Significance
The current paradigm in liquid biopsies focuses on circulating tumor cell (CTC) count and genomics to provide clinically actionable information. We push beyond current capabilities to introduce a functional assay that quantifies protease secretion from live CTCs with single-cell resolution. In this assay, an integrated microfluidic device captures CTCs from blood and washes and encapsulates them into nanoliter-scale droplets with fluorogenic substrates within minutes, maintaining physiologic conditions. Our data support the presence of outlier CTCs with high protease activity that may drive metastasis or immune evasion. We reveal an intriguing correlation between CTC protease activity and metastatic progression. Such functional liquid biopsy approaches provide avenues to understand the metastatic process and potential for companion diagnostics.
Keywords: protease, circulating tumor cells, cell secretion, microfluidics, liquid biopsy
Abstract
Tumor cells are hypothesized to use proteolytic enzymes to facilitate invasion. Whether circulating tumor cells (CTCs) secrete these enzymes to aid metastasis is unknown. A quantitative and high-throughput approach to assay CTC secretion is needed to address this question. We developed an integrated microfluidic system that concentrates rare cancer cells >100,000-fold from 1 mL of whole blood into ∼50,000 2-nL drops composed of assay reagents within 15 min. The system isolates CTCs by size, exchanges fluid around CTCs to remove contaminants, introduces a matrix metalloprotease (MMP) substrate, and encapsulates CTCs into microdroplets. We found CTCs from prostate cancer patients possessed above baseline levels of MMP activity (1.7- to 200-fold). Activity of CTCs was generally higher than leukocytes from the same patient (average CTC/leukocyte MMP activity ratio, 2.6 ± 1.5). Higher MMP activity of CTCs suggests active proteolytic processes that may facilitate invasion or immune evasion and be relevant phenotypic biomarkers enabling companion diagnostics for anti-MMP therapies.
The presence of circulating tumor cells (CTCs) has been correlated with the aggressive spread of a tumor and negative prognosis in breast, colon, and prostate cancer. However, current methods to identify CTC number based on immunofluorescence (IF) staining have led to mixed success in predicting treatment effectiveness and assisting in clinical treatment decisions (1–4). For example, high levels of surface marker used to identify CTCs following an initial therapy used as impetus to switch therapy early, did not lead to an increase in overall survival (5). Heterogeneity in CTC phenotype is likely one contributing factor in the discrepancies between CTC counts and prognosis. Not all CTCs are expected to possess a phenotype optimized for extravasation and spread, which is hypothesized to include the following: high motility, deformability needed to squeeze through cell and extracellular matrix (ECM) layers, periods of low cell adhesiveness followed by adhesion in a new metastatic site, and/or high protease secretion needed to degrade ECM barriers (6, 7).
Cell-secreted proteolytic enzymes, such as matrix metalloproteases (MMPs), that cleave ECM proteins are implicated in cancer invasion of neighboring tissues and metastasis. For example, immunohistochemistry (IHC) of invasive tumors has shown high levels of MMP2, MMP9, MMP13, and MT-1 (8, 9). Although these studies document the presence of MMPs, IHC does not provide information on functional state. MMPs initially exist in an inactive form. The interaction of several types of proteases leads to MMP activation and ECM degradation. An understanding of the functional state of MMPs produced by tumor cells, neighboring stromal cells, tumor-associated leukocytes, and cells that escape the tumors would lead to a better understanding of cooperative interactions involved in metastasis.
CTCs may also employ MMPs to assist in forming new metastatic sites. CTCs initially dislodge from a tumor site, traverse across the ECM of the basement membrane, before squeezing between endothelial cells to enter the bloodstream or lymphatic vessels. These CTCs can then invade new sites and organs through the reverse process of extravasation. Analysis of metastatic tumors and patient blood serum has shown significantly higher levels of MMPs, suggesting that the level of MMPs produced by CTCs could serve as a functional marker of cells that have metastasis-enabling properties (10).
In addition, the measurement of MMPs or other proteases secreted by CTCs can potentially improve prognosis of cancer aggressiveness and help determine the relative importance of CTC protease secretion in extravasation, immune evasion, and metastasis. CTCs that are secreting active proteases are expected to be executing cellular processes required for viability and may be primed for tissue invasion or immune suppression, while those cells that are not actively secreting may have lost viability or have adopted a quiescent state. Understanding the activity of MMPs and other proteases secreted by CTCs may also provide new, more selective, targets for antimetastatic therapies, or new therapies being developed to prevent proteolytic shedding of immune activating cell surface proteins (e.g., MICA and MICB) by cancer cells (11), whereby the measurement approaches described could serve in a companion diagnostic role to indicate when targeted treatment is warranted.
Measuring the secretions of individual CTCs is extremely challenging given the rarity of CTCs, the large background of proteases in body fluid samples, and the significant dilution of the few secreted molecules from single cells when isolated into a large volume of solution. Techniques to increase the concentration of single-cell secretions enhance sensitivity by confining cells into a small volume of surrounding fluid using microchannels, microwells, or droplet microfluidics (12–16). However, microwell and drop-generating systems are generally not integrated with purification or solution exchange operations. Integrated sample preparation is critical to reduce background and cell-to-cell cross-contamination, reduce operation time to maintain physiological cell state, and prevent significant cell loss in transfer steps (12, 13, 17, 18). Jing et al. (13) highlight the importance of washing out background proteases in media or plasma around cells to obtain a cell-specific signal without substantial background fluorescence; however, due to the continuous generation of droplets, a large number of empty droplets are made during the entire sample-processing time, which is not suited for rare cell analysis. An integrated system that can isolate CTCs from blood, wash away contaminating blood cells and plasma, introduce new reagents, and encapsulate them into droplets without manual transfer steps would enable the study of protease activity of CTCs.
We have developed size-based purification and encapsulation of cells (SPEC), a technique that integrates functions of isolation, reagent exchange, and encapsulation for single-cell secretion analysis of rare cells. The integrated device performs vortex trapping of large rare CTCs, followed by single-cell encapsulation in a pristine fluorogenic reporter solution using an extreme throughput droplet generator to measure proteases secreted by individual cells. Integration enables a process from whole-blood sample to isolated CTCs in under 15 min, better preserving the physiologic state of analyzed cells. We characterized the sensitivity of the system to collagenase enzymes and found sensitivity down to ∼7 molecules per droplet. We evaluated the differences in MMP secretion across a range of cancer cell lines and other circulating cells as well as demonstrated sensitivity sufficient to identify pharmacological interference of MMP secretion. Finally, we evaluated the secretions of CTCs and other circulating cells from late-stage prostate cancer patients. Although heterogeneous, some CTCs from cancer patients actively secrete MMPs over a 3-h time period after isolation, and on average CTCs are associated with more MMP activity compared with leukocytes in the same patient (2.6 ± 1.5, average ratio of CTC/leukocyte intensity). These results indicate that CTCs may secrete higher levels of these enzymes than normal circulating cells in situ and open up the capability to study secretions from these unique cells.
Results
Cell Trapping and Subsequent Encapsulation into Droplets.
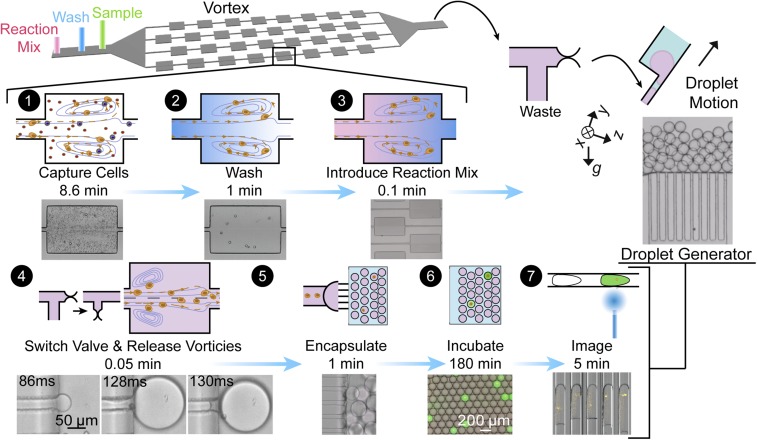
The CTC isolation component of our device consists of a series of channels that expand and contract to form reservoirs, within which stable laminar vortices develop under flow. Larger cells, such as CTCs, are stably trapped within the microvortices that form in the reservoirs, while smaller red and white blood cells enter but do not form stable limit cycles or orbits (19). The performance of this vortex system has been described extensively in other reports (6, 20). We then exchange solutions while under continuous flow to wash out plasma proteins and leave pure cells within a continuously exchanging reaction buffer (fluorogenic broad spectrum MMP-cleavable peptide substrate), minimizing any nonspecific signals (Movie S1). The trapped cells are released along with the solution of MMP-cleavable substrate into an inline connected droplet generator where they are encapsulated into uniform microdroplets without any manual transfer steps (Fig. 1 and Movie S2). The entire process, from cells starting in whole blood to encapsulation in droplets along with MMP detection reagent, is complete in less than 15 min.
Fig. 1.
Size-based purification and encapsulation of cells (SPEC) followed by fluorescence analysis of enzyme secretion (1). Large cells are trapped in microvortices, while smaller cells and molecules are washed away with a wash buffer (2). An MMP-cleavable peptide substrate solution is introduced through another fluid exchange (3). We dissipate the vortices by lowering the flow rates and release captured cells into the substrate solution. A pinch valve is opened to the droplet generator in synchrony with vortex dissipation (4). The droplets float away from the generation region due to buoyancy differences with the oil (5). The cells can then be incubated and imaged in the large reservoir section of the droplet generator. An imaging cytometer can also be used to image the droplets and contained cells in flow (6 and 7).
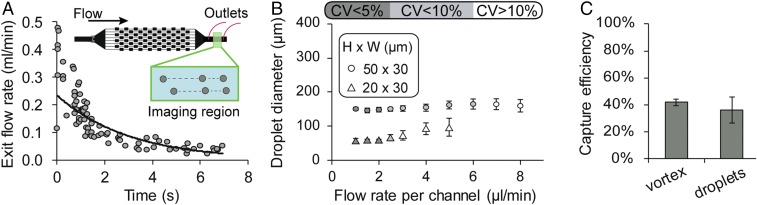
Due to the seamless transition of fluid and cells, we have minimal loss of cells through all steps of the process (Fig. 2C). The initial concentration step in microvortices dominates the yield of cells with an efficiency of >40% for spiked cancer cells. Further concentration is achieved through single-cell encapsulation, in which the overall efficiency is decreased slightly but remains >35%. Through this series of automated steps on chip, we transfer rare cells from 20 mL of diluted blood volume (1 mL of whole blood) into individual droplets with a volume of ∼2 nL, and thus increase the concentration of trapped cells and their secreted molecules by six orders of magnitude. Importantly, the first vortex-based concentration step achieves an initial enrichment of the diluted sample, such that a small number of droplets (<50,000) contains the captured cells, thus reducing analysis time.
Fig. 2.
Vortex-based cell release and step emulsification. (A) The flow rate decays quickly when the pressure is released to dissipate the vortices. Beads tracked in the exit region of the vortex were used to calculate the decay. (B) Droplet generation remains stable and monodisperse at flow rates from 0.001 to 0.002 mL/min per channel but becomes polydisperse beyond these flow rates. (C) There is minimal loss between the cells captured in the vortex device and the cells in the droplets, because of the continuous flow integrated system.
We developed a droplet generator design that operates in a highly parallel manner, with low flow rate sensitivity and without any oil coflow, to be compatible with the variable flow rates during release of cells from the vortex device. The flow rate decays exponentially at the exit channel over time as the vortices dissipate, indicating that the operating conditions should span an order of magnitude in flow rates from ∼0.03 to 0.3 mL/min (Fig. 2A). The droplet generator stably produces relatively monodisperse droplets (coefficient of variation, 4%) in the flow rate range from 0.001 to 0.002 mL/min per channel with dimensions of 50-µm height and 30-µm width (Fig. 2B). The droplet generator included 100 channels, to generate drops over the release flow rates while maintaining uniform size. Note that this device shares features with the design concurrently reported by Stolovicki et al. (21), such as the use of buoyancy of drops to drive their flow away from the junctions where drops are generated, avoiding coalescence without an oil flow.
Cell-secreted proteases and the fluorescent reaction products accumulate in the 2-nL droplets during an incubation period in an on-chip reservoir that minimizes the motion of drops (Movie S3). No detectable cross talk of the fluorescent reaction products is observed over a 3-h incubation period (SI Appendix, Fig. S1), suggesting the signal is dominated by individual cell secretions. We observe individual cells, because Poisson encapsulation statistics govern occupancy when we consider 10–1,000 captured cells are released into ∼50,000 droplets. The encapsulation rate and low cross talk ensure a low background intensity of the empty drops and provide a sensitive and proportional metric for what each cell is secreting. The cells settle to the bottom of the droplets during incubation, allowing facile imaging of both cells and accumulated fluorescent product with a fluorescent microscope.
Assay Characterization.
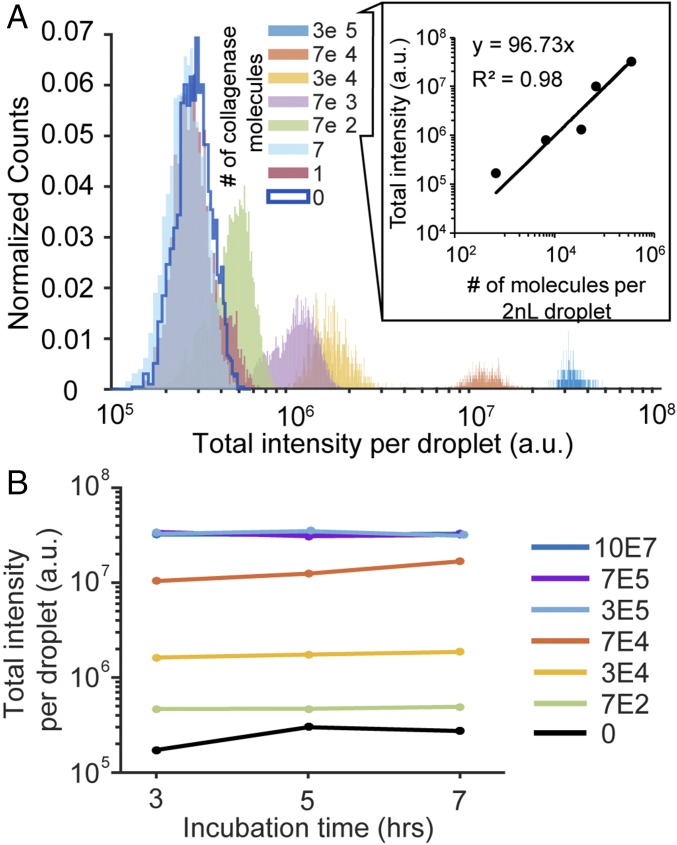
To characterize our protease secretion assay in droplets, we first used purified collagenase (MMP1) introduced with the FRET-based MMP-cleavable peptide substrate in droplets. We evaluated the time course of enzyme activity, detection limits, and repeatability of the assay using known concentrations of collagenase. Over the course of 3–7 h, droplets loaded with an average of 700 collagenase molecules and 0.5% diluted MMP substrate reveal that enzyme activity saturates after 3 h (Fig. 3). The plateau in response of the collagenase molecules between 3 and 7 h below the maximum possible signal from higher concentrations of collagenase suggests the collagenase becomes inactivated over time, potentially through a self-digestion process (22). As concentration of collagenase increases, the signal is distinct from ∼7 molecules to ∼3 × 105 collagenase molecules per drop, and saturates beyond this point. These results indicate that the substrate is in excess when less than ∼3 × 105 enzymes are used in the reaction. For our subsequent studies, we assume single cells release levels of proteases within this detection regime. The linear correlation between intensity and number of molecules per droplet suggests that the fluorescence intensity of each droplet is a good proxy for the time-averaged number of molecules secreted or presented on the surface of single cells (Fig. 3B).
Fig. 3.
MMP assay performance. (A) We test the detection limits of the assay using serial dilutions of known concentrations of collagenase. A linear correlation exists between droplet intensity and number of molecules reacted for greater than 700 molecules per droplet up to 300,000 molecules per droplet, indicating a large dynamic range for detection. These correlations can be used to estimate the time-averaged number of molecules secreted by single cells. (B) The signal becomes saturated for concentrations of greater than 300,000 molecules per droplet, and there is little time dependence of the signal beyond 3 h.
Detection of Single-Cell Protease Secretion.
We initially tested the system using cancer cell lines and endothelial cells, which both can be present in circulation and express MMPs (23, 24). Following vortex capture, solution exchange, and encapsulation, cells maintained membrane integrity as indicated by the positive intracellular calcein signal. Because of the highly effective washing process immediately before encapsulation, empty droplets in a cell encapsulation experiment are truly negative with minimal variation in intensity (138,400 ± 27,700), similar to measuring empty droplets including substrate alone (156,400 ± 24,500) (SI Appendix, Fig. S2). This result indicated that we could use empty drops as an internal negative control to normalize the intensity of each cell-containing droplet, which could account for variations from the optical system or autofluorescence of the device. Droplets with intensities above the normalized baseline value of 1, have a positive signal above background. The variation in baseline causes some nonsecreting cells to have a lower than 1 normalized value.
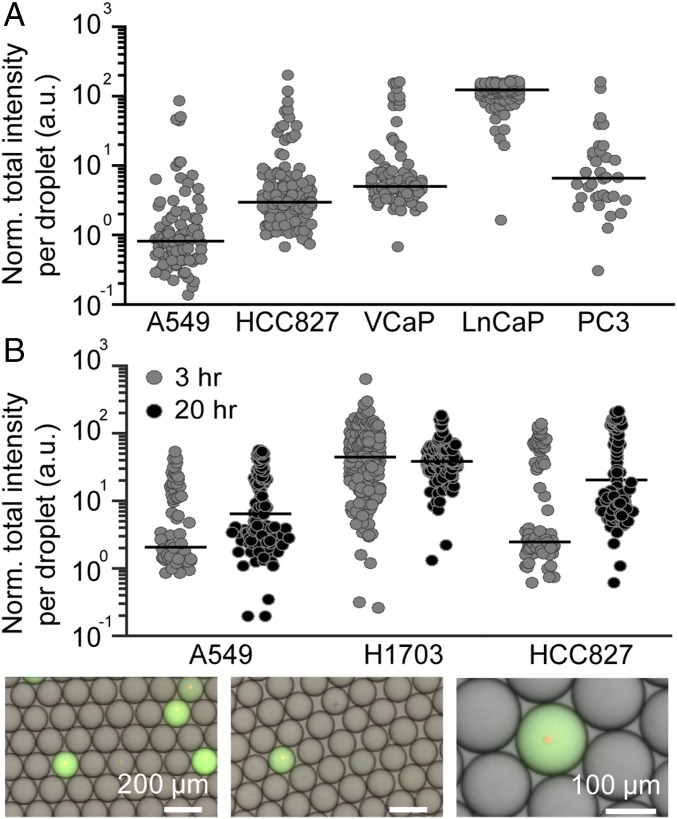
The fluorescence intensity of droplets encapsulating single cells possess a nonnormal distribution with variation in the distribution shape depending on cell line. The gini index was used to quantify the heterogeneity in intensity in drops that represents the time-averaged level of MMP secretion in the cell populations (25). Cancer cell lines were generally observed to have high heterogeneity in MMP activity, with a gini index ranging from 0.7 to 1 with a number of high-secreting outlier cells (normalized median intensity, 28 ± 53, and outliers up to two orders of magnitude higher than the median) (Fig. 4A). Cell size variations in a sample do not appear to explain these larger changes in MMP activity. When analyzing the relationship between cell size and MMP secretion, we found no correlation (SI Appendix, Fig. S3). The distribution and median values of MMP activity for cell lines remains comparable over different passages. A549 normalized median intensity remained around 2 ± 1 across passages and batches of MMP substrates with intensity distributions possessing gini indices between 0.7 and 0.8 (SI Appendix, Fig. S4). Additionally, we have found that vortex processing yielded no significant effect on MMP activity levels compared with standard cellular processing with centrifugations and washes when normalized on a per-cell basis (SI Appendix, Fig. S5).
Fig. 4.
(A) MMP secretion levels vary across cell lines. Lung cancer cell lines (A549 and HCC827) and prostate cancer cell lines (VCaP, LnCaP, and PC3) secrete varying levels of MMPs. Only droplets with single viable cells were measured, and intensity was normalized as a ratio of empty drop levels. (B) Lung cancer cells were interrogated for MMP secretion at 3 and 20 h.
Cell secretions and signal accumulates in droplets over time; however, a shorter time window ensures a cellular phenotype closer to the initial in situ or in vitro surface-attached conditions and avoids signal saturation. The activity of the highest secreting cells in Fig. 4A (LnCaP cells) appears saturated in agreement with intensity levels of ∼200-fold background associated with saturation for collagenase activity (Fig. 3B). For LnCaP cells, which have the highest MMP activity, 6% secrete enough MMPs to reach saturation. Following a 20-h incubation time, fluorescence intensity of drops increases slightly (approximately fourfold) compared with 3 h; however, the background fluorescence also increases, potentially due to peptide hydrolysis or a degree of fluorescent molecule leakage out of the droplets. Considering these factors, results of all subsequent studies were collected within the shorter 3-h incubation window.
Detecting Modulation of Single-Cell MMP Secretion.
We conducted a series of experiments to up- and down-regulate MMP secretion and determined the ability to capture these phenotypes with our single-cell assay. MMP up-regulation can be achieved through the exposure of endothelial cells to histamine. Histamine interacts with the H2 receptor on the endothelial cell surface and has been found to trigger MMP secretion (26). Our assay shows a 58% increase in normalized signal for cells treated with 10 µM histamine (SI Appendix, Fig. S6A). Up-regulation of the transcription factor SNAIL has also been shown to modulate MMP-9 and MMP-2 secretion and transition cells toward a mesenchymal phenotype (27). We observed that MMP-9 secretion from SNAIL-overexpressing cells are either up-regulated or remain the same in this single-cell assay (SI Appendix, Fig. S7). Cell cycle synchronization did not affect the heterogeneity of MMP secretion; however, serum starvation led to decreased heterogeneity (SI Appendix, Fig. S8).
We also characterized cells subjected to pharmacological inhibition of MMP secretion and observed a significant decrease in intensity in our assay (SI Appendix, Fig. S6B). The expected effect of secretion inhibitors monensin and brefeldin is down-regulation of MMP secretion. Prostate cancer cells, PC3, that were encapsulated into droplets containing the drug mixture show significant decrease in fluorescence intensity within droplets. We observe a 40% decrease in the median intensity in drug-treated cells compared with vehicle-treated cells.
CTCs from Prostate Cancer Patients Secrete MMPs.
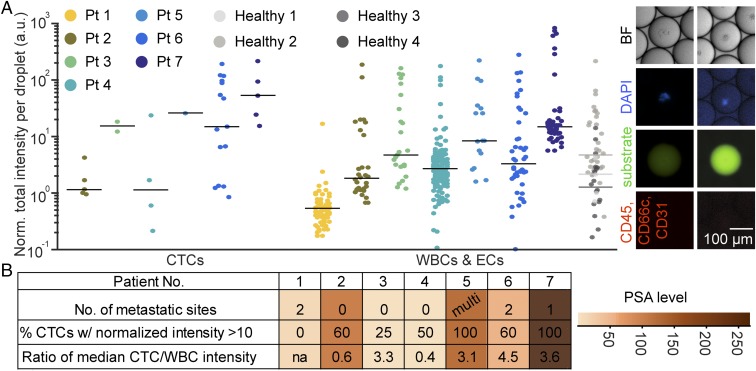
We processed samples from seven metastatic castration-resistant prostate cancer patients. Six out of seven patient samples contained CTCs, and 87% of these CTCs secreted MMPs, leading to fluorescence signals above baseline (Fig. 5). One sample, which contained no CTCs, corresponded with clinical results of no new metastases (patient 1). Patients with lower levels of prostate-specific antigen (PSA) (patients 2 and 4) and in a state of response to treatment (patient 3) had CTCs that generally were found to secrete lower levels of MMPs and had a lower CTC-to-WBC activity ratio. Samples from patients with radiographic progression to the bone and lymph node correlated with a higher proportion of CTCs secreting one order of magnitude above baseline levels (patients 6 and 7). These patients also had the highest levels of blood PSA (SI Appendix, Table S1).
Fig. 5.
CTCs were collected into microdroplets from seven prostate cancer patients and four healthy volunteers. (A) Cells that were negative for CD31, CD66c, and CD45 and positive for prostate-specific membrane antigen or had a large nucleus were classified as CTCs. Six out of seven patient samples were observed to have CTCs with above-background levels of MMP secretion. (B) The ratios of CTC to leukocyte MMP activity levels were higher in patients with progressive disease and correlated with higher PSA levels.
In addition to secretion from CTCs, leukocytes and clusters of RBCs and nonnucleated components obtained from blood of cancer patients were found to secrete comparable amounts of MMPs to CTCs (SI Appendix, Fig. S9). CTC population level behavior appears to be dominated by a few high-secreting cells. We also see a positive correlation between the highest levels of MMPs secreted by CTCs and the MMPs secreted by the same patient’s WBCs and endothelial cells (ECs). This may indicate that there are patient-dependent baseline differences in MMP secretion levels, or overall inflammation is increased in highly metastatic patients, leading to higher levels of leukocyte MMP secretion. When normalizing median intensity from MMPs secreted by CTCs by the same metrics for leukocytes for each patient, we find that CTC levels were on average 2.6-fold higher than leukocytes. When segmenting patients by progression, a 4.1-fold increase on average is seen in patients with signs of metastatic progression, while a 1.9-fold increase is seen in other patients.
Out of all of the CTCs analyzed, 56% were found to exhibit one order of magnitude above baseline level MMP activity, suggesting viable cells were isolated that continued to actively secrete during incubation. To test this hypothesis, we conducted in vitro experiments in which vortex-trapped cancer cells were exposed to radioimmunoprecipitation assay buffer that permeabilized the cell membrane and removed proteins on the cell surface and internal to the cell. When analyzed with our assay, the cell “ghosts” trapped in the vortices had MMP activity signals 10-fold below background levels (SI Appendix, Fig. S10). While we cannot rule out signal below this level in live CTCs, signal above this level should indicate a live cell using active cellular processes. The 44% of low or nonsecreting CTCs may be nonviable or in a quiescent state. Additionally, 12.5% of the CTCs are outliers yielding MMP activity levels 100-fold greater than background. Our findings that population-level behaviors are dominated by high-secreting outliers is also found during single-cell secretion measurements using other platforms, such as nanowells (28). Understanding the existence of outlier cells is important for accurate diagnosis, which would avoid using conventional bulk or cross talk-prone methods that average out these outlier effects. Such high MMP-secreting outlier cells would be of interest to select and better understand transcriptomic and phenotypic differences in future studies of metastasis and cancer immunology.
Discussion
The SPEC platform has the ability to purify cancer cells from large volumes of blood in a high-throughput manner, wash these captured cells, exchange solutions around them, and encapsulate them into microdroplets, all in one integrated device. This proof-of-concept device enables a single-cell resolution assay for MMPs secreted by live CTCs and other circulating cells from prostate cancer patient samples. CTCs, leukocytes, and cancer cell lines were all found to have a wide range of MMP activity levels, with high secreting outlier cells. Activated leukocytes secrete MMPs at the sites of tissue inflammation, such as a tumor, and may be released into circulation in cancer patients (29). This may explain why some subsets of WBCs and ECs from patient samples had high MMP activity levels compared with WBCs and ECs from healthy samples. The range of WBC levels across patients indicates a characteristic baseline secretion level may exist and vary among patients. The median CTC MMP activity from six of seven patients with detectable CTCs was found to be 2.6 ± 1.5 times higher than for the WBCs and ECs from the same patients. The patients with high levels of PSA (patients 2, 5, 6, and 7) had CTCs possessing an even higher ratio of the CTC to WBC MMP activity level than this average, except for patient 2 who was responding to treatment. Obtaining increased numbers of CTCs will be valuable in increasing the accuracy of these phenotypic measurements and can be easily achieved by processing larger volumes (15 mL is easily accessible compared with 5 mL used here) with higher-throughput and higher-efficiency vortex chips (6, 30). Nevertheless, by random sampling of cells from patient 7, we found that even a small number of CTCs (>5) can provide useful metrics (coefficient of variation less than 50% of the population mean) (SI Appendix, Fig. S11). Although this is a small study with variations in the patient population treatment regimen, these results suggest that the relative increase in MMP activity of CTCs normalized to a WBC baseline can indicate the presence of active malignant processes, which could inform prognosis.
Besides CTCs, nucleated cells and platelets isolated from blood secrete MMPs (SI Appendix, Fig. S9). A large majority of platelets from clinical samples secrete more MMPs than healthy samples, which may also be related to an increased number of activated platelets in circulation for vascularization of growing tumors (31). These results indicate that cellular components of blood other than CTCs may also be of interest in studying the mechanism of metastasis.
Moving beyond the broad-spectrum MMP substrate we use in this study and applying this technology to study subcategories of cancer-specific proteases, such as Cathepsin D and MMP9, may allow better elucidation of proteolytic pathways linked to aggressive or patient-specific disease. These factors would allow higher precision or individualized antiprotease therapy. Currently, we are limited by the number of FRET peptide probes commercially available. However, new approaches for optimizing cleavable peptides with higher specificity will enable even better understanding of the metastatic process and development of antimetastasis drugs (32, 33). Ultimately, phenotypic liquid biopsies, such as those assaying protease secretions or cell deformability, can provide more detailed clinical information and directly inform the use of antimetastatic and antiimmune evasion therapies targeting hallmarks of invasive tumor phenotypes.
Materials and Methods
A detailed description of materials and methods can be found in SI Appendix. All blood samples were obtained following University of California, Los Angeles, IRB-approved protocol IRB#11-001798 and deidentified.
Vortex–Droplet Generator Device Operation.
We dilute blood 20 times and process it through the VortexHE device to trap CTCs. After cell trapping, we wash out background molecules and use solution exchange to introduce a FRET peptide-based broad-spectrum MMP substrate. We divert captured cells to the second outlet of the pinch valve leading to a droplet generator for cell encapsulation. The reaction between protease and substrate occurs inside the droplets for 3 h. Following incubation, we analyze the fluorescent intensity in each droplet using a fluorescent microscope or an imaging flow cytometer.
Cell Line Experiments and Immunostaining.
All cell lines were grown using American Type Culture Collection-recommended methods. Cells were counted with a hemocytometer and diluted to 100 cells per mL in PBS, and 5 mL of this cell solution was processed for spiking experiments.
Device Fabrication.
Standard photolithography and soft lithography techniques were used to fabricate device molds and polydimethylsiloxane casting.
Supplementary Material
Acknowledgments
We thank the nurses, all donors, and Dr. Oladunni Adeyiga for their contributions toward blood sample collection. We acknowledge partial support for this work through sponsored research from Vortex Biosciences, National Cancer Institute (NCI) Innovative Molecular Analysis Technologies (IMAT) Program R33CA177456, the NCI Early Detection Research Network NCI 1U01CA214182, and Merit Review Research Funds from the US Department of Veteran Affairs.
Footnotes
Conflict of interest statement: The Regents of the University of California have filed a patent for the integrated vortex capture and droplet generator device of which D.D.C. and M.D. are inventors.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1803884115/-/DCSupplemental.
References
- 1.Cristofanilli M, et al. Circulating tumor cells: A novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23:1420–1430. doi: 10.1200/JCO.2005.08.140. [DOI] [PubMed] [Google Scholar]
- 2.Pierga J-Y, et al. Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin Cancer Res. 2008;14:7004–7010. doi: 10.1158/1078-0432.CCR-08-0030. [DOI] [PubMed] [Google Scholar]
- 3.Danila DC, et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:7053–7058. doi: 10.1158/1078-0432.CCR-07-1506. [DOI] [PubMed] [Google Scholar]
- 4.Galletti G, et al. Circulating tumor cells in prostate cancer diagnosis and monitoring: An appraisal of clinical potential. Mol Diagn Ther. 2014;18:389–402. doi: 10.1007/s40291-014-0101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alix-Panabières C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6:479–491. doi: 10.1158/2159-8290.CD-15-1483. [DOI] [PubMed] [Google Scholar]
- 6.Che J, et al. Classification of large circulating tumor cells isolated with ultra-high throughput microfluidic vortex technology. Oncotarget. 2016;7:12748–12760. doi: 10.18632/oncotarget.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tse HTK, et al. Quantitative diagnosis of malignant pleural effusions by single-cell mechanophenotyping. Sci Transl Med. 2013;5:212ra163. doi: 10.1126/scitranslmed.3006559. [DOI] [PubMed] [Google Scholar]
- 8.Upadhyay J, et al. Membrane type 1-matrix metalloproteinase (MT1-MMP) and MMP-2 immunolocalization in human prostate: Change in cellular localization associated with high-grade prostatic intraepithelial neoplasia. Clin Cancer Res. 1999;5:4105–4110. [PubMed] [Google Scholar]
- 9.Zhang B, et al. Tumor-derived matrix metalloproteinase-13 (MMP-13) correlates with poor prognoses of invasive breast cancer. BMC Cancer. 2008;8:83. doi: 10.1186/1471-2407-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groblewska M, et al. Serum levels and tissue expression of matrix metalloproteinase 2 (MMP-2) and tissue inhibitor of metalloproteinases 2 (TIMP-2) in colorectal cancer patients. Tumour Biol. 2014;35:3793–3802. doi: 10.1007/s13277-013-1502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrari de Andrade L, et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell–driven tumor immunity. Science. 2018;359:1537–1542. doi: 10.1126/science.aao0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jing T, et al. Jetting microfluidics with size-sorting capability for single-cell protease detection. Biosens Bioelectron. 2015;66:19–23. doi: 10.1016/j.bios.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Jing T, et al. Single cell analysis of leukocyte protease activity using integrated continuous-flow microfluidics. Anal Chem. 2016;88:11750–11757. doi: 10.1021/acs.analchem.6b03370. [DOI] [PubMed] [Google Scholar]
- 14.Ng EX, Miller MA, Jing T, Chen C-H. Single cell multiplexed assay for proteolytic activity using droplet microfluidics. Biosens Bioelectron. 2016;81:408–414. doi: 10.1016/j.bios.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Chen C-H, et al. Multiplexed protease activity assay for low-volume clinical samples using droplet-based microfluidics and its application to endometriosis. J Am Chem Soc. 2013;135:1645–1648. doi: 10.1021/ja307866z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C-H, et al. Enhancing protease activity assay in droplet-based microfluidics using a biomolecule concentrator. J Am Chem Soc. 2011;133:10368–10371. doi: 10.1021/ja2036628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao G. Modelling mammalian cellular quiescence. Interface Focus. 2014;4:20130074. doi: 10.1098/rsfs.2013.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazutis L, et al. Single-cell analysis and sorting using droplet-based microfluidics. Nat Protoc. 2013;8:870–891. doi: 10.1038/nprot.2013.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haddadi H, Di Carlo D. Inertial flow of a dilute suspension over cavities in a microchannel. J Fluid Mech. 2017;811:436–467. [Google Scholar]
- 20.Dhar M, et al. High efficiency vortex trapping of circulating tumor cells. Biomicrofluidics. 2015;9:064116. doi: 10.1063/1.4937895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stolovicki E, Ziblat R, Weitz DA. Throughput enhancement of parallel step emulsifier devices by shear-free and efficient nozzle clearance. Lab Chip. 2017;18:132–138. doi: 10.1039/c7lc01037k. [DOI] [PubMed] [Google Scholar]
- 22.Matsushita O, Yoshihara K, Katayama S, Minami J, Okabe A. Purification and characterization of Clostridium perfringens 120-kilodalton collagenase and nucleotide sequence of the corresponding gene. J Bacteriol. 1994;176:149–156. doi: 10.1128/jb.176.1.149-156.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aalinkeel R, et al. Overexpression of MMP-9 contributes to invasiveness of prostate cancer cell line LNCaP. Immunol Invest. 2011;40:447–464. doi: 10.3109/08820139.2011.557795. [DOI] [PubMed] [Google Scholar]
- 24.Atkinson JM, et al. Membrane type matrix metalloproteinases (MMPs) show differential expression in non-small cell lung cancer (NSCLC) compared to normal lung: Correlation of MMP-14 mRNA expression and proteolytic activity. Eur J Cancer. 2007;43:1764–1771. doi: 10.1016/j.ejca.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Jiang L, Chen H, Pinello L, Yuan G-C. GiniClust: Detecting rare cell types from single-cell gene expression data with gini index. Genome Biol. 2016;17:144. doi: 10.1186/s13059-016-1010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doyle JL, Haas TL. Differential role of β-catenin in VEGF and histamine-induced MMP-2 production in microvascular endothelial cells. J Cell Biochem. 2009;107:272–283. doi: 10.1002/jcb.22123. [DOI] [PubMed] [Google Scholar]
- 27.Lin C-Y, et al. Matrix metalloproteinase-9 cooperates with transcription factor Snail to induce epithelial-mesenchymal transition. Cancer Sci. 2011;102:815–827. doi: 10.1111/j.1349-7006.2011.01861.x. [DOI] [PubMed] [Google Scholar]
- 28.Yao X, et al. Functional analysis of single cells identifies a rare subset of circulating tumor cells with malignant traits. Integr Biol. 2014;6:388–398. doi: 10.1039/c3ib40264a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 30.Lemaire CA, et al. Fast and label-free isolation of circulating tumor cells from blood: From a research microfluidic platform to an automated fluidic instrument, VTX-1 liquid biopsy system. SLAS Technol. 2018;23:16–29. doi: 10.1177/2472630317738698. [DOI] [PubMed] [Google Scholar]
- 31.Falcinelli E, Giannini S, Boschetti E, Gresele P. Platelets release active matrix metalloproteinase-2 in vivo in humans at a site of vascular injury: Lack of inhibition by aspirin. Br J Haematol. 2007;138:221–230. doi: 10.1111/j.1365-2141.2007.06632.x. [DOI] [PubMed] [Google Scholar]
- 32.Schuerle S, Dudani JS, Christiansen MG, Anikeeva P, Bhatia SN. Magnetically actuated protease sensors for in vivo tumor profiling. Nano Lett. 2016;16:6303–6310. doi: 10.1021/acs.nanolett.6b02670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwon EJ, Dudani JS, Bhatia SN. Ultrasensitive tumour-penetrating nanosensors of protease activity. Nat Biomed Eng. 2017;1:0054. doi: 10.1038/s41551-017-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.