Abstract
Pesticides are a mixture of chemical substances used to kill pests. Apart from their toxicity to pests, thy affect nontarget organisms. They also generate free radicals producing reactive oxygen species (ROS) which can disturb cellular pathways by inhibiting various enzymes or receptors. Pesticides also induce oxidative DNA damage, DNA adducts, and single or double strand DNA breaks. Various mechanisms of DNA repair deal with such damages and help to maintain cell integrity. Alteration in DNA repair genes modulates the individual's susceptibility towards DNA repair and various diseases. Biological monitoring provides a useful tool for the estimation of genetic risk in populations exposed to pesticides. Large numbers of evidences show that occupational exposure to pesticides in agricultural workers has been associated with an increased incidence of various diseases such as cancer, Parkinson's disease, Alzheimer's disease, reproductive disorders, and birth defects. In this review, we have discussed occupational pesticide exposure, various mechanisms of DNA damage caused by pesticides, DNA repair mechanisms, biomonitoring tools, and various diseases caused by pesticide exposure.
Keywords: DNA repair, genetic DNA damage, health effects, pesticide exposure, reactive oxygen species
INTRODUCTION
Pesticides are a complex mixture of substances used to kill, eliminate, or repel insects, weeds, rodents, fungi, or other organisms. They are being extensively used around the world. Millions of people are exposed to pesticides both environmentally and occupationally. Although exposure to pesticides causes hazardous genetic effects, they are still being used in agriculture.[1] Despite their benefit in increasing crop yield and reduction in postharvest losses in agriculture, their extensive use results in the accumulation of pesticide in food residue, in soil, in runoff water, etc. Pesticides show bio-magnification by entering into the food chain due to high levels of exposure and slow biodegradation.[2] Thus, pesticides pose a threat to public health and the country's economy.
Pesticides exposure is not limited to pests only but poses a serious threat to the surrounding and nontarget species, including humans. In developing countries, like India, occupational pesticide exposure occurs during preparation, mixing, loading, and application of pesticides. Most commonly, pesticide exposure occurs during mixing and loading. Agricultural workers use high dose of pesticide than that recommended by the manufacturer and sprinkle pesticides with bare hands to cut the overhead cost. Agricultural workers use such practices due to unawareness about the adverse health effects of pesticides.[3] Production workers, formulators, sprayers, mixers, loaders, and agricultural farm workers are among the high risk group of individuals who are exposed to pesticides. Long-term exposure to pesticides can cause harm to human health resulting into disturbance in various organs systems of the body such as nervous system, reproductive system, respiratory system, immune system, and cardiovascular system.[2]
In India, a large number of people are exposed occupationally to numerous kinds of pesticides during agricultural practice.[2] Types of pesticides exposure depend upon the class of organisms designed to control (weedicide, fungicide, rodenticide, and herbicide) or by the chemical structure (organochlorine, organophosphates, carbamates, chlorinated hydrocarbons, and dipyridyls). Organophosphates and organochlorines are types of pesticides widely used in agriculture. Round up (Isopropylamine salt of N-(phosphonomethyl) glycine), profenfos (O-4-bromo-2-chlorophenyl-O-ethyl S-propyl phosphorothioate), and dichlorovos (2,2-Dichlorovinyl dimethyl phosphate) are widely used organophosphates in India.
Since pesticides and their residues pose serious concerns to the environment and human health in terms of toxicity, it is important to review the occupational pesticide exposure, DNA damage, and health implications of pesticides on exposed agricultural workers.
PESTICIDE EXPOSURE AND REACTIVE OXIDATIVE DAMAGE
Occupational exposure to pesticides causes production of reactive oxidative species (ROS). These are free radicals with an unpaired electrons such as superoxide anion (O2•−), hydroxyl radical (• OH), as well as nonradical molecules like hydrogen peroxide (H2O2) and singlet oxygen (1 O2). The presence of unpaired electrons makes the molecule highly reactive.[4] Normally, free radicals are neutralized by antioxidants. However, disturbance in the balance between free radicals and antioxidants leads to damage, causing oxidative stress. Oxidative stress disturbs the cell signalling pathway as ROS were considered as the most important messengers in redox signalling. Oxidative stress leads to the development of various diseases such as diabetes, neurodegeneration, schizophrenia, respiratory disorders, aging, cancer, immunodeficiency syndromes, and hypertension.[5]
Pesticide + O2 → Pesticide + O2•−
O2− + O2−→H2O2 →• OH
•OH + DNA → DNA → DNA (adduct), modified base (8-(8)
Reduction of molecular oxygen (O2) by pesticides exposure results in the production of highly reactive ROS, subsequently leading to DNA damage [Figure 1].
Figure 1.
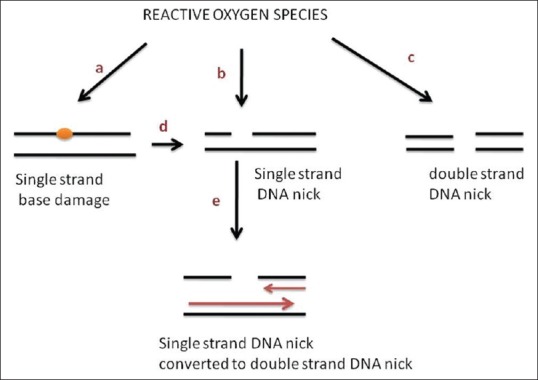
Reactive oxygen species (ROS) can lead to numerous types of DNA damage (a-c), a single-stranded (ss) DNA nick, as in d. A subset of nicks might continue to be present during DNA synthesis, they might be converted to double-stranded (ds) DNA breaks (e). ROS attack can also lead directly to dsDNA breaks (c)
Most cellular components are attacked by ROS causing oxidation and fragmentation of nucleic acids, proteins, and lipids. ROS that flee detoxification causes DNA damage. Damaged DNA causes activation of poly (ADP-ribose) enzyme causing splitting of NAD+, and due to this the level of NAD+ becomes negligible, leading to loss of cell function. DNA damage leads to the formation of various modified bases (including thymine glycol, 5,6-dihydroxycytosine, 8-hydroxyguanine, 2,6-diamino-4-hydroxy-5-formamidopyrimidine), sugar breakdown products such as erythrose, 2-deoxypentonic acid lactone, 2-deoxypentose-4-ulose, base-free sites, and strand breaks.[6,7] Free radicals attack sugars and base moieties, resulting into oxidative DNA damage, including changes or losses of nucleic acids. These ROS species are also formed as byproducts of normal cell metabolism as well as during exposure to mutagens such as ionizing radiation, UVA-radiation or H2O2 and pesticides.[8] Free radicals and ROS are considered the major factor of chronic diseases in humans.[9,10]
MECHANISM OF ACTION OF PESTICIDES
Mechanism of action of pesticide is extremely interesting. It varies due to difference in the uptake and distribution of individual pesticide. There is vast information available about the biological process; however, limited data is available about the toxicology of pesticides on the biological processes. The basic defence mechanism the cell adopts against the pesticides is antioxidant agents. These agents have the potential to donate electron to neutralize free radicals. As discussed earlier, organochlorines and organophosphates are major types of pesticides used in agriculture; apart from these two, pyrethroids, fungicides, and herbicides are also potent pesticides causing adverse effects. Different pesticides have different modes of action.
Pesticides attack nervous system as a mechanism of toxicity because the central nervous system of insects is similar to mammals. These inhibit enzyme action or enzyme production via alteration in the ion channels. Organophosphates (OPs) are a brilliant example of pesticide inducing ion channel dysfunction; these interrupt the passage of acetylcholine. OPs hinder the acetyl-cholinesterase (ACHE) hydrolysis, the enzyme that regulates a neurotransmitter, thus affecting the nervous system.[11] OPs form covalent bond with ACHE through phosphorylation of the serine hydroxyl group of ACHE; resulting in inactivation of ACHE and accumulation of acetylcholine in nervous system. Acetylcholine accumulation affects the receptors on the nervous system via overstimulation of muscarinic and nicotinic receptors.[12] This overstimulation of muscarinic and nicotinic receptors leads to tachycardia, sinus bradycardia, hypertension, hypotension, variation in heart rate, and heart muscle contraction, as well as cyanosis and increased serum levels of creatinine and lactate dehydrogenase[13]
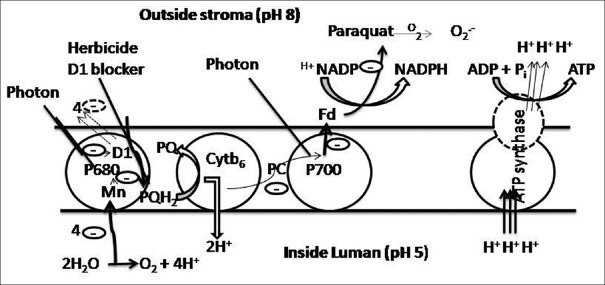
Pesticide can inhibit photosynthesis process. Herbicide, a type of pesticide, binds to (QA) D1 protein and inhibits electron transport by acting as nonreducible analog of plastoquinone and blocks the PQH2 [Figure 2]. Paraquat, a commonly known herbicide interferes with photosynthesis via generation of free radical O2.-. Free radicals disturb the electron gradient system, thus, affecting the transport of electron to the final molecule and hindering the process of energy generation[14]
Pesticides cause inhibition of cell division by affecting the tubulin and microtubules that separates chromosomes during division[15]
Herbicides such as phenylamides inhibit the incorporation of uridine into RNA synthesis by hydrolyzation of the nucleotide base. All three types of RNA polymerases I, II, and III polymerases have different sensitivity toward the toxic effects of these pesticides. RNA polymerase II is extremely sensitive to α-amanitin and organochlorines, whereas RNA polymerase I is less sensitive. However, RNA polymerase III is not affected by α-amanitin. Inhibition of RNA synthesis results in impaired protein synthesis, thus, affecting the enzymatic function
In addition to the production of harmful macromolecules, pesticides affect the molecular and biochemical processes, in which the activity of DNA repair enzymes is hindered and finally reduced DNA repair capacity.[16,17,18]
Figure 2.
The blockage of electron transport chain by paraquat. Paraquat generates free radicals which inhibit electron transfer, thus, hindering electron transport chain to generate energy
Many such evidences have provided the scientists the mechanism of pesticides and development of diseases. It is considered that all the above mechanisms work synergistically rather than individually.
DNA DAMAGE CAUSED BY PESTICIDES
Various chemical agents which are used as pesticides cause DNA damage.[19] DNA damage is an alteration in the chemical structure and sequence of DNA. Pesticide exposure also produces DNA lesions which result in blocking of genome replication and transcription. If these lesions are not repaired or are repaired incorrectly, they lead to mutations or wide-scale genome aberrations threatening cell or organism viability. DNA repair proteins play a crucial role in rectifying the DNA damage and recognize DNA replication errors. Organisms have the ability to repair such DNA damages; however, the efficiency of DNA repair depends not only upon the dose, duration, and type of damaging agent but also upon the species.[20] Significant studies have reported cytogenetic damage in agricultural workers, floriculturists, vineyard cultivators, cotton field workers, and others exposed to different types of pesticides.[21]
REPAIR OF DNA DAMAGE
DNA repair systems play a vital role in protecting the genome from the oxidative and alkylative DNA damage caused by pesticides. Several different DNA repair systems exist, however, the major pathway for repairing such DNA damage is base excision repair (BER). BER pathway specifically targets “nonbulky” base adducts and single strand breaks, such as those induced by ROS, methylation, deamination, and hydroxylation.[22] It removes the damaged base by glycosylase creating an AP site. These lesions are further processed mainly by AP endonucleases, which cleave the DNA backbone generating a 5'-terminal deoxyribose-phosphate moiety. After that, the gap is filled by polymerases in mammalian cells.[23]
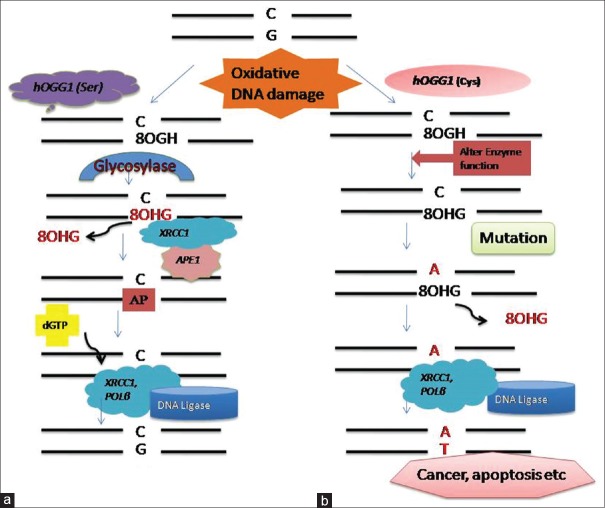
The key players in the BER system are XRCC1, APE1, and OGG1proteins. Oxidative DNA damage caused by pesticides is repaired by hOGG1. It recognizes the damaged oxidative base and recruits other proteins to perform their requisite functions along with mammalian X-ray cross-complementing group 1 (XRCC1). It helps in activating single-strand repair protein complex in vitro and at the site of chromosomal breakage. XRCC1 is a multi-domain protein that functions as the central scaffolding protein.[24] Itinteracts with damaged DNA forming a ternary complex composed of PARP, POL β, and gapped DNA.[25] Interaction with POL β (DNA polymerase β) occur through its N-terminal domain (NTD), DNA ligase III through a C-terminal BRCT-II (breast cancer susceptibility protein-1 homology C-terminal) domain, and with PARP through a unique central BRCT-I domain.[26,27,28] XRCC1 coordinates its activity through protein–protein interactions and two intervening linker regions between these globular domains.[29] The linker region between the NTD and BRCT1 has a region for the binding of other proteins including Apex, OGG1,[30] and PCNA.[31] XRCC1 also stabilizes ligase III and helps in the stimulation of PNK (polynucleotide kinase) activity. DNA glycosylases remove damaged bases by cleavage of the N-glycosylic bond between the base and the deoxyribose moieties of the nucleotide residues creating an AP site. The AP site is then restored by endonuclease and repaired using other strand as template strand by DNA polymerase and ligases.[32] However, polymorphic gene and incorporation of mutation alter DNA repair capacity by producing inefficient enzyme leading increased genotoxicity and accumulation of damaged DNA, which can results into several diseases [Figure 3]. Repair systems are associated with various kinds of syndromes.[33] It has been reported that NER is associated with three syndromes – Cockayne syndrome (point mutation in CSA or CSB gene and is aTCR specific disorder), xeroderma pigmentosum (point mutation in one of seven genes XPA-XPG), and trichothiodystrophy (point mutation). These syndromes are thought to be induced by sun causing skin cancer, impairment of nervous system, dysmyelination, and dwarfism. XPD and XPB mutations also lead to these diseases. In DSBRP, defects in NBS1 gene is linked with Nijmegen breakage syndrome and variation in MRE11 leads to ataxia telangiectasia-like disorder causing cancer predisposition, chromosome instability, and immunodeficiency.[33]
Figure 3.
Repair of oxidative DNA damage caused by pesticides. Oxidative damage is repaired by a set of base excision genes, i.e., hOGG1, APE1, and XRCC1. (a) First the damage base is recognized by OGG1 gene which removes the oxidative base creating an AP site. APE1 repairs the AP site. XRCC1 recruits β-DNA Polymerase to synthesize new base, and the nick is sealed by DNA ligase. (b) However, mutation in these genes modulates the repair capacity. Polymorphic genes alter gene function by producing alternate enzyme leading to incorporation of mutated base which can results in cancer or apoptosis etc
HEALTH EFFECTS OF PESTICIDES
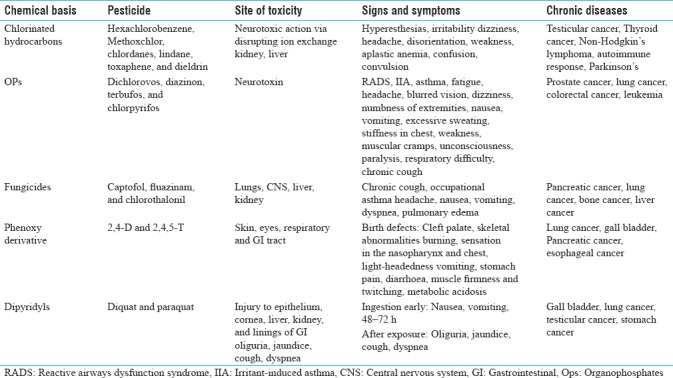
Pesticides target enzymes and systems in pests which may be identical to systems or enzymes in human beings, and therefore, pose risks to human health and the environment. Random and widespread use of pesticides gives rise to various health problems. The health effects caused by the pesticides are not static as pests develop resistance to newly used pesticides and can lead to toxicities. Disturbance in the human system triggers cell-cycle arrest or cell death resulting in acute or chronic health effects. These effects range from an acute allergy to various cancers [Table 1].[34] Chronic diseases induced by pesticide exposure usually show slow development of disease but have long-term effects, which results in increased rate of mortality in the world. The acute effects are headache and nausea while the more serious effects include inflammatory diseases (arthritis, vasculitis, glomerulonephritis), heart stroke, hemochromatosis, pre-eclampsia, and even death.[5,11] Pesticide carcinogenicity is influenced by various factors such as age, sex, smoking history, diet, education level, individual susceptibility, dose and duration of pesticide exposure, as well as absorption pathway of pesticides.[35] Pesticides affect the genetic material directly via stimulation of structural and functional DNA damage, or indirectly disrupting the cellular mitochondria, endoplasmic reticulum, and other factors involved in maintaining cell integrity.
Table 1.
Health effects of pesticide exposure
CARCINOGENESIS
Pesticides through ROS play an important role in the prognosis of cancer.[36] Numerous investigations have proposed the participation of free radicals in mutation, transformations, and carcinogenesis. Free radicals attack the double bond of pyrimidine base, abstracting hydrogen from sugar, resulting in cellular mutagenesis. Pesticide-exposed farmers are at a risk of specific cancers, including leukemia,[37] non-Hodgkin's lymphoma,[38] soft tissue sarcoma,[39] multiple myeloma,[40] stomach and prostate malignancies,[41,42] colorectal, endocrine glands, and brain cancers.[21] Pesticides formulations can mimic hormones resulting in hormonal disruption; pesticides mimic the female hormone estrogen which can block male hormones resulting in hormone-related cancers such as breast and testicular cancers.[43,44] Based on evidences presented by many scientists, pesticide exposure is linked to lympho-hematopoietic cancer by alachlor, colorectal cancer by aldicarb, and breast cancer by chlorpyrifos.[43] Carcinogenicity tests need to be applied to detect the carcinogenic effects of pesticides in both males and females.
CARDIOVASCULAR DISEASES
Pesticides play a role in lipid peroxidation which causes heart-related diseases. Polyunsaturated fatty acids are a major part of lipoproteins in the blood. Oxidation of these lipid components through lipid peroxidation via free radicals produced by pesticides play a key role in atherosclerosis.[45] Lipid peroxidation is a free radical process involving a source of free radical which can act as second messenger or can directly react with other biomolecules leading to biochemical lesions.[46] Chronic exposure to pesticides is linked to an increased risk of coronary artery diseases (CAD).[47]
IMMUNOTOXICITY
Pesticides are potential immunotoxic agents. They affect the immune system in two ways, either by decreasing or increasing the immune response. Immunocompetence, i.e., decreased capacity of immune system to neutralize the foreign particle can cause serious infections as well as increase the risk of cancer while immuno-enhancement, i.e., increased expression of immune response may result into hypersensitivity, autoimmune diseases such as rheumatoid arthritis and autoimmune thyroid diseases.[48] Immune factors response differently on exposure to different kinds of pesticide; for example, chloropyriphos exposure show increased atopy and antibiotic sensitivity, whereas decrease proliferation of CD5 cell in response to mitogens. Similarly, exposure to chlorodane increases hypersensitivity, allergic rhinitis, sinusitis, bronchitis, while decreases the proliferation of CD45RA/T4 cells in response to mitogens, leading to various disease conditions.[49]
BIRTH DEFECTS
Birth defects are functional and structural abnormalities that occur at the time of birth or before birth, leading to physical and mental disorders. Chemicals that cause birth defects are known as teratogen. Pesticides such as DDT and phenoxyherbicides are teratogens that can increase the incidence of congenital disorders.[50,51] Parental exposure to pesticides can also cause developmental toxicity in child such as increased risk of limb anomalies, orofacial cleft/birth marks (trifluralin, 2,4-D), preterm birth, low birth weight, and learning difficulties in child.[52]
REPRODUCTIVE DISORDERS
Pesticides may cause reproductive effects (infertility, malfunctions)[53,54] and developmental problems.[55] Many pesticides such as DDT, chloropyriphos, lindane, malathion, and aldricarb are known to cause reproductive disorders, with long-term effects. Pesticides disrupt the endocrine system causing change in the secretion, binding, transportation, and action of endocrine hormone, leading to adverse effects. Biological differences between male and female hormones are responsible for the difference in susceptibility towards the toxic effects of pesticides.[56] Studies have found a positive association of pesticide exposure with miscarriage (chlorophenoxy, sulfonyl user, and Dicamba), infertility (1,2-dibromo-3-chloropropane and DBCP), reduced sperm count/motility/count (DBCP), preterm delivery (atrazine and 4-(2,4-dichlorophenoxyl) butric acid), still birth (carbamates), and faulty spermatogensis (carbaryl).[57]
NEUROLOGICAL DISORDERS
Pesticides such as organophosphate, cyclodienes, pyrethrins, and organochlorines are known to attack nervous system. Both central and peripheral nervous systems are affected by pesticide exposure resulting into many chronic diseases such as Parkinson's disease, Alzheimer's disease, and amyotrophic lateral sclerosis (ALS). Many studies have found high incidence of such diseases in pesticide-exposed workers.[58,59,60,61] Pesticides affect the nerve signal transmission by altering the function of ion channels. Increased influx of sodium ions or decreased efflux of chloride ions depolarizes the neural membrane resulting into neuronal hyper-excitability.[55]
OTHER DISORDERS
Pesticide exposed workers have been associated with diabetes,[62] chronic respiratory diseases,[63] chronic nephropathesis,[64] autoimmune diseases,[65] hyperglycemia/diabetes,[66] and other many disorders. It is important to biomonitor the population exposed to pesticides as pesticides cause serious health hazards.
BIO-MONITORING
Biomonitoring is referred as the direct assessment of a pesticide, its metabolite, or its product in the biological sample such as blood or urine. Moreover, serum, plasma, amniotic fluid,[67] breast milk,[68] and hair[69] can be used. Blood is considered to be most reliable source for the estimation of parent pesticide whereas urine can be used to measure the metabolites of a pesticide. For example, organophosphates are metabolized into dialkyl phosphate moiety and TCPy (3,56-trichloropyridino) from chlorpyrifos and analyzed by chromatography.[68] In blood, measurement needs to be done within 24 hours of exposure because pesticides get metabolized and eliminated through urine.
To estimate the occupational exposure of pesticides with health effects, various biomarkers of exposure are used.[34] Pesticides can also be detected in the biological sample by analytical techniques such as chromatographic techniques, for example, gas chromatography,[68] high-performance liquid chromatography (HPLC), and mass spectroscopy[70] to precisely determine the urinary metabolites and concentrations of blood body burden of various class of pesticides including organophosphates, carbamates, organochlorines, pyrethroids, etc.[68]
Apart from these, various cytogenetic markers such as micronuclei tests and comet assays are also used to estimate the DNA damage.[71,72,73] These are the most commonly used, reliable, cost-effective, sensitive, realistic, and practical analysis tools for the detection of DNA damage. Micronucleus is referred to as the third nucleus formed during the metaphase of mitosis having an acentric chromosome which does not integrate in the anaphase at opposite poles. However, in comet assay, single strand and double strand breakage is estimated through the comet tail length and intensity. Comets are formed under the influence of electric current in which undamaged DNA nucleus remains intact while damaged DNA show migration of broken strands towards positive side forming a tail around the nucleus and intensity of the tail signifying the number of damage strands.
SUMMARY
Pesticides are chemicals broadly used in agriculture. These chemicals cause adverse effects to the ecosystem as well as human beings. It is known that oxidative stress due to ROS produced by pesticides interfere with DNA and its repair mechanism, leading to mutation and consequently diseases. DNA repair mechanisms help to rectify the DNA damage caused by pesticides. Adverse health effects due to pesticides range from acute to chronic diseases such as cancer, birth defects, neurological defects, reproductive defects, and immunotoxicity. Numerous evidences led scientists to investigate the mechanism by which pesticide exposure cause such diseases. In the present review, pesticides are considered to be the critical risk factor for the development of such diseases. Chiefly, pesticide-exposed agricultural workers are at a higher risk of these diseases because of high exposure of pesticides. This appears to be the major challenge in epidemiology-related studies to elucidate the mechanism of pesticides and their effective prevention strategy. A broad strategy for minimizing disease risk from pesticide includes the study of pesticide characteristics along with the potent active ingredient of the mutagens as well as its mechanism of action. Several epidemiological and experimental studies have strengthened the fact that there is a potential risk of pesticide exposure on human health. Disease risk can be controlled via limiting the pesticide exposure. Complete understanding of the mechanism of specific pesticide will help to prevent the risk of such exposure. Although some attempts have been made in this regard, more comprehensive studies are required to find a preventive approach from the hazardous effects of pesticides.
Meanwhile, health care providers should highlight the significance of minimizing personal exposures to all pesticides to minimize disease risk. The present review recommends the management of pesticide use, regulations about the retail of most hazardous chemicals including pesticides, prevention of misuse of the pesticides and enlightenment programs on safety precautions while handling pesticides such as wearing gloves, mask, and boots, changing clothes, and maintaining proper hygiene. Awareness about the toxic effects of pesticides to the pesticides workers, distributors, and general public will help to reduce the disease risk. Government should strictly act on the exposure of hazardous pesticides especially, which are highly susceptible to women and children. These can be effective approaches for preventing the symptoms and diseases related to occupational pesticide exposure.
Financial support and sponsorship
The work is supported by a grant from Department of Science and Technology – Science and Engineering Research Board (DST-SERB), New Delhi, India (Grant No. YSS/2015/000870).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Griffith J, Duncan RC, Konefal J. Pesticide poisonings reported by Florida citrus fieldworkers. J Environ Sci Health B. 1985;20:701–27. doi: 10.1080/03601238509372505. [DOI] [PubMed] [Google Scholar]
- 2.Nair RS, Paulmurugan R, Wilsanand V. Genotoxic effects of commonly used pesticides of South India in human lymphocytes. Enivromedia. 2005;24:7–12. [Google Scholar]
- 3.Mamane A, Raherison C, Tessier JF, Baldi I, Bouvier G. Environmental exposure to pesticides and respiratory health. Eur Respir Rev. 2015;24:462–73. doi: 10.1183/16000617.00006114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma AD, Mallick AR, Ghosh AK. Free radicals and their role in different clinical conditions: An overview. Int J Pharm Sci Res. 2010;1:185–92. [Google Scholar]
- 5.Pandey AK, Nagpure NS, Trivedi SP, Kumar R, Kushwaha B. Profenofos induced DNA damage in freshwater fish, Channa punctatus (Bloch) using alkaline single cell gel electrophoresis. Mutat Res. 2011;726:209–14. doi: 10.1016/j.mrgentox.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H. Free radical-induced damage to DNA: Mechanisms and measurement. Free Radic Biol Med. 2002;32:1102–15. doi: 10.1016/s0891-5849(02)00826-2. [DOI] [PubMed] [Google Scholar]
- 7.Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA damage and disease: Induction, repair and significance. Mutat Res. 2004;567:1–61. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Lindahl T, Barnes DE. Repair of endogenous DNA damage. Cold Spring Harb Symp Quant Biol. 2000;65:127–33. doi: 10.1101/sqb.2000.65.127. [DOI] [PubMed] [Google Scholar]
- 9.Lioi MB, Scarfì MR, Santoro A, Barbieri R, Zeni O, Di Berardino D, et al. Genotoxicity and oxidative stress induced by pesticide exposure in bovine lymphocyte cultures in vitro. Mutat Res. 1998;403:13–20. doi: 10.1016/s0027-5107(98)00010-4. [DOI] [PubMed] [Google Scholar]
- 10.Paz-y-Miño C, Bustamante G, Sánchez ME, Leone PE. Cytogenetic monitoring in a population occupationally exposed to pesticides in Ecuador. Environ Health Perspect. 2002;110:1077–80. doi: 10.1289/ehp.110-1241062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yadav AS, Sehrawat G. Evaluation of genetic damage in farmers exposed to pesticide mixtures. Int J Hum Genet. 2011;11:105–9. [Google Scholar]
- 12.Kamanyire R, Karalliedde L. Organophosphate toxicity and occupational exposure. Occup Med (Lond) 2004;54:69–75. doi: 10.1093/occmed/kqh018. [DOI] [PubMed] [Google Scholar]
- 13.Saadeh AM, Farsakh NA, al-Ali MK. Cardiac manifestations of acute carbamate and organophosphate poisoning. Heart. 1997;77:461–4. doi: 10.1136/hrt.77.5.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laskay G, Lokas A. Counteracting the effects of paraquat on photosynthesis by chlorogenic acid. Acta Biol Szeged. 2011;55:101–3. [Google Scholar]
- 15.Stenersen J. Chemical Pesticides: Mode of Action and Toxicology. Boca Raton, Florida: CRC Press; 2004. [Google Scholar]
- 16.Martignoni E, Blandini F, Godi L, Desideri S, Pacchetti C, Mancini F, et al. Peripheral markers of oxidative stress in Parkinson's disease. The role of L-DOPA. Free Radic Biol Med. 1999;27:428–37. doi: 10.1016/s0891-5849(99)00075-1. [DOI] [PubMed] [Google Scholar]
- 17.Shimura-Miura H, Hattori N, Kang D, Miyako K, Nakabeppu Y, Mizuno Y, et al. Increased 8-oxo-dGTPase in the mitochondria of substantia Nigral neurons in Parkinson's disease. Ann Neurol. 1999;46:920–4. [PubMed] [Google Scholar]
- 18.Sofic E, Lange KW, Jellinger K, Riederer P. Reduced and oxidized glutathione in the substantia Nigra of patients with Parkinson's disease. Neurosci Lett. 1992;142:128–30. doi: 10.1016/0304-3940(92)90355-b. [DOI] [PubMed] [Google Scholar]
- 19.Islas-González K, González-Horta C, Sánchez-Ramírez B, Reyes-Aragón E, Levario-Carrillo M. In vitro assessment of the genotoxicity of ethyl paraoxon in newborns and adults. Hum Exp Toxicol. 2005;24:319–24. doi: 10.1191/0960327105ht534oa. [DOI] [PubMed] [Google Scholar]
- 20.Hart RW, Hall KY, Daniel FB. DNA repair and mutagenesis in mammalian cells. Photochem Photobiol. 1978;28:131–55. doi: 10.1111/j.1751-1097.1978.tb07689.x. [DOI] [PubMed] [Google Scholar]
- 21.Bolognesi C, Creus A, Ostrosky-Wegman P, Marcos R. Micronuclei and pesticide exposure. Mutagenesis. 2011;26:19–26. doi: 10.1093/mutage/geq070. [DOI] [PubMed] [Google Scholar]
- 22.Laws ER., Jr Evidence of antitumorigenic effects of DDT. Arch Environ Health. 1971;23:181–4. doi: 10.1080/00039896.1971.10665983. [DOI] [PubMed] [Google Scholar]
- 23.Boesch P, Weber-Lotfi F, Ibrahim N, Tarasenko V, Cosset A, Paulus F, et al. DNA repair in organelles: Pathways, organization, regulation, relevance in disease and aging. Biochim Biophys Acta. 2011;1813:186–200. doi: 10.1016/j.bbamcr.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Loizou JI, El-Khamisy SF, Zlatanou A, Moore DJ, Chan DW, Qin J, et al. The protein kinase CK2 facilitates repair of chromosomal DNA single-strand breaks. Cell. 2004;117:17–28. doi: 10.1016/s0092-8674(04)00206-5. [DOI] [PubMed] [Google Scholar]
- 25.Caldecott KW. XRCC1 and DNA strand break repair. DNA Repair (Amst) 2003;2:955–69. doi: 10.1016/s1568-7864(03)00118-6. [DOI] [PubMed] [Google Scholar]
- 26.Dianova II, Sleeth KM, Allinson SL, Parsons JL, Breslin C, Caldecott KW, et al. XRCC1-DNA polymerase beta interaction is required for efficient base excision repair. Nucleic Acids Res. 2004;32:2550–5. doi: 10.1093/nar/gkh567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor RM, Thistlethwaite A, Caldecott KW. Central role for the XRCC1 BRCT I domain in mammalian DNA single-strand break repair. Mol Cell Biol. 2002;22:2556–63. doi: 10.1128/MCB.22.8.2556-2563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Moréra S, Bates PA, Whitehead PC, Coffer AI, Hainbucher K, et al. Structure of an XRCC1 BRCT domain: A new protein-protein interaction module. EMBO J. 1998;17:6404–11. doi: 10.1093/emboj/17.21.6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Callebaut I, Labesse G, Durand P, Poupon A, Canard L, Chomilier J, et al. Deciphering protein sequence information through hydrophobic cluster analysis (HCA): Current status and perspectives. Cell Mol Life Sci. 1997;53:621–45. doi: 10.1007/s000180050082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marsin S, Vidal AE, Sossou M, Ménissier-de Murcia J, Le Page F, Boiteux S, et al. Role of XRCC1 in the coordination and stimulation of oxidative DNA damage repair initiated by the DNA glycosylase hOGG1. J Biol Chem. 2003;278:44068–74. doi: 10.1074/jbc.M306160200. [DOI] [PubMed] [Google Scholar]
- 31.Fan J, Otterlei M, Wong HK, Tomkinson AE, Wilson DM., 3rd XRCC1 co-localizes and physically interacts with PCNA. Nucleic Acids Res. 2004;32:2193–201. doi: 10.1093/nar/gkh556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goode EL, Ulrich CM, Potter JD. Polymorphisms in DNA repair genes and associations with cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11:1513–30. [PubMed] [Google Scholar]
- 33.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–74. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 34.Alavanja MC, Bonner MR. Occupational pesticide exposures and cancer risk: A review. J Toxicol Environ Health B Crit Rev. 2012;15:238–63. doi: 10.1080/10937404.2012.632358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aprea MC. Environmental and biological monitoring in the estimation of absorbed doses of pesticides. Toxicol Lett. 2012;210:110–8. doi: 10.1016/j.toxlet.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Purdue MP, Lan Q, Kricker A, Vajdic CM, Rothman N, Armstrong BK, et al. Vitamin D receptor gene polymorphisms and risk of non-Hodgkin's lymphoma. Haematologica. 2007;92:1145–6. doi: 10.3324/haematol.11053. [DOI] [PubMed] [Google Scholar]
- 37.Daniels JL, Olshan AF, Savitz DA. Pesticides and childhood cancers. Environ Health Perspect. 1997;105:1068–77. doi: 10.1289/ehp.971051068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spinelli JJ, Ng CH, Weber JP, Connors JM, Gascoyne RD, Lai AS, et al. Organochlorines and risk of non-Hodgkin lymphoma. Int J Cancer. 2007;121:2767–75. doi: 10.1002/ijc.23005. [DOI] [PubMed] [Google Scholar]
- 39.Kogevinas M, Kauppinen T, Winkelmann R, Becher H, Bertazzi PA, Bueno-de-Mesquita HB, et al. Soft tissue sarcoma and non-Hodgkin's lymphoma in workers exposed to phenoxy herbicides, chlorophenols, and dioxins: Two nested case-control studies. Epidemiology. 1995;6:396–402. [PubMed] [Google Scholar]
- 40.Dennis LK, Lynch CF, Sandler DP, Alavanja MC. Pesticide use and cutaneous melanoma in pesticide applicators in the agricultural heath study. Environ Health Perspect. 2010;118:812–7. doi: 10.1289/ehp.0901518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bolognesi C. Genotoxicity of pesticides: A review of human biomonitoring studies. Mutat Res. 2003;543:251–72. doi: 10.1016/s1383-5742(03)00015-2. [DOI] [PubMed] [Google Scholar]
- 42.Band PR, Abanto Z, Bert J, Lang B, Fang R, Gallagher RP, et al. Prostate cancer risk and exposure to pesticides in British Columbia farmers. Prostate. 2011;71:168–83. doi: 10.1002/pros.21232. [DOI] [PubMed] [Google Scholar]
- 43.Ibarluzea JM, Fernández MF, Santa-Marina L, Olea-Serrano MF, Rivas AM, Aurrekoetxea JJ, et al. Breast cancer risk and the combined effect of environmental estrogens. Cancer Causes Control. 2004;15:591–600. doi: 10.1023/B:CACO.0000036167.51236.86. [DOI] [PubMed] [Google Scholar]
- 44.Giannandrea F. Long-term pesticide exposure and the risk of testicular cancer. Occup Med (Lond) 2012;62:309–10. doi: 10.1093/occmed/kqs040. [DOI] [PubMed] [Google Scholar]
- 45.Fokina KV, Bezuglyĭ VP. Role of chlora and organophosphate pesticide compleses in the etiology of cerebral atherosclerosis. Vrach Delo. 1978;4:19–23. [PubMed] [Google Scholar]
- 46.Akhgari M, Abdollahi M, Kebryaeezadeh A, Hosseini R, Sabzevari O. Biochemical evidence for free radical-induced lipid peroxidation as a mechanism for subchronic toxicity of malathion in blood and liver of rats. Hum Exp Toxicol. 2003;22:205–11. doi: 10.1191/0960327103ht346oa. [DOI] [PubMed] [Google Scholar]
- 47.Zamzila AN, Aminu I, Niza S, Razman MR, Hadi MA. Chronic organophosphate pesticide exposure and coronary artery disease: Finding a bridge. IIUM Research, Invention and Innovation Exhibition. 2011 [Google Scholar]
- 48.Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223–43. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 49.Corsini E, Liesivuori J, Vergieva T, Van Loveren H, Colosio C. Effects of pesticide exposure on the human immune system. Hum Exp Toxicol. 2008;27:671–80. doi: 10.1177/0960327108094509. [DOI] [PubMed] [Google Scholar]
- 50.Hamlin HJ, Guillette LJ., Jr Birth defects in wildlife: The role of environmental contaminants as inducers of reproductive and developmental dysfunction. Syst Biol Reprod Med. 2010;56:113–21. doi: 10.3109/19396360903244598. [DOI] [PubMed] [Google Scholar]
- 51.Ngo AD, Taylor R, Roberts CL, Nguyen TV. Association between agent orange and birth defects: Systematic review and meta-analysis. Int J Epidemiol. 2006;35:1220–30. doi: 10.1093/ije/dyl038. [DOI] [PubMed] [Google Scholar]
- 52.Association of Farmworker Opportunity Programs. [Last accessed on 2018 Feb 20]. Available from: http://www.afop.org/health-safety/pesticide-safety/pregnancy-pesticide .
- 53.Hanke W, Jurewicz J. The risk of adverse reproductive and developmental disorders due to occupational pesticide exposure: An overview of current epidemiological evidence. Int J Occup Med Environ Health. 2004;17:223–43. [PubMed] [Google Scholar]
- 54.Lauria L, Settimi L, Spinelli A, Figà-Talamanca I. Exposure to pesticides and time to pregnancy among female greenhouse workers. Reprod Toxicol. 2006;22:425–30. doi: 10.1016/j.reprotox.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 55.Baldi I, Lebailly P, Mohammed-Brahim B, Letenneur L, Dartigues JF, Brochard P, et al. Neurodegenerative diseases and exposure to pesticides in the elderly. Am J Epidemiol. 2003;157:409–14. doi: 10.1093/aje/kwf216. [DOI] [PubMed] [Google Scholar]
- 56.García AM. Pesticide exposure and women's health. Am J Ind Med. 2003;44:584–94. doi: 10.1002/ajim.10256. [DOI] [PubMed] [Google Scholar]
- 57.Kumar S. Occupational exposure associated with reproductive dysfunction. J Occup Health. 2004;46:1–9. doi: 10.1539/joh.46.1. [DOI] [PubMed] [Google Scholar]
- 58.Ascherio A, Chen H, Weisskopf MG, O'Reilly E, McCullough ML, Calle EE, et al. Pesticide exposure and risk for Parkinson's disease. Ann Neurol. 2006;60:197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- 59.Parrón T, Requena M, Hernández AF, Alarcón R. Association between environmental exposure to pesticides and neurodegenerative diseases. Toxicol Appl Pharmacol. 2011;256:379–85. doi: 10.1016/j.taap.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 60.Bonvicini F, Marcello N, Mandrioli J, Pietrini V, Vinceti M. Exposure to pesticides and risk of amyotrophic lateral sclerosis: A population-based case-control study. Ann Ist Super Sanita. 2010;46:284–7. doi: 10.4415/ANN_10_03_10. [DOI] [PubMed] [Google Scholar]
- 61.Vinceti M, Bottecchi I, Fan A, Finkelstein Y, Mandrioli J. Are environmental exposures to selenium, heavy metals, and pesticides risk factors for amyotrophic lateral sclerosis? Rev Environ Health. 2012;27:19–41. doi: 10.1515/reveh-2012-0002. [DOI] [PubMed] [Google Scholar]
- 62.Airaksinen R, Rantakokko P, Eriksson JG, Blomstedt P, Kajantie E, Kiviranta H, et al. Association between type 2 diabetes and exposure to persistent organic pollutants. Diabetes Care. 2011;34:1972–9. doi: 10.2337/dc10-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hernández AF, Parrón T, Alarcón R. Pesticides and asthma. Curr Opin Allergy Clin Immunol. 2011;11:90–6. doi: 10.1097/ACI.0b013e3283445939. [DOI] [PubMed] [Google Scholar]
- 64.Siddharth M, Datta SK, Bansal S, Mustafa M, Banerjee BD, Kalra OP, et al. Study on organochlorine pesticide levels in chronic kidney disease patients: Association with estimated glomerular filtration rate and oxidative stress. J Biochem Mol Toxicol. 2012;26:241–7. doi: 10.1002/jbt.21416. [DOI] [PubMed] [Google Scholar]
- 65.Parks CG, Walitt BT, Pettinger M, Chen JC, de Roos AJ, Hunt J, et al. Insecticide use and risk of rheumatoid arthritis and systemic lupus erythematosus in the women's health initiative observational study. Arthritis Care Res (Hoboken) 2011;63:184–94. doi: 10.1002/acr.20335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abdollahi M, Donyavi M, Pournourmohammadi S, Saadat M. Hyperglycemia associated with increased hepatic glycogen phosphorylase and phosphoenolpyruvate carboxykinase in rats following subchronic exposure to Malathion. Comp Biochem Physiol C Toxicol Pharmacol. 2004;137:343–7. doi: 10.1016/j.cca.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 67.Koutroulakis D, Sifakis S, Tzatzarakis MN, Alegakis AK, Theodoropoulou E, Kavvalakis MP, et al. Dialkyl phosphates in amniotic fluid as a biomarker of fetal exposure to organophosphates in Crete, Greece; association with fetal growth. Reprod Toxicol. 2014;46:98–105. doi: 10.1016/j.reprotox.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 68.Barr DB. Biomonitoring of exposure to pesticides. J Chem Health Safety. 2008;6:20–9. [Google Scholar]
- 69.Smith-Baker C, Saleh MA. Hair as a marker for pesticides exposure. J Environ Sci Health B. 2011;46:648–53. doi: 10.1080/03601234.2012.597701. [DOI] [PubMed] [Google Scholar]
- 70.Duca RC, Salquebre G, Hardy E, Appenzeller BM. Comparison of solid phase- and liquid/liquid-extraction for the purification of hair extract prior to multi-class pesticides analysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;955-956:98–107. doi: 10.1016/j.jchromb.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 71.Fairbairn DW, Olive PL, O'Neill KL. The comet assay: A comprehensive review. Mutat Res. 1995;339:37–59. doi: 10.1016/0165-1110(94)00013-3. [DOI] [PubMed] [Google Scholar]
- 72.Grover P, Danadevi K, Mahboob M, Rozati R, Banu BS, Rahman MF, et al. Evaluation of genetic damage in workers employed in pesticide production utilizing the comet assay. Mutagenesis. 2003;18:201–5. doi: 10.1093/mutage/18.2.201. [DOI] [PubMed] [Google Scholar]
- 73.Møller P, Knudsen LE, Loft S, Wallin H. The comet assay as a rapid test in biomonitoring occupational exposure to DNA-damaging agents and effect of confounding factors. Cancer Epidemiol Biomarkers Prev. 2000;9:1005–15. [PubMed] [Google Scholar]