Significance
Parental health and diet at the time of conception determine the development and life-long disease risk of their offspring. While the association between poor maternal diet and offspring health is well established, the underlying mechanisms linking paternal diet with offspring health are poorly defined. Possible programming pathways include changes in testicular and sperm epigenetic regulation and status, seminal plasma composition, and maternal reproductive tract responses regulating early embryo development. In this study, we demonstrate that paternal low-protein diet induces sperm-DNA hypomethylation in conjunction with blunted female reproductive tract embryotrophic, immunological, and vascular remodeling responses. Furthermore, we identify sperm- and seminal plasma-specific programming effects of paternal diet with elevated offspring adiposity, metabolic dysfunction, and altered gut microbiota.
Keywords: developmental programming, maternal responses, metabolic disorder, paternal diet, semen quality
Abstract
The association between poor paternal diet, perturbed embryonic development, and adult offspring ill health represents a new focus for the Developmental Origins of Health and Disease hypothesis. However, our understanding of the underlying mechanisms remains ill-defined. We have developed a mouse paternal low-protein diet (LPD) model to determine its impact on semen quality, maternal uterine physiology, and adult offspring health. We observed that sperm from LPD-fed male mice displayed global hypomethylation associated with reduced testicular expression of DNA methylation and folate-cycle regulators compared with normal protein diet (NPD) fed males. Furthermore, females mated with LPD males display blunted preimplantation uterine immunological, cell signaling, and vascular remodeling responses compared to controls. These data indicate paternal diet impacts on offspring health through both sperm genomic (epigenetic) and seminal plasma (maternal uterine environment) mechanisms. Extending our model, we defined sperm- and seminal plasma-specific effects on offspring health by combining artificial insemination with vasectomized male mating of dietary-manipulated males. All offspring derived from LPD sperm and/or seminal plasma became heavier with increased adiposity, glucose intolerance, perturbed hepatic gene expression symptomatic of nonalcoholic fatty liver disease, and altered gut bacterial profiles. These data provide insight into programming mechanisms linking poor paternal diet with semen quality and offspring health.
There is growing evidence that paternal diet, physiology, and environmental factors impact on sperm quality, postfertilization development, and adult offspring health (1). Studies into mechanisms of paternal programming have identified both direct (sperm quality, epigenetic status, and DNA integrity) and indirect (seminal plasma composition) pathways linking paternal fitness with offspring health (2). Elevated body mass index in men is associated with reduced serum testosterone (3), increased sperm-DNA fragmentation (4), and poor postfertilization development (5). Animal models of paternal high-fat-diet–induced obesity and diabetes also reveal significant increases in sperm-DNA fragmentation (6), impaired embryo development (7), and offspring glucose intolerance and defective insulin secretion (8). Mirroring the programming effects of paternal high-fat diet, paternal undernutrition impacts significantly on embryonic metabolism, fetal growth, and adult cardiometabolic health (9–11).
Epigenetic mechanisms are potential mediators linking paternal environment to sperm quality and adverse offspring development. Changes in the patterns of histone variants (12), DNA methylation (13), and RNA transcripts (14) have been observed in sperm from infertile men. However, it is still unknown whether sperm epigenetic changes are a direct cause or consequence of male infertility. In mice, diets high in fat or low in folates have detrimental effects on sperm-DNA methylation and tRNA-derived small RNA (tsRNA) levels impacting on fetal development and adult offspring glucose tolerance (15, 16). Separate from sperm, seminal plasma can influence postfertilization development through its central role in initiating maternal reproductive tract immunological responses, essential in the establishment and maintenance of pregnancy (17, 18). In mice, absence of seminal plasma at the time of conception retards preimplantation embryo development, programming hypertension, obesity, and glucose intolerance in adult offspring (19).
While our understanding of the impact of suboptimal paternal diet on offspring health is increasing, the mechanisms underlying these changes remain to be defined fully. To date, studies have focused on individual sperm-specific epigenetic changes (DNA methylation, histone modifications, and RNA populations) or seminal plasma-mediated programming of offspring health in isolation. However, the precise and relative sperm- and seminal-plasma specific contributions to offspring programming in vivo are unknown. Male mice in this study were fed normal or low-protein diets as described previously (10, 11). In this model, the low-protein diet does not affect male reproductive fitness (testis weight, sperm number, pregnancy rates, or litter size) but programs elevated offspring growth and perturbed cardiometabolic health. Here, we report that paternal diet modifies sperm-DNA methylation levels and cytokine-associated uterine inflammation and vasculogenesis. Furthermore, we characterize sperm- and seminal plasma-mediated effects of paternal diet on adult offspring growth, adiposity, and metabolic function, including measures of nonalcoholic fatty liver disease and gut microbiota population.
Results
Paternal LPD Leads to Sperm Global DNA Hypomethylation.
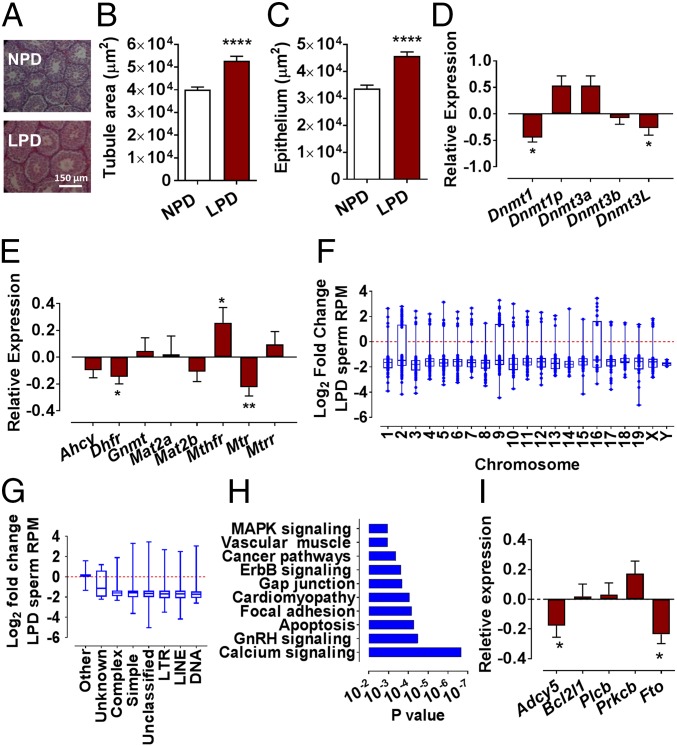
We fed male mice either a control normal protein diet (NPD 18% casein) or isocaloric low-protein diet (LPD; 9% casein) for a minimum of 8 wk (see SI Appendix, Table S1 for composition). In line with our previous studies (10, 11), LPD had no impact on male body weight, testis weight, or the total number of mature epididymal sperm collected (SI Appendix, Fig. S1). However, analysis of testicular histology revealed increased mean tubule and seminiferous epithelium area (Fig. 1 A–C; P ≤ 0.0001). Testes from LPD-fed males displayed reduced expression of the de novo and regulatory DNA methyltransferases Dnmt1 and Dnmt3L, respectively (Fig. 1D; P = 0.023 and 0.05, respectively) and altered folate-cycle enzymes Dhfr (dihydrofolate reductase), Mthfr (methylenetetrahydrofolate reductase), and Mtr (methionine synthase) expression (Fig. 1E; P = 0.036, 0.022, and 0.0012, respectively).
Fig. 1.
Paternal LPD impacts on testicular morphology and epigenetic status. Representative histological images of NPD and LPD testes (A) with area of seminiferous tubules (B) and epithelium (C). Relative NPD and LPD testicular transcript expression of DNA methyltransferases (D) and folate-cycle enzymes (E). LPD sperm methylation status relative to NPD sperm (Log2 fold differences of reads per million, RPM) (F). DNA-repetitive elements’ distribution of differentially methylated loci (G). Pathway analysis of differentially methylated loci between NPD and LPD sperm (H). Relative NPD and LPD sperm transcript expression of differentially methylated genes (I). n = 8 males per dietary group. n = 2 pools of NPD and LPD sperm for F-I. Transcript expression in NPD tissues normalized to 0. Data are mean ± SEM for (B, E, and I). Data are mean ± SEM. *P < 0.05, **P < 0.01, ****P < 0.0001.
Analysis of sperm-DNA methylation status revealed LPD sperm to be comparatively hypomethylated compared with NPD sperm (Fig. 1F). We identified 972 hypomethylated loci in LPD sperm but only 99 hypermethylated loci (Log2 reads per million fold-change <0 or >1, respectively). (See Dataset S1 file for all differentially methylated loci.) LPD differentially methylated loci were present on all chromosomes (Fig. 1F) and associated within several different classes of DNA-repetitive elements (Fig. 1G). Pathway analysis identified calcium-signaling, GnRH-signaling, and apoptosis pathways to be the most enriched, with 24, 14, and 7 genes showing differentially methylated loci, respectively (P < 0.0001) (Fig. 1H). Previously, we identified differential transcript expression of several calcium-signaling, apoptosis, and metabolic regulators (Adcy5, Bcl2l1, Plcb, Prkcb, and Fto) in hearts of offspring derived from LPD-fed males (10). Each gene displayed differential methylation in LPD sperm (Dataset S1). However, in stud sperm, Adcy5 and Fto were the only genes with reduced expression (Fig. 1I; P = 0.05 and 0.043, respectively). Finally, we observed no impact of paternal LPD on sperm capacitation (SI Appendix, Fig. S1) or seminal plasma cytokine levels (SI Appendix, Table S2).
LPD Semen Blunts Maternal Uterine Cytokine and Immunological Responses.
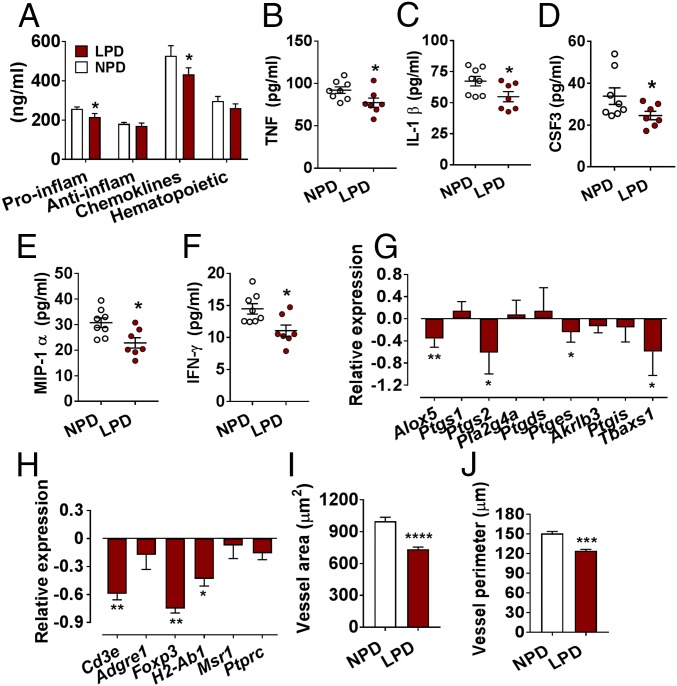
We demonstrated previously that paternal LPD reduced expression of multiple AMPK pathway genes in day 3.5 blastocysts (11). To determine if changes in embryonic gene expression were associated with an altered maternal uterine environment, we analyzed uterine cytokines at day 3.5 post coitus. Here, we observed significant reductions in proinflammatory cytokines and chemokines in females mated with LPD-fed males (Fig. 2A). Specifically, we observed reductions in the levels of TNF, IL-1β, CSF3 MIP-1α, and IFN-γ (Fig. 2 B–F; P < 0.05). Expression analysis of genes involved in the prostaglandin synthesis pathway revealed reductions in the expression of arachidonate 5-lipoxygenase (Alox5), prostaglandin-endoperoxide synthase 2 (Ptgs2), prostaglandin E synthase (Ptges), and thromboxane-A synthase (Tbxas1) in females mated with LPD males (Fig. 2G; P < 0.05). Furthermore, we observed significant reductions in uterine expression of CD3 antigen, epsilon polypeptide (Cd3e), forkhead box P3 (Foxp3), and histocompatibility 2, class II antigen A, beta 1 (H2-Ab1) (Fig. 2H; P < 0.05). Finally, analysis of uterine vascular morphology at day 3.5 post coitus revealed significant reductions in uterine blood vessel area and perimeter (Fig. 2 I and J; P < 0.001) in females mated with LPD-fed males.
Fig. 2.
Paternal LPD modifies maternal preimplantation uterine environment. Impact of paternal LPD on maternal uterine cytokine and chemokine levels (A), TNF (B), IL-1β (C), CSF3 (D), MIP-1α (E), IFN-γ (F), prostaglandin synthesis (G), and immune-response (H) gene transcript levels; NPD uteri normalized to 0. Impact of paternal NPD and LPD on female uterine blood vessel area (I) and vessel perimeter (J). n = 8 females per treatment group, each mated with a separate male. Data are mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Sperm- and Seminal Plasma-Specific Effects on Offspring Health.
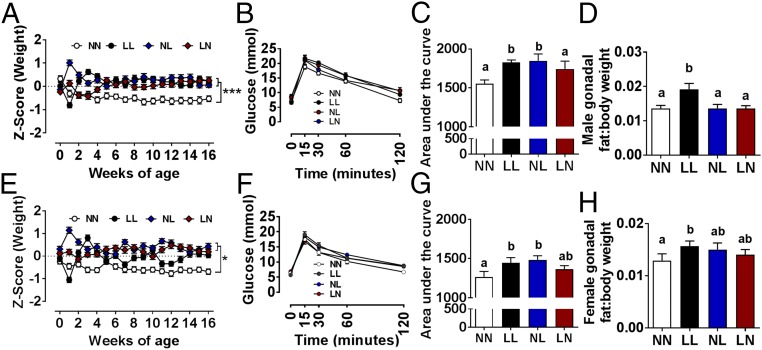
Our results indicated paternal diet programs offspring development through both sperm (direct genomic or epigenetic effects on the embryo) and seminal plasma (perturbed maternal uterine environment) mechanisms. To define the relative sperm and seminal plasma programming contribution to offspring health, we combined artificial insemination and vasectomized male mating. We generated four groups of offspring mice termed NN (NPD sperm and NPD seminal plasma), LL (LPD sperm and LPD seminal plasma), NL (NPD sperm and LPD seminal plasma), and LN (LPD sperm and NPD seminal plasma). No significant difference in maternal gestational weight gain or gestation length, litter size, or weight at birth were observed between treatment groups (SI Appendix, Table S3). For all treatment groups, we observed that approximately one third of inseminated females did not become pregnant (as defined by sustained weight gain over the first 10 d of gestation). Similarly, only two thirds of pregnant females went on to deliver live offspring. However, both pregnancy and live birth rates were similar for all groups. Postnatally, LL, NL, and LN males all became significantly heavier then NN offspring from 5 wk of age and remained heavier for up to 16 wk of age (Fig. 3A; P < 0.001). Female offspring displayed a growth profile similar to that of the males, with the LL, NL, and LN offspring all becoming heavier than the NN females (Fig. 3E; P < 0.05). Furthermore, LN females displayed a significantly heavier growth profile than LL females (P = 0.009). Analysis of 15-wk-old offspring glucose tolerance revealed elevated responses in LL males and females at 30 min post bolus (P = 0.013 and 0.023, respectively); LL males, LN males, and LN females at 60 min (P = 0.04, 0.05 and 0.024, respectively); and LN males, NL males, LL females, LN females, and NL females at 120 min (P = 0.003, 0.004, 0.00, 0.001, and 0.0032, respectively) compared with NN offspring (Fig. 3 B and F). Analysis of mean area under the glucose tolerance test curve revealed increased responses in LL and NL males (P = 0.022 and 0.012, respectively) and females (P = 0.034 and 0.010, respectively) compared with NN offspring (Fig. 3 C and G). At the time of cull (16 wk of age), male and female LL offspring displayed increased gonadal:body weight ratios compared with NN offspring (P = 0.021 and 0.046, respectively; Fig. 3 D and H).
Fig. 3.
Offspring growth and metabolic health are affected in a sperm- and seminal plasma-specific manner. NN (NPD sperm and LPD seminal plasma), LL (LPD sperm and LPD seminal plasma), NL (NPD sperm and LPD seminal plasma), and LN (LPD sperm and NPD seminal plasma) offspring Z-score growth profiles in males (A) and females (E). Male (B and C) and female (F and G) offspring glucose tolerance test responses and area under the glucose tolerance test curves. Male (D) and female (H) offspring gonadal adipose:body weight ratios. n = 14–37 males per treatment group and 14–37 females per treatment group. Data are mean ± SEM. Differences at P < 0.05 between groups are represented as different letters. *P < 0.05, ***P < 0.001.
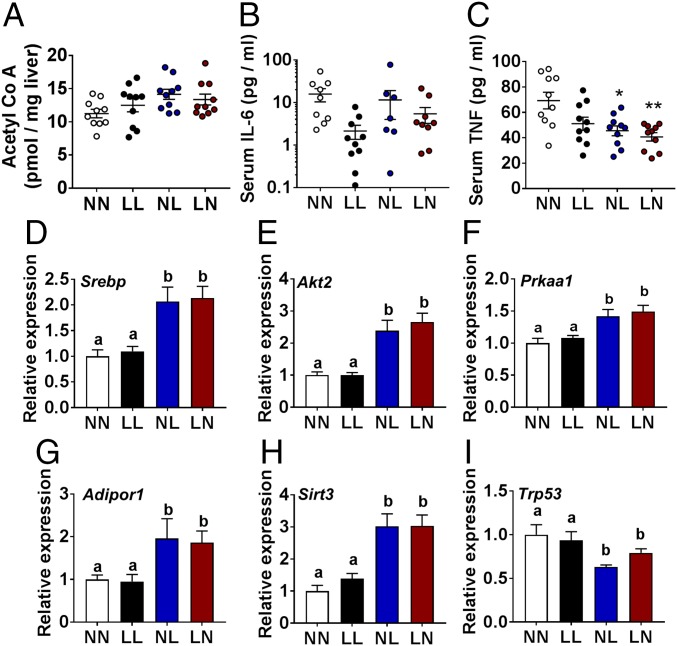
Increased obesity and poor glucose regulation are central markers for nonalcoholic fatty liver disease (NAFLD). As we observed no impact of offspring sex on any of the NAFLD parameters measured, data for male and female offspring within each treatment group were combined. Liver Acetyl-CoA and serum IL-6 showed no difference between treatment groups (Fig. 4 A and B). However, NL and LN offspring displayed a reduced level of serum TNF compared with NN offspring (P = 0.011 and 0.002, respectively; Fig. 4C). NL and LN offspring also displayed increased hepatic expression of the central NAFLD-regulating genes adiponectin 1 receptor (Adipor1; P = 0.019 and 0.033, respectively), AKT serine/threonine kinase 2 (Akt2; P = 0.001 and >0.001, respectively), protein kinase, AMP-activated, alpha 1 catalytic subunit (Prkaa1; P = 0.002 and >0.001, respectively), sirtuin 3 (Sirt3; P > 0.001 for both), and sterol regulatory element binding transcription factor 1 (Srebf1; P = 0.001 and 0.002, respectively) (Fig. 4 D–H) compared with NN offspring. Furthermore, NL and LN offspring displayed reduced expression of the cellular tumor antigen p53 (Trp53; Fig. 4I; P = 0.002 and 0.032, respectively) compared with NN offspring. Interestingly, there were no significant differences between the LL and NN offspring for any of the genes studied.
Fig. 4.
Analysis of offspring nonalcoholic fatty liver disease phenotype. NN (NPD sperm and NPD seminal plasma), LL (LPD sperm and LPD seminal plasma), NL (NPD sperm and LPD seminal plasma), and LN (LPD sperm and NPD seminal plasma) adult offspring liver Acetyl CoA (A), serum IL-6 (B), and serum TNF-α (C) levels. Adult offspring liver transcript expression of Adipor1 (D), Akt2 (E), Prkaa1 (F), Trp53 (G), Sirt3 (H), and Srebf1 (I). n = 5 males and females per treatment group, each from different litters. NPD expression normalized to 1 in D–I. Data are mean ± SEM. Differences at P < 0.05 between groups are represented as different letters. *P < 0.05, **P < 0.01.
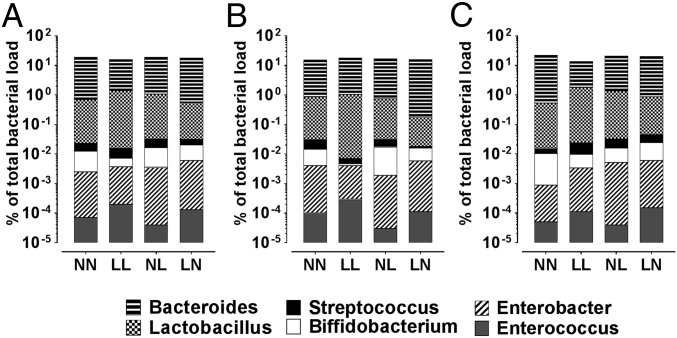
Finally, using universal primers, we observed no significant difference in mean total bacterial content between treatment groups or sexes (Fig. 5 A–C). However, analysis of individual bacterial genus revealed LL males to have a higher Enterococcus profile compared with NN and NL males (P = 0.049 and 0.024, respectively; Fig. 5B). We also observed that LL offspring displayed higher levels of Lactobacillus compared with LN offspring (Fig. 5A; P = 0.043). Analysis of fecal Streptococcus levels revealed NN and LN males to have significantly higher profiles compared with LL and NL males (Fig. 5B). Finally, LL offspring displayed a significantly reduced profile of fecal Biffidobacterium compared with NL and LN offspring (Fig. 5A; P = 0.041 and 0.024, respectively). No difference in fecal Enterobacter or Bacteroides was observed between groups. As only six specific bacterial groups within the total population were analyzed within the current study, further sequencing of the bacterial 16s ribosomal region would be required to determine fully how paternal diet affects offspring gut microbiota.
Fig. 5.
Analysis of offspring microbiota. Offspring gut bacterial profiles in males and females combined (A), males only (B), and females only (C). n = 10 males and females per treatment group.
Discussion
While the association between poor paternal diet and offspring metabolic dysfunction is now established, the underlying mechanisms remain poorly defined. We now show that feeding male mice an LPD results in perturbed testicular epigenetic status and globally hypomethylated sperm. Furthermore, in females mated with LPD males, we observe blunted uterine immunological signaling responses and vascular remodeling. Using artificial insemination with vasectomized male mating we show that offspring growth, metabolism, adiposity, and gut bacteria are programmed by paternal diet in a sperm- or seminal plasma-specific manner. Significantly, all offspring derived from either LPD sperm and/or seminal plasma developed phenotypic characteristics of metabolic dysfunction. These results provide insights into links between poor paternal diet at the time of conception and the programming of metabolic health in his offspring.
Diets high in fat and/or protein (28% protein) have been shown to impair testicular steroidogenesis, increasing rates of apoptosis and sperm-DNA damage in rodents (20, 21). In response to LPD, we observed increased mean testicular seminiferous tubule and epithelium area. However, the mechanisms underlying these changes in tubule morphology remain unknown at present. We also observed paternal LPD perturbed testicular expression of the folate-cycle enzymes Dhfr, Mthfr, and Mtr and of the DNA methyltransferases Dnmt1, Dnmt3L. In rodents, maternal LPD has been associated with elevated plasma homocysteine levels, modifying s-adenosylmethionine levels for epigenetic regulation of gene expression (22, 23). Male Mthfr knockout mice display altered testicular histology, loss of germ cell number, and reduced sperm-DNA methylation profiles (15, 24), while pharmacological reduction in cellular Dnmt1/3L levels impact negatively on spermatogenesis and sperm-DNA methylation levels (25, 26). Therefore, disruption of the testicular folate cycle in LPD males may impact the provision of methyl donors for the establishment of appropriate epigenetic marks within the developing sperm, although this remains to be confirmed.
To determine the impact of paternal LPD on sperm epigenetic status, we performed global analysis of sperm-DNA methylation. We observed that sperm from LPD-fed males displayed global hypomethylation aligned within gene body locations. However, minimal changes in DNA methylation at promoter regions or CpG islands were detected. Similar minimal impacts on promoter DNA methylation (as determined by MeDIP-Seq) have been observed in sperm from mice fed LPD (9). However, gene body methylation status was not reported. In sperm, DNA methylation at non–CpG-dense gene–body regions has been reported and correlates positively with gene expression (27). It is believed to regulate gene transcription from alternative intragenic promoters (28). Therefore, further studies are required to elucidate fully the impact of paternal diet on additional sperm epigenetic modifications including histone modifications (29) and RNAs (15) in addition to DNA methylation.
Previously, we have shown offspring of LPD males display cardiovascular dysfunction associated with reduced cardiac expression of calcium-signaling (Adcy5, Plcb, and Prkcb), fat mass- and obesity-associated (Fto), and antiapoptotic (Bcl2l1) genes (10). As Adcy5, Bcl2l1, Plcb, and Fto displayed differential methylation in LPD sperm, we assessed their expression at the transcript level. While Adcy5 and Fto expression were reduced in LPD sperm, no difference in Bcl2l1, Plcb, or Prkcb was observed. These observations indicate a nondirect association between DNA methylation and other epigenetic mechanisms in sperm (9). Future studies will define further the associations between paternal diet, testicular function, and sperm epigenetic status.
We also observed that paternal LPD suppressed normal maternal uterine inflammatory responses. Following mating, deposition of seminal plasma within the female reproductive tract initiates secretion of embryotrophic and immune-modulatory cytokines as well as initiating regulatory T cell expansion and leukocyte infiltration (30). Disruption to the balance of oviductal and uterine-signaling molecules during preimplantation development has been linked to perturbed embryonic and offspring development (19). We observed reduced uterine TNF, IL-1β, IFN-γ, MIP-1α, and CSF3 levels following mating with LPD males. IL-1β has been linked to embryo development with levels correlated to blastocyst cell number in humans (31). Uterine MIP-1α and CSF3 regulate paternal antigen-presenting cells, which are essential for uterine immune response suppression ahead of blastocyst implantation (32), while uterine natural killer cell-derived IFN-γ and TNF regulate the remodeling of the uterine vasculature. We also observed significantly decreased uterine expression of Alox5, Ptgs2, Ptges, Tbxas1 genes in females mated with LPD males. ALOX5 regulates the expression of proinflammatory cytokines including TNF (33) and IL-1β (34), while Ptges is involved in the maternal recognition of pregnancy (30). These observations may be explained by blunted uterine inflammatory and regulatory T cell (Tregs) mediated responses post mating. Our observation of significantly reduced uterine expression of Cd3e, Foxp3, and H2-Ab1 supports this interpretation. H2-Ab1 is involved in the presentation of paternal antigens by dendritic cells to T cells via the T cell receptor (TCR). Signaling via Cd3e, a component of the TCR, results in the activation of Treg cells and the induction of Foxp3 gene transcription (35). However, further studies are needed to characterize uterine Foxp3+ Treg cell populations.
Our data reveal the impact of paternal diet on both sperm epigenetic status and the preimplantation uterine environment. As both mechanisms have the potential to program postfertilization development and offspring health, we define their respective impacts on adult offspring well-being. We combined artificial insemination with vasectomized male mating to generate four groups of offspring (NN, LL, NL, LN; where the first and second letters denote the diet of the sperm and seminal plasma, respectively). All offspring generated using LPD sperm and/or seminal plasma displayed characteristics associated with metabolic syndrome including elevated adiposity, glucose intolerance, nonalcoholic fatty liver disease, and altered gut bacterial profiles. We observed that the greatest impacts on offspring phenotype occurred when the dietary origin of the sperm and seminal plasma are mismatched (NL and LN groups). This was most evident in our analysis of offspring nonalcoholic fatty liver disease (NAFLD). NAFLD is the most common form of chronic liver disease in Western societies and is characterized typically by increased levels of inflammatory markers, hepatic lipids, metabolites, and apoptosis (36). We observed no impact of paternal diet on hepatic acetyl-CoA or serum IL-6 levels, while levels of the proinflammatory cytokine TNF were reduced in NL and LN offspring. Studies have shown that obese prepubescent children display lower levels of serum TNF than nonobese children (37). These studies suggest that in younger individuals the relationship between obesity and inflammatory status may be different to that in adults. As our offspring were only 16 wk of age, they may only be at an early stage of NAFLD before the chronic elevation of proinflammatory markers. Furthermore, we observed significantly increased expression of Adipor1, Akt2, Prkaa1, Sirt3, and Srebf1 in NL and LN offspring, while Tp53 was decreased. Such patterns have been linked with obesity (38), increased hepatic fatty acid synthesis (39), and increased lipid deposition (40).
There is increasing interest in the interaction between lifestyle, gut microbiota, and liver function (41). Our initial analysis of offspring gut bacterial profiles revealed significant changes in the levels of Enterococcus, Lactobacillus, Streptococcus, and Biffidobacterium between groups. Collectively and individually, these bacteria are involved in the metabolism of carbohydrates, fatty acids, and bile acids (42). Probiotic treatment of obese children with Lactobacillus and Biffidobacterium has been shown to reduce the severity of NAFLD (43). It is interesting to speculate if the changes in offspring gut microbiota were established in early neonatal life or occurred in response to their perturbed metabolic status. Recent studies have established seminal plasma possess its own microbiota, which can be modified by paternal high-fat diet (44). Whether the seminal microbiota can influence the maternal reproductive tract microbiota and so alter offspring gut microbiota at birth remains to be established. However, this identifies another mechanism by which paternal diet could influence the health and metabolism of offspring.
In contrast to our previous study (10), we observed no effect of paternal LPD on weight at birth, but we did observe a significant effect on postnatal growth affecting both male and female offspring. One key difference between the current study and our previous studies is the use of superovulation and artificial insemination. Ovarian hyperstimulation can impact negatively on embryo quality, endometrial receptivity, and perinatal outcomes (45, 46), masking or exaggerating offspring phenotypic effects in a sex-specific manner separate to other programming mechanisms, for example, paternal diet (10) and absence of seminal plasma (19). In the current study we are not able to define the potential impact of the superovulation and/or artificial insemination techniques used on offspring health. However, as all treatment groups were generated using the same manipulation techniques, we believe that differences in postnatal phenotype between treatment groups can be attributed to the dietary status of the male from which the sperm and/or seminal plasma originated.
Our study provides insights into the mechanism linking paternal nutrition to the early origins of offspring health. Separate studies have shown that offspring metabolic health can be impaired either via sperm-specific programming of the preimplantation embryo (15, 47) or seminal plasma-specific modifications to the uterine environment (19). Our study suggests that a mismatch in the origin of the sperm and seminal plasma between dietary treatments has a greater impact on offspring metabolic health than when both semen components originate from the same suboptimal dietary treatment group. We hypothesize that when both sperm and seminal plasma originate from the same dietary background, the seminal plasma-primed uterine environment is appropriate for the sperm-programmed preimplantation embryo, impacting minimally on offspring health. When the seminal plasma-mediated programming of the embryo and uterus is mismatched, adaptive changes initiated within the embryo/fetus elevate metabolic disease risk in adult life (1). However, further studies are needed to determine precisely how mismatched sperm and seminal plasma affect offspring metabolic health.
We believe our study has relevance for both human- and large animal-assisted reproduction where embryos are routinely generated and develop in a seminal plasma-free environment and where embryos are transferred to a nonseminal plasma-primed uterus. This is an important context for this phase of our work. It will, therefore, be of interest to determine in humans and large animals the extent to which a mismatch between the programming of the embryo and the optimal priming of the uterus direct the development and well-being of offspring.
Materials and Methods
Animal Treatments.
All procedures were conducted in accordance with the UK Home Office Animal (Scientific Procedures) Act 1986 and local ethics committees. Virgin 8-wk-old males, both intact (n = 32 in total) and vasectomized (n = 16 in total), and 5- to 9-wk-old female C57BL/6 mice (Harlan Ltd) were maintained either at the University of Nottingham’s Bio Support Unit or at Aston University’s biomedical research facility. Males were allocated either control normal protein diet (NPD; 18% casein) or isocaloric low-protein diet (LPD; 9% casein) for a minimum of 8 wk as described previously (10, 11) and detailed in SI Appendix, Table S1.
Stud Sperm MBD2-Seq Analysis.
Methylated DNA from NPD and LPD male epididymal sperm was isolated ahead of library preparation, sequencing, and bioinformatics analysis detailed in SI Appendix, Supplementary Materials and Methods.
Uterine and Seminal Plasma Cytokine Array.
Virgin 7- to 9-wk-old C57BL/6 females were caged overnight with NPD or LPD studs. At day 3.5 post coitus, whole uteri were homogenized for the analysis of cytokine levels using a Bio-Plex Pro Mouse Cytokine 23-plex immunoassay (Bio-Rad Laboratories) and analyzed as detailed in SI Appendix, Supplementary Materials and Methods. Using the same Bio-Plex assay, we also analyzed stud male seminal plasma cytokine levels as detailed in SI Appendix, Supplementary Materials and Methods.
Paternal and Maternal Tissue Histology.
Day 3.5 post coitus uteri from females mated with NPD- or LPD-fed males and whole NPD- and LPD-fed male testes were processed into paraffin wax and sectioned and analyzed as detailed in SI Appendix, Supplementary Materials and Methods.
Stud Sperm Capacitation Assay.
Motile epididymal sperm were isolated from NPD and LPD males for analysis of capacitation by chlortetracycline (CTC) staining and cholesterol efflux as detailed in SI Appendix, Supplementary Materials and Methods.
Offspring Generation.
Female C57BL/6 mice were superovulated and artificially inseminated using sperm from NPD- or LPD-fed males as detailed in SI Appendix, Supplementary Materials and Methods. Following artificial insemination, females were housed overnight with an NPD- or LPD-fed vasectomized male. Four groups of offspring were generated, termed NN (NPD sperm and NPD seminal plasma), LL (LPD sperm and LPD seminal plasma), NL (NPD sperm and LPD seminal plasma), and LN (LPD sperm and NPD seminal plasma). A minimum of eight litters per treatment group were generated.
Offspring Glucose Tolerance Test.
Offspring glucose tolerance at 15 wk of age was determined as described previously (10). Offspring were fasted overnight before an i.p. glucose bolus (2 g/kg body weight in PBS) as detailed in SI Appendix, Supplementary Materials and Methods.
Offspring Tissue Sampling.
Offspring were culled at 16 wk of age for the collection and analysis of tissues as detailed in SI Appendix, Supplementary Materials and Methods.
RNA and DNA Extraction for Gene Expression and Bacterial Profile Analysis.
RNA was extracted from stud sperm, day 3.5 post coitus maternal uterine tissue, and offspring kidney tissues, and DNA was extracted from offspring fecal samples as detailed in SI Appendix, Supplementary Materials and Methods. RT-qPCR was performed using primer sequences provided in SI Appendix, Table S5 and as detailed in SI Appendix, Supplementary Materials and Methods.
Statistical Analyses.
Stud male (excluding MBD-Seq data) and maternal data were analyzed using independent samples or repeated measures t tests, where appropriate (SPSS version 23). For each litter, only one sperm donor male and one vasectomized male were used, and no male was used more than once. All offspring data were analyzed using a multilevel random effects regression model (SPSS version 23) (10) with paternal origin of litter incorporated as a random effect in addition to litter size and body weight as detailed in SI Appendix, Supplementary Materials and Methods.
Supplementary Material
Acknowledgments
We acknowledge technical support and advice from Dr. Wing Yee Kwong, Dr. Victoria Wright, and Dr. Sunir Malla and the biomedical research facilities at the University of Nottingham and Aston University. Dr. A.J.W. was supported by a University of Nottingham Advanced Research Fellowship and an Aston Research Centre for Healthy Ageing (ARCHA) Research Fellowship. Professor K.D.S. was supported by a Society for Reproduction and Fertility Academic Scholarship Fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 9827.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1806333115/-/DCSupplemental.
References
- 1.Fleming TP, et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet. 2018;391:1842–1852. doi: 10.1016/S0140-6736(18)30312-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucas ES, Watkins AJ. The long-term effects of the periconceptional period on embryo epigenetic profile and phenotype; the paternal role and his contribution, and how males can affect offspring’s phenotype/epigenetic profile. Adv Exp Med Biol. 2017;1014:137–154. doi: 10.1007/978-3-319-62414-3_8. [DOI] [PubMed] [Google Scholar]
- 3.Tunc O, Bakos HW, Tremellen K. Impact of body mass index on seminal oxidative stress. Andrologia. 2011;43:121–128. doi: 10.1111/j.1439-0272.2009.01032.x. [DOI] [PubMed] [Google Scholar]
- 4.Campbell JM, Lane M, Owens JA, Bakos HW. Paternal obesity negatively affects male fertility and assisted reproduction outcomes: A systematic review and meta-analysis. Reprod Biomed Online. 2015;31:593–604. doi: 10.1016/j.rbmo.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Yang Q, et al. Effect of paternal overweight or obesity on IVF treatment outcomes and the possible mechanisms involved. Sci Rep. 2016;6:29787. doi: 10.1038/srep29787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakos HW, Mitchell M, Setchell BP, Lane M. The effect of paternal diet-induced obesity on sperm function and fertilization in a mouse model. Int J Androl. 2011;34:402–410. doi: 10.1111/j.1365-2605.2010.01092.x. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell M, Bakos HW, Lane M. Paternal diet-induced obesity impairs embryo development and implantation in the mouse. Fertil Steril. 2011;95:1349–1353. doi: 10.1016/j.fertnstert.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 8.Ng SF, et al. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature. 2010;467:963–966. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- 9.Carone BR, et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143:1084–1096. doi: 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watkins AJ, Sinclair KD. Paternal low protein diet affects adult offspring cardiovascular and metabolic function in mice. Am J Physiol Heart Circ Physiol. 2014;306:H1444–H1452. doi: 10.1152/ajpheart.00981.2013. [DOI] [PubMed] [Google Scholar]
- 11.Watkins AJ, et al. Paternal low protein diet programs preimplantation embryo gene expression, fetal growth and skeletal development in mice. Biochim Biophys Acta. 2017;1863:1371–1381. doi: 10.1016/j.bbadis.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Hammoud SS, et al. Genome-wide analysis identifies changes in histone retention and epigenetic modifications at developmental and imprinted gene loci in the sperm of infertile men. Hum Reprod. 2011;26:2558–2569. doi: 10.1093/humrep/der192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montjean D, et al. Methylation changes in mature sperm deoxyribonucleic acid from oligozoospermic men: Assessment of genetic variants and assisted reproductive technology outcome. Fertil Steril. 2013;100:1241–1247. doi: 10.1016/j.fertnstert.2013.06.047. [DOI] [PubMed] [Google Scholar]
- 14.Ostermeier GC, Dix DJ, Miller D, Khatri P, Krawetz SA. Spermatozoal RNA profiles of normal fertile men. Lancet. 2002;360:772–777. doi: 10.1016/S0140-6736(02)09899-9. [DOI] [PubMed] [Google Scholar]
- 15.Chen Q, et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science. 2016;351:397–400. doi: 10.1126/science.aad7977. [DOI] [PubMed] [Google Scholar]
- 16.Lambrot R, et al. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat Commun. 2013;4:2889. doi: 10.1038/ncomms3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharkey DJ, Macpherson AM, Tremellen KP, Robertson SA. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol Hum Reprod. 2007;13:491–501. doi: 10.1093/molehr/gam028. [DOI] [PubMed] [Google Scholar]
- 18.Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol. 2012;188:2445–2454. doi: 10.4049/jimmunol.1102736. [DOI] [PubMed] [Google Scholar]
- 19.Bromfield JJ, et al. Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring. Proc Natl Acad Sci USA. 2014;111:2200–2205. doi: 10.1073/pnas.1305609111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vendramini V, Cedenho AP, Miraglia SM, Spaine DM. Reproductive function of the male obese Zucker rats: Alteration in sperm production and sperm DNA damage. Reprod Sci. 2014;21:221–229. doi: 10.1177/1933719113493511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao JL, Zhao YY, Zhu WJ. A high-fat, high-protein diet attenuates the negative impact of casein-induced chronic inflammation on testicular steroidogenesis and sperm parameters in adult mice. Gen Comp Endocrinol. 2017;252:48–59. doi: 10.1016/j.ygcen.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135:1382–1386. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- 23.Petrie L, Duthie SJ, Rees WD, McConnell JM. Serum concentrations of homocysteine are elevated during early pregnancy in rodent models of fetal programming. Br J Nutr. 2002;88:471–477. doi: 10.1079/BJN2002695. [DOI] [PubMed] [Google Scholar]
- 24.Chan D, et al. Strain-specific defects in testicular development and sperm epigenetic patterns in 5,10-methylenetetrahydrofolate reductase-deficient mice. Endocrinology. 2010;151:3363–3373. doi: 10.1210/en.2009-1340. [DOI] [PubMed] [Google Scholar]
- 25.La Salle S, et al. Loss of spermatogonia and wide-spread DNA methylation defects in newborn male mice deficient in DNMT3L. BMC Dev Biol. 2007;7:104. doi: 10.1186/1471-213X-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oakes CC, Kelly TL, Robaire B, Trasler JM. Adverse effects of 5-aza-2′-deoxycytidine on spermatogenesis include reduced sperm function and selective inhibition of de novo DNA methylation. J Pharmacol Exp Ther. 2007;322:1171–1180. doi: 10.1124/jpet.107.121699. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi H, et al. Contribution of intragenic DNA methylation in mouse gametic DNA methylomes to establish oocyte-specific heritable marks. PLoS Genet. 2012;8:e1002440. doi: 10.1371/journal.pgen.1002440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulis M, Queirós AC, Beekman R, Martín-Subero JI. Intragenic DNA methylation in transcriptional regulation, normal differentiation and cancer. Biochim Biophys Acta. 2013;1829:1161–1174. doi: 10.1016/j.bbagrm.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Siklenka K, et al. Disruption of histone methylation in developing sperm impairs offspring health transgenerationally. Science. 2015;350:aab2006. doi: 10.1126/science.aab2006. [DOI] [PubMed] [Google Scholar]
- 30.Robertson SA, Prins JR, Sharkey DJ, Moldenhauer LM. Seminal fluid and the generation of regulatory T cells for embryo implantation. Am J Reprod Immunol. 2013;69:315–330. doi: 10.1111/aji.12107. [DOI] [PubMed] [Google Scholar]
- 31.Taşkın EA, Baltacı V, Cağıran G, Aytaç R. Detection of IL-1β in culture media supernatants of pre-implantation human embryos; its relation with embryo grades and development. Gynecol Endocrinol. 2012;28:296–298. doi: 10.3109/09513590.2011.631627. [DOI] [PubMed] [Google Scholar]
- 32.Orsi NM, Ekbote UV, Walker JJ, Gopichandran N. Uterine and serum cytokine arrays in the mouse during estrus. Anim Reprod Sci. 2007;100:301–310. doi: 10.1016/j.anireprosci.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Lin HC, et al. 5-Lipoxygenase inhibitors attenuate TNF-α-induced inflammation in human synovial fibroblasts. PLoS One. 2014;9:e107890. doi: 10.1371/journal.pone.0107890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monteiro AP, et al. Pivotal role of the 5-lipoxygenase pathway in lung injury after experimental sepsis. Am J Respir Cell Mol Biol. 2014;50:87–95. doi: 10.1165/rcmb.2012-0525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li MO, Rudensky AY. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat Rev Immunol. 2016;16:220–233. doi: 10.1038/nri.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alkhouri N, Feldstein AE. Noninvasive diagnosis of nonalcoholic fatty liver disease: Are we there yet? Metabolism. 2016;65:1087–1095. doi: 10.1016/j.metabol.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zabaleta J, et al. Inverse correlation of serum inflammatory markers with metabolic parameters in healthy, Black and White prepubertal youth. Int J Obes. 2014;38:563–568. doi: 10.1038/ijo.2013.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carazo A, et al. Hepatic expression of adiponectin receptors increases with non-alcoholic fatty liver disease progression in morbid obesity in correlation with glutathione peroxidase 1. Obes Surg. 2011;21:492–500. doi: 10.1007/s11695-010-0353-2. [DOI] [PubMed] [Google Scholar]
- 39.Kohjima M, et al. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int J Mol Med. 2008;21:507–511. [PubMed] [Google Scholar]
- 40.Leavens KF, Easton RM, Shulman GI, Previs SF, Birnbaum MJ. Akt2 is required for hepatic lipid accumulation in models of insulin resistance. Cell Metab. 2009;10:405–418. doi: 10.1016/j.cmet.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2014;7:17–44. doi: 10.3390/nu7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jayasinghe TN, Chiavaroli V, Holland DJ, Cutfield WS, O’Sullivan JM. The new era of treatment for obesity and metabolic disorders: Evidence and expectations for gut microbiome transplantation. Front Cell Infect Microbiol. 2016;6:15. doi: 10.3389/fcimb.2016.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Famouri F, Shariat Z, Hashemipour M, Keikha M, Kelishadi R. Effects of probiotics on nonalcoholic fatty liver disease in obese children and adolescents. J Pediatr Gastroenterol Nutr. 2017;64:413–417. doi: 10.1097/MPG.0000000000001422. [DOI] [PubMed] [Google Scholar]
- 44.Javurek AB, et al. Consumption of a high-fat diet alters the seminal fluid and gut microbiomes in male mice. Reprod Fertil Dev. 2017;29:1602–1612. doi: 10.1071/RD16119. [DOI] [PubMed] [Google Scholar]
- 45.Watkins AJ, et al. Mouse embryo culture induces changes in postnatal phenotype including raised systolic blood pressure. Proc Natl Acad Sci USA. 2007;104:5449–5454. doi: 10.1073/pnas.0610317104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santos MA, Kuijk EW, Macklon NS. The impact of ovarian stimulation for IVF on the developing embryo. Reproduction. 2010;139:23–34. doi: 10.1530/REP-09-0187. [DOI] [PubMed] [Google Scholar]
- 47.Grandjean V, et al. RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Sci Rep. 2015;5:18193. doi: 10.1038/srep18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.