Significance
Formation of viable sex cells, such as eggs and sperm in humans, occurs during a special type of cell division (meiosis), in which parental chromosomes (homologs) must separate from each other. In most species this process requires a physical connection between homologs; these connections, called “cross-overs,” arise from DNA breaks, which occur at high frequency at special sites called “hotspots.” Successful meiosis requires that DNA double-strand breaks (DSBs) and the resulting cross-overs be carefully controlled. We describe here a physical mechanism for control of DSBs between distant hotspots via the 3D clustering of hotspots bound by their determinant proteins. Based on these results, we propose a physical mechanism for crossover interference, which was discovered over 100 y ago but whose mechanism has remained elusive.
Keywords: meiosis, crossover interference, DNA break interference, DSB hotspot clustering, S. pombe
Abstract
Viable gamete formation requires segregation of homologous chromosomes connected, in most species, by cross-overs. DNA double-strand break (DSB) formation and the resulting cross-overs are regulated at multiple levels to prevent overabundance along chromosomes. Meiotic cells coordinate these events between distant sites, but the physical basis of long-distance chromosomal communication has been unknown. We show that DSB hotspots up to ∼200 kb (∼35 cM) apart form clusters via hotspot-binding proteins Rec25 and Rec27 in fission yeast. Clustering coincides with hotspot competition and interference over similar distances. Without Tel1 (an ATM tumor-suppressor homolog), DSB and crossover interference become negative, reflecting coordinated action along a chromosome. These results indicate that DSB hotspots within a limited chromosomal region and bound by their protein determinants form a clustered structure that, via Tel1, allows only one DSB per region. Such a “roulette” process within clusters explains the observed pattern of crossover interference in fission yeast. Key structural and regulatory components of clusters are phylogenetically conserved, suggesting conservation of this vital regulation. Based on these observations, we propose a model and discuss variations in which clustering and competition between DSB sites leads to DSB interference and in turn produces crossover interference.
During meiosis, the diploid chromosome set in somatic cells is reduced to a haploid set to form gametes for sexual reproduction. Successful chromosome segregation requires that the two parental homologs segregate from each other at the first meiotic division, in sharp contrast to mitotic divisions, in which sister chromatids segregate from each other. Meiotic homolog segregation requires their mutual recognition. In most eukaryotic species this entails formation, by homologous genetic recombination, of a physical connection in regions of extensive DNA sequence identity. In addition to these connections (called “cross-overs”), cohesion between sister chromatids, by cohesin protein complexes, is required to form tension between homologs, which facilitates the onset of proper homolog segregation (1). A crossover too near another may leave too little cohesion to effectively hold the homologs together and thereby provide the necessary tension. Consequently, most species have evolved a mechanism of communication along chromosomes to prevent cross-overs from occurring too near each other. This phenomenon, called “crossover interference,” was discovered over 100 y ago (2), but its mechanism has remained elusive.
Homologous recombination, including crossover formation, occurs at high frequency during meiosis and is initiated by the formation of DNA double-strand breaks (DSBs) by Spo11 (or its homolog) and several essential partner proteins (Fig. 1A) (3). Repair of a DSB by interaction with the homolog can lead to a crossover, measured genetically by the formation of reciprocal recombinants between homologs appropriately marked. A hierarchical combination of factors shapes the genome-wide topography of meiotic DSBs. Like cross-overs, DSBs are controlled in both frequency and distribution along chromosomes (3). Short chromosomal intervals with especially high frequency of DSBs (called “DSB hotspots”) are separated by cold regions with relatively low DSB frequency. In the fission yeast Schizosaccharomyces pombe, studied here, DSB hotspots occur roughly 30–50 kb apart, and nearly all are bound with high specificity by three small, putatively coiled-coil proteins, Rec25, Rec27, and Mug20, which are also required for the formation of nearly all DSBs at nearly all hotspots (4). These hotspot-determinant proteins appear to form a complex with Rec10, which is required for all meiotic DSB formation and recombination genome-wide. These four proteins, with other chromosomal components, form linear elements (LinEs) related to the synaptonemal complex (SC) of other species (4, 5). Thus, LinE proteins dictate the formation of both DSBs at hotspots and chromosomal structures. We report here that these functions are related and have a common purpose—the physical coordination of DSB formation to prevent their overabundance along chromosomes.
Fig. 1.
DSB hotspots compete with each other over ∼200-kb regions. (A) Rec12 (Spo11 homolog; green ball) forms DSBs, remains covalently linked to 5′ ends, and is removed by an endonuclease to expose 3′ single-stranded tails (30, 58). Tails invade homologous DNA to form joint DNA molecules, resolved to form cross-overs as shown or non–cross-overs. Each line is ssDNA, blue and red from each parent; dashed lines indicate newly synthesized DNA. (B) DSBs are reduced at hotspots within ∼100 kb of the Rec25, Rec27-dependent ade6-3049 hotspot (4, 10, 24). Shown is DSB frequency, measured by ChIP-chip of Rec12-DNA covalent complexes, on part of Chr 3 with (ade6-3049; red line) or without (ade6-3057; blue line) a hotspot. (C) DSB frequency is increased only on the chromosome with hotspot alteration. (Upper) Natural hotspot mbs1 on Chr 1 (mbs1+ vs. mbs1-20 deletion). (Lower) ade6 hotspot on Chr 3 (ade6-3049 vs. ade6-3057). (SI Appendix, Fig. S1) (11). Individual points (+) are microarray values at other hotspots ≤50 kb of each side of the compared hotspot. Heat maps (densest in magenta) indicate densities of other points. Scales, log10. (D) Competition extends ∼100 kb on each side of a hotspot. Hotspot peaks surrounding mbs1 or ade6-3049 were integrated in the presence or absence of mbs1 or ade6-3049; each hotspot’s DSB ratio was plotted against its distance from mbs1 or ade6-3049. Values >1 indicate more breakage in the absence of each hotspot. Data were averaged (blue line) using a 50-kb sliding window in 25-kb steps. Median ratio is 0.95 (solid red line); dashed red lines indicate median ± two SD.
We find that DSB hotspots bound by LinE proteins form 3D clusters that encompass chromosomal regions of ∼200 kb. We also observe competition between DSB hotspot sites and interference between DSBs occurring on the same molecule, with both these effects extending over similar physical distances as hotspot clustering. We propose a model in which limiting the number of breaks within a cluster produces both DSB interference and hotspot competition; we present evidence that this limitation is imposed by the DNA damage-response protein kinase Tel1 (an ATM homolog, a tumor suppressor) (6–8). Because DSBs give rise to cross-overs, this proposed model leads to a mechanism for crossover interference and provides a means of communicating the DNA state between distant points regardless of chromosome physical size.
Results
Hotspots for Meiotic DSB Formation Compete with Each Other over ∼200-kb Regions.
To assess communication between DSB hotspots along a chromosome, we first determined the effect of adding or deleting a hotspot on the frequency of DSBs at nearby hotspots. We assayed genome-wide the immediate product of DSB formation—DNA covalently linked to Rec12 (Spo11 homolog) (Fig. 1A)—by immunoprecipitation of Rec12-DNA complexes and hybridization to tiling microarrays (9). By comparing two strains with and without the single base pair mutation ade6-3049, which creates a strong DSB hotspot (10), we observed that DSBs at hotspots flanking ade6-3049 were reduced, compared with the nonhotspot control, as far as ∼100 kb to each side (Fig. 1B). Conversely, when we deleted the natural hotspot mbs1 on a different chromosome (11), DSBs became readily detectable at minor hotspots, previously barely visible, up to ∼100 kb on each side of mbs1 (SI Appendix, Fig. S1). In both experiments DSBs far from the manipulated hotspot, or on the other two chromosomes, arose at nearly the same frequency (Fig. 1C). This effect, called “DSB competition,” is locally limited to an ∼200-kb region encompassing the break site (Fig. 1D). This interval size is similar to the average distance between DSBs [∼60 DSBs across the 12,600-kb genome (12)]. This outcome is consistent with only one DSB being made per ∼200-kb region (within a “cluster” as discussed below). DSB competition has also been reported in budding yeast but over somewhat shorter distances (up to ∼70 kb) (13–17). In both yeasts, however, these distances correspond to ∼25–35 cM, somewhat less than the genetic distance (50 cM) resulting from one crossover (Discussion).
In Fission Yeast, DSB Hotspots Compete in cis but Not in trans.
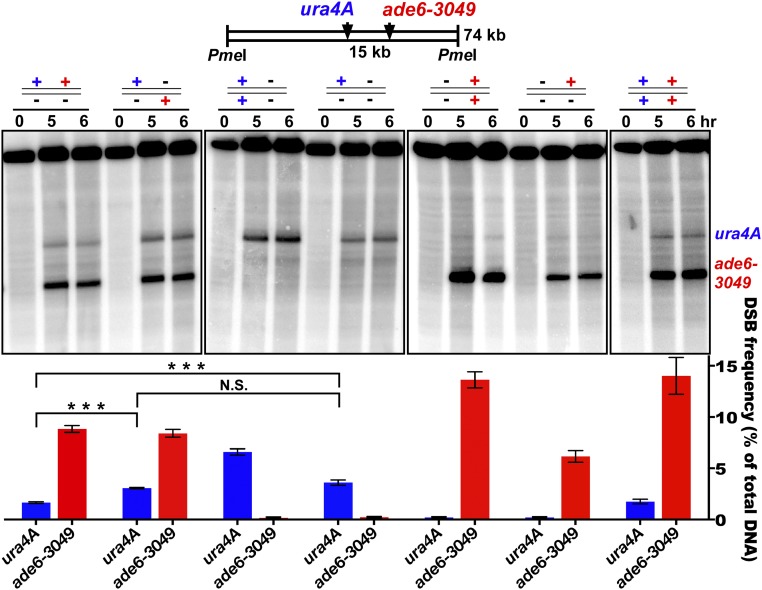
To test if this form of communication (hotspot competition) occurs along only one of the two parental homologs (in cis) or also extends between homologs (in trans), we tested the effect of having two nearby hotspots on the same or on different homologs (Fig. 2). Our first test used two previously studied hotspots, ade6-3049 and an inserted copy of ura4+ about 15 kb away (called “ura4A”) (18, 19). When alone and heterozygous, ura4A gave 3.6% DSBs. When ade6-3049 was added only in trans, this DSB level was not significantly changed (3.1% DSBs; P > 0.07 by unpaired t test). However, when ade6-3049 was added only in cis, the DSB level of ura4A was significantly reduced by about half, to 1.65% DSBs (P < 0.0005 by unpaired t test). Therefore, ade6-3049 competed against ura4A significantly more in cis than in trans (1.65% vs. 3.1% DSBs at ura4A; P < 0.0001 by unpaired t test) and may, in fact, not compete at all in trans. Furthermore, each individual hotspot showed about twice the DSB level when homozygous as when heterozygous, suggesting that there is little if any self-competition in trans. Additional comparisons of the data show that the stronger hotspot, ade6-3049, was not significantly competed by the weaker hotspot, ura4A. In a second assay of DSB competition, using two artificial hotspots about 45 kb apart, we also found competition in cis but not in trans. In this case the slightly weaker hotspot competed against the slightly stronger one (SI Appendix, Fig. S2). In Saccharomyces cerevisiae DSB competition occurs both along and between the two homologs (13–17, 20). In S. pombe we conclude that DSB competition occurs along only one homolog.
Fig. 2.
DSB competition acts along a homolog (in cis) but not between homologs (in trans). ura4A (blue) and ade6-3049 (red) DSB hotspots on Chr 3 were on the same (in cis; leftmost lane set) or different (in trans, second lane set) parental homologs. +, hotspot present; −, hotspot absent. DSBs were assayed at the indicated times after induction of meiosis in rad50S [wild-type DSB distribution (59)]. Data (mean ± SEM; n = 3 or 4) show the percent of DNA broken at the indicated hotspot (assay probe at the right end of the PmeI fragment). The fourth lane set from the left (no ade6 hotspot) shows that the ura4A hotspot is reduced by ade6-3049 in cis (first lane set; ***P < 0.0005 by unpaired t test) but not in trans [second lane set; P > 0.07 by unpaired t test; N.S. (not significant)]. The first two lane sets show that ade6-3049 reduces the ura4 hotspot more in cis than in trans (***P < 0.0001 by unpaired t test). See SI Appendix, Table S3 for individual data and SI Appendix, Fig. S2 for additional competitive pairs.
Interference of DSB Formation Along One DNA Molecule Is also Limited to ∼200-kb Regions.
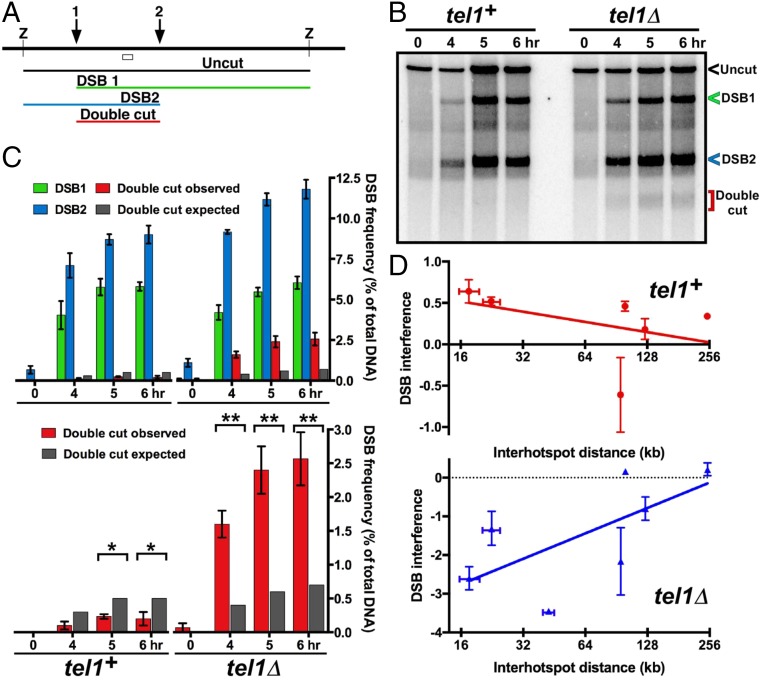
The analysis above showed that DSB hotspots compete with one another on the same homolog but not between homologs. To assess the ability of a DSB to interfere with DSB formation at another site on a given chromatid (i.e., along one DNA molecule), we assayed the frequency of DNA molecules broken at each of two nearby hotspots. DSB frequency was assayed by Southern blot hybridization of DNA extracted from meiotic cells, cut by an appropriate restriction enzyme, and separated by gel electrophoresis. A radioactive probe homologous to DNA located between the two chosen hotspots, about 15 kb apart, allowed concurrent assay (i.e., on the same Southern blot hybridization) of the frequency of DNA broken at one or the other or both hotspots (Fig. 3A). We found that doubly broken DNA was three to five times less frequent than expected from breakage at the two sites independently (i.e., the product of the frequency of breakage at each individual site) (Fig. 3 B and C and SI Appendix, Fig. S3). Thus, breakage at one site interferes with breakage at the other on the same DNA molecule. We observed DSB interference under multiple conditions for inducing meiosis (21, 22) and between several additional hotspot pairs on different chromosomes, ranging from ∼15–125 kb apart (Fig. 3D and SI Appendix, Figs. S3–S5). The extent of DSB interference, like that of DSB competition, decreased with distance, however, and became markedly less at ∼200 kb (Figs. 1 and 3 and SI Appendix, Figs. S3–S5 and Table S1).
Fig. 3.
Interference of DSB formation at nearby hotspots depends on Tel1 DNA damage-response protein kinase. (A) Scheme for assaying DSB frequency at either one or both hotspots (1 and 2) by Southern blot hybridization of DNA cut by restriction enzyme Z; the open box indicates the probe position. (B) DSB interference between two hotspots about 15 kb apart near the left end of Chr 2. (C) The observed doubly broken fragment (red bars) is less frequent than expected from independent breakage at the two hotspots (dark gray bars) in wild type but is more frequent than expected in tel1Δ. See SI Appendix, Figs. S3–S5 and Table S1 for additional data. (D) DSB interference is positive in tel1+ but negative in tel1Δ mutants. Error bars indicate SEM or range (SI Appendix, Table S1).
DSB interference and DSB competition may reflect the same phenomenon (Discussion), but their assays are different: Interference assays one DNA molecule encompassing two hotspots, whereas DSB competition assays all four chromatids (DNA molecules) at a site distant from the one genetically altered. They are also conceptually distinct: Interference indicates that breakage at two nearby hotspots on the same DNA molecule occurs less frequently than expected from independent breakage at the two sites. Competition indicates that the overall frequency of breakage across a region is dependent on the presence or absence of another nearby hotspot. Because DSB interference and competition have similar distance dependencies and could plausibly stem from the same phenomenon, we infer that they do; but because their assays are different and could be mechanistically different, we refer to them with different terms.
Tel1 DNA Damage-Response Protein Kinase Controls DSB and Crossover Interference.
In a mutant (tel1Δ) lacking the Tel1 protein kinase (ATM homolog) important for the DNA damage response (6), DSB interference was eliminated (Fig. 3), as reported in budding yeast (8). In fact, in fission yeast we observed more doubly broken DNA than expected from independent breakage, indicating negative DSB interference. [Interference (I) is quantitatively defined as 1 – CoC, where CoC (coefficient of coincidence) = RD/R1⋅R2; RD is the frequency of double events, and R1 and R2 are the frequencies of the individual events. Thus, observing more double events than expected from independence yields negative interference.] Negative interference was observed in tel1Δ mutants over distances from ∼15 kb to 125 kb but not significantly at 250 kb (Fig. 3 C and D and SI Appendix, Figs. S3–S5 and Table S1). These results support the notion that protein phosphorylation by Tel1 kinase is involved in controlling communication along meiotic chromosomes (Discussion) (8).
Furthermore, double cross-overs in the two adjacent genetic intervals ura2–leu2–lys7, each with a strong DSB hotspot ∼15 kb apart (4, 12), were also observed at a higher-than-expected frequency in the tel1Δ mutant (I = −0.85 ± 0.072, n = 16; P < 0.0001 by one-sample t test) (Table 1 and SI Appendix, Table S2). Consistent with this result, we observed negative DSB interference between these two DSB hotspots in the tel1Δ mutant (SI Appendix, Fig. S5). This negative crossover interference shows that cross-overs in fission yeast can be formed within a limited chromosomal region in a concerted fashion in tel1Δ. In tel1+ we observed a low but significant level of positive crossover interference (I = 0.28 ± 0.033, n = 17; P < 0.0001 by one-sample t test) (Table 1 and SI Appendix, Table S2); a previous report also found low but significant positive interference (0.33 and 0.29) in two intervals tested (23). Thus, cross-overs appear not to be formed in a simple random (independent) fashion as previously supposed in this yeast (Discussion) (23). As expected, no significant crossover interference, either positive or negative, was observed between intervals on different chromosomes in tel1+ (I = 0.02 ± 0.066, n = 16; P = 0.99 by one-sample t test) (SI Appendix, Table S2B) or in tel1Δ (I = −0.10 ± 0.059, n = 16; P = 0.06 by one-sample t test) (SI Appendix, Table S2C). This outcome bolsters the significance of the observed positive and negative interference between adjacent chromosomal intervals in tel1+ and tel1Δ, respectively. These results further show that recombination sites along a chromosome, but not between different chromosomes, indeed communicate regionally in individual meiotic cells, as also demonstrated by DSB competition and DSB interference in wild-type populations (Figs. 1–3, Table 1, and SI Appendix, Figs. S1–S5 and Tables S1–S3). Thus, in tel1 mutants both DSBs and cross-overs manifest negative interference, a result of coordinated action along the chromosome.
Table 1.
Cross-overs show strong negative interference in tel1 mutants
| Strain | Crossover interval 1, %* | Crossover interval 2, %† | Double crossover observed, % | Double crossover expected, % | Interference‡ |
| tel1+ | 3.76 ± 0.26 | 17.5 ± 2.1 | 0.49 ± 0.081 | 0.67 ± 0.11 | 0.26 ± 0.051§ |
| tel1Δ | 3.02 ± 0.14 | 8.0 ± 0.54 | 0.47 ± 0.048 | 0.245 ± 0.025 | −0.90 ± 0.048 |
In five (tel1+) and eight (tel1Δ) independent crosses, crossover recombination was assayed in the ura2 – leu2 – lys7 intervals (1 and 2, respectively), each of which contains a strong Rec25, Rec27-bound DSB hotspot (4, 12). Data are mean ± SEM. See SI Appendix, Table S2A for individual data and SI Appendix, Table S2 B and C for additional data. Bold face indicates mean values.
Interval 1 is ura2 – leu2 on Chr 1.
Interval 2 is leu2 – lys7 on Chr 1.
Interference = 1 – (double crossover observed/double crossover expected).
The near-zero values for most intervals reported by Munz (23) may not be significantly different from the value reported here, since many fewer recombinants were reported by Munz than were observed here. Two intervals showed weak but significant positive interference (I = 0.33 and 0.29).
Hotspots Physically Cluster over ∼200-kb Chromosomal Regions via DSB Hotspot-Determining Proteins.
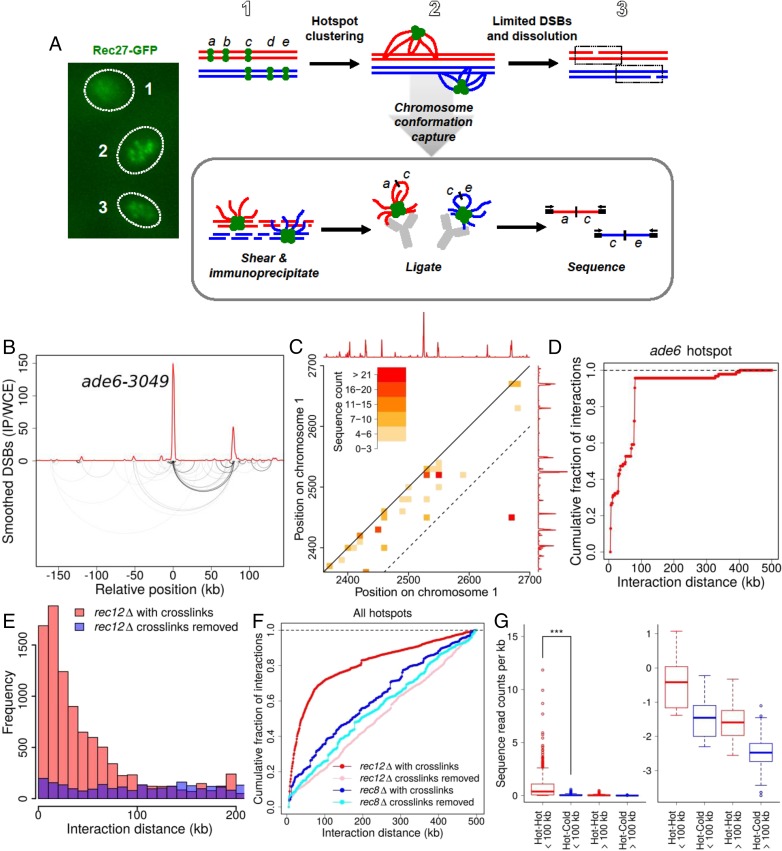
DSB competition and DSB interference indicate that breakage at one hotspot reduces or eliminates breakage at another hotspot over a limited chromosomal region. We hypothesized that these effects result from physical interaction of hotspots within this region. To test this hypothesis, we assessed the close physical association of hotspots bound by LinE proteins, which bind hotspots with high specificity, are required for the formation of most DSBs at nearly all hotspots, and form nuclear foci visible by light microscopy of live cells (Fig. 4A) (4, 24). We assayed the relative proximity (clustering) of hotspot DNA using a modification of the chromosome-conformation-capture (3C) technique, related to chromatin interaction analysis by paired-end tag sequencing (ChIA-PET) (25, 26), which relies on DNA ligation frequency as a proxy for distance. We cross-linked the DNA and closely bound proteins in meiotic cells, extracted and mechanically sheared the chromatin, and immunoprecipitated the hotspot-determinant proteins Rec25 or Rec27 (fused to appropriate epitope tags) to enrich for hotspot interactions involving these proteins. We ligated the closely apposed DNA ends and used paired-end sequencing to determine the genomic locations of the ends ligated together (SI Appendix, Fig. S6).
Fig. 4.
Physical clustering of DSB hotspots is limited to an ∼200-kb chromosomal region. (A) Scheme for determining clustering of DSB hotspots bound by LinE proteins, such as Rec27-GFP, which form a limited number of foci (or clusters of foci) in meiotic nuclei (Left) (31), perhaps corresponding to the steps in the Upper row. DNA within each cluster is cross-linked to a tagged LinE protein and analyzed as indicated in the box (see text). (B) Analysis of DNA bound by Rec27-GFP, which binds DSB hotspots with high specificity (4), shows preferential ligation of the ade6-3049 hotspot DNA to another hotspot ∼80 kb away (lower arcs; darker lines indicate greater frequency). Ligated sequences were omitted if neither end mapped to a hotspot. The red line indicates DSB frequency relative to genome median, determined by microarray hybridization (4). See also SI Appendix, Fig. S7. (C) Standard contact heat-map of ligations (hot–hot and hot–cold) in the 2,350–2,700 kb region of Chr 1. DSB frequency relative to the genome median (red line, on a linear scale) is from ref. 12. The dashed line indicates positions 100 kb apart on the chromosome; note that most of the intense interactions are within this limit, with the exception of ligations between two hotspots about 250 kb apart. See also SI Appendix, Figs. S8–S10. (D) Summation of all ligations between the ade6-3049 hotspot and DNA within 500 kb of each side. (E) Distance between pairs of sites ligated among all genomic hotspots with chromatin cross-links maintained until after ligation (red bars) or with cross-links removed just before ligation (purple bars; blue where these values are greater than those with maintained cross-links). (F) Summation of ligations between all genomic hotspots with chromatin cross-links maintained until after ligation (red line) or with cross-links removed just before ligation (pink line). Preferential ligations between nearby hotspots are much less frequent and extend greater distances in the absence of the Rec8 cohesin subunit with (dark blue line) or without (light blue line) maintenance of chromatin cross-links (see also SI Appendix, Fig. S13). (G, Left) Ligations between hotspots <100 kb apart are more frequent than those >100 kb apart (***P < 0.001 by unpaired t test). (Right) The same data are shown on a log10 scale for clarity of low levels. Data are the number of ligations per kilobase; mean values (thick horizontal bars) are flanked by the 25th and 75th percentiles (boxes) and 95th percentiles (whiskers). See SI Appendix, Fig. S6D for additional data with Rec25 and with another tag for immunoprecipitation and SI Appendix, Figs. S6–S13 for additional data.
This methodology differed in two important ways from typical 3C procedures, which likely would have failed to detect the relatively infrequent physical interactions between hotspots anticipated from our analyses above. First, after cross-linking the chromatin, we randomly sheared it by sonication rather than digesting it with a site-specific nuclease (27). This modification disrupted the compacted nuclear organization, which would have produced the abundant, short-range interactions observed in standard 3C analyses. Second, as noted above, we immunoprecipitated a hotspot-binding protein (Rec27 or Rec25) to enrich for specific protein–DNA complexes of interest (DSB hotspots). These two modifications reduced the more abundant, widespread interactions in standard 3C analyses, which likely would have obscured the rare hotspot-specific interactions studied here (see below). The ends of the sheared, immunoprecipitated DNA were then ligated and prepared for paired-end deep sequence analysis; the sequences in each pair were independently mapped to the fission yeast genome (28). Genome-wide analyses showed that many readily ligated ends came from two hotspot sites that were up to ∼100 kb apart on the linear genome sequence but must have been close in 3D space in the nucleus at the time of cross-linking. Similar procedures were used in S. pombe for different chromosomal proteins but, as expected, with markedly different results (26, 29) (see below).
Preferential ligation between nearby hotspot DNAs is exemplified by the ade6-3049 hotspot bound by Rec27-GFP (4). Of the ends ligated to ade6-3049, ∼40% came from another Rec27-bound hotspot ∼80 kb away, shown by the arcs connecting two interacting, well-separated chromosomal sites in Fig. 4B. Similar results were observed at several hotspot pairs on a different chromosome (SI Appendix, Fig. S7A). Preferential hotspot–hotspot interaction is also shown by a conventional “contact map” (Fig. 4C and SI Appendix, Figs. S7–S10) in which the most intense interactions are at points (squares) connecting two hotspots. Summation of all of the DNA ends ligated to the ade6-3049 hotspot showed a rapid accumulation over the first 100 kb followed by a much slower accumulation of ligated ends farther apart (Fig. 4D). Averaging all 603 hotspots (i.e., including even very minor ones) in the genome (12) refined the pattern and demonstrated preferential ligation of hotspots to sites <100 kb away (Fig. 4 E and F). Ligations were most frequent between hotspots 10–20 kb apart and became less frequent with increasing interhotspot distance (Fig. 4E). Nearly all cross-link–dependent ligations were between DSB hotspots 100 kb apart or less on the given chromosome (Fig. 4E and SI Appendix, Fig. S11). This feature is also evident in the contact map: Most of the intense interactions occur within ∼100 kb of the diagonal representing the chromosome (Fig. 4C and SI Appendix, Figs. S7–S10). Most of the longer-range (>100 kb), less frequent ligations likely result from random association of DNA ends across the genome. As expected, they accumulate at constant, low rate with linear chromosomal distance, as do ligations observed when Rec27-DNA cross-links were removed just before ligation (Fig. 4F). Also as expected, ligations between sites on different chromosomes were much less frequent, per kilobase, than ligations within 100 kb on the same chromosome (SI Appendix, Fig. S11).
To determine if this proximity-dependent association is hotspot-specific, we partitioned the genome into DSB-hot and DSB-cold regions and calculated the frequency of ligation events per kilobase. Ligation of hotspot DNA to other nearby hotspot DNA (i.e., <100 kb away) was on average ∼14 times more frequent than ligation to either proximal cold-region DNA or distant hotspot DNA (>100 kb away) and was >100 times more frequent than ligation to distant cold-region DNA (P < 0.001 for each comparison) (Fig. 4G and SI Appendix, Fig. S11A). Note that ∼77% of the chromosomal DNA is in DSB-cold regions by the cutoff used here (12); cross-linking and ligation of random neighboring DNA would predict only 0.23 times as many ligations of a hotspot to another hotspot as to any chromosomal point at random. Thus, in the nuclear 3D space hotspot DNA is much closer to other hotspot DNA nearby on the linear genome than to other DNA, as shown by the standard contact maps in Fig. 4C and SI Appendix, Figs. S7–S10 and S12A. As expected, LinE-bound DNA ligations are discontinuous (site-specific) across a genomic region, whereas standard Hi-C and ChIA-PET ligations are continuous (monotonically decreasing with distance) across the same genomic region (SI Appendix, Fig. S12) (26, 29). As deduced from the data analysis above, most pairs of ligated chromosomal sites occur <100 kb apart, and the most highly enriched pairs are between hotspots <100 kb apart (Fig. 4 and SI Appendix, Fig. S11). We note, however, that there are rare exceptions to this general rule: A few DSB hotspots are not fully dependent on Rec27 (4), some ligations occurred between hotspots >100 kb apart, and some hotspots were ligated at only low frequency (Fig. 4C and SI Appendix, Figs. S7–S10). We take the more general, abundant ligations preferentially between DSB hotspots <100 kb apart (Fig. 4G) as evidence for clustering of these sites by the hotspot-determinant protein Rec27.
Note that our results differ markedly from those obtained by Hi-C or the analysis of cohesin and condensin proteins in S. pombe involving similar procedures of chromatin cross-linking and sonication followed by immunoprecipitation and DNA ligation (Fig. 4 and SI Appendix, Fig. S12) (26, 29). These two proteins preferentially bind to and organize certain chromosomal intervals of up to ∼80 kb (cohesin) or ∼300 kb (condensin). Each protein appears to bind a fixed preferred site at the edge of the interval and one of many other sites within the adjacent interval, suggesting chromatin loops with one fixed and one variable chromosomal point. Alternatively, all the sites in an interval may be in a compact structure that allows ligation between any two points within the interval. By contrast, our results show ligations between well-separated, isolated points—LinE-bound DSB hotspots. (The similarity of these interval sizes and those of clusters may reflect related factors influencing their formation.)
Our hotspot clustering analyses were done in the absence of DSB formation (i.e., in the absence of Rec12) (30), indicating that the interactions occur without, and therefore likely before, DSB formation and are not due to DNA repair processes. This result is expected because binding of Rec27 to hotspots is independent of Rec12 (4), as is focus formation by other LinE proteins Rec10 and Mug20 (31, 32). Similar results were obtained using a different epitope tag (FLAG) and a different antibody for immunoprecipitation of Rec27 and when Rec25 immunoprecipitates were analyzed (SI Appendix, Fig. S6D), indicating the robustness of these results. The meiosis-specific cohesin subunit Rec8 is required for Rec27 to form most foci, bind to nearly all hotspots, and form DSBs at them (4). As expected, preferential ligations were much rarer in the absence of Rec8 than in the absence of Rec12 and were considerably farther apart on average (Fig. 4F and SI Appendix, Fig. S13). This outcome is expected, because these remaining (Rec8-independent, Rec27-bound) DSB hotspots are located on average >400 kb from each other (4). Focus formation of LinE proteins Rec10, Rec25, and Rec27 appeared normal in tel1Δ mutants (SI Appendix, Fig. S14), suggesting that clusters form independently of Tel1.
In summary, we observed close physical interactions between DSB sites over limited chromosomal distances with two LinE proteins, independent of the tag used but largely dependent on Rec8 and the maintenance of cross-links before ligation.
Discussion
Our results presented here show that DSB hotspot competition and interference extend along chromosomes for distances (∼200 kb) very similar to that of hotspot clustering (∼200 kb). This observation and the coordinate effects of genetic mutations on these features strongly suggest that clusters provide the physical basis for DSB competition and DSB interference. In other words, DSB hotspots communicate along chromosomes via the formation of physical interactions between the DSB hotspots that are bound by their determinant proteins. We discuss the implications of our results for regulating the formation not only of DSBs but also the cross-overs arising from them.
Evidence for Hotspot–Hotspot Interactions Forming Localized Clusters of LinE-Bound Hotspots.
Our data here (Fig. 4 and SI Appendix, Figs. S6–S13) indicate that DSB hotspots over a limited chromosomal region are located sufficiently close to each other to be cross-linked by formaldehyde, to remain associated during sonication and immunoprecipitation, and to allow ligation of the DNA ends produced by sonication and “polishing.” This standard procedure is an established method for determining the association of distant chromosomal sites in 3D space (25). We employed these standard procedures, with modifications that have been previously used (26, 29), to show that meiotic DSB hotspots within a limited chromosomal region (∼200 kb) are more frequently associated with each other than are hotspots farther apart or on another chromosome and that within that limited region a hotspot is associated much more frequently with other hotspot DNA than with cold-region (nonhotspot) DNA. Detecting the enhanced hotspot–hotspot interactions (which form clusters) depends on cross-linking and thus these interactions are not random; the spatial and hotspot-specific preferences also show that these interactions are neither random nor due to normal chromosome compaction. Furthermore, these interactions require the Rec8 cohesin subunit, which is required for loading of the LinE proteins Rec25 and Rec27 (31), the proteins we analyzed for clustering. Collectively, these observations show that LinE-bound DSB hotspots form clusters over a limited chromosomal region.
Relations Between DSB Hotspot Clustering, DSB Competition, and DSB Interference.
The genetic and physical data presented here indicate that DSB hotspot clusters are closely related to DSB competition and DSB interference. (As noted above, competition and interference may reflect the same phenomenon but are conceptually distinct and are observed by distinct assays.) First, all three phenomena occur over approximately the same distance, ∼200 kb (Figs. 1, 3, and 4). This is also the average distance between DSBs (12). Second, the LinE proteins Rec25 and Rec27 bind DSB hotspots with high specificity and are required for DSB formation at nearly all these hotspots (4). These proteins form readily visible, colocalizing foci in meiotic cells (4, 31) and, by the type of 3C analysis used here (related to ChIA-PET) (Fig. 4), form clusters of hotspot sites located across chromosomal regions of ∼200 kb. Third, genetic removal of the meiosis-specific cohesin subunit Rec8 greatly reduces binding of Rec27 to hotspots, formation of microscopic foci, formation of DSBs at hotspots, and formation of hotspot clusters (Fig. 4) (4, 31). Fourth, the ade6-3049 single base pair mutation creates a strong binding site for LinE proteins and a strong LinE-dependent DSB hotspot; it also imparts localized cluster formation, DSB competition, and DSB interference (Figs. 1–4) (4, 10, 24). Fifth, the Tel1 protein kinase imparts both DSB interference and crossover interference; in its absence, both become negative (Fig. 3, Table 1, and SI Appendix, Figs. S1–S5). These results are consistent with a causal connection between these two phenomena. Collectively, these observations demonstrate that these features arise from a common source, the clustering of LinE-bound DSB hotspots and the limiting of DSB formation within these clusters. We discuss below the mechanistic implications of hotspot clustering.
Molecular Basis of DSB Hotspot Competition and DSB Interference Within a Hotspot Cluster.
Limiting the number of DSBs that can occur within a cluster can explain both hotspot competition between chromatids and DSB interference between hotspots on a single DNA molecule. Hotspot sites within a cluster compete for the limited number of DSBs that can be formed in a cluster. Adding a new strong hotspot, such as ade6-3049, will reduce the overall frequency of breakage at nearby existing hotspots. Similarly, limiting the number of DSBs within a cluster means that a break at one site will reduce the probability of a second break at another nearby site on the same DNA molecule within the cluster, resulting in DSB interference.
How might DSB formation be limited within hotspot clusters? We propose that formation of the first DSB within a cluster physically alters the proteins, DNA, or both within a cluster such that a second DSB is formed at reduced frequency or perhaps not at all, in which case one cluster produces one DSB. This proposal is consistent with ∼60 DSBs per meiosis (12) being distributed by competition and interference across the 12.6-Mb fission yeast genome and with previous conclusions of a limit, at a given locus, of only one DSB per homolog pair in many meiotic cells in budding yeast (20, 33, 34). For example, modification of one or more proteins in the cluster may change the protein’s activity and thereby prevent further DSB formation. Specifically, when the first DSB is formed, the Tel1 protein kinase may be activated and then phosphorylate, and thereby inactivate, a component of the DSB-forming complex [Rec12 and its half-dozen essential partner proteins (35)]. This feature readily accounts for Tel1 and its kinase activity being required for DSB interference and suppression of nearby cross-overs in fission yeast (Fig. 3, Table 1, SI Appendix, Figs. S2–S5 and Table S2), for full levels of DSB and crossover interference in budding yeast (8, 34), and for strong restriction of DSB formation in mice (7). We note that Tel1 is not required, however, for focus formation by the LinE proteins Rec25, Rec27, or Rec10 (SI Appendix, Fig. S14). This result suggests that Tel1 acts to control DSB formation after hotspots are clustered by the LinE proteins. Binding and clustering of the DSB machinery in the absence of Tel1 may also explain strong negative interference of both DSBs and cross-overs in the absence of Tel1 (Fig. 3, Table 1, and SI Appendix, Figs. S2–S5 and Tables S1 and S2). The close proximity of the uncontrolled DSB-promoting factors may result in frequent breakage at multiple sites in a cluster and thus the formation of multiple, closely spaced cross-overs.
An alternative (or additional) mechanism to limit DSB formation is a limiting component, e.g., the active-site protein Rec12, within each cluster such that only one DSB can be formed. Finally, the conformation of the DSB-forming complex may change and prevent further DSB formation, as is the case for the recombination hotspot-activator RecBCD enzyme of Escherichia coli (36).
DSB Interference as a Basis of Crossover Interference.
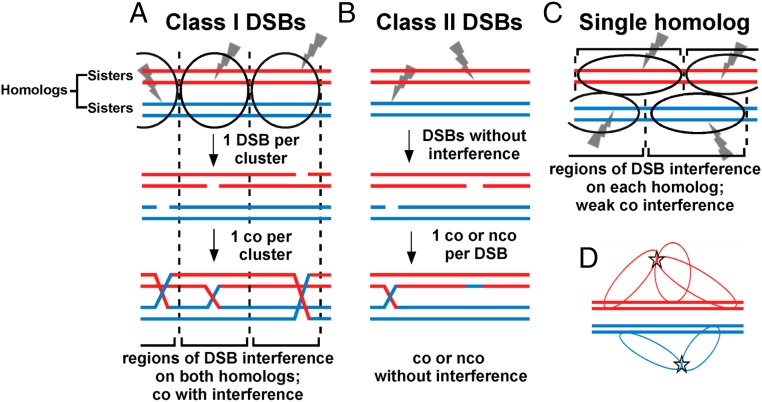
Closely related to DSB interference is crossover interference, the occurrence of closely spaced double cross-overs less frequently than expected under independence. Given that DSBs are the precursors to cross-overs, DSB interference would be expected to give rise to crossover interference. Therefore, based on our results here, we propose the following physical basis for meiotic crossover interference and the model in Fig. 5. This model may apply to some but not all species, for there are clearly variations in the mechanism and control of meiotic recombination among species. Furthermore, in addition to DSB interference there may be other mechanisms of crossover interference such as those proposed by others (37–41). In species with strong crossover interference, we propose that potential sites of DSB formation form clusters encompassing both homologs, and a single DSB is made in each cluster (Fig. 5A). (Note that in any given cell these potential sites are only the sites in a chromosomal region bound by hotspot-determinant proteins; they are not all the sites in a chromosomal region at which DSBs are made in the population of cells.) Consequently, no more than one crossover can arise in the clustered region, which would encompass a genetic distance of no more than 50 cM, the genetic distance resulting from one crossover. If clusters are more or less randomly distributed across the genome in a population, interference would be nearly complete at short genetic distances (a few centimorgans) but would disappear as distances approach 50 cM. At short distances, both genetic intervals for crossover assays would be in the same cluster, whereas at longer distances they would be in, or extend over, two or more clusters, which act independently. This is the outcome observed in some species (41), which we propose have clusters and DSBs regulated as in Fig. 5A. Most eukaryotes have extensive heterochromatic regions devoid of DSBs around their centromeres (42). We suppose that DSB hotspot clusters do not form in or across such pericentric regions, thereby explaining why crossover interference does not occur across the centromere in some cases.
Fig. 5.
Model for crossover interference based on DSB interference among clustered DSB hotspots. Each horizontal line is one sister chromatid (dsDNA molecule), red for one parental homolog (pair of sister chromatids) and blue for the other. Ovals indicate clusters, within which one DSB (gray lightning bolt) occurs. (A) In species with complete interference, clusters of activated DSB hotspots on both homologs form in a limited chromosomal region; only one DSB (class I) is made in each cluster. Consequently, no more than one crossover is made in that region, resulting in crossover interference. In a population of cells, clusters are distributed more or less randomly; interference is thus complete in short genetic intervals but becomes less in longer genetic intervals and is negligible in genetic intervals equal to or greater than that resulting from one crossover (50 cM). (B) In some species, class II DSBs are also formed but are not cluster-controlled and consequently do not manifest interference. Crossover interference is incomplete but is greater if class I DSBs outnumber class II DSBs. (C) In some species, such as fission yeast, clusters form between activated DSB hotspot sites on one homolog, not two. Consequently, DSBs (class I) manifest interference (on one DNA molecule), but cross-overs manifest only weak interference, since DSBs form independently on the two homologs (Discussion). (D) Flexible chromatin loops enable very distant DSB hotspots bound by their activating protein determinants to form a cluster. DSB hotspots on chromatin loops could extend from the chromosomal axis (central thick lines, each a dsDNA molecule or sister chromatid) and thereby allow activated DSB hotspots to form a cluster (star), even though they might be at the extremities of the chromosome. Clusters might form over both homologs (as in A) or over only one homolog (as in C).
Variation of crossover interference in other species can be accounted for by two classes of DSBs and by clusters encompassing both homologs or only one (Fig. 5 B and C). Two classes of cross-overs have been identified previously in various species (34, 43), those manifesting interference (class I) and those manifesting weak or no interference (class II). We propose that these crossover classes correspond to two classes of DSBs, as follows. The first DSB made in a cluster (here called “class I”) is unique in that it prevents the formation of further DSBs of its type in that cluster. Any additional DSBs (here called “class II”) made subsequently are repaired to either noninterfering cross-overs or non–cross-overs. Class II DSBs may arise either within clusters (after class I DSBs are formed) or outside clusters; the latter case seems simpler and accounts for DSBs formed independently of hotspot determinants (4, 12). In species with incomplete crossover interference, both class I and class II cross-overs occur. The designation of the first DSB as class I, and thus the designation of an interfering crossover, could be made either before or at the time of that DSB formation; both possibilities are compatible with the general scheme proposed here. We further note that if DSB formation is weakened, e.g., by alteration of the DSB-forming protein Spo11 (44), fewer class II DSBs are made after the first (class I) DSB is made. Thus, the frequency of interfering cross-overs, the major class, is maintained despite fewer total DSBs being made and at the expense of fewer non–cross-overs from class II DSBs. This feature, called “crossover homeostasis” (44), is thereby accounted for, as is the concurrent loss of crossover interference and crossover homeostasis in certain mutants (45).
In the two species reported to have weak or no crossover interference (S. pombe and the fungus Aspergillus) (Table 1 and SI Appendix, Table S2) (23), we propose that most or all DSBs are interfering (class I) but occur in clusters encompassing only one homolog (Fig. 5C) rather than both homologs, as in species shown in Fig. 5A (33, 34). This feature may reflect incomplete synaptonemal complex formation between homologs in S. pombe and Aspergillus (5); the SC has long been associated with crossover interference (46, 47). Although S. pombe LinE proteins form structures similar to the lateral elements of the SC, S. pombe has no obvious orthologs of the SC central elements which connect homologs in other species. The apparent absence of central element components is consistent with the proposal that S. pombe makes clusters encompassing only one homolog. Cluster formation on only one homolog also accounts for DSB competition acting along a homolog (in cis) but not between homologs (in trans) in S. pombe (Fig. 2 and SI Appendix, Fig. S2). If clusters on the two homologs are independently distributed, there would be DSB interference, as assayed on single DNA molecules, but only weak crossover interference, as observed (Fig. 3, Table 1, SI Appendix, Figs. S3–S5 and Tables S1 and S2). Because DSBs could arise independently on each homolog even in a short interval, double cross-overs would be observed. However, the absence of two DSBs on each individual homolog (i.e., only one DSB arising per pair of sister chromatids) would reduce the frequency of total DSBs in a cluster-size interval relative to their frequency under complete independence; consequently, there would be weak crossover interference only over short intervals, as observed (Table 1 and SI Appendix, Table S2). Thus, this proposal solves the seeming paradox of strong (positive) DSB interference without strong (positive) crossover interference in fission yeast. It also explains the paradoxical observation of strong negative crossover interference (I = −0.85) in the absence of Tel1 (Table 1 and SI Appendix, Table S2). The concerted formation of both DSBs and cross-overs in tel1 mutants indicates that S. pombe has, like species manifesting (positive) crossover interference, the basic mechanism of communication over a long distance along a chromosome.
To our knowledge, only one publication has discussed DSB interference as the basis for crossover interference. Berchowitz and Copenhaver (48) discussed DSB competition and crossover interference extending over about 70 kb in S. cerevisiae (16, 49), but they dismissed the possibility that DSB interference might explain crossover interference over much larger (megabase) regions, as observed in most multicellular species. However, the flexibility of loops in chromatin provides a basis for the long-distance communication between sites bound by DSB hotspot determinants (i.e., not over entire, continuous regions) along chromosomes discussed above. We propose that the potential sites at which DSBs could be made occur on loops emanating from the chromosomal axis and that the protein-bound sites form a cluster (Fig. 5D). These potential sites are limited to the few bound to their protein determinants in a particular cell; they are not all the sites in the included chromosomal region at which DSBs are made in the population. In S. pombe, only sites bound by LinE proteins in a particular cell would form a cluster. Note that the chromosomal regions between these sites are not proposed to be in the cluster; the majority of the chromosome can remain in the extended linear form of the axis or synaptonemal complex. In a 20-μm-diameter nucleus, such as that of a human oocyte (50), two loops of about 60 kb would allow sites at the ends of a 100-Mb chromosome to cluster in this way. (60 kb is for B-form DNA; nucleosome-containing DNA would require about 200 kb, but each of these DNA lengths is a small fraction of the entire chromosome and would likely be invisible by ordinary microscopy.) Thus, we see no difficulty in DSB site clustering, and consequently DSB interference, being the basis for crossover interference even for large chromosomes.
Clusters have been previously discussed in connection with crossover interference. Stahl (51) proposed that “recombination nodules,” thought to be protein complexes that promote meiotic recombination, might be swept into “piles” (clusters) to impose crossover interference. Stahl et al. (52) similarly proposed that “crossover intermediates,” presumably joint DNA molecules, or “attempts,” presumably crossover and non–crossover events combined, might form finite clusters in an interval. To our knowledge, 3D clustering of DSB sites has not been previously proposed.
1D clusters of DSBs along a chromosome, like birds clustered along a telephone wire, have been observed and discussed extensively (e.g., refs. 8 and 34). These clusters are physically and conceptually distinct from the 3D clusters of DSB hotspots discussed here, (Fig. 5). Garcia et al. (8, 53) proposed that DSB hotspots along several clustered chromatin loops are physically separate but subject to Tel1-independent DSB competition and that DSB hotspots along one loop are separate but subject to Tel1-dependent DSB interference. Anderson et al. (34) proposed a model for crossover interference incorporating 1D clusters of DSBs regulated by the synaptonemal initiation complex (SIC) and Tel1. In this model, DSBs are made independent of either factor; an SIC binds a DSB and prevents, in a Tel1-dependent manner, further nearby DSB formation, resulting in Tel1-dependent crossover interference. The fundamental basis of crossover interference in this model is SIC positioning (by an unspecified mechanism, but see below), whereas in our model it is 3D clustering of DSB hotspots (by LinE complex formation) and limitation of LinE-dependent DSBs.
Comparison of DSB Hotspot Clusters and Beam-Film Meshworks as the Basis of Long-Distance Chromosomal Communication.
The conservation of communication along meiotic chromosomes invites a comparison of two models proposed to account for such communication—the “beam-film” model for crossover interference (37) and the clustering model for DSB and crossover interference presented here (Fig. 5). The beam-film model posits that mechanical stress on the chromosomes, partially dependent on topoisomerase II (45), builds up until a crossover is formed, locally relieving the stress and thereby discouraging a second, nearby crossover. Chromosomes are proposed to fold in three dimensions and form a “meshwork” of contacts, also unspecified, between distant sites within which stress is built up and relieved (45). Our clustering model and data reported here provide a structural and mechanistic basis for several of these concepts, with clusters taking the place of meshworks. One significant difference between the two models, however, is the level at which interference operates. The beam-film model posits that stress is relieved by crossing over, not by DSB formation, and therefore interference applies to cross-overs but not to DSBs. In contrast, in our hotspot 3D clustering model (Fig. 5) interference applies to DSBs and therefore also to the cross-overs that result from them. Data from both S. cerevisiae (8) and our current study in S. pombe clearly demonstrate the existence of DSB interference, supporting the clustering model.
Materials and Methods
Materials.
S. pombe strains are listed in SI Appendix, Table S4 with their genotypes, sources, and the figures or tables in which data from them are presented. Growth media were rich yeast extract liquid (YEL; Difco), yeast extract agar (YEA; Difco), appropriately supplemented Edinburgh minimal media (EMM2), pombe minimal glutamate (PMG), nitrogen base agar (NBA; Difco), sporulation agar (SPA), and malt extract agar (MEA; Difco) (54). Reagents for the preparation and analysis of DNA are as described in ref. 55. Oligonucleotides are listed in SI Appendix, Table S5. Meiotic chromatin was immunoprecipitated (4) using anti-GFP (Roche) or anti-FLAG (Invitrogen) antibodies. DNA ligations used T4 DNA ligase (New England Biolabs). Amplification of immunoprecipitated DNA was carried out using either a Sequenase version 2.0 DNA Sequencing Kit (Affymetrix) or a REPLI-g Single Cell Kit (Qiagen).
Analysis of Meiotic Recombination.
Crosses were conducted on supplemented SPA and analyzed as described in ref. 54 and SI Appendix, Table S2.
Preparation and Analysis of Meiotic DNA.
S. pombe cultures were grown and induced for meiosis as described (55, 56). For analysis of DSBs by Southern blot hybridization in Fig. 3 and SI Appendix, Figs. S3 and S4, DNA was extracted with phenol-chloroform from cells in liquid buffer (57) and analyzed as described (55). (Cells were not embedded in agarose plugs before DNA extraction to avoid diffusion and thus loss of the small doubly broken DNA fragments from the plugs.) For analysis of DSBs by Southern blot hybridization in Fig. 2 and SI Appendix, Figs. S2 and S5, cells were embedded in agarose, and DNA was extracted and analyzed as described (55). The genomic regions analyzed and the restriction enzymes used were as follows: in Fig. 2 and SI Appendix, Figs. S3 and S4, 1.26–1.34 Mb on chromosome (Chr) 3, cut with PmeI; in Fig. 3 and SI Appendix, Fig. S2, 0.94–0.99 Mb on Chr 2, cut with AvrII; in SI Appendix, Fig. S5A, 0.52–1.02 Mb on Chr 1, cut with NotI; SI Appendix, Fig. S5B, 4.08–5.08 Mb on Chr 1, cut with NotI; in SI Appendix, Fig. S5C, 3.03–4.53 Mb (right end) on Chr 2, cut with NotI; in SI Appendix, Fig. S5D, 2.72–3.60 Mb on Chr 1, cut with NotI.
Analysis of DNA Proximity by Chromosome-Conformation Capture.
The analysis of DNA proximity by chromosome-conformation capture is described in the SI Appendix.
Supplementary Material
Acknowledgments
We thank the Fred Hutchinson Cancer Research Center Shared Resources Group for expert DNA sequence analyses; Paul Russell for the tel1::kanMX6 allele; Yoshinori Watanabe for the cnp20::2FLAG-kanMX6 allele; José Ayte for the pat1-as1 allele; Emily Higuchi for strains and information on DSB and crossover interference; Gabriel Cortez, Hannah Sue Hults, and Mai-Chi Nguyen for technical assistance; Sue Amundsen, Harmit Malik, Peter Kushner, Michael Lichten, Mridula Nambiar, Tom Petes, Rasi Subramaniam, Stephen Tapscott, Jeetu Thakur, and SaraH Zanders for comments on the manuscript; and Greg Copenhaver for discussions about crossover interference. This work was supported by NIH Grants R01 GM031693, R01 GM032194, and R35 GM118120 (to G.R.S.) and P30 CA015704 to the Fred Hutchinson Cancer Research Center. Data on DSB competition, DSB interference, and hotspot 3D clustering and a model for crossover interference based on these data were reported by G.R.S. at the 2011 European Molecular Biology Organization Conference on Meiosis and at subsequent annual meetings.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE119921).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1801920115/-/DCSupplemental.
References
- 1.Petronczki M, Siomos MF, Nasmyth K. Un ménage à quatre: The molecular biology of chromosome segregation in meiosis. Cell. 2003;112:423–440. doi: 10.1016/s0092-8674(03)00083-7. [DOI] [PubMed] [Google Scholar]
- 2.Sturtevant AH. The behavior of the chromosomes as studied through linkage. Z Indukt Abstamm Vererbungsl. 1915;13:234–287. [Google Scholar]
- 3.Lam I, Keeney S. Mechanism and regulation of meiotic recombination initiation. Cold Spring Harb Perspect Biol. 2014;7:a016634. doi: 10.1101/cshperspect.a016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowler KR, Gutiérrez-Velasco S, Martín-Castellanos C, Smith GR. Protein determinants of meiotic DNA break hot spots. Mol Cell. 2013;49:983–996. doi: 10.1016/j.molcel.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loidl J. S. pombe linear elements: The modest cousins of synaptonemal complexes. Chromosoma. 2006;115:260–271. doi: 10.1007/s00412-006-0047-7. [DOI] [PubMed] [Google Scholar]
- 6.Langerak P, Russell P. Regulatory networks integrating cell cycle control with DNA damage checkpoints and double-strand break repair. Philos Trans R Soc Lond B Biol Sci. 2011;366:3562–3571. doi: 10.1098/rstb.2011.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lange J, et al. ATM controls meiotic double-strand-break formation. Nature. 2011;479:237–240. doi: 10.1038/nature10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia V, Gray S, Allison RM, Cooper TJ, Neale MJ. Tel1(ATM)-mediated interference suppresses clustered meiotic double-strand-break formation. Nature. 2015;520:114–118. doi: 10.1038/nature13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cromie GA, et al. A discrete class of intergenic DNA dictates meiotic DNA break hotspots in fission yeast. PLoS Genet. 2007;3:e141. doi: 10.1371/journal.pgen.0030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steiner WW, Smith GR. Optimizing the nucleotide sequence of a meiotic recombination hotspot in Schizosaccharomyces pombe. Genetics. 2005;169:1973–1983. doi: 10.1534/genetics.104.039230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cromie GA, Rubio CA, Hyppa RW, Smith GR. A natural meiotic DNA break site in Schizosaccharomyces pombe is a hotspot of gene conversion, highly associated with crossing over. Genetics. 2005;169:595–605. doi: 10.1534/genetics.104.037176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowler KR, Sasaki M, Milman N, Keeney S, Smith GR. Evolutionarily diverse determinants of meiotic DNA break and recombination landscapes across the genome. Genome Res. 2014;24:1650–1664. doi: 10.1101/gr.172122.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jessop L, Allers T, Lichten M. Infrequent co-conversion of markers flanking a meiotic recombination initiation site in Saccharomyces cerevisiae. Genetics. 2005;169:1353–1367. doi: 10.1534/genetics.104.036509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan QQ, Xu F, White MA, Petes TD. Competition between adjacent meiotic recombination hotspots in the yeast Saccharomyces cerevisiae. Genetics. 1997;145:661–670. doi: 10.1093/genetics/145.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robine N, et al. Genome-wide redistribution of meiotic double-strand breaks in Saccharomyces cerevisiae. Mol Cell Biol. 2007;27:1868–1880. doi: 10.1128/MCB.02063-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu TC, Lichten M. Factors that affect the location and frequency of meiosis-induced double-strand breaks in Saccharomyces cerevisiae. Genetics. 1995;140:55–66. doi: 10.1093/genetics/140.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu L, Kleckner N. Sequence non-specific double-strand breaks and interhomolog interactions prior to double-strand break formation at a meiotic recombination hot spot in yeast. EMBO J. 1995;14:5115–5128. doi: 10.1002/j.1460-2075.1995.tb00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zahn-Zabal M, Lehmann E, Kohli J. Hot spots of recombination in fission yeast: Inactivation of the M26 hot spot by deletion of the ade6 promoter and the novel hotspot ura4-aim. Genetics. 1995;140:469–478. doi: 10.1093/genetics/140.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baur M, et al. The meiotic recombination hot spot ura4A in Schizosaccharomyces pombe. Genetics. 2005;169:551–561. doi: 10.1534/genetics.104.033647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuda T, Kugou K, Sasanuma H, Shibata T, Ohta K. Targeted induction of meiotic double-strand breaks reveals chromosomal domain-dependent regulation of Spo11 and interactions among potential sites of meiotic recombination. Nucleic Acids Res. 2008;36:984–997. doi: 10.1093/nar/gkm1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerra-Moreno A, Alves-Rodrigues I, Hidalgo E, Ayté J. Chemical genetic induction of meiosis in Schizosaccharomyces pombe. Cell Cycle. 2012;11:1621–1625. doi: 10.4161/cc.20051. [DOI] [PubMed] [Google Scholar]
- 22.lino Y, Yamamoto M. Negative control for the initiation of meiosis in Schizosaccharomyces pombe. Proc Natl Acad Sci USA. 1985;82:2447–2451. doi: 10.1073/pnas.82.8.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munz P. An analysis of interference in the fission yeast Schizosaccharomyces pombe. Genetics. 1994;137:701–707. doi: 10.1093/genetics/137.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martín-Castellanos C, et al. A large-scale screen in S. pombe identifies seven novel genes required for critical meiotic events. Curr Biol. 2005;15:2056–2062. doi: 10.1016/j.cub.2005.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295:1306–1311. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- 26.Kim KD, Tanizawa H, Iwasaki O, Noma K. Transcription factors mediate condensin recruitment and global chromosomal organization in fission yeast. Nat Genet. 2016;48:1242–1252. doi: 10.1038/ng.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fullwood MJ, Ruan Y. ChIP-based methods for the identification of long-range chromatin interactions. J Cell Biochem. 2009;107:30–39. doi: 10.1002/jcb.22116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood V, et al. The genome sequence of Schizosaccharomyces pombe. Nature. 2002;415:871–880, and erratum (2003) 421:94. doi: 10.1038/nature724. [DOI] [PubMed] [Google Scholar]
- 29.Tanizawa H, Kim KD, Iwasaki O, Noma KI. Architectural alterations of the fission yeast genome during the cell cycle. Nat Struct Mol Biol. 2017;24:965–976. doi: 10.1038/nsmb.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cervantes MD, Farah JA, Smith GR. Meiotic DNA breaks associated with recombination in S. pombe. Mol Cell. 2000;5:883–888. doi: 10.1016/s1097-2765(00)80328-7. [DOI] [PubMed] [Google Scholar]
- 31.Davis L, Rozalén AE, Moreno S, Smith GR, Martin-Castellanos C. Rec25 and Rec27, novel linear-element components, link cohesin to meiotic DNA breakage and recombination. Curr Biol. 2008;18:849–854. doi: 10.1016/j.cub.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Estreicher A, Lorenz A, Loidl J. Mug20, a novel protein associated with linear elements in fission yeast meiosis. Curr Genet. 2012;58:119–127. doi: 10.1007/s00294-012-0369-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, Kim KP, Kleckner NE, Storlazzi A. Meiotic double-strand breaks occur once per pair of (sister) chromatids and, via Mec1/ATR and Tel1/ATM, once per quartet of chromatids. Proc Natl Acad Sci USA. 2011;108:20036–20041. doi: 10.1073/pnas.1117937108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson CM, Oke A, Yam P, Zhuge T, Fung JC. Reduced crossover interference and increased ZMM-independent recombination in the absence of Tel1/ATM. PLoS Genet. 2015;11:e1005478. doi: 10.1371/journal.pgen.1005478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cromie GA, Smith GR. Meiotic recombination in Schizosaccharomyces pombe: A paradigm for genetic and molecular analysis. In: Egel R, Lankenau D-H, editors. Recombination and Meiosis: Models, Means, and Evolution, Genome Dynamics and Stability. Springer; Berlin: 2008. pp. 195–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor AF, et al. Control of RecBCD enzyme activity by DNA binding- and Chi hotspot-dependent conformational changes. J Mol Biol. 2014;426:3479–3499. doi: 10.1016/j.jmb.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kleckner N, et al. A mechanical basis for chromosome function. Proc Natl Acad Sci USA. 2004;101:12592–12597. doi: 10.1073/pnas.0402724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.King JS, Mortimer RK. A polymerization model of chiasma interference and corresponding computer simulation. Genetics. 1990;126:1127–1138. doi: 10.1093/genetics/126.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujitani Y, Mori S, Kobayashi I. A reaction-diffusion model for interference in meiotic crossing over. Genetics. 2002;161:365–372. doi: 10.1093/genetics/161.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hultén MA. On the origin of crossover interference: A chromosome oscillatory movement (COM) model. Mol Cytogenet. 2011;4:10. doi: 10.1186/1755-8166-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foss E, Lande R, Stahl FW, Steinberg CM. Chiasma interference as a function of genetic distance. Genetics. 1993;133:681–691. doi: 10.1093/genetics/133.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nambiar M, Smith GR. Repression of harmful meiotic recombination in centromeric regions. Semin Cell Dev Biol. 2016;54:188–197. doi: 10.1016/j.semcdb.2016.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de los Santos T, et al. The Mus81/Mms4 endonuclease acts independently of double-Holliday junction resolution to promote a distinct subset of crossovers during meiosis in budding yeast. Genetics. 2003;164:81–94. doi: 10.1093/genetics/164.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martini E, Diaz RL, Hunter N, Keeney S. Crossover homeostasis in yeast meiosis. Cell. 2006;126:285–295. doi: 10.1016/j.cell.2006.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang L, et al. Topoisomerase II mediates meiotic crossover interference. Nature. 2014;511:551–556. doi: 10.1038/nature13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Egel-Mitani M, Olson LW, Egel R. Meiosis in Aspergillus nidulans: Another example for lacking synaptonemal complexes in the absence of crossover interference. Hereditas. 1982;97:179–187. doi: 10.1111/j.1601-5223.1982.tb00761.x. [DOI] [PubMed] [Google Scholar]
- 47.Sym M, Roeder GS. Crossover interference is abolished in the absence of a synaptonemal complex protein. Cell. 1994;79:283–292. doi: 10.1016/0092-8674(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 48.Berchowitz LE, Copenhaver GP. Genetic interference: Don’t stand so close to me. Curr Genomics. 2010;11:91–102. doi: 10.2174/138920210790886835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohta K, Wu TC, Lichten M, Shibata T. Competitive inactivation of a double-strand DNA break site involves parallel suppression of meiosis-induced changes in chromatin configuration. Nucleic Acids Res. 1999;27:2175–2180. doi: 10.1093/nar/27.10.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lintern-Moore S, Peters H, Moore GP, Faber M. Follicular development in the infant human ovary. J Reprod Fertil. 1974;39:53–64. doi: 10.1530/jrf.0.0390053. [DOI] [PubMed] [Google Scholar]
- 51.Stahl FW. Genetic recombination: Thinking about it in phage and fungi. In: Heslop-Harrison JS, Flavell RB, editors. The Chromosome. Bios Scientific Publishers; Oxford: 1993. pp. 1–9. [Google Scholar]
- 52.Stahl FW, et al. Does crossover interference count in Saccharomyces cerevisiae? Genetics. 2004;168:35–48. doi: 10.1534/genetics.104.027789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cooper TJ, Garcia V, Neale MJ. Meiotic DSB patterning: A multifaceted process. Cell Cycle. 2016;15:13–21. doi: 10.1080/15384101.2015.1093709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith GR. Genetic analysis of meiotic recombination in Schizosaccharomyces pombe. In: Keeney S, editor. Meiosis, Methods in Molecular Biology. Humana; Totowa, NJ: 2009. pp. 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hyppa RW, Smith GR. Using Schizosaccharomyces pombe meiosis to analyze DNA recombination intermediates. In: Keeney S, editor. Meiosis, Methods in Molecular Biology. Humana; Totowa, NJ: 2009. pp. 235–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cipak L, Polakova S, Hyppa RW, Smith GR, Gregan J. Synchronized fission yeast meiosis using an ATP analog-sensitive Pat1 protein kinase. Nat Protoc. 2014;9:223–231. doi: 10.1038/nprot.2014.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beach DH, Klar AJS. Rearrangements of the transposable mating-type cassettes of fission yeast. EMBO J. 1984;3:603–610. doi: 10.1002/j.1460-2075.1984.tb01855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Milman N, Higuchi E, Smith GR. Meiotic DNA double-strand break repair requires two nucleases, MRN and Ctp1, to produce a single size class of Rec12 (Spo11)-oligonucleotide complexes. Mol Cell Biol. 2009;29:5998–6005. doi: 10.1128/MCB.01127-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hyppa RW, Cromie GA, Smith GR. Indistinguishable landscapes of meiotic DNA breaks in rad50+ and rad50S strains of fission yeast revealed by a novel rad50+ recombination intermediate. PLoS Genet. 2008;4:e1000267. doi: 10.1371/journal.pgen.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.