Significance
Megakaryocyte progenitors (MkPs), derived from hematopoietic stem cells (HSCs), play major roles in hemostasis, thrombosis, inflammation, and vascular biology through generating platelets. However, the regulatory factors involved in MkP differentiation from HSCs are largely unknown. Here, we utilized a unique genomic approach, including the microarray gene expression commons platform, CRISPR/Cas9-mediated gene deletion, lentivirus-mediated gene overexpression, as well as multicolor flow cytometry and functional assays, and identified 10 genes that are highly expressed in MkPs and required for and can promote MkP generation from HSCs. In addition, we found inhibition of histone deacetylase activity increased MkP differentiation. Our results will not only shed light on the regulations of MkPs, but also facilitate efficient generation of MkPs and platelets for clinical applications.
Keywords: megakaryocyte progenitor, transcription factors, screening, gene editing
Abstract
Different combinations of transcription factors (TFs) function at each stage of hematopoiesis, leading to distinct expression patterns of lineage-specific genes. The identification of such regulators and their functions in hematopoiesis remain largely unresolved. In this study, we utilized screening approaches to study the transcriptional regulators of megakaryocyte progenitor (MkP) generation, a key step before platelet production. Promising candidate genes were generated from a microarray platform gene expression commons and individually manipulated in human hematopoietic stem and progenitor cells (HSPCs). Deletion of some of the candidate genes (the hit genes) by CRISPR/Cas9 led to decreased MkP generation during HSPC differentiation, while more MkPs were produced when some hit genes were overexpressed in HSPCs. We then demonstrated that overexpression of these genes can increase the frequency of mature megakaryocytic colonies by functional colony forming unit-megakaryocyte (CFU-Mk) assay and the release of platelets after in vitro maturation. Finally, we showed that the histone deacetylase inhibitors could also increase MkP differentiation, possibly by regulating some of the newly identified TFs. Therefore, identification of such regulators will advance the understanding of basic mechanisms of HSPC differentiation and conceivably enable the generation and maturation of megakaryocytes and platelets in vitro.
Derived from megakaryocytes, platelets play a major role in hemostasis, thrombosis, inflammation, and vascular biology, and platelet transfusions are frequently utilized to prevent thrombocytopenia, which can result from cancer therapy, trauma, sepsis, as well as blood disorders (1). Unfortunately, the supply of these short-lived platelets currently come with the high cost of maintaining quality donors, the extensive testing protocols to prevent contamination or recipient infection, and the generation of alloantibodies to the platelets which limit the donor pool. Another promising strategy is to transplant ex vivo-generated megakaryocytes (2–5), or megakaryocyte progenitor cells (MkPs), the direct precursor for megakaryocyte, which have proliferation capacity and engraftment potential and may therefore provide a better clinical alternative to standard transfusions, or as a target for activity inducers (6, 7). Although MkPs were identified many years ago (7), the regulatory factors involved in their differentiation from hematopoietic stem and progenitor cells (HSPCs) are largely unknown.
During hematopoiesis, transcription factors (TFs) control induction and maintenance of the expression of lineage-specific genes and suppression of competing gene expression of other lineages (8–14). MkPs are originally derived from hematopoietic stem cells (HSCs) through a well-documented stepwise differentiation (15, 16). To date, only a few TFs have been reported to be involved in this process, including AML1, FLI1, GABPA, GATA1, RUNX1, NFE2, SCL, GATA2, MYB, and LMO2 (17–19). The bipotent megakaryocyte-erythroid progenitors (MEPs) can directly give rise to MkPs and erythroid progenitors (EPs), which further develop into megakaryocytes and erythrocytes, respectively (20). MkPs and EPs share some TFs, including GATA1, FOG1, SCL, and GFI1b, but also have several lineage-specific genes, such as KLF1 (erythroid) and GABPA, FLI1, and RUNX1 (megakaryocytic) (20, 21). They coordinate the prevention of progenitor maintenance and the activation of downstream lineage-specific genes and the combination of some of those genes have recently been reported to either convert human and murine fibroblasts to MkPs (22, 23) or promote megakaryocyte generation from human pluripotent stem cell (hPSC) lines (13). Although the results are encouraging, identification of megakaryocyte-unique master regulators, especially those involved in MEP differentiation to MkP, will enable avenues for MkP and platelet generation and for mechanistic study of their regulation.
The CRISPR/Cas9 adaptive immune system, originally found in bacteria to confer resistance to foreign genetic elements, was demonstrated to mediate efficient and precise cleavage at endogenous genomic loci in human cells (24, 25). Single-guide RNA (sgRNA) can be synthesized to target the specific genomic loci, and Cas9 can induce DNA double-strand breaks (DSBs), which may generate insertion/deletion mutations and result in a loss-of-function allele. Therefore, using an sgRNA library to modify specific genomic loci by CRISPR/Cas9 suggests a way to interrogate gene function on a large scale (26–28).
In this study, we report a strategy to identify regulators for MkP generations by genetic manipulation. Sixty candidate genes were first generated from the gene expression commons (GEXC) microarray platform based on their high expression level in MkPs and low in MEPs and EPs. Then CRISPR/Cas9-mediated gene knockout as a negative screen and lentiviral-mediated gene overexpression as a positive way were utilized to determine gene functions on the modulation of generation of MkPs from HSPCs. By gene expression analysis, multicolor flow cytometry, colony forming unit-megakaryocyte (CFU-Mk) functional assay, 10 regulatory genes (the hit genes) from 60 candidates were identified. Furthermore, we showed the hit genes could promote the generation of megakaryocytes as well as platelets. Finally, we found that inhibition of histone deacetylase (HDAC) activity could also promote MkP differentiation, possibly by regulating some of the hit genes.
Results
Identification of Candidate TFs for MkPs from Gene Expression Commons.
To generate the candidate gene list, we used GEXC (29) developed in our laboratory. GEXC is a platform for profiling absolute expression of any gene using a large number (>10,000) of varied microarray datasets (30–32). Based on our previous work on mouse hematopoietic hierarchy, we generated a comprehensive “mouse hematopoiesis and stroma” model in GEXC, composed of lineage-specific genes for HSPCs and mature populations in adult mouse bone marrow (BM), spleen, and thymus.
Since there are no data available for human MkPs in GEXC and we believe the critical regulators are conserved between mouse and human, we used the mouse model to generate the MkP-specific candidate TF list and evaluated their expression pattern in human HSPCs. In addition to TFs, other proteins, such as coactivators, HDACs, and methylases, which do not have DNA-binding domains, but are essential for gene regulation, are also considered. In this case, the genesets for transcription regulators in GEXC were targeted with the search term of “inactive in MEP and EP, while active in MkP” (Fig. 1A and SI Appendix, Fig. S1A). By this search, we obtained a list of genes, which were ranked by geneset activity in MkPs.
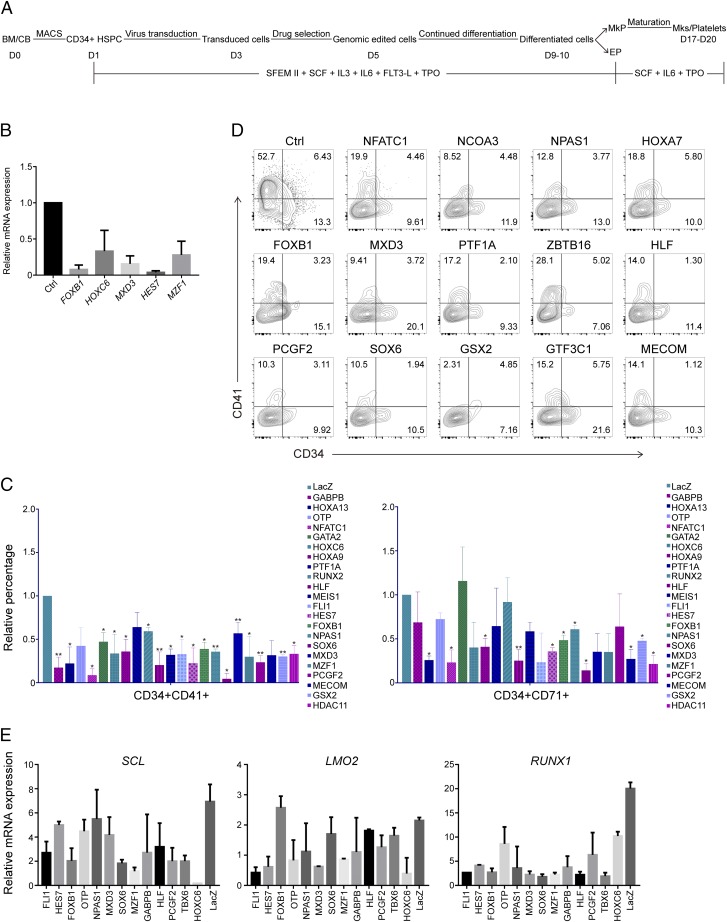
Fig. 1.
Experiment design for the knockout screening strategy. (A) Expression of some of the candidate TFs, including Fli1, Nfatc1, Mzf1, Mxd3, Hes7, and Pcgf2, in the mouse hematopoietic system as determined in GEXC. (B) Expression analysis of some of the candidate genes in human bone marrow MEPs, EPs, and MkPs by real-time PCR. n = 3; error bar indicates SD.
We then selected the 60 top-ranked genes to further identify their roles in megakaryopoiesis (SI Appendix, Table S1). To our expectation, there are some previously reported hematopoietic-specific TFs, such as Fli1, Gata2, Meis1, Pbx1, Smad5, and Mecom, while most of the other listed genes have not been reported in hematopoietic lineages. Fli1 is critical for megakaryopoiesis and has been shown to drive cell lines to develop megakaryocytic features and its overexpression inhibits erythroid development (33, 34). Therefore, we choose Fli1 as a positive control in our following experiments.
We next validated the expression pattern of some candidate genes in human MEPs, MkPs, and EPs by real-time PCR. Those three populations were isolated from human bone marrow mononuclear cells (35). The results showed most of the candidate genes, such as FLI1, HOXC6, MXD3, MEIS1, ERG, PRDM16, ZBTB16, PCGF2, GSX2, HDAC11, NPAS1, TBX6, and FOXB1 have a higher expression level in MkP than in MEP and/or EP cells, suggesting a conserved expression pattern in those populations between mouse and human (Fig. 1B).
Establishment of the Screen System.
The rarity of the MkP population (0.01%) in total bone marrow nucleated blood cells and the lack of suitable cell line models hinder the identification of signals that regulate human megakaryopoiesis. Bone marrow CD34+ HSPCs can differentiate into MkPs and megakaryocytes. However, the differentiation is heterogeneous and inefficient. To establish a repeatable protocol for screening, we used a serum-free hematopoietic expansion medium supplemented with cytokines, and tested CD34+CD41+ (MkP/megakaryocyte marker) cells and CD41+ (megakaryocyte marker) cells, CD34+CD71+ (EP/erythrocyte marker) cells, and CD71+ (erythrocyte marker) cells (15, 35) to determine the differentiation efficiency. Flow cytometry analysis of cell mixtures after 7–10 d of differentiation showed the five cytokines TPO, SCF, FLT3, IL3, and IL6 in serum-free expansion medium II (SFEMII) can lead to the highest percentage of CD34+CD41+ MkP cells and CD41+ megakaryocyte cells. Under the five-cytokine mixture culture condition, CD34+CD41+ cells represented ∼10% of the cell population after in vitro differentiation (SI Appendix, Fig. S1B), and the cell can expand 50- to 100-fold. Therefore, the five-cytokine mixture was used for the differentiation from HSPCs into MkPs, and then TPO, SCF, and IL6 are used for megakaryocyte maturation and platelet generation in an additional 1- to 2-wk culture (Fig. 2A). The additional culture will expand cells by an additional 50- to 100-fold and finally each megakaryocyte can give rise to thousands of platelets.
Fig. 2.
Identification of key TFs for human megakaryopoiesis by knockout screening. (A) Scheme of the screening process. (B) Confirmation of knockout for some of the candidate TFs by real-time PCR. n = 3; error bar indicates SD. (C) Summary of the FACS analysis for the increase/decrease of MkP (CD34+CD41+)- or EP (CD34+CD71+)-enriched population after CRISPR/Cas9-mediated knockout of candidate TFs in CD34+ HSPC culture. Here, the percentage of the control cell-derived MkP or EP is set to 1, and percentage from gene knockout cells was normalized to that. n = 3; error bar indicates SD. (D) FACS plot showed that the MkP population (CD34+CD41+) decreased after knockout of the hit TFs. (E) MkP gene expression analysis after knockout of the hit TFs. n = 3; error bar indicates SD. *P < 0.05, **P < 0.01.
We then used lentiviral-mediated transduction to deliver gene edits in HSPCs. However, the transduction efficiency was reportedly very low and it becomes an obstacle to manipulate hematopoietic cells for basic research and disease treatment (36, 37). We performed several optimization steps on the viral gene delivery strategy to improve the transduction efficiency. Firstly, we used retronectin to enhance transduction by facilitating colocalization of viral particles and hematopoietic cells. Secondly, viruses were highly concentrated and applied to CD34+ cells with an optimal mulitiplicity of infection. In addition, spin-mediated multiple-round virus incubation was used. Such optimization strategies eventually enabled ∼90% transduction efficiency after puromycin selection, as demonstrated in the following experiments where human CD34+ cells were treated with lentivirus-expressing EGFP (SI Appendix, Figs. S1C and S4A).
Negative Screen for MkP Essential Genes by CRISPR-Mediated Knockout.
Specific sgRNAs were designed either by the sgRNA designer tool online (crispr.mit.edu) or selected from the Human GeCKO Lentiviral sgRNA Library (26, 27), which are designed to target all of the isoforms of candidate genes. Synthesized sgRNA for human genes were cloned individually into the all-in-one CRISPR lentiviral vector (SI Appendix, Table S1). We then used real-time PCR to analyze the CRISPR-mediated knockout of candidate genes in human CD34+ cells. The results showed that most of the genes decreased their expression levels after CRISPR-mediated gene knockout, compared with the control group (Fig. 2B). For example, HOXC6 and MZF1 expression decreased by around 60% while FOXB1 and HES7 expression are almost completely suppressed. Those results suggested that we can successfully manipulate gene expression in human CD34+ cells and therefore used this optimized strategy to screen genes by CRISPR in hematopoietic cells.
CRISPR-mediated gene knockout in human CD34+ HSPCs cells were used for the primary loss-of-function screen. sgRNA targeting Escherichia coli LacZ locus delivered by the same viral system was used as a nonsense interruption control (38). FLI1 was used as a positive control, the knockout of which induced significant change in CD34+CD41+ cells (Fig. 2C). The screening experiment showed that deletion of some genes resulted in the deficiency of differentiation from HSPCs into MkPs, suggesting these genes may be critical for megakaryocyte development (Fig. 2C and SI Appendix, Figs. S2 and S3A). The flow cytometry data showed that, after deleting HOXC6, NFATC1, GSX2, or MXD3 et al., both CD34+/CD41+ and CD34+/CD71+ cells from human CD34+ HSPCs were dramatically reduced. For example, after NFATC1 knockout, the generation of CD34+/CD41+ is only 4% and CD34+/CD71+ is only 10.7%, compared with LacZ control of 7% and 15%, respectively (Fig. 2D and SI Appendix, Fig. S3B). The cutoff level for this screen is based on CD34+CD41+ reduction compared with virus vehicle control, and we got about 30 hits (Fig. 2C and SI Appendix, Fig. S2). We excluded genes that have already been reported to be essential for MkPs development, such as GATA2, MEIS1, MECOM, PBX1, ZBTB16, and we generated a final list of 20 genes for further study. Most of the genes on the list, to our knowledge, have not been reported to be functional in human hematopoietic cells, especially MkP differentiation from HSPCs.
To determine the gene expression changes after TF knockout (20), we analyzed SCL, LMO2, and RUNX1, which are essential transcription factors for MkPs. Real-time PCR results showed, in the knockout cells, these genes decreased compared with LacZ controls (Fig. 2E). FLI1 deletion led to a decrease of over 50% of SCL, LMO2, and RUNX1 gene expression, while HOXC6 and MZF1 deletion can lead up to 80% lost.
Together, primary screen results suggested that knockout of some candidate genes can lead to reduction of MkP differentiation from HSPCs, indicating that these genes might be involved in the regulation of MkP cell fate decision during hematopoiesis.
Gain-of-Function Screen of the Narrowed Candidate Genes.
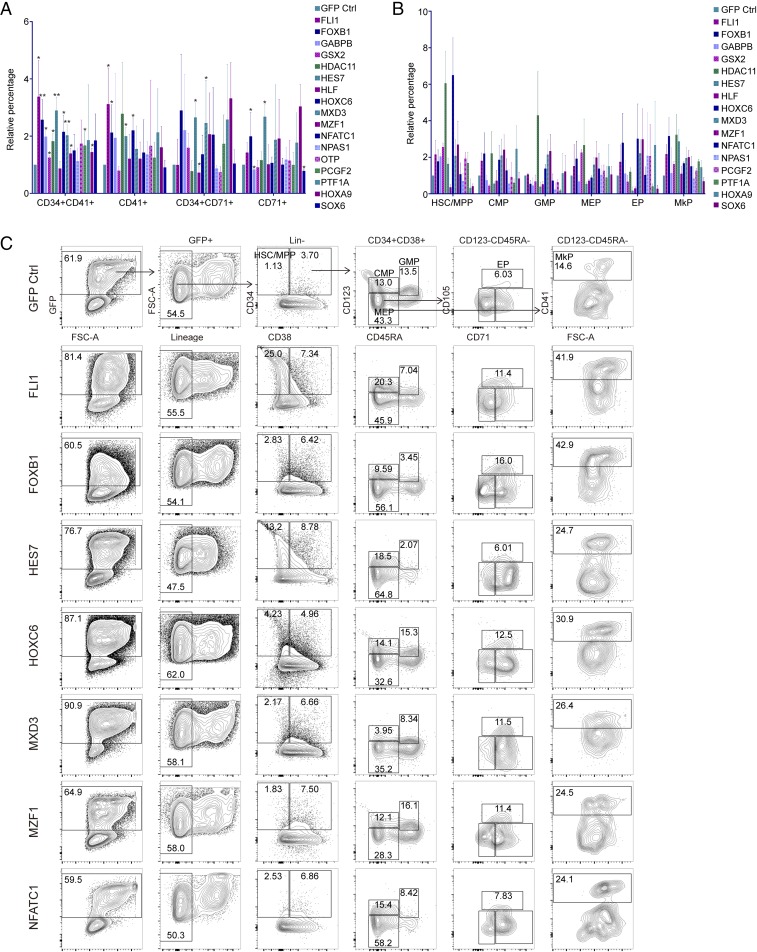
Next, we performed ectopic expression of the candidate TFs in HSPCs to further explore their regulatory function in MkP generation. Genes were expressed under the murine sarcoma cell virus (MSCV) promoter, which exhibits activity in hematopoietic cells and embryonic stem cells (39, 40), and GFP in the same construct can be an indicator for transduced cells. We used the empty viral vehicle without gene as a control. Overexpression of individual genes in human CD34+ cells was confirmed by GFP expression (SI Appendix, Fig. S4A), and some were also confirmed by real-time PCR analysis. (SI Appendix, Fig. S4B). Overexpression of these TFs led to significant changes in the production of MkP/megakaryocytes and EP/erythrocytes. FLI1 specifically increased CD34+CD41+ cells by 3- to 5-fold but not CD34+CD71+ cells. HES7, FOXB1, HOXC6, and MXD3 promoted the generation of CD34+CD41+ cells by more than 2-fold (Fig. 3A and SI Appendix, Fig. S4 C and D), and GABPB, HDAC11, MZF1, NFATC1, NPAS1, OTP, PCGF2, PTF1A, HOXA9, and SOX6 led to an increase of CD34+CD41+ cells by 1.5- to 2-fold. HLF showed minor effects.
Fig. 3.
Some hit TFs can promote MkP differentiation when overexpressed. (A and B) Summary of the FACS analysis for the increase/decrease of MkP (CD34+CD41+)- or EP (CD34+CD71+)-enriched population (A) or HSPC population (B) after lentiviral vector-mediated overexpression of some hit TFs in CD34+ HSPC culture. Here, the percentage of the control cell-derived MkP, EP, or other HSPCs is set to 1, and percentage from gene overexpressing cells was normalized to that. n = 3; error bars indicate SDs. (C) FACS plot to show changes of HSPC populations after lentiviral vector-mediated overexpression. Error bar indicates SD. *P < 0.05, **P < 0.01.
To explore the lineage-specific gene expression changes in those cells, expression levels of SCL, LMO2, and RUNX1 were analyzed. When FLI1 was overexpressed in CD34+ cells, SCL and RUNX1 increased dramatically by tens of folds. Consistently, overexpression of the other narrowed genes showed comparable effects to FLI1, such as HOXA9, MZF1, SOX6, and PCGF2. We then investigated more lineage-specific gene expressions by real-time PCR, including genes involved in erythroid differentiation (KLF1 and EPOR), those in MkP differentiation (FLI1 and GABPA), and those in both lineages (GATA1, NFE2, GATA2, and MYB). Erythroid genes have low expression levels in all of the samples and overexpression of narrowed genes did not lead to significant changes, while the MkP genes were obviously up-regulated in most of the samples (SI Appendix, Fig. S4E).
Stepwise differentiation from HSCs to MkPs included several stages, such as HSCs, multipotent progenitors (MPPs), common myeloid progenitors (CMPs), and MEPs. Different populations can be identified by multiple cell surface markers. Flow cytometry showed that overexpression of HOXC6 increased HSC/MPP, CMP, MEP, EP, and MkP populations compared with the control group. Importantly, no changes on MEP and granulocyte-monocyte progenitors (GMPs) were observed, suggesting HOXC6 may function mostly in early stage progenitors, such as HSCs, MPPs, and CMPs. FOXB1 and MZF1 increased MEPs as well as MkPs and EPs at the expense of GMP, suggesting they function in CMP stage. The function of HES7 may be at both HSC and MkP stages as its overexpression dramatically increased only HSCs and MkPs but not CMPs, MEPs, or EPs. However, some of the genes did not increase MkP generation, such as GABPB, HLF, and SOX6 (Fig. 3 B and C).
To examine whether the MkPs generated when the selected TFs were overexpressed are functional progenitors, we investigated their ability to generate megakaryocyte colonies (CFU-Mk) in semisolid media optimized for megakaryocyte colony growth. After 2–3 wk, 5,000 BM CD34+ cells expanded in the control group and overexpression group were assayed for CFU-Mk content. FLI1-overexpressing cells generated a fourfold increase in megakaryocyte colonies compared with the control group, which indicates that overexpression of FLI1 generates more megakaryocyte precursors. HOXC6, HDAC11, NPAS1, and NFATC1 overexpression also resulted in an approximate twofold change (SI Appendix, Fig. S5A).
We then used chemical compounds to further confirm the function of some regulators. Of all of the final 10 genes, HES7 is the downstream effector of the Notch pathway, and Notch antagonists DAPT and DBZ, the ligand DLL4 could mimic the function of HES7 knockout and overexpression, respectively. NFATC1 is one of the five members of the NFAT family, and upon activation by calcium, it translocates to the nucleus, where it targets various genes, including the cytokine gene IL2 (41). The small molecule cyclosporin A is a well-known inhibitor for NFATC1. Therefore, we treated HSPCs with DLL4, DAPT, DBZ, or cyclosporine A in the differentiation culture and found that MkP differentiation was greatly inhibited by adding DAPT, DBZ, or cyclosporine A, but increased by DLL4 (SI Appendix, Fig. S5B).
MkPs generate megakaryocytes, which then produce platelets. During maturation, megakaryocytes become polyploid, show increased protein and membrane levels, and then extend branches. After that, one megakaryocyte will finally release thousands of platelets in blood vessels. To determine the production of platelets with gene overexpression, we extended the differentiation with an additional 1–2 wk to induce MkPs into megakaryocytes and then platelets with the cytokine mixture TPO, SCF, and IL6. After that, we used flow cytometry to analyze platelet generation. During the culture, we observed large-sized polyploid megakaryocytes by Giemsa staining which then became tiny-sized platelets (SI Appendix, Fig. S5D). We used forward/side scatter (FSC/SSC) to gate out small size platelets and then analyzed their surface markers, including CD41 [glycoprotein (GP) IIb], CD42a (GP IX), and CD61 (GP IIIa). Compared with the control group, gene overexpression generated an approximate one- to fivefold increase in CD41+/CD42a+ and CD61+ cells, including HES7, HDAC11, MXD3, NFATC1, PCGF2, and FLI1. Significantly, HES7 can promote platelet generation to 34.3% CD41 and CD42a dual positive cells (SI Appendix, Fig. S5C).
Taken together, our results suggested that MZF1, GSX2, HOXC6, HDAC11, HES7, FOXB1, MXD3, HOXA9, NFATC1, and PCGF2 are potential regulators of MkP generation.
HDAC Regulation of MkPs and the Hit TFs.
HDACs are a class of enzymes regulating DNA deacetylation. They are therefore associated with a variety of transcriptional repressors that control cellular differentiation and proliferation. HDAC inhibition has been reported to regulate proliferation and expansion of HSCs in vitro (42). We thus believe it is important to examine whether HDACs function in MkP generation and regulate the newly identified TFs.
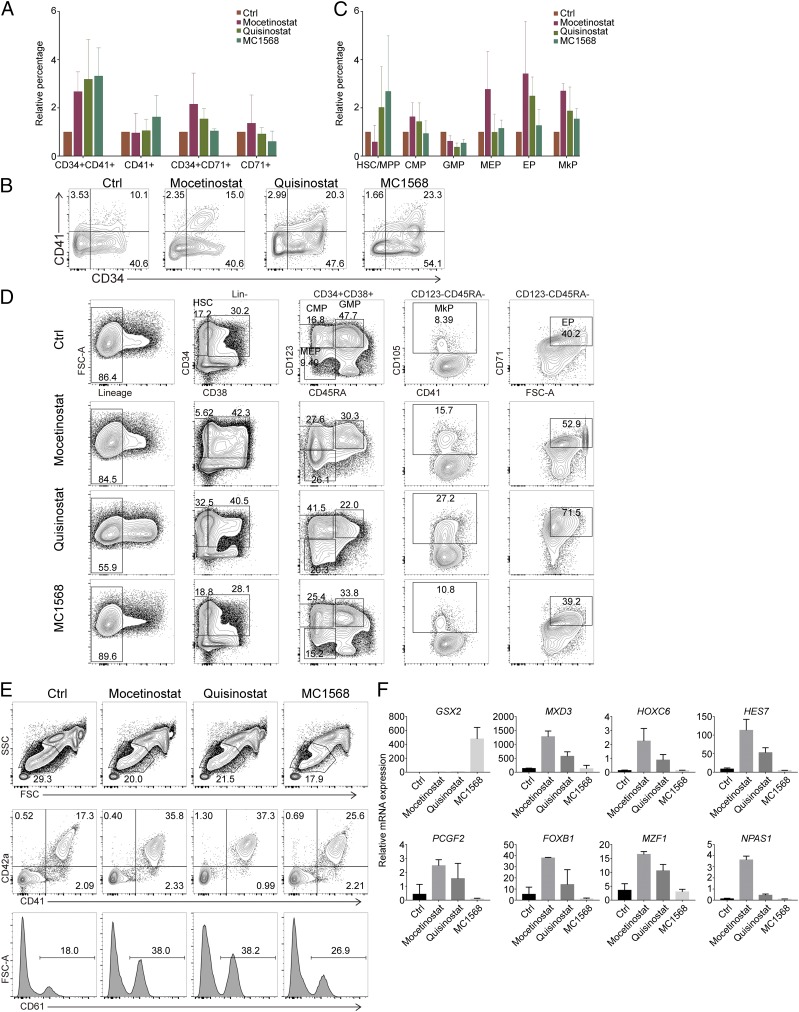
To answer this question, we first determined whether HDAC family members regulated MkP generation. The expression of the HDAC family members, including HDAC1-11, was analyzed by GEXC, in mouse hematopoiesis models. Except for those not expressed in MEPs, MkPs, or EPs (HDACs 4, 6, and 9), other HDACs fall into two distinct expression patterns in MEP, MkP, and EP populations (SI Appendix, Fig. S6A). HDACs 5 and 11 are expressed in MkPs rather than MEPs or EPs, according to GEXC; real-time PCR revealed similar expression patterns in human populations (SI Appendix, Fig. S6B). Knocking out of HDAC11 by CRISPR in human CD34+ cells showed a decrease of CD34+CD41+ MkP population while overexpression of HDAC11 increased this population, suggesting it could positively regulate MkP generation (Figs. 2C and 3A).
Other HDACs, including 1, 2, 3, 7, 8, and 10, showed higher expression levels in MEP and/or EP than in MkPs (SI Appendix, Fig. S6 A and B), which indicates that during MEP differentiation into MkPs, the HDAC family may repress MkP essential genes and thus their inhibition would promote MkP generation. To test this hypothesis, effects of HDAC inhibitors, including Quisinostat, Mocetinostat, and MC1568, were examined in our in vitro culture model. Quisinostat (JNJ-26481585) 2HCl is a novel second-generation HDAC inhibitor with the highest potency for class I HDAC1 and modestly potent to HDAC2, -4, -10, and -11. MC1568 is a selective inhibitor of class IIa HDACs (including HDAC4, -5, -7, and -9). Mocetinostat is an orally available inhibitor that selectively targets class I HDAC1 and -2. As expected, 10 nM Quisinostat, 2 μM Mocetinostat, and 5 μM MC1568 increased CD34+CD41+ populations from human CD34+ cells (Fig. 4 A and B and SI Appendix, Fig. S6C). Multiple color flow cytometry showed MkP increased after HDAC inhibitor treatment (Fig. 4 C and D). However, different HDAC inhibitors perform a bit differently. Quisinostat showed the most significant effect. Real-time PCR analysis showed the treatment increases most known MkP-specific TFs but not EP-related genes such as KLF1 and EPOR (SI Appendix, Fig. S6D).
Fig. 4.
HDAC inhibitors promote MkP differentiation. (A and B) Summary (A) or flow cytometry plot (B) for the increase/decrease of MkP (CD34+CD41+)- or EP (CD34+CD71+)-enriched population after different HDAC inhibitor treatment in CD34+ HSPC culture. Here, the percentage of the control cell-derived MkP- and EP-enriched populations is set to 1, and percentage from other treatments was normalized to that. n = 3; error bar indicates SD. (C and D) Summary (C) or flow cytometry plot (D) for the increase/decrease of different HSPC populations after different HDAC inhibitor treatment in CD34+ HSPC culture. Here, the percentage of the control cell-derived HSPC populations is set to 1, and percentage from other treatments was normalized to that. n = 3; error bar indicates SD. (E) FACS analysis of platelet surface markers CD41, CD42a, and CD61 after HDAC inhibitor treatment. (F) Expression analysis of some of the hit TF changes in HDAC inhibitor-treated cells by real-time PCR. n = 3; error bar indicates SD.
We then determined whether HDAC inhibitor treatment could affect platelet production. Since cord blood represents a large and readily available source of hematopoietic cells, we used HDAC inhibitors to treat cord blood mononuclear cells during their differentiation into platelets. Flow cytometry showed that CD41/CD42a+ cells increased one- to threefold while CD61+ cells increased correspondingly after HDAC inhibitor treatment of HSPCs. Mocetinostat can lead to more than 30% CD41+CD42a+ cells as well as CD61+ cells (Fig. 4E).
We then examined if HDACs could regulate our newly identified genes. The real-time PCR results showed GSX2, MXD3, HOXC6, and HES7 are highly increased by tens of folds by the inhibition of HDACs, indicating that HDACs might repress their expressions during hematopoiesis. PCGF2, FOXB1, and MZF1 have modest increase while no effect was observed for NPAS1 and HOXA9 (Fig. 4F). These results suggested that those newly identified genes might be downstream of HDAC modification.
Discussion
In this study, we combined CRISPR and functional studies to identify candidate factors that regulate MkP generation from HSPCs. The screening initiated with a solid candidate gene list generated from GEXC, a platform to analyze absolute gene expression levels based on microarray data. Real-time PCR was used to confirm expression pattern of those candidate genes in human MkPs. After that, CRISPR-mediated individual gene knockout, lentivirus-mediated gene overexpression, lineage-specific gene expression analysis, and functional in vitro colony-forming unit assay were used for the multiple rounds of screening. Using this strategy, we tested 60 candidates revealing 10 genes that conferred a regulatory role during HSPC differentiation to MkPs. Of these, some genes are highly up-regulated after HDAC inhibition, suggesting that they might be downstream of HDAC modification. Those results can provide an investigation of TFs as regulators in MkP generation and make it possible to change differentiation trends from MEP into its progenies.
Interestingly, of all of the genes identified, only a few of them have been shown to be involved in megakaryopoiesis, including FLI1 and MZF1. Most other genes, although they performed similar functions to FLI1 when deleted or overexpressed, have been reported only in either other hematopoietic lineages or in tissues outside of the hematopoietic system, etc., opening the possibility that other lineage genes might also be expressed in megakaryocytes. FLI1 belongs to the Ets gene family of transcription factors, and is well known for its function in erythrocyte and megakaryocyte development. Inactivation of FLI1 leads to defective megakaryopoiesis and abnormal erythroid development, while overexpression of FLI1 in the K562 cell line promotes its differentiation toward the megakaryocytic lineage (43). Nuclear factor of activated T-cells, cytoplasmic 1 (NFATC1) is first described in T cells. NFATC1 is one of the five members of the NFAT family, and upon activation by calcium, it translocates to the nucleus, where it targets various genes, including the cytokine gene IL2 (41). It is a target of inhibition by immunosuppressive agents cyclosporine and FK506, working through calcineurin and FKBP, respectively, and was later found to be critical for other tissue development, such as cardiac development (44–47). The NFAT family is reported to negatively regulate megakaryopoiesis in mouse (48). Here, we identified its expression and function in human MkP generation from HSCs and their further differentiation into megakaryocytes. One toxicity of chronic cyclosporine A administration can be thrombocytopenia (49). In addition, these studies have identified some factors that have not been reported to be involved in megakaryocyte development (SI Appendix, Table S2), demonstrating that our method cannot only validate already known genes, but also help reveal genes critical for MkP differentiation. In one scenario, cell-specific inducers of the appropriate platelet production pathway might be found to be a specific and safe alternative to TPO.
Taken together, our results presented here should facilitate the identification of regulators for MkP differentiation from HSPCs, which will help the development of protocols for generation of MkP and platelets by manipulating these genes/pathways for clinical use. Furthermore, this method could be readily applied to any other cell lineages for identification of critical regulators. Given the need of platelets in many pathological situations, understanding the regulation of platelet generation is an important research field with important clinical applications.
Materials and Methods
Cell culture, virus production, cell transduction, colony-forming unit assay, flow cytometry, RNA isolation, and real-time PCR were done as described in SI Appendix.
GEXC (https://gexc.riken.jp/) provides dynamic range of each gene by metaanalysis of thousands of microarray data. Transcription factor expressions in the mouse hematopoietic system in GEXC were analyzed in cell populations of hematopoietic stem, progenitor, and mature populations in adult mouse bone marrow, spleen, and thymus.
Supplementary Material
Acknowledgments
We thank Tal Raveh for her help in editing this manuscript; Terry Storm and Tejaswitha Naik for laboratory management; Patty Lovelace for help with flow cytometry; and the FACS core at Stanford Institute for Stem Cell Biology and Regenerative Medicine. This work is supported by National Institutes of Health (NIH) Grants U01-HL099999-07 and R01-CA086065 (to I.L.W.) and the Ludwig Foundation. F.Z. is a Seibel Scholar of the Seibel Stem Cell Institute. This work is also supported by NIH Pathway to Independence Award R00CA201075 (to M.F.) and the Damon Runyon–Dale F. Frey Award for Breakthrough Scientists DFS-22-16 (to M.F.).
Footnotes
The authors declare no conflict of interest.
See Commentary on page 9818.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1805434115/-/DCSupplemental.
References
- 1.Sim X, Poncz M, Gadue P, French DL. Understanding platelet generation from megakaryocytes: Implications for in vitro-derived platelets. Blood. 2016;127:1227–1233. doi: 10.1182/blood-2015-08-607929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uchida N, Aguila HL, Fleming WH, Jerabek L, Weissman IL. Rapid and sustained hematopoietic recovery in lethally irradiated mice transplanted with purified Thy-1.1lo Lin-Sca-1+ hematopoietic stem cells. Blood. 1994;83:3758–3779. [PubMed] [Google Scholar]
- 3.Uchida N, et al. High doses of purified stem cells cause early hematopoietic recovery in syngeneic and allogeneic hosts. J Clin Invest. 1998;101:961–966. doi: 10.1172/JCI1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Na Nakorn T, Traver D, Weissman IL, Akashi K. Myeloerythroid-restricted progenitors are sufficient to confer radioprotection and provide the majority of day 8 CFU-S. J Clin Invest. 2002;109:1579–1585. doi: 10.1172/JCI15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, et al. Comparative analysis of human ex vivo-generated platelets vs megakaryocyte-generated platelets in mice: A cautionary tale. Blood. 2015;125:3627–3636. doi: 10.1182/blood-2014-08-593053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNiece I, et al. Ex vivo expanded peripheral blood progenitor cells provide rapid neutrophil recovery after high-dose chemotherapy in patients with breast cancer. Blood. 2000;96:3001–3007. [PubMed] [Google Scholar]
- 7.Nakorn TN, Miyamoto T, Weissman IL. Characterization of mouse clonogenic megakaryocyte progenitors. Proc Natl Acad Sci USA. 2003;100:205–210. doi: 10.1073/pnas.262655099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orkin SH, Zon LI. Hematopoiesis: An evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pimanda JE, Göttgens B. Gene regulatory networks governing haematopoietic stem cell development and identity. Int J Dev Biol. 2010;54:1201–1211. doi: 10.1387/ijdb.093038jp. [DOI] [PubMed] [Google Scholar]
- 10.Laslo P, et al. Multilineage transcriptional priming and determination of alternate hematopoietic cell fates. Cell. 2006;126:755–766. doi: 10.1016/j.cell.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 11.McNagny KM, Sieweke MH, Döderlein G, Graf T, Nerlov C. Regulation of eosinophil-specific gene expression by a C/EBP-Ets complex and GATA-1. EMBO J. 1998;17:3669–3680. doi: 10.1093/emboj/17.13.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nerlov C, Graf T. PU.1 induces myeloid lineage commitment in multipotent hematopoietic progenitors. Genes Dev. 1998;12:2403–2412. doi: 10.1101/gad.12.15.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye M, Graf T. Early decisions in lymphoid development. Curr Opin Immunol. 2007;19:123–128. doi: 10.1016/j.coi.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Spooner CJ, Cheng JX, Pujadas E, Laslo P, Singh H. A recurrent network involving the transcription factors PU.1 and Gfi1 orchestrates innate and adaptive immune cell fates. Immunity. 2009;31:576–586. doi: 10.1016/j.immuni.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seita J, Weissman IL. Hematopoietic stem cell: Self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med. 2010;2:640–653. doi: 10.1002/wsbm.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanjuan-Pla A, et al. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature. 2013;502:232–236. doi: 10.1038/nature12495. [DOI] [PubMed] [Google Scholar]
- 17.Doré LC, Crispino JD. Transcription factor networks in erythroid cell and megakaryocyte development. Blood. 2011;118:231–239. doi: 10.1182/blood-2011-04-285981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tijssen MR, et al. Genome-wide analysis of simultaneous GATA1/2, RUNX1, FLI1, and SCL binding in megakaryocytes identifies hematopoietic regulators. Dev Cell. 2011;20:597–609. doi: 10.1016/j.devcel.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pimanda JE, et al. Gata2, Fli1, and Scl form a recursively wired gene-regulatory circuit during early hematopoietic development. Proc Natl Acad Sci USA. 2007;104:17692–17697. doi: 10.1073/pnas.0707045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klimchenko O, et al. A common bipotent progenitor generates the erythroid and megakaryocyte lineages in embryonic stem cell-derived primitive hematopoiesis. Blood. 2009;114:1506–1517. doi: 10.1182/blood-2008-09-178863. [DOI] [PubMed] [Google Scholar]
- 21.Wilson NK, et al. Combinatorial transcriptional control in blood stem/progenitor cells: Genome-wide analysis of ten major transcriptional regulators. Cell Stem Cell. 2010;7:532–544. doi: 10.1016/j.stem.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Pulecio J, et al. Direct conversion of fibroblasts to megakaryocyte progenitors. Cell Rep. 2016;17:671–683. doi: 10.1016/j.celrep.2016.09.036. [DOI] [PubMed] [Google Scholar]
- 23.Moreau T, et al. Large-scale production of megakaryocytes from human pluripotent stem cells by chemically defined forward programming. Nat Commun. 2016;7:11208, and erratum (2017) 8:15076. doi: 10.1038/ncomms11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cong L, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mali P, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11:783–784. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shalem O, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–87. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang T, Wei JJ, Sabatini DM, Lander ES. Genetic screens in human cells using the CRISPR-Cas9 system. Science. 2014;343:80–84. doi: 10.1126/science.1246981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seita J, et al. Gene expression commons: An open platform for absolute gene expression profiling. PLoS One. 2012;7:e40321. doi: 10.1371/journal.pone.0040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen JY, et al. Hoxb5 marks long-term haematopoietic stem cells and reveals a homogenous perivascular niche. Nature. 2016;530:223–227. doi: 10.1038/nature16943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan CK, et al. Identification and specification of the mouse skeletal stem cell. Cell. 2015;160:285–298. doi: 10.1016/j.cell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beerman I, et al. Proliferation-dependent alterations of the DNA methylation landscape underlie hematopoietic stem cell aging. Cell Stem Cell. 2013;12:413–425. doi: 10.1016/j.stem.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 33.Jackers P, Szalai G, Moussa O, Watson DK. Ets-dependent regulation of target gene expression during megakaryopoiesis. J Biol Chem. 2004;279:52183–52190. doi: 10.1074/jbc.M407489200. [DOI] [PubMed] [Google Scholar]
- 34.Athanasiou M, Mavrothalassitis G, Sun-Hoffman L, Blair DG. FLI-1 is a suppressor of erythroid differentiation in human hematopoietic cells. Leukemia. 2000;14:439–445. doi: 10.1038/sj.leu.2401689. [DOI] [PubMed] [Google Scholar]
- 35.Mori Y, Akashi K, Weissman IL. Identification of human erythroid lineage-committed progenitors. Rinsho Ketsueki. 2016;57:585–591. doi: 10.11406/rinketsu.57.585. [DOI] [PubMed] [Google Scholar]
- 36.Miyoshi H, Smith KA, Mosier DE, Verma IM, Torbett BE. Transduction of human CD34+ cells that mediate long-term engraftment of NOD/SCID mice by HIV vectors. Science. 1999;283:682–686. doi: 10.1126/science.283.5402.682. [DOI] [PubMed] [Google Scholar]
- 37.Genovese P, et al. Targeted genome editing in human repopulating haematopoietic stem cells. Nature. 2014;510:235–240. doi: 10.1038/nature13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malina A, et al. Repurposing CRISPR/Cas9 for in situ functional assays. Genes Dev. 2013;27:2602–2614. doi: 10.1101/gad.227132.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klug CA, Cheshier S, Weissman IL. Inactivation of a GFP retrovirus occurs at multiple levels in long-term repopulating stem cells and their differentiated progeny. Blood. 2000;96:894–901. [PubMed] [Google Scholar]
- 40.Uchida N, et al. HIV, but not murine leukemia virus, vectors mediate high efficiency gene transfer into freshly isolated G0/G1 human hematopoietic stem cells. Proc Natl Acad Sci USA. 1998;95:11939–11944. doi: 10.1073/pnas.95.20.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Northrop JP, et al. NF-AT components define a family of transcription factors targeted in T-cell activation. Nature. 1994;369:497–502. doi: 10.1038/369497a0. [DOI] [PubMed] [Google Scholar]
- 42.Chaurasia P, Gajzer DC, Schaniel C, D’Souza S, Hoffman R. Epigenetic reprogramming induces the expansion of cord blood stem cells. J Clin Invest. 2014;124:2378–2395. doi: 10.1172/JCI70313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Athanasiou M, et al. Increased expression of the ETS-related transcription factor FLI-1/ERGB correlates with and can induce the megakaryocytic phenotype. Cell Growth Differ. 1996;7:1525–1534. [PubMed] [Google Scholar]
- 44.Friedman J, Weissman I. Two cytoplasmic candidates for immunophilin action are revealed by affinity for a new cyclophilin: One in the presence and one in the absence of CsA. Cell. 1991;66:799–806. doi: 10.1016/0092-8674(91)90123-g. [DOI] [PubMed] [Google Scholar]
- 45.Liu J, et al. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 46.Friedman J, Trahey M, Weissman I. Cloning and characterization of cyclophilin C-associated protein: A candidate natural cellular ligand for cyclophilin C. Proc Natl Acad Sci USA. 1993;90:6815–6819. doi: 10.1073/pnas.90.14.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ke H, Zhao Y, Luo F, Weissman I, Friedman J. Crystal structure of murine cyclophilin C complexed with immunosuppressive drug cyclosporin A. Proc Natl Acad Sci USA. 1993;90:11850–11854. doi: 10.1073/pnas.90.24.11850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zaslavsky A, et al. The calcineurin-NFAT pathway negatively regulates megakaryopoiesis. Blood. 2013;121:3205–3215. doi: 10.1182/blood-2012-04-421172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tejaswi C, Mohanan S, Murugaiyan R, Karthikeyan K. Double trouble: Cyclosporine-induced thrombocytosis in a patient with methotrexate toxicity: Are they related? J Pharmacol Pharmacother. 2015;6:160–162. doi: 10.4103/0976-500X.162005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.