells are critical for allergic and inflammatory responses in which the peptide substance P (SP) and the cytokine IL-33 are involved. SP (0.01–1 μM) administered together with IL-33 (30 ng/mL) to human cultured LAD2 mast cells stimulates a marked increase (P < 0.0001) in secretion of the proinflammatory cytokine IL-1β. Preincubation of LAD2 (30 min) with the SP receptor (NK-1) antagonists L-733,060 (10 μM) or CP-96345 (10 µM) inhibits (P < 0.001) secretion of IL-1β stimulated by either SP (1 μM) or SP together with IL-33 (30 ng/mL). Surprisingly, secretion of IL-1β stimulated by IL-33 is inhibited (P < 0.001) by each NK-1 antagonist. Preincubation with an antibody against the IL-33 receptor ST2 inhibits (P < 0.0001) secretion of IL-1β stimulated either by IL-33 or together with SP. The combination of SP (1 μM) with IL-33 (30 ng/mL) increases IL-1β gene expression by 90-fold in LAD2 cells and by 200-fold in primary cultured mast cells from human umbilical cord blood. The combination of SP and IL-33 increases intracellular levels of IL-1β in LAD2 by 100-fold and gene expression of IL-1β and procaspase-1 by fivefold and pro-IL-1β by twofold. Active caspase-1 is present even in unstimulated cells and is detected extracellularly. Preincubation of LAD2 cells with the natural flavonoid methoxyluteolin (1–100 mM) inhibits (P < 0.0001) secretion and gene expression of IL-1β, procaspase-1, and pro-IL-1β. Mast cell secretion of IL-1β in response to SP and IL-33 reveals targets for the development of antiinflammatory therapies.
Mast cells are immune cells that do not circulate but exist in vascularized tissues and have multiple diverse functions (1–3). Mast cells are best known for their critical role in allergic reactions (4–8) via activation by allergens of the high-affinity IgE receptor FcεRI (9). Mast cells are also stimulated by the peptide substance P (SP) (10–12) initially characterized by Chang and Leeman (13) and shown to participate in inflammatory processes (14–17).
Mast cells, when stimulated, secrete preformed molecules stored in their granules that include histamine, tryptase (18), and many proinflammatory cytokines and chemokines synthesized de novo (19–22). Even though many immune cells secrete IL-1β (23), the ability of human mast cells to secrete IL-1β has not been previously investigated.
IL-33 is a member of the IL-1 family of cytokines and has emerged as an early warning sign (dubbed “alarmin”) (24) in autoimmune or inflammatory process (25–27). IL-33 is secreted by fibroblasts and endothelial cells (28). IL-33 augments the effect of IgE on the secretion of histamine from mast cells and basophils (24, 29) by “priming” them (30). We recently showed that stimulation of human mast cells by SP given together with IL-33 markedly increases secretion and gene expression of another proinflammatory cytokine, TNF (12). We also reported that this response is inhibited by the natural flavonoid methoxyluteolin (5,7,3′,4′-tetramethoxyflavone) (12, 31, 32).
IL-1β is a key proinflammatory cytokine secreted mostly by macrophages that plays an important role in immune and inflammatory diseases (33). IL-1β is present in the cytoplasm in a biologically inactive proform that requires activation via proteolytic cleavage by caspase-1. This protease is also present in the cytoplasm in a proform and is activated by the multiprotein complex known as “inflammasome” [Nod-like receptor pyrin domain containing protein 3 (NLRP3) and Apoptosis-associated speck-like protein containing CARD (ASC)] (34, 35).
The data presented in this report show that when SP and IL-33 are administered together a marked increase in the secretion of IL-1β from human cultured mast cells occurs. Preincubation with NK-1 antagonists inhibits not only the combined effect of SP and IL-33 but also the effect of IL-33 given alone. SP and IL-33, when administered together, also stimulate gene expression of pro-IL-1β and procaspase 1, components required for the synthesis of IL-1β. Both active caspase-1 and the mature form of IL-1β are present in unstimulated human mast cells. These effects are all inhibited by methoxyluteolin, which could be used for the treatment of inflammatory diseases.
Results
SP and IL-33 Administered Together Stimulate a Marked Secretion of IL-1β.
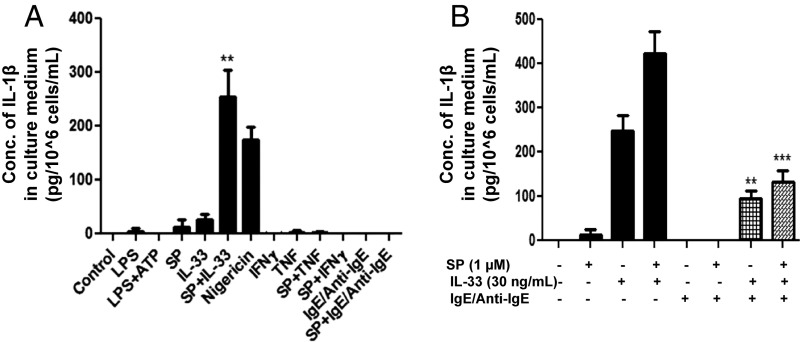
Administration of SP (1 μM) and IL-33 (30 ng/mL) together for 24 h stimulates a 100-fold (P < 0.01) increase in the secretion of IL-1β from LAD2 cells compared with unstimulated cells and a 10-fold increase compared with cells treated by IL-33 alone (Fig. 1A). In contrast, stimulation of LAD2 cells by SP (1 μM) alone for 24 h results in the secretion of 15 pg⋅10−6 cells⋅mL IL-1β (P = 0.15), and stimulation by IL-33 (30 ng/mL) alone results in the secretion of 35 pg⋅10−6 cells⋅mL IL-1β (P = 0.09), neither of which is significant (Fig. 1).
Fig. 1.
(A) SP and IL-33 stimulate IL-1β secretion. LAD2 cells (1 × 105 cells per well) were seeded in a 96-well culture plate and stimulated with LPS (100 ng/mL), ATP (5 μM), SP (1 μΜ), IL-33 (30 ng/mL), nigericin (10 µM), TNF (50 ng/mL), IFN-γ (100 U), IgE (1 µg/mL)/anti-IgE (5 µg/mL), or their combinations as shown for 24 h. Control cells were treated with culture medium only (n = 3, **P < 0.01 compared with unstimulated controls). (B) IgE/anti-IgE decrease the SP- and IL-33–stimulated secretion of IL-1β. LAD2 cells (1 × 105 cells per well) were seeded in a 96-well culture plate, were preincubated with human IgE (1 µg/mL) overnight, and were stimulated the next day with anti-IgE (10 ng/mL) for 2 h and/or SP (1 µM) and IL-33 (30 ng/mL) for 24 h. Supernatant fluids were collected at the end of the incubation period and were assayed for IL-1β using ELISA (n = 3, **P < 0.01 and ***P < 0.001 compared to SP alone or to SP+IL-33, respectively). Conc, concentration.
LAD2 cells stimulated for 24 h either with known triggers of IL-1β secretion from macrophages [LPS (100 ng/mL), ATP (5 μM), nigericin (10 μM), TNF (50 ng/mL), IFN-γ (100 U)] or with the trigger of mast cell secretion [IgE (1 µg/mL)/anti-IgE (10 µg/mL)] do not result in the secretion of IL-1β (Fig. 1A). Even though stimulation with IgE/anti-IgE has no effect on IL-1β secretion, preincubation of LAD2 cells with IgE/anti-IgE significantly (P < 0.001) decreases the secretion of IL-1β stimulated by the combination of SP and IL-33 (Fig. 1B). Surprisingly, preincubation with IgE alone significantly increased the secretion of IL-1β when cells were stimulated with IL-33. These results suggest that there may be an interaction between ST2 or NK-1, or both, and FcεRI.
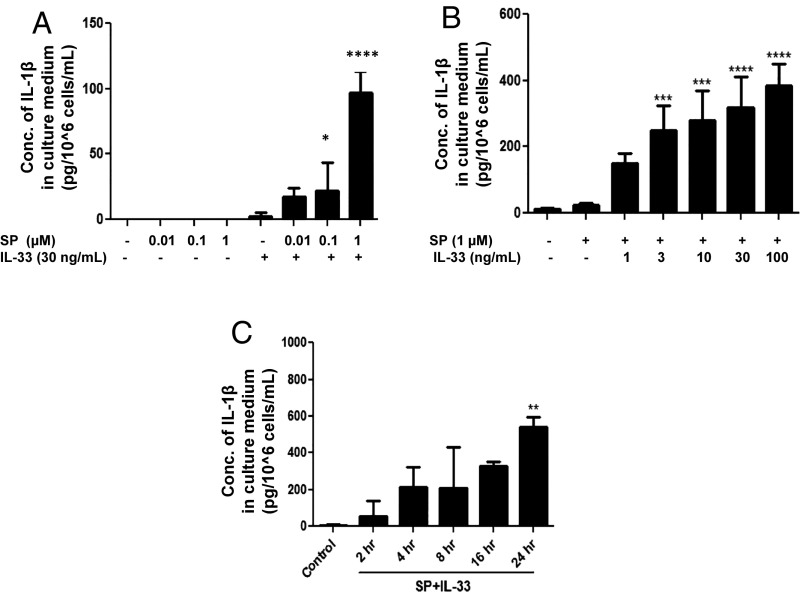
To select which dose results in maximal secretion of IL-1β, LAD2 cells were treated with various concentrations of SP, IL-33, or their combination. Stimulation by SP alone does not trigger the secretion of IL-1β at 0.01–0.1 µM but has a small effect at 1 µM (Fig. 2A). The combination of SP (1 μM) and IL-33 (30 ng/mL) causes maximal (P < 0.0001) secretion of IL-1β from LAD2 cells (Fig. 2B) as well as intracellular levels of IL-1β (Fig. 2A); therefore, this combination was selected for further studies. A time-course study showed that 24 h of stimulation yields the highest amount of IL-1β secretion (Fig. 2C), but some secretion of IL-1β was measurable even at 2 h of stimulation, illustrating the rapidity of the response.
Fig. 2.
Selection of the optimal doses to study IL-1β secretion stimulated by SP and IL-33 when administered in combination. (A and B) LAD2 cells (1 × 105 cells per well) were stimulated with SP (0.01–1 μΜ) (n = 3, *P < 0.05 and ****P < 0.0001 compared with SP alone) (A) or IL-33 (1–100 ng/mL) (B) and their combination as shown for 24 h (n = 3, ***P < 0.001 and ****P < 0.0001 compared with SP alone). (C) Time-dependent study of IL-1β secretion. LAD2 cells (1 × 105 cells per well) were stimulated with the combination of SP (1 μΜ) and IL-33 (30 ng/mL) for 2–24 h. Supernatant fluids were collected at the end of the incubation period. IL-1β secretion was assayed using ELISA (n = 3, **P < 0.01). Conc, concentration.
SP and IL-33 Administered Together Induce IL-1β Gene Expression.
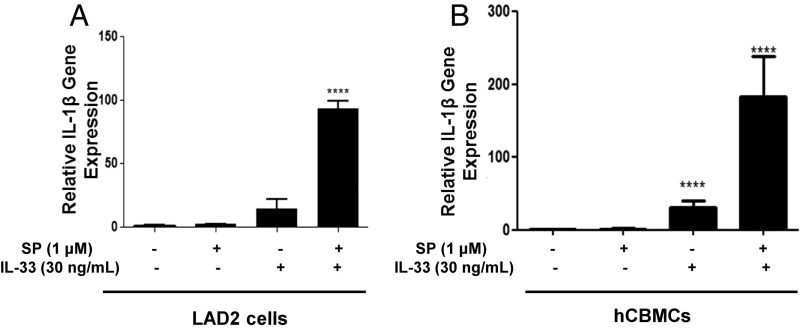
LAD2 and primary human umbilical cord blood mast cells (hCBMCs) were stimulated with SP and IL-33 for 6 h, and the expression of the IL-1β gene was measured. SP (1 μM) and IL-33 (30 ng/mL) administered together for 6 h increase IL-1β gene expression, as measured by qPCR, by 90-fold (P < 0.0001) in LAD2 cells and by 200-fold (P < 0.0001) in hCBMCs (Fig. 3).
Fig. 3.
SP and IL-33 markedly enhance IL-1β gene expression and secretion. LAD2 cells (1 × 106 cells per well) (A) and hCBMCs (0.3 × 106 cell per well) (B) were seeded in a 12-well culture plate and were stimulated with SP (1 μM), IL-33 (30 ng/mL), or their combination for 6 h. IL-1β mRNA expression levels were measured by qRT-PCR and were normalized to human GAPDH endogenous control (n = 3, ****P < 0.0001).
NK-1 Receptor Antagonists Inhibit Secretion of IL-1β Stimulated by SP and IL-33.
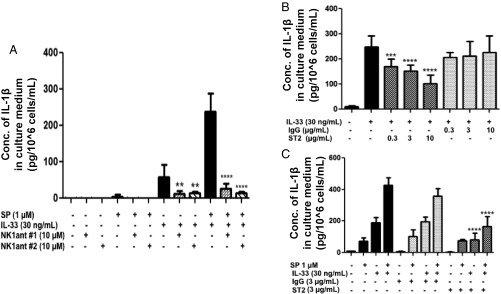
Preincubation of LAD2 cells with each of the neurokinin 1 (NK-1) receptor antagonists [L-733,060 (10 μM) or CP-96345 (10 μM)] for 30 min and followed by stimulation for 24 h with SP (1 μM) alone, IL-33 (30 ng/mL) alone, or their combination significantly (P < 0.001) inhibits the secretion of IL-1β (Fig. 4A). Surprisingly, the antagonists also (P < 0.001) inhibit the effect of IL-33 when given alone (Fig. 4A), suggesting an interaction between NK-1 and ST2.
Fig. 4.
NK-1 receptor antagonists inhibit IL-1β secretion. (A) LAD2 cells were pretreated with NK-1R antagonists L-733,060 (10 μM) (n = 3, **P < 0.01 and ****P < 0.0001 compared with IL-33 alone) and CP-96345 (10 µM) (n = 3, ****P < 0.0001 compared with SP and IL-33) for 30 min and then were stimulated with SP (1 μM), IL-33 (30 ng/mL), or their combination for 24 h. Antibody against ST2 inhibits IL-1β secretion. (B) LAD2 cells (1 × 105 cells per well) were seeded in a 96-well culture plate and were preincubated with an antibody against ST2, the IL-33 receptor (ST2, 0.3–10 µg/mL), or a nonspecific antibody not recognizing ST2 (IgG control) (0.3–10 µg/mL) for 2 h and then were stimulated with IL-33 (30 ng/mL) for 24 h (n = 3, ***P < 0.01, ****P < 0.0001 compared with IL33 alone). (C) LAD2 cells were preincubated with anti-ST2 neutralizing antibody (3 ng/mL) or IgG control (3 ng/mL) for 2 h and then were stimulated with SP (1 μΜ), IL-33 (30 ng/mL), or their combination for 24 h. Collected supernatant fluids were assayed by IL-1β ELISA (n = 3, ****P < 0.0001 compared with SP and IL-33). Conc, concentration.
An Antibody Against ST2 Inhibits Secretion of IL-1β Stimulated by the Combination of SP and IL-33.
LAD2 cells were pretreated with an anti-ST2 antibody (3 µg/mL) or with a nonspecific antibody that does not recognize ST2 (3 µg/mL) for 2 h and then were stimulated for 24 h with IL-33 (30 ng/mL) alone (Fig. 4B) or with either SP (1 μM) alone (Fig. 4C) or the combination of both SP and IL-33 (Fig. 4C). Preincubation with the anti-ST2 antibody inhibits (P < 0.0001) secretion of IL-1β in response to either IL-33 alone or the combination of both SP and IL-33 but not in response to stimulation by SP alone (Fig. 4C).
The Combination of SP and IL-33 Stimulates Synthesis of pro-IL-1β.
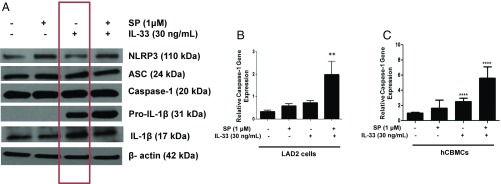
IL-33 (30 ng/mL) alone or in combination with SP (1 μM) stimulates pro-IL-1β protein expression (Fig. 5A) but does not affect the inflammasome protein (NLRP3 or ASC) levels (Fig. 5A). An important finding is that active forms of capsase-1 (p20 kDa) and IL-1β (p17 kDa) are present even in unstimulated LAD2 cells (Fig. 5A).
Fig. 5.
Expression of NLRP3 inflammasome components and the mature form of IL-1β. (A) LAD2 cells (1 × 106 cells per well) were seeded in a 12-well culture plate and were stimulated with SP (1 μΜ), IL-33 (30 ng/mL), or their combination for 24 h. Cell lysates were collected after 24 h, and protein levels of the NLRP3 inflammasome components (NLRP3, ASC, caspase-1), pro-IL-1β, and active IL-1β (p17) were measured by Western blot, using β-actin as loading control (shown in a representative gel of n = 3). SP and IL-33 increase caspase-1 gene expression. (B) LAD2 cells (1 × 106 cells per well), (n = 3, **P < 0.01 compared with unstimulated cells). (C) hCMBCs (0.3 × 106 cell per well) were seeded in a 12-well culture plate and were stimulated by SP (1 µM), IL-33 (30 ng/mL), or their combination for 6 h. Caspase-1 gene expression was measured by qRT-PCR and normalized to human GAPDH endogenous control (n = 3, ***P < 0.001 compared with unstimulated cells).
The Combination of SP and IL-33 Increases Caspase-1 Gene Expression and Activity.
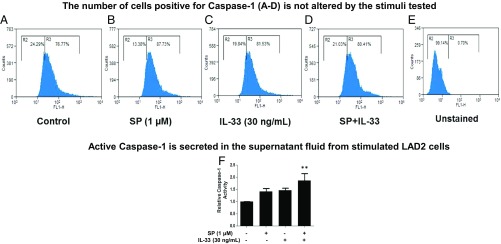
The combination of SP (1 μM) and IL-33 (30 ng/mL) significantly (P < 0.01) increases caspase-1 gene expression by fivefold in both LAD2 cells (Fig. 5B) and primary hCBMCs (Fig. 5C). The presence of active caspase-1 in unstimulated and stimulated LAD2 cells was also investigated using the FLICA assay. Untreated control LAD2 cells make up over 5% of active caspase-1+ cells (Fig. 6A). The percentage of active caspase-1+ cells does not change after stimulation with SP (1 μM) alone, IL-33 (30 ng/mL) alone, or their combination (Fig. 6 B–D). The combination of SP and IL-33 increases capsase-1 activity in the culture medium by twofold (Fig. 6F).
Fig. 6.
Active caspase-1 is constitutively present. (A–E) LAD2 cells (0.25 × 106 cells per well) were stimulated with SP (1 μΜ), IL-33 (30 ng/mL), or their combination for 24 h. Stimulated cells were incubated with the FLICA (FL-1H) caspase-1 probe for 1 h, and active caspase-1+ cells (counts are on the vertical axis) were assessed by flow cytometry. Each panel is representative of three experiments. SP and IL-33 increase caspase-1 activity. (F) LAD2 cells (0.5 × 105 cells per well) were stimulated with SP (1 μΜ), IL-33 (30 ng/mL), or their combination for 24 h; after stimulation, 50 μM YVAD-AFC substrate was added to cells and incubated for 2 h. Caspase-1 activity in the supernatant fluids was determined by measuring fluorescence at the 405-nm wavelength. The fold-increase in caspase-1 activity was determined by comparing the values to the untreated control samples (n = 3, **P < 0.01 compared with SP and IL-33).
Methoxyluteolin Inhibits the Secretion of IL-1β.
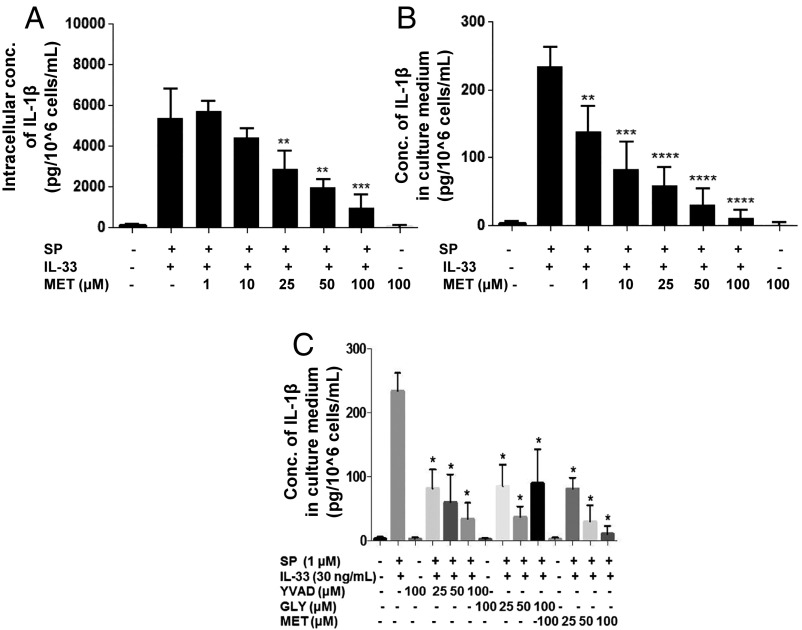
Preincubation with methoxyluteolin significantly (P < 0.01) inhibits the secretion (Fig. 7B) and intracellular levels (Fig. 7A) of IL-1β (Fig. 7). Preincubation with the known inflammasome inhibitors [the selective irreversible caspase-1 inhibitor AC-YVAD-CMK (25–100 μM) or the potassium channel blocker glybenclamide (25–100 μM)] (36) reduces the secretion of IL-1β but to a much smaller degree than methoxyluteolin (Fig. 7C).
Fig. 7.
Methoxyluteolin inhibits the secretion of IL-1β. LAD2 cells (1 × 105 cells per well) were seeded in a 96-well culture plate, were pretreated with methoxyluteolin (MET, 1–100 μM) for 2 h, and then were stimulated with the combination of SP (1 μM) and IL-33 (30 ng/mL) for 24 h. LAD2 cells were also pretreated with AC-YVAD-CMK (YVAD 25–100 μM) or glybenclamide (GLY 25–100 μM). Control cells were treated with 0.1% DMSO, the highest concentration corresponding to that of 100 μM methoxyluteolin. Collected lysates (A) and supernatant fluids (B and C) were assayed for IL-1β using ELISA (n = 3, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 compared with SP and IL-33). Conc, concentration.
Methoxyluteolin Inhibits Gene and Protein Expression of IL-1β.
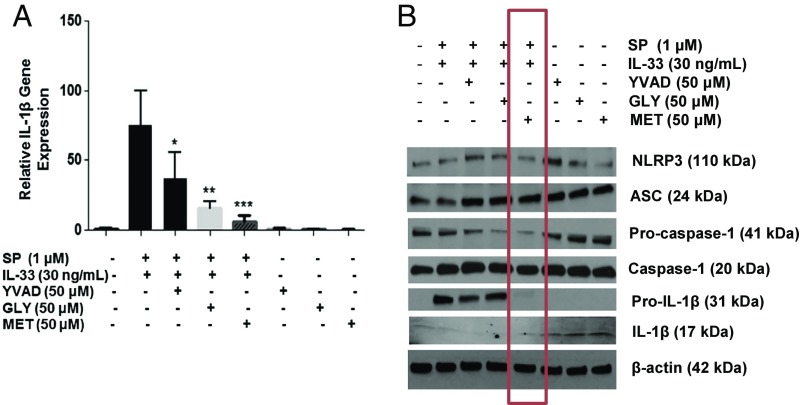
Methoxyluteolin completely (P < 0.05) inhibits gene (Fig. 8A) and protein (Fig. 8B) expression of IL-1β. The inflammasome inhibitors partially reduce gene (Fig. 8A) and protein (Fig. 8B) expression of IL-1β. Expression of the inflammasome proteins NLRP3 and ASC is not affected (Fig. 8B).
Fig. 8.
Methoxyluteolin inhibits gene and protein expression of IL-1β as well as protein expression of procaspase I and pro-IL-1β. LAD2 cells (1 × 106 cells per well) were seeded in a 12-well culture plate, were preincubated with methoxyluteolin (MET, 50 μM), AC-YVAD-CMK (YVAD, 50 μM), or glybenclamide (GLY, 50 μM), and then were stimulated with the combination of SP (1 μM) and IL-33 (30 ng/mL) for 6 h. (A) Gene expression of IL-1β was measured by qRT-PCR and normalized to human GAPDH endogenous control. (n = 3, *P < 0.05, **P < 0.01, *** and P < 0.001 compared to SP and IL-33). (B) Protein expression of NLRP3, ASC, procaspase I, pro-IL-1β, and IL-1β were assayed by Western blot analysis using β-actin as the loading control. The red rectangle identifies the effect of methoxyluteolin.
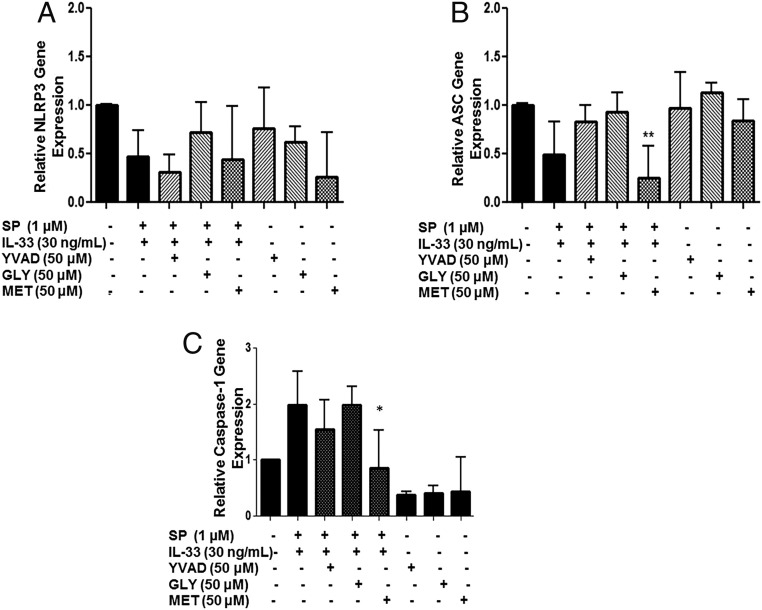
Methoxyluteolin Inhibits Gene Expression of Procaspase 1 and pro-IL-1β.
Only methoxyluteolin, but not the inflammasome inhibitors, significantly (P < 0.05) inhibits protein expression not only of IL-1β but also of procaspase I and pro-IL-1β (Fig. 8B), components necessary for the synthesis of IL-1β. Only methoxyluteolin also inhibits caspase-1 gene expression (Fig. 9C). Gene expression of the inflammasome proteins NLRP3 and ASC is not affected (Fig. 9 A and B).
Fig. 9.
Methoxyluteolin decreases caspase-1 gene expression. LAD2 cells (1 × 106 cells per well) were seeded in a 12-well culture plate, were preincubated with AC-YVAD-CMK (YVAD 50 μM), glybenclamide (GLY 50 μM), or methoxyluteolin (MET 50 μM), and then were stimulated with the combination of SP (1 µM) and IL-33 (30 ng/mL) for 6 h. The gene expression of the inflammasome components NLRP3 (A) and ASC (B) and caspase-1 gene expression (C) were measured by qRT-PCR and normalized to human GAPDH endogenous control (n = 3, *P < 0.05 and **P < 0.01 compared to SP and IL-33).
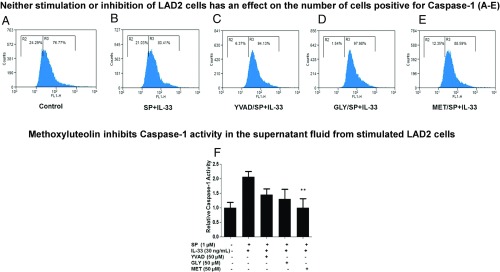
Methoxyluteolin Inhibits Procaspase 1 Activity.
Pretreatment with either methoxyluteolin or the inflammasome inhibitors has no effect on the percentage of active caspase-1+ cells (Fig. 10 A–E). Only methoxyluteolin inhibits caspase-1 activity in the culture medium (Fig. 10F).
Fig. 10.
(A–E) Methoxyluteolin and inflammasome inhibitors do not decrease the percentage of active caspase-1–expressing human mast cells. LAD2 cells (0.25 × 106 cells per well) were preincubated with AC-YVAD-CMK (YVAD, 50 μM), glybenclamide (GLY, 50 μM), or methoxyluteolin (MET, 50 μM) and then were stimulated with SP (1 μΜ) and IL-33 (30 ng/mL) for 24 h. Stimulated cells were incubated with the FLICA caspase-1 probe for 1 h, and active capsase-1+ cells were assessed by flow cytometry. Each panel is representative of three experiments. (F) Methoxyluteolin decreases caspase-1 activity. LAD2 cells (0.5 × 105 cells per well) were preincubated with AC-YVAD-CMK (YVAD, 50 μM), glybenclamide (GLY, 50 μM), or methoxyluteolin (MET, 50 μM) and then were stimulated with SP (1 μΜ) and IL-33 (30 ng/mL) for 24 h. After stimulation, 50 μM YVAD-AFC substrate was added to cells and incubated for 2 h. Caspase-1 activity in the supernatant fluids was determined by measuring fluorescence at 405 nm. Fold-increase in caspase-1 activity was determined by comparing the values to the untreated control samples (n = 3, **P < 0.01 compared with SP and IL-33).
Discussion
Human mast cells synthesize and secrete 1,000-fold more IL-1β than controls in response to the combination of SP and IL-33 as early as 2 h. This impressive amount contrasts starkly with previous reports that unstimulated human foreskin skin mast cells produced a threefold increase in the secretion of IL-1β in response to phorbol 12-myristate 13-acetate (PMA) but not to IgE receptor cross-linking (37). PMA plus the ionophore A23187 also stimulated a sixfold increase in IL-1β secretion from the immature mast cell leukemic (HMC-1) cell line (38).
The effects we report occur via activation of the high-affinity NK-1 receptor previously shown to be expressed by human mast cells (12). The smallest amount of SP required for a response was 0.1 μΜ (Fig. 2), but we chose to use 1 μΜ for maximal effect, as we previously showed for TNF secretion (12). One explanation for the use of this seemingly high amount of SP in our experiments is that SP may be degraded during the assay procedure by chymase secreted from human mast cells, as reported previously (39).
Preincubation with the two different SP receptor antagonists L-733,060 or CP-96345 significantly inhibits IL-1β secretion stimulated by the combined administration of SP and IL-33 and also inhibits IL-1β secretion in response to IL-33 alone, suggesting that there may be some receptor–receptor interaction between NK-1 and ST2. The same SP receptor antagonists also inhibited the secretion of TNF from human cultured mast cells in response to IL-33 (12). The notion of NK-1–ST2 interactions is consistent with our previous finding that NK-1 coimmunoprecipitated with ST2 (12).
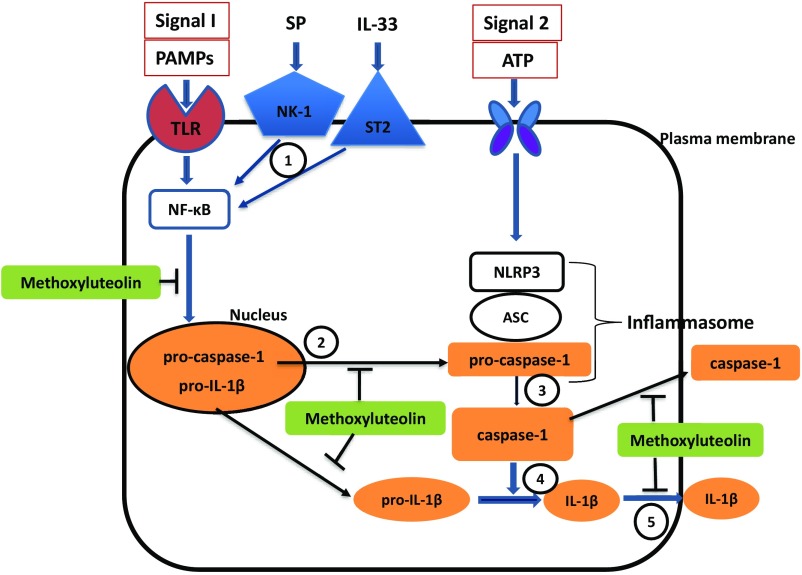
Here we report that IL-33 by itself and in combination with SP stimulates gene expression and synthesis not only of IL-1β, but also of pro-IL-1β and caspase-1. It is known that IL-1β is present in the cytoplasm in a biologically inactive form (pro-IL-1β) and is activated via proteolytic cleavage by caspase-1, which also is present in a proform (procaspase I) and is activated by the NLRP3 inflammasome (34, 35). NLRP3 activation requires two signals (Fig. 1) (40, 41). Signal 1 can be pathogen-associated molecular patterns, environmental agents (silica, asbestos), or endogenous danger signals (27, 42) that induce the transcriptional factor NF-κB, which then stimulates gene expression of the NLRP3 components and pro-IL-1β (Fig. 11). Signal 2 can be reactive oxygen species, potassium ion efflux, or calcium ions influx (43) and initiates the recruitment and assembly of the adaptor ASC together with the NLRP3 protein and procapsase-1, subsequently cleaving the inactive procaspase-1 into active caspase-1. When activated, caspase-1 then cleaves pro-IL-1β into the active mature IL-1β, which is then secreted extracellularly (Fig. 11) (43, 44). The fact that unstimulated mast cells contain pre-IL-1β and active caspase-1 implies that mast cells can respond rapidly to danger signals (27) without requiring induction of their respective genes. The results reported are of additional importance given that none of the known triggers of the NLRP3 inflammasome (i.e., LPS, ATP, TNF, and IFN-γ) can stimulate IL-1β secretion from cultured human mast cells.
Fig. 11.
Diagrammatic representation of the stimulatory effect of SP and IL-33 on IL-1β synthesis and secretion and the proposed point of inhibition of methoxyluteolin. Our evidence indicates that (1) SP and IL-33 activate their respective receptors and stimulate synthesis of procaspase-1 and pro-IL-1β, possibly via NF-κB, activation, which is inhibited by methoxyluteolin. (2) Procaspase-1 and pro-IL-1β are released from the nucleus, a process that could also be inhibited by methoxyluteolin. (3) In the cytoplasm, caspase-1, which is already active, converts pro-IL-1β to active IL-1β. (4) Some of the procaspase-1 is converted to caspase-1, but this may be a minor contribution since the NLRP3 inflammasome does not seem to be involved. (5) IL-1β and active caspase-1 are then secreted extracellularly, a process that is also inhibited by methoxyluteolin. Orange boxes and ovals indicate facts supported by our findings. Open boxes and ovals indicate pathways not supported by our data. The (T) attached to methoxyluteolin indicates possible points of inhibition.
As reported here, stimulation of mast cells by SP and IL-33 alone or in combination does not affect the gene or protein expression of NLRP3 and ASC proteins. These findings raise the possibility that the NLRP3 inflammasome (signal 2) may not be required for the synthesis of IL-1β in human mast cells, at least in response to SP and IL-33 (Fig. 11).
IL-1β is known to be involved in the regulation of the innate immune response (33, 45) and is mandatory for autoinflammatory diseases known as “cryopyrin-associated periodic syndromes” (CAPS) (25, 26, 28, 46, 47) IL-1β could also be important in diseases involving mast cells and SP (22, 48) and may be involved in multiple other diseases that involve mast cells, including asthma (49), rheumatoid arthritis (50), multiple sclerosis (51), and psoriasis (52–54). In fact, gene expression and activity of caspase-1 were reported to be increased in lesional psoriatic epidermis (55, 56).
Even though anti–IL-1β antibody, soluble IL-1R, and IL-1RA are available for the treatment of inflammatory diseases involving IL-1β (57, 58), there are no specific clinically available drugs that could prevent IL-1β synthesis and secretion from any cell type, including mast cells (59–62). Hence, it is clinically desirable to develop molecules that could prevent the secretion of IL-1β, especially from mast cells.
The fact that methoxyluteolin significantly inhibits the impressive gene expression and synthesis of IL-1β reported here is noteworthy. It was previously reported that the methoxyluteolin structural analog luteolin (5,7,3′,4′-tetratetraxydroxyflavone) inhibited the limited IL-1β secretion from HMC-1 cells stimulated by PMA and the ionophore A23187 (38). The precise biochemical step(s) by which methoxyluteolin exerts its inhibitory effect on 1L-1β synthesis and secretion is not presently known. We had previously reported that methoxyluteolin inhibits NF-κB activation stimulated by SP in human mast cells (31). Other potential sites of action of methoxyluteolin are presented in Fig. 11. Methoxyluteolin could inhibit the release of procaspase-1 and/or pro-IL-1β from the nucleus since flavonoids have been reported to enter the nucleus (63). Another possibility is that methoxyluteolin could inhibit how the secretory vesicles fuse with the plasma membrane to secrete IL-1β (Fig. 11). One study using cultured rat basophil leukemia (RBL-1) cells reported that a number of polyphenolic compounds, including luteolin, interfered with this secretory process (64). The concentrations (1–100 μM) shown to be effective in this report have previously been reported to inhibit human cultured mast cells (31, 32). The smallest concentration of luteolin that could be attained in the blood of individuals on ordinary dietary intake was estimated to be 0.1 μM (65), which is 10 times higher than the smallest concentration (1 μM) showing significant inhibitory activity in our studies. This higher concentration could be reached by administration of dietary supplements containing pure luteolin or methoxyluteolin.
In conclusion, IL-33 by itself, and more impressively SP and IL-33 administered together, are identified as stimuli of IL-1β synthesis and secretion from human mast cells. That mast cells contain caspase-1 and IL-1β in their active forms even before stimulation of gene expression is consistent with the data showing a rapid secretion (2 h) of IL-1β. Together, these findings provide insights into the complex interplay among SP, IL-33, and mast cells, thus expanding our understanding of their role in inflammation and in the regulation of IL-1β secretion. The unique inhibitory ability of methoxyluteolin illustrates possibilities for the development of antiinflammatory therapies.
Materials and Methods
SP, LPS, ATP, and AC-YVAD-CMK were purchased from Sigma-Aldrich. Recombinant human IL-33, recombinant human TNF, and recombinant human IFN-γ were obtained from R&D Systems. Nigericin was purchased from Enzo Life Sciences, and glybenclamide was purchased from InvivoGen. Human recombinant IgE and anti-IgE were purchased from EMD Millipore. The NK-1 antagonist L-733,060 was purchased from Sigma-Aldrich. The proteasome inhibitor PS 341 was obtained from Tocris Biosciences. Tetramethoxyflavone was obtained from Pharma Science Nutrients (Hangzhou Skyherb Technologies Co., Ltd.). The RNeasy Mini Kit was from Qiagen, Inc., and the iScript cDNA synthesis kits were purchased from Bio-Rad. Taqman gene-expression primers/assays for NLRP3 (Hs00918082_m1), PYCARD (Hs01547324_gH), CASP1 (Hs00354836_m1), IL1B (Hs01555410_m1), and GAPDH (the endogenous control) (4310884E) were purchased from Applied Biosystems. ELISA kits for IL-1β (DY201), IL-33 (DY3625), and TNF (DY210) were purchased from R&D Systems. Rabbit anti-human primary antibodies for NLRP3, pro-IL-1β, caspase-1, and β-actin were purchased from Cell Signaling Technology, and mouse anti-human ASC and cleaved IL-1β were obtained from Santa Cruz Biotechnology. The FLICA Caspase-1 Activity Assay was purchased from ImmunoChemistry Technologies, and Ac-YVAD-AFC was obtained from Santa Cruz Biotechnology.
Culture of Human Mast Cells.
LAD2 cells, derived from a human mast cell leukemia (66), were kindly supplied by Dr. A. Kirshenbaum (NIH, Bethesda) and were cultured in StemPro-34 medium (Invitrogen) supplemented with 100 U/mL penicillin/streptomycin and 100 ng/mL recombinant human stem cell factor (rhSCF; StemGen), kindly supplied by Swedish Orphan Biovitrum AB. Cells were maintained at 37 °C in a humidified incubator at an atmosphere of 95% O2/5% CO2. LAD2 cells double within 2 wk in the presence of 100 ng/mL stem cell factor (SCF), showing slow proliferation rates. Even though LAD2 cells are an immortalized proliferating cell line, this cell culture closely resembles CD34+-derived primary human mast cells due to its ability to respond to SCF and express functional FcεRI receptors (66). The LAD2 mast cells have been repeatedly shown to behave like immature primary human mast cells (31, 67, 68). Our results were also validated using primary hCBMCs (12, 69) which give more reproducible results than normal primary mast cells derived from skin (70). Mononuclear cells were isolated by layering heparin-treated cord blood onto Lymphocyte Separation Medium (MP Biomedicals). CD34+ progenitor cells were isolated by positive selection of AC133 (CD133+/CD34+) cells using magnetic cell sorting (CD133 Microbead Kit; Miltenyi Biotech). For the first 6 wk, CD34+ progenitor cells were cultured in Iscove’s modified Dulbecco’s medium (Life Technologies) supplemented with 1% insulin-transferrin-selenium, 50 ng/mL IL-6, 0.1% β-mercaptoethanol, 1% penicillin/streptomycin, and 100 ng/mL rhSCF. After 6 wk, the cells were cultured in Iscove’s modified Dulbecco medium supplemented with 10% FBS, 50 ng/mL IL-6, 0.1% β-mercaptoethanol, 1% penicillin/streptomycin, and 100 ng/mL rhSCF. These hCBMCs were cultured for at least 12 wk before being used for experiments.
Cell viability was measured by Trypan blue (0.4%) exclusion (11) or by propidium iodide at all SP and IL-33 concentrations tested.
Mast Cell Treatments.
LAD2 cells and/or hCBMCs were stimulated with various concentration of SP (0.01–1 µM; Sigma-Aldrich) and IL-33 (1–30 ng/mL; R&D Systems) alone or in combination. In some experiments LAD2 cells were stimulated with human IgE (1 µg/mL; EMD Millipore) overnight and then were triggered with anti-IgE (10 ng/mL; Life Technologies). In other experiments, LAD2 cells were pretreated with the NK-1 antagonists L-733,060 (10 μM; Sigma-Aldrich) and CP-96345 (10 µM; Tocris Biosciences), a goat anti-human ST2-neutralizing antibody (0.3 µg/mL–10 μg/mL; R&D Systems), or nonspecific goat anti-human IgG antibody (0.3 µg/mL–10 μg/mL; R&D Systems), and the proteasome inhibitor PS 341 (1–50 µM; Tocris Biosciences); these agents were washed off after preincubation was competed. Methoxyluteolin (1–100 µM) was obtained (98.5% purity) from Hangzhou Skyherb Technologies. Co., Ltd., and was used during the preincubation period and also during stimulation. Silencer Select siRNA targeting either NK-1 or ST2 receptors, as well as control scramble siRNA (10–100 nM; Life Technologies) were used in Lipofectamine RNAiMAX and Opti-MEM medium (Life Technologies) to treat LAD2 cells for 72–96 h to inhibit gene expression of respective receptors.
IL-1β and TNF Assays.
LAD2 cells (1 × 105 cells/0.5 mL in each well) were treated with various concentration of SP (0.01–1 µM) and IL-33 (30 ng/mL) for 24 h. Control cells were treated with the same volume of culture medium. Supernatant fluids were collected and assayed using IL-1β and TNF DuoSet ELISA kits (R&D Systems). These ELISA kits had 6.3% cross-reactivity with human recombinant pro-IL-1β according to the manufacturer’s instructions.
RNA Isolation and qRT-PCR.
Mast cells were stimulated with SP (1 μΜ, 6 h), IL-33 (30 ng/mL, 6 h), or their combination. Total mRNA was extracted with an RNeasy Mini kit (Qiagen Inc.) in accordance with the manufacturer’s instructions. An iScript cDNA synthesis kit (Bio-Rad) was used for reverse-transcription of each mRNA sample. qRT-PCR was performed using Taqman gene-expression assays for IL-1β, NLRP3, ASC, and caspase-1 (Applied Biosystems). Samples were run at 45 cycles using a real-time PCR system (7300; Applied Biosystems). Relative mRNA levels were determined from standard curves run with each experiment. The mRNA gene expressions were normalized to GAPDH endogenous control (Applied Biosystems).
Western Blot Analysis.
LAD2 cells (1 × 106 cells) were preincubated with glybenclamide (50 µM), AC-YVAD-CMK (50 µM), or methlut (50 µM) for 2 h and then were stimulated with SP (1 μM), IL-33 (30 ng/mL), or their combination for 24 h. The reaction was stopped by the addition of ice-cold PBS. Cells were washed once with PBS and then were lysed using protein lysis radio-immunoprecipitation (RIPA) buffer (Sigma-Aldrich) in the presence of protease and phosphatase inhibitor mixtures (Thermo Fisher Scientific, Inc.). Total protein concentration was determined by the bicinchoninic acid assay (Thermo Fisher Scientific, Inc.) method using BSA as the standard. The total cellular proteins (20-μg aliquots) were separated using 4–20% Mini Protean TGX gels (Bio-Rad) under SDS denaturing conditions and were electrotransferred onto PVDF membranes (Bio-Rad). Blocking was carried out with 5% BSA in Tris-buffered saline containing 0.05% Tween-20. The membranes were probed with the following primary antibodies at 1:1,000 dilutions: NLRP3, pro-IL-1β, caspase-1, β-actin (Cell Signaling Technology), ASC, and cleaved IL-1β (Santa Cruz Biotechnology). For the loading control β-actin was probed. For detection, the membranes were incubated with the appropriate secondary HRP-conjugated antibody (Cell Signaling Technology) at a 1:1,000 dilution, and the blots were visualized with enhanced chemiluminescence using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Inc.).
FLICA Caspase-1 Activity Assay.
LAD2 cells (2.5 × 105) were treated with SP (1 μΜ), IL-33 (30 ng/mL), or their combination for 24 h, centrifuged at 500 × g for 5 min, and washed in Apoptosis Wash Buffer (ImmunoChemistry Technologies). Cells were resuspended in 300 μL of 1× FLICA-YVAD (ImmunoChemistry Technologies) fluorescent caspase-1–binding probe and were incubated for 1 h at 37 °C. Propidium iodide staining was used as negative control. Following the incubation, unreacted substrate was removed by washing the cells with Apoptosis Wash Buffer. Finally, cells were resuspended in 300 μL of Apoptosis Wash Buffer for flow cytometry analysis. Active caspase-1 was determined using a FACSCalibur flow cytometer (BD Biosciences).
Caspase-1 Activity Assay.
LAD2 cells (5 × 104) were stimulated with SP (1 μΜ), IL-33 (30 ng/mL), or their combination for 24 h in black 96-well plates (Corning, Inc.). After stimulation, 50 μM YVAD-AFC was added to cells and incubated for 2 h at 37 °C (Ac-YVAD-AFC; Santa Cruz Biotechnology). Fluorescence was measured by a SpectraMax Gemini XPS fluorometer (Molecular Devices) using an excitation wavelength of 400 nm and an emission wavelength of 505 nm. The medium background was subtracted from raw fluorescent values, and then the values were normalized to control untreated cells.
Statistics.
All experiments were performed in triplicate and were repeated at least three times (n = 3). Data are presented as mean ± SD. Results were analyzed using the unpaired, two-tailed, Student’s t test. Significance of comparisons between conditions is denoted by *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Acknowledgments
We thank Drs. D. Metcalfe and A. S. Kirshenbaum (NIH) for providing the LAD2 human mast cells and Swedish Orphan Biovitrum for their generous gift of rhSCF. We also thank Dr. V. Iyer (Tufts Medical Center) for help with collection of the umbilical cord blood, and Dr. A. Degterev (Tufts University School of Medicine) for helpful suggestions during the course of the experiments. This work was supported in part by Pfizer ASPIRE Rheumatology and Dermatology Award WI194875, a Translational Grant from the National Psoriasis Foundation, and an anonymous grant (to T.C.T.).
Footnotes
Conflict of interest statement: T.C.T. is the recipient of US patent no. 7,906,153 covering the use of flavonoids in neuroinflammatory conditions.
References
- 1.Rodewald HR, Dessing M, Dvorak AM, Galli SJ. Identification of a committed precursor for the mast cell lineage. Science. 1996;271:818–822. doi: 10.1126/science.271.5250.818. [DOI] [PubMed] [Google Scholar]
- 2.Kitamura Y, Ito A. Mast cell-committed progenitors. Proc Natl Acad Sci USA. 2005;102:11129–11130. doi: 10.1073/pnas.0505073102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmetzer O, Valentin P, Church MK, Maurer M, Siebenhaar F. Murine and human mast cell progenitors. Eur J Pharmacol. 2016;778:2–10. doi: 10.1016/j.ejphar.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Galli SJ, Tsai M. Mast cells in allergy and infection: Versatile effector and regulatory cells in innate and adaptive immunity. Eur J Immunol. 2010;40:1843–1851. doi: 10.1002/eji.201040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373:163–172. doi: 10.1056/NEJMra1409760. [DOI] [PubMed] [Google Scholar]
- 6.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachelet I, Levi-Schaffer F, Mekori YA. Mast cells: Not only in allergy. Immunol Allergy Clin North Am. 2006;26:407–425. doi: 10.1016/j.iac.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivera J, Fierro NA, Olivera A, Suzuki R. New insights on mast cell activation via the high affinity receptor for IgE. Adv Immunol. 2008;98:85–120. doi: 10.1016/S0065-2776(08)00403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Church MK, el-Lati S, Caulfield JP. Neuropeptide-induced secretion from human skin mast cells. Int Arch Allergy Appl Immunol. 1991;94:310–318. doi: 10.1159/000235393. [DOI] [PubMed] [Google Scholar]
- 11.Theoharides TC, et al. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci USA. 2010;107:4448–4453. doi: 10.1073/pnas.1000803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taracanova A, et al. SP and IL-33 together markedly enhance TNF synthesis and secretion from human mast cells mediated by the interaction of their receptors. Proc Natl Acad Sci USA. 2017;114:E4002–E4009. doi: 10.1073/pnas.1524845114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang MM, Leeman SE. Isolation of a sialogogic peptide from bovine hypothalamic tissue and its characterization as substance P. J Biol Chem. 1970;245:4784–4790. [PubMed] [Google Scholar]
- 14.Mashaghi A, et al. Neuropeptide substance P and the immune response. Cell Mol Life Sci. 2016;73:4249–4264. doi: 10.1007/s00018-016-2293-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Connor TM, et al. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201:167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- 16.Hökfelt T, Pernow B, Wahren J. Substance P: A pioneer amongst neuropeptides. J Intern Med. 2001;249:27–40. doi: 10.1046/j.0954-6820.2000.00773.x. [DOI] [PubMed] [Google Scholar]
- 17.Douglas SD, Leeman SE. Neurokinin-1 receptor: Functional significance in the immune system in reference to selected infections and inflammation. Ann N Y Acad Sci. 2011;1217:83–95. doi: 10.1111/j.1749-6632.2010.05826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 19.Cao J, Cetrulo CL, Theoharides TC. Corticotropin-releasing hormone induces vascular endothelial growth factor release from human mast cells via the cAMP/protein kinase A/p38 mitogen-activated protein kinase pathway. Mol Pharmacol. 2006;69:998–1006. doi: 10.1124/mol.105.019539. [DOI] [PubMed] [Google Scholar]
- 20.Artuc M, Hermes B, Steckelings UM, Grützkau A, Henz BM. Mast cells and their mediators in cutaneous wound healing–Active participants or innocent bystanders? Exp Dermatol. 1999;8:1–16. doi: 10.1111/j.1600-0625.1999.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 21.Puxeddu I, Ribatti D, Crivellato E, Levi-Schaffer F. Mast cells and eosinophils: A novel link between inflammation and angiogenesis in allergic diseases. J Allergy Clin Immunol. 2005;116:531–536. doi: 10.1016/j.jaci.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Theoharides TC, et al. Mast cells and inflammation. Biochim Biophys Acta. 2012;1822:21–33. doi: 10.1016/j.bbadis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: Back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moulin D, et al. Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine. 2007;40:216–225. doi: 10.1016/j.cyto.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Saluja R, et al. The role of the IL-33/IL-1RL1 axis in mast cell and basophil activation in allergic disorders. Mol Immunol. 2015;63:80–85. doi: 10.1016/j.molimm.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Theoharides TC, Petra AI, Taracanova A, Panagiotidou S, Conti P. Targeting IL-33 in autoimmunity and inflammation. J Pharmacol Exp Ther. 2015;354:24–31. doi: 10.1124/jpet.114.222505. [DOI] [PubMed] [Google Scholar]
- 27.Theoharides TC. Danger signals and inflammation. Clin Ther. 2016;38:996–999. doi: 10.1016/j.clinthera.2016.03.036. [DOI] [PubMed] [Google Scholar]
- 28.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: The new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 29.Silver MR, et al. IL-33 synergizes with IgE-dependent and IgE-independent agents to promote mast cell and basophil activation. Inflamm Res. 2010;59:207–218. doi: 10.1007/s00011-009-0088-5. [DOI] [PubMed] [Google Scholar]
- 30.Halova I, et al. Changing the threshold–Signals and mechanisms of mast cell priming. Immunol Rev. 2018;282:73–86. doi: 10.1111/imr.12625. [DOI] [PubMed] [Google Scholar]
- 31.Weng Z, Patel AB, Panagiotidou S, Theoharides TC. The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. J Allergy Clin Immunol. 2015;135:1044–52.e5. doi: 10.1016/j.jaci.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel AB, Theoharides TC. Methoxyluteolin inhibits neuropeptide-stimulated proinflammatory mediator release via mTOR activation from human mast cells. J Pharmacol Exp Ther. 2017;361:462–471. doi: 10.1124/jpet.117.240564. [DOI] [PubMed] [Google Scholar]
- 33.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 34.Latz E. The inflammasomes: Mechanisms of activation and function. Curr Opin Immunol. 2010;22:28–33. doi: 10.1016/j.coi.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanaja SK, Rathinam VA, Fitzgerald KA. Mechanisms of inflammasome activation: Recent advances and novel insights. Trends Cell Biol. 2015;25:308–315. doi: 10.1016/j.tcb.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamkanfi M, et al. Glyburide inhibits the cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Babina M, et al. Comparative cytokine profile of human skin mast cells from two compartments–Strong resemblance with monocytes at baseline but induction of IL-5 by IL-4 priming. J Leukoc Biol. 2004;75:244–252. doi: 10.1189/jlb.0403157. [DOI] [PubMed] [Google Scholar]
- 38.Jeon IH, et al. Anti-inflammatory and antipruritic effects of luteolin from Perilla (P. frutescens L.) leaves. Molecules. 2014;19:6941–6951. doi: 10.3390/molecules19066941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okamura Y, et al. The dual regulation of substance P-mediated inflammation via human synovial mast cells in rheumatoid arthritis. Allergol Int. 2017;66S:S9–S20. doi: 10.1016/j.alit.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Dowling JK, O’Neill LA. Biochemical regulation of the inflammasome. Crit Rev Biochem Mol Biol. 2012;47:424–443. doi: 10.3109/10409238.2012.694844. [DOI] [PubMed] [Google Scholar]
- 41.Lu A, Wu H. Structural mechanisms of inflammasome assembly. FEBS J. 2015;282:435–444. doi: 10.1111/febs.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: A sensor for metabolic danger? Science. 2010;327:296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- 43.Jo EK, Kim JK, Shin DM, Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol. 2016;13:148–159. doi: 10.1038/cmi.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franchi L, Núñez G. Immunology. Orchestrating inflammasomes. Science. 2012;337:1299–1300. doi: 10.1126/science.1229010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dinarello CA. Setting the cytokine trap for autoimmunity. Nat Med. 2003;9:20–22. doi: 10.1038/nm0103-20. [DOI] [PubMed] [Google Scholar]
- 46.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 48.Robuffo I, et al. Mast cell in innate immunity mediated by proinflammatory and antiinflammatory IL-1 family members. J Biol Regul Homeost Agents. 2017;31:837–842. [PubMed] [Google Scholar]
- 49.Kim SR, et al. NLRP3 inflammasome activation by mitochondrial ROS in bronchial epithelial cells is required for allergic inflammation. Cell Death Dis. 2014;5:e1498. doi: 10.1038/cddis.2014.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruscitti P, et al. Monocytes from patients with rheumatoid arthritis and type 2 diabetes mellitus display an increased production of interleukin (IL)-1β via the nucleotide-binding domain and leucine-rich repeat containing family pyrin 3(NLRP3)-inflammasome activation: A possible implication for therapeutic decision in these patients. Clin Exp Immunol. 2015;182:35–44. doi: 10.1111/cei.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inoue M, Shinohara ML. NLRP3 inflammasome and MS/EAE. Autoimmune Dis. 2013;2013:859145. doi: 10.1155/2013/859145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamura Y, et al. Critical role for mast cells in interleukin-1β-driven skin inflammation associated with an activating mutation in the nlrp3 protein. Immunity. 2012;37:85–95. doi: 10.1016/j.immuni.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carlström M, Ekman AK, Petersson S, Söderkvist P, Enerbäck C. Genetic support for the role of the NLRP3 inflammasome in psoriasis susceptibility. Exp Dermatol. 2012;21:932–937. doi: 10.1111/exd.12049. [DOI] [PubMed] [Google Scholar]
- 54.Johnston A, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109–120. doi: 10.1016/j.jaci.2016.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johansen C, Moeller K, Kragballe K, Iversen L. The activity of caspase-1 is increased in lesional psoriatic epidermis. J Invest Dermatol. 2007;127:2857–2864. doi: 10.1038/sj.jid.5700922. [DOI] [PubMed] [Google Scholar]
- 56.Niebuhr M, Baumert K, Heratizadeh A, Satzger I, Werfel T. Impaired NLRP3 inflammasome expression and function in atopic dermatitis due to Th2 milieu. Allergy. 2014;69:1058–1067. doi: 10.1111/all.12428. [DOI] [PubMed] [Google Scholar]
- 57.Dubois EA, Rissmann R, Cohen AF. Rilonacept and canakinumab. Br J Clin Pharmacol. 2011;71:639–641. doi: 10.1111/j.1365-2125.2011.03958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruzicka T, Mihara R. Anti-interleukin-31 receptor A antibody for atopic dermatitis. N Engl J Med. 2017;376:2093. doi: 10.1056/NEJMc1704013. [DOI] [PubMed] [Google Scholar]
- 59.Finn DF, Walsh JJ. Twenty-first century mast cell stabilizers. Br J Pharmacol. 2013;170:23–37. doi: 10.1111/bph.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siebenhaar F, Redegeld FA, Bischoff SC, Gibbs BF, Maurer M. Mast cells as drivers of disease and therapeutic targets. Trends Immunol. 2018;39:151–162. doi: 10.1016/j.it.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 61.Weng Z, et al. Quercetin is more effective than cromolyn in blocking human mast cell cytokine release and inhibits contact dermatitis and photosensitivity in humans. PloS One. 2012;7:e33805. doi: 10.1371/journal.pone.0033805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vieira Dos SR, et al. Topical sodium cromoglicate relieves allergen- and histamine-induced dermal pruritus. Br J Dermatol. 2010;162:674–676. doi: 10.1111/j.1365-2133.2009.09516.x. [DOI] [PubMed] [Google Scholar]
- 63.Notas G, et al. Quercetin accumulates in nuclear structures and triggers specific gene expression in epithelial cells. J Nutr Biochem. 2012;23:656–666. doi: 10.1016/j.jnutbio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 64.Yang Y, et al. Polyphenols differentially inhibit degranulation of distinct subsets of vesicles in mast cells by specific interaction with granule-type-dependent SNARE complexes. Biochem J. 2013;450:537–546. doi: 10.1042/BJ20121256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cao J, Zhang Y, Chen W, Zhao X. The relationship between fasting plasma concentrations of selected flavonoids and their ordinary dietary intake. Br J Nutr. 2010;103:249–255. doi: 10.1017/S000711450999170X. [DOI] [PubMed] [Google Scholar]
- 66.Kirshenbaum AS, et al. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res. 2003;27:677–682. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 67.Zhang B, Asadi S, Weng Z, Sismanopoulos N, Theoharides TC. Stimulated human mast cells secrete mitochondrial components that have autocrine and paracrine inflammatory actions. PLoS One. 2012;7:e49767. doi: 10.1371/journal.pone.0049767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guhl S, Babina M, Neou A, Zuberbier T, Artuc M. Mast cell lines HMC-1 and LAD2 in comparison with mature human skin mast cells–Drastically reduced levels of tryptase and chymase in mast cell lines. Exp Dermatol. 2010;19:845–847. doi: 10.1111/j.1600-0625.2010.01103.x. [DOI] [PubMed] [Google Scholar]
- 69.Zhang B, et al. Human mast cell degranulation and preformed TNF secretion require mitochondrial translocation to exocytosis sites: Relevance to atopic dermatitis. J Allergy Clin Immunol. 2011;127:1522–31.e8. doi: 10.1016/j.jaci.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Theoharides TC. Skin mast cells: Are we missing the forest for the trees? Exp Dermatol. 2016;25:422–423. doi: 10.1111/exd.13008. [DOI] [PubMed] [Google Scholar]